Abstract
In the hospital department dedicated to COVID-19-patient, infection prevention and control measures were upgraded. Therefore, the cross-transmission of other micro-organisms was thought unlikely to occur. However, we report an outbreak of NDM-5-producing Escherichia. coli in a 12-beds ICU dedicated to COVID-19 patients. This outbreak involved 6 patients of which 5 were asymptomatic carriers and 1 was infected. Several findings might have contributed to cross-transmission including the multiple-bedroom configuration of the department, uncomplete compliance for standard and contact precautions, overwork due to the burden of the disease, lack of training of staff for the care of ICU-patients, and misuse of gloves. Furthermore, as infection prevention and control measures were thought to be applied, contact patients were not screened for eXDR carriage. Applying rigorously standard and contact precautions and performing screening in contact patients when indicated must be the rules in COVID-19 wards.
Key Words: Coronavirus, Pandemic, eXDR, CPE, SARS-CoV-2, Infection prevention and control
INTRODUCTION
Due to its high morbidity and mortality, coronavirus disease (COVID-19) has led to a dramatic increase in the number of hospitalized patients in intensive care units (ICU).1 In order to limit the spread of SARS-CoV-2 to other patients and health care workers, infection prevention and control (IPC) measures (ie, contact and respiratory precautions) should be rigorously applied.2 , 3 Furthermore, the hospitals were organized with specific departments dedicated to SARS-CoV-2-infected patients (COVID-19-departments). As this context implies upgraded IPC measures, the risk of hospital-transmission of other micro-organisms such as extremely drug-resistant micro-organisms (eXDR), that is, carbapenemase-producing Enterobacterales (CPE) and vancomycin-resistant Enterococcus might be reduced in COVID-19-departments. However, these units also experienced overwork due to the rapid increase of patients' admissions and shortages in personal protective equipment (PPE) which could play a role in hospital-transmissions.4 , 5 In this particular context, we describe an outbreak of an NDM-5-producing Escherichia coli occurring in an ICU dedicated to COVID-19-patients.
METHODS
CPE screening
As recommended by French guidelines, CPE carriage was screened in all contact patients, and those with a previous history of hospitalization outside of France within the past year.6 CPE screenings were performed on rectal swabs and processed using both a multiplex PCR (Xpert Carba v2, Cepheid, Sunnyvale, CA) as recommended by the manufacturer and a culture on selective chromogenic media (ChromID CarbaSmart, BioMérieux, Marcy-l'Etoile, France). For culture, agar plates were incubated in aerobic atmosphere at 35°C for 24 hours. Suspected colonies were identified using MALDI-TOF mass spectrometry and carbapenemase confirmation using an immunochromatographic assay (NG Rapid test Carba-5, Eurobio, Les Ulis, France) and a multiplex PCR (Xpert Carba v2). Antibiotic susceptibility testing was performed and interpreted as previously described.7 , 8
Identification of contact and investigation
Contacts patients were defined as those for whom inpatient care involved sharing paramedical health care workers with one or more carrier patients for at least 24 hours and where there has been direct contact between the health care workers and the patients.6 Three consecutive CPE screening separated by at least 5 days were performed in each contact patients as recommended.6 Discharged patients were considered lost out to follow-up. Rectal swabs were processed by a molecular method and/or a selective culture as described in the previous section.
Whole-genome sequencing and genomic data analysis
NDM-5-producing E coli isolates were sequenced using Illumina technology as previously described.9 Total DNA was extracted from colonies using the Ultraclean Microbial DNA Isolation Kit (MO BIO Laboratories, Ozyme, Saint-Quentin, France) following the manufacturer's instructions. The DNA library was prepared as described.9 De novo assembly and read mappings were performed using CLC Genomics Workbench v12.0 (Qiagen, Les Ulis, France). The acquired antimicrobial resistance genes were identified using Resfinder server v3.1 (https://cge.cbs.dtu.dk/services/ResFinder/) and CARD database (https://card.mcmaster.ca). Phylogeny was performed using CSIphylogeny v1.4 server (www.cge.cbs.dtu.dk/services/CSIPhylogeny/) and visualised using FigTree software v1.4.3 (www.tree.bio.ed.ac.uk). Single nucleotide polymorphisms (SNPs) analysis was performed on whole genomes using CSIphylogeny V1.4 with parameters as follow select min depth at SNP position at 10X, minimum distance between SNPs at 10 bp, minimum SNP quality at 30.
RESULTS
Implementation of additional COVID-ICU
Foch hospital displays a 22-beds ICU. The first COVID-19-patient was hospitalized on March 3, 2020. As the number of COVID-19 strongly increased, almost all the beds were switched for COVID-19-patients on March 15, 2020. As all ICU-beds were occupied on March 26, 2020, 3 additional COVID-ICUs were created on March 27 (12 beds in a postanesthesia care unit [PACU]), March 31 (10 beds in operating rooms) and April 3 (8 beds in a cardiology ICU). All ICU were single-beds except the PACU which was an open-space with multibeds, all beds being separated by screens. In all additional ICU, medical and paramedical staffs were those working in original units. The health care worker: patient ratio was similar to the pre-COVID period with a minimum ratio of 1/8, 1/2 and 1/6 for physicians, nurses, and caregivers per patient. But, the burden of care for these patients in prone position was high. All staff wears anytime a FFP2 standard mask, gown, apron, gloves, eye protection, and mobcap. Gloves and aprons were changed for each different patient care while all other PPE were change twice-a-day. Cleaning was performed at least twice a day in all COVID-19 departments. Excreta management was handled using a bedpan washer but without the use of bedpan protective bags. Due to overwork, the infection control team was unable to check daily the application of contact and respiratory IPC. Misuse of gloves was reported, mainly in outdoor staff. Furthermore, in order to prevent shortages of PPE, paintball, and work building gowns were used. On April 27, 2020 as the number of patients requiring mechanical ventilation strongly decreased the COVID-19-ICU located in the PACU was closed.
Investigation of the outbreak
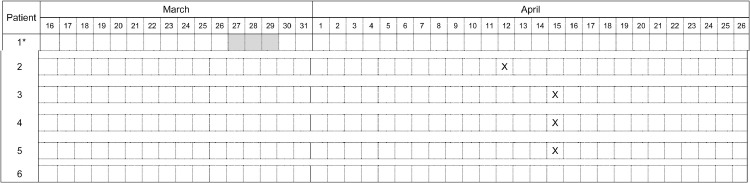
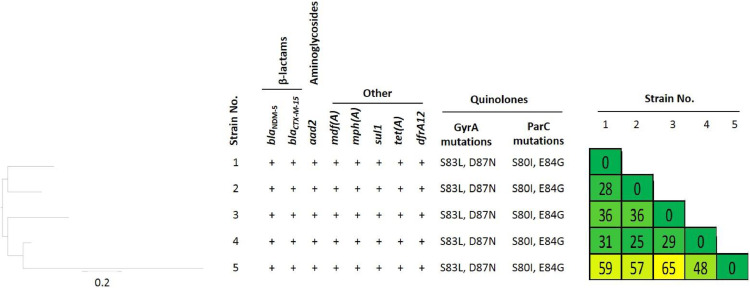
On March 27, the index case, a known NDM-5-producing E coli carrier presenting a COVID-19, was transferred to the PACU from the main ICU to ensure kidney replacement and ECMO for another patient of the hospital (Fig 1 ). His last positive CPE screening was performed on March 15, 2020. As part of a case-by-case risk analysis, it was considered that specific COVID-19-IPC, which was applied for all patients of the unit, was sufficient to prevent the transmission of the CPE. In this context, and considering the overwork of nurses, the contacts patients were not screened for CPE carriage. The index patient was discharged on March 29. On April 12, a protected distal bronchial sample performed in a patient hospitalized in the PACU since March 2, growth of an NDM-5-producing E coli. The patient had no history of hospitalization or travel during the past year. Although he was not hospitalized at the same time as the index case, the hypothesis of a cross-transmission was raised. The infection control team reinforces advice and quick training of the medical and paramedical team. Thirty-one contact patients of the index and the secondary cases were identified. Twenty-two patients were screened once, while 3 were screened 3 times in a row. About a half of all screening were performed in the presence of eXDR carriers. Seven were lost out of follow-up. Three carriers were identified on March 15. All the cases were grouped together in one place of the multibedroom PACU. They were reported as eXDR carrier on the medical software. Additional screening performed 5- and 10-days later did not identify further carriers. But, an additional carrier was identified on the admission screening performed in another hospital on April 26, 2020. Unfortunately, due to overwork, the strain isolated from this patient was not recovered for whole-genome sequencing. Overall, the NDM-5-producing E. coli outbreak involved 6 patients. The index case (patient No. 1), was a known carrier when admitted in the PACU. Four patients (No. 2 to 5), all close contact of the index patient, were identified within the PACU. The remaining one (patient No. 6), also a close contact of patient No. 1, was screened positive for the first time after his transfer in another hospital. Whole-genome sequencing analysis revealed that all analyzed NDM-5-producing E. coli isolates (n = 5) belonged to the same sequence type (ST), ST-361. In addition, the phylogenetic analysis demonstrated that all strains were clonally related with an average of 41.4 ± 12.7NPs along their whole genome (Fig 2 ). On April 30, 2020, all but 2 patients were discharged from hospital. All patients were colonized except one that presented ventilator-associated pneumonia related to the eXDR strain. He recovered with a combination of aztreonam plus ceftazidime-avibactam and colimycin.
Fig 1.
Temporal relation between NDM-5-producing E. coli carriers in the PACU transformed in ICU-COVID, March-April 2020. *: index case. X: date of first CPE positive sample. The index case was known as an NDM-5-producing E. coli carrier before his admission in the ICU-COVID. Hospital stay in the ICU-COVID are highlighted in gray. Patient 6 was screened positive on admission in another hospital on May 5, 2020.
Fig 2.
Phylogenetic tree and SNP matrix of phylogenetic analysis of NDM-5-producing E. coli ST-361. Scale bar on tree indicates the number of single-nucleotide polymorpisms per position of common sequences. SNPs matrix was obtained from CSI phylogeny (https://cge.cbs.dtu.dk/services/CSIPhylogeny/).
DISCUSSION
The prevention of micro-organisms transmission required a rigorous application of IPC measures,6 which should be implemented according to a case-by-case analysis risk. In the atypical context of the COVID-19 pandemic, we considered the risk of cross-transmission was low, as contact precautions were upgraded and applied to all patients of the COVID-department. Consequently, we decided not to screen contact patients. This strategy was not effective, as an outbreak involving 6 cases occurs but resolved when screening of contact patients was applied.
Several findings might have contributed to the spread of the NDM-5-producing E. coli strain. First, the geographical configuration of the PACU transformed in a multiple bedroom COVID-ICU might have favored the transmission of micro-organisms by inoculation of the environment. However, cleaning was performed at least twice a day and it was previously demonstrated that applying contact precautions in a multiple bedroom was noninferior to a strategy of contact precautions in a single bedroom.10 But, in the present outbreak, patient's beds were closely located. This latter finding suggests that incomplete compliance of standard and contact precautions was probably a cause of the present outbreak. The overwork due to the burden in care could have led to less vigilance of medical and paramedical staff and punctual low compliance for contact precaution.
Understaffing which can be assessed by the health care worker: patient ratio was previously associated with a higher risk of eXDR cross-transmission.4 Despite sufficient staff, the burden of care for these patients in prone positions was high. Then, the implementation of COVID-19 IPC measures that are more restrictive than those for eXDR could have falsely reassured medical and paramedical staff. The lack of training has also been associated with lower adherence to IPC measures.11 In the present context, medical and paramedical staff was mainly those of the PACU which were not usually incharge of ICU patients. We introduced the practice of wearing gloves and gowns anytime in order to protect staff. However, misuse of PPE has been previously associated with an increased risk of cross-transmission.12 , 13 Specifically, an outbreak of Imipenem-resistant Acinetobacter baumanii related to the misuse of gloves was previously described.14 Moreover, the shortages in medical PPE has led to the use of probably unsuitable alternative PPE. Unfortunately, due to the overwork of the ITC team, we were unable to audit the compliance for specific IPC measures despite the misuse of gloves were reported.
Last, antimicrobial exposure was previously associated with hospital acquisition of CPE strains and with an outbreak of MRSA.4 , 15 , 16 However, as the outbreak occurs in an additional COVID department which was opened for 20 days, we were not able to compare antibiotic consumption to baseline use. Finally, as eXDR cross-transmission occurred, we could hypothesize cross-transmission of other micro-organisms, such as MRSA, ESBL-producing Enterobacterales or C difficile occurred in this department. In a previous report, Yap et al described an outbreak of MRSA in an ICU in the context of SARS-infected patients in 2003 in Hong Kong.16
In conclusion, despite the implementation of upgraded ICP measures in the context of the COVID-19 pandemic, CPE outbreak may occur in COVID-19 departments. Several changes could have contributed to this outbreak such as overwork, misuse of PPE, and low compliance for standard and contact precaution. We believe the absence of screening in contact-patient has probably led to a delay in the identification of the outbreak. Therefore, we emphasize the need for applying rigorously standard precaution at all time. Applying contact precaution for eXDR carriers and performing screening in contact patients must be the rule.
ACKNOWLEDGMENTS
We thank all the teams at Foch Hospital involved in the care of COVID and non-COVID patients.
Footnotes
Conflict of interest: The authors state they have no conflict of interest to declare.
References
- 1.Kinross P, Suetens C, Dias JG, et al. Rapidly increasing cumulative incidence of coronavirus disease (COVID-19) in the European Union/European Economic Area and the United Kingdom, 1 January to 15 March 2020. Eurosurveillance. 2020;25:1–5. doi: 10.2807/1560-7917.ES.2020.25.11.2000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Coronavirus Disease (COVID-19) technical guidance: infection prevention and control. 2020. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/infection-prevention-and-control. Accessed April 16, 2020.
- 3.Farfour E, Ballester M-C, Lecuru M, et al. COVID-19: before stopping specific infection prevention and control measures, be sure to exclude the diagnosis. J Hosp Infect. 2020;105:375–376. doi: 10.1016/j.jhin.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legeay C, Thépot-Seegers V, Pailhoriès H, Hilliquin D, Zahar JR. Is cohorting the only solution to control carbapenemase-producing Enterobacteriaceae outbreaks? A single-centre experience. J Hosp Infect. 2018;99:390–395. doi: 10.1016/j.jhin.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Rowan NJ, Laffey JG. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic – Case study from the Republic of Ireland. Sci Total Environ. 2020;725 doi: 10.1016/j.scitotenv.2020.138532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.HCSP. Actualisation des recommandations relatives à la maîtrise de la diffusion des bactéries hautement résistantes aux antibiotiques émergentes (BHRe) 2019. 2019. Available at:https://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=758. Accessed October 8, 2020.
- 7.CA-SFM. Comité de l'antibiogramme de la Société Française de Microbiologie: recommandations 2019v2.0 mai. 2019 Available at: https://www.sfm-microbiologie.org/wp-content/uploads/2019/05/CASFM2019_V2.0_MAI.pdf. Accessed October 8, 2020.
- 8.Emeraud C, Escaut L, Boucly A, et al. Aztreonam plus clavulanate, tazobactam, or avibactam for treatment of infections caused by metallo-lactamase-producing gram-negative bacteria. Antimicrob Agents Chemother. 2019;63:63–68. doi: 10.1128/AAC.00010-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girlich D, Bonnin RA, Bogaerts P, et al. Chromosomal amplification of the blaOXA-58 carbapenemase gene in a Proteus mirabilis clinical isolate. Antimicrob Agents Chemother. 2017;61:16–26. doi: 10.1128/AAC.01697-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kluytmans-van den Bergh MFQ, Bruijning-Verhagen PCJ, Vandenbroucke-Grauls CMJE, et al. Contact precautions in single-bed or multiple-bed rooms for patients with extended-spectrum β-lactamase-producing Enterobacteriaceae in Dutch hospitals: a cluster-randomised, crossover, non-inferiority study. Lancet Infect Dis. 2019;19:1069–1079. doi: 10.1016/S1473-3099(19)30262-2. [DOI] [PubMed] [Google Scholar]
- 11.Houghton C, Meskell P, Delaney H, et al. Barriers and facilitators to healthcare workers’ adherence with infection prevention and control (IPC) guidelines for respiratory infectious diseases: a rapid qualitative evidence synthesis. Cochrane Database Syst Rev. 2020;4 doi: 10.1002/14651858.CD013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loveday HP, Lynam S, Singleton J, Wilson J. Clinical glove use: Healthcare workers’ actions and perceptions. J Hosp Infect. 2014;86:110–116. doi: 10.1016/j.jhin.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Wilson J, Prieto J, Singleton J, O'Connor V, Lynam S, Loveday H. The misuse and overuse of non-sterile gloves: application of an audit tool to define the problem. J Infect Prev. 2015;16:24–31. doi: 10.1177/1757177414558673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye D, Shan J, Huang Y, et al. A gloves-associated outbreak of imipenem-resistant Acinetobacter baumannii in an intensive care unit in Guangdong, China. BMC Infect Dis. 2015;15:179–188. doi: 10.1186/s12879-015-0917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mathers AJ, Vegesana K, German-Mesner I, et al. Risk factors for Klebsiella pneumoniae carbapenemase (KPC) gene acquisition and clinical outcomes across multiple bacterial species. J Hosp Infect. 2020;104:456–468. doi: 10.1016/j.jhin.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yap FHY, Gomersall CD, Fung KSC, et al. Increase in Methicillin-Resistant Staphylococcus aureus acquisition rate and change in pathogen pattern associated with an outbreak of severe acute respiratory syndrome. Clin Infect Dis. 2004;39:511–516. doi: 10.1086/422641. [DOI] [PMC free article] [PubMed] [Google Scholar]