ABSTRACT
Nuclear movement and positioning play a role in developmental processes throughout life. Nuclear movement and positioning are mediated primarily by linker of nucleoskeleton and cytoskeleton (LINC) complexes. LINC complexes are comprised of the inner nuclear membrane SUN proteins and the outer nuclear membrane (ONM) KASH proteins. In Arabidopsis pollen tubes, the vegetative nucleus (VN) maintains a fixed distance from the pollen tube tip during growth, and the VN precedes the sperm cells (SCs). In pollen tubes of wit12 and wifi, mutants deficient in the ONM component of a plant LINC complex, the SCs precede the VN during pollen tube growth and the fixed VN distance from the tip is lost. Subsequently, pollen tubes frequently fail to burst upon reception. In this study, we sought to determine if the pollen tube reception defect observed in wit12 and wifi is due to decreased sensitivity to reactive oxygen species (ROS). Here, we show that wit12 and wifi are hyposensitive to exogenous H2O2, and that this hyposensitivity is correlated with decreased proximity of the VN to the pollen tube tip. Additionally, we report the first instance of nuclear Ca2+ peaks in growing pollen tubes, which are disrupted in the wit12 mutant. In the wit12 mutant, nuclear Ca2+ peaks are reduced in response to exogenous ROS, but these peaks are not correlated with pollen tube burst. This study finds that VN proximity to the pollen tube tip is required for both response to exogenous ROS, as well as internal nuclear Ca2+ fluctuations.
KEYWORDS: Male fertility, pollen tube termination, nuclear envelope, LINC complex, nuclear calcium, reactive oxygen species
Introduction
Nuclear movement and positioning are vital for cellular processes and development in opisthokonts, for example for p-cell and hypodermal cell development in C. elegans and eye disc development in D. melanogaster [reviewed in 1, 2]. In vertebrates, nuclear movement and positioning are required for myofiber development and formation of neuromuscular junctions [1]. Nuclear movement in opisthokonts is primarily mediated by two groups of proteins: the outer nuclear membrane (ONM) Klarsicht/ANC-1/Syne Homology (KASH) proteins, and the inner nuclear membrane (INM) Sad1/Unc-84 (SUN) proteins [2]. SUN and KASH proteins bind in the nuclear envelope (NE) lumen and form the core of Linker of Nucleoskeleton and Cytoskeleton (LINC) complexes [1]. KASH proteins, which have variable cytoplasmic domains, interact directly or indirectly with a variety of cytoskeletal elements, while SUN proteins primarily interact with Lamin A/C [2].
While plants have homologs of SUN proteins, they lack homologs of opisthokont KASH proteins. Approximately 40 amino acids of the C-terminus of animal KASH proteins extend into the NE lumen and terminate in a consensus ‘PPPX’ motif [2]. Plant KASH proteins were first identified in Arabidopsis, through identification of a transmembrane domain, a shorter luminal tail domain (9–15 aa), and a similar characteristic C-terminal tail, terminating in a consensus ‘XVPT’ motif [3,4]. Homologs of Arabidopsis SUN and KASH proteins have subsequently been found in a variety of land plant species [5] and functionally investigated in Z. mays and M. truncatula [4,6,7,8]. The first identified plant KASH proteins, WPP-Interacting Proteins (WIPs) 1, 2 and 3, along with their ONM interaction partners the WPP-Interacting Tail Anchored Proteins 1 and 2 (WITs), form a LINC complex with Arabidopsis SUN1 and SUN2 [3]. This complex binds a motor protein, Myosin XI–i, and is involved in moving nuclei in root and leaf cells [9]. In pollen, the WIT-WIP-SUN complex is involved in male fertility. The WIP triple mutant wip123 and WIT double mutant wit12 have a random seed-loss phenotype [4]. Loss of either the WIP or WIT protein family, as well as loss of both, results in about 50% reduction in seed set [10]. While pollen tube growth is not significantly altered in these mutants, the distance of the pollen vegetative nucleus (VN) from the growing pollen tube tip is increased [10]. The resulting mutant pollen tubes have defects in pollen tube termination, with pollen tube overgrowth at the site of ovules frequently observed [10].
During pollen germination and subsequent pollen tube growth in many angiosperm species, the two sperm cells (SCs) are physically connected to the VN, and collectively named the male germ unit (MGU) [11]. As the pollen tube grows, the MGU maintains a fixed distance from the advancing pollen tube tip, with the VN leading the SCs [12]. The fact that the VN and SCs migrate as a unit has been proposed to be important for efficient movement of the SCs to the ovule [13,15]. A mutant deficient in two helix-loop-helix transcription factors, drop1 drop2, transports only the VN and is phenotypically near normal, including pollen tube rupture after entering the ovule [16]. This suggests that reception and termination signaling can proceed in the absence of the SCs.
In contrast, wip and wit mutant pollen tubes that transport SCs, but lead to a partial loss of the VN at the pollen tube tip, have defects in pollen tube termination [10]. Upon mutant pollen germination, the SCs emerge first, followed by the VN, a reversal in MGU order compared to wildtype [10]. Wip and wit mutant pollen tubes grow at normal rates, but the distance of the VN to the pollen tube tip increases over time [10]. Frequently, the VN is not observed at the pollen tube tip, and the corresponding pollen tubes either stall at the entrance to an ovule or continue to grow past the synergids but remain intact [10,17]. SUN1 and SUN2 mutant combinations of varying severity are defective in male gametophyte development [18] or show reduced seed set [19]. A combination of knockdown, knockout, and a dominant-negative approach presented the same MGU order defects seen in wip and wit mutants, indicating a role for SUNs in this process [19]. How the requirement for the WIT-WIP-SUN complex, and, by extension, the tip-located VN, relates to the known signaling steps of pollen tube reception and termination is not known.
Pollen tube reception at the ovule involves intricate interactions between the pollen tube and the synergid cells, leading to pollen tube growth arrest and burst, and the release of the SCs [20,21]. Several signaling components of this step on the female side have been identified (for review see [22]). Loss of function mutants of the synergid cell surface receptor kinase FERONIA lead to failed WT pollen tube burst upon arrival at a fer mutant ovule, and subsequent pollen tube overgrowth [23]. FERONIA is required for an increase in reactive oxygen species (ROS) production by the synergid cells upon pollen tube arrival and a synergid calcium (Ca2+) fluctuation that precedes a corresponding Ca2+ increase in the pollen tube tip prior to burst [24,25]. In vitro-induction of ROS leads to pollen tube growth arrest and rupture in a Ca2+-dependent manner, suggesting pollen tube-synergid crosstalk involves a synergid-triggered Ca2+-ROS signal that leads to pollen tube termination and SC release [26].
How signals from the synergids are perceived by the pollen tube remains an open question. Two FERONIA-related proteins, ANXUR1 and ANXUR2, are specifically expressed in pollen, and the double mutant shows premature growth arrest and burst, but whether they are involved in signal reception by the pollen tube is not known [27]. A second pair of pollen tube receptors (BUPS1 and BUPS2) has a similar function, protecting the pollen tube from premature burst. BUPS1 and BUPS2 bind both pollen-expressed and female-expressed Rapid Alkalinization Factor (RALF) peptide ligands, and interaction with female-expressed RALF34 releases the protection from burst by BUPS1 and BUPS2 [28]. Thus, an interplay between ovule-derived and pollen-derived peptide ligands might enable the pollen tube to respond to the synergid environment created by FERONIA and related pathways [28]. The pollen tube response pathway downstream of plasma membrane-associated signaling is less well understood.
Here, we hypothesized that the WIT-WIP-SUN LINC complex is required for ROS-induced pollen tube rupture. We report a decrease in Ca2+-dependent ROS-induced pollen tube rupture of WIP and WIT locus mutants grown semi-in vivo. The hyposensitivity to ROS correlates with an increased distance of the VN from the pollen tube tip. In addition, we provide a first report of nuclear Ca2+ fluctuations in a gametophytic nucleus and show that the patterns during growth and ROS-mediated pollen tube burst are altered in the mutants, dependent on VN positioning.
Materials and methods
Plant materials
Arabidopsis thaliana (Columbia-0 ecotype) was germinated on Murashige and Skoog medium plates (Caisson Laboratories) containing 1% sucrose under constant light. Plants at the two-leaf stage were transplanted to soil and grown at an average temperature of 22–23°C under a 16-hour light/8-hour dark regime. The wit1-1 (GABI-Kat 470E06) wit2-1 (SALK_127765) (wit12) double null mutant was reported by [29] and the quintuple null mutant wip1-1 (SAIL_390_A08) wip2-1 (SALK_052226) wip3-1 (GABI-Kat 459H07) wit1-1 wit2-1 (‘wifi’) mutant was reported by [3]. Heterozygous male sterility-1 (ms-1) was obtained from the Arabidopsis Biological Resource Center (https://abrc.osu.edu/).
Cloning
All primers used in cloning and construct generation are outlined in Supplemental Table 1. The pollen-specific promoter LAT52 was cloned from the LAT52pro::GFP construct in the binary plasmid pMDC107 as previously reported [10]. Restriction sites for enzymes SacI and SpeI were added to the 5ʹ and 3ʹ ends, respectively. The amplified fragment was digested with the appropriate restriction enzymes. The LAT52 promoter fragment was isolated, purified with the QIAquik PCR Purification kit (Qiagen) and subsequently ligated into a pH2GW7 vector [30].
The Yellow Cameleon 3.6 (YC3.6) calcium (Ca2+) sensor N-terminally tagged with the SV40 nuclear localization signal (NLS) was cloned from a UBQ10pro::NLS-YC3.6 [31]. The R-GECO1 Ca2+ sensor was amplified from CMVpro::R-GECO1 [32]. NLS-YC3.6 and R-GECO1 were cloned into pENTR/D-TOPO vectors (Life Technologies) and then moved to LAT52pro::pH2GW7 via the Gateway® LR reaction (Life Technologies).
Generation of transgenic plants
Binary vectors containing Ca2+ sensors were transformed into either Agrobacterium tumefaciens strains ABI or GV3101 by triparental mating [33]. Arabidopsis plants were transformed using the Agrobacterium-mediated floral dip method [34]. The Col-0 ecotype (WT), wit12 and wifi backgrounds were used for the transformation. Transgenic plants were isolated on MS plates supplemented with using 30 µg/mL hygromycin, and positive transformants were confirmed by confocal microscopy. One homozygous T2 transgenic line for each background was used for all assays described here.
Semi in-vivo pollen germination and ROS-induced pollen tube rupture
Pollen germination media (PGM) containing 5 mM KCl, 5 mM CaCl2, 1 mM Ca(NO3)2, 1 mM MgSO4, 10% sucrose, 0.01% boric acid, pH 7.5 [35] was heated with 0.4% (w/v) agarose, pipetted onto a glass slide, and allowed to solidify before use. Anthers at time of anthesis were used to pollinate stigmas of ms-1 flowers at developmental stage 14 [10]. Two hours after pollination, stigmas were excised and placed horizontally onto the solid PGM agar pad. The stigmas were incubated for an additional 3 or 5 hours in a humidity chamber [35]. Elongating pollen tubes were imaged using a 4 x objective with 5 times digital zoom. At time of imaging, pollen tubes were treated with 10 µL of liquid PGM (control) or with 10 µL of liquid PGM containing 6 mM H2O2. Immediately following treatment, single plane images of pollen tubes were acquired every 10 seconds for 10 minutes with confocal microscopy (Eclipse C90i; Nikon). In addition, after this 10-minute time course, images were acquired at different focal planes to ensure that all pollen tubes could be identified and counted. To quantify the percentage of rupture events, the total number of intact and germinated pollen tubes were tabulated prior to and 10 minutes after treatment. The number of pollen tube discharge events was then counted. For the Ca2+ depletion experiments, pollen was germinated on low-Ca2+ PGM medium (5 mM KCl, 100 µM CaCl2, 1 mM MgSO4, 10% sucrose, 0.01% boric acid, pH 7.5). Pollen tubes were then treated with 10 µL of low-Ca2+ PGM containing 100 µM GdCl3 and after a 10-minute incubation 10 µL of low-Ca2+ PGM with 12 mM H2O2 was added.
Time to rupture quantification
The time to rupture was quantified using data collected in Figure 1d. For each experiment, the 10-minute videos were split into 1-minute intervals and the number of pollen tubes that burst during each interval was recorded. That number was then divided by the total number of pollen tubes that ruptured over the course of the 10-minute video and represented as percent. The average percent rupture for each time point was plotted.
Figure 1.
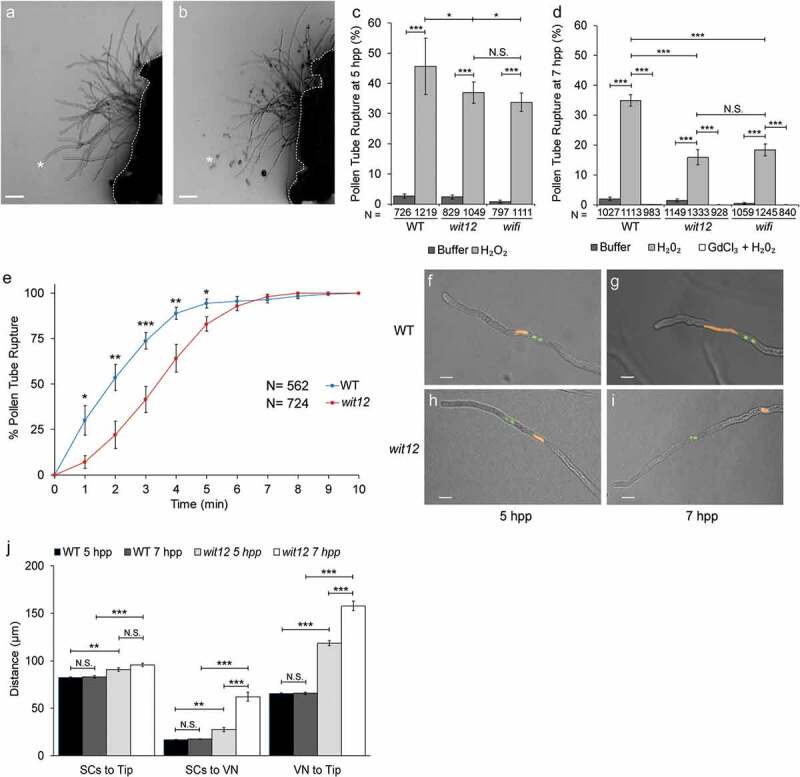
Ca2+-dependent ROS-induced pollen tube burst correlates with nuclear position.
(a–b), Semi-in vivo germinated WT pollen expressing Lat52pro::R-GECO1 Ca2+ sensor. Size bars = 50 µm. (a), Pollen tubes prior to incubation with the ROS generating compound H2O2. (b), Pollen tubes following incubation with H2O2. Several tubes have ruptured as indicated by cytoplasm outside the pollen tube. The asterisks in a and b mark the same pollen tube before and after rupture. The white dotted line marks the base of the stigma. (c–d), ROS induced pollen tube rupture in semi-in vivo germinated pollen at 5 hours (c) and 7 hours (d) post pollination (hpp). Values represent the average number of ruptured pollen tubes observed when treated with buffer, H2O2, or H2O2 and a Ca2+ channel inhibitor, gadolinium(III) chloride (GdCl3 + H2O2). Bars are standard error. N equals total number of pollen tubes. * P ≤ 0.05; **P ≤ 0.01; *** P ≤ 0.001 for Student’s t-test; N.S. indicates no significance by Student’s t-test. (e), The time to ROS induced pollen tube rupture in semi-in vivo germinated WT and wit12 pollen at 7 hpp. Bars are standard error. N equals total number of pollen tubes. * P ≤ 0.05; **P ≤ 0.01; *** P ≤ 0.001 for Student’s t-test. (f–i), The localization of the VN (orange) and SCs (green) was examined in elongating pollen tubes at 5 and 7 hpp. The VN and SCs were visualized using UBQ10pro::NLS-mCherry and MGH3pro::GFP respectively. The position of the VN tip in WT remains close to the pollen tube tip at 5 (f) and 7 hpp (g). The VN in wit12 pollen increases in distance from the pollen tube tip over time. The nucleus is closer at 5 hpp (h) than at 7 hpp (i). Scale bars = 10 µm. (j), The position of the SCs and VN relative to the tip and distance between the SCs and VN was quantified for WT and wit12 pollen tubes at both time points. N = 150 pollen tubes. Bars are standard error. **P ≤ 0.01; *** P ≤ 0.001 for Student’s t-test.
Measurement of positions of male germ unit components within the pollen tube
WT and wit12 lines expressing both the VN marker UBQ10pro::NLS-mCherry and SC marker MGH3pro::GFP were germinated using the semi-in vivo method on PGM agar pads, as described above. Elongated pollen tubes were imaged using a 40X water immersion objective. Images of pollen at 5 and 7 hpp were acquired at several different focal planes to ensure the MGU and pollen tube tip were in focus. Distances from the relative center of the sperm cells (SCs) and vegetative nucleus (VN) to the tip of the pollen tube were measured using the NIS Elements analysis software (Nikon).
Ca2+ sensor imaging
Pollen expressing either the R-GECO1 Ca2+ sensor or the NLS-YC3.6 nuclear Ca2+ sensor was germinated as previously described [36]. A square was drawn onto a glass slide with a grease pencil (Staples). An isolated, pollinated stigma was placed in the center of the square. The square was filled with liquid PGM until the media began to mound. The slide was placed in a high humidity chamber for 5 hours to allow pollen tube elongation [36,37]. Individual elongating pollen tubes were imaged under a coverslip with a 40 x water immersion objective with 2 x digital zoom. NLS-YC3.6 sensor was excited with 457 nm, and fluorescence emission was detected between 465 and 505 nm (CFP) and between 530 nm and 570 (cpVenus). R-GECO1 was excited with 561 nm, and its emission was detected between 620 and 650 nm. Time-lapse movies of the pollen tube tip and VN were generated by acquiring an image every 3 seconds for a total of 10 minutes. To quantify the Ca2+ fluctuations, a region of interest (ROI) was defined using the binary editor function of the NIS Elements analysis software. For cytoplasmic Ca2+, an ROI was drawn proximal to the pollen tube tip. For nuclear Ca2+, an ROI was drawn around the VN. The mean fluorescence intensity was obtained for the defined ROI for every image captured in the time-lapse dataset and graphed as a function of time. For NLS-YC3.6, the YFP/CFP ratio for each time point was calculated by dividing the mean fluorescence intensity of YFP by the mean fluorescence intensity of CFP.
Peak number and standard deviation measurement
Changes in Ca2+ sensor fluorescence signals during pollen tube growth were quantified based on the number of peaks and the standard deviation of the RFP signal for R-GECO1 and the YFP/CFP ratio for NLS-YC3.6. Cytoplasmic peaks were determined based on an RFP fluorescence intensity increase of 150 A.U. or higher from the baseline, while nuclear Ca2+ peaks were determined based on a YFP/CFP ratio increase of 0.25 A.U. or higher. The average fluorescence intensity or YFP/CFP ratio and the standard deviation for each 200-frame time-lapse movie was calculated. All 20 standard deviations were then averaged to determine the mean standard deviation for R-GECO1 in WT, R-GECO1 in wit12, NLS-YC3.6 in WT, and NLS-YC3.6 in wit12. Nuclear Ca2+ fluctuations after addition of H2O2 were analyzed using a similar peak determination as described above. However, a nuclear Ca2+ peak was determined based on a YFP/CFP ratio increase of 0.50 A.U. or higher.
Circularity index measurement
WT and wit12 pollen tubes expressing Lat52pro::NLS-YC3.6 were grown using the semi-in vivo method and imaged at 7 hpp with a confocal microscope (Eclipse C90i, Nikon). Images were taken using a 40 x water immersion objective and acquired using NIS-Elements AR version 3.2. At least 150 pollen vegetative nuclei were visualized for each line by creating z-stack sections (3 μm) to capture the entire nucleus. A maximum intensity projection of each nucleus was generated, and ImageJ was used to calculate the circularity index of each nucleus.
Results
Ca2+-dependent ROS-induced pollen tube burst correlates with nuclear position
This study uses two previously described Arabidopsis mutant lines ‘wit12’ (T-DNA insertions in WIT1 and WIT2) and ‘wifi’ (T-DNA insertions in WIP1, WIP2, WIP3, WIT1, and WIT2) that have defects in MGU trafficking and pollen tube rupture [10]. Pollen tubes were tested for their sensitivity to external application of hydrogen peroxide (H2O2) by performing semi-in vivo pollen tube rupture assays at 5 hours post pollination (hpp) and at 7 hpp, as described previously [26]. Pollen tube rupture events were defined by the appearance of cytoplasmic material at the end of the pollen tube (Figure 1a,b), as described before [26]. At 5 hpp, approximately 45% of Columbia-0 wild type (WT) pollen tubes treated with H2O2 burst, corroborating prior studies [26]. In contrast, a reduced number of rupture events were observed in wit12 and wifi pollen, with a burst frequency of 37% and 34%, respectively (Figure 1c).
When the experiment was extended by another 2 hours (7 hpp), efficiency of burst dropped in WT pollen tubes to 35% but dropped substantially more in wit12 (16%) and wifi (18%). At both 5 hpp and 7 hpp, the two mutants behaved similarly (Figure 1c,d). This suggests that semi-in vivo pollen tube burst in wit12 and wifi is hyposensitive to external ROS, and that this effect increases over time. When pollen tubes were grown on low calcium (Ca2+) media and treated with the Ca2+ channel inhibitor gadolinium(III) chloride prior to the application of H2O2, all three lines behaved similarly with few to no rupture events observed (Figure 1d). In addition, we analyzed time to response of pollen tubes rupture within 10 minutes after addition of H2O2 (Figure 1e). The data show that there was a delay in response to H2O2 application in the wit12 pollen tube population compared to WT.
Together, these data show that wit12 and wifi are similarly hyposensitive to ROS-induced pollen tube rupture, that this hyposensitivity increases over time post-pollination, and that while pollen tubes burst less frequently in wit12 and wifi, the burst is still dependent on Ca2+.
Because mutations in WIP and WIT affect the mobility of the VN during pollen tube growth, we tested if there is a connection between nuclear position and the ROS response. Because wit12 and wifi pollen tubes showed a comparable response to ROS, as well as comparable male fertility defects [10], we proceeded with the wit12 mutant only. WT and wit12 pollen tubes concurrently expressing the VN (UBQ10pro::NLS-mCherry) and SC (MGH3pro::GFP) fluorescent markers were germinated using the semi-in vivo method and the nuclear position was examined. In WT, the VN remained at a constant distance of approximately 65 µm from the pollen tube tip, regardless of when the pollen tubes were examined (Figure 1f,g). By contrast, at 5 hpp the VN of wit12 pollen tubes was positioned, on average, 118 µm from the pollen tube tip (Figure 1h,j). This distance was further increased to an average of 157 µm at 7 hpp (Figure 1i,j). The separation between VN and SCs in wit12 also increased between 5 hpp and 7 hpp (Figure 1j). Taken together, these data indicate a correlation between VN-tip distance and responsiveness to exogenous ROS in the pollen-tube rupture mechanism.
Cytoplasmic Ca2+ fluctuations are not disrupted in wit12 pollen tubes.
Given inhibition of Ca2+ channels prevents pollen tube burst in both WT and wit12, we next sought to determine if Ca2+ signaling was disrupted in wit12. Transgenic lines expressing the cytoplasmic Ca2+ sensor R-GECO1 under the pollen-specific promoter Late Anther Tomato 52 (LAT52) [38] were used in time-lapse imaging of WT and wit12 pollen tubes at 7 hpp (Figure 2a,b). Transgenic pollen was germinated using the semi-in vivo method. The fluorescence was measured at the tips of growing WT and wit12 pollen tubes using a region of interest (ROI)-based analysis method in the NIS Elements analysis software (see Materials and Methods). Qualitatively, both WT and wit12 pollen tubes exhibited a fluorescence pattern (Figure 2c,d) consistent with what has been previously reported for growing Arabidopsis pollen tubes [39]. To determine differences in Ca2+ oscillations between WT and wit12, the graphs were quantified in two ways. First, the number of peaks over the 10-minute imaging period was counted (Figure 2e). A peak was recorded if an RFP fluorescence intensity both increased and decreased at least 150 A.U. compared to the baseline (see Materials and Methods). WT and wit12 exhibited similar peak numbers, with an average of 7 peaks within 10 minutes of imaging (Figure 2e). Second, we calculated the average fluorescence intensity and standard deviation of each graph (Figure 2f,g). Here, standard deviation was used as an indicator of how much the fluorescence intensity fluctuated over time compared to the baseline (average fluorescence intensity), with a high standard deviation indicating more fluctuation and a low standard deviation indicating little, if any, fluctuation. Similar standard deviations between WT and wit12 were observed and correspond with peak frequency (Figure 2g). We observed no significant differences in average fluorescence intensity between pollen tubes (Figure 2f), indicating that expression levels did not significantly influence our results. These data suggest that the cytoplasmic Ca2+ oscillations at the pollen tube tip of wit12 are not significantly altered during elongation.
Figure 2.
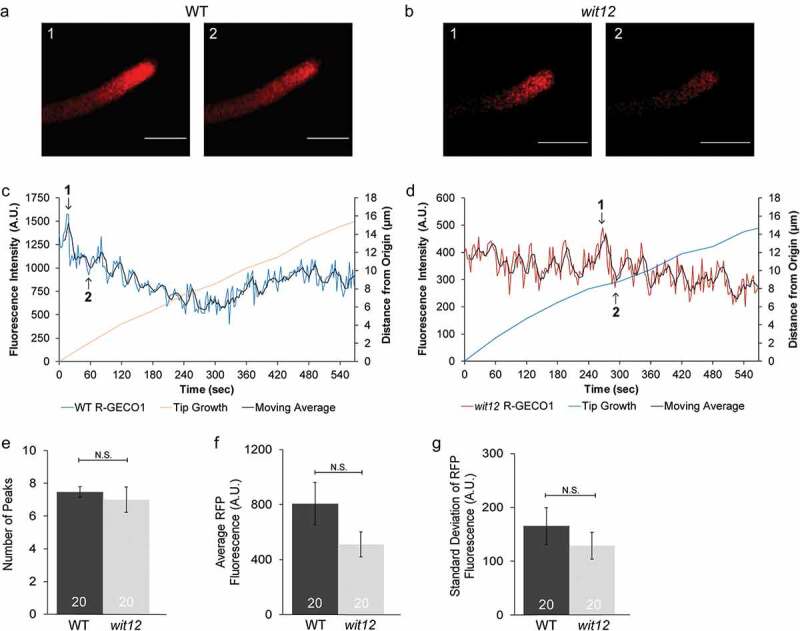
Cytoplasmic Ca2+ fluctuations are not disrupted in wit12 pollen tubes.
(a–g), The cytoplasmic Ca2+ sensor R-GECO1 (Lat52pro::R-GECO1) was used to measure Ca2+ oscillations in elongating pollen tubes. (a,b), Images of changes in cytoplasmic Ca2+ oscillations during growth for a WT pollen tube (a) and a wit12 pollen tube (b). Images in a and b correspond to time points 1 and 2, shown in (c) and (d). Scale bar = 10 µm. (c), Cytoplasmic Ca2+ oscillations measured at the pollen tube tip of WT pollen are represented as fluorescence intensity in the blue line. The red line marks the pollen tube growth from the origin at the start of imaging. The black line represents a 4-point rolling average of the data used to highlight the overall trend. Numbers in the graph correspond to the image numbers in (a). (d), Cytoplasmic Ca2+ fluctuations at the tip of elongating wit12 pollen. Fluorescence intensity is depicted by the red line. The blue line marks the pollen tube growth from the origin at the start of imaging. The black line represents a 4-point rolling average of the data used to highlight the overall trend. Numbers in the graph correspond to the image numbers in (b). (e–g), Cytoplasmic Ca2+ oscillations were quantified based on the number of peaks (e), the average fluorescence intensity for the entire time-lapse movie (f), and the standard deviation of the fluorescence intensity for the entire time-lapse movie (g). Twenty pollen tubes were analyzed in each case. Bars are standard error. N.S. indicates no significant difference by Student’s t-test.
Pollen nuclear Ca2+ fluctuations correlate with the position of the nucleus
ROS-induced rupture is impaired in wit12 but is still Ca2+ -dependent. Given that cytoplasmic Ca2+ oscillations were not altered in wit12 but the position of the VN was, we hypothesized that a nuclear Ca2+ signal may play a role in ROS-induced pollen tube rupture. Increasing the distance of the VN from the pollen tube tip might thus alter a nuclear Ca2+ signal. Nuclear Ca2+ fluctuations have been described as compounds of signal transduction pathways in a variety of biological systems, notably during root symbioses [40,42]. After nodulation (Nod) or mycorrhizal (Myc) factor perception, pronounced Ca2+ oscillations in and around the nucleus are observed, which are required for the transcriptional response of the host plant [43]. Examples of nuclear Ca2+ spiking were also found in relation to biotic and abiotic stress as well as root development; however, in these cases single or few spikes were observed instead of pronounced oscillations [43,44].
To observe nuclear Ca2+, we generated transgenic WT and wit12 plants expressing the VN-localized Ca2+ sensor NLS-YC3.6, driven by the LAT52 promoter (Figure 3a-c). Several attempts to create a wit12 line expressing the NLS-R-GECO sensor driven by the LAT52 promoter resulted only in genetically unstable progeny. Therefore, NLS-YC3.6 was used here as nuclear Ca2+ sensor. We will refer to changes in nuclear Ca2+ as fluctuations instead of oscillations because of their irregular nature. WT nuclei exhibited clear Ca2+ fluctuations at 7 hpp (Figure 3d). In contrast, the Ca2+ fluctuations observed in elongating wit12 pollen tubes at 7 hpp exhibited a range of patterns. In some cases, the number of Ca2+ fluctuations was reminiscent of those observed in WT (not shown). In other cases, the number of Ca2+ fluctuations was reduced compared to WT (Figure 3e, red line) or no fluctuations were observed (Figure 3e, black line).
Figure 3.
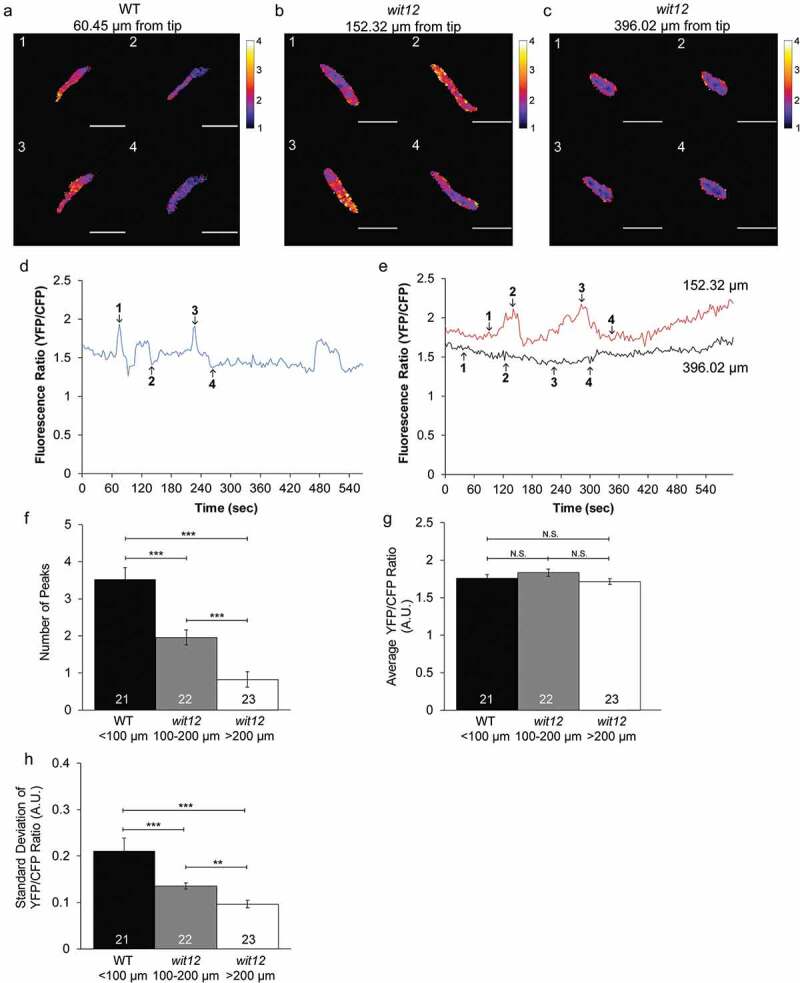
Pollen nuclear Ca2+ fluctuations correlate with the position of the nucleus.
(a–h), The nucleus-localized Ca2+ sensor (Lat52pro::NLS-YC3.6) was used to measure Ca2+ fluctuations in the VNs of elongating pollen tubes (a–c), Ratio images of changes in nuclear Ca2+ fluctuations during growth for a WT pollen tube with the VN positioned 60 µm from the tip (a), a wit12 pollen tube with the VN positioned 152 µm from the tip (b), a wit12 pollen tube with the VN positioned 396 µm from the tip (c). Scale bar = 10 µm. (d), A representative nuclear Ca2+ graph for a WT nucleus 60 µm from the pollen tube tip, presented as FRET ratio of YFP to CFP fluorescence intensity, is shown as a blue line. Numbers in the graph correspond to the image numbers in (a). (e), A representative nuclear Ca2+ graph for a wit12 nucleus 152 µm from the pollen tube tip, presented as a FRET ratio of YFP to CFP fluorescence intensity, is shown as a red line. A representative nuclear Ca2+ graph for a wit12 nucleus 396 µm from then pollen tube tip, presented as a FRET ratio of YFP to CFP fluorescence intensity, is shown as a black line. Numbers in the graph correspond to the image numbers in (b) and (c), respectively. (f–h), Nuclear Ca2+ fluctuations were quantified based on the number of peaks (f), the average YFP/CFP ratio for the entire time-lapse movie (g), and the standard deviation of the YFP/CFP ratio for the entire time-lapse movie (h). At least 20 pollen tubes were analyzed per background. Columns were split based on the distance of the VN from the pollen tube tip. Bars are standard error. N.S. indicates no significant difference by Student’s t-test. * P < 0.05; **P < 0.01; *** P < 0.001.
We next tested if the variation in nuclear Ca2+ fluctuations in wit12 was dependent on the distance of the VN to the pollen tube tip. As in Figure 2, we used peak number and standard deviation to quantify differences in Ca2+ fluctuations. A Ca2+ peak was defined as a signal that both increased and decreased at least 0.25 A.U. For each 10-minute movie generated, the distance between the VN and tip was also recorded. WT nuclei exhibited Ca2+ fluctuations with an average of 3.5 peaks per 10 minutes. All VNs were positioned between 50 and 100 µm from the tip (Figure 3f-h), consistent with data presented in Figure 1j. VNs in wit12 were distributed over a range of tip distances, but never below 100 µm. wit12 VNs located between 100 µm and 200 µm from the pollen tube tip exhibited more WT-like Ca2+ fluctuations, although the average frequency of peaks per 10 minutes was reduced to 2 peaks (Figure 3f,g). wit12 VNs located further than 200 µm from the pollen tube tip exhibited fewer Ca2+ fluctuations, on average showing only 1 peak (Figure 3f,g). The differences in standard deviation correspond with peak frequency (Figure 3h), with the standard deviation decreasing as the distance of the VN to the pollen tube tip increases. No significant differences in average YFP/CFP ratios between pollen tubes were observed, indicating that expression levels did not influence our results (Figure 3g). These data show that nuclear Ca2+ fluctuates during pollen tube growth and that the frequency of these fluctuations is correlated with the proximity of the VN to the pollen tube tip.
Finally, we examined how the cytoplasmic and nuclear Ca2+ levels are altered in response to H2O2. WT pollen tubes that ruptured exhibited an increase in the cytoplasmic calcium signal at the apex of the pollen tube prior to rupture (Suppl. Fig. S1, top row). This increase then propagated down the shank while the signal at the apex remained elevated (Suppl. Fig. S1 and Suppl. Movie 1). These observations are in agreement with what was described previously [26]. In wit12 pollen tubes, a similar propagation of the Ca2+ signal was observed prior to burst, suggesting that there is no significant difference in the H2O2-induced tip Ca2+ response (Suppl. Fig. S1, bottom row and Suppl. Movie 2).
Nuclear Ca2+ fluctuations after ROS treatment are altered in wit12
Next, we described the nuclear Ca2+ fluctuations of WT and wit12 pollen in response to H2O2. Pollen tubes, expressing the NLS-YC3.6 sensor, were treated with H2O2 at 7 hpp. A Ca2+ peak was defined as a signal that both increased and decreased at least 0.5 A.U., or that increased at least 0.5 A.U. immediately prior to rupture. A range of nuclear Ca2+ peaks were recorded, from a peak height of 7 A.U. to the lowest peak counted of 0.5 A.U. For illustration purposes, we show example ratiometric images of nuclear Ca2+ fluctuations prior to pollen tube rupture and corresponding graphs for WT (Figure 4a-d) and wit12 (Figure 4e-h). The first example for both WT (Figure 4a,b) and wit12 (Figure 4e,f) shows pollen tubes that exhibited a peak prior to rupture. The second example for both WT (Figure 4c,d) and wit12 (Figure 4g,h) shows pollen tubes without a Ca2+ peak prior to rupture. Sixty percent of WT pollen tubes that burst showed a peak, compared to 50% that did show a peak but did not burst (Figure 4i). Thirty-five percent of wit12 pollen tubes that burst and 39% of wit12 pollen tubes that did not burst showed a peak (Figure 4i). Thus, overall fewer wit12 pollen tubes showed a post-H2O2 nuclear Ca2+ peak compared to WT, but this reduction was not correlated with whether the pollen tubes burst or did not burst.
Figure 4.
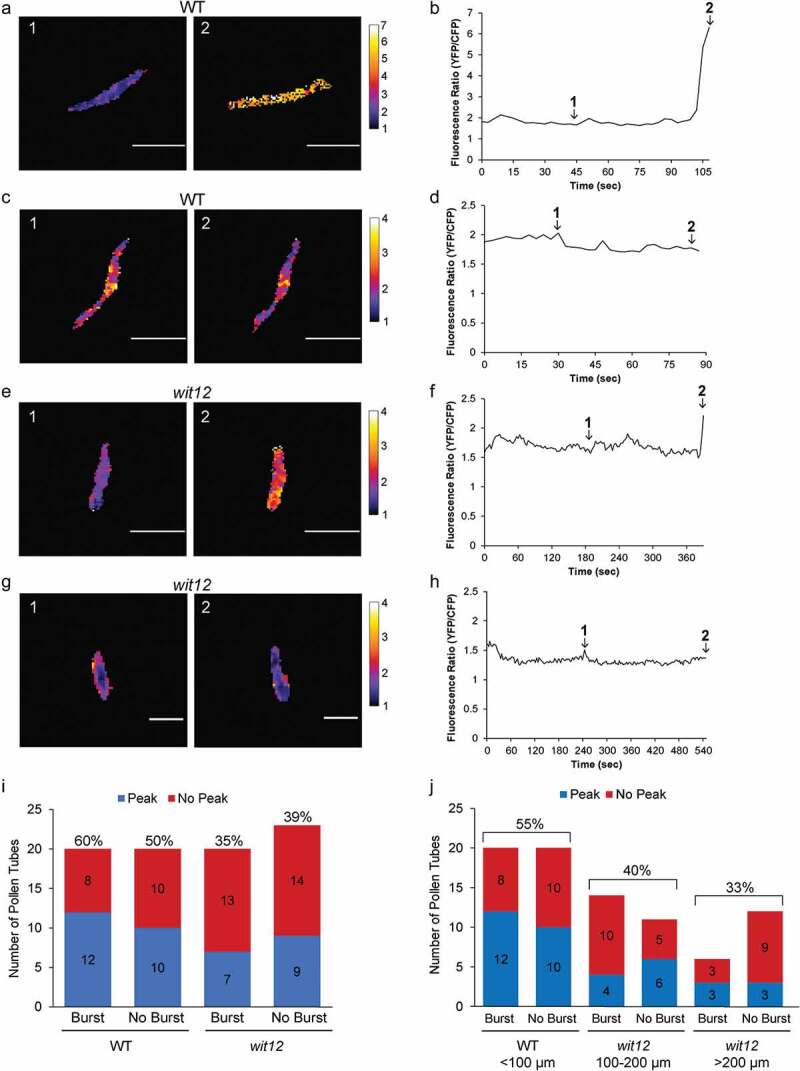
Post-ROS Ca2+ fluctuations.
(a–j), The nucleus-localized Ca2+ sensor (Lat52pro::NLS-YC3.6) was used to measure Ca2+ fluctuations in the VNs after addition of exogenous H2O2. (a–h), Example ratiometric images of nuclear Ca2+ fluctuations prior to pollen tube rupture and corresponding graphs. Scale bar = 10 µm. (a,b), A WT pollen tube with a nuclear Ca2+ peak at 6.5 A.U. prior to rupture. (c,d), A WT pollen tube without a nuclear Ca2+ peak prior to rupture. (e,f), A wit12 pollen tube with a nuclear Ca2+ peak at 2.5 A.U. prior to rupture. (g,h), A wit12 pollen tube without a nuclear Ca2+ peak prior to rupture. Images designated as 1 and 2 in (a,c,e,g) are indicated as time points 1 and 2 in graphs (b,d,f) and (h–j), quantification of presence or absence of peaks for pollen tubes that burst and pollen tubes that fail to burst for both WT and wit12. The numbers of pollen tubes with or without a peak for each condition is shown in the bar graph. (i), WT is compared to the entire wit12 population. The percent of pollen tubes that presented a nuclear Ca2+ peak is shown above each bar. (j), wit12 columns were split based on the distance of the VN from the pollen tube tip. The percent of pollen tubes that presented a nuclear Ca2+ peak, with burst and no burst combined for each group, is shown above each bar.
Regardless of whether the pollen tube ruptured, 55% of WT pollen tubes had a Ca2+ peak while only 37% of wit12 pollen tubes had a Ca2+ peak (Figure 4i,j). When the wit12 population was split up based on the distance of the VN from the pollen tube tip, 40% of VNs positioned between 100 µm and 200 µm from the tip showed a Ca2+ peak, compared to only 33% VNs located over 200 µm from the tip (Figure 4j). In addition, of the 20 wit12 pollen tubes that did burst, 14 had the VN between 100 µm and 200 µm and only 6 over 200 µm from the tip. Among the 23 non-bursting wit12 pollen tubes, the VN was over 200 µm from the tip in 12 cases, again suggesting that the capacity to undergo H2O2-induced burst correlates with the position of the VN.
Together, these data suggest that there is a correlation between ROS-induced nuclear Ca2+ peaks and VN position, as well as between ROS-induced burst and VN position, but there is no correlation between nuclear Ca2+ peaks in individual pollen tubes and the capacity of the specific pollen tube to rupture.
Discussion
The male fertility defect of mutants in WIP or WIT loci is based on reduced pollen tube burst. Duan et al. [26], have shown that exogenous H2O2 mimics the high-ROS environment of the synergids and leads to in vitro pollen tube burst in a calcium-dependent manner [26]. We show here that pollen tube burst based on exogenous H2O2 application is reduced to about 50% in the wit12 mutant, consistent with the reported reduction in in vivo male fertility [10]. The wit12 mutant response to H2O2 is still Ca2+-dependent, and the mutant shows no observable defect in pollen tube tip Ca2+ oscillations [39] or in the known H2O2-induced Ca2+ increase at the pollen tube tip [26]. Thus, the reduced response to H2O2 lies likely downstream of the known ROS-Ca2+ interplay at the plasma membrane that precedes pollen tube burst [45].
The reduced response to H2O2 correlates with the distance of the VN from the pollen tube tip. One hypothesis consistent with this phenomenon is that a tip-based, diffusible signal needs to be perceived by the VN for the orchestration of the pollen tube response. A relationship between position of the nucleus and the strength of a signaling pathway has been shown in animals, for example in the notch signaling pathway [46]. In this case, a diffusible compound is involved and the signal reaching the nucleus is dampened by increased distance. Reception of the signal in this system also requires a LINC complex for nuclear movement [10,46]. In Arabidopsis pollen, mutants in a family of transcription factors (MYB97/101/120) show similar pollen tube reception defects as those observed in wit12 and wifi mutants [17,47]. A diffusible signal might thus be upstream of the VN gene expression profile required for late stages of pollen tube growth.
Ca2+ as a diffusible second messenger is well established in both animal and plant systems [48,49] and was thus tested here as a possible signal. We found that neither the previously described Ca2+ fluctuations during pollen tube growth [39] nor the H2O2-induced rapid and expanding increase of tip-based Ca2+ [26] were measurably affected in the wit12 mutant. While nuclear Ca2+ oscillations have now been described in a variety of biological systems [43], nuclear Ca2+ has, to our knowledge, not been addressed in the pollen tube nucleus. Using the ratiometric fluorescent Ca2+ sensor YC3.6 fused to an NLS, we describe here distinct nuclear Ca2+ fluctuations both during pollen tube growth and during response to H2O2. In both cases, the nuclear Ca2+ fluctuations were dampened in the wit12 mutant and were further reduced in those pollen tubes with a nucleus positioned further away from the pollen tube tip. This suggests that the proximity of the VN to the pollen tube tip, and thus – by extension – to the cytoplasmic Ca2+ signal, is required for the establishment of the nuclear Ca2+ patterns.
Dual, rhythmic Ca2+ oscillations, at both the nuclear and cytoplasmic surface of the nuclear envelope, are a hallmark during root symbioses, and their coordination and crosstalk are an area of active investigation [40,42]. However, a number of other stimuli lead to single or few, less rhythmic calcium peaks, similar to the ones observed here [43,44]. For example, using dual cytoplasmic and nucleoplasmic GECO-based calcium sensors, Kelner et al. [50] found after cold shock and NaCl treatment in Arabidopsis root epidermal cells both a cytoplasmic and a nucleoplasmic transient calcium increase, which could be distinguished by the specific time delays between the cytoplasmic and nuclear peaks [50]. This is consistent with a study by Huang et al. [51], who showed that cytoplasmic and nuclear calcium responses to osmotic and salt stress can occur independent from each other [51]. Several earlier studies also suggest that plant nuclei can generate Ca2+ oscillations independent of changes in cytoplasmic Ca2+, and that an increase of the extranuclear Ca2+ concentration is not sufficient to trigger Ca2+ increases in isolated nuclei [52,53].
Together, this makes it unlikely that the nuclear Ca2+ increases observed here in the growing pollen tube ‘merely’ reflect the known cytoplasmic Ca2+ fluctuations by passive Ca2+ influx, especially in light of the fact that the frequency of fluctuation differs markedly between nucleus and cytoplasm (compare Figures 2 and 3). They are thus more likely indicative of a separate Ca2+ release from nuclear storage, e.g. the nuclear envelope lumen. Nevertheless, a cytoplasmic Ca2+ signal could be required to directly or indirectly activate a nuclear Ca2+ signal [43], and the distance of the nucleus from the tip could, therefore, dampen this response. Newly developed dual Ca2+ sensors [50] will now be better equipped to resolve the timing relationship between cytoplasmic and nuclear Ca2+ signatures and the relationship between the spread of cytoplasmic Ca2+ in the pollen tube shank and the position of the nucleus. In addition, specific nuclear and cytoplasmic Ca2+ quenching, as demonstrated by Huang et al. [51], can now be used to address the interdependence of the two signals.
The relationship at the single-cell level between the VN Ca2+ peaks and H2O2-induced pollen tube burst remains currently unclear. It is possible that the VN senses cytoplasmic Ca2+ signatures, including those elevated by H2O2, and that the nuclear Ca2+ peaks trigger unknown downstream signals, but that these are unrelated to pollen tube burst. Discovering and investigating male gametophyte-expressed nuclear envelope-associated Ca2+ channels will therefore now be crucial to address if the VN Ca2+ fluctuations are caused by nuclear envelope Ca2+ release and – if so – which pollen tube processes are affected by their disruption.
The movement of the vegetative nucleus through the pollen tube is one of the longest nuclear migrations in plants [54]. Nuclei move over significant distances also in other elongated plant cells, for example in root hairs and root epidermal cells. Notably, plant nuclei in these cell types are hyper-elongated and it has been proposed that this shape might protect the nuclei from physical strain during migration [54]. The elongated shape requires the WIT-WIP-SUN LINC complex, plant lamin-like CRWN proteins [19] and other nuclear envelope-associated proteins [55]. In the respective mutants, both nuclear shape and nuclear movement are affected, and this correlation is so strong that nuclear shape change has been used as a primary genetic screen to identify nuclear movement mutants [9]. Building on prior work [10], we show here that loss of the WIT-WIP-SUN LINC complex leads to a defect in nuclear migration in pollen tubes (see Figure 1). In addition, we noted that wit12 nuclei appeared less elongated than WT nuclei, which were thin and highly stretched (see Figures 1f-i, 3a-c, 4a-g, S2A). When quantified by calculating the circularity index for WT and wit12 vegetative nuclei, it became apparent that wit12 nuclei are indeed shorter and more circular than WT nuclei, consistent with similar nuclear shape changes observed in the wit12 sporophyte (Suppl. Fig. S2B). This reduced elongation might be a consequence of reduced forces acting on the wit12 VN, which might be released from an active transport process in the pollen tube.
A number of older studies in species other than Arabidopsis suggest that cytoskeletal motors might be involved in pollen VN transport. Galanthus nivalis pollen tubes show the VN preceding the SC, similar to Arabidopsis. Depolymerization of microtubules affects this order and leads to an increased distance between the VN and the pollen tube tip [56]. In addition, depolymerizing Lilium henryi F-actin leads to a contraction of the elongated VN, and a greater distance between the VN and SCs [57]. Inhibitor experiments like the ones described in these studies are difficult to use in a way that allows to clearly separate effects on pollen tube elongation and VN movement. Instead, a search for Arabidopsis pollen-expressed motor proteins that bind to the WIT-WIP-SUN LINC complex and have an effect on VN shape, VN movement and male fertility would be fruitful to dissect the important mechanism of keeping the VN close to the pollen tube tip during all stages of pollen tube development. It is formally possible that the dampened ROS and Ca2+ responses in wit12 are not a result of increased distance between VN and pollen tube tip, but stem from an unknown role of the WIT-WIP-SUN LINC complex other that nuclear shape and movement control. Identifying motor mutants that recapitulate the ROS and Ca2+-related effects described here could therefore functionally tie the role of the LINC complex in nuclear movement to its roles in ROS response and nuclear Ca2+ response.
Supplementary Material
Acknowledgments
This work was funded by a National Science Foundation grant to I.M. (NSF-1613501). We would like to thank all members of the Meier lab for many fruitful discussions throughout this work. We thank Dr. Keith Slotkin (Donald Danforth Plant Science Center) for gifting the LAT52pro::GFP construct, Dr. Melanie Krebs (Universität Heidelberg) for gifting UBQ10pro::NLS-YC3.6, Dr. Simon Gilroy (University of Wisconsin-Madison) for gifting 35Spro::YC3.6 and Dr. Anna Dobritsa (The Ohio State University) for critically reading this manuscript.
Funding Statement
This work was supported by a grant from the National Science Foundation to I.M. [NSF-1613501].
Disclosure statement
No potential conflict of interest was reported by the authors.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NM_001160797 (WIP1), NM_125004 (WIP2), NM_112181 (WIP3), NM_121177 (WIT1), and NM_105565 (WIT2).
Author contributions
M.M., A.K., and I.M. designed experiments; M.M. and A.K. performed experiments; M.M., A.K., N.R.G., and I.M. developed methods, interpreted experiments, wrote, and edited the manuscript. I.M. supervised the work and provided funding.
Supplemenatry material
Supplemental data for this article can be accessed here.
References
- [1].Bone CR, Starr DA.. Nuclear migration events throughout development. J Cell Sci. 2016;129:1951–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol. 2010;26:421–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhou X, Graumann K, Evans DE, et al. Novel plant SUN-KASH bridges are involved in RanGAP anchoring and nuclear shape determination. J Cell Biol. 2012;196:203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhou X, Graumann K, Wirthmueller L, et al. Identification of unique SUN-interacting nuclear envelope proteins with diverse functions in plants. J Cell Biol. 2014;205:677–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Poulet A, Probst AV, Graumann K, et al. Exploring the evolution of the proteins of the plant nuclear envelope. Nucleus. 2017;8:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Graumann K. Evidence for LINC1-SUN associations at the plant nuclear periphery. PLoS One. 2014;9:e93406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gumber HK, McKenna JF, Estrada AL, et al. Identification and characterization of genes encoding the nuclear envelope LINC complex in the monocot species. J Cell Sci. 2019;132. DOI: 10.1242/jcs.221390 [DOI] [PubMed] [Google Scholar]
- [8].Newman-Griffis AH, Del Cerro P, Charpentier M, et al. Medicago LINC Complexes Function in Nuclear Morphology, Nuclear Movement, and Root Nodule Symbiosis. Plant Physiol. 2019;179:491–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tamura K, Iwabuchi K, Fukao Y, et al. MyosinXI-i links the nuclear membrane to the cytoskeleton to control nuclear movement and shape in Arabidopsis. Curr Biol. 2013;23:1776–1781. [DOI] [PubMed] [Google Scholar]
- [10].Zhou X, Meier I. Efficient plant male fertility depends on vegetative nuclear movement mediated by two families of plant outer nuclear membrane proteins. Proc Natl Acad Sci USA. 2014;111:11900–11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Borg M, Brownfield L, Twell D. Male gametophyte development: a molecular perspective. J Exp Bot. 2009;60:1465–1478. [DOI] [PubMed] [Google Scholar]
- [12]. Li H-J, Meng J-G, Yang W-C. Multilayered signaling pathways for pollen tube growth and guidance. Plant Reprod. 2018;31:31–41. [DOI] [PubMed] [Google Scholar]
- [13].Dumas C, Knox R, Gaude T. The spatial association of the sperm cells and vegetative nucleus in the pollen grain of Brassica. Protoplasma. 1985;124:168–174. [Google Scholar]
- [14].McCue AD, Cresti M, Feijó JA, et al. Cytoplasmic connection of sperm cells to the pollen vegetative cell nucleus: potential roles of the male germ unit revisited. J Exp Bot. 2011;62:1621–1631. [DOI] [PubMed] [Google Scholar]
- [15].Russell S, Cass D. Ultrastructure of the sperms of Plumbago zeylanica 1. Cytology and association with the vegetative nucleus. Protoplasma. 1981;107:85–107. [Google Scholar]
- [16].Zhang J, Huang Q, Zhong S, et al. Sperm cells are passive cargo of the pollen tube in plant fertilization. Nat Plants. 2017;3:17079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].AR L, KM B, Woroniecka K, et al. Three MYB transcription factors control pollen tube differentiation required for sperm release. Curr Biol. 2013;23:1209–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Varas J, Graumann K, Osman, et al. Absence of SUN1 and SUN2 proteins in Arabidopsis thaliana leads to a delay in meiotic progression and defects in synapsis and recombination. Plant J. 2015;81:329–346. [DOI] [PubMed] [Google Scholar]
- [19].Zhou X, NR G, Meier I. Plant nuclear shape is independently determined by the SUN-WIP-WIT2-myosin XI-i complex and CRWN1. Nucleus. 2015;6:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dresselhaus T, Franklin-Tong N. Male-female crosstalk during pollen germination, tube growth and guidance, and double fertilization. Mol Plant. 2013;6:1018–1036. [DOI] [PubMed] [Google Scholar]
- [21].Kessler SA, Grossniklaus U. She’s the boss: signaling in pollen tube reception. Curr Opin Plant Biol. 2011;14:622–627. [DOI] [PubMed] [Google Scholar]
- [22].Johnson MA, Harper JF, Palanivelu R. A fruitful journey: pollen tube navigation from germination to fertilization. Annu Rev Plant Biol. 2019;70:809–837. [DOI] [PubMed] [Google Scholar]
- [23].Escobar-Restrepo J-M, Huck N, Kessler S, et al. The FERONIA receptor-like kinase mediates male-female interactions during pollen tube reception. Science. 2007;317:656. [DOI] [PubMed] [Google Scholar]
- [24].Iwano M, Ngo QA, Entani T, et al. Cytoplasmic Ca2+ changes dynamically during the interaction of the pollen tube with synergid cells. Development. 2012;139:4202–4209. [DOI] [PubMed] [Google Scholar]
- [25].Ngo QA, Vogler H, Lituiev DS, et al. A calcium dialog mediated by the FERONIA signal transduction pathway controls plant sperm delivery. Dev Cell. 2014;29:491–500. [DOI] [PubMed] [Google Scholar]
- [26].Duan Q, Kita D, EA J, et al. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nature Commun. 2014;5:3129. [DOI] [PubMed] [Google Scholar]
- [27].Boisson-Dernier A, Roy S, Kritsas K, et al. Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development. 2009;136:3279–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ge Z, Bergonci T, Zhao Y, et al. pollen tube integrity and sperm release are regulated by RALF-mediated signaling. Science. 2017;358:1596–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhao Q, Brkljacic J, Meier I. Two distinct interacting classes of nuclear envelope-associated coiled-coil proteins are required for the tissue-specific nuclear envelope targeting of Arabidopsis RanGAP. Plant Cell. 2008;20:1639–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Takagi S, Islam MS, Iwabuchi K. Chapter four – dynamic behavior of double-membrane-bounded organelles in plant cells. Int Rev Cell Mol Biol. 2011;286:181–222. [DOI] [PubMed] [Google Scholar]
- [31].Krebs M, Held K, Binder A, et al. FRET-based genetically encoded sensors allow high-resolution live cell imaging of Ca2+ dynamics. Plant J. 2011;69:181–192. [DOI] [PubMed] [Google Scholar]
- [32].Zhao Y, Araki S, Wu J, et al. An expanded palette of genetically encoded Ca2⁺ indicators. Science. 2011;333:1888–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wise AA, Liu Z, Binns AN. Three methods for the introduction of foreign DNA into Agrobacterium. Methods Mol Biol. 2006;343:43–54. [DOI] [PubMed] [Google Scholar]
- [34].SJ C, AF B. Floral dip: a simplified method for Agrobacterium -mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. [DOI] [PubMed] [Google Scholar]
- [35].Palanivelu R, Preuss D. Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol. 2006;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Boavida LC, McCormick S. Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J. 2007;52:570–582. [DOI] [PubMed] [Google Scholar]
- [37].Johnson MA, Kost B. Pollen Tube Development. Methods Mol Biol. 2010;655:155–176. [DOI] [PubMed] [Google Scholar]
- [38].Eyal Y, Curie C, McCormick S. Pollen specificity elements reside in 30 bp of the proximal promoters of two pollen-expressed genes. Plant Cell. 1995;7:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Iwano M, Entani T, Shiba H, et al. Fine-Tuning of the Cytoplasmic Ca(2+) concentration is essential for pollen tube growth. Plant Physiol. 2009;150:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Capoen W, Sun J, Wysham D, et al. Nuclear membranes control symbiotic calcium signaling of legumes. Proc Natl Acad Sci USA. 2011;108:14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Charpentier M, Sun J, TV M, et al. Nuclear-localized cyclic nucleotide–gated channels mediate symbiotic calcium oscillations. Science. 2016;352:1102. [DOI] [PubMed] [Google Scholar]
- [42].Liang Y, Tóth K, Cao Y, et al. Lipochitooligosaccharide recognition: an ancient story. New Phytol. 2014;204:289–296. [DOI] [PubMed] [Google Scholar]
- [43].Charpentier M. Calcium signals in the plant nucleus: origin and function. J Exp Bot. 2018;69:4165–4173. [DOI] [PubMed] [Google Scholar]
- [44].Leitão N, Dangeville P, Carter R, et al. Nuclear calcium signatures are associated with root development. Nat Commun. 2019;10:4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang M, Zhang X, Gao X. ROS in the Male-Female Interactions During Pollination: function and Regulation. Front Plant Sci. 2020;11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Del Bene F, AM W, BA L, et al. Regulation of neurogenesis by interkinetic nuclear migration through an apical-basal notch gradient. Cell. 2008;134:1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Liang Y, Tan ZM, Zhu L, et al. MYB97, MYB101 and MYB120 function as male factors that control pollen tube-synergid interaction in Arabidopsis thaliana fertilization. PLoS Genet. 2013;9:e1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. [DOI] [PubMed] [Google Scholar]
- [49].Dodd AN, Kudla J, Sanders D. The language of calcium signaling. Annu Rev Plant Biol. 2010;61:593–620. [DOI] [PubMed] [Google Scholar]
- [50].Kelner A, Leitão N, Chabaud M, et al. Dual color sensors for simultaneous analysis of calcium signal dynamics in the nuclear and cytoplasmic compartments of plant cells. Front Plant Sci. 2018;9:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Huang F, Luo J, Ning T, et al. Cytosolic and nucleosolic calcium signaling in response to osmotic and salt stresses are independent of each other in roots of. Front Plant Sci. 2017;8:1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mazars C, Bourque S, Mithöfer A, et al. Calcium homeostasis in plant cell nuclei. New Phytol. 2009;181:261–274. [DOI] [PubMed] [Google Scholar]
- [53].Pauly N, MR K, Thuleau P, et al. Control of free calcium in plant cell nuclei. Nature. 2000;405:754–755. [DOI] [PubMed] [Google Scholar]
- [54].Griffis AH, Groves NR, Zhou X, et al. Nuclei in motion: movement and positioning of plant nuclei in development, signaling, symbiosis, and disease. Front Plant Sci. 2014;5:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Goto C, Tamura K, Fukao Y, et al. The novel nuclear envelope protein KAKU4 modulates nuclear morphology in Arabidopsis. Plant Cell. 2014;26:2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Heslop-Harrison J, Heslop-Harrison Y, Cresti M, et al. Cytoskeletal elements, cell shaping and movement in the angiosperm pollen tube. J Cell Sci. 1988;91:49–60. [Google Scholar]
- [57].Heslop-Harrison J, Heslop-Harrison Y. Conformation and movement of the vegetative nucleus of the angiosperm pollen tube: association with the act in cytoskeleton. J Cell Sci. 1989;93:299–308. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


