Abstract
Malignant tumors are often associated with eosinophilic pleural effusion. Here, we encountered a case of interleukin‐5 (IL‐5)‐producing malignant pleural mesothelioma with eosinophilic pleural effusion. The patient was a 50‐year‐old male. He had a history of a cough for several weeks and had visited a local doctor. Left pleural effusion was noted, and the patient was referred to our hospital. He was diagnosed with malignant pleural mesothelioma by pleural biopsy, with eosinophilic pleural effusion. IL‐5 in the pleural effusion increased, and tumor cells were IL‐5‐positive by immunostaining. There have been few reports of IL‐5‐producing tumors, and this is the first report of IL‐5‐producing malignant pleural mesothelioma. Host‐tumor cell interactions cause eosinophilic pleural effusion. In patients with eosinophilic pleural effusion, malignant pleural effusion should be considered. It is necessary to clarify the pathophysiology of malignant tumors and eosinophils.
Keywords: Eosinophilic pleural effusion, IL‐5‐producing tumor, immunostaining, malignant pleural mesothelioma
This is the first report of IL‐5‐producing malignant pleural mesothelioma with eosinophilic pleural effusion.
Introduction
Eosinophilic pleural effusion is defined as pleural fluid with a nucleated cell count containing more than 10% eosinophils. 1 , 2 These effusions are relatively rare. It is estimated that approximately 10% of exudative pleural effusions are eosinophilic. 2 The pathogenesis of eosinophilic pleural effusion is still not well understood. The most common cause of eosinophilic pleural effusion has been reported to be malignancy (26%), followed by idiopathic (25%) and pneumonia (13%), according to a meta‐analysis of 687 cases. 3
We encountered a case of interleukin‐5 (IL‐5)‐producing malignant pleural mesothelioma with eosinophilic pleural effusion. There have been few reports of IL‐5‐producing tumors, and this is the first report of IL‐5‐producing malignant pleural mesothelioma.
Case report
A 50‐year‐old man complained of cough and sputum for several weeks and visited a local doctor. At that time, chest X‐ray revealed a large amount of unilateral pleural effusion, and the patient was referred to our hospital. He had smoked two packets of cigarettes per day for 35 years. His profession was painting, and asbestos exposure was considered likely. Breathing sounds in the left lung were diminished. Blood tests showed elevated white blood cells (12000/μL), platelets (41.1 × 104/μL), and cytokeratin 19 fragments (7.6 ng/mL). The percentage of eosinophils in the peripheral blood was 3%. Chest X‐ray (Fig 1a) and contrast‐enhanced computed tomography (CT) (Fig 1b) showed moderate left pleural effusion. A total of 850 mL of pleural effusion was drained. Pleural effusion was exudate. The total number of cells was 1342/μL. Cell classification was 4% neutrophils, 47% eosinophils, 36% lymphocytes, and 10% monocytes. On pleural effusion cytology, many atypical cells with prominent eosinophilic nucleoli were found to be solitary to aggregated. From the immunocytochemical study, calretinin, thrombomodulin, D2‐40, EMA, and E‐cadherin were positive (Fig 2a), and TTF‐1, Napsin A, CEA, and MOC‐31 were negative. Malignant pleural mesothelioma was suspected. Thus, using cell blocks prepared from the pleural effusion, p16 homozygous deletion was confirmed by p16 fluorescence in situ hybridization because 45% of the tumor cells showed loss of both p16 gene signals. Thus, malignant pleural mesothelioma was considered. Thoracoscopic biopsy of parietal pleura confirmed the diagnosis because of the tumor invasion to the pleura and adipose tissue (Fig 2b). Biphasic malignant pleural mesothelioma with 70% epithelioid cells and 30% sarcomatoid cells was diagnosed. After examination, including 18‐fluorodeoxyglucose positron emission tomography (FDG‐PET/CT) (Fig 1c,d), the patient was clinically diagnosed with malignant pleural mesothelioma cT3N2M0 stage III.
Figure 1.
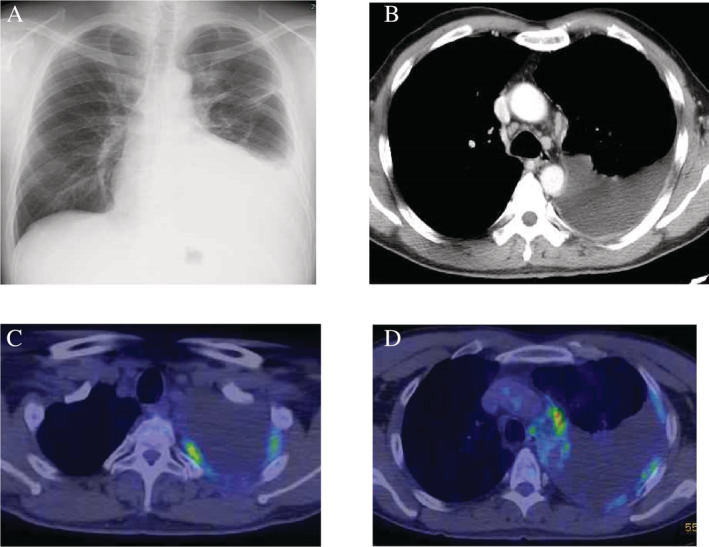
(A) Chest X‐ray; and (B) contrast‐enhanced computed tomography (CT) at first admission. (C, D) 18‐fluorodeoxyglucose positron emission tomography (FDG‐PET/CT): An irregular tumor was present in the left pleura, and FDG uptake was observed. Maximum standardized uptake value (SUVmax) was 6.0.
Figure 2.
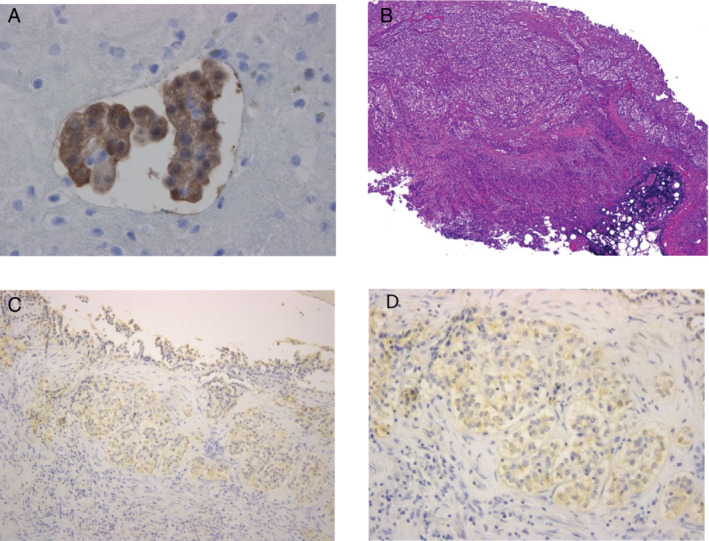
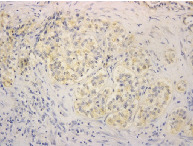
(A) On pleural effusion cytology, aggregated tumor cells were seen, and were immunocytochemically stained with calretinin (magnification x400). (B) Pleural biopsy revealed that tumor cells invaded the parietal pleura involving the adipose tissue. (Hematoxylin‐eosin staining, magnification x40). (C, D) Immunostaining of tumor nests showed positive staining for IL‐5 (magnification x100 and x200 in (C) and (D), respectively).
IL‐5 and granulocyte/macrophage colony‐stimulating factor (GM‐CSF) were measured by ELISA. IL‐5 in the pleural fluid was 23.5 pg./mL, and GM‐CSF in the pleural effusion was 8 pg/mL or less. IL‐5 and GM‐CSF in the peripheral blood were 3.9 pg/mL or less and 8 pg/mL or less, respectively. Immunostaining of the tumor tissue showed positive staining for IL‐5 (Fig 2c, d). Thus, IL‐5 production from malignant pleural mesothelioma cells was suggested.
The patient was diagnosed with biphasic malignant pleural mesothelioma. Chemotherapy (cisplatin and pemetrexed) was performed at a monthly intervals for a total of three cycles. However, his condition progressed, and he was transferred to a hospice and died about a year later.
Discussion
Eosinophilic pleural effusion is caused by pleural damage, malignancy, pulmonary embolism, drug‐induced infection, chronic eosinophilic pneumonia, or rheumatoid arthritis. 4 In a review of 153 pleural fluid samples demonstrating eosinophilic pleural effusion, 47 were associated with malignancy, of which 23 were associated with lung cancer. 5 Malignant pleural mesothelioma was present in only two of 343 eosinophilic pleural effusions. 6
The recruitment of eosinophils into tissues is mediated by such factors as IL‐3, IL‐5, eotaxins, GM‐CSF, and regulated on activation, normal T cell expressed and secreted. In addition, eosinophil survival in the pleural space is considered to be mediated by cytokines such as IL‐3, IL‐5, and GM‐CSF. 7 , 8 IL‐5 has emerged as a central controlling cytokine for eosinophilia production, activation, and recruitment. 9
In the present study, we measured cytokines and found that IL‐5 in pleural fluid was higher than in peripheral blood. Immunostaining of tumor tissue showed positive staining for IL‐5, suggestive of IL‐5 production from malignant pleural mesothelioma cells. Reports of IL‐5‐producing tumors are uncommon, and we found only three previously reported cases. 10 , 11 , 12
The functional role of eosinophils in human cancers remains to be clarified. One study suggested that tumor‐associated eosinophilia may provide a modest survival benefit in cancer patients. 13 However, blood and bone marrow eosinophilia is more commonly found in aggressive tumors and is generally considered an adverse prognostic feature. 14 Intrapleural administration of IL‐2 caused marked eosinophilia in the pleural fluid. 15 IL‐5 augments cytotoxicity of eosinophils, as measured by their improved activity against various human tumor cells line. This cytotoxicity was significantly inhibited upon the addition of an antibody to IL‐5. 16 In cancer immunotherapy, particularly in therapies using immune checkpoint inhibitors, eosinophils have been shown to be a potential predictive marker for a beneficial clinical response. 17 , 18 Host‐tumor cell interactions cause eosinophilic pleural effusion. 19 Moreover, recent findings suggest that eosinophils play pleiotropic and opposing roles in the tumor microenvironment. 17
In conclusion, here, we encountered a case of IL‐5‐producing malignant pleural mesothelioma with eosinophilic pleural effusion. This is the first report of malignant pleural mesothelioma producing IL‐5. In patients with eosinophilic pleural effusion, malignant pleural effusion should be considered. In the future, it is necessary to elucidate the pathophysiology of malignant tumors and eosinophils.
Disclosure
The authors declare no conflicts of interest.
References
- 1. Hirsch A, Ruffie P, Nebut M, Bignon J, Chretien J. Pleural effusion: Laboratory tests in 300 cases. Thorax 1979; 34 (1): 106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rubins JB, Rubins HB. Etiology and prognostic significance of eosinophilic pleural effusions. A prospective study. Chest 1996; 110 (5): 1271–4. [DOI] [PubMed] [Google Scholar]
- 3. Oba Y, Abu‐Salah T. The prevalence and diagnostic significance of eosinophilic pleural effusions: A meta‐analysis and systematic review. Respiration 2012; 83 (3): 198–208. [DOI] [PubMed] [Google Scholar]
- 4. Martinez‐Garcia MA, Cases‐Viedma E, Cordero‐Rodriguez PJ et al Diagnostic utility of eosinophils in the pleural fluid. Eur Respir J 2000; 15 (1): 166–9. [DOI] [PubMed] [Google Scholar]
- 5. Krenke R, Nasilowski J, Korczynski P et al Incidence and aetiology of eosinophilic pleural effusion. Eur Respir J 2009; 34 (5): 1111–7. [DOI] [PubMed] [Google Scholar]
- 6. Adelman M, Albelda SM, Gottlieb J, Haponik EF. Diagnostic utility of pleural fluid eosinophilia. Am J Med 1984; 77 (5): 915–20. [DOI] [PubMed] [Google Scholar]
- 7. Kalomenidis I, Mohamed KH, Lane KB et al Pleural fluid levels of vascular cell adhesion molecule‐1 are elevated in eosinophilic pleural effusions. Chest 2003; 124 (1): 159–66. [PubMed] [Google Scholar]
- 8. Nakamura Y, Ozaki T, Kamei T et al Factors that stimulate the proliferation and survival of eosinophils in eosinophilic pleural effusion: relationship to granulocyte/macrophage colony‐stimulating factor, interleukin‐5, and interleukin‐3. Am J Respir Cell Mol Biol 1993; 8 (6): 605–11. [DOI] [PubMed] [Google Scholar]
- 9. Dent LA, Strath M, Mellor AL, Sanderson CJ. Eosinophilia in transgenic mice expressing interleukin 5. J Exp Med 1990; 172 (5): 1425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Balian A, Bonte E, Naveau S et al Intratumoral production of interleukin‐5 leading to paraneoplastic peripheral eosinophilia in hepatocellular carcinoma. J Hepatol 2001; 34 (2): 355–6. [DOI] [PubMed] [Google Scholar]
- 11. Fridlender ZG, Simon HU, Shalit M. Metastatic carcinoma presenting with concomitant eosinophilia and thromboembolism. Am J Med Sci 2003; 326 (2): 98–101. [DOI] [PubMed] [Google Scholar]
- 12. Pandit R, Scholnik A, Wulfekuhler L, Dimitrov N. Non‐small‐cell lung cancer associated with excessive eosinophilia and secretion of interleukin‐5 as a paraneoplastic syndrome. Am J Hematol 2007; 82 (3): 234–7. [DOI] [PubMed] [Google Scholar]
- 13. Samoszuk M. Eosinophils and human cancer. Histol Histopathol 1997; 12 (3): 807–12. [PubMed] [Google Scholar]
- 14. Isaacson NH, Rapoport P. Eosinophilia in malignant tumors; its significance. Ann Intern Med 1946; 25 (6): 893–902. [DOI] [PubMed] [Google Scholar]
- 15. Nakamura Y, Ozaki T, Yanagawa H, Yasuoka S, Ogura T. Eosinophil colony‐stimulating factor induced by administration of interleukin‐2 into the pleural cavity of patients with malignant pleurisy. Am J Respir Cell Mol Biol 1990; 3 (4): 291–300. [DOI] [PubMed] [Google Scholar]
- 16. Rivoltini L, Viggiano V, Spinazzè S et al In vitro anti‐tumor activity of eosinophils from cancer patients treated with subcutaneous administration of interleukin 2. Role of interleukin 5. Int J Cancer 1993; 54 (1): 8–15. [DOI] [PubMed] [Google Scholar]
- 17. Reichman H, Karo‐Atar D, Munitz A. Emerging roles for eosinophils in the tumor microenvironment. Trends Cancer 2016; 2 (11): 664–75. [DOI] [PubMed] [Google Scholar]
- 18. Simon SCS, Utikal J, Umansky V. Opposing roles of eosinophils in cancer. Cancer Immunol Immunother 2019; 68 (5): 823–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stathopoulos GT, Sherrill TP, Karabela SP et al Host‐derived interleukin‐5 promotes adenocarcinoma‐induced malignant pleural effusion. Am J Respir Crit Care Med 2010; 182 (10): 1273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


