Abstract
E-cadherin, a central component of the adherens junction (AJ), is a single-pass transmembrane protein that mediates cell-cell adhesion. The loss of E-cadherin surface expression, and therefore cell-cell adhesion, leads to increased cell migration and invasion. Treatment of colorectal (CRC)-derived cells (SW-480, HT-29) with 2 mM metformin promoted a redistribution of cytosolic E-cadherin to de novo formed puncta along the length of the contacting membranes of these cells. Metformin also promoted translocation from the cytosol to the plasma membrane of p120-catenin, another core component of the AJs. Furthermore, E-cadherin and p120-catenin co-localized with β-catenin at cell-cell contacts. Western blot analysis of lysates of CRC-derived cells revealed a substantial metformin-induced increase in the level of p120-catenin as well as E-cadherin phosphorylation on Ser838/840, a modification associated to β-catenin/E-cadherin interaction. These modifications in E-cadherin, p120-catenin and β-catenin localization suggest that metformin induces rebuilding of AJs in CRC-derived cells. Those modifications were accompanied by the inhibition of focal adhesion kinase (FAK) as revealed by a significant decrease in the phosphorylation of FAK at Tyr397 and paxillin at Tyr118. These changes were associated to a reduction in the numbers, but an increase in the size, of focal adhesions (FAs) and by the inhibition of cell migration. Overall, these observations indicate that metformin targets multiple pathways associated to colorectal cancer development and progression.
1. INTRODUCTION
Despite recent developments in the detection and treatment of colorectal cancer (CRC), this disease is still one of the main causes of cancer-related mortality in the developed world (Ferlay et al., 2013; Siegel, Miller, & Jemal, 2019). Therefore, an understanding of the mechanisms involved in CRC development and progression is needed in order to identify novel targets and therapeutic agents.
Several epidemiological studies indicate that metformin, a widely prescribed antidiabetic drug, exerts a protective effect on different cancers including CRC (Bradley et al., 2018; Chang et al., 2018; Gonzalez et al., 2017; Ikhlas & Ahmad, 2017; Jackson & Garcia-Albeniz, 2018; Klil-Drori, Azoulay, & Pollak, 2017; Kobiela et al., 2018). Importantly, a recent randomized trial showed that low-dose metformin administration to non-diabetic patients markedly decreased metachronous colorectal adenoma/polyp formation (Higurashi et al., 2016). Accordingly, the elucidation of the mechanism(s) by which metformin acts as a chemopreventive agent is of major significance. Although inhibition of the mTORC1 pathway through AMPK-mediated phosphorylation is a prominent mechanism for the anti-proliferative effects of metformin in a variety of cell types (Howell et al., 2017; Rozengurt, Sinnett-Smith, & Kisfalvi, 2010; Soares, Ni, Kisfalvi, Sinnett-Smith, & Rozengurt, 2013) other mechanisms remain unclear, especially those implicated in suppressing cell migration.
Adherens junctions (AJs) are dynamic multiprotein structures that mediate cell-cell adhesions via their binding to the actin cytoskeleton (Bruser & Bogdan, 2017). AJs are also related to CRC growth and distant metastasis (Venhuizen, Jacobs, Span, & Zegers, 2019). The core components of the AJs in epithelial cells are members of the cadherin and catenin families (Daulagala, Bridges, & Kourtidis, 2019). Indeed, expression or surface localization of E-cadherin, a key component of the AJs of the colonic epithelium, is frequently lost or its function disrupted in CRC (Kourtidis, Lu, Pence, & Anastasiadis, 2017; Petrova, Schecterson, & Gumbiner, 2016). β-Catenin, another AJs component that interacts with E-cadherin (Berx & van Roy, 2009; Nelson, 2008), is also frequently absent in the surface of tumor cells, a factor that contributes to diminished cell-cell adhesion and deregulated Wnt signaling (Heuberger & Birchmeier, 2010; Valenta, Hausmann, & Basler, 2012). Another key element of the AJs is p120-catenin, a member of the catenin family that also interacts with E-cadherin and stabilizes the AJs. Recent studies from our laboratory showed that exposure to metformin induce translocation of β-catenin to the plasma membrane (Amable et al., 2019). Accordingly, we examined whether metformin induces re-distribution of E-cadherin and p120-catenin to the plasma membrane in CRC-derived cells.
Focal adhesions (FAs) are integrin-containing multiprotein complexes that connect the cell to the extracellular matrix (ECM). These highly dynamic structures play a key role in cell adhesion, spreading and migration (Berrier & Yamada, 2007). Different lines of evidence support the notion that a crosstalk between AJs and FAs is central to the changes that mediate cell-cell and cell-ECM interactions (Avizienyte & Frame, 2005; Brunton, MacPherson, & Frame, 2004; Canel, Serrels, Frame, & Brunton, 2013; Cicchini et al., 2008; Serrels, Canel, Brunton, & Frame, 2011). Consequently, we hypothesized that metformin coordinately induces rebuilding AJs and down-regulation FA function in CRC-derived cells, thereby leading to inhibition of cell migration. In support of this hypothesis, the results show that treatment of the human CRC-derived SW-480 and HT-29 cells with metformin promoted robust plasma membrane translocation of E-cadherin, p120-catenin and β-catenin, which co-localized along the surface of contacting cells. These changes were accompanied by metformin-induced inhibition of focal adhesion kinase (FAK) and ERK signaling and reduction in the numbers, but an increase in the size, of focal adhesions (FAs). Finally, we show that treatment with metformin suppressed cell migration.
2. Materials and methods
2.1. Cell culture
The human colorectal adenocarcinoma-derived cell lines SW-480 and HT-29 were obtained from the American Type Culture Collection (Manassas, Virginia, USA) and maintained as previously described (Amable et al., 2019). Cells were passed no longer than six months after removal from liquid nitrogen.
2.2. Immunocytochemistry, Western blot, subcellular fractionation and cell imaging
Immunocytochemistry and indirect immunofluorescence were done as previously described (Rey, Young, Cantrell, & Rozengurt, 2001). Western blot analysis was performed as reported (Rey et al., 2001). The immunoblots signals were acquired with a GeneGnome XRQ chemiluminescence imaging system (Syngene) and the intensity of the detected bands quantified with GeneTools software (Syngene)(Martinez-Leon et al., 2019). The Subcellular Protein Fractionation Kit for Cultured Cells from Thermo Scientific (part # 78840), Massachusetts, USA, a widely employed kit to separate cytosolic and membrane fractions -enriched in integral and membrane-associated proteins (Frescas et al., 2017; Y. Kim et al., 2018; Liao et al., 2011; Rosenberg et al., 2018; Roy, Placzek, & Scanlan, 2012; Schernthaner-Reiter, Trivellin, & Stratakis, 2018)- was employed according to the manufacturer’s recommendations. α-Tubulin and the plasma membrane Ca2+ ATPase (PMCA) were used as cytosolic and membrane markers (Li et al., 2007; Sepulveda, Hidalgo-Sanchez, & Mata, 2004) and as controls for loading normalization of the proteins examined in the cytosolic and membrane fractions, i. e. E-cadherin, β-catenin and p120-catenin. Images analysis to assess co-localization of E-cadherin/β-catenin and E-cadherin/p120-catenin was performed using Mander’s overlap coefficient (R) (Manders, Verbeek, & Aten, 1993) as implemented by CoLocalizer Pro v 1.4 (CoLocalization Research Software, Tokyo, Japan). The overlap coefficients have values between 0 and 1; a value of 0 indicates that there is no co-localization, whereas a value of 1.0 means there is complete co-localization. A total of 5 fields, each one containing at least 10 cells, were analyzed per condition as described (Rey, Young, Papazyan, Shapiro, & Rozengurt, 2006). Quantification of FAs number and area (n= 30 cells/condition) was performed as previously described (Horzum, Ozdil, & Pesen-Okvur, 2014).
2.3. Cell wound closure assay
Cells (3×105 cells/ml) were seeded into ibidi-Treat μ-Dish35mm, high Culture-Insert (Ibidi GmbH, Martinsried, Germany) and 24 h later the culture inserts were detached leaving a cell-free gap of 500 μm. The cultures were washed with pre-warmed DMEM and incubated with DMEM plus 10% Fetal Bovine Serum with or without 2.0 mM metformin. Differential contrast phase images were captured every 24 h during 4 days (SW-480 cells) or 5 days (HT-29 cells). Wound-healing/cell migration was quantified with the Wound Healing Automated Cellular Analysis application (Ibidi GmbH, Martinsried, Germany).
2.5. Statistical analysis
Western blots, FAs numbers and size, and cell wound closure assay values represent the mean ± SE and their statistical analysis was performed with Welch’s unequal variances t-tests (Ruxton, 2006; Welch, 1947) applying multiple-testing-correction of the p-values with the Bonferroni’s method (Dunn, 1958). To analyse changes in the protein content proportion/percentage in membrane and cytoplasmic fractions we utilized beta regressions (Douma & Weedon, 2019) since they originate from continuous variables, applying multiple-testing-correction of the p-values with the Bonferroni’s method (Dunn, 1958).
2.6. Materials
Antibodies were obtained from Thermo Fisher Scientific (Waltham, Massachusetts, USA): anti-FAK, anti-phospho FAK Tyr397, anti-phospho FAK Ser910, anti-p120-catenin, anti-paxillin, anti-phospho paxillin Tyr118, Alexa Fluor-conjugated anti-rabbit or anti-mouse IgGs; Abcam Inc. (Cambridge, UK): anti-α-tubulin, anti-E-cadherin, anti-phospho E-cadherin Ser838/840, anti-Ca2+ pan PMCA ATPase; Cell Signaling Technology (Danvers, Massachusetts, USA): anti β-catenin, anti-phospho ERK1/2 Thr202/Tyr204; Santa Cruz Biotech (Dallas, Texas, USA): anti-E-cadherin, anti-ERK 2; GE Healthcare (Little Chalfont, Buckinghamshire, UK): horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgGs. Metformin was from Sigma-Aldrich (St. Louis, Missouri, USA). All other reagents were of the highest grade commercially available.
3. Results and discussion
3.1. Metformin promotes the plasma membrane translocation and co-localization of E-cadherin, β-catenin and p120-catenin
In order to determine whether metformin induces redistribution of E-cadherin from the cytosol to the surface of CRC-derived cells, SW-480 cells were treated with 2 mM metformin and its localization examined by indirect immunofluorescence. As shown in Fig. 1A, E-cadherin was present in the cytoplasm of control cells in a punctuate pattern as well as in their periphery. Treatment with metformin promoted a noticeable redistribution of cytosolic E-cadherin to de novo formed puncta along the length of the contacting membranes. Next, we determined the effect of metformin on the intracellular localization of β-catenin. In agreement with our previous results (Amable et al., 2019), metformin also induced the redistribution of β-catenin to the plasma membrane, specifically at the periphery of contacting cells (Fig. 1A), a localization that coincided with the distribution of E-cadherin (Fig. 1A, insets). Similar intracellular redistribution of E-cadherin and β-catenin was elicited by metformin in HT-29 cells (Fig. 1A), a human CRC-derived cell line with a genetic background distinct from SW-480 cells (Ahmed et al., 2013; Gayet et al., 2001).
Figure 1.
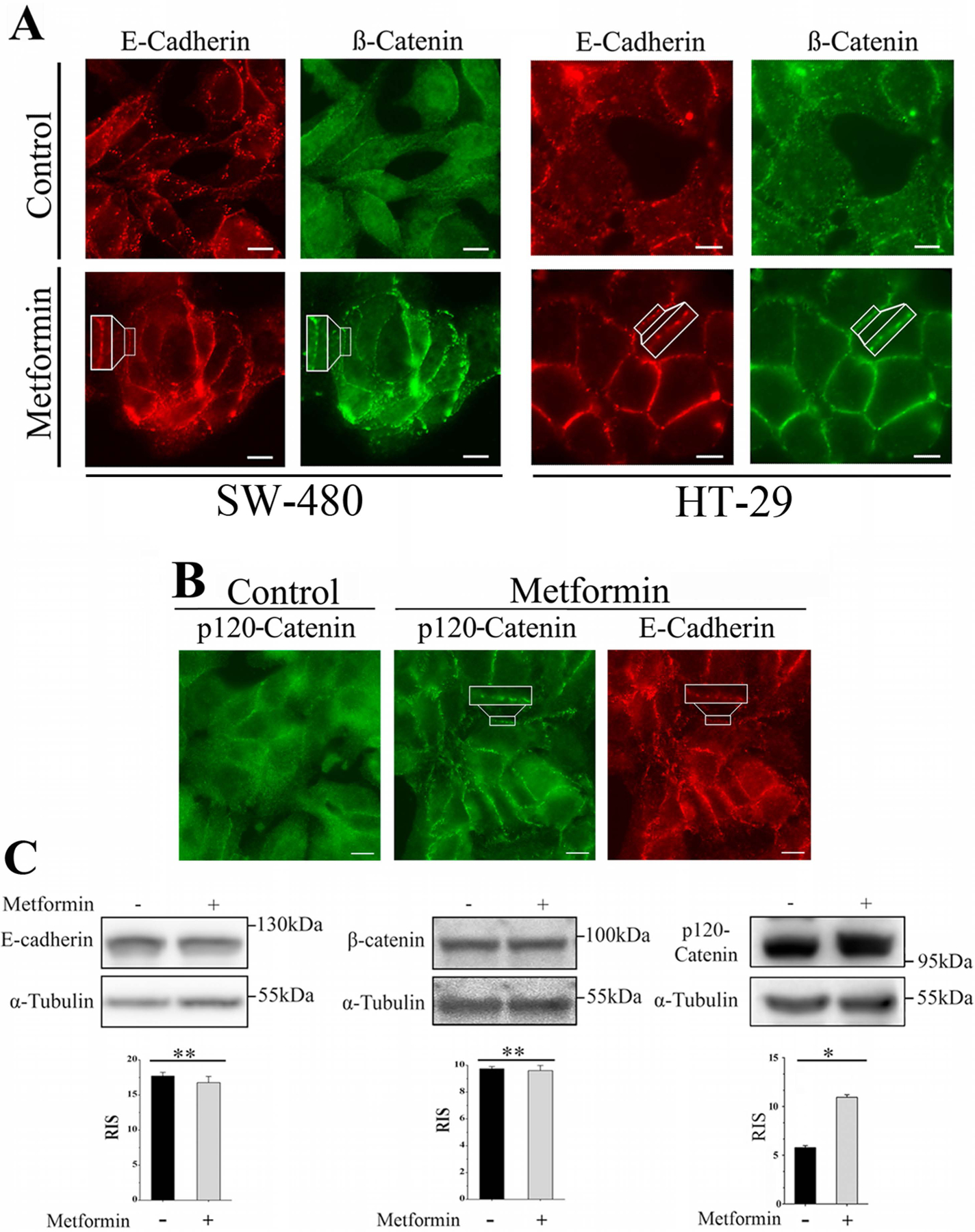
Metformin promotes the plasma membrane translocation of E-cadherin, β-catenin and p120-catenin. (A) SW-480 or HT-29 cells preincubated for 16 h with 2.0 mM metformin were processed for immunofluorescence using a murine monoclonal antibody against E-cadherin and a rabbit polyclonal antibody against β-catenin followed by Alexa Fluor 568 or Alexa Fluor 488 conjugated anti-mouse and anti-rabbit IgGs, respectively. The images are representative of four independent experiments. Bar: 10 μm. (B) SW-480 cells preincubated for 16 h with 2.0 mM metformin were processed for immunofluorescence using a murine monoclonal antibody against p120-catenin and a rabbit antibody against E-cadherin followed by Alexa Fluor 488 or Alexa Fluor 568 conjugated anti-mouse and anti-rabbit IgGs, respectively. The images are representative of four independent experiments. Bar: 10 μm. (C) Total SW-480 cells lysates incubated for 16 h with 2.0 mM metformin were analyzed by Western blot using antibodies against E-cadherin, β-catenin, p120-catenin and α-tubulin. Bars represent the mean ± SE relative cellular content E-cadherin (four experiments), β-catenin (three experiments), and p120-catenin (three experiments) normalized by α-tubulin. p (Bonferroni) **< 0.01, *<0.05. RIS= Relative Intensity Signal.
p120-Catenin is a protein that regulates E-cadherin endocytosis by preventing the binding of endocytic adaptors to its juxtamembrane domain (Bruser & Bogdan, 2017; Cadwell, Su, & Kowalczyk, 2016; Kourtidis, Ngok, & Anastasiadis, 2013; Kowalczyk & Nanes, 2012; Nanes et al., 2012). In view of the results presented in Fig. 1A, we examined whether metformin also influenced the intracellular distribution of p120-catenin. As illustrated by the images presented in Fig. 1B, most of p120-catenin in control cells was detected in the cytoplasm with a minor fraction at the cell periphery consistent with plasma membrane localization. In contrast, a marked p120-catenin shift from the cytosol to the plasma membrane was observed in metformin-treated cells where it co-localized with E-cadherin at discrete regions along the plasma membrane (Fig. 1B, insets). Interestingly, we did not find significant changes in the protein expression level of either E-cadherin or β-catenin in metformin-treated cells, but we detected a ~2-fold increment in the level of p120-catenin (Fig. 1C).
In order to assess the level of co-localization of E-cadherin, β-catenin and p120-catenin promoted by metformin, we performed quantitative image analysis of cells incubated with antibodies against E-cadherin/β-catenin or E-cadherin/p120-catenin followed by Alexa Fluor-conjugated anti-rabbit or anti-mouse IgGs as previously described (Rey et al., 2006). E-cadherin and β-catenin displayed overlap coefficients (R) of 0.8742 ± 0.079 (mean ± SD) for metformin-treated (n=50 cells) versus 0.3513 ± 0.042 for untreated cells (n=50 cells) whereas p120-catenin and E-cadherin increased their overlap coefficients from 0.286 ± 0.062 for untreated cells (n= 50) to 0.687 ± 0.093, for metformin-treated cells (n= 50). Collectively, these results support the notion that metformin promotes the plasma membrane redistribution and co-localization of E-cadherin, β-catenin and p120-catenin.
To further support the observation that metformin promoted the redistribution of E-cadherin, β-catenin and p120-catenin to the plasma membrane, SW-480 cells were fractionated and the distribution of these proteins examined in the cytosolic and membrane fractions of non-treated or metformin-treated cultures. In agreement with the results presented in Fig. 1, metformin promoted a marked redistribution of E-cadherin, β-catenin and p120-catenin to the membrane fraction (Fig. 2A).
Figure 2.
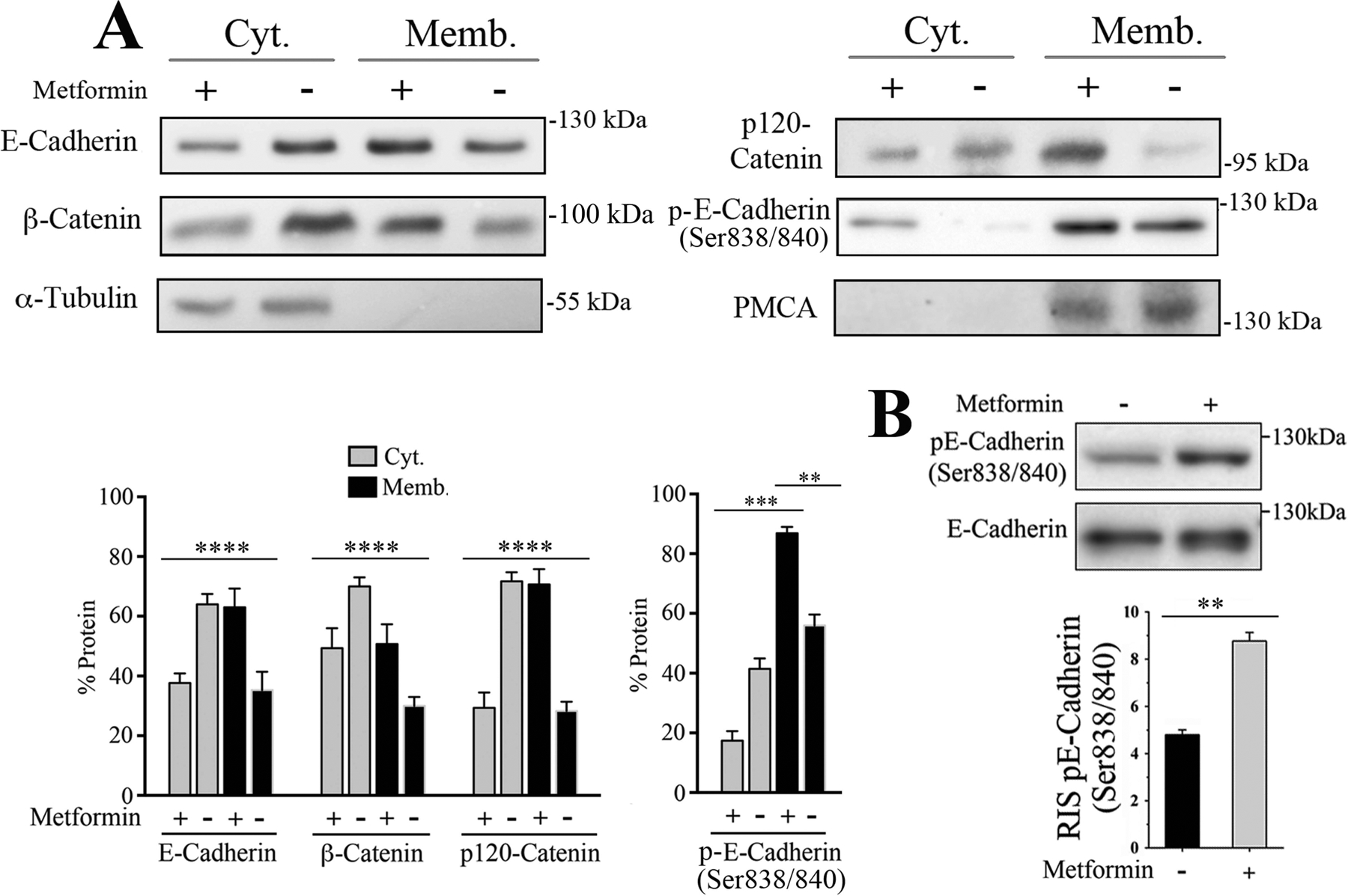
Metformin promotes the intracellular redistribution of E-cadherin, β-catenin and p120-catenin and E-cadherin Ser838/840 phosphorylation. (A) SW-480 cells incubated for 16 with 2.0 mM metformin underwent subcellular fractionation and the content of E-cadherin, β-catenin, p120-catenin and E-cadherin phosphorylated at Ser838/840 determined in the cytosolic and membrane fractions of three independent experiments by Western blot. Bars represent the mean ± SE as percentage of the total (Cyt. + memb.) of each examined protein, with or without metformin treatment. p(Bonferroni) ****<0.0001, ***< 0.001, **<0.01. α-Tubulin and the plasma membrane Ca2+ ATPase (PMCA) were used as cytosolic and membrane markers and as controls for loading normalization. (B) Total SW-480 cells lysates incubated for 16 with 2.0 mM metformin were analyzed by Western blot using rabbit antibodies against E-cadherin phospho-Ser838/840 (pE-Cadherin Ser838/840) and a murine monoclonal antibody against E-cadherin. Bars represent the mean ± SE relative cellular content of three independent experiments normalized by α-tubulin. p (Bonferroni) **< 0.01. RIS= Relative Intensity Signal.
The phosphorylation of the cytoplasmic tail of E-cadherin at Ser838/840 promotes β-catenin binding and interaction stability (Choi, Huber, & Weis, 2006; Dupre-Crochet et al., 2007; Ishiyama & Ikura, 2012; Lickert, Bauer, Kemler, & Stappert, 2000; McEwen, Maher, Mo, & Gottardi, 2014; Pokutta & Weis, 2007; Serres et al., 2000). In view of our results, we examined whether the redistribution of E-cadherin and its co-localization with β-catenin to distinct regions of the plasma membrane in response to metformin was associated with the phosphorylation of E-cadherin cytoplasmic tail. Western blot analysis of cytosolic and membrane fractions and from total cell lysates revealed that E-cadherin phosphorylated at Ser838/840 was predominantly present in the membrane fraction (Fig. 2A) and that metformin promoted a substantial increase in E-cadherin Ser838/840 phosphorylation (Fig. 2A–B). Interestingly, the amino acid sequence surrounding Ser838/840 conforms to a GSK3β recognition site, a kinase that is activated in human colorectal adenocarcinoma-derived cells as a result of metformin treatment (Amable et al., 2019).
3.2. Metformin inhibits FAK signaling and reorganize the FAs
Since crosstalk between AJs and FAs is central to the changes that mediate cell-cell and cell-ECM interactions (Avizienyte & Frame, 2005; Brunton et al., 2004; Canel et al., 2013; Cicchini et al., 2008; Serrels et al., 2011), we next determined whether metformin treatment also modifies the size, number or function of the FAs. FAK, an integral component of the FAs that regulates their maturation and turnover (Lawson et al., 2012; Tomar, Lawson, Ghassemian, & Schlaepfer, 2012), is frequently activated in different types of cancers including CRC (Ashton et al., 2010; Golubovskaya et al., 2012; Sulzmaier, Jean, & Schlaepfer, 2014a; Tai, Lai, Peng, Ding, & Shen, 2016). Accordingly, we first examined the effect of metformin upon FAK activation by examining Tyr397 phosphorylation, an auto-phosphorylation site that regulates downstream signaling (Brunton & Frame, 2008; Jacamo, Jiang, Lunn, & Rozengurt, 2007; Mitra & Schlaepfer, 2006).
Metformin-treated cells showed a significant decrease in FAK Tyr397 phosphorylation (Fig. 3A) suggesting that cell exposure to metformin inhibited FAK catalytic activaty. In order to further support this conclusion, we examined Tyr118 phosphorylation in paxillin, a direct FAK substrate that also forms part of the FAs (Horton et al., 2015). As Fig. 3B shows, metformin inhibited Tyr118 phosphorylation. Moreover, metformin also inhibited FAK Ser910 phosphorylation (Fig. 3C), a modification associated to paxillin/FAK interaction, cell spreading and migration (Chu et al., 2011; Luo, Matthews, Robinson, & Jones, 2019; Vincent & Settleman, 1997). Since FAK Ser910 phosphorylation is mediated by ERK (Hunger-Glaser, Fan, Perez-Salazar, & Rozengurt, 2004; Hunger-Glaser, Salazar, Sinnett-Smith, & Rozengurt, 2003; Jiang, Sinnett-Smith, & Rozengurt, 2007) and ERK signaling is implicated in promoting cell migration (Samson et al., 2019), we examined whether metformin also inhibits this pathway. In agreement with results reported in other cell types (Ming et al., 2014; Zhou et al., 2016), exposure to metformin effectively blocked ERK activity in CRC-derived cells (Fig. 3D).
Figure 3.
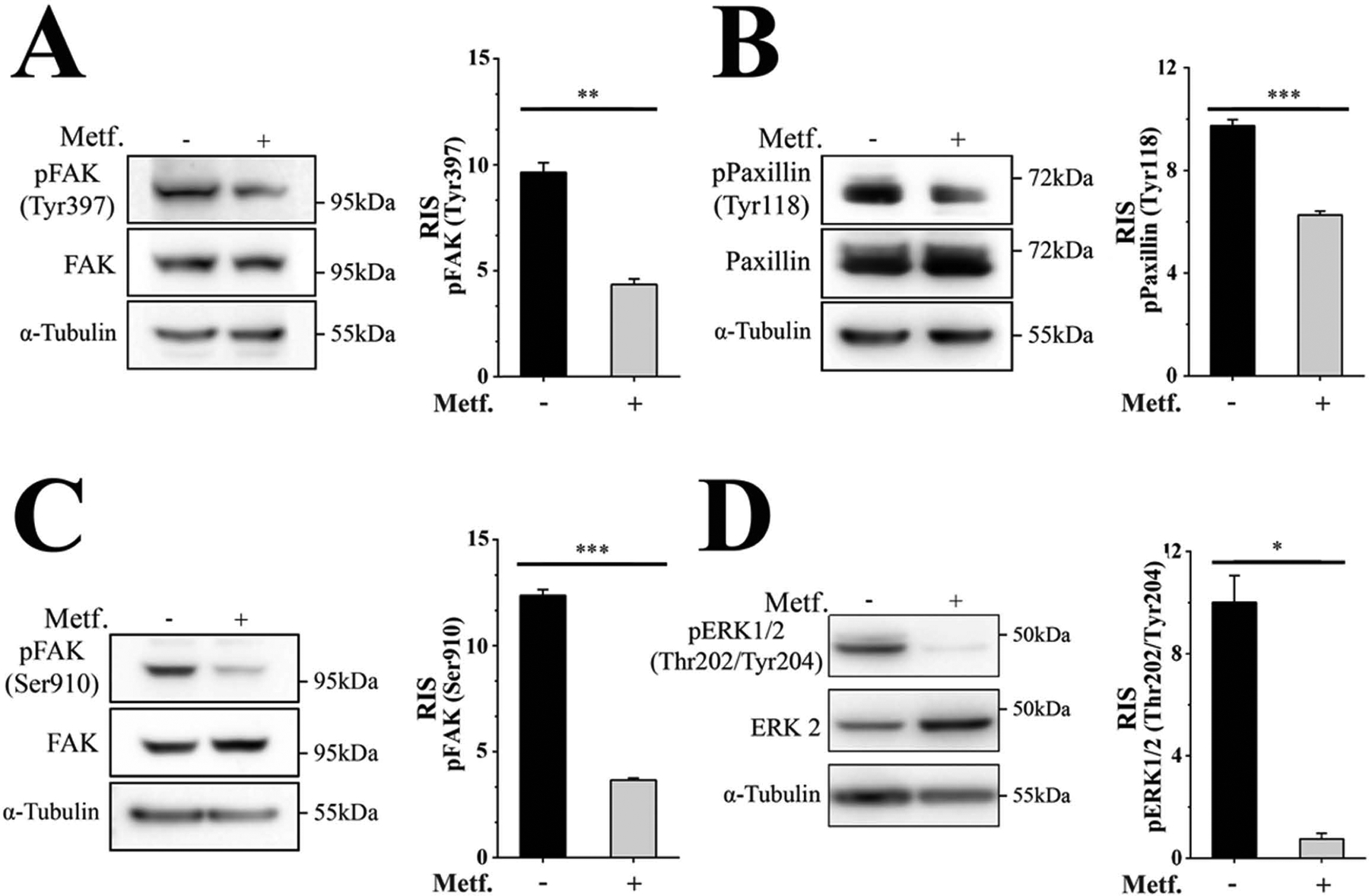
Metformin inhibits FAK and ERK signaling. SW-480 cells incubated for 16 with 2.0 mM metformin were analyzed in three independent experiments by Western blot using rabbit and murine antibodies against FAK phospho-Tyr397, FAK phospho-Ser910, FAK, paxillin phospho-Tyr118, paxillin, ERK1/2 phospho-Thr202/Tyr204, ERK2 and α-tubulin. Bars represent the mean ± SE for each phosphorylated protein normalized by the total counterpart, i. e. FAK, paxillin and ERK2. p(Bonferroni) ***< 0.001, **<0.01, *<0.05. RIS= Relative Intensity Signal.
FAs increase their size upon FAK inhibition or depletion very likely by a mechanism that interferes with FAK-induced FAs turnover (Ilic et al., 1995; Iwanicki et al., 2008; D. H. Kim & Wirtz, 2013; Plotnikov, Pasapera, Sabass, & Waterman, 2012). Because our results indicated that metformin inhibited FAK signaling, we examined whether its inhibition led to FAs structural changes. Untreated or metformin-treated SW-480 cells processed for indirect immunofluorescence were co-stained with antibodies against FAK and paxillin in order to locate and determine FAs numbers and size. As Fig. 4 shows, numerous and small FAs were present in control cells whereas metformin-treated cells showed fewer and larger FAs. Quantitative images analysis (n=30 cells/condition) corroborated that metformin treatment was associated to FAs numbers reduction and size enlargement. Overall, these results imply that metformin reduces the number of FAs while increases their size by a mechanism that involves FAK signaling inhibition.
Figure 4.
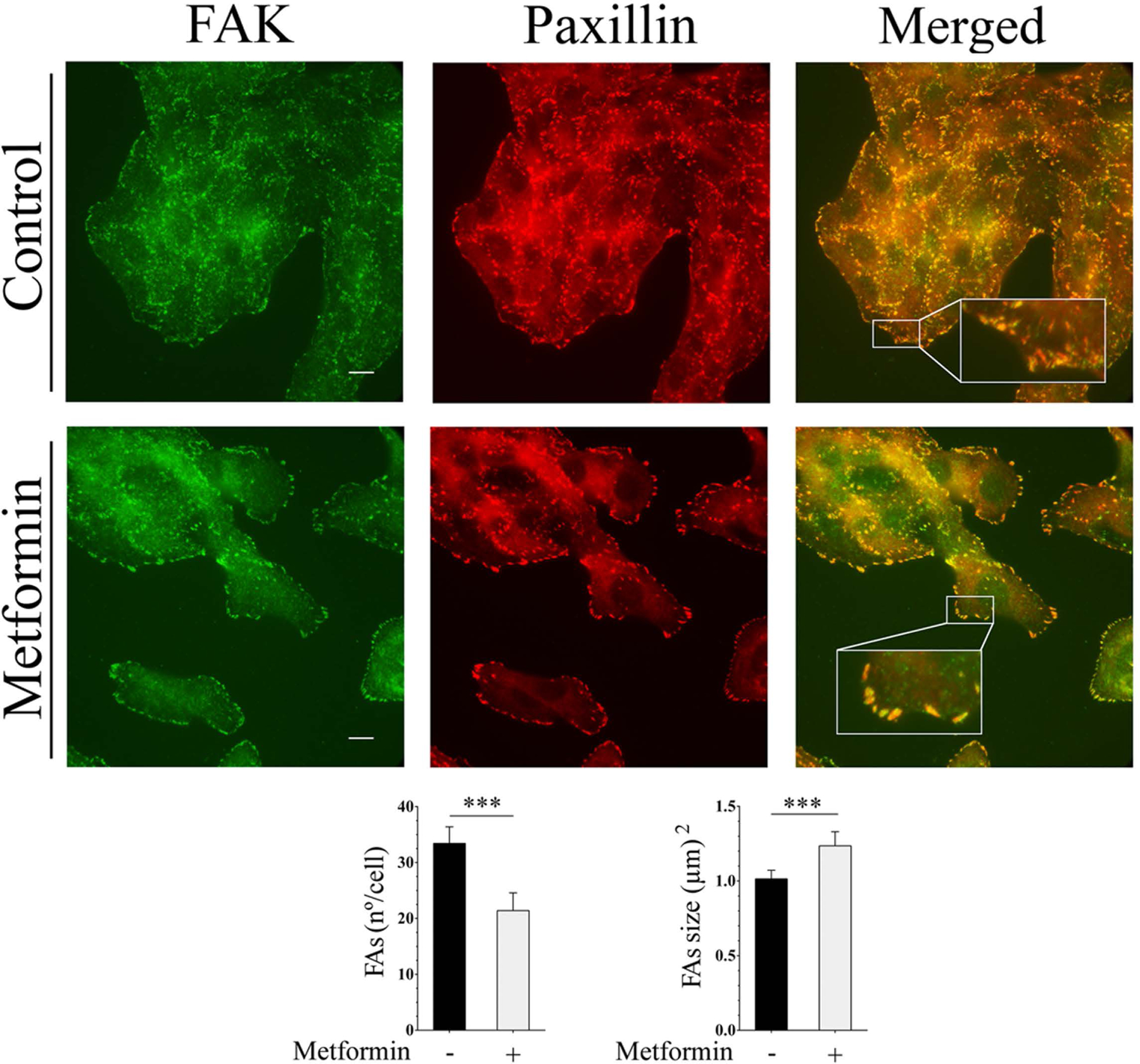
Metformin stimulates the reorganization of FAs. SW-480 cells preincubated for 16 h with 2.0 mM metformin were processed for immunofluorescence analysis using a murine monoclonal antibody against FAK and a rabbit antibody against paxillin followed by Alexa Fluor 488 or Alexa Fluor 568 conjugated anti-mouse and anti-rabbit IgGs, respectively. Bar: 10 μm. FAs numbers and size quantification was performed as described under Materials and Methods. Bars represent the mean ± SE (n=30 cells/condition). p(Bonferroni) ***≤ 0.001.
3.3. Metformin inhibits cell migration
AJs de novo formation and FAK signaling inhibition in response to metformin led us to examine whether these effects would reduce cell migration. In order to examine this possibility, HT-29 and SW-480 cells were seeded into μ-Dish system (Nyegaard, Christensen, & Rasmussen, 2016) and incubated with or without metformin. Differential contrast phase images of the cultures were captured every 24 h during 5 days (HT-29 cells) or 4 days (SW-480 cells). As shown in Fig. 5, the effect of metformin on HT-29 cells started to be evident after 24 h, being greater at 120 h when the wound was completely closed in control cells but remained approximately 40–45% open in metformin-treated cells. A noticeable effect of metformin upon wound closure was evident in SW-480 cells at 72 h reaching a gap of 30–35% at 96 h when the wound was close in control cells further supporting the notion that metformin inhibits CRC-derived cell migration.
Figure 5.
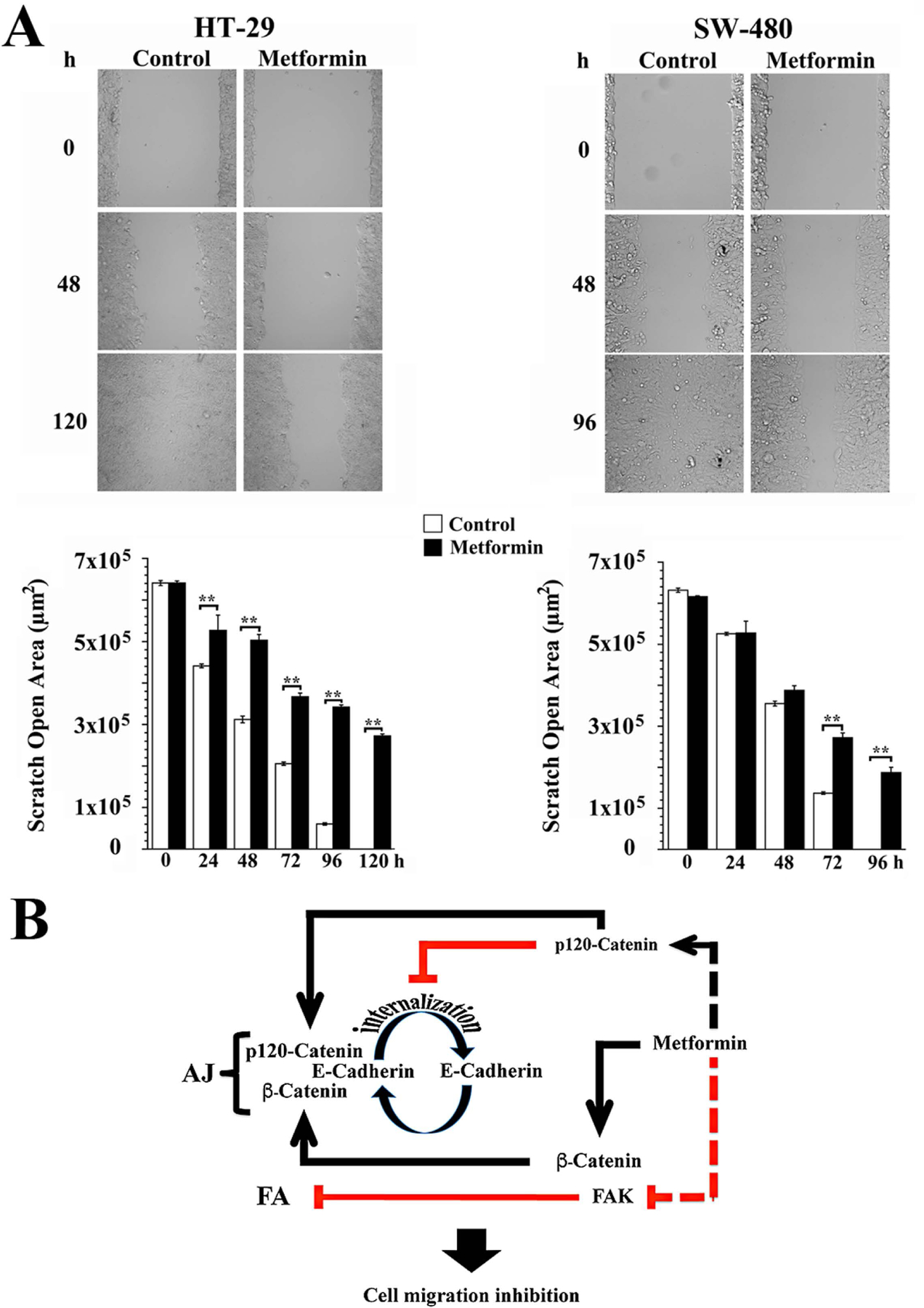
Metformin inhibits cell migration. (A) HT-29 and SW-480 cells were incubated during 120 h and 96 h, respectively, in the presence of 2.0 mM metformin and images acquired every 24 h. Cell migration was quantified using the Wound Healing Automated Cellular Analysis System application (Ibidi GmbH). Values represent the mean ± SE of the scratch open area (μm2) of three independent experiments. p(Bonferroni) **<0.01. (B) Schematic representation of a simplified model of metformin-associated cell migration inhibition. Metformin treatment promotes the up-regulation and plasma membrane translocation of p120-catenin where interacts with E-cadherin and inhibits its endocytosis thereby facilitating the redistribution of E-cadherin/β-catenin to the plasma membrane and AJs rebuilding. Metformin-mediated inhibition of FAK catalytic activity leads to FAs reorganization very likely through a modification of FAs turnover. All these effects converge in the inhibition of CRC-derived cell migration.
4. Concluding remarks
AJs are dynamic multiprotein structures that play a critical role in cell-cell adhesion and are disrupted in inflammation and cancer. E-cadherin, β-catenin and p120 constitute the core elements of AJs. Indeed, several lines of evidence indicate that β‐catenin regulates E-cadherin cell surface availability and function (Ishiyama & Ikura, 2012; McEwen et al., 2014; Pokutta & Weis, 2007; Valenta et al., 2012). Here, we report that treatment of CRC-derived cells with metformin induces redistribution of E-cadherin to the plasma membrane that coincided with the translocation of β-catenin to the same compartment. Moreover expression at the plasma membrane was dramatically enhanced by metformin. Importantly, β-catenin, E-cadherin and p120-catenin co-localized along the length of contacting membranes suggesting that metformin stimulated the formation of AJs. Within this context, and considering the inhibitory role of p120-catenin in cadherin endocytosis and recycling (Bruser & Bogdan, 2017), it is plausible that the increase in the level of p120-catenin induced by metformin treatment facilitates the rebuilding of AJs. TJs and desmosomes, together with AJs, form an apical junction complex that control epithelial barrier function, cell-cell adhesion and signaling (Mehta, Nijhuis, Kumagai, Lindsay, & Silver, 2015; Shigetomi & Ikenouchi, 2019). Interestingly, several studies indicate that AMPK signaling exerts a protective effect on intestinal barrier function by a mechanism that stimulates the formation of TJs (Chen et al., 2018; Peng, Li, Green, Holzman, & Lin, 2009; Wu, Wang, Liu, Shan, & Wang, 2018; Zhang, Li, Young, & Caplan, 2006). Since TJs assembly is coupled to AJs formation (Campbell, Maiers, & DeMali, 2017), it is conceivable that the formation of AJs in response to metformin contributes to TJs assembly and intestinal barrier recovery after injury.
FAK occupies a central node in various signaling pathways that control tumor growth and metastasis (Canel et al., 2010; Sulzmaier, Jean, & Schlaepfer, 2014b; Tai, Chen, & Shen, 2015). For example, FAK null mice fibroblasts showed a reduced rate of migration associated to FAs reorganization (Ilic et al., 1995) while FAK deficient cancer cells display large FAs and reduced motility (Chan, Cortesio, & Huttenlocher, 2009; Hsia et al., 2003; Huttenlocher & Horwitz, 2011; Webb et al., 2004). Very little is known about the impact of metformin on FAK regulation. Previous studies reported that metformin inhibited FAK phosphorylation in ovarian (Erices et al., 2017) and prostatic cancer cells (Yu et al., 2017) but its impact on CRC-derived cells was unknown. Here, we found that metformin markedly reduced FAK autophosphorylation at Tyr397, a site that plays a critical role in FAK signaling. In line with this conclusion, metformin also inhibited the phosphorylation of the FA-associated protein paxillin at Tyr118, a residue targeted by FAK/Src (Zhao & Guan, 2011). Based on our findings and current literature, we propose a scheme model (Fig. 5B) to explain the inhibitory effect of metformin on CRC-derived cells migration. In this simplified model, metformin treatment promotes the up-regulation and plasma membrane translocation of p120-catenin. Once in the plasma membrane, p120-catenin interacts with E-cadherin and inhibits its endocytosis thereby facilitating the redistribution of E-cadherin/β-catenin to the plasma membrane and therefore AJs rebuilding. Inhibition of FAK catalytic activity and Tyr397 phosphorylation in response to metformin leads to FAs reorganization very likely through a modification of FAs turnover. All these effects converge in the inhibition of CRC-derived cell migration. Further experiments expressing non- and phosphorylatable forms of E-cadherin and FAK in CRC-derived cells with the corresponding endogenous proteins knocked-out, as well as studies on metformin-mediated p120-catenin up-regulation, are needed to reach a better understanding of the molecular mechanisms mediating metformin effects on AJ and FA.
Previous studies with metformin in cultured cells have used concentrations 10–100-fold higher than those found in the serum of type 2 diabetes mellitus patients receiving the recommended doses of this drug leading to conclusions that remain controversial (He and Wondisford, 2015), a caveat that does not apply to our studies since we employed metformin concentrations within the range of those found in human and rodent intestines after administration of therapeutic doses of metformin (Bailey, Wilcock, & Scarpello, 2008; Paleari et al., 2018; Wilcock & Bailey, 1994). Collectively, the results presented here indicate that metformin targets key pathways associated to CRC development, progression and dissemination.
ACKNOWLEDGEMENTS
Support from the Sistema Nacional de Microscopía de la Secretaría de Ciencia, Tecnología e Innovación Productiva, Argentina, and the Microscopy and Imaging Core of the INIGEM is gratefully acknowledged.
FUNDING
This study was supported by PICT 2013-0891 and PICT 2019-00064 from the Fondo para la Investigación Científica y Tecnológica, Secretaría de Ciencia Tecnología e Innovación Productiva, Argentina. E.R. is supported by a National Institutes of Health grant DK041301 and VA Merit Review I01BX003801. E. M. L. and G. A. are recipients of fellowship awards from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest with the contents of this article.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknaes M, Hektoen M, … Lothe RA (2013). Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis, 2, e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amable G, Martinez-Leon E, Picco ME, Di Siervi N, Davio C, Rozengurt E, & Rey O (2019). Metformin inhibits β-catenin phosphorylation on Ser-552 through an AMPK/PI3K/Akt pathway in colorectal cancer cells. International Journal of Biochemistry and Cell Biology, 112, 88–94. [DOI] [PubMed] [Google Scholar]
- Ashton GH, Morton JP, Myant K, Phesse TJ, Ridgway RA, Marsh V, … Sansom OJ (2010). Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Developmental Cell, 19(2), 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avizienyte E, & Frame MC (2005). Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Current Opinion in Cell Biology, 17(5), 542–547. [DOI] [PubMed] [Google Scholar]
- Bailey CJ, Wilcock C, & Scarpello JH (2008). Metformin and the intestine. Diabetologia, 51(8), 1552–1553. [DOI] [PubMed] [Google Scholar]
- Berrier AL, & Yamada KM (2007). Cell-matrix adhesion. Journal of Cellular Physiology, 213(3), 565–573. [DOI] [PubMed] [Google Scholar]
- Berx G, & van Roy F (2009). Involvement of members of the cadherin superfamily in cancer. Cold Spring Harbor Perspectives in Biology, 1(6), a003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MC, Ferrara A, Achacoso N, Ehrlich SF, Quesenberry CP Jr., & Habel LA (2018). A cohort study of metformin and colorectal cancer risk among patients with Diabetes Mellitus. Cancer Epidemiology, Biomarkers and Prevention, 27(5), 525–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton VG, & Frame MC (2008). Src and focal adhesion kinase as therapeutic targets in cancer. Current Opinion in Pharmacology, 8(4), 427–432. [DOI] [PubMed] [Google Scholar]
- Brunton VG, MacPherson IR, & Frame MC (2004). Cell adhesion receptors, tyrosine kinases and actin modulators: a complex three-way circuitry. Biochemical and Biophysical Acta, 1692(2–3), 121–144. [DOI] [PubMed] [Google Scholar]
- Bruser L, & Bogdan S (2017). Adherens Junctions on the move-membrane trafficking of E-cadherin. Cold Spring Harbor Perspectives in Biology, 9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell CM, Su W, & Kowalczyk AP (2016). Cadherin tales: Regulation of cadherin function by endocytic membrane trafficking. Traffic, 17(12), 1262–1271. [DOI] [PubMed] [Google Scholar]
- Campbell HK, Maiers JL, & DeMali KA (2017). Interplay between tight junctions & adherens junctions. Experimental Cell Research, 358(1), 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canel M, Serrels A, Frame MC, & Brunton VG (2013). E-cadherin-integrin crosstalk in cancer invasion and metastasis. Journal of Cell Science, 126(Pt 2), 393–401. [DOI] [PubMed] [Google Scholar]
- Canel M, Serrels A, Miller D, Timpson P, Serrels B, Frame MC, & Brunton VG (2010). Quantitative in vivo imaging of the effects of inhibiting integrin signaling via Src and FAK on cancer cell movement: effects on E-cadherin dynamics. Cancer Research, 70(22), 9413–9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KT, Cortesio CL, & Huttenlocher A (2009). FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. Journal of Cell Biology, 185(2), 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YT, Tsai HL, Kung YT, Yeh YS, Huang CW, Ma CJ, … Wang JY (2018). Dose-dependent relationship between metformin and colorectal cancer occurrence among Patients with Type 2 Diabetes-A nationwide cohort study. Translational Oncology, 11(2), 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Wang J, You Q, He S, Meng Q, Gao J, … Xu Q (2018). Activating AMPK to restore tight junction assembly in intestinal epithelium and to attenuate experimental colitis by metformin. Frontiers in Pharmacology, 9, 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Huber AH, & Weis WI (2006). Thermodynamics of β-catenin-ligand interactions: the roles of the N- and C-terminal tails in modulating binding affinity. The Journal of Biological Chemistry, 281(2), 1027–1038. [DOI] [PubMed] [Google Scholar]
- Chu M, Iyengar R, Koshman YE, Kim T, Russell B, Martin JL, … Samarel AM (2011). Serine-910 phosphorylation of focal adhesion kinase is critical for sarcomere reorganization in cardiomyocyte hypertrophy. Cardiovascular Research, 92(3), 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, … Tripodi M (2008). TGFβ-induced EMT requires focal adhesion kinase (FAK) signaling. Experimental Cell Research, 314(1), 143–152. [DOI] [PubMed] [Google Scholar]
- Daulagala AC, Bridges MC, & Kourtidis A (2019). E-cadherin beyond structure: A Signaling hub in colon homeostasis and disease. International Journal of Molecular Science, 20(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douma JC, & Weedon JT (2019). Analysing continuous proportions in ecology and evolution: A practical introduction to β and Dirichlet regression. Methods in Ecology and Evolution, 10, 1412–1430. [Google Scholar]
- Dunn OJ (1958). Estimation of the means for dependent variables. Annals of Mathematical Statistics, 29(4), 1095–1111. [Google Scholar]
- Dupre-Crochet S, Figueroa A, Hogan C, Ferber EC, Bialucha CU, Adams J, … Fujita Y (2007). Casein kinase 1 is a novel negative regulator of E-cadherin-based cell-cell contacts. Molecular and Cellular Biology, 27(10), 3804–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erices R, Cubillos S, Aravena R, Santoro F, Marquez M, Orellana R, … Owen GI (2017). Diabetic concentrations of metformin inhibit platelet-mediated ovarian cancer cell progression. Oncotarget, 8(13), 20865–20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, … Bray F (2013). Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. European Journal of Cancer, 49(6), 1374–1403. [DOI] [PubMed] [Google Scholar]
- Frescas D, Roux CM, Aygun-Sunar S, Gleiberman AS, Krasnov P, Kurnasov OV, … Gudkov AV (2017). Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody. Proceedings of the National Academy of Sciences of the United States of America, 114(9), E1668–E1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayet J, Zhou XP, Duval A, Rolland S, Hoang JM, Cottu P, & Hamelin R (2001). Extensive characterization of genetic alterations in a series of human colorectal cancer cell lines. Oncogene, 20(36), 5025–5032. [DOI] [PubMed] [Google Scholar]
- Golubovskaya VM, Figel S, Ho BT, Johnson CP, Yemma M, Huang G, … Cance WG (2012). A small molecule focal adhesion kinase (FAK) inhibitor, targeting Y397 site: 1-(2-hydroxyethyl)-3, 5, 7-triaza-1-azoniatricyclo [3.3.1.1(3,7)]decane; bromide effectively inhibits FAK autophosphorylation activity and decreases cancer cell viability, clonogenicity and tumor growth in vivo. Carcinogenesis, 33(5), 1004–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez N, Prieto I, Del Puerto-Nevado L, Portal-Nunez S, Ardura JA, Corton M, … Ortiz A (2017). 2017 update on the relationship between diabetes and colorectal cancer: epidemiology, potential molecular mechanisms and therapeutic implications. Oncotarget, 8(11), 18456–18485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuberger J, & Birchmeier W (2010). Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harbor Perspectives in Biology, 2(2), a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higurashi T, Hosono K, Takahashi H, Komiya Y, Umezawa S, Sakai E, … Nakajima A (2016). Metformin for chemoprevention of metachronous colorectal adenoma or polyps in post-polypectomy patients without diabetes: a multicentre double-blind, placebo-controlled, randomised phase 3 trial. The Lancet. Oncology, 17(4), 475–483. [DOI] [PubMed] [Google Scholar]
- Horton ER, Byron A, Askari JA, Ng DHJ, Millon-Fremillon A, Robertson J, … Humphries MJ (2015). Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nature Cell Biology, 17(12), 1577–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horzum U, Ozdil B, & Pesen-Okvur D (2014). Step-by-step quantitative analysis of focal adhesions. Methods X, 1, 56–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell JJ, Hellberg K, Turner M, Talbott G, Kolar MJ, Ross DS, … Manning BD (2017). Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metabolism, 25(2), 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, … Schlaepfer DD (2003). Differential regulation of cell motility and invasion by FAK. Journal of Cell Biology, 160(5), 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunger-Glaser I, Fan RS, Perez-Salazar E, & Rozengurt E (2004). PDGF and FGF induce focal adhesion kinase (FAK) phosphorylation at Ser-910: dissociation from Tyr-397 phosphorylation and requirement for ERK activation. Journal of Cellular Physiology, 200(2), 213–222. [DOI] [PubMed] [Google Scholar]
- Hunger-Glaser I, Salazar EP, Sinnett-Smith J, & Rozengurt E (2003). Bombesin, lysophosphatidic acid, and epidermal growth factor rapidly stimulate focal adhesion kinase phosphorylation at Ser-910: requirement for ERK activation. The Journal of Biological Chemistry, 278(25), 22631–22643. [DOI] [PubMed] [Google Scholar]
- Huttenlocher A, & Horwitz AR (2011). Integrins in cell migration. Cold Spring Harbor Perspectives in Biology, 3(9), a005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikhlas S, & Ahmad M (2017). Metformin: Insights into its anticancer potential with special reference to AMPK dependent and independent pathways. Life Sciences, 185, 53–62. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, … Yamamoto T (1995). Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature, 377(6549), 539–544. [DOI] [PubMed] [Google Scholar]
- Ishiyama N, & Ikura M (2012). The three-dimensional structure of the cadherin-catenin complex. Subcellular Biochemistry, 60, 39–62. [DOI] [PubMed] [Google Scholar]
- Iwanicki MP, Vomastek T, Tilghman RW, Martin KH, Banerjee J, Wedegaertner PB, & Parsons JT (2008). FAK, PDZ-RhoGEF and ROCKII cooperate to regulate adhesion movement and trailing-edge retraction in fibroblasts. Journal of Cell Science, 121(Pt 6), 895–905. [DOI] [PubMed] [Google Scholar]
- Jacamo R, Jiang X, Lunn JA, & Rozengurt E (2007). FAK phosphorylation at Ser-843 inhibits Tyr-397 phosphorylation, cell spreading and migration. Journal of Cellular Physiology, 210(2), 436–444. [DOI] [PubMed] [Google Scholar]
- Jackson JW, & Garcia-Albeniz X (2018). Studying the effects of nonindicated medications on cancer: Etiologic versus action-focused analysis of epidemiologic data. Cancer Epidemiology, Biomarkers & Prevention 27(5), 520–524. [DOI] [PubMed] [Google Scholar]
- Jiang X, Sinnett-Smith J, & Rozengurt E (2007). Differential FAK phosphorylation at Ser-910, Ser-843 and Tyr-397 induced by angiotensin II, LPA and EGF in intestinal epithelial cells. Cell Signaling, 19(5), 1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, & Wirtz D (2013). Focal adhesion size uniquely predicts cell migration. FASEB Journal, 27(4), 1351–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Park J, Kim S, Kim M, Kang MG, Kwak C, … Kim VN (2018). PKR senses nuclear and mitochondrial signals by Interacting with endogenous double-stranded RNAs. Molecular Cell, 71(6), 1051–1063 [DOI] [PubMed] [Google Scholar]
- Klil-Drori AJ, Azoulay L, & Pollak MN (2017). Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nature Reviews: Clinical Oncology, 14(2), 85–99. [DOI] [PubMed] [Google Scholar]
- Kobiela J, Dobrzycka M, Jedrusik P, Kobiela P, Spychalski P, Sledzinski Z, & Zdrojewski T (2018). Metformin and colorectal cancer - A systematic review. Experimental and Clinical Endocrinology & Diabetes, 127 (07), 445–454. [DOI] [PubMed] [Google Scholar]
- Kourtidis A, Lu R, Pence LJ, & Anastasiadis PZ (2017). A central role for cadherin signaling in cancer. Experimental Cell Research, 358(1), 78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtidis A, Ngok SP, & Anastasiadis PZ (2013). p120-catenin: an essential regulator of cadherin stability, adhesion-induced signaling, and cancer progression. Progress in Molecular Biology and Translational Science, 116, 409–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk AP, & Nanes BA (2012). Adherens junction turnover: regulating adhesion through cadherin endocytosis, degradation, and recycling. Subcellular Biochemistry, 60, 197–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson C, Lim ST, Uryu S, Chen XL, Calderwood DA, & Schlaepfer DD (2012). FAK promotes recruitment of talin to nascent adhesions to control cell motility. Journal of Cell Biology, 196(2), 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CC, Chiang TC, Wu TS, Pacheco-Rodriguez G, Moss J, & Lee FJ (2007). ARL4D recruits cytohesin-2/ARNO to modulate actin remodeling. Molecular Biology of the Cell, 18(11), 4420–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao A, Broeg K, Fox T, Tan SF, Watters R, Shah MV, … Liu X (2011). Therapeutic efficacy of FTY720 in a rat model of NK-cell leukemia. Blood, 118(10), 2793–2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lickert H, Bauer A, Kemler R, & Stappert J (2000). Casein kinase II phosphorylation of E-cadherin increases E-cadherin/β-catenin interaction and strengthens cell-cell adhesion. The Journal of Biological Chemistry, 275(7), 5090–5095. [DOI] [PubMed] [Google Scholar]
- Luo L, Matthews JD, Robinson BS, & Jones RM (2019). Vibrio parahaemolyticus VopA is a potent inhibitor of cell migration and apoptosis in the intestinal epithelium of Drosophila melanogaster. Infectection and Immunity, 87(3), e00669–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manders EMM, Verbeek FJ, & Aten JA (1993). Measurement of colocalization of objects in dual-color confocal images. Journal of Microscopy, 169(3), 375–382. [DOI] [PubMed] [Google Scholar]
- Martinez-Leon E, Amable G, Jacamo R, Picco ME, Anaya L, Rozengurt E, & Rey O (2019). Protein kinase D1 inhibition interferes with mitosis progression. Journal of Cell Physiology, 234(11), 20510–20519. [DOI] [PubMed] [Google Scholar]
- McEwen AE, Maher MT, Mo R, & Gottardi CJ (2014). E-cadherin phosphorylation occurs during its biosynthesis to promote its cell surface stability and adhesion. Molecular Biology of the Cell, 25(16), 2365–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta S, Nijhuis A, Kumagai T, Lindsay J, & Silver A (2015). Defects in the adherens junction complex (E-cadherin/β-catenin) in inflammatory bowel disease. Cell Tissue Research, 360(3), 749–760. [DOI] [PubMed] [Google Scholar]
- Ming M, Sinnett-Smith J, Wang J, Soares HP, Young SH, Eibl G, & Rozengurt E (2014). Dose-Dependent AMPK-dependent and independent mechanisms of berberine and metformin inhibition of mTORC1, ERK, DNA synthesis and proliferation in pancreatic cancer cells. PLoS One, 9(12), e114573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, & Schlaepfer DD (2006). Integrin-regulated FAK-Src signaling in normal and cancer cells. Current Opinion in Cell Biology, 18(5), 516–523. [DOI] [PubMed] [Google Scholar]
- Nanes BA, Chiasson-MacKenzie C, Lowery AM, Ishiyama N, Faundez V, Ikura M, … Kowalczyk AP (2012). p120-catenin binding masks an endocytic signal conserved in classical cadherins. Journal of Cell Biology, 199(2), 365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ (2008). Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochemical Society Transactions, 36(Pt 2), 149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyegaard S, Christensen B, & Rasmussen JT (2016). An optimized method for accurate quantification of cell migration using human small intestine cells. Metabolic Engineering Communications, 3, 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paleari L, Burhenne J, Weiss J, Foersch S, Roth W, Parodi A, … DeCensi A (2018). High Accumulation of metformin in colonic tissue of subjects with diabetes or the metabolic syndrome. Gastroenterology, 154(5), 1543–1545. 0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng L, Li ZR, Green RS, Holzman IR, & Lin J (2009). Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. The Journal of Nutrition, 139(9), 1619–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova YI, Schecterson L, & Gumbiner BM (2016). Roles for E-cadherin cell surface regulation in cancer. Molecular Biology of the Cell, 27(21), 3233–3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov SV, Pasapera AM, Sabass B, & Waterman CM (2012). Force fluctuations within focal adhesions mediate ECM-rigidity sensing to guide directed cell migration. Cell, 151(7), 1513–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, & Weis WI (2007). Structure and mechanism of cadherins and catenins in cell-cell contacts. Annual Review of Cell and Developmental Biology, 23, 237–261. [DOI] [PubMed] [Google Scholar]
- Rey O, Young SH, Cantrell D, & Rozengurt E (2001). Rapid protein kinase D translocation in response to G protein-coupled receptor activation: dependence on protein kinase Cε. The Journal of Biological Chemistry, 276(35), 32616–32626. [DOI] [PubMed] [Google Scholar]
- Rey O, Young SH, Papazyan R, Shapiro MS, & Rozengurt E (2006). Requirement of the TRPC1 Cation Channel in the Generation of Transient Ca2+ Oscillations by the Calcium-sensing Receptor. The Journal of Biological Chemistry, 281(50), 38730–38737. [DOI] [PubMed] [Google Scholar]
- Rosenberg S, Simeonova I, Bielle F, Verreault M, Bance B, Le Roux I, … Sanson M (2018). A recurrent point mutation in PRKCA is a hallmark of chordoid gliomas. Nature Communications, 9(1), 2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy G, Placzek E, & Scanlan TS (2012). ApoB-100-containing lipoproteins are major carriers of 3-iodothyronamine in circulation. The Journal of Biological Chemistry, 287(3), 1790–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E, Sinnett-Smith J, & Kisfalvi K (2010). Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clinical Cancer Research, 16(9), 2505–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruxton GD (2006). The unequal variance t-test is an underused alternative to Student’s t-test and the Mann–Whitney U test. Behavorial Ecology, 17(4), 688–690. [Google Scholar]
- Samson SC, Elliott A, Mueller BD, Kim Y, Carney KR, Bergman JP, … Mendoza MC (2019). p90 ribosomal S6 kinase (RSK) phosphorylates myosin phosphatase and thereby controls edge dynamics during cell migration. The Journal of Biological Chemistry, 294(28), 10846–10862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schernthaner-Reiter MH, Trivellin G, & Stratakis CA (2018). Interaction of AIP with protein kinase A (cAMP-dependent protein kinase). Human Molecular Genetics, 27(15), 2604–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda MR, Hidalgo-Sanchez M, & Mata AM (2004). Localization of endoplasmic reticulum and plasma membrane Ca2+-ATPases in subcellular fractions and sections of pig cerebellum. European Journal of Neuroscience, 19(3), 542–551. [DOI] [PubMed] [Google Scholar]
- Serrels A, Canel M, Brunton VG, & Frame MC (2011). Src/FAK-mediated regulation of E-cadherin as a mechanism for controlling collective cell movement: insights from in vivo imaging. Cell Adhesion and Migration, 5(4), 360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serres M, Filhol O, Lickert H, Grangeasse C, Chambaz EM, Stappert J, … Schmitt D (2000). The disruption of adherens junctions is associated with a decrease of E-cadherin phosphorylation by protein kinase CK2. Experimental Cell Research, 257(2), 255–264. [DOI] [PubMed] [Google Scholar]
- Shigetomi K, & Ikenouchi J (2019). Cell adhesion Sstructures in epithelial cells are formed in dynamic and cooperative ways. Bioessays, e1800227. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, & Jemal A (2019). Cancer statistics, 2019. CA: A Cancer Journal for Clinicians, 69(1), 7–34. [DOI] [PubMed] [Google Scholar]
- Soares HP, Ni Y, Kisfalvi K, Sinnett-Smith J, & Rozengurt E (2013). Different patterns of Akt and ERK feedback activation in response to rapamycin, active-site mTOR inhibitors and metformin in pancreatic cancer cells. PLoS One, 8(2), e57289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzmaier FJ, Jean C, & Schlaepfer DD (2014a). FAK in cancer: mechanistic findings and clinical applications. Nature Reviews Cancer, 14(9), 598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YL, Chen LC, & Shen TL (2015). Emerging roles of focal adhesion kinase in cancer. Biomed Research International, 2015, doi: 10.1155/2015/690690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YL, Lai IR, Peng YJ, Ding ST, & Shen TL (2016). Activation of focal adhesion kinase through an interaction with β4 integrin contributes to tumorigenicity of colon cancer. FEBS Letters, 590(12), 1826–1837. [DOI] [PubMed] [Google Scholar]
- Tomar A, Lawson C, Ghassemian M, & Schlaepfer DD (2012). Cortactin as a target for FAK in the regulation of focal adhesion dynamics. PLoS One, 7(8), e44041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenta T, Hausmann G, & Basler K (2012). The many faces and functions of β-catenin. EMBO Journal, 31(12), 2714–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venhuizen JH, Jacobs FJC, Span PN, & Zegers MM (2019). p120 and E-cadherin: Double-edged swords in tumor metastasis. Semininars in Cancer Biology doi: 10.1016/j.semcancer.2019.07.020 [DOI] [PubMed] [Google Scholar]
- Vincent S, & Settleman J (1997). The PRK2 kinase is a potential effector target of both Rho and Rac GTPases and regulates actin cytoskeletal organization. Molecular and Cellular Biology, 17(4), 2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, & Horwitz AF (2004). FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nature Cell Biology, 6(2), 154–161. [DOI] [PubMed] [Google Scholar]
- Welch BL (1947). The generalisation of student’s problems when several different population variances are involved. Biometrika, 34(1–2), 28–35. [DOI] [PubMed] [Google Scholar]
- Wilcock C, & Bailey CJ (1994). Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica; the fate of foreign compounds in biological systems, 24(1), 49–57. [DOI] [PubMed] [Google Scholar]
- Wu W, Wang S, Liu Q, Shan T, & Wang Y (2018). Metformin protects against LPS-induced intestinal barrier dysfunction by activating AMPK pathway. Molecular Pharmaceutics, 15(8), 3272–3284. [DOI] [PubMed] [Google Scholar]
- Yu T, Wang C, Yang J, Guo Y, Wu Y, & Li X (2017). Metformin inhibits SUV39H1-mediated migration of prostate cancer cells. Oncogenesis, 6(5), e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li J, Young LH, & Caplan MJ (2006). AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proceedings of the National Academy of Sciences of the United States of America, 103(46), 17272–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, & Guan JL (2011). Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Advanced Drug Delivery Reviews, 63(8), 610–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Yu J, Wang A, Liu SH, Sinnett-Smith J, Wu J, … Brunicardi FC (2016). Metformin restrains pancreatic duodenal homeobox-1 (PDX-1) function by inhibiting ERK signaling in pancreatic ductal adenocarcinoma. Current Molecular Medicine, 16(1), 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]


