Abstract
Background
Histologically, SCLC are classified as pure (P‐SCLC) and combined subtypes (C‐SCLC). Currently, few studies compare the clinicopathological characteristics and explore the treatment strategies applied to them.
Methods
Between July 2005 and April 2016, the clinical records of 297 postoperative patients with pathologically confirmed SCLC were retrospectively analyzed. Kaplan‐Meier method and Cox regression model were separately used for stratified univariate and multivariate survival analysis.
Results
A total of 46 cases (15.5%) of C‐SCLCs and 251 cases (85.5%) of pure SCLCs (P‐SCLCs) were included in this study. The average age of C‐SCLCs was a little higher than that of P‐SCLCs (59.65 ± 8.72 vs. 56.56 ± 10.12; P = 0.053). More patients had a history of smoking in C‐SCLC (78.3% vs. 63.3%; P = 0.074). The five‐year overall survival (OS) rate for P‐SCLCs and C‐SCLCs was 65.1% and 56.7%, respectively (P = 0.683). For P‐SCLC, stage and an intervention of prophylactic cranial irradiation (PCI) were independent factors that affected OS. In C‐SCLCs cases, performing sublobectomy was an independent risk factor for poor prognosis.
Conclusions
We identified no significant difference in clinical characteristics and outcome between C‐SCLCs and P‐SCLCs. However, the factors affecting the prognosis of the two subtypes were slightly inconsistent. For C‐SCLCs, the extent of resection had a greater impact on survival, and lobectomy combined with systemic lymph node dissection should therefore be performed as extensively as possible. In addition, PCI was beneficial in improving the SCLC OS rate.
Key points
This study demonstrated the prognosis of C‐SCLCs did not significantly differ from that of P‐SCLCs, but was more susceptible to the extent of resection. Patients with C‐SCLC who underwent limited resection had a significantly increased risk of shorter OS.
This study highlighted the importance of performing lobectomy for resectable C‐SCLC patients. This study also proved the benefit of PCI in improving the OS rate for both P‐SCLC and C‐SCLC patients.
Keywords: Combined small cell lung cancer, prognosis, small cell lung cancer, survival analysis, treatment

We performed a retrospective study to compare the clinicopathological characteristics and explore treatment strategies of pure and combined SCLC and found the prognosis of C‐SCLCs were more susceptible to extent of resection than P‐SCLCs. Patients with C‐SCLC who underwent limited resection had a significantly increased risk of shorter overall survival. Based on these results, we recommend a lobectomy should be performed, especially in resectable C‐SCLC patients.
Introduction
Small cell lung cancer (SCLC) accounts for approximately 13%–15% of lung cancer and is the major histological type of neuroendocrine tumors in the lung. 1 , 2 Most SCLCs are pure SCLC (P‐SCLC), while some can be combined with additional components of any histological types of non‐small cell lung cancer (NSCLC), which is defined as combined small cell lung cancer (C‐SCLC), according to the World Health Organization (WHO) in 2001. 3 The combined NSCLC histological types include adenocarcinoma, squamous cell carcinoma, large cell carcinoma, large cell neuroendocrine carcinoma, or any other rare component, such as giant cell carcinoma or sarcoma‐like cancer, etc. 3 Incidence of C‐SCLC has been previously reported to range from 2% to 28% in different studies. 4 , 5 , 6 The heterogeneous results of these studies may be affected by variations in sampling methods, sample quantity, sample size, and sample integrity, etc. 7 Recently, the number of cases diagnosed as C‐SCLC continues to increase as surgical resection plays an increasingly prominent role in multimodal therapy of early to middle stage SCLC. However, there are limited studies on C‐SCLC and clinical standardization for C‐SCLC is lacking, because most of the previous studies regarded combined and pure SCLC as a whole. 8 , 9 To gain more knowledge on the diagnosis, treatment and prognosis of C‐SCLC, we performed a retrospective study to compare the clinical characteristics, current status of treatment, prognosis and related prognostic factors between C‐SCLC and P‐SCLC patients.
Methods
Patient selection and clinical data collection
Patients with histologically confirmed SCLC after surgical resection of lung cancer and systematic lymph node dissection from July 2005 to April 2016 were collected from the medical record system of the Cancer Hospital of the Chinese Academy of Medical Sciences (CHCAMS). Patients who underwent concurrent chemoradiotherapy as a first treatment were excluded. This study was approved by the Ethics Committee and Institutional Review Boards, and all patients were exempt from an informed consent due to the retrospective nature of the study. The clinical staging criteria was according to the 2009 American Joint Committee on Cancer (AJCC) seventh edition TNM and the Veterans Administration Lung Study Group (VALSG) staging systems. 10 , 11 Staging inspection and postoperative monitoring was conducted by physical examination, computed tomography (CT) of the chest, positron emission computer tomography (PET‐CT), neck and abdomen ultrasound, brain magnetic resonance imaging (MRI) and bone scanning imaging. All archived formalin‐fixed, paraffin‐embedded (FFPE) sections were reviewed by two experienced clinical pathologists specializing in chest tumor pathology to confirm the diagnosis of C‐SCLC or P‐SCLC, and identify the additional histological components of C‐SCLC.
The following data of clinicopathological characteristics were retrieved: age, gender, smoking history, tumor laterality, TNM stage, VALSG stage, lymph node status, pathological subtype, additional histological type in cases with C‐SCLC, and treatment history. The involved lymph nodes were pathologically confirmed. The follow‐up information was acquired by regular patient visits or telephone calls and was complete until 28 February 2019. In general, patients were recommended for outpatient review every month for the first six months after surgery. The follow‐up periods and intervals were then determined according to tumor status and treatment recomended by physicians. Recurrence‐free survival (RFS) was defined as the time between the start of surgery and observation of intrathoracic recurrence and/or distant metastasis of the tumor. Since most patients did not meet the conditions for second biopsy, the status of recurrence was evaluated by chest and abdominal CT, PET‐CT, brain MRI and bone scanning imaging. Intrathoracic recurrence included intrathoracic lymph nodes metastasis and local failures defined as recurrence involving the bronchial stump or staple line. Distant metastasis was defined as the presence of new lesions in other organs evaluated by imaging. In the case of patients with no recurrence during follow‐up, the endpoint of RFS was the last follow‐up or death. Overall survival (OS) was defined as the time from the start of surgery to death or the last follow‐up. The primary endpoint of this study was OS, and the second endpoint was RFS.
Statistical analysis
For continuous normal distribution variables, the mean ± standard deviation was calculated, and the Student's t‐test was applied to show the significance of difference between groups. For categorical variables, the percentage was calculated, and the Fisher's exact test or the Chi‐square test was applied to determine significance of difference. The Kaplan‐Meier (KM) method was used to estimate the probability of RFS and OS, with the Log‐rank test performed to evaluate significance. The Cox proportional hazards model was used for multifactor analysis for RFS and OS. Both the univariable and multivariable analysis were performed on cases with pure and combined SCLC. All tests were bilateral and P < 0.05 was considered statistically significant. All statistical analysis was performed using SPSS software version 25.0.
Results
Comparison of clinicopathological characteristics
A total of 297 SCLC patients were included in the study. Among these, 46 were confirmed as C‐SCLC (15.5%) and 251 as P‐SCLC (84.5%). Among 46 cases of C‐SCLCs, four patients underwent bronchoscopic biopsies before surgery, only one patient was diagnosed with C‐SCLC, and the other three patients were misdiagnosed as P‐SCLC, whereas among 251 P‐SCLCs, 24 cases underwent biopsy, and their diagnoses were confirmed by biopsy and surgery. The additional components in C‐SCLC were mainly squamous cell carcinoma with 19 cases (41.3%), followed by 18 (39.1%) cases of adenocarcinoma, four (8.7%) cases of large cell carcinoma, two (4.3%) cases of large cell neuroendocrine carcinoma, one (2.1%) case of carcinoid tumor, one case of carcinoid tumor combined with large cell neuroendocrine carcinoma, and one case of adenosquamous carcinoma. The age of 297 patients overall ranged from 19 to 82 years, with an average age of 57.04 ± 9.97 years. The average age of C‐SCLC patients was slightly higher than that of P‐SCLC patients (59.65 ± 8.72 vs. 56.56 ± 10.12; P = 0.053) (Table 1). There were 210 (70.7%) males and 87 (29.3%) females in total. Both the C‐SCLC and P‐SCLC groups consisted of significantly more men than women, but no significant difference existed in sex ratio. There was 195 (65.7%) patients who had a history of smoking. The proportion of smoking patients with C‐SCLC was a little higher than those with P‐SCLC, but the difference did not reach statistical significance (78.3% vs. 63.3%; P = 0.074) (Table 1). According to the AJCC seventh edition TNM staging system, 87 (29.3%) patients were stage I, 85 (28.6%) stage II, 115 (38.7%) stage III, and 10 (3.4%) stage IV. In parallel to the VALSG staging system, 286 (96.3%) patients had limited disease, and 11 (3.7%) were extensive stage disease. As for the patients who had extensive stage disease who received surgery, we carefully checked the original case records and found that there were 11 patients, including four with brain metastases, four with bone metastases, two with pleural metastases and one with bilateral supraclavicular lymph node metastasis. The two patients with pleural metastasis were accidentally discovered during surgery, while the other four patients with brain and four with bone metastases were found after surgery had been performed. In comparison, the distribution of the stage by the two staging systems was not significantly different between the two subtypes (TNM stage P = 0.469; VALSG stage P = 0.682) (Table 1).
Table 1.
Clinical characteristics of 251 P‐SCLC and 46 C‐SCLC patients
| Total | P‐SCLC | C‐SCLC | |||
|---|---|---|---|---|---|
| Characteristics | (n = 297) | (n = 251) | (n = 46) | P‐value | SMD |
| Age (years) | 57.04 ± 9.97 | 56.56 ± 10.12 | 59.65 ± 8.72 | 0.053 | 0.327 |
| Age group | |||||
| ≤60 years | 188 (63.3) | 164 (65.3) | 24 (52.2) | 0.124 | 0.270 |
| >60 years | 109 (36.7) | 87 (34.7) | 22 (47.8) | ||
| Gender | |||||
| Female | 87 (29.3) | 76 (30.3) | 11 (23.9) | 0.486 | 0.144 |
| Male | 210 (70.7) | 175 (69.7) | 35 (76.1) | ||
| Smoking | 195 (65.7) | 159 (63.3) | 36 (78.3) | 0.074 | 0.333 |
| Tumor laterality | |||||
| Left | 146 (49.2) | 126 (50.2) | 20 (43.5) | 0.498 | 0.135 |
| Right | 151 (50.8) | 125 (49.8) | 26 (56.5) | ||
| TNM stage (Seventh) | |||||
| I | 87 (29.3) | 78 (31.1) | 9 (19.6) | 0.469 | 0.269 |
| II | 85 (28.6) | 70 (27.9) | 15 (32.6) | ||
| III | 115 (38.7) | 95 (37.8) | 20 (43.5) | ||
| IV | 10 (3.4) | 8 (3.2) | 2 (4.3) | ||
| VALSG stage | |||||
| Extensive | 11 (3.7) | 9 (3.6) | 2 (4.3) | 0.682 | 0.039 |
| Limited | 286 (96.3) | 242 (96.4) | 44 (95.7) | ||
| Lymph node metastasis | 183 (61.6) | 153 (61.0) | 30 (65.2) | 0.703 | 0.088 |
| Treatment | |||||
| S | 35 (11.8) | 32 (12.7) | 3 (6.5) | 0.485 | 0.223 |
| S + CTx | 166 (55.9) | 140 (55.8) | 26 (56.5) | ||
| S + CTx + RT | 96 (32.3) | 79 (31.5) | 17 (37.0) | ||
| Extent of resection † | |||||
| Lobectomy | 236 (79.5) | 200 (79.7) | 36 (78.3) | 0.623 | 0.162 |
| Pneumonectomy | 20 (6.7) | 18 (7.2) | 2 (4.3) | ||
| Sublobectomy | 19 (6.4) | 15 (6.0) | 4 (8.7) | ||
| CTx before S | 27 (9.1) | 25 (10.0) | 2 (4.3) | 0.278 | 0.219 |
| Chemotherapy | 262 (88.2) | 219 (87.3) | 43 (93.5) | 0.339 | 0.212 |
| Radiation | 96 (32.3) | 79 (31.5) | 17 (37.0) | 0.576 | 0.116 |
| PCI | 66 (22.2) | 59 (23.5) | 7 (15.2) | 0.294 | 0.211 |
| Chemo‐regmen ‡ | |||||
| SCLC regimen | 167 (56.2) | 150 (59.8) | 17 (37.0) | <0.001 * | 1.069 |
| NSCLC regimen | 9 (3.0) | 0 (0.0) | 9 (19.6) | ||
| SCLC + NSCLC regimen | 7 (2.4) | 3 (1.2) | 4 (8.7) | ||
| No chemotherapy | 35 (11.8) | 32 (12.7) | 3 (6.5) | ||
| Follow‐up § | |||||
| DM | 72 (24.2) | 64 (25.5) | 8 (17.4) | 0.687 | 0.228 |
| IR | 38 (12.8) | 31 (12.4) | 7 (15.2) | ||
| IR + DM | 22 (7.4) | 20 (8.0) | 2 (4.3) | ||
| No recurrence | 152 (51.2) | 129 (51.4) | 23 (50.0) | ||
AJCC, American Joint Committee on Cancer; C‐SCLC, combined small cell lung cancer; CTx, chemotherapy; DM, distant metastasis; IR, intrathoracic recurrence; PCI, prophylactic cranial irradiation; P‐SCLC, pure small cell lung cancer; RT, radiotherapy; S, surgery; SMD, standard mean difference; VALSG, Veterans Administration Lung Study Group.
22 patients were excluded because the surgical information was unavailable.
79 patients were excluded because the chemotherapy regimen was unknown.
13 patients were excluded because the sites of recurrence was not recorded.
P < 0.05 is indicated by bold italics.
Method of surgical resection and treatment
All patients underwent surgical resection and systematic lymph node dissection, which included 236 (79.5%) lobectomies, 19 (6.4%) sublobectomies (including segmental resection and wedge resection), 20 (6.7%) pneumonectomies, and 22 (7.4%) without recorded extent of resection. For treatment strategies, 35 (11.8%) patients were treated with surgery alone, 166 (55.9%) received surgery and chemotherapy, and 96 (32.3%) underwent surgery combined with chemotherapy and chest radiotherapy. The majority (88.2%) of patients overall were treated comprehensively with at least two treatments. The treatment options for C‐SCLCs and P‐SCLCs were basically uniform. Platinum‐based combined chemotherapy regimen (etoposide combined with cisplatin/carboplatin, etc) was used for adjuvant chemotherapy. Of note, nine (19.6%) patients with C‐SCLC were treated with regimens sensitive to NSCLC (paclitaxel or vinorelbine combined with platinum). A total of 59 patients with P‐SCLC and seven patients with C‐SCLC received PCI (23.5% vs. 15.2%; P = 0.294) (Table 1).
Patterns of relapse and survival analysis
The follow‐up period for the entire cohort was 1.0–166.6 months, with a median follow‐up time of 46.8 months. By the end of follow‐up, 145 (48.8%) patients had relapsed and 112 (37.7%) patients were deceased. The most common form of relapse was distant metastasis (24.2%), followed by intrathoracic recurrence (12.8%), and both intrathoracic and distant recurrence (7.4%). In terms of form of relapse, there was no significant difference between P‐SCLC and C‐SCLC (P = 0.687) (Table 1). During the follow‐up, 45 (15.1%) patients were lost. The overall one‐, three‐, and five‐year RFS rates were 72.4%, 54.6%, and 51.8%, respectively; the one‐, three‐, and five‐year OS rates were 93.8%, 71.2%, and 63.8%, respectively. The median RFS was 82.9 months (95% confidence interval [CI]: 44.19–121.61), and the median OS was not reached. Univariate analysis of 297 SCLCs revealed that there was no significant difference in RFS and OS between the two subtypes (Table S1). The five‐year RFS rates of 251 P‐SCLC and 46 C‐SCLC were 52.5% vs. 47.6% (P = 0.944) (Table S1), and the five‐year OS rates were 65.1% vs. 56.7% (P = 0.683) (Table S1). The multivariate analysis also indicated histologic subtype had insignificant effects on prognosis (Figs [Link], [Link]).
For 251 cases of P‐SCLC, univariate analysis indicated that male, smoking, TNM stage III or IV, lymph node metastasis and pneumonectomy were risk factors for postoperative recurrence (Table 2); TNM stage III or IV, VALSG extensive stage and lymph node metastasis were risk factors for poor OS. The OS of patients with PCI was significantly better than that of patients without PCI. The five‐year OS were 78.9% and 60.7%, respectively (P = 0.023) (Table 3). For 46 cases of C‐SCLC, univariable analysis showed that lymph node metastasis and the extent of surgical resection had an impact on RFS (Table 2), VALSG and TNM staging, lymph node metastasis, and the extent of resection had an impact on OS (Table 3). However, the use of different adjuvant chemotherapy regimens (RFS P = 0.980, OS P = 0.840) (Tables 2, 3) did not show significant impact on prognosis. Variables with univariate analysis were included in the Cox proportional hazard model for multivariate analysis. As for tumor relapse, TNM stage greater than stage I was an independent risk factor for P‐SCLCs (Fig 1). Females had more favorable RFS than males, and the five‐year RFS rates of females and males were 65.7% and 46.5%, respectively (hazard ratio [HR] 0.64, 95% CI: 0.41–0.99; P = 0.044) (Fig 1). For OS, stage III by TNM staging (HR 3.50, 95% CI: 2.01–6.09; P < 0.001) (Fig 2) and extensive stage by VALSG staging (HR 14.75, 95% CI: 1.85–117.57; P = 0.011) (Fig 2) were independent risk factors for P‐SCLCs. Performing PCI was an independent predictive factor for P‐SCLCs (HR 0.43, 95% CI: 0.24–0.76; P = 0.004) (Fig 2). In cases of C‐SCLC, extensive stage (HR 110.01, 95% CI: 1.99–6078.24; P = 0.022) (Fig 2) and receiving sublobectomy (HR 6.43 95% CI: 1.49–27.69; P = 0.012) (Fig 2) were independent risk factors for poor OS.
Table 2.
Univariable analysis for RFS in 251 P‐SCLC and 46 C‐SCLC patients
| P‐SCLC | C‐SCLC | |||||
|---|---|---|---|---|---|---|
| Clinical factors | (n = 251) | Five‐year RFS% | P‐value | (n = 46) | Five‐year RFS% | P‐value |
| Gender | ||||||
| Male | 175 (69.7) | 46.5 | 0.006 * | 35 (76.1) | 49.7 | 0.647 |
| Female | 76 (30.2) | 65.7 | 11 (23.9) | 39.8 | ||
| Age | ||||||
| ≤60 years | 164 (65.3) | 52.5 | 0.688 | 24 (52.2) | 48.7 | 0.653 |
| >60 years | 87 (34.7) | 54.4 | 22 (47.8) | 47.8 | ||
| Smoking history | ||||||
| Yes | 159 (63.3) | 47.1 | 0.033 * | 36 (78.3) | 45.0 | 0.463 |
| No | 92 (36.6) | 61.4 | 10 (21.7) | 60.0 | ||
| Tumor laterality | ||||||
| Left | 126 (50.1) | 52.8 | 0.691 | 20 (43.5) | 44.1 | 0.536 |
| Right | 125 (49.8) | 52.2 | 26 (56.5) | 49.7 | ||
| VALSG staging | ||||||
| Limited | 242 (96.4) | 52.9 | 0.183 | 44 (95.7) | 48.8 | 0.112 |
| Extensive | 9 (3.2) | 41.7 | 2 (4.3) | 0.0 | ||
| AJCC seventh staging | ||||||
| I | 78 (31.1) | 72.9 | <0.001 * | 9 (19.6) | 87.5 | 0.103 |
| II | 70 (27.9) | 56.0 | 15 (32.6) | 27.2 | ||
| III | 95 (37.8) | 34.7 | 20 (43.5) | 45.0 | ||
| IV | 8 (3.2) | 46.9 | 2 (4.3) | 0.0 | ||
| Lymph node metastasis | ||||||
| Yes | 153 (61.0) | 43.0 | <0.001 * | 30 (65.2) | 34.7 | 0.031 * |
| No | 98 (39.0) | 68.0 | 16 (34.8) | 79.1 | ||
| Extent of resection † | ||||||
| Lobectomy | 200 (79.7) | 56.5 | 0.012 * | 36 (78.3) | 58.0 | 0.012 * |
| Sublobectomy | 15 (6.0) | 50.9 | 4 (8.7) | 25.0 | ||
| Pneumonectomy | 18 (7.2) | 41.3 | 2 (4.3) | 0.0 | ||
| Treatment mode | ||||||
| S | 32 (12.7) | 66.8 | 0.007 * | 3 (6.5) | 50.0 | 0.827 |
| S + CTx | 140 (55.8) | 56.0 | 26 (56.5) | 51.7 | ||
| S + CTx + RT | 79 (31.5) | 41.0 | 17 (37) | 41.2 | ||
| Chemo‐regmen ‡ | ||||||
| SCLC regimen | 150 (59.8) | 44.2 | 0.940 | 17 (37) | 55.0 | 0.980 |
| NSCLC regimen | 0 (0.0) | — | 9 (19.6) | 50.0 | ||
| SCLC+NSCLC regimen | 3 (1.2) | 33.3 | 4 (8.7) | 33.3 | ||
| No chemotherapy | 32 (12.7) | 66.8 | 3 (6.5) | 50 | ||
| PCI | ||||||
| Yes | 59 (23.5) | 58.9 | 0.267 | 7 (15.2) | 57.1 | 0.752 |
| No | 192 (76.5) | 50.5 | 39 (84.8) | 46.0 | ||
AJCC, American Joint Committee on Cancer; C‐SCLC, combined small cell lung cancer; CTx, chemotherapy; PCI, prophylactic cranial irradiation; P‐SCLC, pure small cell lung cancer; RFS, recurrence‐free survival; RT, radiotherapy; S, surgery; VALSG, Veterans Administration Lung Study Group.
18 and four patients with P‐SCLC and C‐SCLC respectively were excluded because the surgical information was unavailable.
66 and 13 patients with P‐SCLC and C‐SCLC were excluded respectively because the chemotherapy regimen was unknown.
P < 0.05 is indicated by bold italics.
Table 3.
Univariable analysis for OS in 251 P‐SCLC and 46 C‐SCLC patients
| P‐SCLC | C‐SCLC | |||||
|---|---|---|---|---|---|---|
| Clinical factors | (n = 251) | Five‐year OS% | P‐value | (n = 46) | Five‐year OS% | P‐value |
| Gender | ||||||
| Male | 175 (69.7) | 61.4 | 0.090 | 35 (76.1) | 58.1 | 0.862 |
| Female | 76 (30.2) | 73.9 | 11 (23.9) | 50.0 | ||
| Age | ||||||
| ≤60 years | 164 (65.3) | 66.8 | 0.322 | 24 (52.2) | 66.0 | 0.184 |
| >60 years | 87 (34.7) | 61.2 | 22 (47.8) | 47.0 | ||
| Smoking history | ||||||
| Yes | 159 (63.3) | 69.6 | 0.305 | 36 (78.3) | 53.3 | 0.399 |
| No | 92 (36.6) | 62.5 | 10 (21.7) | 71.4 | ||
| Tumor laterality | ||||||
| Left | 126 (50.1) | 65.2 | 0.997 | 20 (43.5) | 44.9 | 0.204 |
| Right | 125 (49.8) | 65.0 | 26 (56.5) | 64.2 | ||
| VALSG staging | ||||||
| Limited | 242 (96.4) | 66.0 | 0.022 * | 44 (95.7) | 59.4 | <0.001 * |
| Extensive | 9 (3.2) | 38.1 | 2 (4.3) | 0.0 | ||
| AJCC seventh staging | ||||||
| I | 78 (31.1) | 76.9 | <0.001 * | 9 (19.6) | 87.5 | <0.001 * |
| II | 70 (27.9) | 75.8 | 15 (32.6) | 48.8 | ||
| III | 95 (37.8) | 50.5 | 20 (43.5) | 54.0 | ||
| IV | 8 (3.2) | 43.8 | 2 (4.3) | 0.0 | ||
| Lymph node metastasis | ||||||
| Yes | 153 (61.0) | 58.0 | 0.003 * | 30 (65.2) | 44.6 | 0.030 * |
| No | 98 (39.0) | 76.2 | 16 (34.8) | 84.4 | ||
| Extent of resection † | ||||||
| Lobectomy | 200 (79.7) | 68.8 | 0.690 | 36 (78.3) | 68.1 | <0.001 * |
| Sublobectomy | 15 (6.0) | 55.6 | 4 (8.7) | 25.0 | ||
| Pneumonectomy | 18 (7.2) | 70.6 | 2 (4.3) | 0.0 | ||
| Treatment mode | ||||||
| S | 32 (12.7) | 67.7 | 0.221 | 3 (6.5) | 50.0 | 0.862 |
| S + CTx | 140 (55.8) | 67.5 | 26 (56.5) | 61.2 | ||
| S + CTx + RT | 79 (31.5) | 60.6 | 17 (37) | 51.8 | ||
| Chemo‐regmen ‡ | ||||||
| SCLC regimen | 150 (59.8) | 59.9 | 0.870 | 17 (37) | 61.4 | 0.840 |
| NSCLC regimen | 0 (0.0) | — | 9 (19.6) | 66.7 | ||
| SCLC + NSCLC regimen | 3 (1.2) | 33.3 | 4 (8.7) | 27.2 | ||
| No chemotherapy | 32 (12.7) | 67.7 | 3 (6.5) | 50.0 | ||
| PCI | ||||||
| Yes | 59 (23.5) | 78.9 | 0.023 * | 7 (15.2) | 80.0 | 0.107 |
| No | 192 (76.5) | 60.7 | 39 (84.8) | 51.4 | ||
AJCC, American Joint Committee on Cancer; C‐SCLC, combined small cell lung cancer; CTx, chemotherapy; OS, overall survival; PCI, prophylactic cranial irradiation; P‐SCLC, pure small cell lung cancer; RT, radiotherapy; S, surgery; VALSG, Veterans Administration Lung Study Group.
18 and four patients with P‐SCLC and C‐SCLC respectively were excluded because of the surgical information was unavailable.
66 and 13 patients with P‐SCLC and C‐SCLC were excluded respectively because the chemotherapy regimen was unknown.
P < 0.05 is indicated by bold italics.
Figure 1.

Multivariate analysis for recurrence‐free survival (RFS) in 251 cases of pure small cell lung cancer (P‐SCLC) patients (blue line) and 46 cases of combined small cell lung cancer (C‐SCLC) patients (yellow line). CI, confidence interval; HR, hazard ratio. HR1 and P1 represent the parameters for pure SCLC, HR2 and P2 represent the parameters for combined SCLC.  , pure SCLC;
, pure SCLC;  , combined SCLC.
, combined SCLC.
Figure 2.
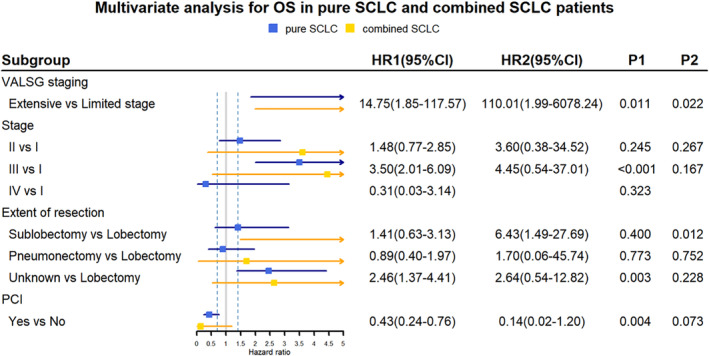
Multivariate analysis for overall survival (OS) in 251 cases of P‐SCLC patients (blue line) and 46 cases of C‐SCLC patients (yellow line). CI, confidence interval; HR, hazard ratio. HR1 and P1 represent the parameters for pure SCLC, HR2 and P2 represent the parameters for combined SCLC.  , pure SCLC;
, pure SCLC;  , combined SCLC.
, combined SCLC.
Discussion
In our cohort, C‐SCLC accounted for 15.5% of all SCLC, which is in the range of reported incidence rate of 2% to 28%. 4 , 5 , 6 Due to thorough pathological assessment of surgically resected specimens, the detection rate of C‐SCLC was relatively higher compared with other studies that included patients with biopsy or cytological samples. The additional components of C‐SCLC in our cohort were mainly squamous cell carcinoma and adenocarcinoma with an incidence of 41.3% and 39.1%, respectively. This observation is consistent with other published studies. 12 , 13 In a few studies which used a Caucasian cohort, large cell carcinoma was reported to be the most common component, where inconsistency may be explained by ethnic differences. 4 , 6 A previous study reported that the C‐SCLC was mainly located in the upper lobe, 14 and in another study that it often appeared as peripheral lung cancer. 5 The incidence of peripheral lesions of C‐SCLCs and other subtypes (including oat cell or intermediate and mixed small cell/large cell) detected by chest x‐ray was 56% and 14%, respectively. 5 In clinical diagnosis, if imaging suggests the presence of peripheral SCLC, the possibility of C‐SCLC should be considered. 5 However, some studies have reported that C‐SCLCs are mainly the central type. 13 A possible explanation in the study by Hajmanoochehri et al. was the disproportionate inclusion of C‐SCLCs with squamous cell carcinoma, and most squamous cell carcinomas were the central type. 15 We could not retrieve data concerning the specific localization of tumors to assess the proportion of peripheral and central localization. However, we found that the two subtypes were basically uniform for the distribution of the left and right lung lobes, with a proportion of approximately 50% for both, and different laterality did not affect prognosis in the two subtypes (P‐SCLC P = 0.997, C‐SCLC P = 0.204) (Table 3).
For C‐SCLC, two basic questions remain: Are there any differences in clinical characteristics and prognosis of C‐SCLC compared with P‐SCLC? Should different treatment strategies be applied for the two subtypes? 14 For the first question, we found that the average age of patients with C‐SCLC was higher than that for patients with P‐SCLC, and there were more patients with a smoking history. Although the statistics of the two factors did not reach the threshold for significance, the standard mean difference (SMD) of both factors was greater than 0.1 (age SMD = 0.327, smoking SMD = 0.333) (Table 1). Currently, there is no universal consensus for the threshold of SMD to indicate the significant imbalance between different groups, 16 but a standard difference less than 0.1 has been taken to indicate a negligible difference in the mean or prevalence of a covariate between treatment groups. 17 Therefore, we believed there was a slightly different distribution of patients for smoking history and age between P‐SCLCs and C‐SCLCs, which may be caused by different pathogenesis in the two subtypes. No concordant agreements have been reached in the analysis of recurrence and prognosis of C‐SCLC and P‐SCLC. Some believe that the prognosis of C‐SCLC is better than that of P‐SCLC, 6 while some disagree. 18 Moon et al. studied 184 cases of C‐SCLCs in a 1:3 matched dataset on the basis of the Surveillance, Epidemiology, and End Results (SEER) database and compared the effect of the histological subtype of SCLC on treatment outcomes and prognosis. Their study determined that C‐SCLCs had a more favorable prognosis before and after matching in univariate analysis for lung cancer‐specific survival (CSS) than noncombined SCLCs, but there was no significant difference for CSS in multivariate survival analysis, indicating that the histologic subtype was not an independent prognostic factor. 14 Our research results are partially consistent with this study: there was no statistical difference for both RFS and OS of the two subtypes, in both univariate (RFS P = 0.944, OS P = 0.683) (Table S1) and multivariate survival analysis (RFS P = 0.204, OS P = 0.878) (Figs [Link], [Link]). The inconsistencies in different studies may be due to: (i) the low incidence and detection rate of C‐SCLC, resulting in a small sample size and low statistical power; (ii) interpatient heterogeneity due to the additional histological type; and (iii) differences in distribution of disease stage and treatment methods.
According to the National Comprehensive Cancer Network (NCCN) and Chinese Society of Clinical Oncology (CSCO), radical surgical treatment is recommended for patients with T1‐2N0M0 SCLC. Several studies have shown that the prognosis was improved in SCLC patients with surgical resection compared to those without surgery in different stages, especially for patients with stage I or without lymph node involved. 19 , 20 For patients with pathologically confirmed positive lymph nodes, the OS of patients who received lobectomy plus adjuvant chemotherapy was superior to those who received standard of care of concurrent chemoradiotherapy. 19 Therefore, surgery‐based comprehensive treatment in SCLC should be emphasized in the future. Our study shows that the extent of resection had a more significant impact on C‐SCLC (Figs 2, 4). The prognosis of C‐SCLC patients with sublobectomy was significantly worse than those with lobectomy, with five‐year OS rates of 25% and 68.1%, respectively. (P < 0.001) (Table 3). Moreover, performing sublobectomy is an independent risk factor for poor OS in C‐SCLC after adjusting the stage and PCI. (HR 6.43, 95% CI: 1.49–27.69; P = 0.012) (Fig 2). As for pure SCLC, the OS was not significantly affected by the extent of resection, with five‐year OS rates 55.6%, 68.8% and 70.6% for patients who received sublobectomy, lobectomy and pneumonectomy, respectively (P = 0.690) (Table 3, Fig 4a). Thus, lobectomy and systemic lymph node dissection should be performed as extensively as possible, especially for C‐SCLC patients who are eligible to receive surgery. The different impact of extent of resection on prognosis of C‐SCLC and P‐SCLC may be attributed to the different biological behavior of the two subtypes. For C‐SCLC, different cell components may be derived from a common precursor with stemness. 18 Therefore, we speculate that there are more cancer stem cells (CSC) in the component of C‐SCLC, and it may be more likely to cause postoperative CSC survival if not completely resected, compared with P‐SCLC. In addition, C‐SCLC is more heterogeneous, which may lead to enhanced metastatic capacity due to the paracrine signaling involved in the interaction across different tumor clones. 18 Thus, the scope of surgical resection should be considered as appropriate to ensure complete resection for C‐SCLC.
Figure 4.
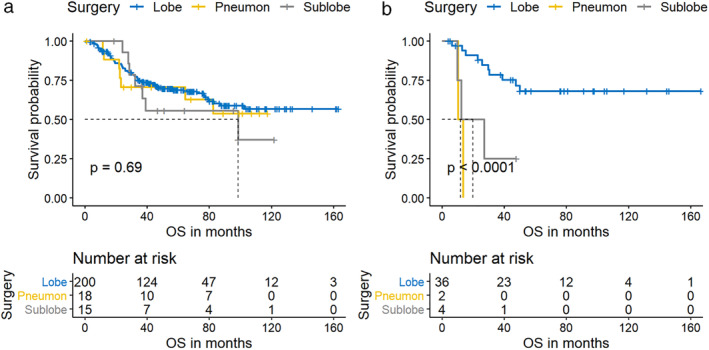
The effect of extent of resection on overall survival (OS) in pure small cell lung cancer (P‐SCLC) (a)  , Lobe;
, Lobe;  , Pneumon;
, Pneumon;  , Sublobe and combined small cell lung cancer (C‐SCLC) (b)
, Sublobe and combined small cell lung cancer (C‐SCLC) (b)  , Lobe;
, Lobe;  , Pneumon;
, Pneumon;  , Sublobe patients. The extent of resection had a more significant effect on the OS of C‐SCLC, patients with C‐SCLC who underwent limited resection had a significantly increased risk of shorter OS.
, Sublobe patients. The extent of resection had a more significant effect on the OS of C‐SCLC, patients with C‐SCLC who underwent limited resection had a significantly increased risk of shorter OS.
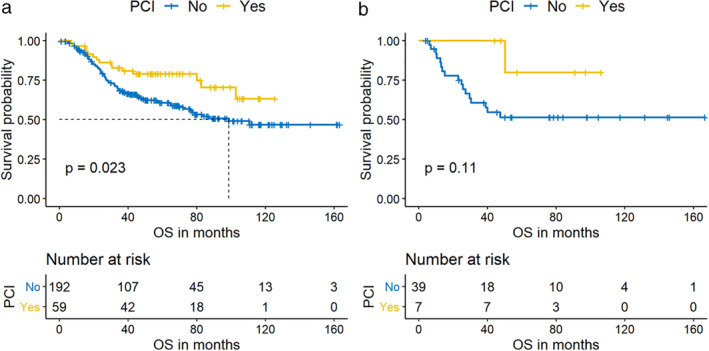
Currently, C‐SCLCs are treated based on SCLC guidelines. 14 In our cohort, 17 (37.0%) C‐SCLC patients were treated with platinum‐based doublets sensitive to SCLC, with 14 (30.4%) of cases using etoposide and three (6.5%) cases using irinotecan. Of note, nine (19.5%) C‐SCLC patients were treated with platinum‐based doublet with paclitaxel or vinorelbine, which are more sensitive to NSCLC. The regimen of 13 cases of C‐SCLC patients was not available. There was no significant difference in RFS and OS among the groups using different regimens for C‐SCLC (RFS P = 0.980, OS P = 0.840) (Tables 2, 3). Since there was an incompleteness of first and posterior line treatment information, we could not accurately evaluate the curative effect of different regimens. In addition, PCI can significantly improve the OS of P‐SCLC, and the five‐year OS rate was 78.9% vs. 60.7% (HR 0.43, 95% CI: 0.24–0.76; P = 0.004) (Figs 2, 3), but for C‐SCLC, PCI did not reach statistical significance in both univariate (P = 0.107) (Table 3, Fig 3b) and multivariate analysis (P = 0.073) (Fig 2). However, the five‐year OS rate of patients with C‐SCLC who received PCI was obviously better than those without PCI, with 80.0% vs. 51.4% respectively (Table 3).
Figure 3.
The effect of prophylactic cranial irradiation (PCI) on overall survival (OS) in pure small cell lung cancer (P‐SCLC) (a)  , No;
, No;  , Yes and combined small cell lung cancer (C‐SCLC) (b)
, Yes and combined small cell lung cancer (C‐SCLC) (b)  , No;
, No;  , Yes patients. The OS was better for both P‐SCLC (a) and C‐SCLC (b) patients with PCI compared with those without PCI.
, Yes patients. The OS was better for both P‐SCLC (a) and C‐SCLC (b) patients with PCI compared with those without PCI.
Due to the rarity of C‐SCLC in clinics, we could not include as many patients with C‐SCLC as P‐SCLC, which is one of the limitations of this study. On the other hand, given that our research was retrospective, we could not exclude the inherent selection bias for patients who were in a relatively better physical condition to receive PCI and lobectomies. In addition, since the subsequent treatment information after adjuvant treatment was incomplete, the response to the different regimens was difficult to accurately evaluate. However, our study was based on the real‐world condition. In the future, more prospective researches are expected to confirm the conclusions of this study.
In conclusion, by reviewing the clinical characteristics, treatment modes and prognosis, this retrospective study revealed different risk factors for relapse and prognosis of P‐SCLCs and C‐SCLCs. Male and TNM stages greater than stage I were demonstrated to be independent risk factors for recurrence in P‐SCLC patients, while sublobar resection a risk factor for shorter RFS in C‐SCLC patients. TNM staging, VALSG staging, and PCI were independent factors affecting OS in P‐SCLC patients, while sublobar resection proved to be an independent risk factor for poor prognosis in C‐SCLC patients. Based on our findings, we recommend that a multidisciplinary comprehensive treatment model including surgery should be adopted for C‐SCLC where possible. Surgical resection should follow lobectomy and systematic lymph node dissection when physical condition permits. In addition, PCI will improve the OS rate of SCLCs. Thus, PCI should be performed in selected patients in good physical condition as much as possible.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1 Univariable analysis for RFS and OS in total 297 SCLC patients.
Figure S1 Multivariate analysis for recurrence‐free survival (RFS) in overall 297 small cell lung cancer (SCLC) patients.
Figure S2 Multivariate analysis for overall survival (OS) in overall 297 small cell lung cancer (SCLC) patients.
Acknowledgments
We would like to acknowledge China Cancer Foundation Beijing Hopes Marathon Fund Project (LC2017A20, 2017‐2020).
References
- 1. Govindan R, Page N, Morgensztern D et al Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: Analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol 2006; 24: 4539–44. [DOI] [PubMed] [Google Scholar]
- 2. Rekhtman N. Neuroendocrine tumors of the lung: An update. Arch Pathol Lab Med 2010; 134: 1628–38. [DOI] [PubMed] [Google Scholar]
- 3. Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumours. Eur Respir J 2001; 18: 1059–68. [DOI] [PubMed] [Google Scholar]
- 4. Nicholson SA, Beasley MB, Brambilla E et al Small cell lung carcinoma (SCLC): A clinicopathologic study of 100 cases with surgical specimens. Am J Surg Pathol 2002; 26: 1184–97. [DOI] [PubMed] [Google Scholar]
- 5. Mangum MD, Greco FA, Hainsworth JD, Hande KR, Johnson DH. Combined small‐cell and non‐small‐cell lung cancer. J Clin Oncol 1989; 7: 607–12. [DOI] [PubMed] [Google Scholar]
- 6. Babakoohi S, Fu P, Yang M, Linden PA, Dowlati A. Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer 2013; 14: 113–9. [DOI] [PubMed] [Google Scholar]
- 7. Fraire AE, Johnson EH, Yesner R, Zhang XB, Spjut HJ, Greenberg SD. Prognostic significance of histopathologic subtype and stage in small cell lung cancer. Hum Pathol 1992; 23: 520–8. [DOI] [PubMed] [Google Scholar]
- 8. Takei H, Kondo H, Miyaoka E et al Surgery for small cell lung cancer: A retrospective analysis of 243 patients from Japanese lung Cancer registry in 2004. J Thorac Oncol 2014; 9: 1140–5. [DOI] [PubMed] [Google Scholar]
- 9. Takenaka T, Takenoyama M, Inamasu E et al Role of surgical resection for patients with limited disease‐small cell lung cancer. Lung Cancer 2015; 88: 52–6. [DOI] [PubMed] [Google Scholar]
- 10. Micke P, Faldum A, Metz T et al Staging small cell lung cancer: Veterans administration lung study group versus International Association for the Study of Lung Cancer—what limits limited disease? Lung Cancer 2002; 37: 271–6. [DOI] [PubMed] [Google Scholar]
- 11. Edge SB, Compton CC. The American joint committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–4. [DOI] [PubMed] [Google Scholar]
- 12. Zakowski MF. Pathology of small cell carcinoma of the lung. Semin Oncol 2003; 30: 3–8. [DOI] [PubMed] [Google Scholar]
- 13. Men Y, Hui Z, Liang J et al Further understanding of an uncommon disease of combined small cell lung cancer: Clinical features and prognostic factors of 114 cases. Chin J Cancer Res 2016; 28: 486–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moon SW, Seo JH, Jeon HW, Moon MH. Effect of histological subtype and treatment modalities on T1‐2 N0‐1 small cell lung cancer: A population‐based study. Thorac Cancer 2019; 10: 1229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hajmanoochehri F, Mohammadi N, Zohal MA, Sodagar A, Ebtehaj M. Epidemiological and clinicopathological characteristics of lung cancer in a teaching hospital in Iran. Asian Pac J Cancer Prev 2014; 15: 2495–500. [DOI] [PubMed] [Google Scholar]
- 16. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011; 46: 399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Normand ST, Landrum MB, Guadagnoli E et al Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: A matched analysis using propensity scores. J Clin Epidemiol 2001; 54: 387–98. [DOI] [PubMed] [Google Scholar]
- 18. Zhao X, McCutcheon JN, Kallakury B et al Combined small cell carcinoma of the lung: Is it a single entity? J Thorac Oncol 2018; 13: 237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wakeam E, Acuna SA, Leighl NB et al Surgery versus chemotherapy and radiotherapy for early and locally advanced small cell lung cancer: A propensity‐matched analysis of survival. Lung Cancer 2017; 109: 78–88. [DOI] [PubMed] [Google Scholar]
- 20. Xu L, Zhang G, Song S, Zheng Z. Surgery for small cell lung cancer: A surveillance, epidemiology, and end results (SEER) survey from 2010 to 2015. Medicine 2019; 98: e17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Univariable analysis for RFS and OS in total 297 SCLC patients.
Figure S1 Multivariate analysis for recurrence‐free survival (RFS) in overall 297 small cell lung cancer (SCLC) patients.
Figure S2 Multivariate analysis for overall survival (OS) in overall 297 small cell lung cancer (SCLC) patients.


