Abstract
Background
Bone metastasis (BoM) is common in patients with advanced non‐small cell lung cancer (NSCLC) and considered as one of the negative prognostic factors. However, the impact of BoM on clinical outcomes of patients with advanced NSCLC treated with immune checkpoint inhibitors (ICIs) remains unclear.
Methods
A total of 103 patients treated with ICI monotherapy and 101 patients treated with ICIs combined with chemotherapy or antiangiogenesis therapy were retrospectively analyzed. The differences in progression‐free survival (PFS), overall survival (OS) and objective response rate (ORR) between BoM+ and BoM− were investigated.
Results
Of those 101 patients who received combination therapy, no significant difference between BoM− and BoM+ in terms of both median PFS and median OS (median PFS, 10.1 vs. 12.1 months, P = 0.6; median OS, NR vs. 24.6 months, P = 0.713) was determined. In contrast, of the 103 patients who received ICI monotherapy, BoM+ patients had an inferior PFS (4.2 vs. 6.7 months, P = 0.0484) and OS (12.5 vs. 23.9 months, P = 0.0036) compared with BoM− patients. The univariate and multivariate analysis in the ICI monotherapy group also identified BoM as an independent factor attenuating the efficacy of ICI monotherapy. Of all BoM+ patients who received ICI monotherapy, neither palliative radiotherapy nor bisphosphonate drugs improved OS (palliative radiotherapy: 12.5 vs. 16.7 months, P = 0.487; bisphosphonate drugs: 12.5 vs. 9.7 months, P = 0.568).
Conclusions
BoM attenuated the efficacy of ICI monotherapy in patients with advanced NSCLC. Of BoM+ patients who received ICI monotherapy, neither palliative radiotherapy nor bisphosphonate drugs improved OS. Other therapeutic strategies are needed for patients with BoM.
Keywords: Bone metastasis, immune checkpoint inhibitor, non‐small cell lung cancer, outcomes
Bone metastasis is common in patients with advanced non‐small cell lung and considered as one of the negative prognostic factors, but the impact of bone metastases on clinical outcomes of immune checkpoint inhibitors remains unclear. Our study demonstrated that bone metastases attenuated the efficacy of ICIs monotherapy in patients with advanced NSCLC. Of patients with bone metastases who received ICIs monotherapy, neither palliative radiotherapy nor bisphosphonate drugs improved OS.

Introduction
Advancement of immunotherapy has revolutionized the therapeutic landscape of patients with advanced non‐small cell lung cancer (NSCLC). 1 Immune checkpoint inhibitor (ICI) treatment, targeting the programmed death‐1 (PD‐1)/PD‐ligand 1 (PD‐L1) pathway, are now standard of care for both first‐line (pembrolizumab in combination with chemotherapy or monotherapy in PD‐L1 tumor proportion score [TPS] ≥50%) and second‐line settings (pembrolizumab in PD‐L1 TPS ≥ 1%, nivolumab or atezolizumab in unselected patients) for patients with advanced NSCLC and without EGFR/ALK molecular alterations. 2 , 3 , 4 , 5 , 6 , 7
However, together with the potential clinical applications of ICIs, a series of unanswered questions has emerged which needs to be resolved urgently. 8 One of those is that the objective response rate (ORR) is only around 20% in unselected advanced NSCLC patients, and the efficacy varies greatly across individuals. 9 Even in a highly selected patient population (PD‐L1 TPS ≥ 50%), accounting for approximately 30% of total patients, it has been reported that only 44.8% of patients achieved an objective response from ICI treatment. 10 Therefore, to discriminate the certain factors that influence the efficacy of ICIs is critically important. Previous studies have demonstrated that intratumoral PD‐L1 expression, tumor mutation burden (TMB), and the amount and location of tumor infiltrating lymphocytes (TILs) are associated with the effectiveness of ICI treatment under certain circumstances. 11 , 12 , 13 Furthermore, a number of clinical parameters have also shown influences on ICI therapy, including Eastern Cooperative Oncology Group Performance Status (ECOG PS), smoking history and malignant pleural effusion. 14 , 15 , 16 Therefore, to determine the influencing factors and then distinguish the dominant group is one of the important challenges in clinical practice.
Recent studies have focused on the impact of specific metastatic sites, such as liver, brain or bone metastases (BoM) on the efficacy of ICI treatment. In a retrospective study conducted in 215 patients with advanced or recurrent NSCLC who received ICI therapy, liver metastases was found to be independently associated with shorter progression‐free survival (PFS) and overall survival (OS). 17 Another study evaluated the impact of brain metastases on nivolumab monotherapy in 73 patients with advanced NSCLC and found similar PFS and OS in patients with and without brain metastases. 18 These findings suggest that metastases to different organs may have diverse influences on the efficacy of ICI treatment, and this may due to the tumor microenvironment (TME) in different organs. 19
BoM are common in advanced NSCLC patients, and considered as one of the negative prognostic factors. Skeletal‐related events (SREs) caused by BoM such as bone pain, pathologic fracture, hypercalcemia, and spinal cord compression have been reported to occur in 30%–60% of all lung cancer patients with BoM, 20 and have a detrimental impact on the survival of patients with advanced NSCLC. 21 Clinically, palliative radiotherapy to BoM sites, bisphosphonate administration and denosumab (a monoclonal antibody targeting RANKL) are major treatment strategies for patients with BoM. 22 Because the bone marrow environment includes vast immune cell composition of lymphocytes and myeloid cells, which exhibit both antitumor and tumor promoting effects, we hypothesized that BoM may influence the response to ICI treatment in patients with advanced NSCLC. 23 , 24 Interestingly, Schmid et al. retrospectively analyzed the organ‐specific response to nivolumab in patients with NSCLC and found that nivolumab appeared to be more active in patients with lymph node metastases compared with other organ sites such as liver, adrenals and bone. Moreover, nine out of 12 patients with BoM had progressive bone lesions at the time of overall tumor progression. 25 Thus, we performed the present study to investigate whether BoM influences the efficacy of ICI treatment in patients with advanced NSCLC.
Methods
Patient characteristics
This was a retrospective study. A total of 204 patients diagnosed with advanced NSCLC who commenced ICI‐based treatment from July 2015 to June 2019 in Shanghai Pulmonary Hospital, Tongji University regardless of treatment lines were identified. Overall, 103 of these patients received ICI monotherapy, and the remaining101 patients were administered ICIs combined with chemotherapy or antiangiogenesis agents. Patient characteristics were collected which mainly included: gender, age, smoking history, stage of disease, pathologic types, metastatic organ sites, ECOG PS, treatment lines, treatment strategies, treatment initiation date, best response to treatment, therapeutic regimens, dates of tumor progression and death or last follow‐up. We used pack year (PY) for identification of smoking history, and one PY meant an average of 20 cigarettes per day for one year. Never smokers were defined as patients with a smoking history of <100 cigarettes within their lifetime, light smokers were defined as 5–29 PY and heavy smokers were defined as ≥30 PY. ORRs were assessed by the investigators according to the Response Evaluation Criteria In Solid Tumors (RECIST; version 1.1). 26 Follow‐up time was defined as the time from ICI treatment initiation to 4 December 2019. BoM assessments were detected by bone scintigraphy, MRI or PET/CT.
This study was approved by the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine. All patients provided their written informed consent before treatment.
Statistical analysis
Investigator‐assessed ORR, PFS and OS were evaluated. PFS was defined as from the treatment initiation date of ICIs to the date of physician assessment of disease progression or death, and OS was calculated from the treatment initiation date of ICIs to the date of death or last follow‐up in surviving participants. The Chi‐square statistic was used to measure the association between patient characteristics and ORR. PFS and OS were estimated using the Kaplan‐Meier method and survival curves were compared with log‐rank test or Breslow test. Univariate and multivariate Cox proportional hazards regression analyses were performed to determine independent prognostic factors for disease survival. All variables with significant association in univariate analysis (by Kaplan–Meier with a P‐value < 0.1) were included in the multivariate model (Cox regression) to determine their independent effects. A P‐value < 0.05 was considered statistically significant. Data were analyzed using the SPSS 25.0 (SPSS Inc., Chicago, Illinois) software package.
Results
Patient characteristics
A total of 216 patients with advanced NSCLC received ICI treatment at Shanghai Pulmonary Hospital from July 2015 to June 2019. Among them, 204 patients were evaluated for an objective response and enrolled into this study. Among the 204 patients, 103 had been treated with ICI monotherapy, and the remaining 101 patients with ICIs combined with chemotherapy (72/101, 71.3%) or antiangiogenesis agents (28/101, 27.7%) or both (1/101, 0.9%). Of all these patients, 67 (32.8%) had BoM at baseline of ICI treatment, and 67 (32.8%) had squamous carcinoma histology. A total of 69 patients (33.8%) received ICI treatment as first‐line therapy, and the remainder (66.2%) received ICI treatment as second or subsequent line therapy. All the distributions of clinicopathologic characteristics were a better balance of patients between BoM+ and BoM− in two groups and are shown in Table 1.
Table 1.
Characteristics in treatment groups
| All patients | Immunomonotherapy | Combination therapy | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BoM− (N/%) | BoM+ (N/%) | P‐value | BoM− (N/%) | BoM+ (N/%) | P‐value | BoM− (N/%) | BoM+ (N/%) | P‐value | |
| Age at diagnosis | 0.349 | 1.000 | 0.136 | ||||||
| <65 years | 86 (62.8) | 47 (70.1) | 40 (58.0) | 20 (58.8) | 46 (67.6) | 27 (81.8) | |||
| ≥65 years | 51 (37.2) | 20 (29.9) | 29 (42.0) | 14 (41.2) | 22 (32.4) | 6 (18.2) | |||
| Gender | 0.070 | 0.220 | 0.130 | ||||||
| Male | 120 (87.6) | 51 (76.1) | 62 (89.9) | 27 (79.4) | 58 (85.3) | 24 (72.7) | |||
| Female | 17 (12.4) | 16 (23.9) | 7 (10.1) | 7 (20.6) | 10 (14.7) | 9 (27.3) | |||
| Smoking history | 0.052 | 0.059 | 0.344 | ||||||
| Heavy | 64 (46.7) | 21 (31.3) | 39 (56.5) | 12 (35.3) | 25 (36.8) | 9 (27.3) | |||
| Never/light | 73 (53.3) | 46 (68.7) | 30 (43.5) | 22 (64.7) | 43 (63.2) | 24 (72.7) | |||
| Pathology | 0.319 | 0.535 | 0.200 | ||||||
| Squamous carcinoma | 48 (35.0) | 19 (28.4) | 29 (42.0) | 15 (44.1) | 19 (27.9) | 4 (12.1) | |||
| Nonsquamous carcinoma | 77 (56.2) | 45 (67.2) | 33 (47.8) | 18 (52.9) | 44 (64.7) | 27 (81.8) | |||
| NSCLC | 12 (8.8) | 3 (4.4) | 7 (10.1) | 1 (2.9) | 5 (7.4) | 2 (6.1) | |||
| Metastasis to other organ | 1.000 | 0.664 | 0.694 | ||||||
| Yes | 95 (69.3) | 46 (68.7) | 46 (66.7) | 21 (61.8) | 49 (72.1) | 25 (75.8) | |||
| No | 42 (30.7) | 21 (31.3) | 23 (33.3) | 13 (38.2) | 19 (27.9) | 8 (24.2) | |||
| ECOG performance status | 1.000 | 1.000 | 0.327 | ||||||
| 0–1 | 129 (94.2) | 63 (94.0) | 61 (88.4) | 31 (91.2) | 68 (100) | 32 (97) | |||
| ≥2 | 8 (5.8) | 4 (6.0) | 8 (11.6) | 3 (8.8) | 0 (0) | 1 (3) | |||
| PD‐L1 expression | 0.352 | 0.917 | 0.067 | ||||||
| Negtive | 18 (13.1) | 7 (10.4) | 9 (13.0) | 5 (14.7) | 9 (13.2) | 2 (6.1) | |||
| 1%–49% | 15 (10.9) | 3 (4.5) | 6 (8.7) | 2 (5.9) | 9 (13.2) | 1 (3.0) | |||
| ≥50% | 9 (6.6) | 3 (4.5) | 4 (5.8) | 3 (8.8) | 5 (7.4) | 0 (0) | |||
| Not known | 95 (69.3) | 54 (80.6) | 50 (72.5) | 24 (70.6) | 45 (66.2) | 30 (90.9) | |||
| Treatment line (s) | 0.529 | 0.347 | 0.833 | ||||||
| 1 | 44 (32.1) | 25 (37.3) | 7 (10.1) | 6 (17.6) | 37 (54.4) | 19 (57.6) | |||
| ≥2 | 93 (67.9) | 42 (62.7) | 62 (89.9) | 28 (82.4) | 31 (45.6) | 14 (42.4) | |||
| Gene mutation | 0.180 | 0.876 | 0.065 | ||||||
| Wild‐type | 82 (59.9) | 44 (65.7) | 37 (53.6) | 21 (61.8) | 45 (66.2) | 23 (69.7) | |||
| KRAS | 12 (8.8) | 10 (14.9) | 6 (8.7) | 3 (8.8) | 6 (8.8) | 7 (21.2) | |||
| Other mutation | 2 (1.5) | 1 (1.5) | 1 (1.4) | 0 (0) | 1 (1.5) | 1 (3.0) | |||
| Unknown | 41 (29.9) | 12 (17.9) | 25 (36.2) | 10 (29.4) | 16 (23.5) | 2 (6.1) | |||
| Combined treatment | 0.818 | ||||||||
| Chemotherapy | — | — | — | — | 49 (72.1) | 23 (69.7) | |||
| Antiangiogenesis | — | — | — | — | 19 (27.9) | 10 (30.3) | |||
Correlation between bone metastases and outcomes
We first evaluated the PFS and OS in all patients according to BoM and no differences of PFS and OS were observed between BoM− and BoM+ (Fig 1). The homogeneity of treatment strategies was the first factor we considered that might confound the results, and we divided the patients into two subgroups: the ICI monotherapy group and the ICI combination treatment group. Notably, we found significant differences in terms of PFS and OS between BoM− and BoM+ in the ICI monotherapy group, but not in patients who received ICI combination treatment. As illustrated in Fig 2a, patients with BoM− and BoM+ had similar PFS (10.1 vs. 12.1 months, P = 0.600), OS (not reached [NR] vs. 24.6 months, P = 0.713), and ORR (39.7% vs. 36.4%, P = 0.746) after the ICI combination treatment. However, patients with BoM+ who received ICI monotherapy had significantly shorter PFS (4.2 vs. 6.7 months, P = 0.048)and OS (12.5 vs. 23.9 months, P = 0.004) than those with BoM−. However, we did not find significant differences of ORR (29.4% vs. 31.9%, P = 0.826) between BoM− and BoM+, as illustrated in Fig 2b.
Figure 1.
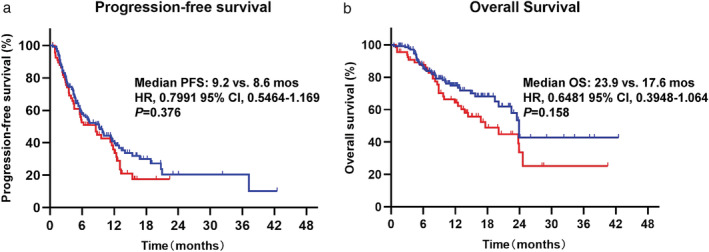
The analyses of progression‐free survival (PFS) and overall survival (OS) in all patients according to BoM (bone metastasis). Progression‐free survival:  , BoM‐;
, BoM‐;  , BoM+. Overall survival:
, BoM+. Overall survival:  , BoM‐;
, BoM‐;  , BoM+.
, BoM+.
Figure 2.
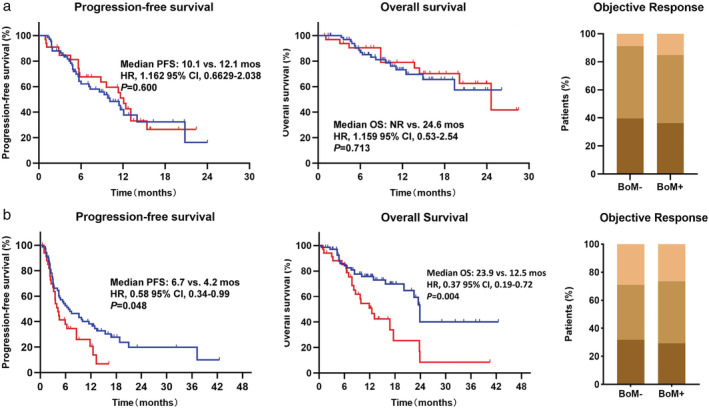
(a) The analyses of progression‐free survival (PFS), overall survival (OS) and obective response rate (ORR) in patients receiving immune‐checkpoint inhibitor (ICI) combination therapy according to BoM. Progression‐free survival:  , BoM‐;
, BoM‐;  , BoM+. Overall survival:
, BoM+. Overall survival:  , BoM‐;
, BoM‐;  , BoM+. Objective Response:
, BoM+. Objective Response:  , PD;
, PD;  , SD;
, SD;  , PR. (b) The analyses of PFS, OS and ORR in patients receiving ICI monotherapy according to BoM. Progression‐free survival:
, PR. (b) The analyses of PFS, OS and ORR in patients receiving ICI monotherapy according to BoM. Progression‐free survival:  , BoM‐;
, BoM‐;  , BoM+. Overall survival:
, BoM+. Overall survival:  , BoM‐;
, BoM‐;  , BoM+. Objective Response:
, BoM+. Objective Response:  , PD;
, PD;  , SD;
, SD;  , PR.
, PR.
Impact of palliative radiotherapy and bisphosphonate administration on clinical outcomes
Palliative radiotherapy and bisphosphonate administration are currently two major therapeutic methods for patients with BoM+. In order to define the impact of palliative radiotherapy on bone and bisphosphonate administration on the survival, we further evaluated the efficacy of all BoM+ patients in the ICI monotherapy group according to palliative radiotherapy/bisphosphonate administration. Among 34 BoM+ patients in the ICI monotherapy group, nine patients (26.5%) received palliative radiotherapy to BoM sites, 11 patients (32.4%) received bisphosphonate administration and five patients (14.7%) received both. As shown in Fig 3, there were no significant differences of OS in BoM+ patients between those receiving palliative radiotherapy or bisphosphonate administration or those not receiving treatment (palliative radiotherapy median OS: 12.5 vs. 16.7 months, P = 0.487, bisphosphonate administration median OS: 12.5 vs. 9.7 months, P = 0.568).
Figure 3.
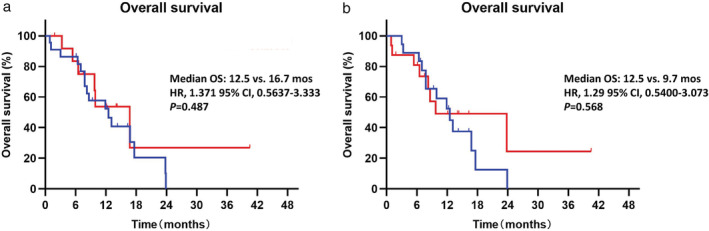
Analyses of overall survival (OS) in all BoM+ (bone metastasis+) patients receiving palliative treatments (radiotherapy of bisphosphonate administration) compared to those not receiving palliative treatments. Overall survival:  , No RT;
, No RT;  , With RT. Overall survival:
, With RT. Overall survival:  , No Bis;
, No Bis;  , With Bis.
, With Bis.
Univariate and multivariate analyses
There may be some other clinical factors that influence the survival of patients, and we therefore performed a univariate analysis for PFS and OS including age, gender, smoking history, pathological types, BoM, other organ metastasis, ECOG PS, treatment line and ICI‐monotherapy or ICI combined with other therapy, as shown in Table 2. In the ICI‐monotherapy group, we found that BoM+ patients had significantly shorter PFS (P = 0.048) and OS (P = 0.004) than BoM− patients. Meanwhile, patients diagnosed with squamous histology had shorter OS (P = 0.037) than nonsquamous histology. Further, ECOG equalled 0–1 patients had longer OS (P = 0.049) than ECOG equalled 2 or higher patients. In all patients, only pathology type, ECOG performance status, and treatment line, but not bone metastasis were associated with OS. These factors with a P‐value under 0.1 were further included in a multivariate analysis of OS, as shown in Table 3. We determined that BoM remained an independent prognostic factor for OS in the ICI‐monotherapy group (HR = 0.458, 95% CI: 0.25–0.838, P = 0.01) after adjusting for the other factors.
Table 2.
Univariate analyses for PFS and OS in ICIs monotherapy group and all patients
| ICIs mono‐therapy | All patients | |||||||
|---|---|---|---|---|---|---|---|---|
| Factors | Median PFS (month) | P‐value | Median OS (month) | P‐value | Median PFS (month) | P‐value | Median OS (month) | P‐value |
| Age (<65 vs. ≥65) | 5.3 vs. 6.7 | 0.516 | 23.5 vs. 20.1 | 0.859 | 8.6 vs. 9.6 | 0.767 | 23.9 vs. 20.1 | 0.864 |
| Gender (male vs. female) | 5.9 vs. 3.6 | 0.589 | 22.5 vs. NR | 0.462 | 9.2 vs. 5.8 | 0.344 | 23.5 vs. NR | 0.691 |
| Smoking history (never/light vs. heavy) | 5.3 vs. 5.9 | 0.702 | 23.9 vs. 22.5 | 0.516 | 8.6 vs. 8.8 | 0.720 | 24.6 vs. 23.5 | 0.271 |
| Pathology (squamous vs. nonsquamous NSCLC) | 4.8 vs. 6.3 | 0.905 | 12.9 vs. 23.9 | 0.037 | 5.9 vs. 9.8 | 0.187 | 15.7 vs. 23.9 | 0.056 |
| Bone metastasis (No vs. Yes) | 6.7 vs. 4.2 | 0.048 | 23.9 vs. 12.5 | 0.004 | 9.2 vs. 8.6 | 0.376 | 23.9 vs. 17.6 | 0.158 |
| Other organ metastasis (No vs. Yes) | 4.4 vs. 5.9 | 0.843 | 22.5 vs. 23.8 | 0.785 | 7.4 vs. 8.8 | 0.738 | 23.5 vs 23.8 | 0.331 |
| ECOG performance status (0–1 vs. ≥2) | 5.9 vs. 2.3 | 0.261 | 22.5 vs. 7.8 | 0.049 | 8.8 vs. 3.2 | 0.127 | 23.8 vs. 7.8 | 0.001 |
| Treatment line (1 vs. ≥2) | 11.8 vs. 4.8 | 0.212 | 23.5 vs. 22.5 | 0.504 | 11.4 vs. 5.9 | 0.011 | NR vs 22.5 | 0.031 |
| Treatment (ICI monotherapy vs. combined therapy) | — | — | — | — | 5.9 vs. 11.4 | 0.026 | 22.5 vs. 24.6 | 0.147 |
Table 3.
Multivariate analyses for OS in ICIs monotherapy group and all patients
| ICI monotherapy | All patients | |||||
|---|---|---|---|---|---|---|
| Factors | P‐value | Hazard ratio | 95% CI | P‐value | Hazard ratio | 95% CI |
| Pathology (squamous vs. nonsquamous NSCLC) | 0.005 | 2.593 | 1.327–5.065 | 0.003 | 2.133 | 1.299–3.501 |
| Bone metastasis (No vs. Yes) | 0.019 | 0.482 | 0.263–0.886 | — | — | — |
| ECOG performance status (0–1 vs. ≥2) | 0.172 | 0.496 | 0.181–1.357 | 0.059 | 0.436 | 0.184–1.032 |
| Treatment line (1 vs. ≥2) | — | — | — | 0.038 | 0.555 | 0.319–0.967 |
Discussion
To date, the impact of BoM on ICI treatment is still not well known. In the current study there were additional important aspects which revealed the following: (i) In the subjects enrolled into the study, 32.8% of patients had BoM before initiation of ICI treatment, which represented a considerable number of patients with advanced NSCLC; none of the previous randomized trials stratified patients according to presence of BoM. (ii) Bone is an immune organ which contains different populations of immune cells, 23 , 27 including regulatory T cells, cytotoxic T cells, B cells, dendritic cells, natural killer T cells, and myeloid‐derived suppressor cells. A previous study has demonstrated that BoM negatively affects ICI treatment in patients with prostate cancer. 28 A recent study also indicated that BoM impairs nivolumab efficacy, with shorter PFS and OS, as well as lower ORR, in both nonsquamous and squamous NSCLC. 29 To our knowledge, our current study, for the first time, investigated the role of BoM both on ICI monotherapy and ICI combination therapy, and we found that BoM had an adverse impact on the clinical outcome of ICI monotherapy in patients with advanced NSCLC.
However, we did not see a significant difference in the combination therapy group according to BoM, so does that mean the combined therapies of ICI and chemotherapy or antiangiogenesis therapy are able to overcome the adverse effects of BoM? Nowadays, a series of ICI combination strategies have been tested in clinical studies in advanced lung cancer patients and some have been approved for clinical practice. KEYNOTE‐189 and KEYNOTE‐407 are two clinical trials which tested the combination of ICIs with chemotherapy in patients with advanced NSCLC compared with standard chemotherapy. Both PFS and OS improved following combination therapy in these two trials. 5 , 6 It has previously been demonstrated that chemotherapy has certain regulatory effects on immune system function, such as immunogenic death of tumor cells and immune cell components, 30 , 31 and it may be one of the mechanisms by which chemotherapy and ICIs have synergistic effects. Antiangiogenesis agents have also been reported to influence the immune system when combined with ICI treatment. 32 In our current study, ICI combination therapy with chemotherapy or antiangiogenesis therapy was shown to improve the efficacy compared with ICI‐monotherapy, and BoM was a poor prognostic factor for patients treated with ICI monotherapy; however, the presence or absence of BoM did not affect the efficacy and prognosis in patients treated with combination therapies.
In the current study we also explored whether adding palliative radiotherapy to bone metastatic sites or bisphosphonate administration would improve the efficacy of ICI treatment; however, we did not observe any synergistic effects, and the two therapeutic strategies had no significant impact on the survival of patients with BoM. However, there is evidence which has been reported in the literature which demonstrates that survival benefits with combined immunotherapy‐radiotherapy strategies have been found in NSCLC patients. 33 For instance, in a phase 2 randomized clinical trial which evaluated the effect of pembrolizumab after stereotactic body radiotherapy versus pembrolizumab alone on tumor response in patients with advanced NSCLC, the subgroup analyses showed the greatest benefit from the addition of radiotherapy in patients with PD‐L1 negative tumors, although the results did not meet the study's prespecified end point criteria for meaningful clinical benefits. 34 Furthermore, a secondary analysis from the KEYNOTE‐001 trial also demonstrated that previous radiotherapy resulted in longer PFS and OS in patients with advanced NSCLC treated with pembrolizumab. 35 However, whether radiotherapy to BoM improved the survival outcomes remains unclear. Nevertheless, a series of prospective clinical studies are ongoing to investigate the synergistic effect of immunotherapy‐radiotherapy strategies in patients with advanced NSCLC. 36 We have demonstrated that concomitant use of bisphosphonates and EGFR‐TKIs brings survival benefits to NSCLC patients with EGFR mutations and bone metastases, 37 , 38 but there are few clinical data on proving the synergistic effect of immunotherapy and bisphosphonates in NSCLC. Interestingly, a recent study revealed that increased Th 17 cells and lack of Th 1 cells in bone marrow confers resistance to ICI treatment in prostate cancer and anti‐TGF‐β antibodies rather than anti‐RANKL antibodies potentiates ICI efficacy by restoring Th 1 lineage polarization, 28 suggesting a potential role of TGF‐β inhibitors in patients with BoM. Furthermore, our study also found that patients treated with ICI combined therapy (either in combination with chemotherapy or antiangiogenesis agents) had numerically longer PFS (12.1 vs. 4.2 months) and OS (24.6 vs. 12.5 months) than those treated with ICI monotherapy in patients with BoM, although statistical analyses were not available. Taken together, additional studies are needed to determine the influence of combined therapy or TGF‐β inhibitors in patients with advanced NSCLC and with BoM.
The limitations of the current study should be acknowledged. First, it was a retrospective study and no patients who did not receive ICIs were set as a control group. Another very important issue is that lack of TMB data and PD‐L1 expression was detected in only a few patients. Moreover, we did not separate BoM+ patients to subgroups according to the metastasis site and quantity because of the lack of subjects. Further additional large cohort studies with a better balance of patients need to be conducted in the future.
In conclusion, our data indicated that BoM could impair the efficacy of ICI monotherapy in patients with advanced NSCLC. Adding palliative radiotherapy to bone metastatic sites or administration of biphosphonates did not seem to improve the efficacy of ICI treatment. Other therapeutic strategies are needed for patents with BoM.
Disclosure
The authors confirm that there are no conflicts of interest.
References
- 1. Tay R, Prelaj A, Califano R. Immune checkpoint blockade for advanced non‐small cell lung cancer: Challenging clinical scenarios. J Thorac Dis 2018; 10: S1494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus Docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gandhi L, Rodriguez‐Abreu D, Gadgeel S et al Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med 2018; 378: 2078–92. [DOI] [PubMed] [Google Scholar]
- 6. Paz‐Ares L, Luft A, Vicente D et al Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med 2018; 379: 2040–51. [DOI] [PubMed] [Google Scholar]
- 7. Socinski MA, Jotte RM, Cappuzzo F et al Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–301. [DOI] [PubMed] [Google Scholar]
- 8. Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity 2020; 52: 17–35. [DOI] [PubMed] [Google Scholar]
- 9. Sharma P, Hu‐Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017; 168: 707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reck M, Rodriguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 11. Ahmadzada T, Kao S, Reid G, Boyer M, Mahar A, Cooper WA. An update on predictive biomarkers for treatment selection in non‐small cell lung cancer. J Clin Med 2018; 7: 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mu CY, Huang JA, Chen Y, Chen C, Zhang XG. High expression of PD‐L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011; 28: 682–8. [DOI] [PubMed] [Google Scholar]
- 13. Tang H, Wang Y, Chlewicki LK et al Facilitating T cell infiltration in tumor microenvironment overcomes resistance to PD‐L1 blockade. Cancer Cell 2016; 29: 285–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adachi Y, Tamiya A, Taniguchi Y et al Predictive factors for progression‐free survival in non‐small cell lung cancer patients receiving nivolumab based on performance status. Cancer Med 2020; 9: 1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kawachi H, Tamiya M, Tamiya A et al Association between metastatic sites and first‐line pembrolizumab treatment outcome for advanced non‐small cell lung cancer with high PD‐L1 expression: A retrospective multicenter cohort study. Invest New Drugs 2020; 38: 211–8. [DOI] [PubMed] [Google Scholar]
- 16. Bilen MA, Shabto JM, Martini DJ et al Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer 2019; 19: 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kitadai R, Okuma Y, Hakozaki T, Hosomi Y. The efficacy of immune checkpoint inhibitors in advanced non‐small‐cell lung cancer with liver metastases. J Cancer Res Clin Oncol 2020; 146: 777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang G, Cheng R, Wang H et al Comparable outcomes of nivolumab in patients with advanced NSCLC presenting with or without brain metastases: A retrospective cohort study. Cancer Immunol Immunother 2020; 69: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oliver AJ, Lau PKH, Unsworth AS et al Tissue‐dependent tumor microenvironments and their impact on immunotherapy responses. Front Immunol 2018; 8: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kuchuk M, Addison CL, Clemons M, Kuchuk I, Wheatley‐Price P. Incidence and consequences of bone metastases in lung cancer patients. J Bone Oncol 2013; 2: 22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res 2008; 466: 729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scagliotti GV, Hirsh V, Siena S. Overall survival improvement in patients with lung cancer and bone metastases treated with denosumab versus zoledronic acid: Subgroup analysis from a randomized phase 3 study. J Thorac Oncol 2012; 7: 1823–9. [DOI] [PubMed] [Google Scholar]
- 23. Zhao E, Xu H, Wang L et al Bone marrow and the control of immunity. Cell Mol Immunol 2012; 9: 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reinstein ZZ, Pamarthy S, Sagar V et al Overcoming immunosuppression in bone metastases. Crit Rev Oncol Hematol 2017; 117: 114–27. [DOI] [PubMed] [Google Scholar]
- 25. Schmid S, Diem S, Li Q et al Organ‐specific response to nivolumab in patients with non‐small cell lung cancer (NSCLC). Cancer Immunol Immunother 2018; 67: 1825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 27. Ahn SB, Lee SB, Singh TD et al Multimodality imaging of bone marrow‐derived dendritic cell migration and antitumor immunity. Transl Oncol 2017; 10: 262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiao S, Subudhi SK, Aparicio A et al Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 2019; 179: e1113. [DOI] [PubMed] [Google Scholar]
- 29. Landi L, D'Inca F, Gelibter A et al Bone metastases and immunotherapy in patients with advanced non‐small‐cell lung cancer. J Immunother Cancer 2019; 7: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tesniere A, Schlemmer F, Boige V et al Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010; 29: 482–91. [DOI] [PubMed] [Google Scholar]
- 31. Sevko A, Michels T, Vrohlings M et al Antitumor effect of paclitaxel is mediated by inhibition of myeloid‐derived suppressor cells and chronic inflammation in the spontaneous melanoma model. J Immunol 2013; 190: 2464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol 2018; 15: 325–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gong X, Li X, Jiang T et al Combined radiotherapy and anti‐PD‐L1 antibody synergistically enhances antitumor effect in non‐small cell lung cancer. J Thorac Oncol 2017; 12: 1085–97. [DOI] [PubMed] [Google Scholar]
- 34. Theelen W, Peulen HMU, Lalezari F et al Effect of Pembrolizumab after stereotactic body radiotherapy vs Pembrolizumab alone on tumor response in patients with advanced non–small cell lung cancer. JAMA Oncol 2019; 11: 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shaverdian N, Lisberg AE, Bornazyan K et al Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non‐small‐cell lung cancer: A secondary analysis of the KEYNOTE‐001 phase 1 trial. Lancet Oncol 2017; 18: 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Spaas M, Lievens Y. Is the combination of immunotherapy and radiotherapy in non‐small cell lung cancer a feasible and effective approach? Front Med 2019; 6: 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huang CY, Wang L, Feng CJ. Bisphosphonates enhance EGFR‐TKIs efficacy in advanced NSCLC patients with EGFR activating mutation: A retrospective study. Oncotarget 2016; 7: 66480–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang G, Cheng R, Zhang Z et al Bisphosphonates enhance antitumor effect of EGFR‐TKIs in patients with advanced EGFR mutant NSCLC and bone metastases. Sci Rep 2017; 7: 42979. [DOI] [PMC free article] [PubMed] [Google Scholar]


