Abstract
We describe a patient with coronavirus disease 2019 (COVID-19) and multiple concomitant thromboses occurring on the 9th day of hospital stay. Thromboses were found in distinct zones of the aorta, as well as in the renal, humeral, and pulmonary arteries. The extensive biological workup performed following this catastrophic thrombotic syndrome found no evidence for underlying prothrombotic disease. In light of current evidence regarding endothelium abnormalities related to COVID-19, this extreme case of catastrophic thrombotic syndrome suggests that COVID-19 can induce severe arterial thrombosis following intense endothelial activation.
Résumé
Nous décrivons le cas d’un patient atteint de la maladie à coronavirus 2019 (COVID-19) et présentant de multiples thromboses concomitantes survenant au 9e jour d'hospitalisation. Les thromboses ont été identifiées dans des zones distinctes de l'aorte, ainsi que dans les artères rénales, humérales et pulmonaires. Un examen biologique approfondi effectué à la suite de ce syndrome thrombotique catastrophique n'a révélé aucun signe de maladie prothrombotique sous-jacente. À la lumière de ces éléments concernant les anomalies de l'endothélium liées à la COVID-19, ce cas extrême de syndrome thrombotique catastrophique suggère que la COVID-19 peut induire une thrombose artérielle sévère suite à une activation endothéliale intense.
We describe a patient with coronavirus disease 2019 (COVID-19) and multiple concomitant thromboses occurring on the 9th day of hospital stay with a negative extensive thrombophilia screening while he was treated with low-dose direct oral anticoagulants since his admission to the hospital.
A 78-year-old hypertensive obese (body mass index: 41 kg/m2) man was hospitalized for fever and dyspnea suspected to be related to COVID-19. He had a history of unprovoked pulmonary embolism in 2016 following a lower-limb phlebitis that was treated with rivaroxaban. At admission, his usual treatment was pravastatin, irbesartan, and omeprazole. Polymerase chain reaction analysis was positive for severe acute respiratory syndrome coronavirus 2. The thoracic computed tomography (CT) scan revealed COVID-19 lesions of 25%-50% of the pulmonary parenchyma without evidence of pulmonary embolism. Initial treatment combined cefotaxime, azithromycine, and rivaroxaban (10 mg/d) as the patient was initially thought to have long-term direct oral anticoagulants following his pulmonary embolism (later shown to have been stopped more than 6 months prior to admission).
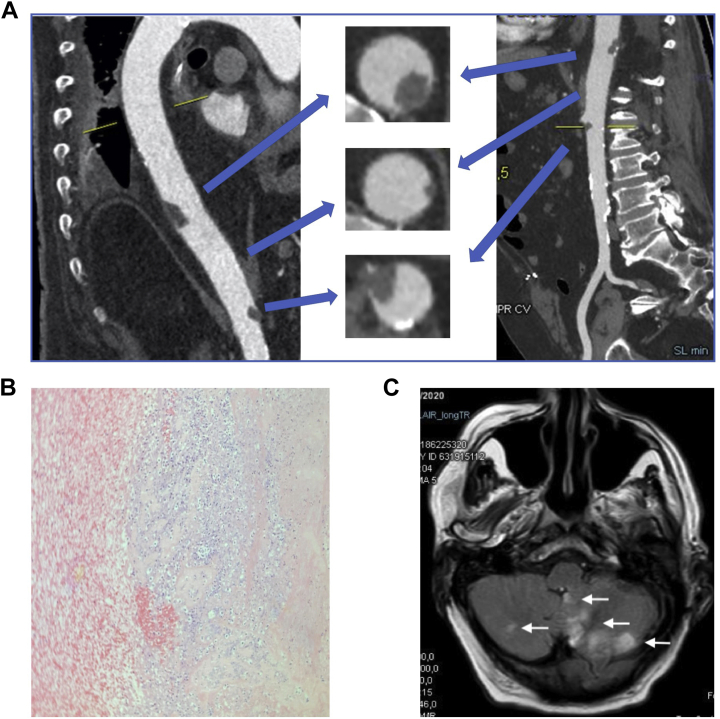
The patient experienced a rapid clinical worsening on day 9 and was transferred to the cardiac intensive care unit. On examination, the patient had evidence of ischemia of the left hand and signs of intestinal obstruction. The combined thoracic and abdominal CT (with intravenous [IV] contrast injection) identified a 2-cm large floating thrombus attached to the posterior wall of the abdominal supra-renal aorta, a small thrombus of the lateral wall of the aorta, and renal ischemia related to a complete occlusion of the right renal artery (Fig. 1A). The patient was diagnosed with intestinal occlusion due to paralytic ileus following the renal embolic event. The thoracic CT also showed an in situ pulmonary thrombosis of the posterobasal segment of the right lower lobe. The vascular CT of the left arm identified a distal thrombosis of the humeral artery. The Doppler exam of the carotids and lower limbs did not identify any other arterial or venous thrombosis. Transthoracic echocardiography identified neither intracardiac thrombus nor intracardiac shunt (negative for a right-to-left shunt using contrast). Transesophageal echocardiography was not performed. Intravenous unfractionated heparin was initiated (and rivaroxaban stopped). The patient underwent Fogarty embolectomy, which successfully removed the left humeral thrombosis (histopathology shown in Fig. 1B).
Figure 1.
(A) Images from the combined thoracic and abdominal computed tomography scan. The top arrows indicate a large aortic floating thrombus attached to the posterior wall of the abdominal supra-renal aorta. The middle arrows indicate the small aortic thrombus of the lateral wall. The bottom arrows indicate the proximal portion of the thrombus occluding the right renal artery. (B) Histopathology view (hematoxylin and eosin stain) of the humeral thrombus showing numerous altered neutrophils in its center (in blue—cell nucleus in deep blue; unaltered cells in pink). (C) Cerebral magnetic resonance imaging findings (FLARE sequence) showing multiple ischemic lesions in the brain stem, cerebellar vermis.
On day 10, while the patient had been on IV unfractionated heparin (with heparinemia within therapeutic range), the patient developed aphasia and motor deficit of the left lower limb. The brain magnetic resonance imaging scan showed an ischemic stroke in the territory of the left posterior inferior cerebellar artery (Fig. 1C). Major gastrointestinal bleeding occurred while heparinemia was ranging between 0.75 and 1.21 UI/ml. Concomitantly, an occlusion of the left palmar arch occurred. The oxygenation status did not worsen, and the patient did not undergo intubation. However, given the severity of the neurologic disorders, the coexistence of severe bleeding (which occurred after the ischemic stroke) and threatening thrombosis, the severe pain induced by digit ischemia, and the underlying frailty favoring rapid clinical worsening (sacral pressure ulcer and bronchial stasis), a thorough discussion with the patient’s family was undertaken. In line with the family’s wishes, palliative care was started on day 12. The patient died on day 19.
At admission, lab tests (Table 1) excluded COVID-19 coagulopathy according to the International Society on Thrombosis and Hemostasis definition (partial thromboplastin time 29.7 seconds). Platelet counts remained greater than 100 G/L despite daily hemograms. The extensive thrombophilia screening we performed was negative. Specifically, we found no evidence for antiphospholipid syndrome (2 negative tests, using 2 techniques, following the International Society on Thrombosis and Hemostasis guidelines).
Table 1.
Most significant lab findings, including thrombophilia screening
| General biological findings |
Coagulation workup | Thrombophilia screening and complement evaluation |
||
|---|---|---|---|---|
| At admission | Most abnormal value (d) | During the hospital stay | ||
| Leucocyte count | 8.84 G/L | 14.7 G/L (D10) | Antithrombine (N 83-125) | 94% |
| Lymphocyte count | 0.59 G/L | 0.41 G/L (D9) | C protein (N 70-140) | 137% |
| Platelet count | 248 G/L | 108 G/L (D4) | S protein (N 74-146) | 88% |
| Hemoglobin | 14.3 g/dL | 7.3 g/dL (D11) | Antiphospholids antibodies | |
| Fibrinogen | 9.3 g/l | / | IgM anticardiolpines (N < 20) | 12.4 U/mL |
| D-dimer | 4169 ng/mL | / | IgG anticardiolpines (N < 20) | 5.8 U/mL |
| Factor V | 118% | / | IgM/ antiBeta2 GP1 (N < 20) | 8.0 UI/mL |
| Albumin | 23 g/L | 21 g/L (D8) | IgG antiBeta2 GP1 (N < 20) | 3.1 UI/mL |
| Bilirubin | 2 μmol/L | 7 μmol/L (D8) | Lupus anticoagulant | 2 negative tests∗ |
| eGFR (CKD-EPI) | 85 ml/min/1.73 m2 | 50 (D10) | Mutation for factor II and V | Negative |
| AST ALT |
48 UI/L 43 UI/L |
175 (D8) 174 (D11) |
Homocysteinemia (N 3.2 to 10.7 μmol/l) | 10 μmol/L |
| NT-proBNP | 1319 pg/mL | C3 (N 0.85-1.8) | 1.40 g/L | |
| Troponine Ic | <0.015 ng/mL | C4 (N 0.13-0.39) | 0.10 g/L | |
| LDH (N [57-241]) | 391 UI/L | CH50 U/mL (N 31.7-71.4) | 53.8 U/mL | |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CKD-EPI, chronic kidney disease epidemiology collaboration; eGFR, estimated glomerular filtration rate; Ig, immunoglobulin; LDH, lactic acid dehydrogenase; NT pro BNP, N-terminal pro brain natriuretic peptide.
Compliant with the International Society of Thrombosis and Hemostasis (ISTH) guidelines.
Our patient suffered from a thrombotic storm/catastrophic thrombotic syndrome1 in the setting of COVID-19, with thrombus found in the aorta, and thromboembolic occlusion of pulmonary, renal, and humeral arteries. In contrast with 3 recently reported cases of embolic events in the setting of COVID-19,2 we found no evidence for antiphospholipid antibodies or other inherited thrombophilia. As our patient had a negative thrombophilia screening, and no atrial fibrillation and/or severe atherosclerosis, we believe that COVID-19 could represent a new etiology of catastrophic thrombotic syndrome. However, given that the patient had a history of previous pulmonary embolism, and thrombophilia screening can be falsely negative while under anticoagulants, we cannot completely rule out a concomitant other thrombotic risk factor. Nonetheless, the trigger for these extensive thromboses could well be the COVID-19–related endotheliitis recently described by Varga and collegues.3 The intensity of this endothelial involvement related to severe acute respiratory syndrome coronavirus 2 prompted Ciceri and colleagues to describe COVID-19 disease as an “endothelial thromboinflammatory syndrome.”4 This intense endothelial thromboinflammatory syndrome could have been the trigger of the catastrophic case we are reporting herein, in the absence of “usual” procoagulant factors. Clinical trials (such as the Weight-Adjusted vs Fixed Low Doses of Low Molecular Weight Heparin For Venous Thromboembolism Prevention in COVID-19 [COVI-DOSE] trial—NCT04373707) investigating the best anticoagulation strategies in this setting are urgently needed.
Novel Teaching Point.
-
•
This unusual case report should raise the attention of clinicians regarding the likelihood of severe thrombotic complications in the setting of COVID-19.
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: The research reported in this paper adhered to relevant guideline.
See page 200 for disclosure information.
References
- 1.Ortel T.L., Erkan D., Kitchens C.S. How I treat catastrophic thrombotic syndromes. Blood. 2015;126:1285–1293. doi: 10.1182/blood-2014-09-551978. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ciceri F., Beretta L., Scandroglio A.M. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): an atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020;22 doi: 10.51893/2020.2.pov2. 95-7. [DOI] [PMC free article] [PubMed] [Google Scholar]