Abstract
PUROSE
Ethiopia has one cobalt radiotherapy (RT) machine to serve a population of more than 100 million. The purpose of this study was to report on patterns of palliative RT of bone metastasis in a severely low-capacity setting.
PATIENTS AND METHODS
Patient and treatment characteristics of patients irradiated for palliation of symptomatic bone metastasis were extracted from a retrospective database of patients treated between May 2015 and January 2018. This database included a random sample of 1,823 of the estimated 4,000 patients who were treated with RT within in the study period. Associations between the applied RT schedule and patient and tumor characteristics were evaluated with the χ2 test. Hypothetical savings of RT sessions and time were compared in the case of a single-fraction policy.
RESULTS
From the database, 234 patients (13%) were treated for bone metastasis. Most patients were ≤ 65 years of age (n = 189; 80%) and female (n = 125; 53%). The most common primary sites were breast (n = 82; 35%) and prostate (n = 36; 15%). Fractionated regimens were preferred over single fraction: 20 Gy in 5 fractions (n = 192; 82.1%), 30 Gy in 10 fractions (n = 7; 3%), and 8 Gy in 1 fraction (n = 28; 12%). Factors associated with single-fraction RT included nonaxial sites of bone metastasis (P < .01) and an address outside Addis Ababa (P ≤ .01). If single-fraction RT would have been given uniformly for bone metastasis, this would have resulted in a 78% reduction in the number of RT sessions and 76% reduction in total RT time.
CONCLUSION
The pattern of palliative RT for bone metastasis in Ethiopia favors fractionated regimens over single fraction. Efforts should be made to adopt evidence-based and cost-effective guidelines.
INTRODUCTION
Cancer burden is increasing in sub-Saharan Africa because there is a shift from communicable diseases to noncommunicable diseases.1 A majority of patients with cancer are presenting with advanced disease when curative treatment is difficult or not feasible.2 Bone is a common site of metastasis in several types of cancer, such as breast and lung cancer, which are common worldwide regardless of a country's development status.3 Radiotherapy (RT) is widely regarded as an effective, efficient, and cost-effective therapeutic approach for symptomatic bone metastasis.4
Context
Key Objective
This article addresses patterns of radiotherapy practice for bone metastasis in Ethiopia, a country with a severe shortage of radiation capacity.
Knowledge Generated
Over a period of 2.5 years, the majority of patients received fractionated radiotherapy regimens (5-10 fractions). Patients with nonspinal metastasis and patients living farther from the radiotherapy center were more likely to receive a single fraction of radiotherapy.
Relevance
The findings reflect a preference toward fractionated regimens for the treatment of bone metastasis in Ethiopia despite the safety and efficacy of a single fraction of radiotherapy in uncomplicated patients. Previous practice surveys across Africa have suggested a preference toward fractioned regimens; however, there is a paucity of audited data. Significant savings of radiation capacity could be achieved by shifting patients with uncomplicated bone metastasis to single-fraction radiotherapy. Implementation of resource-stratified treatment guidelines promotes use of cost-effective treatment strategies.
Despite the fact that the world’s cancer burden in Africa is growing, there is an ongoing shortage of RT capacity. Approximately half of the countries in Africa have no RT centers, and the other half have some RT equipment, but all are below the ideal ratio of RT centers per population as set by the International Atomic Energy Agency (IAEA).5 In Africa, there is an average of 3.8 million people per RT machine; however, the current situation in Ethiopia is even more severe, with one cobalt RT machine for more than 100 million people.6
In Ethiopia, a majority of patients are presenting at an advanced stage and receiving palliative-intent RT. Priority is given to palliative patients; thus, the median waiting time for curative-intent therapy is 5 months.7 The purpose of this study was to describe and highlight the current patterns of palliative RT for symptomatic bone metastasis in a large and populated country in sub-Saharan Africa with severely limited RT capacity to advise institutional guidelines for the most effective and cost-efficient regimens.
PATIENTS AND METHODS
Study Design and Participants
This retrospective study included patients treated with external-beam cobalt RT for bone metastasis between May 2015 and January 2018 in the Oncology Department of Tikur Anbessa Specialized Hospital (TASH) in Addis Ababa, Ethiopia. It is estimated that during the 2.5-year study period, approximately 4,250 patients were treated with RT at the center. A selection of 1,826 patient paper files were randomly chosen from the file room by file attendants. This database included adult and pediatric patients with any malignant diagnosis treated with either curative- or palliative-intent RT. The methods have been described previously.7 Inclusion of all patients treated during the study period was not considered feasible because of difficulty locating paper charts from incomplete registration data in an overcrowded file room.
Inclusion criteria were patients treated with palliative-intent RT for metastatic disease of solid tumors (based on anatomic extent of the disease according to the TNM classification) and whose the primary site of RT was to the appendicular (shoulder or hip girdles and extremities) or axial (skull, spine, and ribs) skeleton.
Data Collection
General demographic information (age, sex, residing region), diagnosis (site of primary tumor), presence of symptoms, and data regarding RT treatment were extracted from the database. Treatment characteristics, including site of treatment, dose, fraction(s), dates of treatment, wait time (time since initial visit to starting treatment), the number of patients who completed the prescribed regimen, and toxicity (type and grade) were recorded. Survival data, performance status, and information regarding surgical intervention were not available in the database because this information was not routinely documented in patient files, nor was there an institutional or national record of vital statistics (deaths) because of large patient numbers and inadequate quantity of workforce.
Study Site
TASH is currently the only comprehensive cancer center in Ethiopia. There is one functional cobalt machine (Theratron Equinox, Best Theratronics, Ottawa, Ontario, Canada). The logistics of providing RT services to a large country with one machine has been previously described.8 The cobalt source was last replaced in 2016, the same year brachytherapy capacity was added. There were six radiation oncologists and 28 residents by the end of the study. The four senior oncologists were trained in South Africa and Egypt, and the others were trained at TASH, which opened a training program in 2013. There were four medical physicists, five radiation therapists, and 26 oncology nurses on staff. On average, 80-100 patients were treated per day.
RT Procedure
Palliative RT is prioritized at TASH, and generally, treatment is started within days of presentation. Patients with suspected bone metastases were evaluated with computed tomography scans or, less commonly, magnetic resonance imaging to confirm the site of disease. RT dose and fractionation were decided by the treating radiation oncologist, because there were no specific institutional guidelines. Radiation planning was two-dimensional radiation based on imaging and examination, using anatomic landmarks to decide on the specific site for radiation treatment. Simple arrangements of radiation fields were used. For vertebral metastasis located on the thoracic and lumbar spine, a single posterior field was typically used. Lesions located on the cervical spine were often treated with two lateral fields to spare the oral cavity. Metastases in the pelvis were treated as either whole pelvis or hemipelvis (anterior-posterior and posterior-anterior fields). Other sites of bone metastases were also treated with a single- or two-field arrangements. Quality assurance was conducted daily on the cobalt machine, but not before each new patient. Any acute toxicities documented by the treating physician (type and grade using Common Terminology Criteria for Adverse Effects, version 4.0) were recorded. This was assessed at the end of treatment and at the recommended 4-week follow-up, although follow-up was often sporadic, and toxicity was not routinely documented.
Statistical Analysis
Categorical variables were expressed as numbers and percentages. Continuous variables were expressed as mean with standard deviation in case of a normal distribution or otherwise as median with interquartile range. To assess the association between patient and tumor characteristics and treatment with single-fraction RT, χ2 test was performed. A P value < .05 was considered statistically significant. Change in fractions and time was calculated by percent change of the summation of fractions and time per fraction in current distribution of dosing regimens (excluding seven patients receiving varied regimens) compared with whether all bone metastases were treated with single-fraction therapy, such as performed by Williams et al9 in 2006. IBM SPSS Statistics (version 24; IBM, Armonk, NY) was used for all statistical analyses.
Ethical Considerations
Ethical approval was obtained from the Addis Ababa University Clinical Oncology Department ethical review board. The data had previously been de-identified and was managed on a secured Microsoft Excel 2018 (v16.18) spreadsheet (Microsoft, Redmond, WA). The study was conducted without individual informed consent, per guidelines at TASH, because the study relied on retrospective data collected as part of routine patient care.
RESULTS
Patient Characteristics, Cancer Distribution, and Baseline Symptoms
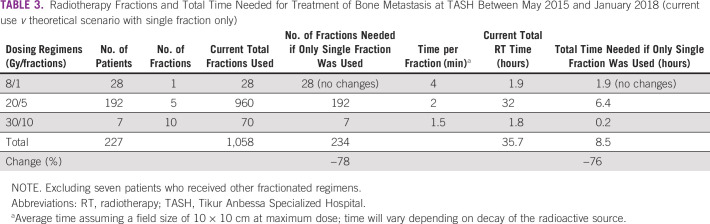
Of 1,823 patients in the database, 1,138 (62.4%) were treated with palliative-intent RT and 685 (37.6%) were treated for curative-intent RT. Of the 1,138 palliative-intent patients, 234 (20.6%) were treated for bone metastasis. Patient and general treatment characteristics are listed in Table 1. The median age at the time of treatment was 50 years (interquartile range, 37-62); 80% of patients were ≤ 65 years of age (n = 189). Females made up 53.4% of patients (n = 125). One third of patients (n = 82) had breast cancer with bone metastasis. More than 80% of patients (n = 190) were reported to be symptomatic (pain, n = 189; pain and neurologic symptoms, n = 4). None of the patients had documented radiologic evidence of cord compression.
TABLE 1.
Characteristics of Patients With Bone Metastasis Treated at TASH Between May 2015 and January 2018
RT Dosing Regimens, Wait Time, and Toxicity
The dosing regimens that were used at TASH for patients with bone metastasis were as follows: 20 Gy in 5 fractions (n = 192; 82%), 8 Gy in 1 fraction (n = 28; 12%), 30 Gy in 10 fractions (n = 7; 3%), and other fractionated regimens (n = 7, 3%). Thus, nearly 90% (n = 206) were treated with fractionated regimens. All four patients with neurologic symptoms were treated with fractionated regimens. Nearly all patients, 99.6% (n = 233) started therapy the same day of their initial visit. All patients (n = 234) completed the prescribed RT regimen without interruption. For four patients (1.7%), grade 1-2 skin and GI toxicity were recorded in the charts.
Variables Associated With a Single-Fraction Regimen
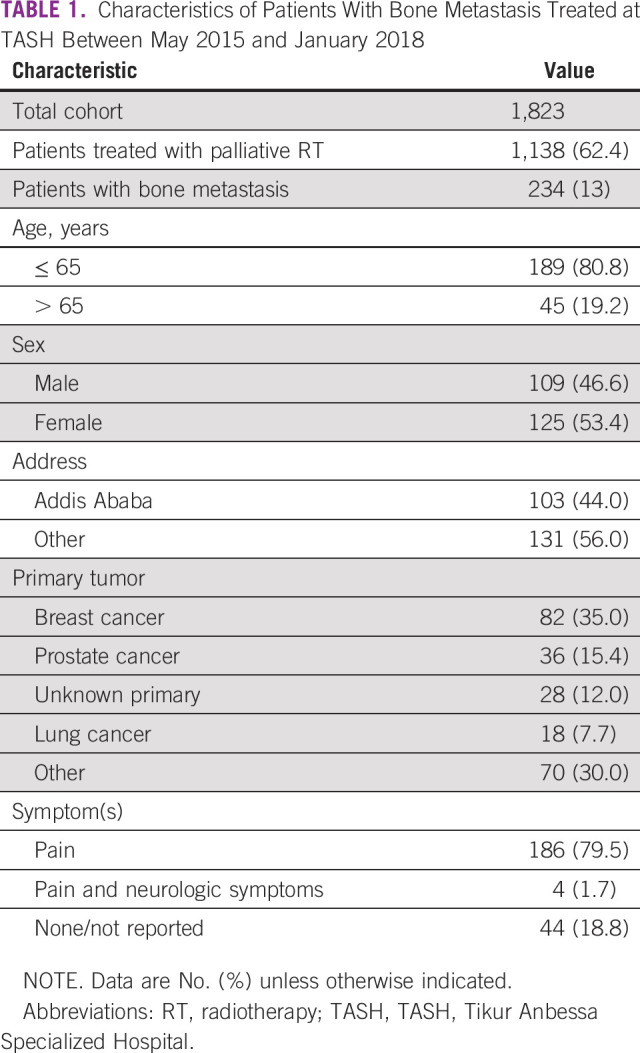
Table 2 shows the association of variables and single-fraction RT. Patients with bony metastasis to the appendicular skeleton were more likely to receive single-fraction RT (P < .01). Furthermore, patients who resided outside Addis Ababa were more likely to be treated with a single fraction (P = .01). Otherwise, there was no association with age, sex, presence of symptoms, and calendar year of treatment.
TABLE 2.
Predictors for Single-Fraction Radiotherapy for Bone Metastasis in Patients Treated at TASH Between May 2015 and January 2018
Number of RT Fractions Needed for Bone Metastasis
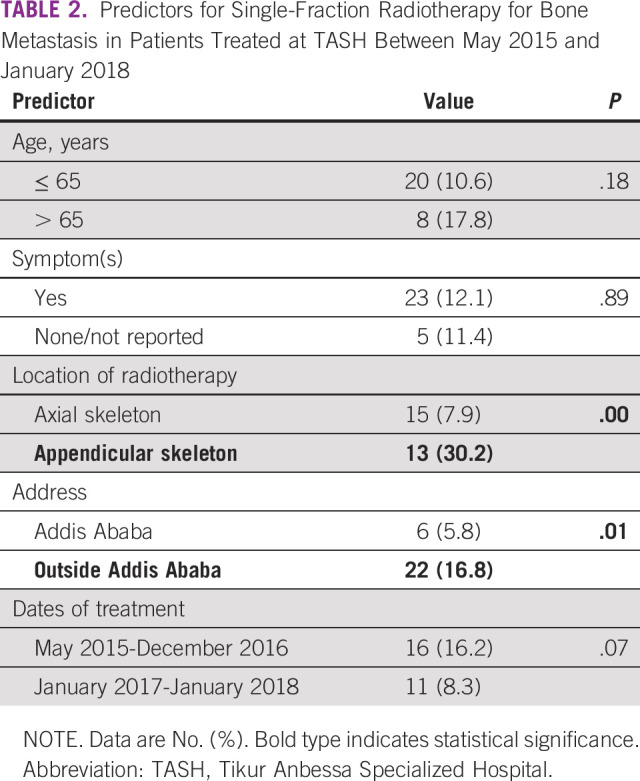
Table 3 demonstrates that if a single-fraction guideline or policy for bone metastasis was adopted in Ethiopia, the total RT fractions/sessions (and therefore hospital visits) needed for bone metastasis would be reduced by 78% (from 1,058 fractions to 234 fractions), and total cobalt RT time needed would be reduced by 76% (from 35.7 hours to 8.5 hours).
TABLE 3.
Radiotherapy Fractions and Total Time Needed for Treatment of Bone Metastasis at TASH Between May 2015 and January 2018 (current use v theoretical scenario with single fraction only)
DISCUSSION
To our knowledge, this is the first study describing the patterns of RT for bone metastasis in Ethiopia. Currently, there are three preferred regimens used: 20 Gy in 5 fractions, 30 Gy in 10 fractions, and 8 Gy in 1 fraction. These regimens are all efficacious and included in US consensus guidelines, whereas the Dutch national guidelines predominantly recommend a single fraction.10,11 There is no institutional policy guiding practice treating bone metastasis in Ethiopia, which is consistent with other countries in Africa. Based on an IAEA survey of 23 RT centers in Africa in 2008, nearly 40% of countries did not have a national or institutional policy for the treatment of bone metastasis, and 18 centers were mainly using fractionated regimens.12 At that time, Ethiopia reported mainly using the 30-Gy-in-10-fractions regimen; thus, practice has shifted to the shorter 20-Gy-in-5-fractions regimen over the last decade. The topic was revisited in 2014 with a survey of 15 RT centers in Africa, and fractionated regimens were still preferred, with a slight increase in the use of a single fraction.13
Our study highlights the ongoing preference for fractionated regimens for bone metastasis in Ethiopia despite severely limited capacity for RT, with long wait times for curative therapy. Single-fraction RT has been shown to be as safe and effective as fractionated regimens in large randomized trials for uncomplicated disease. In the largest study, a Dutch multicenter trial, 1,171 patients were randomly assigned to a single fraction of 8 Gy versus 24 Gy in 6 fractions, showing similar efficacy, time to response, and toxicity.14,15 In the United States, the largest trial included 949 patients with bone metastasis (secondary to breast and prostate cancer), who were randomly assigned to 8 Gy in a single fraction versus 30 Gy in 10 fractions, with similar efficacy (pain relief, use of narcotics, and incidence of subsequent pathologic fracture). Patients with neurologic compromise were excluded.16 A large British trial randomly assigned 765 patients to a single fraction, 20 Gy in 5 fractions, or 30 Gy in 10 fractions with no significant difference in overall response and time to response.17 The major disadvantage of single-fraction RT is a higher rate of retreatment, 18%-25% compared with 7%-9% with fractionated regimens; however, the risk of retreatment is most applicable to those patients with longer life expectancies.14,16 The advantages are that a single fraction is cost effective, more convenient for patients (especially those with long distances to travel for care), and preserves radiation capacity. An analysis of the large randomized Dutch study showed a cost savings of $873 per patient with a single fraction (including retreatments and nonmedical costs) compared with 6 fractions ($2,438 v $3,311 per patient), with fewer RT sessions.14,18 In our study, if institutional guidelines were to adopt 8 Gy in 1 fraction, the number of RT sessions needed for bone metastasis would decrease significantly. This would translate to a large reduction of hospital visits and costs for the patient and of the cobalt machine RT sessions needed to treat bone metastasis. Converting all patients to single fraction is not realistic because not all bony metastases are uncomplicated, but we suggest that if patients without a substantial soft tissue component, fracture, or impending fracture were converted, it would increase capacity to treat curative-intent patients.
Despite the evidence of safety and efficacy, there has been a worldwide reluctance to use the single-fraction regimen.19-23 Bradley et al24 reported factors that influence treatment, including (1) oncologist-related factors (eg, training, level of experience, type of reimbursement); (2) patient-related factors (eg, age, travel distance, tumor type, performance status, presence of skeletal-related event); (3) setting-related factors (eg, waiting lists, institutional policies); (4) attitudes and beliefs; and (5) published evidence. Our study did not survey clinicians on their attitudes and preferences toward fractionated regimens; however, Jeremic et al13 suggested this could be explained by a general lack of confidence in the efficacy of a single fraction. We suggest the culture of the institution was influencing this decision.
In our study, we found a significant association between single-fraction RT and two variables: site of the metastasis and where the patient lives. If the disease was in the appendicular skeleton, single-fraction RT was more likely to be administered. This may be explained by a reluctance to use single fraction in the spine (axial skeleton), a pattern that has been noted in other African countries.13 In one of the sentinel randomized trials showing efficacy of a single fraction, there was an increase in pathologic fractures noted in the single-fraction group (4% v 2%); however, this was not observed in a later randomized trial in 2005.14,16 We also found an association between patients who traveled from outside Addis Ababa to receive treatment and single-fraction RT. This corresponds to patient convenience and cost when traveling to receive treatment.24 It was observed that there was a weak association between single fraction and patients > 65 years of age, although not statistically significant and possibly related to functional status.
Our study was retrospective and therefore limited to data available in the database; we did not have data on variables such as performance status, field size, soft tissue involvement, and fracture or impending fracture, which may have an influence on choice of regimen. We would have preferred to have outcome, toxicity, and re-irradiation data to compare with other studies and confirm that single fraction is as effective and safe in the Ethiopian setting as in higher-resourced settings (where a majority of studies originate). In addition, the database was a random sample of available patient charts rather than a random sample from the list of all patients treated during the study period; thus, it is possible that the data may differ with the addition of the entire sample.
In conclusion, the current pattern of palliative RT for bone metastasis in Ethiopia favors fractionated regimens. Additional efforts are being made to address this issue in Ethiopia, such as prospective research on efficacy and re-irradiation rates of single-fraction RT. Data proving the efficacy of single-fraction RT in the Ethiopian setting could shift practice and lead to the development of an institutional or national protocol for RT dosing for uncomplicated bone metastasis. If this approach proves successful, it could represent a useful contribution to other countries in Africa who share similar patterns of practice. Increased use of single-fraction RT for uncomplicated bone metastasis would reduce the number of RT sessions and RT time needed for bone metastasis, which could increase capacity for curative treatments while providing evidence-based and cost-conscious care.
ACKNOWLEDGMENT
We thank Yvette van Norden for her contributions.
PRIOR PRESENTATION
Preliminary data presented at American Society for Radiation Oncology annual meeting, Chicago, IL, September 15-18, 2019.
SUPPORT
Supported by an unrestricted grant from Varian Medical Systems (L.I.).
AUTHOR CONTRIBUTIONS
Conception and design: Tara J. Rick, Biruk Habtamu, Wondemagegnhu Tigeneh, Aynalem Abreha, Surbhi Grover, Mathewos Assefa, Luca Incrocci
Provision of study materials or patients: Wondemagegnhu Tigeneh, Aynalem Abreha, Mathewos Assefa
Collection and assembly of data: Tara J. Rick, Biruk Habtamu, Luca Incrocci
Data analysis and interpretation: Tara J. Rick, Surbhi Grover, Wilma Heemsbergen, Luca Incrocci
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/go/site/misc/authors.html.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Tara J. Rick
Research Funding: Varian Medical Systems (Inst)
Travel, Accommodations, Expenses: Varian Medical Systems
Luca Incrocci
Research Funding: Varian Medical Systems (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Gouda HN, Charlson F, Sorsdahl K, et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990-2017: Results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7:e1375–e1387. doi: 10.1016/S2214-109X(19)30374-2. [DOI] [PubMed] [Google Scholar]
- 2.Jedy-Agba E, McCormack V, Adebamowo C, et al. Stage at diagnosis of breast cancer in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob Health. 2016;4:e923–e935. doi: 10.1016/S2214-109X(16)30259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzmaurice C, Abate D, Abbasi N, et al. doi: 10.1001/jamaoncol.2019.2996. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol 5:1749-1768, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. J Clin Oncol. 2014;32:2913–2919. doi: 10.1200/JCO.2014.55.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grover S, Xu MJ, Yeager A, et al. A systematic review of radiotherapy capacity in low- and middle-income countries. Front Oncol. 2015;4:380. doi: 10.3389/fonc.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zubizarreta EH, Fidarova E, Healy B, et al. Need for radiotherapy in low and middle income countries – the silent crisis continues. Clin Oncol (R Coll Radiol) 2015;27:107–114. doi: 10.1016/j.clon.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Rick T, Habtamu B, Tigeneh W, et al. Patterns of care of cancers and radiotherapy in Ethiopia. J Glob Oncol. 2019;5:1–8. doi: 10.1200/JGO.19.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rick TJ, Habtamu B, Abreha A, et al. Ethiopia: How the care of 100 million people pivots on a single cobalt teletherapy machine. Int J Radiat Oncol Biol Phys. 2020;106:230–235. doi: 10.1016/j.ijrobp.2019.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Williams MV, James ND, Summers ET, et al. National survey of radiotherapy fractionation practice in 2003. Clin Oncol (R Coll Radiol) 2006;18:3–14. doi: 10.1016/j.clon.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Lutz S, Balboni T, Jones J, et al. Palliative radiation therapy for bone metastases: Update of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7:4–12. doi: 10.1016/j.prro.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Groenen KHJ, van der Linden YM, Brouwer T, et al. The Dutch national guideline on metastases and hematological malignancies localized within the spine; a multidisciplinary collaboration towards timely and proactive management. Cancer Treat Rev. 2018;69:29–38. doi: 10.1016/j.ctrv.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Sharma V, Gaye PM, Wahab SA, et al. Patterns of practice of palliative radiotherapy in Africa, Part 1: Bone and brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:1195–1201. doi: 10.1016/j.ijrobp.2007.07.2381. [DOI] [PubMed] [Google Scholar]
- 13.Jeremic B, Vanderpuye V, Abdel-Wahab S, et al. Patterns of practice in palliative radiotherapy in Africa - case revisited. Clin Oncol (R Coll Radiol) 2014;26:333–343. doi: 10.1016/j.clon.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 14. doi: 10.1016/s0167-8140(99)00110-3. Steenland E, Leer JW, van Houwelingen, et al: The effect of a single fraction compared to multiple fractions on painful metastases: A global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 52:101-109, 1999 [Erratum: Radiother Oncol 53:167, 1999] [DOI] [PubMed] [Google Scholar]
- 15.van der Linden YM, Lok JJ, Steenland E, et al. Single fraction radiotherapy is efficacious: A further analysis of the Dutch Bone Metastasis Study controlling for the influence of retreatment. Int J Radiat Oncol Biol Phys. 2004;59:528–537. doi: 10.1016/j.ijrobp.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 17.Bone Pain Trial Working Party 8 Gy single fraction radiotherapy for the treatment of metastatic skeletal pain: Randomised comparison with a multifraction schedule over 12 months of patient follow-up. Radiother Oncol. 1999;52:111–121. [PubMed] [Google Scholar]
- 18.van den Hout WB, van der Linden YM, Steenland E, et al. Single- versus multiple-fraction radiotherapy in patients with painful bone metastases: Cost-utility analysis based on a randomized trial. J Natl Cancer Inst. 2003;95:222–229. doi: 10.1093/jnci/95.3.222. [DOI] [PubMed] [Google Scholar]
- 19.Roos DE. Continuing reluctance to use single fractions of radiotherapy for metastatic bone pain: An Australian and New Zealand practice survey and literature review. Radiother Oncol. 2000;56:315–322. doi: 10.1016/s0167-8140(00)00250-4. [DOI] [PubMed] [Google Scholar]
- 20.Fairchild A, Barnes E, Ghosh S, et al. International patterns of practice in palliative radiotherapy for painful bone metastases: Evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75:1501–1510. doi: 10.1016/j.ijrobp.2008.12.084. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura N, Shikama N, Wada H, et al. Patterns of practice in palliative radiotherapy for painful bone metastases: A survey in Japan. Int J Radiat Oncol Biol Phys. 2012;83:e117–e120. doi: 10.1016/j.ijrobp.2011.11.075. [DOI] [PubMed] [Google Scholar]
- 22.Popovic M, den Hartogh M, Zhang L, et al. Review of international patterns of practice for the treatment of painful bone metastases with palliative radiotherapy from 1993 to 2013. Radiother Oncol. 2014;111:11–17. doi: 10.1016/j.radonc.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Rutter CE, Yu JB, Wilson LD, et al. Assessment of national practice for palliative radiation therapy for bone metastases suggests marked underutilization of single-fraction regimens in the United States. Int J Radiat Oncol Biol Phys. 2015;91:548–555. doi: 10.1016/j.ijrobp.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 24.Bradley NM, Husted J, Sey MSL, et al. Review of patterns of practice and patients’ preferences in the treatment of bone metastases with palliative radiotherapy. Support Care Cancer. 2007;15:373–385. doi: 10.1007/s00520-006-0161-3. [DOI] [PubMed] [Google Scholar]