Abstract
PURPOSE
Conventional cytotoxic therapies increase the risk of clonal hematopoiesis and select for TP53-mutant clones, which carry a high risk for transformation to therapy-related myelodysplastic neoplasms. In contrast, the effect of immune checkpoint blockade (ICB) on clonal hematopoiesis is unknown.
METHODS
Paired peripheral-blood samples taken before and after treatment with ICB were obtained for 91 patients with either cutaneous melanoma or basal cell carcinoma. Error-corrected sequencing of a targeted panel of genes recurrently mutated in clonal hematopoiesis was performed on peripheral-blood genomic DNA.
RESULTS
The average interval between acquisition of the paired samples was 180 days. Forty-one percent of the patients had clonal hematopoiesis at a variant allele frequency (VAF) > 0.01 in the pretreatment sample. There was near-complete agreement in the distribution and burden of clonal hematopoiesis mutations in the paired blood samples, with 87 of 88 mutations identified across the cohort present in paired samples, regardless of the duration between sample collection. The VAF in the paired samples also showed a high correlation, with an R2 = 0.95 (P < .0001). In contrast to cytotoxic therapy, exposure to ICB did not lead to selection of TP53- or PPM1D-mutant clones. However, consistent with the known effects of DNA-damaging therapy, we identified one patient who had eight unique TP53 mutations in the posttreatment blood sample after receiving two courses of radiation therapy.
CONCLUSION
There was no expansion of hematopoietic clones or selection for clones at high risk for malignant transformation in patients who received ICB, observations that warrant further validation in larger cohorts. These findings highlight an important difference between ICB and conventional cytotoxic therapies and their respective impacts on premalignant genetic lesions.
INTRODUCTION
Clonal hematopoiesis is a phenomenon in which recurrent somatic mutations in hematopoietic stem cells result in selective clonal outgrowth. Clonal hematopoiesis of indeterminate potential (CHIP) refers specifically to the presence of a leukemia-associated driver mutation present in at least 4% of peripheral-blood cells (defined by a variant allele frequency [VAF] of at least 0.02) in an individual without a hematologic malignancy. CHIP is associated not only with an increased risk of myeloid neoplasms, but also with decreased overall survival in both healthy individuals and individuals with cancer.1-3 The strongest known risk factor for the development of CHIP, aside from age, is the receipt of chemotherapy or radiation, increasing the rate of CHIP by 5- to 10-fold relative to age-matched controls.2,3 Exposure to cytotoxic therapy specifically leads to a clonal advantage for hematopoietic stem cells with TP53 or PPM1D mutations, which render the cells resistant to DNA damage and lead to expanded clones bearing these mutations. These mutations are also enriched in therapy-related myelodysplastic syndrome (t-MDS) and acute myeloid leukemia (t-AML), consistent with the finding that TP53-mutant CHIP has a relatively high risk of transforming to leukemia.4-8
Context
Key Objective
Clonal hematopoiesis is common and associated with adverse outcomes, particularly in patients with cancer. Although conventional cytotoxic therapies are known to drive clonal hematopoiesis, particularly in patients with high-risk features such as TP53 mutations, the effect of immune checkpoint blockade (ICB) on clonal evolution is unknown.
Knowledge Generated
Analysis of paired blood samples obtained before and after treatment from 91 patients with ICB revealed few changes in the prevalence, clone size, and mutational landscape of clonal hematopoiesis, suggesting that, in contrast to cytotoxic therapies, ICB does not drive clonal evolution in hematopoietic cells.
Relevance
The absence of clonal evolution after ICB treatment contrasts with chemotherapeutic and radiation modalities, highlighting an important difference in potential long-term adverse outcomes. These findings can also inform our understanding of premalignant genetic lesions in other contexts.
Immune checkpoint blockade (ICB) targeting PD-1 and CTLA-4 is increasingly used for the treatment of a wide range of cancer types. Although ICB and cytotoxic agents have different toxicity and efficacy profiles, little is known about the selective pressure of ICB on CHIP clones.9 If CHIP clones are sensitive to ICB, immunotherapy could decrease the risk of the clinical sequelae of CHIP; if ICB enables clones to expand, ICB could increase the risk of t-MDS and t-AML as does cytotoxic therapy. To address this question directly, we characterized the landscape of somatic mutations using error-corrected sequencing in paired blood samples obtained before and after treatment with ICB in a cohort of patients with metastatic cutaneous melanoma and basal cell carcinoma.
METHODS
The study was approved by institutional review boards at each institution, and all samples were obtained after patient consent. Genomic DNA was isolated from peripheral-blood samples (DNEasy Kit; Qiagen, Hilden, Germany) of patients treated at our institutions. Error-corrected sequencing of all samples was performed by hybrid capture on the genomic DNA samples followed by library preparation with double-stranded unique molecular identifiers using a custom bait set from Twist Bioscience (San Francisco, CA; Data Supplement). Sequencing was performed on the Illumina platform (Illumina, San Diego, CA). After deduplication and consensus sequence calling, mutations were identified using Varscan 2.2.3 and annotated using ANNOVAR. Mutations were scored based on allele fraction, strand bias differential, local noise and mapping quality, and frequency in germline polymorphism databases. These variants were visually inspected in Integrated Genome Viewer (Broad Institute, Cambridge, MA). We required at least three alternative reads to consider a variant for further analysis. To minimize the possibility of inadvertent inclusion of artifactual mutations or cross-sample contamination, we only considered non–hot spot variants that were present in no more than 2 unique individuals. VAF was determined by the ratio of alternative reads to total reads at a specific nucleotide. Mutations were classified as pathogenic based on variant rules as previously described.10
Statistical comparisons were performed using two-sided Mann-Whitney U or χ2 tests, with a P < .05 considered significant. Statistical analyses were performed using Prism (v8.4.2; GraphPad, La Jolla, CA).
RESULTS
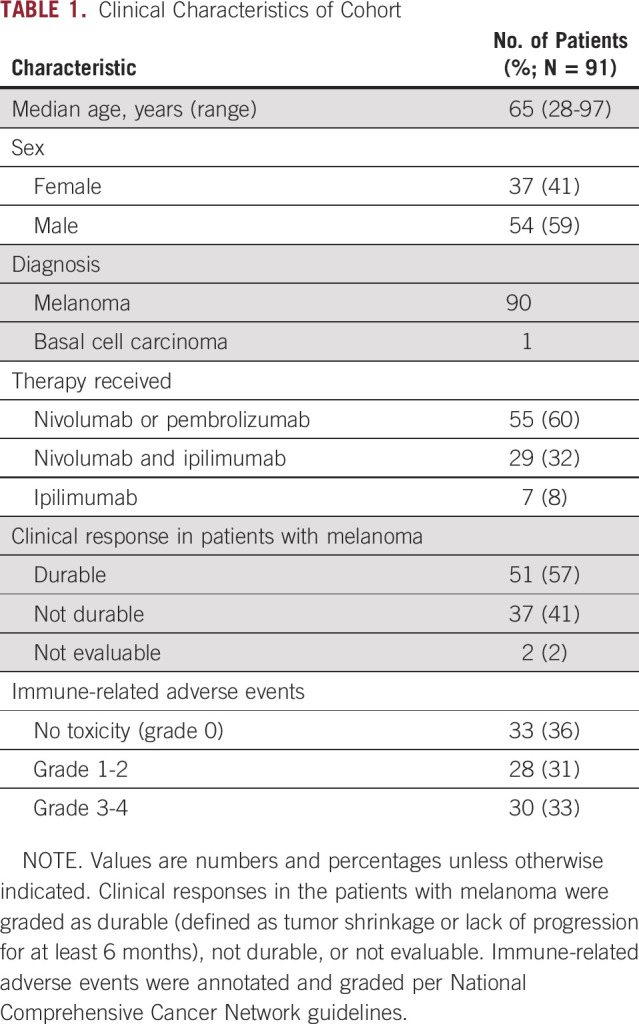
To determine the impact of ICB on clonal somatic hematopoietic mutations, we examined pre- and posttreatment blood samples (median interval, 180 days; interquartile range [IQR], 141-204 days) from 91 patients treated at our institutions for cutaneous melanoma (n = 90) or basal cell carcinoma (n = 1). Patients received ICB with either pembrolizumab or nivolumab (n = 55), ipilimumab (n = 7), or ipilimumab in combination with nivolumab (n = 29; Table 1, Appendix Fig A1A, Data Supplement). The median age of the cohort was 65 years (IQR, 57.0-73.0 years), and the majority of patients were men (59%; Appendix Fig A1B). At baseline, clonal mutations with a VAF > 0.01 were present in 41% of patients, were age associated, and were enriched for C→T single nucleotide variants as expected (Appendix Figs A1C and A1D). The most commonly mutated genes at baseline in the patients with CHIP were DNMT3A and TET2 (Appendix Fig A1E, Data Supplement).
TABLE 1.
Clinical Characteristics of Cohort
The clonal mutations detected and their VAFs were stable after ICB treatment, indicating that ICB does not apply an immediate selective pressure to these clones. TP53 and PPM1D clones did not change in size after ICB as they do after cytotoxic therapy (Fig 1A). Of the 65 mutations with VAF > 0.01 present in both samples, there was a significant correlation between the VAFs in the paired samples (slope, 0.96; R2 = 0.95; P < .0001; Fig 1B). We did not observe any genes in which mutations consistently expanded or contracted over the treatment interval (Figs 1B and 1C). Furthermore, there was no association between the change in VAF and the interval between blood draws (Fig 1D), including in patients with an interval of > 1 year. We expanded our analysis to include all mutations regardless of VAF and found that all but two were identified in the paired sample, meaning that across the entire cohort, 87 (99%) of 88 mutations initially identified with a VAF > 0.01 were present in both of the paired samples.
FIG 1.
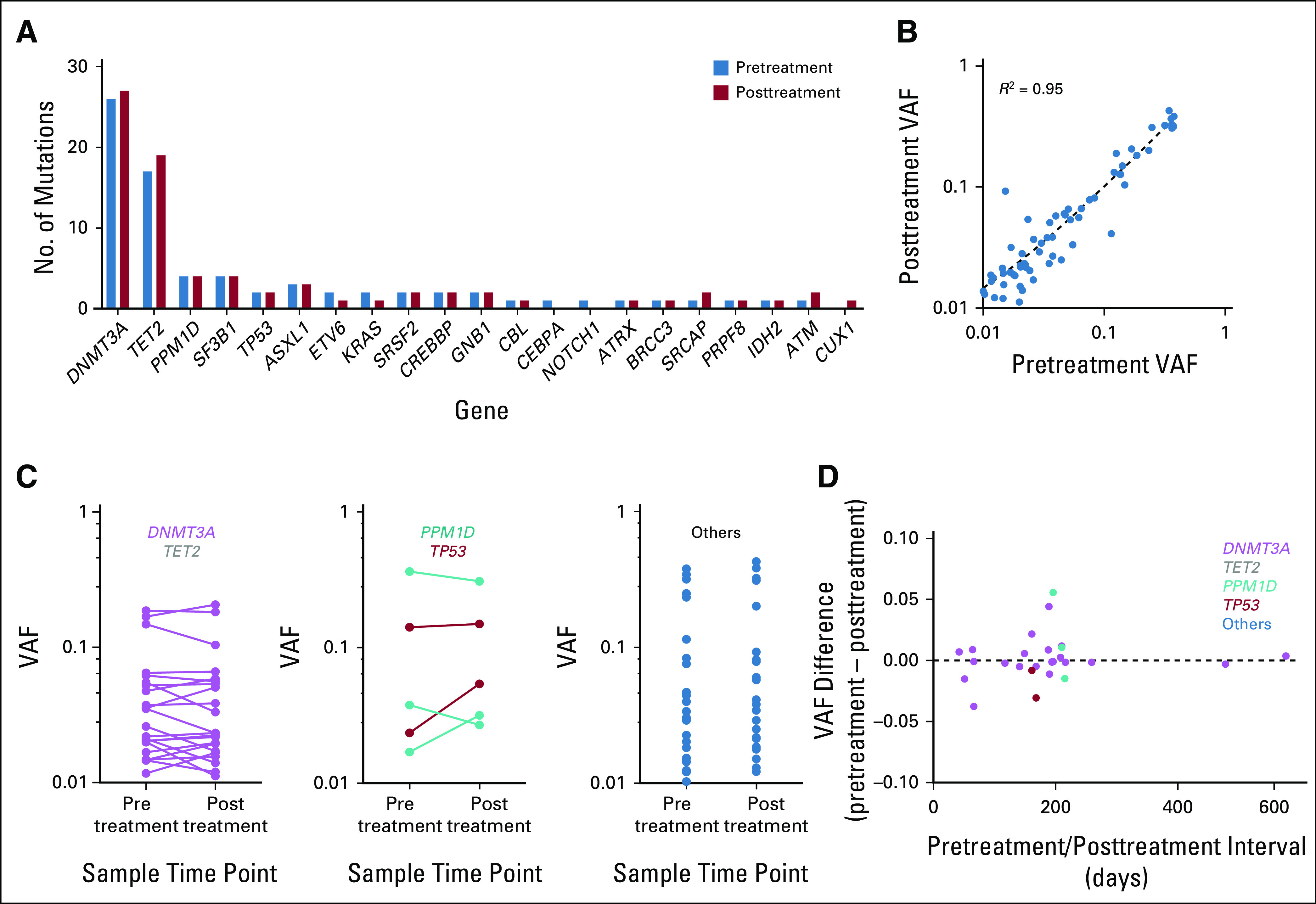
Immune checkpoint blockade does not induce clonal evolution. (A) Frequency of mutations identified in each gene in the pretreatment (blue) and posttreatment (red) samples. (B) Relationship between the variant allele frequency (VAF) of a particular mutation in the pre- and posttreatment samples. (C) Change in VAF for each mutation in the pre- and posttreatment samples stratified by mutation group. (D) Relationship between the change in VAF between pre- and posttreatment samples and interval between sample acquisition.
We found that one patient had eight unique TP53 mutations in the DNA binding domain with VAFs ranging from 0.003 to 0.006 (A129S, C182S, D184indelDY, M133L, Q165K, Q167E, S166L, and T150P), all of which were present in the posttreatment sample only. Upon further investigation, we found that this patient had received both ICB and two rounds of targeted radiation therapy (18 Gy to the left occipital area and 18 Gy to the right precuneus) approximately 2 and 5.5 months after the pretreatment sample, respectively, but both before the posttreatment sample. The emergence of these mutations is consistent with the rapid expansion of TP53-mutant hematopoietic stem-cell clones after exposure to cytotoxic therapy, as previously observed in both human studies of CHIP animal modeling of radiation effects on expansion of TP53-mutant hematopoietic cells.2,11 TP53-mutant clones did not emerge in the two other patients in the cohort who received radiation therapy in the sample interval.
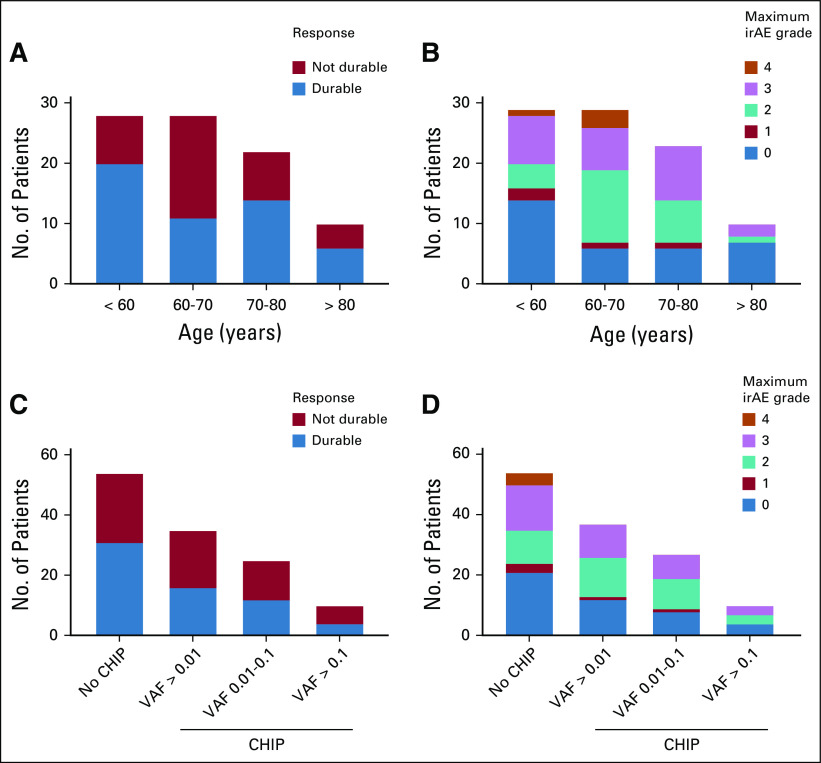
Immune-related adverse events (irAEs) were annotated and graded per National Comprehensive Cancer Network guidelines, and responses in the patients with melanoma were graded as either durable (n = 51; defined as tumor shrinkage or lack of progression for at least 6 months), not durable (n = 37), or not evaluable (n = 2; Appendix Figs A2A and A2B; Data Supplement). In univariable analysis, neither age nor CHIP, regardless of VAF, was significantly associated with response to therapy or irAEs, although the size of the cohort limits identification of small effect sizes (Appendix Fig A2). In the 15 patients for whom clinical sequencing of the solid tumor specimen was available,12 we identified 4 patients in whom reported mutations in the tumor were present in the blood and likely represent CHIP contamination of the solid tumor (Data Supplement), an increasingly recognized and clinically important issue when interpreting solid tumor sequencing.13,14
DISCUSSION
Our data highlight two important differences between the effects of cytotoxic and ICB therapies on the clonal dynamics of hematopoietic cells. First, ICB did not select for cells with mutations in genes involved in the DNA damage response, including TP53, and second, it did not cause expansion of CHIP clones that existed before treatment. Although we cannot rule out the possibility of subtle effects on clonal dynamics that would be identifiable only with a longer period of observation, the lack of expansion was independent of the time interval between sample collection, and the median interval of 180 days exceeds the time that clonal evolution has been observed after chemotherapy or radiation exposure in both human cohorts and mouse models.3,8,11,15 The absence of selection for cells with TP53 mutations is of particular clinical importance given the high risk of transformation of these clones. More generally, larger clones are associated with increased risk of cytopenias, malignancy, and death from cardiovascular disease.4,5,16-18 Our findings also indicated that ICB does not effectively target premalignant clones in the blood, consistent with the poor efficacy of ICB as monotherapy for myeloid malignancies.19 Further efforts to validate these results in larger cohorts are warranted.
Premalignant clones have been observed in a variety of tissues, including the lung, liver, and colon.20-22 Accordingly, the potential of pharmacologic therapies to alter the prevalence and evolution of these lesions has implications for the risk of developing second cancers. In the case of hematopoietic cells, cytotoxic therapies cause the expansion of clones that lead to t-MDS and t-AML. Our findings demonstrate that, in contrast to cytotoxic therapies, ICB does not alter clonal dynamics of somatically mutated cells and is therefore unlikely to alter predisposition to myeloid malignancies
APPENDIX
FIG A1.
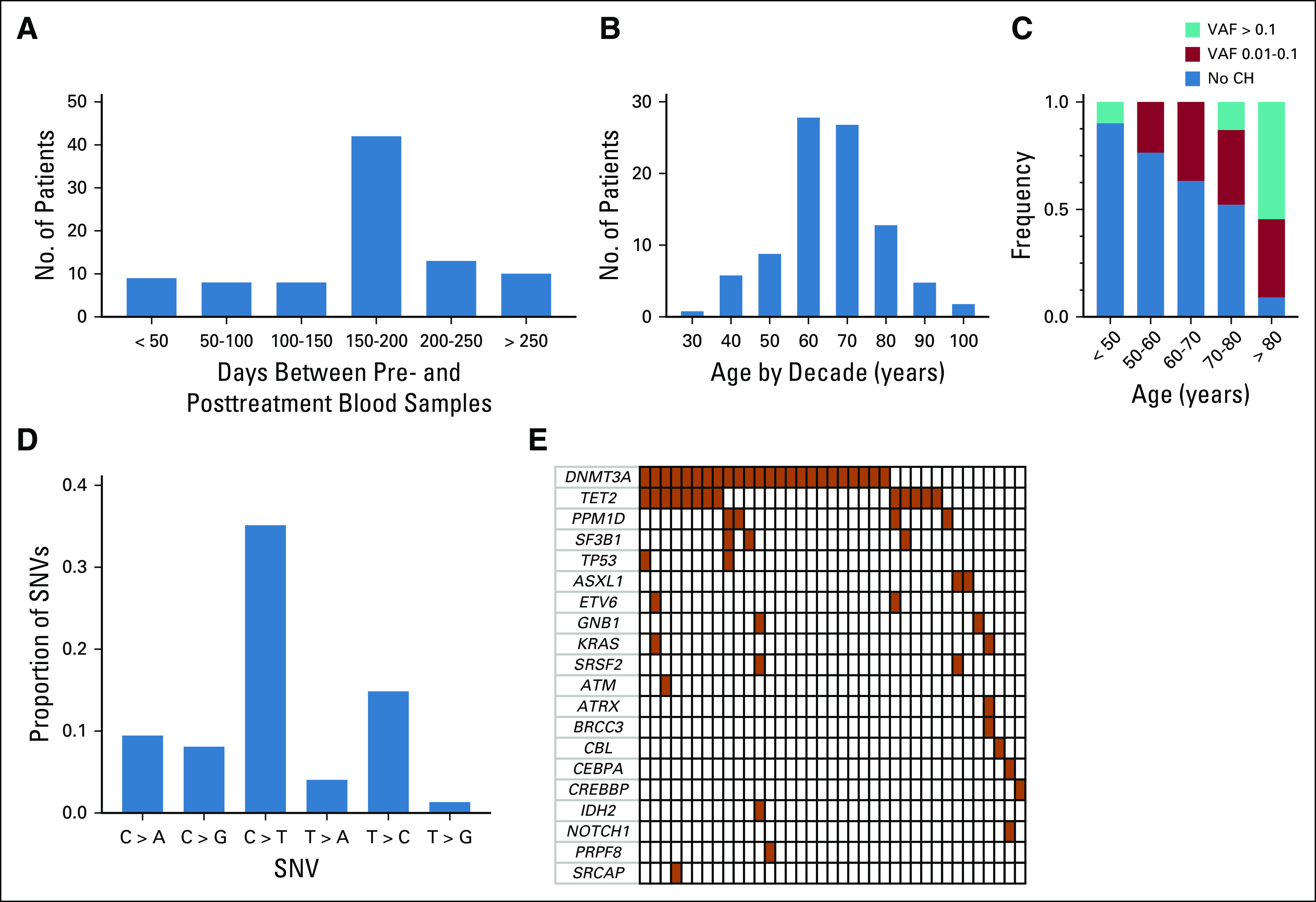
Clonal hematopoietic mutation characteristics identified in cohort. (A) Distribution of time interval between pre- and posttreatment blood draw. (B) Age distribution of cohort by decade. (C) Distribution of variant allele frequencies (VAFs) in different age groups. (D) Frequency of single nucleotide variants (SNVs) in patients with somatic mutations. (E) Co-mutation plot of somatic mutations identified in the pretreatment blood samples. Each column represents a patient, with darkened cells indicating the presence of a mutation. CH, clonal hematopoiesis.
FIG A2.
Association between clonal hematopoiesis and response to immune checkpoint blockade. (A) Clinical response and (B) maximum severity of immune-related adverse events (irAEs) stratified by age. (C) Clinical response and (D) maximum severity of irAEs stratified by clonal hematopoiesis of indeterminate potential (CHIP) status (no CHIP, CHIP with variant allele frequency [VAF] > 0.01, CHIP with VAF 0.01-0.1, and CHIP with VAF > 0.1).
SUPPORT
Supported by National Institutes of Health Grants No. R01HL082945 and P01CA108631, the Howard Hughes Medical Institute, the Edward P. Evans Foundation, the Leukemia and Lymphoma Society (B.L.E.), an Evans Foundation Young Investigator Award (P.G.M.), an American Society of Hematology Research Training Award for Fellows (P.G.M.), a Ruth L. Kirschstein National Research Service Award (A.S.S.), and a Conquer Cancer Foundation Young Investigator Award (A.S.S.).
AUTHOR CONTRIBUTIONS
Conception and design: Peter G. Miller, Christopher J. Gibson, Arnav Mehta, Adam S. Sperling, Nir Hacohen, F. Stephen Hodi, Genevieve M. Boland, Benjamin L. Ebert
Financial support: F. Stephen Hodi, Genevieve M. Boland, Benjamin L. Ebert
Administrative support: Michael P. Manos
Provision of study materials or patients: Dennie T. Frederick, Michael P. Manos, Benchun Miao, Nir Hacohen, Genevieve M. Boland
Collection and assembly of data: Peter G. Miller, Arnav Mehta, Adam S. Sperling, Dennie T. Frederick, Michael P. Manos, F. Stephen Hodi
Data analysis and interpretation: Peter G. Miller, Christopher J. Gibson, Arnav Mehta, Adam S. Sperling, Benchun Miao, F. Stephen Hodi, Benjamin L. Ebert
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Peter G. Miller
Employment: Anthem (I)
Consulting or Advisory Role: Foundation Medicine
Arnav Mehta
Consulting or Advisory Role: Third Rock Ventures
Nir Hacohen
Stock and Other Ownership Interests: BioNTech
Honoraria: Merck
Consulting or Advisory Role: Neon Therapeutics
Patents, Royalties, Other Intellectual Property: Patent portfolio for neoantigen vaccines
F. Stephen Hodi
Employment: Dana-Farber Cancer Institute
Stock and Other Ownership Interests: Apricity, Torque
Consulting or Advisory Role: Merck Sharp & Dohme, Novartis, Genentech, EMD Serono, Sanofi, Bayer, Aduro Biotech, Pfizer, Verastem, Bristol Myers Squibb, Takeda, Surface, Compass Therapeutics, Partners Therapeutics, Pionyr, 7Hills Pharma, Torque, Rheos, Amgen, Boston Pharmaceuticals, Checkpoint Therapeutics, Eisai
Research Funding: Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Genentech (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Patent pending as per institutional policy; patent pending royalties received on MICA-related disorders application to institution per institutional intellectual property policy; angiopoietin-2 biomarkers predictive of anti-immune checkpoint response (Inst); compositions and methods for identification, assessment, prevention, and treatment of melanoma using PD-L1 isoforms; methods of using pembrolizumab and trebananib (Inst)
Travel, Accommodations, Expenses: Novartis, Bristol Myers Squibb
Other Relationship: Bristol Myers Squibb, Genentech
Genevieve M. Boland
Honoraria: Novartis, Takeda Oncology
Consulting or Advisory Role: NW Biotherapeutics
Research Funding: Takeda, Olink Proteomics, Palleon Pharmaceuticals, Takeda Oncology
Other Relationship: Nektar
Benjamin L. Ebert
Stock and Other Ownership Interests: Skyhawk Therapeutics, Exo Therapeutics
Consulting or Advisory Role: Exo Therapeutics, Skyhawk Therapeutics
Research Funding: Celgene, Deerfield Management
Patents, Royalties, Other Intellectual Property: Patents related to the prediction of risk of cardiovascular disease (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–2498. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. doi: 10.1016/j.stem.2017.07.010. Coombs CC, Zehir A, Devlin SM, et al: Therapy-related clonal hematopoiesis in patients with non-hematologic cancers is common and associated with adverse clinical outcomes. Cell Stem Cell 21:374-382.e4, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gibson CJ, Lindsley RC, Tchekmedyian V, et al. Clonal hematopoiesis associated with adverse outcomes after autologous stem-cell transplantation for lymphoma. J Clin Oncol. 2017;35:1598–1605. doi: 10.1200/JCO.2016.71.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abelson S, Collord G, Ng SWK, et al. Prediction of acute myeloid leukaemia risk in healthy individuals. Nature. 2018;559:400–404. doi: 10.1038/s41586-018-0317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai P, Mencia-Trinchant N, Savenkov O, et al. Somatic mutations precede acute myeloid leukemia years before diagnosis. Nat Med. 2018;24:1015–1023. doi: 10.1038/s41591-018-0081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. doi: 10.1016/j.stem.2018.10.004. Hsu JI, Dayaram T, Tovy A, et al: PPM1D mutations drive clonal hematopoiesis in response to cytotoxic chemotherapy. Cell Stem Cell 23:700-713.e6, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kahn JD, Miller PG, Silver AJ, et al. PPM1D-truncating mutations confer resistance to chemotherapy and sensitivity to PPM1D inhibition in hematopoietic cells. Blood. 2018;132:1095–1105. doi: 10.1182/blood-2018-05-850339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong TN, Ramsingh G, Young AL, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2015;518:552–555. doi: 10.1038/nature13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis EJ, Salem JE, Young A, et al. Hematologic complications of immune checkpoint inhibitors. Oncologist. 2019;24:584–588. doi: 10.1634/theoncologist.2018-0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindsley RC, Saber W, Mar BG, et al. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376:536–547. doi: 10.1056/NEJMoa1611604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boettcher S, Miller PG, Sharma R, et al. A dominant-negative effect drives selection of TP53 missense mutations in myeloid malignancies. Science. 2019;365:599–604. doi: 10.1126/science.aax3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia EP, Minkovsky A, Jia Y, et al. Validation of OncoPanel: A targeted next-generation sequencing assay for the detection of somatic variants in cancer. Arch Pathol Lab Med. 2017;141:751–758. doi: 10.5858/arpa.2016-0527-OA. [DOI] [PubMed] [Google Scholar]
- 13.Ptashkin RN, Mandelker DL, Coombs CC, et al. Prevalence of clonal hematopoiesis mutations in tumor-only clinical genomic profiling of solid tumors. JAMA Oncol. 2018;4:1589–1593. doi: 10.1001/jamaoncol.2018.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Severson EA, Riedlinger GM, Connelly CF, et al. Detection of clonal hematopoiesis of indeterminate potential in clinical sequencing of solid tumor specimens. Blood. 2018;131:2501–2505. doi: 10.1182/blood-2018-03-840629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong TN, Miller CA, Jotte MRM, et al. Cellular stressors contribute to the expansion of hematopoietic clones of varying leukemic potential. Nat Commun. 2018;9:455. doi: 10.1038/s41467-018-02858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malcovati L, Gallì A, Travaglino E, et al. Clinical significance of somatic mutation in unexplained blood cytopenia. Blood. 2017;129:3371–3378. doi: 10.1182/blood-2017-01-763425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bick AG, Pirruccello JP, Griffin GK, et al. Genetic interleukin 6 signaling deficiency attenuates cardiovascular risk in clonal hematopoiesis. Circulation. 2020;141:124–131. doi: 10.1161/CIRCULATIONAHA.119.044362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Garcia-Manero G, Tallman MS, Martinelli G, et al: Pembrolizumab, a PD-1 inhibitor, in patients with myelodysplastic syndrome (MDS) after failure of hypomethylating agent treatment. Blood 128:345, 2016 (abstr) [Google Scholar]
- 20.Moore L, Leongamornlert D, Coorens THH, et al. The mutational landscape of normal human endometrial epithelium. Nature. 2020;580:640–646. doi: 10.1038/s41586-020-2214-z. [DOI] [PubMed] [Google Scholar]
- 21.Yoshida K, Gowers KHC, Lee-Six H, et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature. 2020;578:266–272. doi: 10.1038/s41586-020-1961-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunner SF, Roberts ND, Wylie LA, et al. Somatic mutations and clonal dynamics in healthy and cirrhotic human liver. Nature. 2019;574:538–542. doi: 10.1038/s41586-019-1670-9. [DOI] [PMC free article] [PubMed] [Google Scholar]