Abstract
Metabolic reprogramming, including enhanced biosynthesis of macromolecules, altered energy metabolism, and maintenance of redox homeostasis, is considered a hallmark of cancer, sustaining cancer cell growth. Multiple signaling pathways, transcription factors and metabolic enzymes participate in the modulation of cancer metabolism and thus, metabolic reprogramming is a highly complex process. Recent studies have observed that ubiquitination and deubiquitination are involved in the regulation of metabolic reprogramming in cancer cells. As one of the most important type of post-translational modifications, ubiquitination is a multistep enzymatic process, involved in diverse cellular biological activities. Dysregulation of ubiquitination and deubiquitination contributes to various disease, including cancer. Here, we discuss the role of ubiquitination and deubiquitination in the regulation of cancer metabolism, which is aimed at highlighting the importance of this post-translational modification in metabolic reprogramming and supporting the development of new therapeutic approaches for cancer treatment.
Keywords: Ubiquitination, Deubiquitination, Cancer, Metabolic reprogramming
Background
Metabolic pathways are of vital importance in proliferating cells to meet their demands of various macromolecules and energy [1]. Compared with normal cells, cancer cells own malignant properties, such as increased proliferation rate, and reside in environments short of oxygen and nutrient. Correspondingly, metabolic activities are altered in cancer cells to support their malignant biological behaviors and to adapt to stressful conditions, such as nutrient limitation and hypoxia [1]. Cancer metabolism is an old field of research. Warburg effect observed in the 1920s provides a classical example of metabolic reprogramming in cancer [2]. In the past few decades, enhanced biosynthesis of macromolecules, altered energy metabolism, and maintenance of redox homeostasis have been observed to be essential features of cancer metabolism. Altered metabolism in cancer cells have aroused increasing attention and interest [3]. Because of the generality of metabolic alterations in cancer cells, metabolic reprogramming is thought as hallmark of cancer, providing basis for tumor diagnosis and treatment [1]. For instance, the application of 18F-deoxyglucose positron emission tomography is based on tumor cells’ characteristic of increased glucose consumption [4]. Inhibition of some metabolic enzymes, such as L-lactate dehydrogenase A chain (LDH-A), have been observed to regress established tumors [5, 6]. Therefore, research of metabolic reprogramming is of critical importance, which might provide new opportunities for cancer diagnosis and treatment.
Amongst multiple post-translational modification, protein ubiquitination is a common and important process in cells [7, 8]. Ubiquitination and deubiquitination have been observed to be dysregulated in various types of cancers. Genetic and epigenetic aberrations, such as mutation, amplification and deletion, can be the common causes of dysregulated ubiquitination and deubiquitination in cancer cells [9]. Ubiquitination and deubiquitination can also be abnormally regulated by transcriptional, translational or posttranslational mechanisms in cancer cells, exerting oncogenic or anti-cancer roles in carcinogenesis [7, 8, 10]. In recent years, the involvement of ubiquitination and deubiquitination in the regulation of metabolic reprogramming in cancer cells has received a growing body of attention [11]. Given the complexity and importance of both cancer metabolism and protein ubiquitination, the exact roles of protein ubiquitination and deubiquitination in metabolic reprogramming are worth further studies and analyses. The present review will highlight the ubiquitination and deubiquitination system as a regulator of cancer metabolism and discuss future directions focusing on the strategies to improve cancer therapy.
Cancer metabolism
To satisfy nutrient and energy requirements for cells’ survival and growth, metabolic pathways are altered in cancer cells, which is called metabolic reprogramming [1]. Metabolic reprogramming is a highly regulated process [12]. Aberrant activation of mechanistic target of rapamycin complex 1 (mTORC1) is one of the most common alterations in proliferating cancer cells, playing a key role in metabolic reprogramming [12]. Under the stimulation of amino acids, mTORC1 can be activated, which subsequently exerts various biological effects by activation of different downstream targets, such as hypoxia-inducible factor 1 (HIF-1) and sterol regulatory element-binding protein (SREBP) [12, 13]. Proliferating cancer cells require elevated synthesis of protein, lipid and nucleotide. Glycolysis can be upregulated by mTORC1 activation, providing more glycolytic intermediates for biosynthesis of these macromolecules [14]. Moreover, mTORC1 activation promotes glutamine uptake to maintain mitochondrial ATP production [15]. Fatty acids can also supply carbon to the tricarboxylic acid (TCA) cycle to sustain mitochondrial function [16]. PI3K-AKT signaling is the most well-known mechanism for activating mTORC1 [17]. Besides, mTORC1 can be activated or inhibited by various signaling pathways directly or indirectly. For instance, 5′-AMP-activated protein kinase (AMPK) activated by energy shortage is a crucial inhibitor of mTORC1 [18]. What’s more, transcription factor c-Myc and p53 also take part in metabolic reprogramming through transcriptional regulation of metabolism-related genes [19, 20]. Based on multiple regulatory mechanisms, expression or activity of the enzymes involved in glucose, amino acids and fatty acids metabolism are altered, directly contributing to metabolic reprogramming [21]. What’s more, the up-regulation of various metabolic processes in cancer cells triggers accumulation of reactive oxygen species (ROS) [22]. Transcription factors nuclear factor erythroid 2-related factor 2 (NRF2) and HIF-1 play key roles in maintenance of redox homeostasis, keeping ROS in an appropriate level to promote tumor growth rather than inducing damage [23, 24].
Under nutrient rich conditions, activation of mTORC1 supports cancer cells growth. In periods of cellular stress, low levels of amino acids or absent ATP induces mTORC1 inhibition, which subsequently activates a compensatory mechanism named autophagy [25]. Autophagy is a highly regulated pathway essential for cell survival in nutrient-deprived conditions, complementing the classical pathways like glycolysis. Autophagy supplies amino acids by inducing degradation of macromolecules and organelles in lysosome, thereby providing intracellular amino acids supply to fuel the TCA cycle, gluconeogenesis and protein synthesis [26]. However, the interplay between autophagy and glycolysis seems to be complex. Activation of autophagy has been observed to enhance glycolysis [27]. Deficiency of mitophagy can induce mitochondrial dysfunctions, enhancing glycolysis and Warburg effect [28]. Additionally, studies have found that oxidative stress induced by cancer cells can promote aerobic glycolysis and autophagy in cancer associated fibroblasts to obtain recycled nutrients from cancer associated fibroblasts. This phenomenon is called “Reverse Warburg Effect” [29]. Therefore, both the anabolic pathways, such as glycolysis, and the catabolic pathways, such as autophagy, interplay with each other, together contribute to cancer metabolism and supporting cellular growth. Taken together, abnormal alterations of multiple signaling pathways, transcription factors and metabolic pathways synergistically lead to metabolic reprogramming in cancer cells.
Ubiquitination and deubiquitination
Ubiquitination is an ATP-dependent cascade process ligating ubiquitin, a ubiquitously expressed protein consisting of 76 amino acids, to a substrate protein [30]. Ubiquitin-activating enzymes (E1s) initially bind to ubiquitin for activation, and then transfer activated ubiquitin to ubiquitin-conjugating enzymes (E2s). Ubiquitin ligases (E3s) finally transfer ubiquitin from E2 to substrates [30]. According to the number of ubiquitin attaching to one lysine residue in protein, ubiquitination is divided into monoubiquitination (single ubiquitin) and polyubiquitination (a chain of ubiquitin) [31]. In the polyubiquitination chain, ubiquitin can be attached via 7 lysine residues (K6, K11, K27, K29, K33, K48, and K63) or the first methionine (M1) [32]. Different types of ubiquitination lead to disparate fates of substrate proteins. K48-linked polyubiquitination is the most widely studied type, which mainly labels proteins for 26S proteasome-mediated recognition and degradation [32]. K48-linked polyubiquitination also has proteasome independent functions, including regulation of signaling events and transcription, which are possibly determined by the length of the ubiquitin chain [33–35]. K11-linked polyubiquitination is also associated with proteolysis [32]. Ubiquitin-proteasome system is involved in the degradation of more than 80% of proteins in cells [36]. K63-linked polyubiquitination is involved in signaling assemblies [32]. E3 ligases play a key role in the whole process of ubiquitination because of their specificity for substrates. In human genome, there are approximately 1000 E3 ligases, which can be divided into the homology to E6AP C terminus (HECT) domain-containing E3s, the RING-between-RING (RBR) family E3s and the really interesting new gene (RING) finger domain-containing E3s [37]. Deubiquitination is catalyzed by deubiquitinating enzymes (DUBs) to remove ubiquitin from ubiquitinated proteins, thus reversing the ubiquitination process [7]. About 100 DUBs fall into seven subgroups: the ubiquitin-specific proteases (USPs), the ubiquitin C-terminal hydrolases (UCHs), the ovarian tumor proteases (OTUs), the Machado-Josephin domain proteases (MJDs), the JAB1/MPN+/MOV34 (JAMM) domain proteases, the monocyte chemotactic protein-induced proteins (MCPIPs), and the motif interacting with ubiquitin-containing DUB family (MINDY) [10]. Dynamic conversion between ubiquitination and deubiquitination is closely related to various cellular functions and thus, its dysregulation results in multiple disease, such as neurodegenerative diseases and cancer [38]. Understanding of ubiquitination and deubiquitination may provide novel insights into the treatment of these diseases.
Ubiquitination and metabolic signaling pathways
Ubiquitination of mTOR
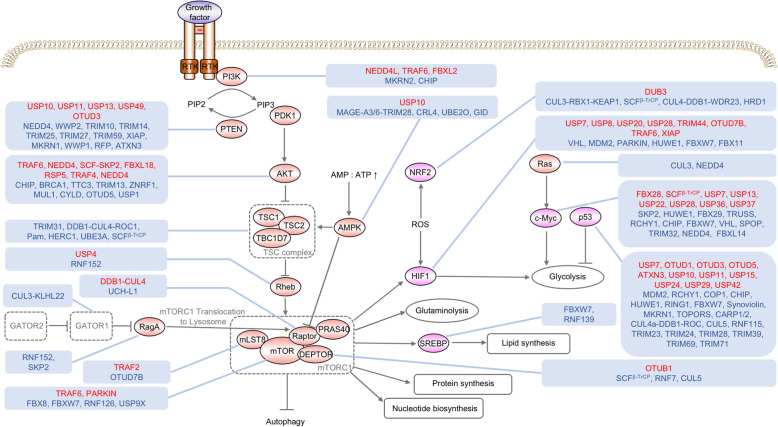
Aberrant activation of mTORC1 is considered as a key feature of metabolic reprogramming. mTORC1 is a complex consisting of mTOR, Raptor, mLST8, PRAS40 and DEPTOR [39]. mTOR is an evolutionarily conserved serine/threonine protein kinase in the PI3K-related kinase superfamily, responsible for the catalytic activity of mTORC1 [40]. Translocation of mTORC1 to lysosome is the premise for its subsequent activation, identified as a critical step in the activation of mTORC1 signaling [41]. Activated RagA is thought to be the main participator in the re-localization of mTORC1 to the lysosomes in amino acid-stimulated cells [41]. Studies have found that E3 ligase TRAF6, which is upregulated in cancer cells, can mediate K63-linked polyubiquitination of mTOR by interacting with p62 under the stimulation of amino acids, promoting the translocation of mTORC1 to the lysosomes and subsequent activation (Fig. 1) [42]. In addition, decreased K48-linked ubiquitination of mTOR by E3 ligase FBX8 and FBXW7 alleviates proteasome-dependent degradation of mTOR, exerting an oncogenic effect in cancer as well [43, 44]. Reduced mTOR ubiquitination is also linked to therapy resistance in cancer. Everolimus is a mTOR inhibitor used in breast cancer patients. Following downregulated phosphorylation of mTOR induced by depletion of dual specificity tyrosine-phosphorylation-regulated kinase 2 (DYRK2), ubiquitination and degradation of mTOR diminish, resulting in everolimus resistance [45]. Non-thermal plasma exerts anti-tumor effect by inducing RNF126 mediated K48-linked polyubiquitination and degradation of mTOR [46]. However, when faced with mitochondrial stress, E3 ligase PARKIN targets mTOR for ubiquitination, which maintains mTORC1 activity instead of affecting mTOR stability, thereby enhancing cell survival [47]. DUB USP9X can negatively modulate mTOR function and mTORC1 activity without changing mTOR protein level [48]. Therefore, different types of ubiquitination and deubiquitination play diverse roles in the regulation of mTOR function.
Fig. 1.
Regulation of signaling pathways and transcription factors associated with cancer metabolism by ubiquitination and deubiquitination. Aberrant activation of signaling pathways like PI3K-AKT-mTORC1, loss of tumor suppressive transcription factors like p53 and activation of oncogenic transcription factors like c-Myc control cancer metabolism. Ubiquitination and deubiquitination indirectly regulate cancer metabolism by modulating these signaling molecules and transcription factors. The ubiquitin ligases and deubiquitinating enzymes in red font positively regulate the activity or expression level of substrate proteins. The ubiquitin ligases and deubiquitinating enzymes in blue font negatively regulate the activity or expression level of substrate proteins. AMP, adenosine monophosphate; ATP, adenosine triphosphate; mTORC1, mTOR complex 1; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-trisphosphate; ROS, reactive oxygen species; RTK, receptor tyrosine kinase; TSC, tuberous sclerosis complex
Ubiquitination of raptor
Raptor is the regulatory protein of mTORC1, maintaining the correct subcellular localization of mTORC1 and allowing the binding of mTORC1 with substrates [49]. DDB1-CUL4 E3 ligase complex is essential for maintaining mTORC1 stability by ubiquitinating Raptor. Displacement of DDB1-CUL4 complex by DUB UCH-L1 can remove K63-linked poly-ubiquitin chains on Raptor, bringing about reduced mTORC1 [49].
Ubiquitination of mLST8
mLST8, also called GβL, is a component of both mTORC1 and mTORC2. mLST8 is associated with the catalytic domain of mTOR and stabilizes its kinase activation loop [50]. mLST8 can be ubiquitinated by TRAF2 through K63-mediated linkage, which breaks the interaction between mLST8 and SIN1 in mTORC2, giving rise to elevated formation of mTORC1. This process can be reversed by DUB OTUD7B, leading to increased mTORC2 formation [50]. The study highlighted the role of ubiquitination and deubiquitination in the balance and competence between mTORC1 and mTORC2 signaling under various conditions.
Ubiquitination of DEPTOR
DEPTOR is an inhibitor of both mTORC1 and mTORC2. mTOR activation can phosphorylate DEPTOR and promote its recognition by SCFβ-TrCP ubiquitin ligase, targeting DEPTOR for polyubiquitination and proteolytic degradation [51, 52]. In tumors with isocitrate dehydrogenase1/2 (IDH1/2) mutations, oncometabolite 2-hydroxyglutarate indirectly promotes DEPTOR polyubiquitination by SCFβ-TrCP, thus activating mTOR [51]. E3 ligase RNF7 exerts oncogenic effect in prostate tumorigenesis by promoting ubiquitination and degradation of DEPTOR [53]. DUB OTUB1 specifically stabilizes DEPTOR via deubiquitination, thereby playing an anticancer role [54]. CUL5 also targets DEPTOR for degradation, which is inhibited during autophagy activation [55]. Autophagy triggered by decreased ubiquitination and degradation of DEPTOR is related to drug resistance in cancer. Anticancer agent MLN4924 causes protective autophagy via inactivation of the CUL E3 ligase and accumulation of DEPTOR, which suppresses its effectiveness [56]. Enhancement of DEPTOR degradation can attenuate autophagy, which has been found to be an effective target in Temozolomide-resistant glioblastoma cells [57].
Ubiquitination of RagA
As we mentioned above, activated RagA plays a key role in the re-localization of mTORC1 to the lysosomes and subsequent activation of mTORC1 [41]. Study found that downregulation of lysosomal E3 ligase RNF152 protected cells from autophagy [58]. RNF152 modifies RagA by K63-linked polyubiquitination and promotes recruitment of RagA inhibitor GATOR1, thus inducing RagA inactivation. Then mTORC1 is released from the lysosomal surface, giving rise to blockade of mTORC1 signaling pathway [58]. Moreover, K63-linked polyubiquitination of RagA can also be mediated by E3 ligase SKP2, which exerts a similar effect with RNF152 [59].
Ubiquitination of GATORs
GATOR1 is a complex consisting of DEPDC5, NPRL2 and NPRL3, while GATOR2 consists of Mios, WDR24, WDR59, Seh1L and sec13. GATOR2 negatively regulates DEPDC5 in GATOR1, which acts as an inhibitor of RagA. Thus, GATOR2 exerts a promoting effect on RagA. Oncogenic E3 ligase CUL3-KLHL22 was observed to mediate K48-linked polyubiquitination of DEPDC5 and target DEPDC5 for degradation under the stimulation of amino acids and promote mTORC1 activation in tumor [60].
Ubiquitination of Rheb
GTP-bound Rheb is the activator of mTORC1 after translocation of mTORC1 to the lysosome. Monoubiquitination of Rheb by the lysosomal E3 ligase RNF152 can enhance its interaction with the tuberous sclerosis complex (TSC) complex, the major inhibitor of Rheb, and can decrease mTORC1 activation [61]. Following phosphorylation by AKT, USP4 can deubiquitinate Rheb to reverse the action of RNF152. Therefore, the dynamic change of ubiquitinating state of Rheb is associated with mTORC1 activation and tumor growth [61].
Ubiquitination of TSC complex
TSC complex, composed of TSC1, TSC2, and TBC1D7, is identified as a major upstream regulator of Rheb, converting GTP-bound Rheb to GDP-bound Rheb, thus inhibiting mTORC1 activation. TRIM31 mediated K48-linked ubiquitination of TSC1-TSC2 complex induces its degradation and promotes growth of hepatocellular carcinoma cells [62]. FBXW5 recruits TSC2 protein to DDB1-CUL4-ROC1 E3 ligase complex, promoting its ubiquitination and degradation [63]. Moreover, E3 ligase Pam, HERC1 and UBE3A were also observed to mediate TSC2 protein ubiquitination and to enhance its degradation [64–66]. Interaction of TSC2 with TSC1 can prevent TSC2 from HERC1 ubiquitin ligase mediated degradation [66], while VPS34 can competitively bind to TSC1, resulting in TSC2 degradation [67]. E3 ubiquitin ligase SCFβ-TrCP can ubiquitinate free TBC1D7 for degradation. Binding to TSC or AKT dependent phosphorylation of free TBC1D7 can prevent TBC1D7 from interaction with SCFβ-TrCP, stabilizing the pool of TBC1D7 [68].
Ubiquitination of PI3K
PI3K-mTORC1 signaling is the crucial signaling pathway that governs metabolic reprogramming and tumor cell growth. PI3K can be activated under the stimulation of growth factors. Activated PI3K phosphorylates PIP2, converting PIP2 to PIP3, which recruits 3-phosphoinositide-dependent protein kinase 1 (PDK1) and Akt to the membrane. PDK1 subsequently activates AKT, a negative regulator of TSC complex, and subsequently activating mTORC1 [69]. Activation of PI3K-mTORC1 signaling can exert various effects on metabolic process and play a key role in the regulation of tumor metabolism [69]. PI3K is a dimeric enzyme composed of a catalytic subunit (p110α, p110β, or p110γ) and a regulatory subunit (p85α, p85β, p55α, p55γ, or p50α) [70]. A dynamic cycle of proteasome-dependent degradation and re-synthesis of PI3Kp110α were observed in activation of PI3K signaling. NEDD4L E3 ligase catalyzes free PI3Kp110α for ubiquitination, leading to its proteasome-dependent degradation and maintenance of PI3K signaling [70]. TRAF6 E3 ligase promotes activation of PI3K pathway in cancer by nonproteolytic polyubiquitination of PI3K catalytic subunit p110α [70]. TRAF6 also directs PI3K recruitment to TGF-β receptor via K63-linked polyubiquitination of PI3Kp85α, which is essential for TGF-β-induced activation of PI3K signaling [71]. What’s more, PI3K regulatory subunit p85α can be ubiquitinated by E3 ligase MKRN2 and E3 ligase complex HSP70-CHIP through K48-mediated linkage, bringing about proteolysis of PI3Kp85α and downregulation of PI3K signaling in cancer [72, 73]. Dephosphorylated free p85β is ubiquitinated by FBXL2 for proteolysis. Decreased free p85β reduces its competition with p85-p110 heterodimers for docking sites on cell membrane, thus upregulating PI3K signaling [74]. Therefore, both the catalytic subunit and the regulatory subunit can be ubiquitinated, exerting various effects on PI3K signaling.
Ubiquitination of PDK1
PDK1 phosphorylates and activates AKT, transducing signal from activated PI3K to AKT. Attenuated ubiquitination and degradation of PDK1 are related to chemoresistance in ovarian cancer [75]. Monoubiquitination of PDK1 in cancer cell lines can be reversed by USP4 catalyzation, the function of which still remains unclear [76].
Ubiquitination of AKT
AKT, a negative regulator of TSC complex, can be activated by PI3K signaling, exerting oncogenic effect by promoting mTORC1 signaling. K63-linked polyubiquitination of AKT catalyzed by TRAF6, NEDD4, SCF-SKP2, FBXL18, RSP5 or TRAF4 E3 ligase is required for cell membrane localization of AKT and is essential in activation of PI3K-AKT-mTOR pathway and subsequent upregulation of glycolysis [77–82]. SETDB1 catalyzed methylation of AKT enhances its K63-linked ubiquitination and activation [83]. After interaction with KDM4B, TRAF6 promotes its ubiquitination of AKT in colorectal cancer, facilitating glucose metabolism and tumor growth [84]. DUB CYLD, OTUD5 and USP1 can reverse K63-linked polyubiquitination of AKT and inhibit its activation [85–87]. Bisdemethoxycurcumin can inhibit hepatocellular carcinoma cell growth by promoting CYLD-mediated deubiquitination of AKT [88]. Enhanced AKT ubiquitination and activation caused by downregulation of DUB OTUD5 give rise to radioresistance in cervical cancer [86]. pAKT ubiquitinated by NEDD4 can regulate its nuclear trafficking to promote tumorigenesis [79]. What’s more, E3 ligases CHIP, BRCA1, TTC3, TRIM13, ZNRF1, MUL1 can modify AKT by K48-linked ubiquitination and promote degradation of AKT to suppress its activation [89–94]. Anticancer agents Rhus coriaria, SC66 and Vitamin C can stimulate ubiquitination and degradation of AKT in cancer cells [95, 96]. When proteasome impairs in cellular stress, E3 ligase MUL1 catalyzes K48-linked ubiquitination of AKT, which subsequently undergoes lysosomal degradation, playing key roles in cellular survival [97]. This process can be reversed by DUB USP7 [97].
Ubiquitination of PTEN
As a negative regulator of PI3K/AKT signaling pathway, phosphatase and tensin homolog (PTEN) converts PIP3 back to PIP2, playing tumor suppressive functions in various cancers. Identified as the E3-ligase of PTEN, NEDD4 not only contributes to monoubiquitination of PTEN for its nuclear transport but also mediates polyubiquitination of PTEN for its proteasomal degradation to activate AKT signaling transduction [98]. Up-regulation of LINC00152 enhances NEDD4 mediated ubiquitination and degradation of PTEN in breast cancer [99]. E3 ligase WWP2, TRIM10, TRIM14, TRIM25, TRIM27, TRIM59 and XIAP can target PTEN for ubiquitination and degradation as well [100–106]. AKT activated MKRN1 E3 ligase also mediates ubiquitination and degradation of PTEN, thus positively regulating PI3K/AKT signaling axis [107]. The E3 ligase WWP1 mediated polyubiquitination can suppress the membrane recruitment and function of PTEN [108]. Ubiquitination via E3 ligase RFP can downregulate PTEN phosphatase activity rather than altering its stability or localization [109]. As for the deubiquitination of PTEN, USP10, USP11, USP13, USP49, and OTUD3 catalyze removal of K48-linked ubiquitin chain on PTEN to enhance protein stability of PTEN and attenuate AKT signaling pathway [110–114]. USP7-induced deubiquitination of PTEN results in its nuclear exclusion rather than regulating its protein stability [115, 116]. DUB ATXN3 suppress PTEN expression by reducing its transcription rather than altering its protein level [117].
Ubiquitination of AMPK
AMPK is an inhibitor of mTORC1 by phosphorylating Raptor and activating TSC2. As an energy sensor, AMPK is crucial in maintenance of NADPH and ATP level in response to reduced intracellular ATP. AMPK is composed of catalytic α and regulatory β and γ subunits [118]. E3 ligase complex MAGE-A3/6-TRIM28 and E3 ligase CRL4A catalyze ubiquitination of AMPKα and target it for degradation, thus reducing autophagy and altering cancer metabolism [118, 119]. UBE2O, an atypical ubiquitin enzyme with both E2 and E3 activities, ubiquitinates AMPKα2 for degradation [120]. CRL4 catalyzed ubiquitination also directs AMPKγ proteolysis [121]. GID ubiquitin ligase mediates ubiquitination and degradation of AMPK as well, leading to decreased autophagy and increased mTOR activity [122]. Ubiquitination of AMPKα can be reversed by USP10 to remove the ubiquitin chain from AMPKα and promote AMPK activation [122].
Ubiquitination of KRAS
As a common mutated oncogene driving tumorigenicity in pancreatic, colon and lung cancers, KRAS enhances expression of glucose transporter type 1 (GLUT1) and controls glycolysis and glutamine metabolism in cancer cells, which is considered to be associated with metabolic reprogramming in primary invasive cancers [123]. Monoubiquitination and diubiquitination of KRAS elevate its GTP loading ability [124]. CUL3-based E3 ligase complex can mediate polyubiquitination and degradation of KRAS [125]. KRAS4B, an alternative splicing of KRAS gene, is targeted by E3 ligase NEDD4 for ubiquitination and proteolysis. However, activated KRAS signaling upregulates NEDD4 expression and prevents NEDD4-mediated KRAS ubiquitination. This in return promotes NEDD4 catalyzed degradation of PTEN to trigger tumor growth [126]. Therefore, modification of signaling molecules by ubiquitination can exert various effects in signaling pathways, thereby regulating metabolic reprogramming in cancer cells.
Ubiquitination and transcription factors
Ubiquitination of HIF-1
HIF-1 is a metabolic-associated transcription factor which can be activated by mTORC1, accumulation of ROS and accumulation of TCA cycle metabolites. Activation of HIF-1 enhances expression of various glycolytic genes including hexokinase 1 (HK1), HK2, LDHA, and pyruvate dehydrogenase kinase isoform1 (PDK1), strengthening glycolytic flux and maintaining redox homeostasis [127]. HIF-1 consists of α and β subunits. Compared with HIF-1β, HIF-1α is unstable and susceptible to ubiquitination. E3 ligase VHL can mediate degradation of HIF-1α under normoxic conditions. Loss of VHL in cancer cells stabilizes HIF-1α, contributing to aerobic glycolysis [128, 129]. E3 ligase MDM2, PARKIN and HUWE1 can catalyze ubiquitination of HIF-1α and targets it for degradation, thus exerting an anti-tumor effect [130–132]. Tumor suppressors PTEN and p53 can enhance MDM2-mediated ubiquitination and degradation of HIF-1α to inhibit tumorigenesis [133–135]. Following phosphorylation by GSK3β, HIF-1α ubiquitination via FBXW7 is increased, which promotes proteolysis of HIF-1α and inhibits tumor growth [136]. Anticancer drug glyceollins and thymoquinone can inhibit tumor growth by elevating ubiquitination and degradation of HIF-1α [137, 138]. E3 ligase FBX11 can reduce the mRNA stability rather than protein stability of HIF-1α [139]. DUB USP28 can antagonize the ubiquitination of HIF-1α by FBXW7 [136]. TRIM44, USP20 and USP7 also stabilizes HIF-1α by deubiquitination, leading to tumor progression under hypoxia [140–143]. DUB OTUD7B can suppress degradation of HIF-1α via proteasome-independent manner [144]. USP8 mediated deubiquitination of HIF-1α was to maintain its expression level in normal conditions [145]. K63-linked polyubiquitination of HIF-1α catalyzed by TRAF6 can protect it from degradation [146]. XIAP modifies HIF-1α by K63-linked polyubiquitination to promote its nuclear retention and enhance HIF-dependent gene expression [147]. In conclusion, ubiquitination is an important regulatory mechanism of HIF-1.
Ubiquitination of c-Myc
Transcription factor c-Myc contributes to metabolic reprogramming through transcriptional regulation of genes participating in metabolism, such as LDHA [148]. c-Myc is an unstable protein susceptible to ubiquitination and proteolysis. E3 ligase SKP2, HUWE1, FBX29, TRUSS, RCHY1, CHIP, FBXW7, VHL, SPOP, TRIM32, NEDD4 and FBXL14 can target c-Myc for ubiquitination and degradation [149–160]. Decreased ubiquitination of c-Myc by VHL is observed to drive aerobic glycolysis in breast cancer cells [159]. Mutation or deletion of these E3 ligase genes induces carcinogenesis by attenuating c-Myc degradation. Anticancer drugs, including lanatoside C, diminish cancer cell growth by upregulating ubiquitination and degradation of c-Myc in cancer [161–163]. Ubiquitination of c-Myc can also be regulated by interaction with other molecules. FBXL16 can competitively bind with c-Myc without inducing ubiquitination of c-Myc, thereby rescuing c-Myc from FBXW7-mediated degradation [164]. Interaction with Evi5 also antagonizes FBXW7-mediated ubiquitination of c-Myc protein in laryngeal squamous cell carcinoma [165]. Phosphorylated ANXA2 interacts with MYC and inhibits ubiquitin-dependent proteasomal degradation of MYC protein in esophageal cancer [166]. LncRNA XLOC_006390, GLCC1 and LINC01638 also block ubiquitination of c-Myc [156, 167, 168]. What’s more, E3 ligase FBX28 mediated non-proteolytic ubiquitination of c-Myc can enhance c-Myc dependent transcription [169]. Ubiquitination of c-Myc by E3 ligase SCFβ-TrCP in G2 phase can stabilize c-Myc to facilitate recovery from an S-phase arrest [170]. Moreover, the DUB USP7, USP13, USP22, USP28, USP36 and USP37 stabilize c-Myc, thereby stimulating tumor growth [155, 157, 171–174].
Ubiquitination of p53
As a critical tumor suppressor participating in cell cycle regulation and apoptosis, p53 was also clarified by recent studies of its participation in metabolic reprogramming. Loss of p53 induces enhancement of glycolysis and maintenance of redox homeostasis in cancer cells [175]. Monoubiquitination, K48-linked and K63-linked polyubiquitination were observed to be common post-translational modification of p53 protein. MDM2/MDMX complex is the major E3 ligases and the main negative regulator of p53, degrading p53 and decreasing transcription of p53 target genes [176]. At the same time, MDM2 is targeted by p53 to form a negative feedback loop for the dynamic regulation of p53 under stressed and unstressed conditions [177]. RCHY1, COP1, CHIP, HUWE1, RING1, FBXW7, Synoviolin, MKRN1, TOPORS, CARP1/2, CUL4a-DDB1-ROC, CUL5, RNF115, TRIM23, TRIM24, TRIM28, TRIM39, TRIM69, TRIM71 can also target p53 for K48-linked polyubiquitination and degradation [178–185]. K48-linked polyubiquitination of p53 can be regulated by various molecules. For instance, MAVS and ATF3 can stabilize p53 by preventing p53 from MDM2-mediated ubiquitination [186, 187]. ACP5 mediated p53 phosphorylation enhanced the ubiquitination and degradation of p53 [188]. p53 protein can be deubiquitinated by DUB OTUD1, OTUD3, OTUD5, ATXN3, USP10, USP11, USP15, USP24, USP29 and USP42 to enhance its function as tumor suppressor under the conditions of high carcinogenicity and genotoxicity [117, 189–198]. DUB USP7 can catalyze deubiquitination of p53 and upregulate level of p53 protein as well [199]. On the other hand, USP7 mediates deubiquitination and stabilization of MDM2 and MDMX, the major E3 ligase of p53 protein, thereby reducing the protein level of p53 [200, 201]. Studies have found that the binding of USP7 with MDM2 is much stronger than that with p53 [200]. USP7 is highly expressed in most cancers, such as breast cancer and colorectal cancer, and plays carcinogenic role by deubiquitinating MDM2 and MDMX [202, 203]. Application of USP7 inhibitors can activate the p53 signaling in cancer cells and play anti-cancer functions [204]. However, USP7 is downregulated in some tumors, such as pulmonary adenocarcinoma, and plays a tumor suppressive role in p53-dependent mechanism [205]. Therefore, USP7 acts in a content-dependent manner and has a paradoxical action on p53 according to different tissues. What’s more, CUL7-mediated K63-linked polyubiquitination of p53 and MDM2 or MSL2-mediated monoubiquitination of p53 are associated with its translocation to the cytoplasm [206]. Anticancer drug HLI98 inhibits p53 degradation via MDM2 to enhance its tumor suppressive function [207]. Therefore, it’s likely for p53 ubiquitination regulation to act as an effective therapeutic method for cancers.
Ubiquitination of NRF2
Accumulation of ROS can also activate NRF2, another essential transcriptional factor for the maintenance of redox homeostasis. KEAP1, a substrate-specific adapter of a CUL3-RBX1 E3 ligase complex, is the binding partner of NRF2 and it negatively controls NRF2 stability. Under normal conditions, the CUL3-RBX1 mediates ubiquitination and degradation of NRF2. Electrophile metabolites formed in oxidative stress prevents CUL3-RBX1-KEAP1 complex from ubiquitinating NRF2, thereby stabilizing NRF2. NRF2 subsequently activates transcription of antioxidant proteins, such as GPXs and TXNs as well as enzymes involved in glutathione and NADPH synthesis [208]. p62, RMP and CDK20 can competitively bind with KEAP1 [209–211]. TRIM25 targets KEAP1 for ubiquitination and degradation [212]. DUB USP15 can stabilize KEAP1 [212]. These events will change the level of NRF2 and affect the transcriptional activity of NRF2 targeted genes. In non-small cell lung cancer, phosphorylation by BMP8A or interaction with PAQR4 can prevent ubiquitination of NRF2 by KEAP1 and stabilize NRF2, resulting in chemotherapy resistance [213, 214]. Besides CUL3-RBX1-KEAP1 complex, E3 ligase SCFβ-TrCP, CUL4-DDB1-WDR23, HRD1 can also ubiquitinate NRF2 and regulate its stability [215–217]. DUB DUB3 removes ubiquitin chain on NRF2 and stabilize NRF2, leading to chemoresistance in colorectal cancer [218].
Ubiquitination of SREBP1
SREBP1 is a transcription factor associated with lipogenic genes [219]. mTORC1 signaling induces enhanced activity of SREBP1 to meet the requirements for fatty acid in proliferating cancer cells [220]. Activation of SREBP1 upregulates fatty acid synthesis as well as lipid import from extracellular space [220]. After phosphorylated by GSK-3, SREBP1 is prone to ubiquitination by E3 ligase FBXW7 and degradation via the ubiquitin-proteasome system [221, 222]. Deacetylation of SREBP by SIRT1 also enhances its ubiquitination and proteolysis [223]. RNF139 can ubiquitinate precursor forms of SREBP1, thereby preventing SREBP1 synthesis [224].
Ubiquitination and autophagy
Ubiquitination of ULK1
In nutrient-deprived conditions, inhibition of mTORC1 subsequently activates autophagy, a highly regulated pathway essential for cell survival [25]. Autophagy complements amino acids by inducing degradation of macromolecules and organelles in lysosomes [26]. ULK1 complex, composed of the ULK1, ATG13 and FIP200, is directly regulated by mTORC1 and is required for autophagy induction. TRAF6 catalyzed K63-linked polyubiquitination of ULK1 can stabilize ULK1 to promote activation of autophagy, which is related to drug resistance in chronic myeloid leukemia patients [225]. Activating molecule in BECN1-regulated autophagy protein 1 (AMBRA1) is a cofactor that interacts with Beclin-1 to regulate autophagy. Decreased phosphorylation of AMBRA1 caused by mTORC1 inactivation will promote its interaction with TRAF6 to upregulate polyubiquitination of ULK1, thereby potentiating autophagy initiation [226]. TRIM16 and TRIM32 also target ULK1 for K63-linked polyubiquitination, which stabilizes ULK1 and increases its phosphorylating activity, respectively [227, 228]. DUB USP1 hydrolyzes the K63-linked ubiquitin chain on ULK1 [229]. Downregulation of USP24 also enhances ULK1 ubiquitination, thereby increasing protein stability and kinase activity of ULK1 [230]. These studies indicate the shift between ubiquitinated ULK1 and deubiquitinated ULK1 is essential for autophagy initiation.
Ubiquitination was also observed to be essential in the regulation of autophagy threshold during autophagy progression. NEDD4L-mediated ULK1 ubiquitination via K27 and K29-linkage assembly induces its proteolysis [231]. Decreased level of ULK1 activates transcription of ULK1 to maintain its basal protein level. Newly synthesized ULK1 will be deactivated by mTOR to ensure a safe threshold of autophagy [231]. CUL3-KLHL20 complex can also downregulate autophagy by targeting activated ULK1 for ubiquitination and proteolysis [232]. ULK1 can be deubiquitinated by USP20, which prevents it from degradation, maintaining its basal level required for the initiation of autophagy [233]. In prolonged starvation, the interaction between USP20 and ULK1 reduces to terminate autophagy [233]. In conclusion, ubiquitination and deubiquitination play essential functions in both autophagy initiation and autophagy progression. Modulating ubiquitination might be an effective treatment for chemoresistant patients with enhanced autophagy in cancer cells.
Ubiquitination of class III PI3K complex
Class III PI3K complex is composed of Beclin-1, ATG14, VPS34 and AMBRA1, essential for the nucleation of the phagophore. Various factors interact with Beclin-1 to regulate autophagy signaling. BCL2 can suppress autophagy by inhibiting Beclin-1 [234]. Starvation induced K63-linked ubiquitination of Beclin-1 by TRAF6 or AMBRA1 can block its interaction with BCL2, thus activating autophagy [235, 236]. AMBRA1 targeted polyubiquitination of Beclin-1 can enhance its association with VPS34 to activate of VPS34 [236]. TRIM50 mediated K63-linked polyubiquitination of Beclin-1 promotes its activation by ULK1 and induces autophagy in starvation [237]. DUB A20 and USP14 limit autophagy by reversing K63-linked ubiquitination of Beclin-1 [235, 238]. NEDD4 and RNF216 ubiquitinate Beclin-1 through K11- and K48- mediated linkage, respectively, which promote its degradation [239, 240]. DUB including USP19, ATXN3, USP10 and USP13 can reverse K11- or K48-ubiquitination of Beclin-1 to rescue it from degradation [241–243]. Beclin-1 also increases deubiquitinating activities of USP10 and USP13, further enhancing autophagy by positive feedback [241].
FBXL20 mediates ubiquitination and proteasome degradation of VPS34 to inhibit autophagy [244]. ATG14 was observed to be ubiquitinated by ZBTB16-CUL3-ROC1 E3 ubiquitin ligase complex for degradation [245].
AMBRA1 is degraded via the action of E3 ligase CUL4 under normal conditions. Activation of ULK1 disassociates CUL4 from AMBRA1, causing stabilization of AMBRA1 to promote autophagy. Disassociation of AMBRA1 with CUL4 can promote AMBRA1 binding to CUL5 to inhibit CUL5-mediated DEPTOR degradation, thereby inducing autophagy [55]. CUL4 can re-associate with AMBRA1 to promote its proteolysis when autophagy terminates, thus regulating autophagy response [55]. What’s more, E3 ligase RNF2 can also catalyze K48-linked ubiquitination and proteolysis of AMBRA1, thus downregulating autophagy [246]. Therefore, all the components of the Class III PI3K complex can be regulated by ubiquitination, which exerts important effects on autophagy.
Ubiquitination of WIPI2
WIPI2 is involved in an early step of the formation of preautophagosomal structures. mTORC1 can mediate phosphorylation of WIPI2. E3 ligase HUWE1 interacts with phosphorylated WIPI2 and catalyzes ubiquitination of phosphorylated WIPI2, which is subsequently targeted for proteolysis, thus inhibiting autophagy flux [247].
Ubiquitination of ATG4
ATG4 contributes to LC3 processing, playing an essential role in the phagophore expansion and autophagosome completion. E3 ligase RNF5 targets ubiquitination and degradation of ATG4B, thus limiting autophagy flux. When cell starves, RNF5 de-associates with ATG4B to induce autophagy [248].
Ubiquitination and metabolic enzymes
Ubiquitination and glucose metabolism
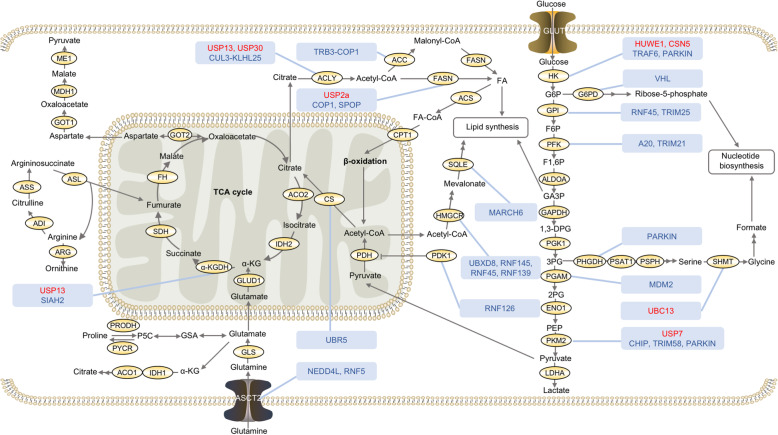
Increased glucose uptake and enhanced glycolytic flux are metabolic characteristics of cancer cells, supplying subsidiary pathways to provide precursors for macromolecule synthesis. Activated AKT was observed to inhibit ubiquitination of HK1, the first rate-limiting enzyme in the glucose metabolism pathway, promoting glycolysis and glioblastoma progression [249]. HUWE1 mediated K63-linked ubiquitination of HK2 promotes its re-localization and activation, enhancing glycolysis and tumor growth (Fig. 2) [250]. TRAF6 mediated K63-linked ubiquitination of HK2 directs HK2 degradation by autophagy, thereby negatively regulating glycolysis [251]. Deubiquitination of HK2 catalyzed by CSN5 can rescue it from degradation and enhance glycolytic flux during hepatocellular cancer metastasis [252]. Mitochondrial HK can also be ubiquitinated by PARKIN, inducing its proteasomal degradation [253].
Fig. 2.
Regulation of metabolic enzymes by ubiquitination and deubiquitination in cancer metabolism. Glycolysis is upregulated to provide more glycolytic intermediates for biosynthesis of macromolecules. Glutamine uptake is enhanced to maintain mitochondrial ATP production. Fatty acids synthesis is increased for membrane biosynthesis. Metabolic enzymes involved in the glucose, fatty acid and amino acid metabolic pathways are under the regulation of ubiquitination and deubiquitination to control cancer metabolism. The ubiquitin ligases and deubiquitinating enzymes in red font positively regulate the activity or expression level of substrate proteins. The ubiquitin ligases and deubiquitinating enzymes in blue font negatively regulate the activity or expression level of substrate proteins. ACC, Acetyl-coenzyme A carboxylase; ACLY, ATP citrate lyase; ACO1/2, Aconitate hydratase 1/2; ACS, Acyl-CoA synthetase; ADI, Arginine deiminase; ALDOA, Fructose-bisphosphate aldolase A; ARG, Arginase; ASCT2, Neutral amino acid transporter B; ASL, Argininosuccinate lyase; ASS, Argininosuccinate synthase; CPT1, Carnitine O-palmitoyltransferase 1; CS, Citrate synthase; ENO 1, Enolase 1; FASN, Fatty acid synthase; FH, Fumarate hydratase; G6PD, Glucose-6-phosphate dehydrogenase; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; GLS1, Glutaminase 1; GLUD1, Glutamate dehydrogenase 1; GLUT1, Glucose transporter type 1; GOT, Aspartate aminotransferase; HK1, Hexokinase 1; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; IDH1/2, Isocitrate dehydrogenase1/2; LDHA, L-lactate dehydrogenase A chain; MDH, Malate dehydrogenase; ME, NADP-dependent malic enzyme; PDH, Pyruvate dehydrogenase; PDK, Pyruvate dehydrogenase kinase; PFK, Phosphofructokinase; PFKFB3, 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3; PFKL, PFK liver type; PFKP, PFK1 platelet isoform; PGAM5, Phosphoglycerate mutase 5; PGK1, Phosphoglycerate kinase 1; PHGDH, D-3-phosphoglycerate dehydrogenase; PKM2, Pyruvate kinase M2; PRODH, Proline dehydrogenase; PSAT1, Phosphoserine aminotransferase 1; PSPH, Phosphoserine phosphatase; PYCR, Pyrroline-5-carboxylate reductase; SDH, Succinate dehydrogenase; SHMT1, Serine hydroxymethyltransferase 1; SQLE, Squalene epoxidase; TCA, Tricarboxylic acid; α-KGDH, α-ketoglutarate dehydrogenase
Phosphofructokinase (PFK), the second rate-limiting enzyme in glycolysis, is a key regulator of glycolytic flux in cancer cells. Decreased A20 mediated ubiquitination and degradation of PFK liver type (PFKL) are related to increased glycolysis during hepatocellular carcinoma progression [254]. Phosphorylation of PFK1 platelet isoform (PFKP) by AKT can prevent it from TRIM21-mediated ubiquitination and degradation, promoting aerobic glycolysis in glioblastoma cells [255].
Pyruvate kinase M2 (PKM2) is the third rate-limiting enzyme of glycolysis. Decreased CHIP catalyzed ubiquitination and degradation of PKM2 are associated with Warburg effect in ovarian cancer cells [256]. Downregulated ubiquitination of PKM2 by TRIM58 is related to progression of osteosarcoma [257]. E3 ligase PARKIN modifies PKM2 by ubiquitination to decrease its enzymatic activity without affecting its stability [258]. PKM2 deubiquitinated by USP7 can strengthen the protein stability of PKM2 [259]. USP20 can also hydrolyze the ubiquitin chain on PKM2, but the detailed function of this deubiquitination is unclear [260].
Other enzymes in glucose metabolism can be also modified by ubiquitination. Glucose-6-phosphate isomerase (GPI), which catalyzes the conversion of glucose-6-phosphate to fructose-6-phosphate, can be ubiquitinated and degraded by E3 ligase RNF45 and TRIM25 [261]. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) is a glycolysis-promoting enzyme catalyzing the conversion between fructose 2,6-bisphosphate and fructose 6-phosphate. ROCK2 interacts with PFKFB3 and attenuates its ubiquitination and degradation in osteosarcoma cells [262]. What’s more, glycolysis during different stages of cell cycle in Hela cells is regulated by E3 ligases. Decreased E3 ligase APC/C-CDH1 can attenuate proteasomal degradation of PFKFB3, promoting glycolysis and transition of late G1 phase into S phase. E3 ligase SCFβ-TrCP activated during S phase can target PFKFB3 for degradation [263]. Similarly, decreased ubiquitination and increased stability of glutaminase 1 (GLS1) were also observed to be mediated by decreased APC/C-CDH1 in mid-to-late G1 [264]. Phosphoglycerate kinase 1 (PGK1) catalyzes the conversion of 1,3-diphosphoglycerate to 3-phosphoglycerate. Decreased ubiquitination of PGK1 leads to chemotherapy resistance in gallbladder cancer cells [265]. Interaction between MetaLnc9 and PGK1 blocks ubiquitin-mediated degradation of PGK1, promoting lung cancer metastasis [266]. Following phosphorylation, phosphoglycerate mutase (PGAM), which converts 3-phosphoglycerate to 2-phosphoglycerate, can be ubiquitinated by MDM2 for degradation [267]. Decreased ubiquitination of PGAM by MDM2 contributes to neoplastic transformation [267]. PDK can be ubiquitinated by RNF126, which targets them for proteasomal degradation, enhancing conversion of pyruvate to acetyl-CoA by pyruvate dehydrogenase (PDH) in cancer cells [268].
Ubiquitination of enzymes involved in the TCA cycle is also associated with cancer progression. Decreased UBR5-mediated ubiquitination of citrate synthase leads to citrate accumulation in hypoxia breast cancer cells, promoting cell migration, invasion, and metastasis [269]. HIF-1 activation under hypoxia condition can promote α-ketoglutarate dehydrogenase (α-KGDH) complex ubiquitination and proteolysis by SIAH2. Decreased α-KGDH activity inhibits glutamine oxidation and promotes glutamine-dependent lipid synthesis for tumor growth [270]. USP13 promotes ovarian cancer progression by deubiquitinating and upregulating α-KGDH [271].
Glucose-6-phosphate dehydrogenase (G6PD) catalyzes the oxidative pentose phosphate pathway, an essential process producing ribose-5-phosphate and NAPDH from G6P. G6PD was observed to be ubiquitinated and degraded by VHL E3 ubiquitin ligase in podocytes [272]. VHL is tumor-suppressor protein [273]. But whether this regulation exists in cancer cells is unclear. Fructose-1,6-biphosphatase (FBP1) is a rate-limiting enzyme of gluconeogenesis. MAGE-TRIM28 mediated ubiquitination and degradation of FBP1 in hepatocellular carcinoma promotes Warburg effect and cancer progression [274]. Phosphoenolpyruvate carboxykinase 1 (PEPCK1) is another rate-limiting enzyme in gluconeogenesis. High glucose in diabetes stimulates PEPCK1 acetylation, which promotes UBR5 mediated ubiquitination and degradation of PEPCK1 [275]. Studies have found that GLUT1 can be ubiquitinated for degradation in diabetes [276]. E3 ubiquitin ligase Malin mediated ubiquitination of glycogen debranching enzyme (AGL) is associated with Lafora and Cori’s disease [277]. But whether ubiquitination of GLUT1, PEPCK1 and AGL participates in tumor progression is still unknown. What’s more, ubiquitination and deubiquitination of other cancer associated metabolic enzymes is still unknown, such as enolase in glycolysis, isocitrate dehydrogenase in the TCA cycle, glycogen phosphorylase in glycogen metabolic process, and pyruvate carboxylase in gluconeogenesis. The relationship between their specific E3 ligases and DUBs and oncogenesis still need exploration.
Ubiquitination and fatty acid metabolism
Fatty acids synthesis is necessary for membrane biosynthesis in proliferating tumor cells. CUL3-KLHL25 E3 ligase can inhibit lipid synthesis and tumor growth by targeting ATP citrate lyase (ACLY) for ubiquitination and proteolysis [278]. However, USP13 and USP30 can mediate deubiquitination of ACLY, increasing the stability of ACLY to promote development of ovarian cancer and hepatocellular carcinoma, respectively [271, 279]. TRB3-COP1 mediates proteolysis of acetyl-coenzyme A carboxylase (ACC) in ubiquitin-dependent manner, inhibiting fatty acid synthesis and stimulating lipolysis [280]. But in breast cancer cells, ACC-alpha (ACCA) interacts with AKR1B10, which prevents ACCA from ubiquitination and proteolysis, thereby promoting de novo fatty acid synthesis and enhancing tumor growth [281]. E3 ligase COP1 can ubiquitinate fatty acid synthase (FASN) with SHP2 as an adapter [282]. E3 ligase SPOP mutation, which is common in prostate cancer, inhibits its ubiquitination of FASN. Increased FASN triggers lipid accumulation and promotes prostate cancer progression [283]. AKT activation promotes deubiquitination of FASN by the USP2a and increased lipogenesis, which promotes hepatocarcinogenesis [284, 285].
3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) is a rate-limiting enzyme in cholesterol biosynthesis. E3 ligases UBXD, RNF145 and RNF45 can mediate sterol-induced ubiquitination and degradation of HMGCR, attenuating cholesterol biosynthesis [286–288]. Hypoxia triggered upregulation of insulin-induced gene 2 can interact with HMGCR and promote its ubiquitination and degradation to avoid unnecessary oxygen consumption [289]. Dysregulated cholesterol metabolism is observed in multidrug resistant cancer cells (MDR), in which decreased E3 ligase RNF139 upregulates HMGCR and induces enhanced cholesterol synthesis [290]. Squalene epoxidase (SQLE), which catalyzes the first oxygenation reaction of cholesterol biosynthesis, can be ubiquitinated and degraded by E3 ligase MARCH6 under stimulation of sterol [291]. Additionally, E3 ligase MYLIP can modulate cellular cholesterol uptake by ubiquitinating LDL receptor which is responsible for cholesterol import [292]. But whether ubiquitination of SQLE and LDL receptor participate in cancer progression is unknown. In addition, ubiquitination and deubiquitination of other enzymes participating in fatty acid metabolism, such as Carnitine O-palmitoyltransferase 1 (CPT1), and their relationship with cancer progression have not been studied yet.
Ubiquitination and amino acid metabolism
In cancer cells, glutamine serves as another important carbon source for the TCA cycle to sustain mitochondrial ATP production [1]. Glutamine uptake increases dramatically in cancer cells. NEDD4L-depleted cancer cells have enhanced neutral amino acid transporter B (ASCT2) stability and glutamine uptake to fuel the mitochondrial metabolism [293]. Promotion of RNF5-targeted ubiquitination and degradation of glutamine carrier proteins ASCT2 and SLC38A2 can improve responsiveness of breast cancer cells to Paclitaxel treatment [294]. Glutamine can be converted via GLS to glutamate, which can subsequently be converted to α-ketoglutarate to fuel the TCA cycle. Succinylation of GLS suppresses its K48-linked ubiquitination and degradation, stabilizing GLS and promoting glutaminolysis in cancer cells [295]. Study also found the function of supranutritional dose of selenite in suppressing tumor progression by promoting APC/C-CDH1 mediated GLS1 ubiquitination and degradation [296]. Ubiquitination of other enzymes involved in glutamine metabolism, such as glutamate dehydrogenase 1 (GLUD1), hasn’t been studied.
3-phosphoglycerate, an intermediate product of glycolysis, can be converted to serine by D-3-phosphoglycerate dehydrogenase (PHGDH). This conversion is subsequently associated with formate production for nucleotide synthesis. Downregulated PARKIN in cancer suppresses ubiquitination of PHGDH and enhances its stability and protein level, thereby activating serine synthesis and promoting cancer progression [297]. Serine hydroxymethyltransferase 1 (SHMT1) is involved in the conversion of serine to glycine. K48-linked ubiquitination of SHMT1 mediates its degradation in the cytoplasm. K63-linked ubiquitination of SHMT1 by UBC13 in the nucleus promotes its nuclear export and prevents it from degradation, promoting tumor progression [298]. What’s more, dysregulation of aspartate and arginine metabolism is also associated with cancer progression [299]. However, ubiquitination and deubiquitination of the enzymes participating in aspartate and arginine metabolism haven’t been studied and are worth attention in the future.
Conclusions
In the past decades, extensive efforts have been made to clarify the molecular mechanisms associated with metabolic reprogramming in cancer. In this review, we highlight the roles of ubiquitination and deubiquitination as modulators of cancer metabolism. Facing metabolic stresses, such as hypoxia, ubiquitination and deubiquitination in cancer cells can be abnormally regulated [10, 270]. On the other hand, dysregulated ubiquitination and deubiquitination play nonnegligible roles in cancer metabolism by involving in the regulation of metabolic reprogramming related signaling pathways, transcription factors as well as metabolic enzymes. For instance, hypoxia induces E3 ubiquitin ligase SIAH2 mediated ubiquitination and proteolysis of α-KGDH, inhibiting glutamine oxidation and promoting glutamine-dependent lipid synthesis to promote tumor growth [270]. Therefore, the interactions between ubiquitination/deubiquitination and cancer metabolism are complex and require more studies. Most studies have focused on the involvement of ubiquitination and deubiquitination in the regulation of signaling pathways and transcription factors, while ubiquitination and deubiquitination of the enzymes involved in glucose, fatty acid and amino acid metabolism are worth more attention in the future.
In the regulation of cancer metabolism and tumor progression, the E3 ubiquitin ligases/DUBs-substrates network is of high complexity. Single E3 ubiquitin ligase or DUB can target numerous substrates, and one molecule can be regulated by multiple E3 ubiquitin ligases or DUBs. For example, FBXW7 acts as a tumor suppressor by targeting mTOR, HIF-1α, c-Myc and SREBP1 for degradation [43, 136, 160, 222]. However, when facing DNA damage, elevated FBXW7 mediates proteasomal degradation of p53, leading to radiotherapy resistance [300]. Amino acids can stimulate subcellular localization of TRAF6 to lysosomes for subsequent K63-linked polyubiquitination and activation of mTOR signaling [42]. However, in starvation induced autophagy, TRAF6 mediates K63-linked polyubiquitination of ULK1, which leads to stabilization of ULK1 and activation of autophagy [225]. Therefore, E3 ligases and DUBs act in a context-dependent manner. Their exact roles in cancer may vary according to their substrates, tissues types, tumor stages, or different metabolic conditions. The study of ubiquitination and deubiquitination in cancer still has a long way to go. For example, whether metabolite levels within cancer cells act as modulators of ubiquitination is ambiguous. Importantly, development of specific drugs that disrupt or enhance specific E3 ligases/DUBs-substrates interactions holds promise for more efficient and less toxic therapeutics.
What’s more, we have observed that decreased ubiquitination and increased stability of the metabolic related molecules, such as PDK1, NRF2, ULK1 and phosphoglycerate kinase 1, are associated with chemoresistance in various cancers. Thereby, modulating the activity of E3 ligases or DUBs could be exploited as a potential strategy for controlling chemoresistance in cancer treatment. Furthermore, various E3 ligases and DUBs have been already identified as potential targets for cancer therapy. Actually, many E3 ligases serve as tumor suppressors by catalyzing ubiquitination and degradation of metabolic related proteins which play oncogenic roles in cancers, indicating that drugs enhancing activities or expression of these E3 ligases should also be emphasized in further researches.
In conclusion, ubiquitination and deubiquitination are suggested to be essential regulators of metabolic reprogramming in cancer cells, demanding more studies in the future with the aim of improving cancer therapy.
Acknowledgements
Not applicable.
Abbreviations
- LDH-A
L-lactate dehydrogenase A chain
- mTORC1
Mechanistic target of rapamycin complex 1
- HIF-1
Hypoxia-inducible factor 1
- SREBP
Sterol regulatory element-binding protein
- TCA
Tricarboxylic acid
- AMPK
5′-AMP-activated protein kinase
- ROS
Reactive oxygen species
- NRF2
Nuclear factor erythroid 2-related factor 2
- HECT
Homology to E6AP C terminus
- RBR
RING-between-RING
- RING
Really interesting new gene
- DUB
Deubiquitinating enzyme
- USP
Ub-specific protease
- UCH
Ub C-terminal hydrolase
- OUT
Ovarian tumor protease
- MJD
Machado-Josephin domain protease
- JAMM
JAB1/MPN+/MOV34
- DYRK2
Dual specificity tyrosine-phosphorylation-regulated kinase 2
- IDH1/2
Isocitrate dehydrogenase1/2
- TSC
Tuberous sclerosis complex
- PDK1
3-phosphoinositide-dependent protein kinase 1
- PTEN
Phosphatase and tensin homolog
- GLUT1
Glucose transporter type 1
- HK1
Hexokinase 1
- PDK
Pyruvate dehydrogenase kinase
- AMBRA1
Activating molecule in BECN1-regulated autophagy protein 1
- PFK
Phosphofructokinase
- PFKL
PFK liver type
- PFKP
PFK1 platelet isoform
- PFKFB3
6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3
- GLS1
Glutaminase 1
- PKM2
Pyruvate kinase M2
- PGK1
Phosphoglycerate kinase 1
- PGAM5
Phosphoglycerate mutase 5
- α-KGDH
α-ketoglutarate dehydrogenase
- G6PD
Glucose-6-phosphate dehydrogenase
- PEPCK1
Phosphoenolpyruvate carboxykinase 1
- PDH
Pyruvate dehydrogenase
- FBP1
Fructose-1,6-biphosphatase
- AGL
Glycogen debranching enzyme
- ACLY
ATP citrate lyase
- ACC
Acetyl-coenzyme A carboxylase
- FASN
Fatty acid synthase
- HMGCR
3-hydroxy-3-methylglutaryl-coenzyme A reductase
- SQLE
Squalene epoxidase
- CPT1
Carnitine O-palmitoyltransferase 1
- MDR
Multidrug resistant
- ASCT2
Neutral amino acid transporter B
- PHGDH
D-3-phosphoglycerate dehydrogenase
- SHMT1
Serine hydroxymethyltransferase 1
Authors’ contributions
T.S., Z.L. and Q.Y. conceived the review. T.S. and Z.L. wrote the first version of the manuscript. Q.Y. revised the manuscript. All of the authors approved the final version of the manuscript.
Funding
This research was supported by National Natural Science Foundation of China (Grant/Award Numbers: 81872125).
Availability of data and materials
All the data obtained and/or analyzed during the current study were available from the corresponding authors on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors give consent for the publication of manuscript in Molecular Cancer.
Competing interests
The authors declare that there is no potential competing interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 3.Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, Yang C, Do QN, Doucette S, Burguete D, et al. Lactate metabolism in human lung tumors. Cell. 2017;171:358–371 e359. doi: 10.1016/j.cell.2017.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreau P, Attal M, Caillot D, Macro M, Karlin L, Garderet L, Facon T, Benboubker L, Escoffre-Barbe M, Stoppa AM, et al. Prospective evaluation of magnetic resonance imaging and [(18)F]Fluorodeoxyglucose positron emission tomography-computed tomography at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 trial: results of the IMAJEM study. J Clin Oncol. 2017;35:2911–2918. doi: 10.1200/JCO.2017.72.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie H, Hanai J, Ren JG, Kats L, Burgess K, Bhargava P, Signoretti S, Billiard J, Duffy KJ, Grant A, et al. Targeting lactate dehydrogenase--a inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014;19:795–809. doi: 10.1016/j.cmet.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YH, Israelsen WJ, Lee D, Yu VWC, Jeanson NT, Clish CB, Cantley LC, Vander Heiden MG, Scadden DT. Cell-state-specific metabolic dependency in hematopoiesis and leukemogenesis. Cell. 2014;158:1309–1323. doi: 10.1016/j.cell.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antao AM, Tyagi A, Kim KS, Ramakrishna S. Advances in deubiquitinating enzyme inhibition and applications in cancer therapeutics. Cancers (Basel). 2020;12. [DOI] [PMC free article] [PubMed]
- 8.Park HB, Kim JW, Baek KH. Regulation of Wnt signaling through ubiquitination and deubiquitination in cancers. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed]
- 9.Mansour MA. Ubiquitination: friend and foe in cancer. Int J Biochem Cell Biol. 2018;101:80–93. doi: 10.1016/j.biocel.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Mennerich D, Kubaichuk K, Kietzmann T. DUBs, hypoxia, and cancer. Trends Cancer. 2019;5:632–653. doi: 10.1016/j.trecan.2019.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Deng L, Meng T, Chen L, Wei W, Wang P. The role of ubiquitination in tumorigenesis and targeted drug discovery. Signal Transduct Target Ther. 2020;5:11. doi: 10.1038/s41392-020-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nat Cell Biol. 2013;15:555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutchak PA, Estill-Terpack SJ, Plec AA, Zhao X, Yang C, Chen J, Ko B, Deberardinis RJ, Yu Y, Tu BP. Loss of a negative regulator of mTORC1 induces aerobic glycolysis and altered fiber composition in skeletal muscle. Cell Rep. 2018;23:1907–1914. doi: 10.1016/j.celrep.2018.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Csibi A, Fendt SM, Li C, Poulogiannis G, Choo AY, Chapski DJ, Jeong SM, Dempsey JM, Parkhitko A, Morrison T, et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell. 2013;153:840–854. doi: 10.1016/j.cell.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikalayeva V, Cesleviciene I, Sarapiniene I, Zvikas V, Skeberdis VA, Jakstas V, Bordel S. Fatty acid synthesis and degradation interplay to regulate the oxidative stress in cancer cells. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed]
- 17.Barker RM, Holly JMP, Biernacka KM, Allen-Birt SJ, Perks CM. Mini review: opposing pathologies in cancer and alzheimer’s disease: does the PI3K/Akt pathway provide clues? Front Endocrinol (Lausanne) 2020;11:403. doi: 10.3389/fendo.2020.00403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holczer M, Hajdu B, Lorincz T, Szarka A, Banhegyi G, Kapuy O. A double negative feedback loop between mTORC1 and AMPK kinases guarantees precise autophagy induction upon cellular stress. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed]
- 19.Zhao XA, Petrashen AP, Sanders JA, Peterson AL, Sedivy JM. SLC1A5 glutamine transporter is a target of MYC and mediates reduced mTORC1 signaling and increased fatty acid oxidation in long-lived Myc hypomorphic mice. Aging Cell. 2019;18. [DOI] [PMC free article] [PubMed]
- 20.Maddocks ODK, Berkers CR, Mason SM, Zheng L, Blyth K, Gottlieb E, Vousden KH. Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 2013;493:542. doi: 10.1038/nature11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D, Fiske BP, Birsoy K, Freinkman E, Kami K, Possemato RL, Chudnovsky Y, Pacold ME, Chen WW, Cantor JR, et al. SHMT2 drives glioma cell survival in ischaemia but imposes a dependence on glycine clearance. Nature. 2015;520:363-+. doi: 10.1038/nature14363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21:363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Liang M, Jiang JX, He RZ, Wang M, Guo XJ, Shen M, Qin RY. Combined inhibition of autophagy and Nrf2 signaling augments bortezomib-induced apoptosis by increasing ROS production and ER stress in pancreatic cancer cells. Int J Biol Sci. 2018;14:1291–1305. doi: 10.7150/ijbs.26776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan MS, Hwang J, Lee K, Choi Y, Seo Y, Jeon H, Hong JW, Choi J. Anti-tumor drug-loaded oxygen nanobubbles for the degradation of HIF-1 alpha and the upregulation of reactive oxygen species in tumor cells. Cancers. 2019;11. [DOI] [PMC free article] [PubMed]
- 25.Follo C, Vidoni C, Morani F, Ferraresi A, Seca C, Isidoro C. Amino acid response by halofuginone in cancer cells triggers autophagy through proteasome degradation of mTOR. Cell Commun Signal. 2019;17. [DOI] [PMC free article] [PubMed]
- 26.Yu L, Chen Y, Tooze SA. Autophagy pathway: cellular and molecular mechanisms. Autophagy. 2018;14:207–215. doi: 10.1080/15548627.2017.1378838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lock R, Roy S, Kenific CM, Su JS, Salas E, Ronen SM, Debnath J. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol Biol Cell. 2011;22:165–178. doi: 10.1091/mbc.e10-06-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C, Lin M, Wu R, Wang X, Yang B, Levine AJ, Hu W, Feng Z. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci U S A. 2011;108:16259–16264. doi: 10.1073/pnas.1113884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Tanowitz HB, Sotgia F, Lisanti MP. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell Biol. 2011;43:1045–1051. doi: 10.1016/j.biocel.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 31.Sewduth RN, Baietti MF, Sablina AA. Cracking the monoubiquitin code of genetic diseases. Int J Mol Sci. 2020;21. [DOI] [PMC free article] [PubMed]
- 32.Baur R, Rape M. Getting close: insight into the structure and function of K11/K48-branched ubiquitin chains. Structure. 2020;28:1–3. doi: 10.1016/j.str.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 33.Yao T, Ndoja A. Regulation of gene expression by the ubiquitin-proteasome system. Semin Cell Dev Biol. 2012;23:523–529. doi: 10.1016/j.semcdb.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flick K, Raasi S, Zhang H, Yen JL, Kaiser P. A ubiquitin-interacting motif protects polyubiquitinated Met4 from degradation by the 26S proteasome. Nat Cell Biol. 2006;8:509–515. doi: 10.1038/ncb1402. [DOI] [PubMed] [Google Scholar]
- 35.Le Cam L, Linares LK, Paul C, Julien E, Lacroix M, Hatchi E, Triboulet R, Bossis G, Shmueli A, Rodriguez MS, et al. E4F1 is an atypical ubiquitin ligase that modulates p53 effector functions independently of degradation. Cell. 2006;127:775–788. doi: 10.1016/j.cell.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 36.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 37.Tang R, Langdon WY, Zhang J. Regulation of immune responses by E3 ubiquitin ligase Cbl-b. Cell Immunol. 2019;340. [DOI] [PMC free article] [PubMed]
- 38.Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–1253. doi: 10.1038/nm.3739. [DOI] [PubMed] [Google Scholar]
- 39.Rogers-Broadway KR, Kumar J, Sisu C, Wander G, Mazey E, Jeyaneethi J, Pados G, Tsolakidis D, Klonos E, Grunt T, et al. Differential expression of mTOR components in endometriosis and ovarian cancer: effects of rapalogues and dual kinase inhibitors on mTORC1 and mTORC2 stoichiometry. Int J Mol Med. 2019;43:47–56. doi: 10.3892/ijmm.2018.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang P, Zhang Q, Tan L, Xu YN, Xie XB, Zhao Y. The regulatory effects of mTOR complexes in the differentiation and function of CD4(+) T cell subsets. J Immunol Res. 2020;2020. [DOI] [PMC free article] [PubMed]
- 41.Rogala KB, Gu X, Kedir JF, Abu-Remaileh M, Bianchi LF, Bottino AMS, Dueholm R, Niehaus A, Overwijn D, Fils ACP, et al. Structural basis for the docking of mTORC1 on the lysosomal surface. Science. 2019;366:468-+. doi: 10.1126/science.aay0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Linares JF, Duran A, Yajima T, Pasparakis M, Moscat J, Diaz-Meco MT. K63 polyubiquitination and activation of mTOR by the p62-TRAF6 complex in nutrient-activated cells. Mol Cell. 2013;51:283–296. doi: 10.1016/j.molcel.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mao JH, Kim IJ, Wu D, Climent J, Kang HC, DelRosario R, Balmain A. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321:1499–1502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang FF, Zhang XJ, Yan YR, Zhu XH, Yu J, Ding Y, Hu JL, Zhou WJ, Zeng ZC, Liao WT, et al. FBX8 is a metastasis suppressor downstream of miR-223 and targeting mTOR for degradation in colorectal carcinoma. Cancer Lett. 2017;388:85–95. doi: 10.1016/j.canlet.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 45.Mimoto R, Nihira NT, Hirooka S, Takeyama H, Yoshida K. Diminished DYRK2 sensitizes hormone receptor-positive breast cancer to everolimus by the escape from degrading mTOR. Cancer Lett. 2017;384:27–38. doi: 10.1016/j.canlet.2016.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Kim SY, Kim HJ, Kim HJ, Kim CH. Non-thermal plasma induces antileukemic effect through mTOR ubiquitination. Cells. 2020;9. [DOI] [PMC free article] [PubMed]
- 47.Park D, Lee MN, Jeong H, Koh A, Yang YR, Suh PG, Ryu SH. Parkin ubiquitinates mTOR to regulate mTORC1 activity under mitochondrial stress. Cell Signal. 2014;26:2122–2130. doi: 10.1016/j.cellsig.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Agrawal P, Chen YT, Schilling B, Gibson BW, Hughes RE. Ubiquitin-specific peptidase 9, X-linked (USP9X) modulates activity of mammalian target of rapamycin (mTOR) J Biol Chem. 2012;287:21164–21175. doi: 10.1074/jbc.M111.328021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hussain S, Feldman AL, Das C, Ziesmer SC, Ansell SM, Galardy PJ. Ubiquitin hydrolase UCH-L1 destabilizes mTOR complex 1 by antagonizing DDB1-CUL4-mediated ubiquitination of raptor. Mol Cell Biol. 2013;33:1188–1197. doi: 10.1128/MCB.01389-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang B, Jie ZL, Joo DH, Ordureau A, Liu P, Gan WJ, Guo JP, Zhang JF, North BJ, Dai XP, et al. TRAF2 and OTUD7B govern a ubiquitin-dependent switch that regulates mTORC2 signalling. Nature. 2017;545:365-+. doi: 10.1038/nature22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carbonneau M, Gagne LM, Lalonde ME, Germain MA, Motorina A, Guiot MC, Secco B, Vincent EE, Tumber A, Hulea L, et al. The oncometabolite 2-hydroxyglutarate activates the mTOR signalling pathway. Nat Commun. 2016;7. [DOI] [PMC free article] [PubMed]
- 52.Chen L, Liu TY, Tu YH, Rong DY, Cao Y. Cul1 promotes melanoma cell proliferation by promoting DEPTOR degradation and enhancing cap-dependent translation. Oncol Rep. 2016;35:1049–1056. doi: 10.3892/or.2015.4442. [DOI] [PubMed] [Google Scholar]
- 53.Tan MJ, Xu J, Siddiqui J, Feng FL, Sun Y. Depletion of SAG/RBX2 E3 ubiquitin ligase suppresses prostate tumorigenesis via inactivation of the PI3K/AKT/mTOR axis. Mol Cancer. 2016;15. [DOI] [PMC free article] [PubMed]
- 54.Zhao LL, Wang XB, Yu Y, Deng L, Chen L, Peng XP, Jiao CC, Gao GL, Tan X, Pan WJ, et al. OTUB1 protein suppresses mTOR complex 1 (mTORC1) activity by deubiquitinating the mTORC1 inhibitor DEPTOR. J Biol Chem. 2018;293:4883–4892. doi: 10.1074/jbc.M117.809533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antonioli M, Albiero F, Nazio F, Vescovo T, Perdomo AB, Corazzari M, Marsella C, Piselli P, Gretzmeier C, Dengjel J, et al. AMBRA1 interplay with cullin E3 ubiquitin ligases regulates autophagy dynamics. Dev Cell. 2014;31:734–746. doi: 10.1016/j.devcel.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Luo ZG, Pan YF, Jeong LS, Liu J, Jia LJ. Inactivation of the cullin (CUL)-RING E3 ligase by the NEDD8-activating enzyme inhibitor MLN4924 triggers protective autophagy in cancer cells. Autophagy. 2012;8:1677–1679. doi: 10.4161/auto.21484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng JB, Zhang Y, Ren X, Li D, Fu HJ, Liu CH, Zhou W, Liu Q, Liu Q, Wu MH. Leucine-rich repeat containing 4 act as an autophagy inhibitor that restores sensitivity of glioblastoma to temozolomide. Oncogene. 2020;39:4551–4566. doi: 10.1038/s41388-020-1312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Deng L, Jiang C, Chen L, Jin J, Wei J, Zhao L, Chen M, Pan W, Xu Y, Chu H, et al. The ubiquitination of rag A GTPase by RNF152 negatively regulates mTORC1 activation. Mol Cell. 2015;58:804–818. doi: 10.1016/j.molcel.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 59.Jin GX, Lee SW, Zhang X, Cai Z, Gao Y, Chou PC, Rezaeian AH, Han F, Wang CY, Yao JC, et al. Skp2-mediated RagA ubiquitination elicits a negative feedback to prevent amino-acid-dependent mTORC1 hyperactivation by recruiting GATOR1. Mol Cell. 2015;58:989–1000. doi: 10.1016/j.molcel.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Ou YH, Yang YY, Li W, Xu Y, Xie YT, Liu Y. KLHL22 activates amino-acid-dependent mTORC1 signalling to promote tumorigenesis and ageing. Nature. 2018;557:585. doi: 10.1038/s41586-018-0128-9. [DOI] [PubMed] [Google Scholar]
- 61.Deng L, Chen L, Zhao LL, Xu Y, Peng XP, Wang XB, Ding L, Jin JL, Teng HQ, Wang YM, et al. Ubiquitination of Rheb governs growth factor-induced mTORC1 activation. Cell Res. 2019;29:136–150. doi: 10.1038/s41422-018-0120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo P, Ma X, Zhao W, Huai W, Li T, Qiu Y, Zhang Y, Han L. TRIM31 is upregulated in hepatocellular carcinoma and promotes disease progression by inducing ubiquitination of TSC1-TSC2 complex. Oncogene. 2018;37:478–488. doi: 10.1038/onc.2017.349. [DOI] [PubMed] [Google Scholar]
- 63.Hu J, Zacharek S, He YJ, Lee H, Shumway S, Duronio RJ, Xiong Y. WD40 protein FBW5 promotes ubiquitination of tumor suppressor TSC2 by DDB1-CUL4-ROC1 ligase. Genes Dev. 2008;22:866–871. doi: 10.1101/gad.1624008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han S, Witt RM, Santos TM, Polizzano C, Sabatini BL, Ramesh V. Pam (protein associated with Myc) functions as an E3 ubiquitin ligase and regulates TSC/mTOR signaling. Cell Signal. 2008;20:1084–1091. doi: 10.1016/j.cellsig.2008.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng L, Ding HR, Lu ZM, Li Y, Pan YQ, Ning T, Ke Y. E3 ubiquitin ligase E6AP-mediated TSC2 turnover in the presence and absence of HPV16 E6. Genes Cells. 2008;13:285–294. doi: 10.1111/j.1365-2443.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- 66.Chong-Kopera H, Inoki K, Li Y, Zhu TQ, Garcia-Gonzalo FR, Rosa JL, Guan KL. TSC1 stabilizes TSC2 by inhibiting the interaction between TSC2 and the HERC1 ubiquitin ligase. J Biol Chem. 2006;281:8313–8316. doi: 10.1074/jbc.C500451200. [DOI] [PubMed] [Google Scholar]
- 67.Mohan N, Shen Y, Dokmanovic M, Endo Y, Hirsch DS, Wu WJ. VPS34 regulates TSC1/TSC2 heterodimer to mediate RheB and mTORC1/S6K1 activation and cellular transformation. Oncotarget. 2016;7:52239–52254. doi: 10.18632/oncotarget.10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Madigan JP, Hou F, Ye LL, Hu JC, Dong AP, Tempel W, Yohe ME, Randazzo PA, Jenkins LMM, Gottesman MM, Tong YF. The tuberous sclerosis complex subunit TBC1D7 is stabilized by Akt phosphorylation-mediated 14–3-3 binding. J Biol Chem. 2018;293:16142–16159. doi: 10.1074/jbc.RA118.003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodriguez-Escudero I, Roelants FM, Thorner J, Nombela C, Molina M, Cid VJ. Reconstitution of the mammalian PI3K/PTEN/Akt pathway in yeast. Biochem J. 2005;390:613–623. doi: 10.1042/BJ20050574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang ZX, Dang TT, Liu TT, Chen S, Li L, Huang S, Fang M. NEDD4L protein catalyzes ubiquitination of PIK3CA protein and regulates PI3K-AKT signaling. J Biol Chem. 2016;291:17467–17477. doi: 10.1074/jbc.M116.726083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hamidi A, Song J, Thakur N, Itoh S, Marcusson A, Bergh A, Heldin CH, Landstrom M. TGF-beta promotes PI3K-AKT signaling and prostate cancer cell migration through the TRAF6-mediated ubiquitylation of p85 alpha. Sci Signal. 2017;10. [DOI] [PubMed]
- 72.Jiang J, Xu YT, Ren HJ, Wudu M, Wang QZ, Song X, Su HB, Jiang XZ, Jiang LH, Qiu XS. MKRN2 inhibits migration and invasion of non-small-cell lung cancer by negatively regulating the PI3K/Akt pathway. J Exp Clin Cancer Res. 2018;37. [DOI] [PMC free article] [PubMed]
- 73.Ko HR, Kim CK, Lee SB, Song J, Lee KH, Kim KK, Park KW, Cho SW, Ahn JY. P42 Ebp1 regulates the proteasomal degradation of the p85 regulatory subunit of PI3K by recruiting a chaperone-E3 ligase complex HSP70/CHIP. Cell Death Dis. 2014;5. [DOI] [PMC free article] [PubMed]
- 74.Kuchay S, Duan SS, Schenkein E, Peschiaroli A, Saraf A, Florens L, Washburn MP, Pagano M. FBXL2-and PTPL1-mediated degradation of p110-free p85 beta regulatory subunit controls the PI(3)K signalling cascade. Nat Cell Biol. 2013;15:472-+. doi: 10.1038/ncb2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu YH, Chang TH, Huang YF, Chen CC, Chou CY. COL11A1 confers chemoresistance on ovarian cancer cells through the activation of Akt/c/EBP beta pathway and PDK1 stabilization. Oncotarget. 2015;6:23748–23763. doi: 10.18632/oncotarget.4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uras IZ, List T, Nijman SMB. Ubiquitin-specific protease 4 inhibits mono-ubiquitination of the master growth factor signaling kinase PDK1. PLoS One. 2012;7. [DOI] [PMC free article] [PubMed]
- 77.Chan CH, Li CF, Yang WL, Gao Y, Lee SW, Feng ZZ, Huang HY, Tsai KKC, Flores LG, Shao YP, et al. The Skp2-SCF E3 ligase regulates Akt ubiquitination, glycolysis, herceptin sensitivity, and tumorigenesis (vol 149, pg 1098, 2012) Cell. 2012;151:913–914. doi: 10.1016/j.cell.2012.10.025. [DOI] [PubMed] [Google Scholar]
- 78.Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, Hur L, Grabiner BC, Lin X, Darnay BG, Lin HK. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325:1134–1138. doi: 10.1126/science.1175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fan CD, Lum MA, Xu C, Black JD, Wang XJ. Ubiquitin-dependent regulation of phospho-AKT dynamics by the ubiquitin E3 ligase, NEDD4–1, in the insulin-like growth factor-1 response. J Biol Chem. 2013;288:1674–1684. doi: 10.1074/jbc.M112.416339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li W, Peng C, Lee MH, Lim D, Zhu F, Fu Y, Yang G, Sheng YQ, Xiao LB, Dong X, et al. TRAF4 is a critical molecule for Akt activation in lung cancer. Cancer Res. 2013;73:6938–6950. doi: 10.1158/0008-5472.CAN-13-0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang JD, Yang ZF, Ou JY, Xia XJ, Zhi F, Cui J. The F-box protein FBXL18 promotes glioma progression by promoting K63-linked ubiquitination of Akt. FEBS Lett. 2017;591:145–154. doi: 10.1002/1873-3468.12521. [DOI] [PubMed] [Google Scholar]
- 82.Liang CX, Liang GY, Zheng XQ, Huang YX, Huang SH, Yin D. RSP5 positively regulates the osteogenic differentiation of mesenchymal stem cells by activating the K63-linked ubiquitination of Akt. Stem Cells Int. 2020;2020. [DOI] [PMC free article] [PubMed]
- 83.Wang GH, Long J, Gao Y, Zhang WN, Han F, Xu C, Sun L, Yang SC, Lan JQ, Hou ZL, et al. SETDB1-mediated methylation of Akt promotes its K63-linked ubiquitination and activation leading to tumorigenesis. Nat Cell Biol. 2019;21:214. doi: 10.1038/s41556-018-0266-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li HJ, Lan JQ, Wang GH, Guo KX, Han CS, Li XL, Hu JB, Cao ZX, Luo XL. KDM4B facilitates colorectal cancer growth and glucose metabolism by stimulating TRAF6-mediated AKT activation. J Exp Clin Cancer Res. 2020;39. [DOI] [PMC free article] [PubMed]
- 85.Lim JH, Jono H, Komatsu K, Woo CH, Lee J, Miyata M, Matsuno T, Xu XB, Huang YX, Zhang WH, et al. CYLD negatively regulates transforming growth factor-beta-signalling via deubiquitinating Akt. Nat Commun. 2012;3. [DOI] [PMC free article] [PubMed]
- 86.Yin FL, He HG, Zhang B, Zheng JH, Wang M, Zhang M, Cui HX. Effect of deubiquitinase ovarian tumor domain-containing protein 5 (OTUD5) on radiosensitivity of cervical cancer by regulating the ubiquitination of Akt and its mechanism. Med Sci Monit. 2019;25:3469–3475. doi: 10.12659/MSM.912904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Goldbraikh D, Neufeld D, Eid-Mutlak Y, Lasry I, Gilda JE, Parnis A, Cohen S. USP1 deubiquitinates Akt to inhibit PI3K-Akt-FoxO signaling in muscle during prolonged starvation. EMBO Rep. 2020;21. [DOI] [PMC free article] [PubMed]
- 88.Qiu CJ, Liu KR, Zhang S, Gao SM, Chen WR, Li DT, Huang YX. Bisdemethoxycurcumin inhibits hepatocellular carcinoma proliferation through Akt inactivation via CYLD-mediated deubiquitination. Drug Des Devel Ther. 2020;14:993–1001. doi: 10.2147/DDDT.S231814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xiang T, Ohashi A, Huang YP, Pandita TK, Ludwig T, Powell SN, Yang Q. Negative regulation of AKT activation by BRCA1. Cancer Res. 2008;68:10040–10044. doi: 10.1158/0008-5472.CAN-08-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suizu F, Hiramuki Y, Okumura F, Matsuda M, Okumura AJ, Hirata N, Narita M, Kohno T, Yokota J, Bohgaki M, et al. The E3 ligase TTC3 facilitates ubiquitination and degradation of phosphorylated Akt. Dev Cell. 2009;17:800–810. doi: 10.1016/j.devcel.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 91.Joo HM, Kim JY, Jeong JB, Seong KM, Nam SY, Yang KH, Kim CS, Kim HS, Jeong M, An S, Jin YW. Ret finger protein 2 enhances ionizing radiation-induced apoptosis via degradation of AKT and MDM2. Eur J Cell Biol. 2011;90:420–431. doi: 10.1016/j.ejcb.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 92.Bae SH, Kim SY, Jung JH, Yoon YM, Cha HJ, Lee HJ, Kim K, Kim J, An IS, Kim J, et al. Akt is negatively regulated by the MULAN E3 ligase. Cell Res. 2012;22:873–885. doi: 10.1038/cr.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Su CH, Wang CY, Lan KH, Li CP, Chao Y, Lin HC, Lee SD, Lee WP. Akt phosphorylation at Thr308 and Ser473 is required for CHIP-mediated ubiquitination of the kinase. Cell Signal. 2011;23:1824–1830. doi: 10.1016/j.cellsig.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 94.Wakatsuki S, Saitoh F, Araki T. ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation. Nat Cell Biol. 2011;13:1415–U1259. doi: 10.1038/ncb2373. [DOI] [PubMed] [Google Scholar]
- 95.Athamneh K, El Hasasna H, Al Samri H, Attoub S, Arafat K, Benhalilou N, Al Rashedi A, Al Dhaheri Y, AbuQamar S, Eid A, Iratni R. Rhus coriaria increases protein ubiquitination, proteasomal degradation and triggers non-canonical Beclin-1-independent autophagy and apoptotic cell death in colon cancer cells. Sci Rep. 2017;7. [DOI] [PMC free article] [PubMed]
- 96.Su X, Shen Z, Yang Q, Sui F, Pu J, Ma JJ, Ma SR, Yao DM, Ji MJ, Hou P. Vitamin C kills thyroid cancer cells through ROS-dependent inhibition of MAPK/ERK and PI3K/AKT pathways via distinct mechanisms. Theranostics. 2019;9:4461–4473. doi: 10.7150/thno.35219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim HJ, Kim SY, Kim DH, Park JS, Jeong SH, Choi YW, Kim CH. Crosstalk between HSPA5 arginylation and sequential ubiquitination leads to AKT degradation through autophagy flux. Autophagy. 2020. [DOI] [PMC free article] [PubMed]
- 98.Trotman LC, Wang XJ, Alimonti A, Chen ZB, Teruya-Feldstein J, Yang HJ, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, et al. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shen X, Zhong JX, Yu P, Zhao QY, Huang T. YY1-regulated LINC00152 promotes triple negative breast cancer progression by affecting on stability of PTEN protein. Biochem Biophys Res Commun. 2019;509:448–454. doi: 10.1016/j.bbrc.2018.12.074. [DOI] [PubMed] [Google Scholar]
- 100.Maddika S, Kavela S, Rani N, Palicharla VR, Pokorny JL, Sarkaria JN, Chen JJ. WWP2 is an E3 ubiquitin ligase for PTEN. Nat Cell Biol. 2011;13:728–U224. doi: 10.1038/ncb2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang H, Wang XX, Zhou CY, Xiao X, Tian C, Li HH, Yin CL, Wang HX. Tripartite motif 10 regulates cardiac hypertrophy by targeting the PTEN/AKT pathway. J Cell Mol Med. 2020;24:6233–6241. doi: 10.1111/jcmm.15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yuan P, Zheng AD, Tang Q. Tripartite motif protein 25 is associated with epirubicin resistance in hepatocellular carcinoma cells via regulating PTEN/AKT pathway. Cell Biol Int. 2020;44:1503–1513. doi: 10.1002/cbin.11346. [DOI] [PubMed] [Google Scholar]
- 103.He R, Liu HX. TRIM59 knockdown blocks cisplatin resistance in A549/DDP cells through regulating PTEN/AKT/HK2. Gene. 2020;747. [DOI] [PubMed]
- 104.Ma L, Yao NH, Chen P, Zhuang ZX. TRIM27 promotes the development of esophagus cancer via regulating PTEN/AKT signaling pathway. Cancer Cell Int. 2019;19. [DOI] [PMC free article] [PubMed]
- 105.Shen WD, Jin ZH, Tong XP, Wang HY, Zhuang LL, Lu XF, Wu SB. TRIM14 promotes cell proliferation and inhibits apoptosis by suppressing PTEN in colorectal cancer. Cancer Manag Res. 2019;11:5725–5735. doi: 10.2147/CMAR.S210782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Themsche C, Leblanc V, Parent S, Asselin E. X-linked inhibitor of apoptosis protein (XIAP) regulates PTEN ubiquitination, content, and compartmentalization. J Biol Chem. 2009;284:20462–20466. doi: 10.1074/jbc.C109.009522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee MS, Jeong MH, Lee HW, Han HJ, Ko A, Hewitt SM, Kim JH, Chun KH, Chung JY, Lee C, et al. PI3K/AKT activation induces PTEN ubiquitination and destabilization accelerating tumourigenesis. Nat Commun. 2015;6. [DOI] [PMC free article] [PubMed]
- 108.Lee YR, Chen M, Lee JD, Zhang J, Lin SY, Fu TM, Chen H, Ishikawa T, Chiang SY, Katon J, et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science. 2019;364. [DOI] [PMC free article] [PubMed]
- 109.Lee JT, Shan J, Zhong JY, Li MY, Zhou B, Zhou A, Parsons R, Gu W. RFP-mediated ubiquitination of PTEN modulates its effect on AKT activation. Cell Res. 2013;23:552–564. doi: 10.1038/cr.2013.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun J, Li TX, Zhao YY, Huang LR, Sun H, Wu H, Jiang XF. USP10 inhibits lung cancer cell growth and invasion by upregulating PTEN. Mol Cell Biochem. 2018;441:1–7. doi: 10.1007/s11010-017-3170-2. [DOI] [PubMed] [Google Scholar]
- 111.Zhang JS, Zhang PJ, Wei YK, Piao HL, Wang WQ, Maddika S, Wang M, Chen DH, Sun YT, Hung MC, et al. Deubiquitylation and stabilization of PTEN by USP13. Nat Cell Biol. 2013;15:1486. doi: 10.1038/ncb2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yuan L, Lv YR, Li HC, Gao HD, Song SS, Zhang Y, Xing GC, Kong XZ, Wang LJ, Li Y, et al. Deubiquitylase OTUD3 regulates PTEN stability and suppresses tumorigenesis (vol 17, pg 1169, 2015). Nat Cell Biol. 2015;17. [DOI] [PubMed]
- 113.Shen WM, Yin JN, Xu RJ, Xu DF, Zheng SY. Ubiquitin specific peptidase 49 inhibits non-small cell lung cancer cell growth by suppressing PI3K/AKT signaling. Kaohsiung J Med Sci. 2019;35:401–407. doi: 10.1002/kjm2.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang H, Wei PT, Lv WW, Han XT, Yang JH, Qin SF. Long noncoding RNA lnc-DILC stabilizes PTEN and suppresses clear cell renal cell carcinoma progression. Cell Biosci. 2019;9. [DOI] [PMC free article] [PubMed]
- 115.Morotti A, Panuzzo C, Crivellaro S, Pergolizzi B, Familiari U, Berger AH, Saglio G, Pandolfi PP. BCR-ABL disrupts PTEN nuclear-cytoplasmic shuttling through phosphorylation-dependent activation of HAUSP. Leukemia. 2014;28:1326–1333. doi: 10.1038/leu.2013.370. [DOI] [PubMed] [Google Scholar]
- 116.Wu Y, Zhou H, Wu K, Lee S, Li RJ, Liu X. PTEN phosphorylation and nuclear export mediate free fatty acid-induced oxidative stress. Antioxid Redox Signal. 2014;20:1382–1395. doi: 10.1089/ars.2013.5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sacco JJ, Yau TY, Darling S, Patel V, Liu H, Urbe S, Clague MJ, Coulson JM. The deubiquitylase Ataxin-3 restricts PTEN transcription in lung cancer cells. Oncogene. 2014;33:4265–4272. doi: 10.1038/onc.2013.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pineda CT, Ramanathan S, Tacer KF, Weon JL, Potts MB, Ou YH, White MA, Potts PR. Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell. 2015;160:715–728. doi: 10.1016/j.cell.2015.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kwon E, Li X, Deng Y, Chang HW, Kim DY. AMPK is down-regulated by the CRL4A-CRBN axis through the polyubiquitination of AMPKalpha isoforms. FASEB J. 2019;33:6539–6550. doi: 10.1096/fj.201801766RRR. [DOI] [PubMed] [Google Scholar]
- 120.Vila IK, Song SJ, Song MS. A new duet in cancer biology: AMPK the typical and UBE2O the atypical. Mol Cell Oncol. 2017;4:e1304846. doi: 10.1080/23723556.2017.1304846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang SJ, Jeon SJ, Nguyen TV, Deshaies RJ, Park CS, Lee KM. Ubiquitin-dependent proteasomal degradation of AMPK gamma subunit by cereblon inhibits AMPK activity. Biochim Biophys Acta Mol Cell Res. 2020;1867. [DOI] [PubMed]
- 122.Liu HZ, Ding J, Kohnlein K, Urban N, Ori A, Villavicencio-Lorini P, Walentek P, Klotz LO, Hollemann T, Pfirrmann T. The GID ubiquitin ligase complex is a regulator of AMPK activity and organismal lifespan. Autophagy. 2019. [DOI] [PMC free article] [PubMed]
- 123.Xu K, Park D, Magis AT, Zhang J, Zhou W, Sica GL, Ramalingam SS, Curran WJ, Deng XM. Small molecule KRAS agonist for mutant KRAS cancer therapy. Mol Cancer. 2019;18. [DOI] [PMC free article] [PubMed]
- 124.Sasaki AT, Carracedo A, Locasale JW, Anastasiou D, Takeuchi K, Kahoud ER, Haviv S, Asara JM, Pandolfi PP, Cantley LC. Ubiquitination of K-Ras enhances activation and facilitates binding to select downstream effectors. Sci Signal. 2011;4. [DOI] [PMC free article] [PubMed]
- 125.Abe T, Umeki I, Kanno S, Inoue S, Niihori T, Aoki Y. LZTR1 facilitates polyubiquitination and degradation of RAS-GTPases. Cell Death Differ. 2020;27:1023–1035. doi: 10.1038/s41418-019-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zeng TL, Wang Q, Fu JY, Lin Q, Bi J, Ding WC, Qiao YK, Zhang S, Zhao WX, Lin HY, et al. Impeded Nedd4–1-mediated Ras degradation underlies Ras-driven tumorigenesis. Cell Rep. 2014;7:871–882. doi: 10.1016/j.celrep.2014.03.045. [DOI] [PubMed] [Google Scholar]
- 127.Dang CV. The interplay between MYC and HIF in the Warburg effect. Ernst Schering Found Symp Proc. 2007:35–53. [DOI] [PubMed]
- 128.Buckley DL, Van Molle I, Gareiss PC, Tae HS, Michel J, Noblin DJ, Jorgensen WL, Ciulli A, Crews CM. Targeting the von Hippel-Lindau E3 ubiquitin ligase using small molecules to disrupt the VHL/HIF-1alpha interaction. J Am Chem Soc. 2012;134:4465–4468. doi: 10.1021/ja209924v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guo Y, Meng X, Ma J, Zheng Y, Wang Q, Wang Y, Shang H. Human papillomavirus 16 E6 contributes HIF-1alpha induced Warburg effect by attenuating the VHL-HIF-1alpha interaction. Int J Mol Sci. 2014;15:7974–7986. doi: 10.3390/ijms15057974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu J, Zhang C, Zhao YH, Yue XT, Wu H, Huang S, Chen J, Tomsky K, Xie HY, Khella CA, et al. Parkin targets HIF-1 alpha for ubiquitination and degradation to inhibit breast tumor progression. Nat Commun. 2017;8. [DOI] [PMC free article] [PubMed]
- 131.Zhang L, Cao J, Dong L, Lin H. TiPARP forms nuclear condensates to degrade HIF-1alpha and suppress tumorigenesis. Proc Natl Acad Sci U S A. 2020;117:13447–13456. doi: 10.1073/pnas.1921815117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Thirusangu P, Vigneshwaran V, Prashanth T, Vijay Avin BR, Malojirao VH, Rakesh H, Khanum SA, Mahmood R, Prabhakar BT. BP-1T, an antiangiogenic benzophenone-thiazole pharmacophore, counteracts HIF-1 signalling through p53/MDM2-mediated HIF-1alpha proteasomal degradation. Angiogenesis. 2017;20:55–71. doi: 10.1007/s10456-016-9528-3. [DOI] [PubMed] [Google Scholar]
- 133.Joshi S, Singh AR, Durden DL. MDM2 regulates hypoxic hypoxia-inducible factor 1alpha stability in an E3 ligase, proteasome, and PTEN-phosphatidylinositol 3-kinase-AKT-dependent manner. J Biol Chem. 2014;289:22785–22797. doi: 10.1074/jbc.M114.587493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chowdhury AR, Long A, Fuchs SY, Rustgi A, Avadhani NG. Mitochondrial stress-induced p53 attenuates HIF-1alpha activity by physical association and enhanced ubiquitination. Oncogene. 2017;36:397–409. doi: 10.1038/onc.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Amelio I, Inoue S, Markert EK, Levine AJ, Knight RA, Mak TW, Melino G. TAp73 opposes tumor angiogenesis by promoting hypoxia-inducible factor 1alpha degradation. Proc Natl Acad Sci U S A. 2015;112:226–231. doi: 10.1073/pnas.1410609111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Flugel D, Gorlach A, Kietzmann T. GSK-3beta regulates cell growth, migration, and angiogenesis via Fbw7 and USP28-dependent degradation of HIF-1alpha. Blood. 2012;119:1292–1301. doi: 10.1182/blood-2011-08-375014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee SH, Jee JG, Bae JS, Liu KH, Lee YM. A group of novel HIF-1alpha inhibitors, glyceollins, blocks HIF-1alpha synthesis and decreases its stability via inhibition of the PI3K/AKT/mTOR pathway and Hsp90 binding. J Cell Physiol. 2015;230:853–862. doi: 10.1002/jcp.24813. [DOI] [PubMed] [Google Scholar]
- 138.Lee YM, Kim GH, Park EJ, Oh TI, Lee S, Kan SY, Kang H, Kim BM, Kim JH, Lim JH. Thymoquinone selectively kills hypoxic renal cancer cells by suppressing HIF-1alpha-mediated glycolysis. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed]
- 139.Ju UI, Park JW, Park HS, Kim SJ, Chun YS. FBXO11 represses cellular response to hypoxia by destabilizing hypoxia-inducible factor-1alpha mRNA. Biochem Biophys Res Commun. 2015;464:1008–1015. doi: 10.1016/j.bbrc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 140.Chen Z, Lin TC, Bi X, Lu G, Dawson BC, Miranda R, Medeiros LJ, McNiece I, McCarty N. TRIM44 promotes quiescent multiple myeloma cell occupancy and survival in the osteoblastic niche via HIF-1alpha stabilization. Leukemia. 2019;33:469–486. doi: 10.1038/s41375-018-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu HT, Kuo YC, Hung JJ, Huang CH, Chen WY, Chou TY, Chen Y, Chen YJ, Chen YJ, Cheng WC, et al. K63-polyubiquitinated HAUSP deubiquitinates HIF-1alpha and dictates H3K56 acetylation promoting hypoxia-induced tumour progression. Nat Commun. 2016;7:13644. doi: 10.1038/ncomms13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Park JJ, Yun JH, Baek KH. Polyclonal and monoclonal antibodies specific for ubiquitin-specific protease 20. Monoclon Antib Immunodiagn Immunother. 2013;32:193–199. doi: 10.1089/mab.2012.0120. [DOI] [PubMed] [Google Scholar]
- 143.Li Z, Wang D, Messing EM, Wu G. VHL protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes HIF-1alpha. EMBO Rep. 2005;6:373–378. doi: 10.1038/sj.embor.7400377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bremm A, Moniz S, Mader J, Rocha S, Komander D. Cezanne (OTUD7B) regulates HIF-1alpha homeostasis in a proteasome-independent manner. EMBO Rep. 2014;15:1268–1277. doi: 10.15252/embr.201438850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Troilo A, Alexander I, Muehl S, Jaramillo D, Knobeloch KP, Krek W. HIF1alpha deubiquitination by USP8 is essential for ciliogenesis in normoxia. EMBO Rep. 2014;15:77–85. doi: 10.1002/embr.201337688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sun H, Li XB, Meng Y, Fan L, Li M, Fang J. TRAF6 upregulates expression of HIF-1alpha and promotes tumor angiogenesis. Cancer Res. 2013;73:4950–4959. doi: 10.1158/0008-5472.CAN-13-0370. [DOI] [PubMed] [Google Scholar]
- 147.Park CV, Ivanova IG, Kenneth NS. XIAP upregulates expression of HIF target genes by targeting HIF1alpha for Lys63-linked polyubiquitination. Nucleic Acids Res. 2017;45:9336–9347. doi: 10.1093/nar/gkx549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A. 1997;94:6658–6663. doi: 10.1073/pnas.94.13.6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee S, Kim W, Ko C, Ryu WS. Hepatitis B virus X protein enhances Myc stability by inhibiting SCF(Skp2) ubiquitin E3 ligase-mediated Myc ubiquitination and contributes to oncogenesis. Oncogene. 2016;35:1857–1867. doi: 10.1038/onc.2015.251. [DOI] [PubMed] [Google Scholar]
- 150.The MULE/HUWE1 E3 ubiquitin ligase is a tumor suppressor. Cancer Discov. 2013;3:OF32. [DOI] [PubMed]
- 151.Koch HB, Zhang R, Verdoodt B, Bailey A, Zhang CD, Yates JR, 3rd, Menssen A, Hermeking H. Large-scale identification of c-MYC-associated proteins using a combined TAP/MudPIT approach. Cell Cycle. 2007;6:205–217. doi: 10.4161/cc.6.2.3742. [DOI] [PubMed] [Google Scholar]
- 152.Choi SH, Wright JB, Gerber SA, Cole MD. Myc protein is stabilized by suppression of a novel E3 ligase complex in cancer cells. Genes Dev. 2010;24:1236–1241. doi: 10.1101/gad.1920310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hakem A, Bohgaki M, Lemmers B, Tai E, Salmena L, Matysiak-Zablocki E, Jung YS, Karaskova J, Kaustov L, Duan SL, et al. Role of Pirh2 in mediating the regulation of p53 and c-Myc. PLoS Genet. 2011;7. [DOI] [PMC free article] [PubMed]
- 154.Paul I, Ahmed SF, Bhowmik A, Deb S, Ghosh MK. The ubiquitin ligase CHIP regulates c-Myc stability and transcriptional activity. Oncogene. 2013;32:1284–1295. doi: 10.1038/onc.2012.144. [DOI] [PubMed] [Google Scholar]
- 155.Fang XG, Zhou WC, Wu QL, Huang Z, Shi Y, Yang KL, Chen C, Xie Q, Mack SC, Wang XX, et al. Deubiquitinase USP13 maintains glioblastoma stem cells by antagonizing FBXL14-mediated Myc ubiquitination. J Exp Med. 2017;214:245–267. doi: 10.1084/jem.20151673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Luo LY, Tang HL, Ling L, Li N, Jia XT, Zhang ZJ, Wang XR, Shi LJ, Yin J, Qiu N, et al. LINC01638 lncRNA activates MTDH-Twist1 signaling by preventing SPOP-mediated c-Myc degradation in triple-negative breast cancer. Oncogene. 2018;37:6166–6179. doi: 10.1038/s41388-018-0396-8. [DOI] [PubMed] [Google Scholar]
- 157.Nicklas S, Hillje AL, Okawa S, Rudolph IM, Collmann FM, van Wuellen T, del Sol A, Schwamborn JC. A complex of the ubiquitin ligase TRIM32 and the deubiquitinase USP7 balances the level of c-Myc ubiquitination and thereby determines neural stem cell fate specification. Cell Death Differ. 2019;26:728–740. doi: 10.1038/s41418-018-0144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen J, Li WJ, Cui K, Ji KY, Xu SX, Xu Y. Artemisitene suppresses tumorigenesis by inducing DNA damage through deregulating c-Myc-topoisomerase pathway. Oncogene. 2018;37:5079–5087. doi: 10.1038/s41388-018-0331-z. [DOI] [PubMed] [Google Scholar]
- 159.Wang SQ, Wang N, Zheng YF, Yang BW, Liu PX, Zhang FX, Li M, Song JX, Chang X, Wang ZY. Caveolin-1 inhibits breast cancer stem cells via c-Myc-mediated metabolic reprogramming. Cell Death Dis. 2020;11. [DOI] [PMC free article] [PubMed]
- 160.Gonzalez-Pecchi V, Kwan AK, Doyle S, Ivanov AA, Du Y, Fu H. NSD3S stabilizes MYC through hindering its interaction with FBXW7. J Mol Cell Biol. 2020;12:438–447. doi: 10.1093/jmcb/mjz098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sriratanasak N, Petsri K, Laobuthee A, Wattanathana W, Vinayanuwattikun C, Luanpitpong S, Chanvorachote P. Novel c-Myc-targeting compound N, N-Bis (5-Ethyl-2-Hydroxybenzyl) methylamine for mediated c-Myc ubiquitin-proteasomal degradation in lung cancer cells. Mol Pharmacol. 2020;98:130–142. doi: 10.1124/mol.120.119719. [DOI] [PubMed] [Google Scholar]
- 162.Fiore D, Piscopo C, Proto MC, Vasaturo M, Dal Piaz F, Fusco BM, Pagano C, Laezza C, Bifulco M, Gazzerro P. N6-Isopentenyladenosine inhibits colorectal cancer and improves sensitivity to 5-fluorouracil targeting FBXW7 tumor suppressor. Cancers. 2019;11. [DOI] [PMC free article] [PubMed]
- 163.Hu Y, Yu K, Wang G, Zhang D, Shi C, Ding Y, Hong D, Zhang D, He H, Sun L, et al. Lanatoside C inhibits cell proliferation and induces apoptosis through attenuating Wnt/β-catenin/c-Myc signaling pathway in human gastric cancer cell. Biochem Pharmacol. 2018;150:280–292. doi: 10.1016/j.bcp.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 164.Morel M, Shah KN, Long WW. The F-box protein FBXL16 up-regulates the stability of C-MYC oncoprotein by antagonizing the activity of the F-box protein FBW7. J Biol Chem. 2020;295:7970–7980. doi: 10.1074/jbc.RA120.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mao CG, Zhou XC, Jiang YD, Wan LJ, Tao ZZ, Guo J. The Evi5 oncogene promotes laryngeal cancer cells proliferation by stabilizing c-Myc protein. Cancer Cell Int. 2020;20. [DOI] [PMC free article] [PubMed]
- 166.Ma S, Lu CC, Yang LY, Wang JJ, Wang BS, Cai HQ, Hao JJ, Xu X, Cai Y, Zhang Y, Wang MR. ANXA2 promotes esophageal cancer progression by activating MYC-HIF1A-VEGF axis. J Exp Clin Cancer Res. 2018;37. [DOI] [PMC free article] [PubMed]
- 167.He J, Li FZ, Zhou Y, Hou XY, Liu SS, Li XC, Zhang YW, Jing XQ, Yang LP. LncRNA XLOC_006390 promotes pancreatic carcinogenesis and glutamate metabolism by stabilizing c-Myc. Cancer Lett. 2020;469:419–428. doi: 10.1016/j.canlet.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 168.Tang JY, Yan TT, Bao YJ, Shen CQ, Yu CY, Zhu XQ, Tian XL, Guo FF, Liang Q, Liu Q, et al. LncRNA GLCC1 promotes colorectal carcinogenesis and glucose metabolism by stabilizing c-Myc. Nat Commun. 2019;10. [DOI] [PMC free article] [PubMed]
- 169.Cepeda D, Ng HF, Sharifi HR, Mahmoudi S, Cerrato VS, Fredlund E, Magnusson K, Nilsson H, Malyukova A, Rantala J, et al. CDK-mediated activation of the SCFFBXO28 ubiquitin ligase promotes MYC-driven transcription and tumourigenesis and predicts poor survival in breast cancer. Embo Mol Med. 2013;5:1067–1086. doi: 10.1002/emmm.201202341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Popov N, Schulein C, Jaenicke LA, Eilers M. Ubiquitylation of the amino terminus of Myc by SCF beta-TrCP antagonizes SCFFbw7-mediated turnover. Nat Cell Biol. 2010;12:973–981. doi: 10.1038/ncb2104. [DOI] [PubMed] [Google Scholar]
- 171.Sun XX, He X, Yin L, Sears R, Dai MS. The nucleolar ubiquitin-specific protease USP36 deubiquitinates and stabilizes c-Myc. Cancer Res. 2015;75. [DOI] [PMC free article] [PubMed]
- 172.Pan J, Deng Q, Jiang C, Wang X, Niu T, Li H, Chen T, Jin J, Pan W, Cai X, et al. USP37 directly deubiquitinates and stabilizes c-Myc in lung cancer. Oncogene. 2015;34:3957–3967. doi: 10.1038/onc.2014.327. [DOI] [PubMed] [Google Scholar]
- 173.Zhang WL, Liu ZK, Wang JM, Tian QB. Knockdown of USP28 enhances the radiosensitivity of esophageal cancer cells via the c-Myc/hypoxia-inducible factor-1 alpha pathway. J Cell Biochem. 2019;120:201–212. doi: 10.1002/jcb.27305. [DOI] [PubMed] [Google Scholar]
- 174.Kim D, Hong A, Park HI, Shin WH, Yoo L, Jeon SJ, Chung KC. Deubiquitinating enzyme USP22 positively regulates c-Myc stability and tumorigenic activity in mammalian and breast cancer cells. J Cell Physiol. 2017;232:3664–3676. doi: 10.1002/jcp.25841. [DOI] [PubMed] [Google Scholar]
- 175.Li X, Wu LM, Zopp M, Kopelov S, Du W. p53-TP53-induced glycolysis regulator mediated glycolytic suppression attenuates DNA damage and genomic instability in fanconi anemia hematopoietic stem cells. Stem Cells. 2019;37:937–947. doi: 10.1002/stem.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20:299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Barak Y, Juven T, Haffner R, Oren M. mdm2 expression is induced by wild type p53 activity. EMBO J. 1993;12:461–468. doi: 10.1002/j.1460-2075.1993.tb05678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bang S, Kaur S, Kurokawa M. Regulation of the p53 family proteins by the ubiquitin proteasomal pathway. Int J Mol Sci. 2020:21. [DOI] [PMC free article] [PubMed]
- 179.Allton K, Jain AK, Herz HM, Tsai WW, Jung SY, Qin J, Bergmann A, Johnson RL, Barton MC. Trim24 targets endogenous p53 for degradation. Proc Natl Acad Sci U S A. 2009;106:11612–11616. doi: 10.1073/pnas.0813177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Banks D, Wu M, Higa LA, Gavrilova N, Quan JM, Ye T, Kobayashi R, Sun H, Zhang H. L2DTL/CDT2 and PCNA interact with p53 and regulate p53 polyubiquitination and protein stability through MDM2 and CUL4A/DDB1 complexes. Cell Cycle. 2006;5:1719–1729. doi: 10.4161/cc.5.15.3150. [DOI] [PubMed] [Google Scholar]
- 181.Luo K, Ehrlich E, Xiao ZX, Zhang WY, Ketner G, Yu XF. Adenovirus E4orF6 assembles with Cullin5-ElonginB-ElonginC E3 ubiquitin ligase through an HIV/SIV Vif-like BC-box to regulate p53. FASEB J. 2007;21:1742–1750. doi: 10.1096/fj.06-7241com. [DOI] [PubMed] [Google Scholar]
- 182.Luo Z, Ye X, Shou F, Cheng Y, Li F, Wang G. RNF115-mediated ubiquitination of p53 regulates lung adenocarcinoma proliferation. Biochem Biophys Res Commun. 2020. [DOI] [PubMed]
- 183.Han YD, Tan Y, Zhao YY, Zhang YC, He XJ, Yu L, Jiang HP, Lu HJ, Tian HY. TRIM23 overexpression is a poor prognostic factor and contributes to carcinogenesis in colorectal cancer. J Cell Mol Med. 2020;24:5491–5500. doi: 10.1111/jcmm.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhang YL, Cui NN, Zheng G. Ubiquitination of P53 by E3 ligase MKRN2 promotes melanoma cell proliferation. Oncol Lett. 2020;19:1975–1984. doi: 10.3892/ol.2020.11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D. Nucleolar Arf sequesters Mdm2 and activates p53. Nat Cell Biol. 1999;1:20–26. doi: 10.1038/8991. [DOI] [PubMed] [Google Scholar]
- 186.Zhang WC, Gong J, Yang H, Wan LM, Peng YM, Wang XL, Sun J, Li F, Geng YQ, Li DY, et al. The mitochondrial protein MAVS stabilizes p53 to suppress tumorigenesis. Cell Rep. 2020;30:725. doi: 10.1016/j.celrep.2019.12.051. [DOI] [PubMed] [Google Scholar]
- 187.Li XY, Guo MQ, Cai L, Du TT, Liu Y, Ding HF, Wang HB, Zhang JR, Chen XG, Yan CH. Competitive ubiquitination activates the tumor suppressor p53. Cell Death Differ. 2020;27:1807–1818. doi: 10.1038/s41418-019-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hu YN, Yu J, Wang Q, Zhang L, Chen XY, Cao Y, Zhao JP, Xu YJ, Jiang DS, Wang Y, Xiong WN. Tartrate-resistant acid phosphatase 5/ACP5 interacts with p53 to control the expression of SMAD3 in lung adenocarcinoma. Mol Ther Oncol. 2020;16:272–288. doi: 10.1016/j.omto.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lim KH, Park JJ, Gu BH, Kim JO, Park SG, Baek KH. HAUSP-nucleolin interaction is regulated by p53-Mdm2 complex in response to DNA damage response. Sci Rep. 2015;5. [DOI] [PMC free article] [PubMed]
- 190.Yuan J, Luo KT, Zhang LZ, Cheville JC, Lou ZK. USP10 Regulates p53 Localization and Stability by Deubiquitinating p53. Cell. 2010;140:384–U121. doi: 10.1016/j.cell.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu WT, Huang KY, Lu MC, Huang HL, Chen CY, Cheng YL, Yu HC, Liu SQ, Lai NS, Huang HB. TGF-beta upregulates the translation of USP15 via the PI3K/AKT pathway to promote p53 stability. Oncogene. 2017;36:2715–2723. doi: 10.1038/onc.2016.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pu Q, Lv YR, Dong K, Geng WW, Gao HD. Tumor suppressor OTUD3 induces growth inhibition and apoptosis by directly deubiquitinating and stabilizing p53 in invasive breast carcinoma cells. BMC Cancer. 2020;20:583. doi: 10.1186/s12885-020-07069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Piao SD, Pei HZ, Huang B, Baek SH. Ovarian tumor domain-containing protein 1 deubiquitinates and stabilizes p53. Cell Signal. 2017;33:22–29. doi: 10.1016/j.cellsig.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 194.Luo JD, Lu ZH, Lu XJ, Chen L, Cao JP, Zhang SY, Ling Y, Zhou XF. OTUD5 regulates p53 stability by deubiquitinating p53. PLoS One. 2013;8. [DOI] [PMC free article] [PubMed]
- 195.Zhang L, Gong F. Involvement of USP24 in the DNA damage response. Mol Cell Oncol. 2016;3:e1011888. doi: 10.1080/23723556.2015.1011888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu J, Chung HJ, Vogt M, Jin Y, Malide D, He L, Dundr M, Levens D. JTV1 co-activates FBP to induce USP29 transcription and stabilize p53 in response to oxidative stress. EMBO J. 2011;30:846–858. doi: 10.1038/emboj.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hock AK, Vigneron AM, Carter S, Ludwig RL, Vousden KH. Regulation of p53 stability and function by the deubiquitinating enzyme USP42. EMBO J. 2011;30:4921–4930. doi: 10.1038/emboj.2011.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ke JY, Dai CJ, Wu WL, Gao JH, Xia AJ, Liu GP, Lv KS, Wu CL. USP11 regulates p53 stability by deubiquitinating p53. J Zhejiang Univ Sci B. 2014;15:1032–1038. doi: 10.1631/jzus.B1400180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- 200.Sheng Y, Saridakis V, Sarkari F, Duan S, Wu T, Arrowsmith CH, Frappier L. Molecular recognition of p53 and MDM2 by USP7/HAUSP. Nat Struct Mol Biol. 2006;13:285–291. doi: 10.1038/nsmb1067. [DOI] [PubMed] [Google Scholar]
- 201.Sarkari F, La Delfa A, Arrowsmith CH, Frappier L, Sheng Y, Saridakis V. Further insight into substrate recognition by USP7: structural and biochemical analysis of the HdmX and Hdm2 interactions with USP7. J Mol Biol. 2010;402:825–837. doi: 10.1016/j.jmb.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 202.An T, Gong Y, Li X, Kong L, Ma P, Gong L, Zhu H, Yu C, Liu J, Zhou H, et al. USP7 inhibitor P5091 inhibits Wnt signaling and colorectal tumor growth. Biochem Pharmacol. 2017;131:29–39. doi: 10.1016/j.bcp.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 203.Xia X, Liao Y, Huang C, Liu Y, He J, Shao Z, Jiang L, Dou QP, Liu J, Huang H. Deubiquitination and stabilization of estrogen receptor alpha by ubiquitin-specific protease 7 promotes breast tumorigenesis. Cancer Lett. 2019;465:118–128. doi: 10.1016/j.canlet.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 204.Fan YH, Cheng J, Vasudevan SA, Dou J, Zhang H, Patel RH, Ma IT, Rojas Y, Zhao Y, Yu Y, et al. USP7 inhibitor P22077 inhibits neuroblastoma growth via inducing p53-mediated apoptosis. Cell Death Dis. 2013;4:e867. doi: 10.1038/cddis.2013.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Masuya D, Huang C, Liu D, Nakashima T, Yokomise H, Ueno M, Nakashima N, Sumitomo S. The HAUSP gene plays an important role in non-small cell lung carcinogenesis through p53-dependent pathways. J Pathol. 2006;208:724–732. doi: 10.1002/path.1931. [DOI] [PubMed] [Google Scholar]
- 206.Kruse JP, Gu W. MSL2 promotes Mdm2-independent cytoplasmic localization of p53. J Biol Chem. 2009;284:3250–3263. doi: 10.1074/jbc.M805658200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Yang YL, Ludwig RL, Jensen JP, Pierre SA, Medaglia MV, Davydov IV, Safiran YJ, Oberoi P, Kenten JH, Phillips AC, et al. Small molecule inhibitors of HDM2 ubiquitin ligase activity stabilize and activate p53 in cells. Cancer Cell. 2005;7:547–559. doi: 10.1016/j.ccr.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 208.Jiang J, Tam LM, Wang PC, Wang YS. Arsenite targets the RING finger domain of Rbx1 E3 ubiquitin ligase to inhibit proteasome-mediated degradation of Nrf2. Chem Res Toxicol. 2018;31:380–387. doi: 10.1021/acs.chemrestox.8b00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Komatsu M, Kurokawa H, Waguri S, Taguchi K, Kobayashi A, Ichimura Y, Sou YS, Ueno I, Sakamoto A, Tong KI, et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat Cell Biol. 2010;12:213–U217. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 210.Wan ZH, Jiang TY, Shi YY, Pan YF, Lin YK, Ma YH, Yang C, Feng XF, Huang LF, Kong XN, et al. RPB5-mediating protein promotes cholangiocarcinoma tumorigenesis and drug resistance by competing with NRF2 for KEAP1 binding. Hepatology. 2020;71:2005–2022. doi: 10.1002/hep.30962. [DOI] [PubMed] [Google Scholar]
- 211.Wang Q, Ma J, Lu Y, Zhang S, Huang J, Chen J, Bei JX, Yang K, Wu G, Huang K, et al. CDK20 interacts with KEAP1 to activate NRF2 and promotes radiochemoresistance in lung cancer cells. Oncogene. 2017;36:5321–5330. doi: 10.1038/onc.2017.161. [DOI] [PubMed] [Google Scholar]
- 212.Liu YF, Tao SS, Liao LJ, Li Y, Li HC, Li ZH, Lin LL, Wan XC, Yang XL, Chen L. TRIM25 promotes the cell survival and growth of hepatocellular carcinoma through targeting Keap1-Nrf2 pathway. Nat Commun. 2020;11. [DOI] [PMC free article] [PubMed]
- 213.Xu PF, Jiang LP, Yang Y, Wu MG, Liu BY, Shi YL, Shen QS, Jiang XL, He YM, Cheng DT, et al. PAQR4 promotes chemoresistance in non-small cell lung cancer through inhibiting Nrf2 protein degradation. Theranostics. 2020;10:3767–3778. doi: 10.7150/thno.43142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yu YP, Cai LC, Wang XY, Cheng SY, Zhang M, Jian WG, Wang TD, Yang JK, Yang KB, Zhang C. BMP8A promotes survival and drug resistance via Nrf2/TRIM24 signaling pathway in clear cell renal cell carcinoma. Cancer Sci. 2020;111:1555–1566. doi: 10.1111/cas.14376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lo JY, Spatola BN, Curran SP. WDR23 regulates NRF2 independently of KEAP1. Plos Genet. 2017;13. [DOI] [PMC free article] [PubMed]
- 216.Cuadrado A. Structural and functional characterization of Nrf2 degradation by glycogen synthase kinase 3/beta-TrCP. Free Radic Biol Med. 2015;88:147–157. doi: 10.1016/j.freeradbiomed.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 217.Wu TD, Zhao F, Gao BX, Tan C, Yagishita N, Nakajima T, Wong PK, Chapman E, Fang DY, Zhang DD. Hrd1 suppresses Nrf2-mediated cellular protection during liver cirrhosis. Genes Dev. 2014;28:708–722. doi: 10.1101/gad.238246.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang Q, Zhang ZY, Du H, Li SZ, Tu R, Jia YF, Zheng Z, Song XM, Du RL, Zhang XD. DUB3 deubiquitinates and stabilizes NRF2 in chemotherapy resistance of colorectal cancer. Cell Death Differ. 2019;26:2300–2313. doi: 10.1038/s41418-019-0303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sun Y, He W, Luo M, Zhou Y, Chang G, Ren W, Wu K, Li X, Shen J, Zhao X, Hu Y. SREBP1 regulates tumorigenesis and prognosis of pancreatic cancer through targeting lipid metabolism. Tumour Biol. 2015;36:4133–4141. doi: 10.1007/s13277-015-3047-5. [DOI] [PubMed] [Google Scholar]
- 220.Bakan I, Laplante M. Connecting mTORC1 signaling to SREBP-1 activation. Curr Opin Lipidol. 2012;23:226–234. doi: 10.1097/MOL.0b013e328352dd03. [DOI] [PubMed] [Google Scholar]
- 221.Zhao XP, Feng DR, Wang Q, Abdulla A, Xie XJ, Zhou J, Sun Y, Yang ES, Liu LP, Vaitheesvaran B, et al. Regulation of lipogenesis by cyclin-dependent kinase 8-mediated control of SREBP-1. J Clin Investig. 2012;122:2417–2427. doi: 10.1172/JCI61462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tu KS, Zheng X, Yin GZ, Zan XF, Yao YM, Liu QG. Evaluation of Fbxw7 expression and its correlation with expression of SREBP-1 in a mouse model of NAFLD. Mol Med Rep. 2012;6:525–530. doi: 10.3892/mmr.2012.953. [DOI] [PubMed] [Google Scholar]
- 223.Walker AK, Yang FJ, Jiang KR, Ji JY, Watts JL, Purushotham A, Boss O, Hirsch ML, Ribich S, Smith JJ, et al. Conserved role of SIRT1 orthologs in fasting-dependent inhibition of the lipid/cholesterol regulator SREBP. Genes Dev. 2010;24:1403–1417. doi: 10.1101/gad.1901210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lee JP, Brauweiler A, Rudolph M, Hooper JE, Drabkin HA, Gemmill RM. The TRC8 ubiquitin ligase is sterol regulated and interacts with lipid and protein biosynthetic pathways. Mol Cancer Res. 2010;8:93–106. doi: 10.1158/1541-7786.MCR-08-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Han SH, Korm S, Han YG, Choi SY, Kim SH, Chung HJ, Park K, Kim JY, Myung K, Lee JY, et al. GCA links TRAF6-ULK1-dependent autophagy activation in resistant chronic myeloid leukemia. Autophagy. 2019;15:2076–2090. doi: 10.1080/15548627.2019.1596492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Nazio F, Strappazzon F, Antonioli M, Bielli P, Cianfanelli V, Bordi M, Gretzmeier C, Dengjel J, Piacentini M, Fimia GM, Cecconi F. mTOR inhibits autophagy by controlling ULK1 ubiquitylation, self-association and function through AMBRA1 and TRAF6. Nat Cell Biol. 2013;15:406. doi: 10.1038/ncb2708. [DOI] [PubMed] [Google Scholar]
- 227.Chauhan S, Kumar S, Jain A, Ponpuak M, Mudd MH, Kimura T, Choi SW, Peters R, Mandell M, Bruun JA, et al. TRIMs and galectins globally cooperate and TRIM16 and galectin-3 co-direct autophagy in endomembrane damage homeostasis. Dev Cell. 2016;39:13–27. doi: 10.1016/j.devcel.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Di Rienzo M, Antonioli M, Fusco C, Liu Y, Mari M, Orhon I, Refolo G, Germani F, Corazzari M, Romagnoli A, et al. Autophagy induction in atrophic muscle cells requires ULK1 activation by TRIM32 through unanchored K63-linked polyubiquitin chains. Sci Adv. 2019;5. [DOI] [PMC free article] [PubMed]
- 229.Raimondi M, Cesselli D, Di Loreto C, La Marra F, Schneider C, Demarchi F. USP1 (ubiquitin specific peptidase 1) targets ULK1 and regulates its cellular compartmentalization and autophagy. Autophagy. 2019;15:613–630. doi: 10.1080/15548627.2018.1535291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Thayer JA, Awad O, Hegdekar N, Sarkar C, Tesfay H, Burt C, Zeng X, Feldman RA, Lipinski MM. The PARK10 gene USP24 is a negative regulator of autophagy and ULK1 protein stability. Autophagy. 2020;16:140–153. doi: 10.1080/15548627.2019.1598754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Nazio F, Cecconi F. Autophagy up and down by outsmarting the incredible ULK. Autophagy. 2017;13:967–968. doi: 10.1080/15548627.2017.1285473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Liu CC, Chen RH. KLHL20 links the ubiquitin-proteasome system to autophagy termination. Autophagy. 2016;12:890–891. doi: 10.1080/15548627.2016.1157243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kim JH, Seo D, Kim SJ, Choi DW, Park JS, Ha J, Choi J, Lee JH, Jung SM, Seo KW, et al. The deubiquitinating enzyme USP20 stabilizes ULK1 and promotes autophagy initiation. EMBO Rep. 2018;19. [DOI] [PMC free article] [PubMed]
- 234.Chang YY, Juhasz G, Goraksha-Hicks P, Arsham AM, Mallin DR, Muller LK, Neufeld TP. Nutrient-dependent regulation of autophagy through the target of rapamycin pathway. Biochem Soc Trans. 2009;37:232–236. doi: 10.1042/BST0370232. [DOI] [PubMed] [Google Scholar]
- 235.Shi CS, Kehrl JH. TRAF6 and A20 regulate lysine 63-linked ubiquitination of beclin-1 to control TLR4-induced autophagy. Sci Signal. 2010;3. [DOI] [PMC free article] [PubMed]
- 236.Xia PY, Wang S, Du Y, Zhao ZN, Shi L, Sun L, Huang GL, Ye BQ, Li C, Dai ZH, et al. WASH inhibits autophagy through suppression of Beclin 1 ubiquitination. EMBO J. 2013;32:2685–2696. doi: 10.1038/emboj.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Fusco C, Mandriani B, Di Rienzo M, Micale L, Malerba N, Cocciadiferro D, Sjottem E, Augello B, Squeo GM, Pellico MT, et al. TRIM50 regulates Beclin 1 proautophagic activity. Biochim Biophys Acta Mol Cell Res. 2018;1865:908–919. doi: 10.1016/j.bbamcr.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 238.Xu DC, Shan B, Sun HW, Xiao J, Zhu KZ, Xie XX, Li XY, Liang W, Lu XJ, Qian LH, Yuan JY. USP14 regulates autophagy by suppressing K63 ubiquitination of Beclin 1. Genes Dev. 2016;30:1718–1730. doi: 10.1101/gad.285122.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Platta HW, Abrahamsen H, Thoresen SB, Stenmark H. Nedd4-dependent lysine-11-linked polyubiquitination of the tumour suppressor Beclin 1. Biochem J. 2012;441:399–406. doi: 10.1042/BJ20111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Xu CF, Feng K, Zhao XN, Huang SQ, Cheng YJ, Qian L, Wang YN, Sun HX, Jin M, Chuang TH, Zhang YY. Regulation of autophagy by E3 ubiquitin ligase RNF216 through BECN1 ubiquitination. Autophagy. 2014;10:2239–2250. doi: 10.4161/15548627.2014.981792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Liu JL, Xia HG, Kim M, Xu LH, Li Y, Zhang LH, Cai Y, Norberg HV, Zhang T, Furuya T, et al. Beclin1 controls the levels of p53 by regulating the deubiquitination activity of USP10 and USP13. Cell. 2011;147:223–234. doi: 10.1016/j.cell.2011.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jin SH, Tian S, Chen YM, Zhang CX, Xie WH, Xia XJ, Cui J, Wang RF. USP19 modulates autophagy and antiviral immune responses by deubiquitinating beclin-1. EMBO J. 2016;35:866–880. doi: 10.15252/embj.201593596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ashkenazi A, Bento CF, Ricketts T, Vicinanza M, Siddiqi F, Pavel M, Squitieri F, Hardenberg MC, Imarisio S, Menzies FM, Rubinsztein DC. Polyglutamine tracts regulate beclin 1-dependent autophagy. Nature. 2017;545:108. doi: 10.1038/nature22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Xiao J, Zhang T, Xu DC, Wang HB, Cai Y, Jin TJ, Liu M, Jin MZ, Wu KJ, Yuan JY. FBXL20-mediated Vps34 ubiquitination as a p53 controlled checkpoint in regulating autophagy and receptor degradation. Genes Dev. 2015;29:184–196. doi: 10.1101/gad.252528.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang T, Dong KY, Liang W, Xu DC, Xia HG, Geng JF, Najafov A, Liu M, Li YX, Han XR, et al. G-protein-coupled receptors regulate autophagy by ZBTB16-mediated ubiquitination and proteasomal degradation of Atg14L. Elife. 2015;4. [DOI] [PMC free article] [PubMed]
- 246.Morel E, Dupont N, Codogno P. Autophagy regulation: RNF2 targets AMBRA1. Cell Res. 2014;24:1029–1030. doi: 10.1038/cr.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wan W, You ZY, Zhou L, Xu YF, Peng C, Zhou TH, Yi C, Shi Y, Liu W. mTORC1-regulated and HUWE1-mediated WIPI2 degradation controls autophagy flux. Mol Cell. 2018;72:303. doi: 10.1016/j.molcel.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 248.Kuang E, Okumura CYM, Sheffy-Levin S, Varsano T, Shu VCW, Qi JF, Niesman IR, Yang HJ, Lopez-Otin C, Yang WY, et al. Regulation of ATG4B stability by RNF5 limits basal levels of autophagy and influences susceptibility to bacterial infection (vol 8, e1003007, 2012). PLoS Genet. 2020;16. [DOI] [PMC free article] [PubMed]
- 249.Liu CH, Zhang Y, She XL, Fan L, Li PY, Feng JB, Fu HJ, Liu Q, Liu Q, Zhao CH, et al. A cytoplasmic long noncoding RNA LINC00470 as a new AKT activator to mediate glioblastoma cell autophagy. J Hematol Oncol. 2018;11. [DOI] [PMC free article] [PubMed]
- 250.Lee HJ, Li CF, Ruan D, He J, Montal ED, Lorenz S, Girnun GD, Chan CH. Non-proteolytic ubiquitination of hexokinase 2 by HectH9 controls tumor metabolism and cancer stem cell expansion. Nat Commun. 2019;10. [DOI] [PMC free article] [PubMed]
- 251.Jiao L, Zhang HL, Li DD, Yang KL, Tang J, Li X, Ji J, Yu Y, Wu RY, Ravichandran S, et al. Regulation of glycolytic metabolism by autophagy in liver cancer involves selective autophagic degradation of HK2 (hexokinase 2) Autophagy. 2018;14:671–684. doi: 10.1080/15548627.2017.1381804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Huang MW, Xiong H, Luo DL, Xu BR, Liu HL. CSN5 upregulates glycolysis to promote hepatocellular carcinoma metastasis via stabilizing the HK2 protein. Exp Cell Res. 2020;388. [DOI] [PubMed]
- 253.Okatsu K, Iemura S, Koyano F, Go E, Kimura M, Natsume T, Tanaka K, Matsuda N. Mitochondrial hexokinase HKI is a novel substrate of the Parkin ubiquitin ligase. Biochem Biophys Res Commun. 2012;428:197–202. doi: 10.1016/j.bbrc.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 254.Feng YL, Zhang Y, Cai Y, Liu RJ, Lu ML, Li TZM, Fu Y, Guo M, Huang HC, Ou YF, Chen YH. A20 targets PFKL and glycolysis to inhibit the progression of hepatocellular carcinoma. Cell Death Dis. 2020;11. [DOI] [PMC free article] [PubMed]
- 255.Lee JH, Liu R, Li J, Zhang CB, Wang YG, Cai QS, Qian X, Xia Y, Zheng YH, Piao YJ, et al. Stabilization of phosphofructokinase 1 platelet isoform by AKT promotes tumorigenesis. Nat Commun. 2017;8. [DOI] [PMC free article] [PubMed]
- 256.Shang Y, He J, Wang Y, Feng Q, Zhang Y, Guo J, Li J, Li S, Wang Y, Yan G, et al. CHIP/Stub1 regulates the Warburg effect by promoting degradation of PKM2 in ovarian carcinoma. Oncogene. 2017;36:4191–4200. doi: 10.1038/onc.2017.31. [DOI] [PubMed] [Google Scholar]
- 257.Yuan P, Zhou YY, Wang R, Chen SY, Wang QQ, Xu ZJ, Liu Y, Yang HL. TRIM58 interacts with pyruvate kinase M2 to inhibit tumorigenicity in human osteosarcoma cells. Biomed Res Int. 2020;2020. [DOI] [PMC free article] [PubMed]
- 258.Liu K, Li FZ, Han HC, Chen Y, Mao ZB, Luo JY, Zhao YM, Zheng B, Gu W, Zhao WH. Parkin regulates the activity of pyruvate kinase M2. J Biol Chem. 2016;291:10307–10317. doi: 10.1074/jbc.M115.703066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Choi HS, Pei CZ, Park JH, Kim SY, Song SY, Shin GJ, Baek KH. Protein stability of pyruvate kinase Isozyme M2 is mediated by HAUSP. Cancers (Basel). 2020;12. [DOI] [PMC free article] [PubMed]
- 260.Kim SR, Kim JO, Lim KH, Yun JH, Han I, Baek KH. Regulation of pyruvate kinase isozyme M2 is mediated by the ubiquitin-specific protease 20. Int J Oncol. 2015;46:2116–2124. doi: 10.3892/ijo.2015.2901. [DOI] [PubMed] [Google Scholar]
- 261.Wang Y, Ha SW, Zhang T, Kho DH, Raz A, Xie Y. Polyubiquitylation of AMF requires cooperation between the gp78 and TRIM25 ubiquitin ligases. Oncotarget. 2014;5:2044–2051. doi: 10.18632/oncotarget.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Deng XQ, Yi X, Deng JY, Zou YQ, Wang SS, Shan WH, Liu P, Zhang ZB, Chen LF, Hao L. ROCK2 promotes osteosarcoma growth and metastasis by modifying PFKFB3 ubiquitination and degradation. Exp Cell Res. 2019;385. [DOI] [PubMed]
- 263.Tudzarova S, Colombo SL, Stoeber K, Carcamo S, Williams GH, Moncada S. Two ubiquitin ligases, APC/C-Cdh1 and SKP1-CUL1-F (SCF)-beta-TrCP, sequentially regulate glycolysis during the cell cycle. Proc Natl Acad Sci U S A. 2011;108:5278–5283. doi: 10.1073/pnas.1102247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Colombo SL, Palacios-Callender M, Frakich N, Carcamo S, Kovacs I, Tudzarova S, Moncada S. Molecular basis for the differential use of glucose and glutamine in cell proliferation as revealed by synchronized HeLa cells. Proc Natl Acad Sci U S A. 2011;108:21069–21074. doi: 10.1073/pnas.1117500108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Cai Q, Wang SH, Jin LY, Weng MZ, Zhou D, Wang JD, Tang ZH, Quan ZW. Long non-coding RNA GBCDRlnc1 induces chemoresistance of gallbladder cancer cells by activating autophagy. Mol Cancer. 2019;18. [DOI] [PMC free article] [PubMed]
- 266.Yu T, Zhao YJ, Hu ZX, Li J, Chu DD, Zhang JW, Li Z, Chen B, Zhang X, Pan HY, et al. MetaLnc9 facilitates lung cancer metastasis via a PGK1-activated AKT/mTOR pathway. Cancer Res. 2017;77:5782–5794. doi: 10.1158/0008-5472.CAN-17-0671. [DOI] [PubMed] [Google Scholar]
- 267.Mikawa T, Maruyama T, Okamoto K, Nakagama H, Lleonart ME, Tsusaka T, Hori K, Murakami I, Izumi T, Takaori-Kondo A, et al. Senescence-inducing stress promotes proteolysis of phosphoglycerate mutase via ubiquitin ligase Mdm2. J Cell Biol. 2014;204:729–745. doi: 10.1083/jcb.201306149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yoshino S, Hara T, Nakaoka HJ, Kanamori A, Murakami Y, Seiki M, Sakamoto T. The ERK signaling target RNF126 regulates anoikis resistance in cancer cells by changing the mitochondrial metabolic flux. Cell Discov. 2016;2. [DOI] [PMC free article] [PubMed]
- 269.Peng MX, Yang D, Hou YX, Liu SQ, Zhao MJ, Qin YL, Chen R, Teng Y, Liu MR. Intracellular citrate accumulation by oxidized ATM-mediated metabolism reprogramming via PFKP and CS enhances hypoxic breast cancer cell invasion and metastasis. Cell Death Dis. 2019;10. [DOI] [PMC free article] [PubMed] [Retracted]
- 270.Sun RC, Denko NC. Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab. 2014;19:285–292. doi: 10.1016/j.cmet.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Han C. Amplification of Usp13 drives ovarian cancer metabolism. Clin Cancer Res. 2019;25:90. doi: 10.1158/1078-0432.CCR-18-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wang M, Hu J, Yan LL, Yang YP, He M, Wu M, Li Q, Gong W, Yang Y, Wang Y, et al. High glucose-induced ubiquitination of G6PD leads to the injury of podocytes. FASEB J. 2019;33:6296–6310. doi: 10.1096/fj.201801921R. [DOI] [PubMed] [Google Scholar]
- 273.Yu F, White SB, Zhao Q, Lee FS. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc Natl Acad Sci U S A. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Jin X, Pan Y, Wang L, Zhang L, Ravichandran R, Potts PR, Jiang J, Wu H, Huang H. MAGE-TRIM28 complex promotes the Warburg effect and hepatocellular carcinoma progression by targeting FBP1 for degradation. Oncogenesis. 2017;6:e312. doi: 10.1038/oncsis.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jiang WQ, Wang SW, Xiao MT, Lin Y, Zhou LS, Lei QY, Xiong Y, Guan KL, Zhao SM. Acetylation regulates gluconeogenesis by promoting PEPCK1 degradation via recruiting the UBR5 ubiquitin ligase. Mol Cell. 2011;43:33–44. doi: 10.1016/j.molcel.2011.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Fernandes R, Carvalho A, Kumagai A, Seica R, Hosoya KI, Terasaki T, Murta J, Pereira P, Faro C. Downregulation of retinal GLUT1 in diabetes by ubiquitinylation. Mol Vis. 2004;10:618–628. [PubMed] [Google Scholar]
- 277.Cheng A, Zhang M, Gentry MS, Worby CA, Dixon JE, Saltiel AR. A role for AGL ubiquitination in the glycogen storage disorders of Lafora and Cori’s disease. Genes Dev. 2007;21:2399–2409. doi: 10.1101/gad.1553207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Zhang C, Liu J, Zhao YH, Yue XT, Wu H, Li J, Shen ZY, Haffty B, Hu WW, Feng ZH. Cullin3-KLHL25 ubiquitin ligase targets ACLY for degradation to inhibit lipid synthesis and tumor progression. Cancer Res. 2017;77. [DOI] [PMC free article] [PubMed]
- 279.Gu L, Zhu Y, Lin X, Lu B, Zhou X, Zhou F, Zhao Q, Prochownik EV, Li Y. The IKKbeta-USP30-ACLY axis controls Lipogenesis and tumorigenesis. Hepatology. 2020. [DOI] [PubMed]
- 280.Qi L, Heredia JE, Altarejos JY, Screaton R, Goebel N, Niessen S, MacLeod IX, Liew CW, Kulkarni RN, Bain J, et al. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science. 2006;312:1763–1766. doi: 10.1126/science.1123374. [DOI] [PubMed] [Google Scholar]
- 281.Ma J, Yan RL, Zu X, Cheng JM, Rao K, Liao DF, Cao DL. Aldo-keto reductase family 1 B10 affects fatty acid synthesis by regulating the stability of acetyl-CoA carboxylase-alpha in breast cancer cells. J Biol Chem. 2008;283:3418–3423. doi: 10.1074/jbc.M707650200. [DOI] [PubMed] [Google Scholar]
- 282.Yu JX, Deng R, Zhu HH, Zhang SS, Zhu CH, Montminy M, Davis R, Feng GS. Modulation of fatty acid synthase degradation by concerted action of p38 MAP kinase, E3 ligase COP1, and SH2-tyrosine phosphatase Shp2. J Biol Chem. 2013;288:3823–3830. doi: 10.1074/jbc.M112.397885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gang XK, Xuan LL, Zhao X, Lv Y, Li F, Wang Y, Wang GX. Speckle-type POZ protein suppresses lipid accumulation and prostate cancer growth by stabilizing fatty acid synthase. Prostate. 2019;79:864–871. doi: 10.1002/pros.23793. [DOI] [PubMed] [Google Scholar]
- 284.Calvisi DF, Wang CM, Ho C, Ladu S, Lee SA, Mattu S, Destefanis G, Delogu S, Zimmermann A, Ericsson J, et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology. 2011;140:1071–U1542. doi: 10.1053/j.gastro.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Graner E, Tang D, Rossi S, Baron A, Migita T, Weinstein LJ, Lechpammer M, Huesken D, Zimmermann J, Signoretti S, Loda M. The isopeptidase USP2a regulates the stability of fatty acid synthase in prostate cancer. Cancer Cell. 2004;5:253–261. doi: 10.1016/S1535-6108(04)00055-8. [DOI] [PubMed] [Google Scholar]
- 286.Menzies SA, Volkmar N, van den Boomen DJ, Timms RT, Dickson AS, Nathan JA, Lehner PJ. The sterol-responsive RNF145 E3 ubiquitin ligase mediates the degradation of HMG-CoA reductase together with gp78 and Hrd1. Elife. 2018;7. [DOI] [PMC free article] [PubMed]
- 287.Jiang LY, Jiang W, Tian N, Xiong YN, Liu J, Wei J, Wu KY, Luo J, Shi XJ, Song BL. Ring finger protein 145 (RNF145) is a ubiquitin ligase for sterol-induced degradation of HMG-CoA reductase. J Biol Chem. 2018;293:4047–4055. doi: 10.1074/jbc.RA117.001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Loregger A, Raaben M, Tan J, Scheij S, Moeton M, van den Berg M, Gelberg-Etel H, Stickel E, Roitelman J, Brummelkamp T, Zelcer N. Haploid mammalian genetic screen identifies UBXD8 as a key determinant of HMGCR degradation and cholesterol biosynthesis. Arterioscler Thromb Vasc Biol. 2017;37:2064. doi: 10.1161/ATVBAHA.117.310002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hwang S, Nguyen AD, Jo Y, Engelking LJ, Brugarolas J, DeBose-Boyd RA. Hypoxia-inducible factor 1alpha activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J Biol Chem. 2017;292:9382–9393. doi: 10.1074/jbc.M117.788562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Gelsomino G, Corsetto PA, Campia I, Montorfano G, Kopecka J, Castella B, Gazzano E, Ghigo D, Rizzo AM, Riganti C. Omega 3 fatty acids chemosensitize multidrug resistant colon cancer cells by down-regulating cholesterol synthesis and altering detergent resistant membranes composition. Mol Cancer. 2013;12. [DOI] [PMC free article] [PubMed]
- 291.Foresti O, Ruggiano A, Hannibal-Bach HK, Ejsing CS, Carvalho P. Sterol homeostasis requires regulated degradation of squalene monooxygenase by the ubiquitin ligase Doa10/Teb4. Elife. 2013;2:e00953. doi: 10.7554/eLife.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zelcer N, Hong C, Boyadjian R, Tontonoz P. LXR regulates cholesterol uptake through idol-dependent ubiquitination of the LDL receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Lee DE, Yoo JE, Kim J, Kim S, Kim S, Lee H, Cheong HS. NEDD4L downregulates autophagy and cell growth by modulating ULK1 and a glutamine transporter. Cell Death Dis. 2020;11. [DOI] [PMC free article] [PubMed]
- 294.Jeon YJ, Khelifa S, Ratnikov B, Scott DA, Feng YM, Parisi F, Ruller C, Lau E, Kim H, Brill LM, et al. Regulation of glutamine carrier proteins by RNF5 determines breast cancer response to ER stress-inducing chemotherapies. Cancer Cell. 2015;27:354–369. doi: 10.1016/j.ccell.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhao S, Wang JM, Yan J, Zhang DL, Liu BQ, Jiang JY, Li C, Li S, Meng XN, Wang HQ. BAG3 promotes autophagy and glutaminolysis via stabilizing glutaminase. Cell Death Dis. 2019;10. [DOI] [PMC free article] [PubMed]
- 296.Zhao JZ, Zhou R, Hui KY, Yang Y, Zhang QY, Ci YL, Shi L, Xu CM, Huang F, Hu Y. Selenite inhibits glutamine metabolism and induces apoptosis by regulating GLS1 protein degradation via APC/C-CDH1 pathway in colorectal cancer cells. Oncotarget. 2017;8:18832–18847. doi: 10.18632/oncotarget.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Liu J, Zhang C, Wu H, Sun XX, Li Y, Huang S, Yue X, Lu SE, Shen Z, Su X, et al. Parkin ubiquitinates phosphoglycerate dehydrogenase to suppress serine synthesis and tumor progression. J Clin Invest. 2020;130:3253–3269. doi: 10.1172/JCI132876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Anderson DD, Eom JY, Stover PJ. Competition between sumoylation and ubiquitination of serine hydroxymethyltransferase 1 determines its nuclear localization and its accumulation in the nucleus. J Biol Chem. 2012;287:4790–4799. doi: 10.1074/jbc.M111.302174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2016;73:377–392. doi: 10.1007/s00018-015-2070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Cui D, Xiong X, Shu J, Dai X, Sun Y, Zhao Y. FBXW7 confers radiation survival by targeting p53 for degradation. Cell Rep. 2020;30:497–509 e494. doi: 10.1016/j.celrep.2019.12.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data obtained and/or analyzed during the current study were available from the corresponding authors on reasonable request.