Abstract
Background
Here, we investigated radiological responses following chemotherapy alone as compared to both radiation/chemotherapy (chemoRT) in patients with thymic epithelial tumors (TETs) who did not receive upfront surgery.
Methods
TETs treated at a tertiary academic cancer center between January 2007 and July 2018 were identified. Patients received chemotherapy or chemoRT as initial therapy and pre‐ and post‐treatment scans were available. Student's t‐test, Wilcoxon rank‐sum tests, and Cox proportional hazards method were used to compare clinical details and survival between groups. The primary outcome was change in tumor size, which was compared between groups using linear mixed‐effects regression models, adjusting for baseline tumor size, age, and histology.
Results
A total of 24 of 114 patients with TETs identified met the inclusion criteria. The majority of patients had 67% thymoma (67%, n = 16) and AJCC8 III–IVA disease (58%, n = 14). Median age was 58.5 years (range: 33–76), median initial tumor volume was 187.1 cc (range: 28.7–653.6) and diameter was 8.5 cm (range: 4.5–14.3). Half of the patients received upfront chemotherapy (n = 12: 83% cisplatin/adriamycin/cyclophosphamide) or chemoRT (n = 12: 58% carboplatin/paclitaxel; median RT dose: 63 Gy [range: 60–70 Gy]). At a median imaging follow‐up of 15 months (range: 0–86): ChemoRT was associated with increased average radiological response compared to chemotherapy alone (volume: −47.0 cc more, P < 0.001; diameter: −0.8 cm more, P = 0.03). In eight patients who received chemotherapy, 33% saw further tumor shrinkage (median volume: −42.3%, P = 0.03; diameter: −3.0%, P = 0.049) with additional radiation/chemoradiation. Median survival increased for patients ultimately receiving surgery versus those who did not (46 month, range: 16–127 vs. 14 month, range: 6–82; P < 0.01).
Conclusions
ChemoRT produced a greater radiologic response compared to chemotherapy alone in patients with TETs not suitable for upfront resection.
Key points
Significant findings of the study
We found that chemoRT was associated with a greater radiologic response compared to patients who received chemotherapy alone.
What this study adds
What this study adds: In patients with TET not amenable to upfront resection, chemoRT may be a feasible strategy for cytoreduction.
Keywords: Induction therapy, radiation therapy, thoracic surgery, thymic carcinoma, thymoma
ChemoRT was associated with a greater radiologic response than chemotherapy alone in patients with upfront unresectable TET
Introduction
Thymic epithelial tumors (TETs), including thymoma and thymic carcinoma, are rare tumors, with an incidence rate of about 3/1,000,000 person‐years. 1 The preferred treatment for these malignancies includes multidisciplinary management leading to a margin‐negative surgical resection. However, when patients with TETs have borderline resectable tumors, chemotherapy is the current recommended neoadjuvant approach to reduce tumor burden in the United States. National Comprehensive Cancer Network (NCCN) guidelines recommend induction cyclophosphamide‐adriamycin‐cisplatin chemotherapy (CAP) every three weeks for thymoma and carboplatin/paclitaxel every three weeks for thymic carcinoma. 2
Little conclusive evidence exists regarding the response rates of chemotherapy compared to the combination of radiation and chemotherapy, either concurrent or consecutive (herein referred to as chemoRT) in the cytoreduction of TETs. Clinical observations in TETs support chemoRT as an effective induction therapy to cytoreduce tumors leading to successful resection. 3 , 4 , 5 In a phase II trial evaluating induction chemoradiation for locally advanced TETs, chemoRT was generally well tolerated by recipients, with 20 out of 21 patients seeing tumor shrinkage. 6 In a retrospective series of 29 patients with unresectable thymic carcinoma, chemoRT was associated with a 50% tumor response rate. 7 While these observations suggest a promising tumor response to chemoRT, further studies are needed to explore the response of TETs to chemoRT as compared to chemotherapy alone.
We undertook this study to compare radiologic responses of locally advanced and advanced TETs that received chemotherapy alone as compared to radiation and chemotherapy as a proxy of the efficacy of each respective treatment modality in cytoreducing tumor bulk. In the subset of patients who subsequently underwent surgery, we explored the differences in tumor pathologic response, progression‐free survival, and overall survival between the two treatment modalities.
Methods
Patient data
This was a retrospective IRB‐approved study (IRB00171161). Patients with thymic malignancies treated at a tertiary academic cancer center between January 2007 and July 2018 were identified from the institutional cancer registry database. Patients were included if: (i) their histology included TETs; (ii) upfront surgery was not indicated; (iii) they received chemotherapy with or without radiation as part of primary treatment; and (iv) they had available baseline and post‐treatment (chemotherapy or radiation) radiologic images. Thus, patients included in the study had locally advanced or metastatic tumors, with primary tumors that were not amenable to upfront surgery.
Baseline patient and tumor, treatment, and outcomes details were obtained retrospectively from chart review. Baseline patient and tumor characteristics included: age, sex, race, histologic type, World Health Organization (WHO) pathologic classification, Masaoka‐Koga stage, and American Joint Committee on Cancer (AJCC eighth edition) tumor stage. 8 Treatment characteristics included: chemotherapy regimen and duration, radiation dose and duration, and receipt of surgery. Treatment outcomes included: radiologic response, pathologic response in patients who underwent subsequent surgery, resection margin status, treatment complications, presence of progression, length of follow‐up, and survival outcome. Baseline pathology slides to confirm TET by a single thoracic pathologist (QKL) were reviewed for all patients. Given treatment over different staging eras, patients were clinically restaged according to the AJCC version 8 staging system using pretreatment diagnostic PET/CT and CT findings. 8 Toxicity grade was reported using the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE v. 4.0).
Radiologic response
Available pre‐ and post‐treatment computed tomography (CT) scans of the chest were all analyzed by a thoracic radiologist (AH). Pretreatment measurements were assessed at the beginning date of chemotherapy or chemoradiotherapy, while post‐treatment measurements were assessed at the first follow‐up visit after completion of treatment. Tumor diameter was measured as the largest one‐dimensional measurement in the axial plane according to Response Evaluation Criteria in Solid Tumors (RECIST) measurement criteria and International Thymic Malignancy Interest Group (ITMIG) guidelines. 9 , 10 The volume of the primary tumor was obtained from the composite of delineated axial tumor volumes using Carestream Vue PACS (Version 12.1.5.7014. Rochester, NY). Radiologic response was defined as the difference between post‐treatment volumetric and one‐dimensional primary tumor measurements and the respective pretreatment measurements.
Pathologic response
For the subset of patients who received chemotherapy or radiation and chemotherapy as induction therapy prior to resection, pathology slides from surgical resection were analyzed by a pathologist (QKL) and assessed for tumor necrosis, tumor fibrosis and percentage of viable cells.
Statistical analysis
Imaging follow‐up was performed at regular 3–6 month intervals at the discretion of the treating physician, and was measured from date of treatment end to the date of last chest CT. Overall follow‐up time was measured from the date of treatment end to the date of last contact or death. The association of baseline characteristics and treatment parameters with treatment response were assessed using Chi‐square and Fisher's exact tests for categorical variables or Student's t‐test and the Wilcoxon rank‐sum test for continuous variables. Overall survival (OS) was calculated from the date of treatment end until the earlier date of death or last follow‐up and presented using the Kaplan Meier curve. Progression‐free survival (PFS) was calculated from date of treatment end to the earliest of date of local, regional, or distant progression, death, or last imaging follow‐up. The association of survival outcomes with independent variables was tested using the log‐rank test and the Cox proportional hazard regression. Spearman rank correlation was used to determine correlation between continuous numeric variables. Linear mixed effects models with a random intercept were used to compare the changes in tumor volume or diameters between the different treatment groups, adjusting for the measures before treatment, age, and histology. The random intercepts accounted for the correlation among the changes for the same patient from different time periods. Statistical analysis was performed using STATA (version 14. College Station, TX). A P‐value <0.05 was considered statistically significant.
Results
Patient cohort
Of the 114 patients with TETs evaluated at our institution during the study time period, 30% (n = 34) received chemotherapy or radiation and chemotherapy as a part of the primary treatment. Of these 34 patients, 70% (n = 24) patients met the criteria for inclusion in this study. A detailed breakdown of these patients is shown in Fig 1. Of the 24 patients with TETs, the median age was 58.5 years (range: 33–76). A majority had AJCC8 stage III–IVA disease (58%, n = 14) and 42% stage IVB disease (n = 10). A majority of the patients had thymomas (67%, n = 16 vs. thymic carcinoma: 33%, n = 8), and received first‐line CAP (63%, n = 15). The median RT dose was 63 Gy (range: 60–70). Median overall follow‐up time was 22.9 months (range: 5.6–126.8).
Figure 1.
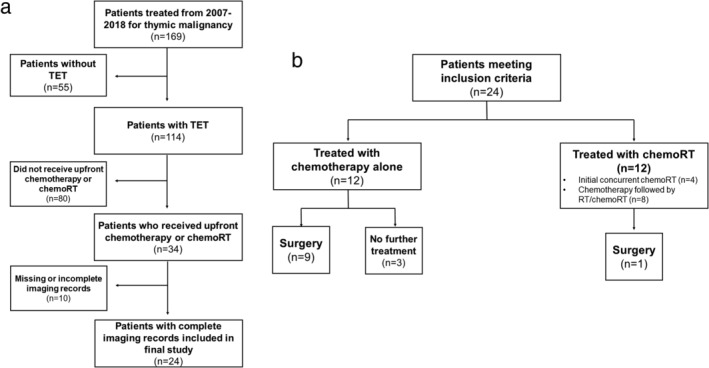
(a) Flow chart of patients who met criteria for the study. (b) Treatment modalities for patients who met criteria. TET, thymic epithelial tumor; chemoRT, receipt of chemotherapy and radiotherapy.
Of these 24 patients, 50% (n = 12) received chemotherapy alone and 50% (n = 12) received chemoRT. In the chemoRT group, 33% (n = 4) received concurrent chemoRT alone, 50% (n = 6) received an initial course of chemotherapy followed by definitive radiation, and 17% (n = 2) received initial chemotherapy followed by chemoRT. In the chemotherapy group, 25% (n = 3) had metastatic (one stage IVA and two stage IVB) disease at presentation and received chemotherapy alone. Of the 10 patients who received subsequent surgery, nine received induction chemotherapy and one received induction chemoradiation.
There were no differences in baseline tumor characteristics between those who received chemotherapy and those who received chemoRT (P > 0.05) (Table 1). Patients who received chemotherapy alone for cytoreduction were more likely to be younger in age, to have received CAP chemotherapy and subsequent surgery compared to patients who received chemoRT (median age: 43 vs. 67 years; CAP: 83% vs. 42%; subsequent surgery: 75% vs. 8%, P ≤ 0.03 for all). This was consistent with our historic institutional preference to give induction chemotherapy to patients with borderline resectable TETs and definitive chemoradiation to patients with unresectable locally advanced TETs. Of the 14 patients who did not receive subsequent surgery (n = 11, chemoRT; n = 3, chemotherapy) all cases of chemoRT did not receive surgery because they were deemed technically inoperable at the time of diagnosis or after initial cytoreductive therapy. There were no cases where patients were denied adjuvant surgery due to the side effects of radiotherapy.
Table 1.
Baseline patient and treatment characteristics of all patients
| Treatment Modality | |||
|---|---|---|---|
| Characteristics | Chemotherapy (n = 12) | Radiation + chemotherapy (n = 12) | P‐value |
| Age (median year, range) | 43 (33–72) | 67 (47–76) | <0.01 |
| Male, n (%) | 6 (50.0) | 8 (66.7) | 0.68 |
| Year of diagnosis (median, range) | 2012 (2007–2017) | 2016 (2006–2017) | 0.07 |
| Histology type, n (%) | 0.20 | ||
| Thymoma | 10 (83.3) | 6 (50.0) | |
| Thymic carcinoma | 2 (16.7) | 6 (50.0) | |
| WHO classification, n (%) | 0.26 | ||
| AB | 0 (0.0) | 1 (8.3) | |
| B1 | 1 (8.3) | 0 (0.0) | |
| B2 | 4 (33.3) | 2 (16.7) | |
| B3 | 5 (41.7) | 2 (16.7) | |
| C | 1 (16.7) | 3 (25.0) | |
| N/A* | 1 (8.3) | 4 (33.3) | |
| Masaoka stage, n (%) | 1.00 | ||
| II | 3 (25.0) | 2 (16.7) | |
| III | 3 (25.0) | 4 (33.3) | |
| IV | 5 (41.7) | 4 (33.3) | |
| N/A* | 1 (8.3) | 2 (16.7) | |
| AJCC8 group stage, n (%) | 0.71 | ||
| IIIA | 2 (16.7) | 4 (33.3) | |
| IIIB | 1 (8.3) | 2 (16.7) | |
| IVA | 3 (25.0) | 2 (16.7) | |
| IVB | 6 (50.0) | 4 (33.3) | |
| Baseline tumor volume in cc (median, range) | 194.5 (28.7–653.6) | 165.0 (44.9–543.9) | 0.95 |
| Baseline tumor diameter in cm (median, range) | 8.4 (4.6–14.3) | 8.5 (4.5–11.0) | 0.98 |
| Radiation dose in Gy (median, range) | N/A | 63.0 (60.0–70.0) | N/A |
| First‐line chemotherapy regimen, n (%) | 0.03 | ||
| CAP | 10 (83.3) | 5 (41.7) | |
| Paclitaxel/carboplatin q3w | 1 (8.3) | 7 (58.3) | |
| Other† | 1 (8.3) | 0 (0.0) | |
| Received subsequent surgery, n(%) | 9 (75.0) | 1 (8.3) | <0.01 |
WHO, World Health Organization; AJCC8, American Joint Committee on Cancer Guidelines, eighth edition; Gy, gray; cc, cubic centimeter; cm, centimeter; CAP, cyclophosphamide, doxorubicin, cisplatin therapy; q3w, every three weeks.
Unclassified due to nonavailable data.
Hyper‐CVAD.
Fisher's exact test and Wilcoxon rank‐sum tests were used to compare categorical and continuous variables, respectively. A P‐value of <0.05 was considered statistically significant.
Radiologic response
Overall, median baseline tumor volume was 187.1 cc (range: 28.7–653.6) and median diameter was 8.5 cm (range: 4.5–14.3). Median imaging follow‐up time was 15.2 months (range: 0.3– 86.2). Median time from the end of treatment until follow‐up imaging was 0.6 months (range: 0–2.1) in the chemotherapy group and 1.3 months (range: 0.3–3.4) in the chemoRT group.
Receipt of chemoRT improved radiologic response when compared to response to chemotherapy alone. On average, multivariate mixed‐effects analysis showed that chemoRT patients had an additional decrease of 47.0 cc (95% confidence interval [CI]: 23.0–71.0; P < 0.001) in tumor volume and 0.8 cm (95% CI: 0.08–1.5; P = 0.03) in tumor diameter compared to chemotherapy‐only patients, controlling for baseline tumor measurements, age, and histology (Table 2). Chemotherapy regimen (cisplatin/adriamcyin/cyclophosphamide vs. carboplatin/paclitaxel) was not associated with a change in radiologic response (P > 0.05; Table S1). Post‐treatment tumor volume and diameter responses were strongly correlated both in terms of percent reduction (Spearman's Rho = 0.73, P < 0.0001) and absolute reduction (Spearman's Rho = 0.58, P < 0.01).
Table 2.
Analysis results of tumor size change from linear mixed‐effects regression models
| Volume change (cc) | Coefficient | 95% CI | P‐value |
|---|---|---|---|
| ChemoRT vs. chemo | −47.0 | (−71.0, −23.0) | <0.001 |
| Initial volume | −0.4 | (−0.6, −0.2) | <0.001 |
| Age | −1.0 | (−3.7, 1.8) | 0.49 |
| Thymoma vs. thymic carcinoma | −33.4 | (−113.1, 46.2) | 0.41 |
| Diameter change (cm) | Coefficient | 95% CI | P‐value |
| ChemoRT vs. chemo | −0.8 | (−1.5, −0.08) | 0.03 |
| Initial diameter | −0.2 | (−0.4, −0.01) | 0.04 |
| Age | 0.01 | (−0.03, 0.05) | 0.54 |
| Thymoma vs. thymic carcinoma | −0.09 | (−1.2, 1.0) | 0.87 |
cc, cubic centimeter; cm, centimeter; CI, confidence interval, chemoRT, radiation and chemotherapy; chemo, chemotherapy.
Linear mixed‐effects regression was used to compare groups, adjusting for tumor size before treatment, age, and histology. A P‐value of <0.05 was considered statistically significant.
One quarter (n = 6) of patients had a partial radiologic response to treatment, defined as a diameter reduction of more than 30% from baseline by RECIST criteria. Both the chemotherapy group and the chemoRT group had 25% (n = 3, out of 12) patients who achieved partial response. In the chemoRT group, the remaining 75% (n = 9) of patients had tumor diameter decrease between 0% and 30%. In the chemotherapy only group, 58% (n = 7) of patients had tumor diameter decrease between 0% and 30% and 17% (n = 2) had progressive disease in the primary tumor.
Eight patients received radiation or chemoradiation after an initial course of chemotherapy. Subsequent radiation therapy further decreased median tumor volume by 39.9 cc (42.3%–95% CI: 17.6%–56.4%, P = 0.03) and median tumor diameter by 1.0 cm (13.3%, 95% CI: 5.0%–23.0%, P = 0.05) compared to post‐chemotherapy measurements (Table 3).
Table 3.
Radiologic response of tumor in patients who received chemotherapy alone, followed by radiation with or without chemotherapy
| Treatment modality | |||
|---|---|---|---|
| Before subsequent radiation | After subsequent radiation or chemoradiation | ||
| n = 8 § median, range | n = 8 § median, range | P‐ value | |
| Tumor volume measurement (cc) | 94.3 (19.8–485.9) | 54.4 (16.5–383.0) | 0.03 |
| Tumor diameter measurement* (cm) | 7.5 (5.6–10.6) | 6.5 (4.2–11.4) | 0.049 |
cc, cubic centimeter; cm, centimeter; CI, confidence interval.
Largest axial diameter across all slices.
Eight patients received treatment with chemotherapy alone, followed by radiation with or without chemotherapy. The imaging response to each respective treatment modality (chemotherapy alone vs. chemotherapy and subsequent follow‐up treatment) were separately analyzed and compared to baseline.
Wilcoxon rank‐sum testing was used to compare tumor size between groups. A P‐value of <0.05 was considered statistically significant.
Pathologic response
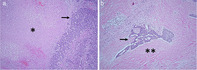
Of the 10 patients who underwent subsequent surgery in our cohort (n = 9 from chemotherapy alone, n = 1 from chemoRT), nine had available pathological slides for re‐review. Baseline and treatment characteristics of this subset with pathologic response results are summarized in Table 4 and Table S2. Median time from the end of treatment to surgery was 2.1 months (range: 1.0– 3.6) in the chemotherapy group and 1.8 months (range: 1.8–1.8) in the chemoRT group. Among these nine patients, the median percent viable tumor remaining was 40% (range: 5%–60%). The only patient to have induction chemoradiation in our cohort had a viable tumor percentage of 30%. Qualitative analysis of slides showed a primarily necrotic change in chemotherapy patients and a primarily fibrotic change in chemotherapy plus radiation patients (Fig 2).
Table 4.
Pathologic response of primary tumor to treatment in patients who received induction therapy before surgery
| Treatment modality | ||
|---|---|---|
| Chemotherapy | Radiation + chemotherapy | |
| n = 8 | n = 1 | |
| % viable tumor (median, range) | 40 (<5–60) | 30 |
| % necrosis (median, range) | 40 (<5–70) | 10 |
| % fibrosis (median, range) | 0 (0–40) | 70 |
| Margin status (n, %) | ||
| Negative | 3 (37.5) | 0 (0.0) |
| Positive | 5 (62.5) | 1 (100.0) |
Figure 2.
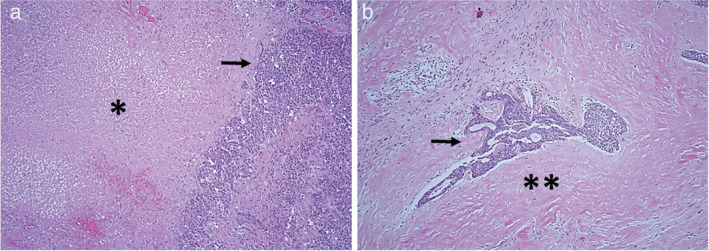
Representative histomorphology of tumors treated with chemotherapy versus a combination of radiation and chemotherapy. (a) Patient who received induction chemotherapy. The tumor shows prominent necrosis, demarcated by *. (b) Patient who received induction radiation with chemotherapy. The tumor shows prominent fibrotic change, demarcated by **. Arrow demarcates residual viable tumor. Both images are taken at 20x magnification on hematoxylin and eosin stained slides.
We also analyzed the pathologic response of primary tumor to treatment in all patients who received induction therapy before surgery (n = 13) at our institution, inclusive of four additional patients identified in the cancer registry database but who did not have baseline radiologic imaging and were therefore not included in the final cohort evaluating radiologic response. Those pathologic results are described in Table S3. The chemoRT group had a statistically higher median rate of fibrosis (70%; range 0%–50%) compared to the chemotherapy only group (0%; range, 0%–40%) (P < 0.01).
Survival
The median OS in the cohort was 22.9 months (range: 5.6–126.8); median PFS was 10.3 months (range: 0.03–68.4). Half (n = 12/24) of patients had local, regional, or distant recurrence or progression. Patients who were able to have subsequent surgery (n = 10) had a median OS of 46 months (range: 16–127) compared to 14 months (range: 6–82) for patients who did not have subsequent surgery (n = 14) (P < 0.01).
Log‐rank tests showed that surgery was associated with a significant improvement in OS (P = 0.02) and a trend towards improvement in PFS (P = 0.06). Cox regression showed a benefit for surgery in OS (hazard ratio (HR = 0.18, 95% CI: 0.03–0.92; P = 0.04) and a trend towards improvement in PFS (HR = 0.28, 95% CI: 0.07–1.11; P = 0.07).
Safety
A total of 10 patients had resection of their primary thymic tumor after induction therapy: nine at our institution and one outside who returned for follow‐up. Of these patients, 70% (n = 7/10) had any grade postoperative complications.
The most common postoperative complication was cardiac arrhythmia, which was seen in five (50%) patients (chemotherapy: 4/9 vs. chemoRT: 1/1). The next most common postoperative complication was diaphragmatic paralysis, seen in three (30%) patients (chemotherapy; 3/9 vs. chemoRT: 0/1).
Discussion
In this series of patients with locally advanced and advanced TET who received chemotherapy or a combination of radiation and chemotherapy as initial therapy, linear mixed‐effects regression showed improved radiologic response in patients treated with chemoRT compared to chemotherapy alone. Furthermore, the addition of radiation after the end of chemotherapy led to further radiologic response of the primary tumor compared to chemotherapy alone. In the subset of patients who received chemotherapy or chemoRT followed by subsequent surgery, we observed a primarily necrotic pathologic response in chemotherapy‐only patients and a primarily fibrotic response in chemoRT patients. Our study showed that patients who received surgery had improved survival outcomes when controlling for patient age and tumor stage.
The median tumor diameter reduction (chemotherapy alone: 1.9 cm, 23.1%; chemoRT: 1.8 cm, 20.3%) observed in our cohort was comparable to the partial responses seen in other induction therapy studies. In two phase II trials of induction chemoradiation before surgery, partial response was seen in 40% (four out of 10) and 48% (10 out of 21), with a median tumor diameter reduction of between 15%–30% by RECIST criteria. 6 , 11 In addition, several prospective trials of solely induction chemotherapy reported combined complete and partial response rates of between 62% and 100%, 12 , 13 , 14 , 15 , 16 albeit in small sample sizes. We uniquely compared the radiologic response to chemotherapy versus radiation and chemotherapy in an indepth fashion, and further evaluated the corresponding pathologic response in a subset of patients that subsequently underwent resection.
With regard to radiologic response as a useful indicator of clinical prognosis, ITMIG guidelines suggest using tumor diameter as the chief tracking parameter, primarily due to standardized software and techniques for measuring one‐dimensional outcomes, as well as the more time‐intensive nature of volumetric measurement. 9 However, some studies argue that volume measurements are more sensitive in detecting disease response or progression. 17 , 18 We found a strong correlation between tumor volume and tumor diameter responses, as well as an improved response in both tumor volume and diameter compared to the combination of radiation and chemotherapy. We recognize that further evaluation of the use of volumetric measurement technique in larger well‐curated TET databases should be pursued.
Several points deserve further consideration. Our study was limited by the retrospective, single‐center nature of the study and a small sample size, subject to multiple statistical testing. In having all pre‐ and post‐treatment scans radiologically re‐reviewed by an expert thoracic radiologist, the sample size of the study was further reduced and analysis was limited to those in which complete radiologic details were available. Many patients (n = 80) were originally excluded from analysis as they did not receive upfront chemotherapy or chemoRT owing to stage of the tumor and/or ability to undergo upfront resection. In addition, our cohort of patients included in this analysis was heterogeneous as it included patients with borderline resectable, locally advanced, and advanced TETs treated with chemotherapy or radiation and chemotherapy. Since chemotherapy alone has historically been the predominant presurgical cytoreductive therapy, receipt of surgery was not distributed evenly between the two treatment groups—only one patient who met the inclusion criteria received chemoRT before surgery. Thus, we could not quantitatively compare surgical outcomes between chemoRT and chemotherapy alone. Finally, given the diverse cohort of patients included in this small cohort, we were unable to assess the impact of clinical details such as WHO tumor grade, and stage on radiologic response. We did, however, use multivariate mixed‐effects analysis that made use of measurements from multiple time points and accounted for age, initial tumor volume, and histology in the radiographic response and is a valid method for small sample size analyses. 19
Our results are useful in that they indicate that chemoradiation may improve radiological response of primary TETs, especially in settings where first‐line chemotherapy agents have failed. Notably, surgical resection has been shown in previous studies to be an independent predictor for survival, even in patients with advanced TETs. 20 Our study suggest that the multidisciplinary oncology team may consider the use of neoadjuvant chemoradiation in patients with borderline‐resectable, locally‐advanced or advanced disease in need of maximal cytoreduction in tumor bulk to potentially improve resectability. However, larger datasets may be necessary to fully elucidate the clinical consequences of these radiologic findings. In light of these results, we hope that future investigators can collaborate to create larger multicenter databases of TET treatment to reach more definitive results concerning the joint use of radiation and chemotherapy as compared to chemotherapy alone to maximize cytoreduction, opportunity for resection, and ultimately, patient outcomes. Since prospective studies of such rare diseases are logistically difficult, pooling several centers' retrospective results may be a more feasible approach towards generalizable results.
Disclosure
The authors declare they have no competing interests. DSE reports personal fees from BeyondSpring Pharmaceuticals, personal fees from Boehringer Ingelheim, personal fees from Bristol‐Myers Squibb, personal fees from Eli Lilly & Co., personal fees from Genentech, personal fees from Guardant Health, Inc., outside the submitted work. KRV reports research funding from Lung Cancer Research Foundation outside the submitted work.
Supporting information
Table S1 Radiographic response of primary tumor to treatment by chemotherapy regimen, overall
Table S2 Tumor and treatment characteristics of n = 9 patients who received induction therapy before surgery
Table S3 Pathologic response of primary tumor to treatment in patients who received induction therapy before surgery
Contributor Information
Robert F. Chu, Email: robert.chu@jhmi.edu
Khinh Ranh Voong, Email: kvoong1@jhmi.edu.
References
- 1. de Jong WK, Blaauwgeers JLG, Schaapveld M, Timens W, Klinkenberg TJ, Groen HJM. Thymic epithelial tumours: A population‐based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008; 44 (1): 123–30. [DOI] [PubMed] [Google Scholar]
- 2. Ettinger DS, Riely GJ, Akerley W et al Thymomas and thymic carcinomas. J Natl Compr Cancer Netw 2013; 11 (5): 562–76. Available from: http://www.jnccn.org/lookup/doi/10.6004/jnccn.2013.0072. [DOI] [PubMed] [Google Scholar]
- 3. Momozane T, Inoue M, Shintani Y et al Trimodality therapy for an advanced thymic carcinoma with both aorta and vena cava invasion. Ann Thorac Surg 2016; 102 (2): e139–41. [DOI] [PubMed] [Google Scholar]
- 4. Akaogi E, Ohara K, Mitsui K et al Preoperative radiotherapy and surgery for advanced thymoma with invasion to the great vessels. J Surg Oncol 1996; 63 (1): 17–22. [DOI] [PubMed] [Google Scholar]
- 5. Detterbeck F, Parsons A. Thymic tumors. Ann Thorac Surg 2004; 77 (5): 1860–9. [DOI] [PubMed] [Google Scholar]
- 6. Korst RJ, Bezjak A, Blackmon S et al Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: A phase II, multi‐institutional clinical trial. J Thorac Cardiovasc Surg 2014; 147 (1): 36–46 e1. [DOI] [PubMed] [Google Scholar]
- 7. Chen Y‐Y, Huang C‐H, Tang Y, Eng H‐L. Concurrent chemoradiotherapy for unresectable thymic carcinoma. Chang Gung Med J 2004; 27 (7): 515–22. [PubMed] [Google Scholar]
- 8. Amin MB, Edge S, Greene F et al AJCC Cancer Staging Manual, 8th edn Springer International Publishing, New York, NY: 2017. [Google Scholar]
- 9. Benveniste MF, Korst RJ, Rajan A, Detterbeck FC, Marom EM. A practical guide from the international thymic malignancy interest group (ITMIG) regarding the radiographic assessment of treatment response of thymic epithelial tumors using modified RECIST criteria. J Thorac Oncol 2014; 9 (9): S119–24. [DOI] [PubMed] [Google Scholar]
- 10. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45 (2): 228–47. [DOI] [PubMed] [Google Scholar]
- 11. Wright CD, Choi NC, Wain JC, Mathisen DJ, Lynch TJ, Fidias P. Induction Chemoradiotherapy followed by resection for locally advanced Masaoka stage III and IVA thymic tumors. Ann Thorac Surg 2008; 85 (2): 385–9. [DOI] [PubMed] [Google Scholar]
- 12. Berruti A, Borasio P, Roncari A, Gorzegno G, Mossetti C, Dogliotti L. Neoadjuvant chemotherapy with adriamycin, cisplatin, vincristine and cyclophosphamide (ADOC) in invasive thymomas: Results in six patients. Ann Oncol 1993; 4 (5): 429–31. [DOI] [PubMed] [Google Scholar]
- 13. Macchiarini P, Chella A, Ducci F et al Neoadjuvant chemotherapy, surgery, and postoperative radiation therapy for invasive thymoma. Cancer 1991; 68 (4): 706–13. [DOI] [PubMed] [Google Scholar]
- 14. Kim ES, Putnam JB, Komaki R et al Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: Final report. Lung Cancer 2004; 44 (3): 369–79. [DOI] [PubMed] [Google Scholar]
- 15. Kunitoh H, Tamura T, Shibata T et al A phase II trial of dose‐dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: Report of a Japan clinical oncology group trial (JCOG 9606). Br J Cancer 2010; 103 (1): 6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riely GJ, Huang J. Induction therapy for locally advanced thymoma. J Thorac Oncol 2010; 5 (10): S323–6. [DOI] [PubMed] [Google Scholar]
- 17. Mozley PD, Bendtsen C, Zhao B et al Measurement of tumor volumes improves RECIST‐based response assessments in advanced lung cancer. Transl Oncol 2012; 5 (1): 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Force J, Rajan A, Dombi E, Steinberg SM, Giaccone G. Assessment of objective responses using volumetric evaluation in advanced thymic malignancies and metastatic non‐small cell lung cancer. J Thorac Oncol 2011; 6 (7): 1267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muth C, Bales KL, Hinde K, Maninger N, Mendoza SP, Ferrer E. Alternative models for small samples in psychological research: Applying linear mixed effects models and generalized estimating equations to repeated measures data. Educ Psychol Meas 2016; 76 (1): 64–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okuma Y, Horio H, Hosomi Y et al The potency of curative‐intent treatment for advanced thymic carcinoma. Lung Cancer 2014; 84 (2): 175–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Radiographic response of primary tumor to treatment by chemotherapy regimen, overall
Table S2 Tumor and treatment characteristics of n = 9 patients who received induction therapy before surgery
Table S3 Pathologic response of primary tumor to treatment in patients who received induction therapy before surgery


