Abstract
PURPOSE
To identify genomic alterations as potential therapeutic targets in extramammary Paget disease (EMPD) of the vulva.
METHODS
We identified all patients with primary vulvar EMPD who were treated at our institution and underwent paired tumor-normal massively parallel sequencing of 410-468 cancer-related genes (MSK-IMPACT assay). EMPD of the vulva samples sequenced from 2014 to 2019 were reviewed and somatic mutations identified, with specific focus on mutations of potential therapeutic targets. Clinical data were abstracted from electronic medical records. Microsatellite instability (MSI) was assessed by MSIscore.
RESULTS
Tumors of 26 patients with EMPD underwent genomic sequencing. At diagnosis, all patients had noninvasive or microinvasive (< 1 mm) disease; invasive disease eventually developed in 2 patients. Primary treatment was surgery for 19 patients (73%) and imiquimod topical therapy for 7 (27%). Seven patients had ≥ 2 surgeries as part of clinical course (1 patient had 5 vulvar resections). Samples had a median of 2 coding mutations in the genes analyzed (range, 0-29). The most common mutations were in PIK3CA (n = 9; 35%), ERBB2 (4 mutations and 3 copy number alterations; 27%), and TP53 (n = 7; 27%). MSIscore was available for 23 samples; all were microsatellite stable. After tumor genomic profiling, a patient who was initially treated with multiple resections and imiquimod was found to have a PIK3CA p.E542K mutation. She underwent PI3K-inhibitor treatment for 18 months before disease progression.
CONCLUSION
EMPD of the vulva has a chronic and relapsing course, often requiring multiple surgical resections. Effective topical treatments are lacking. We identified targetable mutations (PIK3CA or ERBB2) in > 25% of a real-world clinical cohort. Additional prospective research implementing targetable therapies for EMPD treatment is warranted.
Extramammary Paget disease (EMPD) is a rare condition characterized by extreme pruritis and eczematoid-like lesions, most commonly of the skin of the external genitalia. EMPD of the vulva accounts for approximately 65% of EMPD. It occurs most often in White postmenopausal women and may be associated with underlying adenocarcinoma in 10%-30% of cases.1
Context
Key Objective
Extramammary Paget disease (EMPD) of the vulva is a rare, chronic disease leading to substantial morbidity. Mainstay of therapy is surgery, with patients frequently requiring multiple procedures during the course of disease. We sought to identify genomic alterations that may serve as potential therapeutic targets.
Knowledge Generated
The most common mutations noted were in PIK3CA, ERBB2 (both point mutation and oncogenic gene amplification were seen), and TP53. All tumors were microsatellite stable. PIK3CA and ERBB2 point mutations were classified as OncoKB level 3B: candidate predictive biomarkers for drug efficacy.
Relevance
These results suggest the frequent genomic alterations noted in EMPD in genes, including PIK3CA and ERBB2, may make these patients candidates for novel agents targeting these mutations.
The mainstay of treatment of noninvasive EMPD is surgical resection, with some patients requiring multiple resections over the course of the disease.2 Microscopically positive margins after resection are frequent, and multiple studies have shown that disease recurrence is common regardless of margin status.2-4 To spare patients repeated operations for recurrent disease, several nonsurgical modalities have been used to varying degrees of success. Among these, the topical immune-response modifier imiquimod, a toll-like receptor 7 (TLR7) agonist, has shown therapeutic promise as an alternative to surgery or as a perioperative adjunct.1 Other treatment modalities, including radiation therapy and topical and systemic chemotherapy (eg, fluorouracil), have been used in inoperable disease, but their use is limited due to the lack of clinical data outside of case reports.5 Given the frequency of recurrence and morbidity of repeated surgical excision, alternative, conservative treatment options would benefit these patients greatly.
In the search for alternatives to surgery or systemic therapy, prior studies have demonstrated that a subset of intraepithelial and invasive EMPD shows an overexpression of HER2 protein and ERBB2 gene amplification, as well as oncogenic mutations in PIK3CA and AKT1,6-11 which were associated with a more aggressive EMPD phenotype and poorer prognostic factors.
The goal of this study was to prospectively explore the molecular profile of primary noninvasive vulvar EMPD using massively parallel sequencing to identify potential therapeutic targets. We also report on a case of a patient with vulvar EMPD treated with targeted therapy on the basis of her tumor genomic mutations.
METHODS
Institutional review board approval for this retrospective analysis as well as for tumor molecular sequencing was obtained. Tumor and normal DNA were subjected to MSK-IMPACT—Memorial Sloan Kettering Cancer Center Integrated Mutation Profiling of Actionable Cancer Targets—sequencing, which targets 410-468 cancer-related genes (the number of genes analyzed was dependent upon the time when the tumor was subjected to analysis and the version of test available).12,13 Sequencing data analyses were performed, and mutations, copy number variations, and structural rearrangements were identified and annotated using validated bioinformatics approaches, as previously described.14,15 These molecular alterations were further curated using OncoKB (Memorial Sloan Kettering Cancer Center, New York, NY), a precision oncology knowledge base, to identity clinically relevant cancer gene alterations.16 For the quantification of microsatellite instability (MSI), MSIsensor was used, as previously described, and samples with an MSIsensor score ≥ 10 were deemed MSI-high.14,17
All pathology was confirmed by expert gynecologic pathologists. Electronic medical records were queried for all patients for demographics, clinical characteristics, treatment, and follow-up data. These features were integrated with the molecular findings. Research has shown that clinical outcomes are similar for patients with noninvasive and microscopically invasive (< 1 mm depth of invasion) disease, both groups were included in this report.18
RESULTS
Clinical Features
We identified 26 patients with EMPD of the vulva whose tumors had undergone genomic sequencing (Table 1). Median age at diagnosis was 62 years (range, 29-77 years). Seventeen patients (66%) were White, 4 (15%) were Black, and 5 (19%) were Asian. At the time of diagnosis, all patients had noninvasive or microinvasive (< 1 mm depth of invasion) tumors.
TABLE 1.
Clinicopathologic Characteristics of Patients With Extramammary Paget Disease of the Vulva
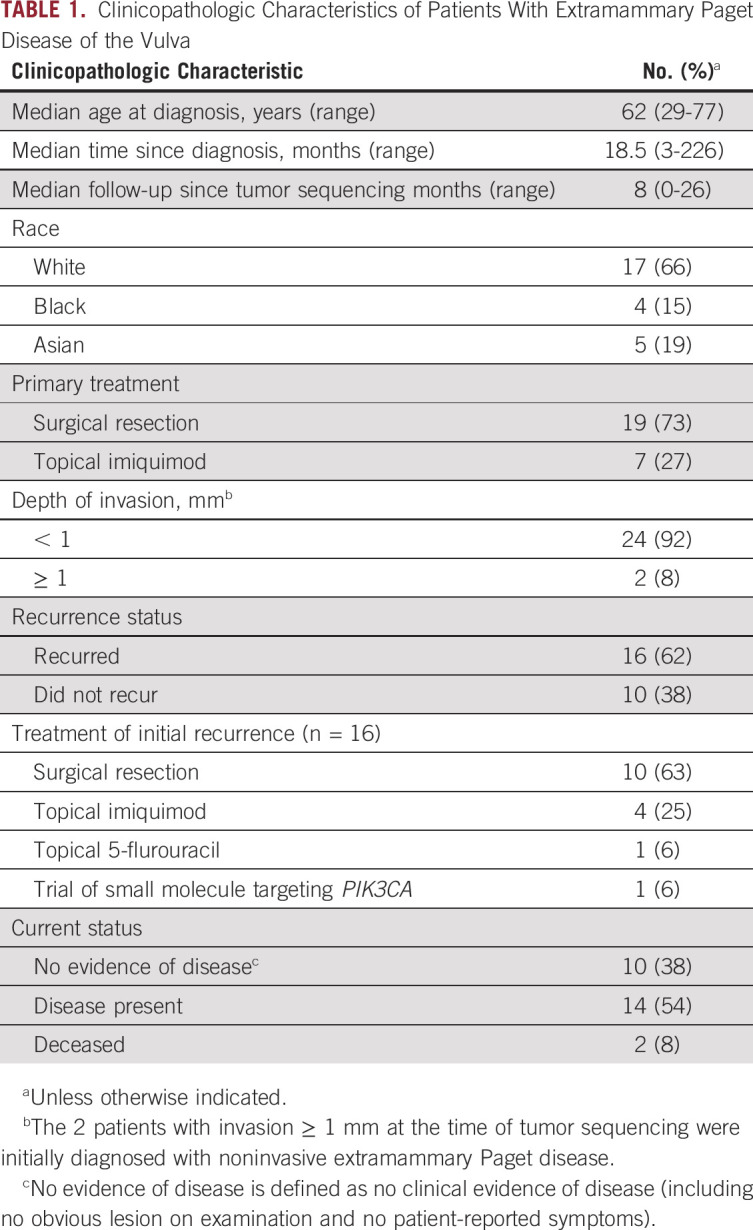
Primary treatment was surgery for 19 patients (73%) and imiquimod topical therapy for 7 (27%). Sixteen patients (62%) had recurrent disease after their initial treatment. Of these, 14 patients (87.5%) underwent a surgical resection at the time of diagnosis, whereas the other 2 (12.5%) were treated with imiquimod topical therapy. Seven patients (27%) had ≥ 2 surgeries as part of their clinical course, and 1 patient had undergone 5 vulvar resections.
Median time since initial diagnosis was 18.5 months (range, 3-226 months). Median follow-up time since tumor sequencing was 8 months (range, 0-26 months). Disease in 2 patients (8%) progressed to invasive adenocarcinoma, likely related to Paget disease, and both patients died of their disease.
Mutational Profiles of Vulvar EMPD
Molecular findings are summarized in Figure 1. The median coding mutation count was 2 (range, 0-29). Median allelic frequency of coding mutations in the samples ranged from 0%-32%. MSI was evaluated in 23 (88%) of the 26 samples. All 23 were microsatellite stable. Mutations were commonly seen in PIK3CA, TP53, and ERBB2.
FIG 1.
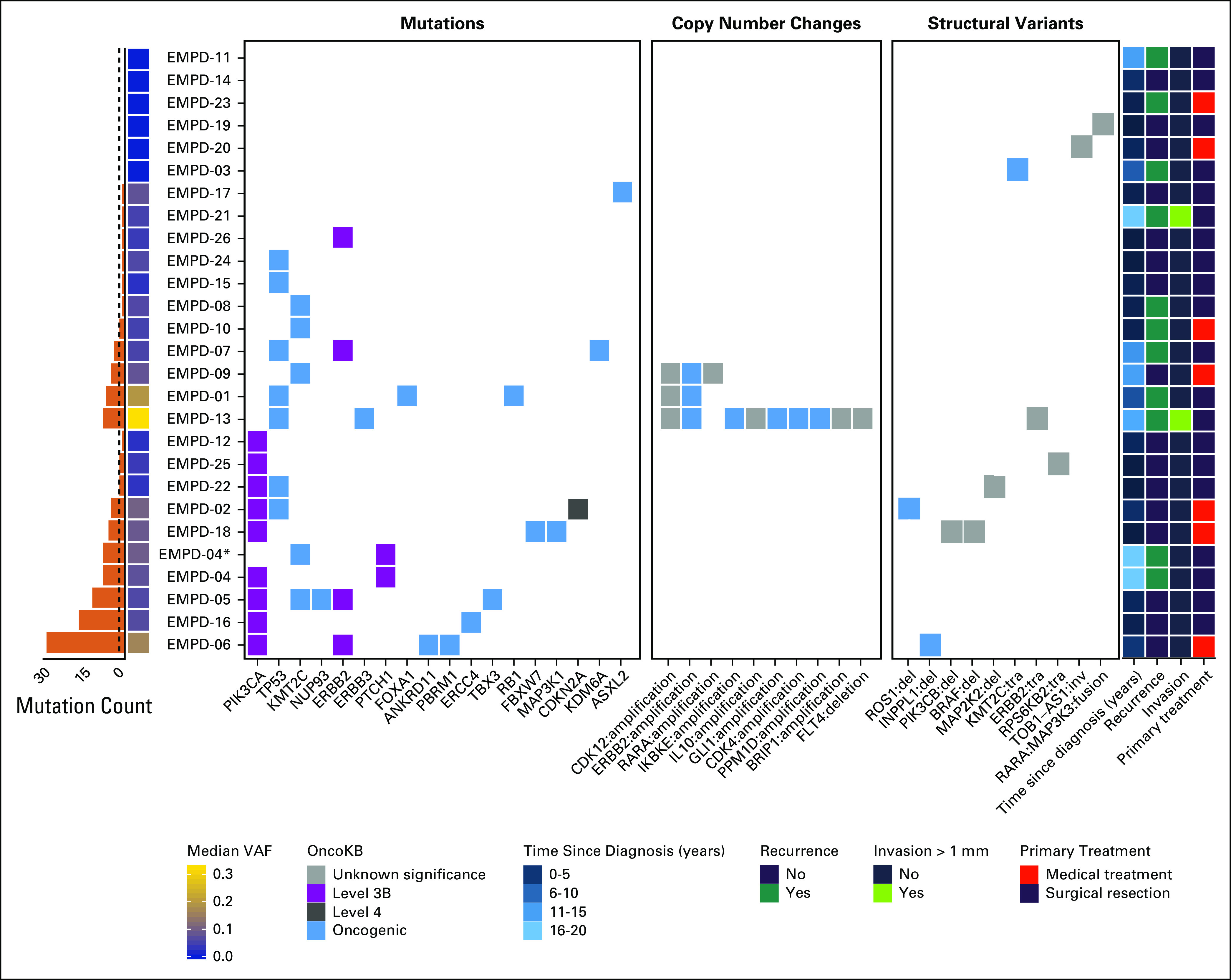
Vulvar extramammary Paget disease (EMPD) tumor sequencing. Tumor and normal DNA from 26 patients underwent MSK-IMPACT sequencing, targeting 410-468 cancer-related genes. All but 1 case (EMPD-21) were sequenced targeting for 468 genes. Mutations, copy number variations, and structural rearrangements were identified and annotated using validated bioinformatics approaches. These molecular alterations were further curated using OncoKB to identity putative clinically relevant cancer gene alterations. For copy number and structural rearrangement events, all variants are shown. For mutations, only those assigned an OncoKB level and/or classified as oncogenic are shown. Mutations of unknown significance are included in the mutation count presented in the bar plot on the left. The dotted line on the bar plot indicates the median mutation count of 2 across all the samples. Median variant allele frequency (VAF) in the samples ranged from 0%-32%. Relevant clinical features are mapped to the molecular results and are included on the right. (*) Metastatic sample for EMPD-04. Amp, amplification; del, deletion; inv, invasion; tra, translocation.
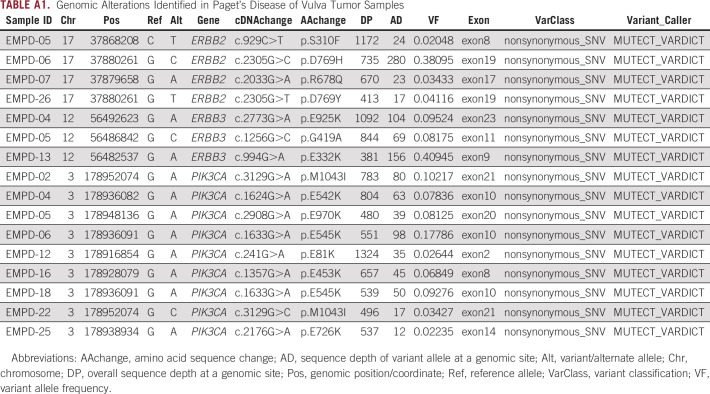
Seven tumors (27%) harbored an oncogenic TP53 mutation. Four tumors (15%) had an ERBB2 mutation, and an additional 3 tumors (12%) had an oncogenic ERBB2 amplification. The point mutations were all classified as OncoKB level 3B, which includes mutations that are candidate predictive biomarkers for US Food and Drug Administration–approved drugs being used off-label or in investigational agents.16 Specific mutations that are characterized as OncoKB level 3B are summarized in Appendix Table A1.
Nine tumors (35%) harbored a PIK3CA mutation. The mutations were all classified as OncoKB level 3B. Of these, 3 tumors (33%) had the hotspot mutations p.E542K or p.E545K. All tumors harboring PIK3CA mutations were noninvasive, and in the time since diagnosis, only 1 patient (EMPD-04, whose tumor harbored a p.E542K mutation) had a recurrence requiring treatment.
Multiple copy number and structural variants were identified in 11 samples (69%). Most of these alterations were variants of uncertain significance, with a few predicted to be oncogenic based on OncoKB but clinically not actionable.
Targeted Treatment of Vulvar EMPD
One patient in whom an oncogenic PIK3CA mutation was identified elected to enroll in a phase II clinical trial for treatment with a novel agent targeting the mutation. She was initially diagnosed with vulvar EMPD at the age of 49 years and was treated with a wide local excision of the tumor. Margins were positive and there was no evidence of invasion. She then had a total vulvectomy 3 years later. Over the next 15 years, she was followed closely with multiple biopsies performed but did not require additional treatment. A symptomatic recurrence in 2015 prompted her to seek treatment, and treatment with topical imiquimod was initiated. Her treatment was limited to 7 months before she self-discontinued for substantial adverse effects and worsening disease.
The patient’s tumor underwent sequencing, and a PIK3CA mutation, p.E542K, was identified. She enrolled in a trial of treatment with a molecule targeting the mutation and had a partial pathologic response with complete symptom resolution. Her disease was well controlled for 19 months before new symptomatic lesions led to drug discontinuation. The patient, once again, was treated with surgical resection. At 10 months since her surgery, she is experiencing minimal symptoms and has no new concerning lesions.
DISCUSSION
Noninvasive EMPD of the vulva is a chronic disease that causes substantial morbidity. The mainstay of treatment is surgical resection, with patients often requiring repeated excisions, leading to disfiguring cosmetic outcomes. Alternatives to surgery include imiquimod and, to a lesser, extent radiation therapy, photodynamic therapy, CO2 laser, topical 5-fluorouracil, and topical bleomycin.19 Topical 5-fluorouracil and bleomycin are highly toxic and are associated with poor response rates.20 Localized radiation therapy can lead to complete regression in up to 80% of patients, but it is also associated with potential adverse effects.21 Topical 5% imiquimod cream has been prospectively shown to be a feasible option for women with recurrent EMPD of the vulva, with a complete response rate of 55%-75%.22,23 Even imiquimod, however, is associated with intolerable adverse effects that can lead to treatment discontinuation. Alternative treatments are needed for this disease.
Over the past decade, many cancers have been successfully treated with targeted therapies on the basis of tumor molecular profiling. Recent publications have reported a subset of EMPD tumors with an overexpression of HER2 protein and ERBB2 gene amplification.6-8 In fact, Barth et al11 reported on a case of a patient with invasive EMPD who responded completely to single-agent trastuzumab. This patient’s tumor was determined to be HER2 positive by immunohistochemistry; however, next-generation sequencing did not identify an ERBB2 mutation or gene amplification. These findings led to an ongoing phase II trial of combining chemotherapy with trastuzumab in advanced EMPD (UMIN Clinical Trials Registry ID No. UMIN000021311). In our cohort, we identified patients with both ERBB2 gene amplifications and mutations. The point mutations identified are all classified as OncoKB level 3B: candidate predictive biomarkers for drug efficacy. Early evidence suggests that patients with EMPD with a somatic ERBB2 mutation would benefit from HER2-targeting agents, similar to those with HER2 gene amplification.24
Another study of EMPD tumors from both male (79%) and female (21%) patients looked at mutations in 10 genes in the RAS/RAF, PI3K/AKT, and WNT pathways, and found mutant RAS and RAF genes in 19% of cases and oncogenic PIK3CA and AKT1 mutations in 35% of cases.9 Interestingly, the majority of EMPD tumors with a PIK3CA and AKT1 mutation had an invasive disease phenotype. The authors postulated that activation of the PI3K/AKT pathway may be a precursor in the development of EMPD. Of note, the upregulation of the PI3K/AKT pathway is an important pathway in a multitude of cancers and may be associated with worse clinical outcomes and resistance to therapies.25-28
Exploring the genomic profile of EMPD in greater depth, Kiniwa et al29 performed whole-exome sequencing on 3 tumors from patients with EMPD and identified recurrent somatic mutations in TP53, PIK3CA, and ERBB2.29 This work contributes to our understanding of the molecular and genetic underpinnings of vulvar EMPD. Similar to previous reports, we identified a subset of patients with PIK3CA mutations and ERBB2 mutations or amplifications.
Multiple PI3K inhibitors are in development, and here we have described a patient who received benefit from such a targeted agent. ERBB2 inhibitors, including tyrosine kinase inhibitors and monoclonal antibodies, have demonstrated particular benefit in patients with breast cancer.30 Although these medications are only approved for tumors with HER2 protein overexpression or ERBB2 gene amplification, evidence suggests that patients with ERBB2 mutation-positive cancers can benefit from ERBB2 targeting agents.24
Similar to treatments aimed at tumor genetic mutations, immunotherapy drugs have become vital to the treatment of a variety of malignancies. Immunotherapy drugs are effective in DNA mismatch repair protein deficient or MSI-high tumors, and pembrolizumab, an antibody targeting anti-programmed cell death protein 1 (PD-1), has been approved by the Food and Drug Administration for use in these cancers. Previous studies have reported conflicting conclusions about programmed death-ligand 1 (PD-L1) expression in EMPD tumors or associated lymphocytes. Mauzo et al31 noted PD-L1 expression in 14% of EMPD tumors and in 83% of the tumor-infiltrating lymphocytes. Conversely, Karpathiou et al32 noted no expression of PD-L1 in any of the 22 EMPD tumors or associated immune infiltrates they examined. Furthermore, Tse et al33 found that no EMPD case in their series was either MSI-high or stained positive for PD-L1.
Although there may be a subset of tumors that express PD-L1, all the tumors that underwent MSI testing in our study were microsatellite stable, and no tumor was MSI-high, likely limiting the utility of immunotherapy in these EMPD tumors. It is also notable that, to date, there is no published literature, to our knowledge, reporting on patients with EMPD responding to checkpoint inhibitors.
Our study is limited by the small number of cases included, making it difficult to draw definitive conclusions. However, vulvar EMPD is a rare disease and our data support findings of previous publications suggesting that some patients with poorly controlled disease may be able to benefit from novel, targeted agents. We believe that although noninvasive EMPD is not lethal, the severity of symptoms and the lack of effective nonsurgical treatment options warrant including patients with EMPD in basket trials evaluating drugs targeting molecular aberrations.
Appendix
TABLE A1.
Genomic Alterations Identified in Paget’s Disease of Vulva Tumor Samples
SUPPORT
This work was funded in part by the National Institutes of Health, National Cancer Institute Memorial Sloan Kettering Cancer Center (Support Grant No. P30 CA008748 [M.M.L.]).
AUTHOR CONTRIBUTIONS
Conception and design: Marina Stasenko, Renee Cowan, Nadeem R. Abu-Rustum, Mario M. Leitao Jr
Provision of study material or patients: Dennis Chi, Mario M. Leitao Jr
Collection and assembly of data: Marina Stasenko, Gowtham Jayakumaran, Dennis S. Chi, Anthony Rossi, Ahmet Zehir, Mario M. Leitao Jr
Data analysis and interpretation: Marina Stasenko, Gowtham Jayakumaran, Renee Cowan, Vance Broach, Anthony Rossi, Ahmet Zehir, Nadeem R. Abu-Rustum, Mario M. Leitao Jr
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Vance Broach
Open Payments Link: https://openpaymentsdata.cms.gov/physician/1162364
Dennis S. Chi
Leadership: C Surgeries
Stock and Other Ownership Interests: Bovie Medical, Verthermia, Intuitive Surgical, TransEnterix
Consulting or Advisory Role: Bovie Medical, Verthemia, Biom 'Up
Anthony Rossi
Honoraria: Allergan, Evolus, Biofrontera, LAM Therapeutics, Cutera, Regeneron
Research Funding: LEO Pharma (Inst), regen (Inst), Skin Cancer Foundation (Inst)
Travel, Accommodations, Expenses: Regeneron
Travis J. Hollman
Consulting or Advisory Role: Sanofi/Aventis
Research Funding: Bristol Myers Squibb
Ahmet Zehir
Honoraria: Illumina
Nadeem R. Abu-Rustum
Honoraria: Prime Oncology
Research Funding: Stryker/Novadaq (Inst), Olympus (Inst), GRAIL (Inst)
Travel, Accommodations, Expenses: Prime Oncology
Mario M. Leitao Jr
Honoraria: Intuitive Surgical
Consulting or Advisory Role: Intuitive Surgical, Johnson & Johnson/Ethicon
Research Funding: KCI
Travel, Accommodations, Expenses: Intuitive Surgical
No other potential conflicts of interest were reported.
REFERENCES
- 1.McDaniel B, Crane JS. 2019. Extramammary Paget disease. in StatPearls. Treasure Island, FL, StatPearls Publishing, [PubMed] [Google Scholar]
- 2.Nitecki R, Davis M, Watkins JC, et al. Extramammary Paget disease of the vulva: A case series examining treatment, recurrence, and malignant transformation. Int J Gynecol Cancer. 2018;28:632–638. doi: 10.1097/IGC.0000000000001189. [DOI] [PubMed] [Google Scholar]
- 3.Black D, Tornos C, Soslow RA, et al. The outcomes of patients with positive margins after excision for intraepithelial Paget’s disease of the vulva. Gynecol Oncol. 2007;104:547–550. doi: 10.1016/j.ygyno.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Onaiwu C.O., Salcedo MP, Pessini SA, et al. Paget’s disease of the vulva: A review of 89 cases. Gynecol Oncol Rep. 2016;19:46–49. doi: 10.1016/j.gore.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edey K.A., Allan E, Murdoch JB, et al. Interventions for the treatment of Paget’s disease of the vulva. Cochrane Database Syst Rev. 2019;6:CD009245. doi: 10.1002/14651858.CD009245.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masuguchi S, Jinnin M, Fukushima S, et al. The expression of HER-2 in extramammary Paget’s disease. Biosci Trends. 2011;5:151–155. doi: 10.5582/bst.2011.v5.4.151. [DOI] [PubMed] [Google Scholar]
- 7.Richter C.E., Hui P, Buza N, et al. HER-2/NEU overexpression in vulvar Paget disease: The Yale experience. J Clin Pathol. 2010;63:544–547. doi: 10.1136/jcp.2010.077446. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka R, Sasajima Y, Tsuda H, et al. Human epidermal growth factor receptor 2 protein overexpression and gene amplification in extramammary Paget disease. Br J Dermatol. 2013;168:1259–1266. doi: 10.1111/bjd.12249. [DOI] [PubMed] [Google Scholar]
- 9.Kang Z, Xu F, Zhang QA, et al. Oncogenic mutations in extramammary Paget’s disease and their clinical relevance. Int J Cancer. 2013;132:824–831. doi: 10.1002/ijc.27738. [DOI] [PubMed] [Google Scholar]
- 10.Kang Z, Xu F, Zhang QA, et al. Correlation of DLC1 gene methylation with oncogenic PIK3CA mutations in extramammary Paget’s disease. Mod Pathol. 2012;25:1160–1168. doi: 10.1038/modpathol.2012.65. [DOI] [PubMed] [Google Scholar]
- 11.Barth P, Dulaimi Al-Saleem E, Edwards KW, et al. Metastatic extramammary Paget’s disease of scrotum responds completely to single agent trastuzumab in a hemodialysis patient: Case report, molecular profiling and brief review of the literature. Case Rep Oncol Med. 2015;2015:895151. doi: 10.1155/2015/895151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng D.T., Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. doi: 10.1038/nm.4333. Zehir A, Benayed R, Shah RH, et al: Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med 23:703-713, 2017 [Erratum: Nat Med 23(8):1004, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. doi: 10.1200/PO.19.00103. Smith ES, Da Cruz Paula A, Cadoo KA, et al: Endometrial cancers in BRCA1 or BRCA2 germline mutation carriers: Assessment of homologous recombination DNA repair defects. JCO Precis Oncol 3:1-11, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weigelt B, Bi R, Kumar R, et al. The landscape of somatic genetic alterations in breast cancers from ATM germline mutation carriers. J Natl Cancer Inst. 2018;110:1030–1034. doi: 10.1093/jnci/djy028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. doi: 10.1200/PO.17.00084. Middha S, Zhang L, Nafa K, et al: Reliable pan-cancer microsatellite instability assessment by using targeted next-generation sequencing data. JCO Precis Oncol 2017:10.1200/PO.17.00084, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crawford D, Nimmo M, Clement PB, et al. Prognostic factors in Paget’s disease of the vulva: A study of 21 cases. Int J Gynecol Pathol. 1999;18:351–359. doi: 10.1097/00004347-199910000-00010. [DOI] [PubMed] [Google Scholar]
- 18. doi: 10.1200/PO.17.00011. Chakravarty D, Gao J, Phillips S, et al: OncoKB: A precision oncology knowledge base. JCO Precis Oncol 1:1-6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wollina U. Extensive invasive extramammary Paget’s disease: Surgical treatment. J Cutan Aesthet Surg. 2013;6:41–44. doi: 10.4103/0974-2077.110098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam C, Funaro D. Extramammary Paget’s disease: Summary of current knowledge. Dermatol Clin. 2010;28:807–826. doi: 10.1016/j.det.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Son S.H., Lee JS, Kim YS, et al. The role of radiation therapy for the extramammary Paget’s disease of the vulva; experience of 3 cases. Cancer Res Treat. 2005;37:365–369. doi: 10.4143/crt.2005.37.6.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowan R.A., Black DR, Hoang LN, et al. A pilot study of topical imiquimod therapy for the treatment of recurrent extramammary Paget’s disease. Gynecol Oncol. 2016;142:139–143. doi: 10.1016/j.ygyno.2016.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawada M, Kato J, Yamashita T, et al. Imiquimod 5% cream as a therapeutic option for extramammary Paget’s disease. J Dermatol. 2018;45:216–219. doi: 10.1111/1346-8138.14117. [DOI] [PubMed] [Google Scholar]
- 24.Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3:224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salvesen H.B., Carter SL, Mannelqvist M, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc Natl Acad Sci USA. 2009;106:4834–4839. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woenckhaus J, Steger K, Sturm K, et al. Prognostic value of PIK3CA and phosphorylated AKT expression in ovarian cancer. Virchows Arch. 2007;450:387–395. doi: 10.1007/s00428-006-0358-3. [DOI] [PubMed] [Google Scholar]
- 27.Ludovini V, Bianconi F, Pistola L, et al. Phosphoinositide-3-kinase catalytic alpha and KRAS mutations are important predictors of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in patients with advanced non-small cell lung cancer. J Thorac Oncol. 2011;6:707–715. doi: 10.1097/JTO.0b013e31820a3a6b. [DOI] [PubMed] [Google Scholar]
- 28.Li SY, Rong M, Grieu F, et al. PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat. 2006;96:91–95. doi: 10.1007/s10549-005-9048-0. [DOI] [PubMed] [Google Scholar]
- 29.Kiniwa Y, Yasuda J, Saito S, et al. Identification of genetic alterations in extramammary Paget disease using whole exome analysis. J Dermatol Sci. 2019;94:229–235. doi: 10.1016/j.jdermsci.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 30. Dean L: Trastuzumab (Herceptin) therapy and ERBB2 (HER2) genotype, in Pratt V, McLeod HL, Rubinstein WS, et al:(eds), Medical Genetics Summaries [Internet]. August 5, 2015. Bethesda, MD, National Center for Biotechnology Information, August 5, 2015. [Google Scholar]
- 31.Mauzo SH, Tetzlaff MT, Milton DR, et al. Expression of PD-1 and PD-L1 in extramammary Paget Disease: Implications for immune-targeted therapy. Cancers (Basel) 2019;11:E754. doi: 10.3390/cancers11060754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karpathiou G, Chauleur C, Hathroubi S, et al. Expression of CD3, PD-L1 and CTLA-4 in mammary and extra-mammary Paget disease. Cancer Immunol Immunother. 2018;67:1297–1303. doi: 10.1007/s00262-018-2189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tse, J, Elvin JA, Vergilio J, et al: Extra-mammary Paget’s disease (EMPD) of the skin: A comprehensive genomic profiling (CGP) study. J Clin Oncol 37:9591, 2019 (15 suppl) [Google Scholar]