Abstract
It is widely recognized that after endocytosis, internalized cargo is delivered to endosomes that act as sorting stations. The limiting membrane of endosomes contain specialized subregions, or microdomains, that represent distinct functions of the endosome, including regions competing for cargo capture leading to degradation or recycling. Great progress has been made in defining the endosomal protein coats that sort cargo in these domains, including Retromer that recycles transmembrane cargo, and ESCRT that degrades transmembrane cargo. In this review, we discuss recent work that is beginning to unravel how such coat complexes contribute to the creation and maintenance of endosomal microdomains. We highlight data that indicates that adjacent microdomains do not act independently but rather interact to cross-regulate. We posit that these interactions provide an agile means for the cell to adjust sorting in response to extracellular signals and intracellular metabolic cues.
Keywords: Endosome, Microdomain, Retromer, RME-8, SNX-1, DNAJC13, SNX1, Actin, mTORC1, HRS, ESCRT, Clathrin, ILV
Endosomes sort membrane-associated cargo
The plasma membrane of eukaryotic cells is a barrier that acts as the interface of a cell with its environment. The complexity of the membrane is vast, with thousands of different lipid species and transmembrane proteins present at any given moment. The cell surface is also quite dynamic in nature, balancing secretion, endocytosis, and endocytic recycling to maintain the size and composition of the plasma membrane. To maintain cellular homeostasis in a changing environment, these transport pathways must also be capable of rapidly varying their rates and selection of cargo as the needs of the cell change.
Endosomes are the major sorting stations for proteins and lipids internalized by endocytosis and those provided by specific pathways from the Golgi. While some endosomal cargo is selected for delivery to the lysosome for degradation, other cargo is selected by any of several recycling pathways for delivery to the plasma membrane, recycling endosomes, or Golgi. For an excellent review on mechanisms of cargo selection in endocytic recycling pathways see Weeratunge et al. [1].
Endosomes are highly dynamic, changing their protein and lipid composition over time in a maturation process from the early endosome to the late endosome [2]. Endosome maturation is centered around the early acting small GTPase Rab5, and the cascade of reactions leading to its eventual replacement by late acting small GTPase Rab7 [3,4]. Throughout this maturation process, the sorting of transmembrane cargo continues. During parts of the maturation process, multiple Rab GTPases are present on individual endosomes, with different Rabs typically residing in restricted microdomains [5,6]. Feedback loops between Rabs and their effectors have been proposed to contribute to this domain formation and segregation [5-8]. Microdomains represent distinct functions of the endosome, such as degradative sorting, fusion capability, and recycling tubule/vesicle elaboration.
Recycling vesicles and tubules bud away from the endosome, removing components to be reused at the plasma membrane or Golgi, while cargo-laden degradative vesicles on the same endosomes bud away from the cytoplasm, toward the interior of the endosome, accumulating as intraluminal vesicles (ILVs). ILVs are usually degraded in lysosomes, or, more rarely, released from the cell surface as exosomes [9]. Two of the best studied peripheral membrane coat complexes mediating recycling and degradation are Retromer and ESCRT, respectively. Retromer creates recycling tubules and vesicles and actively recruits a subset of recycling cargo into these transport carriers [1,10]. ESCRT produces ILVs and recruits ubiquitinated transmembrane cargo destined for degradation into these ILVs (reviewed by Henne [11] and Gatta AT and Carlton [12]).
Actin and recycling
Filamentous actin has long been known to play important roles in several different steps in membrane traffic, and recent work again emphasizes the importance of F-actin in endocytic recycling [13,14]. Traditionally, membrane associated F-actin has been proposed to provide necessary membrane tension during membrane fission events in many contexts [15] and may also promote myosin-based recycling tubule transport [16]. Endosome associated F-actin has also been proposed to extend the lifetime of particular types of recycling tubules during budding, providing time for cargo recruitment [13]. Recently, this idea has been extended, suggesting that F-actin associated with recycling microdomains on endosomes may act as a diffusion barrier that helps corral recycling cargo, helping to prevent loss of recycling cargo to the degradative machinery [16].
The WASH actin nucleation complex is closely associated with Retromer and clearly plays important roles in Retromer-based recycling, whereas other actin nucleation promoting factors, such as WAVE and Formins, also play important roles in retromer independent recycling pathways [17-20]. Of note, recent work in the polarized intestinal epithelia of Caenorhabditis elegans found a requirement for the formin CYK-1 in basolateral recycling of clathrin-independent cargo in the RAB-10 and ARF-6 pathway, whereas the WAVE complex promoted recycling to the Golgi from recycling endosomes, a rarely studied recycling route [18,20]. Finally, C. elegans SAC-1 (independent of its enzymatic activity) was shown to provide necessary inhibition of ARF-6 and PI5-kinase mediated PI(4,5)P2 production on endosomes, preventing overaccumulation of F-actin that can inhibit cargo recycling toward the recycling endosome [18,21].
Functionally opposing microdomains
Retromer and ESCRT are also often found on the same endosomes in separate microdomains [22,23]. The formation and separation of microdomains has been proposed to increase the efficiency of cargo sorting by concentrating cargo and may prevent interference in sorting that could occur if opposing sorting complexes mix [22,24]. Retromer and ESCRT microdomains appear to cross-regulate on early endosomes, whereas Retromer microdomains on late endosomes and lysosomes also contribute to mTORC1-based signaling, and the ESCRT machinery itself may form two adjacent domains to process cargo [22,25,26]. We discuss in the following context our current understanding of strategies the cell uses to form and maintain endosomal microdomains. Physical self-association by oligomerization can partially explain microdomain formation. Additional potential mechanisms, including cross-regulatory interactions between microdomains, cargo interactions, and molecular diffusion barriers are discussed.
Retromer
Retromer is best known for mediating recycling from endosomes to the Golgi, a pathway often referred to as retrograde recycling [27]. Retromer has also been reported to mediate recycling of some transmembrane cargos directly from the endosome to the plasma membrane [28]. Retromer was originally described in yeast as a combined SNX-BAR complex and an associated core cargo-recognition complex, a heterotrimer composed of Vps35, Vps29, and Vps26 [29]. SNX-BARs contain heterodimers of Snx1 or Snx2 with Snx5 or Snx6, whose BAR-domains confer membrane bending/curvature-sensing properties to the complex [30,31]. SNX-BAR dimers form higher order polymers that can cover and bend large regions of membrane to produce tubules [32,33]. Studies in mammalian cells indicate that SNX-BAR complexes connect to the Vps-trimer more loosely, differ in their requirements for recruitment to endosomes from the Vps-trimer, and may recycle some cargo independently of core Retromer [33-38]. In addition, Snx3, which lacks a membrane bending BAR domain, is intimately associated with the Retromer Vps-trimer and may replace SNX-BARs in Retromer for certains cargos, budding vesicles rather than tubules [39]. In mammals, an additional retromer-related complex termed Retriever also interacts with WASH and occupies the recycling microdomain to promote recycling of a subset of cargo proteins [27,40].
WASH subunit FAM21 is typically enriched at sites of recycling tubule constriction that will undergo fission [41]. Over the past few years, it has become clear that the endoplasmic reticulum frequently contacts endosomes, promoting fission and release of Retromer/WASH recycling tubules, among other roles [41]. The ER/endosome contact relevant to Retromer-associated tubules is mediated via the ER protein TMCC1 and endosomal Coronin1C at FAM21/WASH marked endosomal constrictions, defining the site of endosomal recycling tubule fission [41,42]. In another ER contact-driven process on the endosome, Retromer acts to limit WASH-dependent actin accumulation on endosomes via a SNX2/VAP/OSBP-mediated transfer of lipid PI4P to the ER, in a process required for productive recycling [43]. ER resident SAC1, a PI4P phosphatase, can then degrade PI4P in the ER. This PI4P degradation creates a steep chemical gradient between the ER and endosomes termed the phosphoinositide-motive force, allowing for counter transfer of other lipids [44].
Retromer microdomains
The propensity of the SNX-BARs to form higher order polymers likely contributes to the formation of Retromer microdomains, helping to cluster recycling activities within a particular region of the endosomal limiting membrane (Figure 1a) [30,31]. However, recent data also points to a role for SNX-BARs in restricting the growth of ESCRT microdomains via interaction with the Hsc70 cochaperone RME-8, indicating that self-association is not the full story to how microdomains remain separate [22] (Figure 1b). The connection of Retromer to WASH has also been proposed as a driving force in Retromer microdomain cohesion, with the branched actin network assembled by WASH acting as a diffusion barrier, reducing lateral diffusion of Retromer and associated cargo [16].
Figure 1.
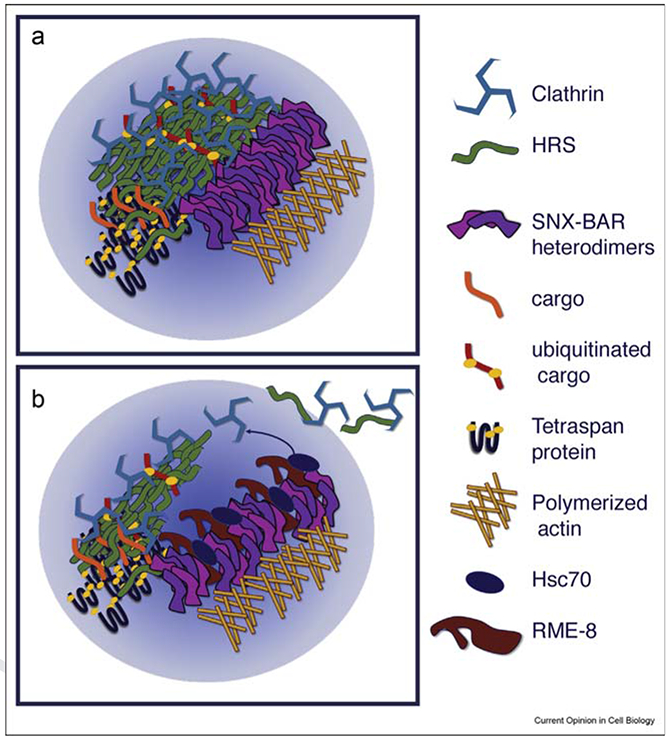
Microdomain formation by self-association and cargo interactions, with maintenance of adjacent microdomain separation by active cross-regulation. Illustration of self-associating machinery and cargo within two adjacent microdomains. Association of ESCRT-0, ubiquitinated cargo, and tetraspan proteins promotes degradative microdomain creation, whereas SNX-BAR and WASH/F-actin promote recycling microdomain formation. Ubiquitinated cargo can aid in ESCRT-0 cross-linking, and ubiquitinated tetraspan proteins can provide ubiquitin in trans for cargo that is unable to be ubiquitinated (a). Illustration of RME-8/Hsc70 anchored by SNX-BAR proteins uncoating ESCRT-0/Clathrin from the degradative microdomain at the interface of adjacent domains, helping to prevent invasion of the recycling domain by degradative machinery (b).
ESCRT
Ubiquitinated transmembrane cargo destined for degradation is captured and sequestered into ILVs by a series of ESCRT complexes (ESCRT-0, -I, -II, -III, and VPS4) [11,45]. Components within ESCRT-0, ESCRT-I, and ESCRT-II recognize degradative cargo via ubiquitin interacting motifs, whereas ESCRT-III forms spiral filaments and works with Vps4 to deform the membrane and produce the ILVs [45,46]. ESCRT-III spirals may also serve as a molecular fence to prevent cargo diffusion because cargo is typically deubiquitinated before sequestration in ILVs, and loss of Ub would cause most cargo to lose affinity for ESCRTs [47].
Until recently in vivo evidence for how the various ESCRT complexes work together has been unclear, but new work provides important insight into the timing and duration of recruitment of individual ESCRT components during ILV formation. Data in yeast on ESCRT-III and Vps4 dynamics indicate that a coordinated and short term residence of this machinery on the endosome of less than a minute likely produces a single ILV [46].
Using CRISPR-Cas9 to tag endogenous components in mammalian cells, another study showed that several ESCRT complexes associated and dissociated from endosomes in waves, with ESCRT-0 component HRS associating with endosomes for a much longer period of time (~150 s), compared with ESCRT-I component TSG101 (for ~27 s), or the latest acting component VPS4B (~7 s). VPS4 arrival and departure likely marks the completion of a single ILV [46,48].
Growth factor signaling has long been associated with elevated ILV formation [49,50]. Importantly, EGF stimulation in these cells extended the duration of all ESCRT component’s association with endosomes, with particularly dramatic effects on TSG101 and VPS4B [48]. This suggested that under EGF stimulation, individual endosomes go through multiple rounds of ILV production to downregulate activated receptors. A related study followed mildly overexpressed fluorescently labeled ESCRT subunits (ESCRT-0, -I, -III, VPS4, and HD-PTP), focusing on cells stimulated by fluorescently labeled EGF [51]. This approach allowed the authors to follow two ESCRT subcomplexes and EGF cargo at the same time, strongly supporting a model in which ILV formation occurs by a concerted action of all of the ESCRT subcomplexes working together in repetitive recruitment waves, with distinctive durations of residence for different subcomplexes [51]. This work also showed that ESCRT recruitment waves begin early in endosome maturation, at the Rab5 positive stage within 5 min of EGF uptake and often continue well into the later Rab7 positive stage [51].
ESCRT microdomains
Several protein interactions likely contribute to ESCRT-0 self-assembly into distinct microdomains on the endosomal limiting membrane (Figure 1). The first is a propensity for the HRS subunit itself to self-associate and form higher order structures [52]. In addition, the C-terminus of HRS binds to clathrin heavy chain, and at least in some cell types clathrin forms a specialized flat lattice on the endosomal limiting membrane [51,53]. Finally, the purified ESCRT-0 HRS/STAM heterotetramer contains a sum total of 8 ubiquitin binding domains, and ESCRT cargo is generally poly-ubiquitinated (K63-linked), suggesting that degradative cargo itself could act to cross-link ESCRT-0 complexes, increasing ESCRT-0 self-association and efficiency [52] (see more on this in the following context). This propensity to self-associate could help to occlude machinery charged with recycling activities or even other ESCRT complexes (I-III).
The view that ESCRT-III assembles adjacent to ESCRT-0, probably assembling spiral polymers at the edges of ESCRT-0 microdomains, is supported by recent work in the C. elegans system [54]. During the C. elegans oocyte-to-embryo transition, where de novo ILV biogenesis occurs in cells that initially lack all ILVs, immunoEM and STED microscopy showed that microdomains of ESCRT-0/HRS were adjacent to, but not precisely overlapping, those labeled by ESCRT-III components IST1 and VPS32 [54]. Analyzing multiple tissues within an intact animal, this work showed that ILVs can form tethered chains, an observation initially described in Arabidopsis [54]. This tethering suggests that ILVs bud continuously from a stable microdomain [54,55].
Transmembrane proteins can contribute to microdomain formation and ESCRT-mediated degradation
Under conditions of low nutrient availability, Saccharomyces cerevisiae internalize and degrade, as opposed to recycle, many of their plasma membrane nutrient transporters. These transporters are not themselves ubiquitinated but rely on a group of tetraspan family Cos proteins to provide ubiquitin in trans [56] (Figure 1). Low cellular NAD+ levels induce the increased production of tetraspan Cos family proteins, that then further interact with the Rsp5 ubiquitin ligase complex, providing ubiquitin in trans for degradative cargo that is not itself ubiquitinated (e.g. GPI-anchored proteins). The Cos proteins clearly self-associate in vivo and in vitro, potentially via their transmembrane domains, and loss of Cos proteins disrupt ESCRT microdomain cohesion and ESCRT-mediated degradation [56]. A poorly understood aspect that deserves greater attention is the role of lipids in microdomain formation and function, and Cos proteins have also been proposed as organizers of lipids, such as sterols and sphingolipids, that facilitate microdomain formation and ILV production [57,58]. Tetraspanins are good candidates to play a similar role in mammalian cells. Although Cos proteins and Tetraspanins lack sequence homology, they have similar overall structure, with four transmembrane domains that contain polar residues, extracellular cysteinerich sequences, and clear self-association, and clustering in membrane microdomains [56,59]. In addition, similar to Cos proteins, tetraspanins can be ubiquitinated and influence endosomal sorting of key cargos such as the EGF-receptor and PMEL-a [60,61].
Balancing the activities of opposing sorting complexes
The juxtaposition of adjacent microdomains with opposing functions on a single endosome provides an intriguing focal point to understand basic trafficking principals. In theory, at least two opposing activities need to be maintained within the same endosome to preserve the ability to sort incoming cargo to multiple destinations. If one sorting complex takes over the entire endosome, sorting would fail and all cargo within that endosome would traffic to the same destination.
Furthermore, homeostasis likely requires a particular balance of recycling and degradation, a balance whose optimum ratio would differ with changing environmental and metabolic conditions. Thus mechanisms that separate and balance opposing sorting activities would help maintain homeostasis and could act as pivot points to rapidly regulate needed changes to sorting over time. Recent research suggests that molecular mechanisms exist, beyond self-association, to enforce microdomain separation and balance the activities of Retromer and ESCRT Mechanisms that promote physically distinct microdomains could include interactions between sorting complexes that cross-regulate assembly or disassembly of opposing microdomains. In the following section, we will discuss recent evidence that supports these possibilities.
RME-8 acts to balance the recycling and degradative domains
A key component of the recycling microdomain is Hsc70 cochaperone RME-8 (also called DNAJC13) [22,23,26,62]. RME-8 is unique in that it is the only endosomally localized Hsc70 cochaperone, a localization that is supported by its N-terminal PI(3)P binding domain [63,64]. DNAJ domain family cochaperones localize and activate the ATPase activity of Hsc70-family chaperones, enabling assembly and/or disassembly of protein complexes [65-67]. On the maturing endosome, RME-8 colocalizes with its binding partner SNX-1 and other Retromer components in the recycling microdomain, separate from ESCRT-0 in the degradative microdomain [22,23,26].
Studies in C. elegans coelomocytes, where single endosomes are large enough to directly visualize functional microdomains by light microscopy, RME-8 and SNX-1 are clearly important for separating the peripheral membrane protein complexes that direct opposing recycling and degradative activities [22]. This is a special property of RME-8 and SNX-1 within the recycling microdomain, as retromer components VPS-35 or SNX-3 do not affect microdomain separation. Current models posit that SNX-1 and RME-8 stimulate Hsc70 to uncoat the adjacent endosomal degradative subdomain, providing a mechanism for the recycling domain to remove encroaching degradative regulators [22]. In the absence of SNX-1 or RME-8, HGRS-1/HRS over-assembles from the cytoplasm onto the endosomal limiting membrane. This HRS over-assembly is accompanied by inappropriate mixing of ESCRT-0 into recycling microdomains, and inappropriate degradation of recycling cargo [22]. In C. elegans, Drosophila, and mammals, loss of RME-8 also leads to overaccumulation of clathrin on endosomal membranes [22,68,69].
Given the known physical interactions between clathrin heavy chain and ESCRT-0 component HRS, and the known ability of Hsc70 to disassemble clathrin lattices, one hypothesis has been that RME-8/Hsc70 directly disassembles endosomal clathrin, which in turn leads to ESCRT-0 disassembly [22,26,53]. Recent observations in mammalian cells suggest the opposite, that HRS requires clathrin binding for ESCRT-0 removal from the endosome, rather than ESCRT-0 assembly on the endosome [51]. Taken together, over-assembly of HRS in the absence of RME-8, or when HRS is modified to block clathrin binding, suggests that clathrin promotes ESCRT-0 disassembly, perhaps acting as a linker for an uncoating activity of RME-8/Hsc70 upon ESCRT-0.
Having endosome bound chaperone activity is likely a powerful mechanism to control the cargo sorting machinery. Much work remains to define the direct endosomal substrates of RME-8/Hsc70 and to define the precise mechanism by which RME-8 affects ESCRT-0. In fact, there may be several substrates for RME-8/ Hsc70 on the endosome. In mammalian cells, RME-8 is known to associate with FAM21, a central component of the WASH actin nucleation complex. Cells depleted of RME-8 accumulate Snx1, Vps35, Vps26, and WASH on apparently stalled endosomal recycling tubules, suggesting that RME-8 may regulate assembly/disassembly dynamics within the recycling microdomain, in addition to providing cross-regulation from Retromer to ESCRT-0 [26,70].
Ubiquitinated cargo required for recycling
Cargo that is not recycled is degraded. Typically, the cargos destined for degradation are decorated with K63-linked ubiquitin chains, which as discussed previously, are recognized and concentrated by the multivalent ubiquitin binding domains of ESCRT-0 [52,71,72]. Suggesting more than just a passive role in cargo sorting, this K63-linked ubiquitination is not only required for many specific cargos to degrade, but is also generally required for ILV formation [47,73,74]. New results in C. elegans suggest that K63 ubiquitination also plays an important role in recycling and microdomain maintenance [24].
UBC-13 is the major E2 ubiquitin-conjugating enzyme generating K63-linked ubiquitin chains in C. elegans, with UBC-13 required for most ESCRT-mediated degradation [71]. Loss of UBC-13 also interferes with Retromer-based recycling of MIG-14/Wls, and produces mixing of ESCRT and Retromer microdomains [24]. These effects of UBC-13 on retromer-based recycling do not seem to be owing to Ub-mediated regulation of recycling factors, as both Retromer-based recycling and microdomain separation are restored in ubc-13 mutants upon forced expression of a single Ub-cargo fusion protein [24]. The observation that the simple addition of ubiquitinated cargo both restores proper recycling of MIG-14/WIs and microdomain separation supports the conclusion that the ubiquitinated cargo itself contributes to the integrity of the ESCRT microdomain, helping to prevent ESCRT-based interference in recycling microdomain function. As discussed previously, the HRS/STAM complex is multivalent for ubiquitin binding sites, hence ubiquitinated cargo likely improves ESCRT self-cohesion, reducing entry of ESCRT into the recycling microdomain, and limiting its inhibition of recycling.
HRS and WASH: further bridging recycling and degradation
In addition to the role HRS plays in seeding and cross-linking a degradative microdomain, a recent report indicates that HRS is important for endosomal recruitment of the WASH complex and F-actin, even though WASH and actin are found in the recycling domain, adjacent to the degradative microdomain enriched in HRS. When HRS is depleted, WASH and actin levels are reduced on EEA-1 positive endosomes [14]. When endosomal actin is present, the simple addition of an actin binding site to a normally degraded cargo is sufficient to sort it for recycling rather than degradation [14]. No evidence of a direct HRS/WASH interaction has been found, hence important questions remain as to how HRS is regulating WASH. Moreover, it is an open question if these results indicate a mechanism by which ESCRT-0 can cross-regulate the recycling microdomain. If so, HRS could be a primary target of cellular regulation that continuously adjusts the balance degradation and recycling [75]. It is interesting to note that HRS has also been reported to interact with retromer SNX-BARs, and SNX-BARs have been implicated in ER-endosome contacts and phosphoinositide regulation, which could influence WASH activity [43].
Microdomain regulation on late endosomes controls TOR signaling
Another example of the apparent coupling of adjacent microdomains involves Retromer, Rab7, and mTORC1/Regulator on late endosomes and lysosomes [25]. In mammalian cells inactive GDP-bound Rab7 is found associated with ER, Golgi, and mitochondrial membranes [76]. Upon activation by GDP-GTP exchange, GTP-Rab7 becomes associated with microdomains on the limiting membrane of late endosomes and lysosomes, often directly adjacent to nutrient sensing mTORC1/Regulator microdomains [25,76]. New studies indicate that core retromer (Vps35/26/29) does not interact with mTORC1 via retromer’s role in recycling transmembrane cargo. Rather retromer provides a necessary down-regulation of Rab7 activity via Rab7-GAP TBC1D5, apparently preventing Rab7 from interfering with microdomain separation, and blocking nutrient activated mTORC1 recruitment on endolysosomal membranes [25,76,77].
This positive Retromer/TOR signaling relationship was also shown to be conserved in yeast and C. elegans [25]. In addition, there is evidence that mTORCl signaling provides positive feedback to the process, as amino acid starvation leads to TFEB-mediated transcriptional upregulation of retromer genes VPS35 and VPS26A [78]. Taken together, these studies suggest that interactions between Retromer, Rab7, and mTORC1 act to balance catabolic activities driven by Rab7, and anabolic activities driven by mTORC1, and suggests that spatial segregation into microdomains provides an important framework for proper function and regulation of signaling.
Summary and future directions
Although functionally specialized endosomal microdomains have been known for quite some time, their mechanisms of formation and disassembly, the relationships of functionally opposing microdomains to one another when present on the same endosome, and even the relative importance of microdomains in general, remains poorly understood. Self-assembly and higher order interactions appear to be key attributes of the molecules that form microdomains. Examples include ESCRT-0/ub-cargo/clathrin complexes, tetraspanins and Cos tetraspan proteins, and oligomerized SNX-BAR proteins. The idea of diffusion barriers in microdomain function is exciting, with proposed examples including polymerized actin domains and ESCRT-III spiral filaments, with more work well warranted to further test these hypotheses.
Proposed mechanisms of cross-regulation include the chaperone mediated uncoating of degradative microdomain components directed by RME-8, as well as the Retromer/RAB7/TFEB interaction in control of mTORC1. In particular, work on cross-regulation suggests that a key function of microdomains is to prevent interference of one process by the presence of machinery promoting a different process. For instance, RME-8 and UBC-13 studies suggest that Retromer may not function properly when mixed with ESCRT, and mTORC1 does not function well when mixed with GTP-bound Rab7. It remains unclear if ESCRT function is impaired by mixing with Retromer. Microdomain based prevention of interference between large macro-molecular assemblies may be as important or more important than efficiencies provided by keeping cofunctioning assemblies in close proximity. It will also be exciting to determine if rapidly fine tuning microdomain cross-regulation is used as a means to deal with rapidly fluctuating trafficking needs of the cell in response to changes in extracellular environment and intracellular metabolism.
A poorly understood aspect that deserves greater attention is the role of lipids in microdomain formation and function. Although sterols, sphingolipids, and LBPA have been proposed to facilitate ILV production, and may influence degradative microdomain cohesion, phosphoinositides are also well placed to play a key role regulating microdomains [57,58]. Phosphoinositide species play a major role in organelle identity, interacting with many endosomal peripheral membrane proteins through low affinity interactions ([79] and reviewed by Dickson and Hille [80]). As discussed previously, two lipids found in nanodomains [81], PI(4,5)P2 and PI(4)P, can contribute to F-actin assembly in recycling microdomains, with PI(4)P as a key regulator of WASH and target of an ER-mediated control mechanism [17,43]. In addition PI(3,5)P2 interacts with the actin filament-binding region of cortactin, inducing release of cortactin from endosomal F-actin networks [82]. Cortactin is likely important as a negative regulator of WASH and as a recruiter of ER tubules during recycling carrier fission.
PI(3)P is canonical for early endosome identity, is an important lipid for both Retromer and ESCRT, and was described early on as enriched in microdomains [83]. Indeed, PX domain proteins, such as SNX-BARs, have the capability to bind to more than one phospholipid species, individually or in combination [79,84]. Thus several phosphoinositide species are well placed to play regulatory roles in microdomains. Given that cells are in a constant state of flux, the local phosphoinositide composition can be altered rapidly by exchange at ER contact sites and local phosphoinositide kinases and phosphatases. It will be of great interest to further analyze the degree to which transient nanodomains of phosphoinositides, and perhaps phosphoinositide mixtures, play an instructive role in endosomal microdomains formation and maintenance. Given that the lipid content of the most popular cultured cell lines used in most trafficking studies may be quite different from that found in the numerous highly specialized cells found in intact organisms, investigating lipids in the context of tissues and whole organisms will be important in future studies.
Finally, integrating information on multiple potential regulators of microdomains should yield new insights that are missed when studied in isolation. This includes a focus on how neighboring microdomains influence one another, and how divergent types of regulators interact. Although Rab GTPases are master regulators of the endosomal system and were among the first proteins to be recognized as markers of endosomal microdomains, the degree to which Rab GTPases influence microdomain formation and separation is still not very well understood, and models of its function would benefit from integration with the roles of coats and lipids. There is a great need to bring diverse observations together into a more coherent model. This will likely require simultaneous monitoring and manipulation of Rab proteins, along with other relevant components such as microdomain-associated coats such as Retromer and ESCRT, and relevant lipids, in systems with sufficient spatial resolution to advance our understanding in living cells.
Acknowledgements
The authors thank the members of the Grant lab for helpful discussions. Our work was supported by National Institutes of Health (NIH) Grants R01GM135326, R01GM067237 and R01GM103995 to B.D.G., and 5F32GM096599 to A.N.
Footnotes
Conflict of interest statement
Nothing declared.
References
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.**.Weeratunge Saroja, Paul B, Collins B: Recognising the signals for endosomal trafficking. Curr Opin Cell Biol 2020.An insightful review of endosomal sorting with an emphasis on how coat complex structure allows cargo recognition, including models of how different sorting nexins combine with retromer and retriever to recognize and sort recycling cargo.
- 2.Huotari J, Helenius A: Endosome maturation. EMBO J 2011, 30: 3481–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rink J, Ghigo E, Kalaidzidis Y, Zerial M: Rab conversion as a mechanism of progression from early to late endosomes. Cell 2005, 122:735–749. [DOI] [PubMed] [Google Scholar]
- 4.Poteryaev D, Datta S, Ackema K, Zerial M, Spang A: Identification of the switch in early-to-late endosome transition. Cell 2010, 141:497–508. [DOI] [PubMed] [Google Scholar]
- 5.Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M: Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol 2000, 149:901–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfeffer SR: Rab GTPase regulation of membrane identity. Curr Opin Cell Biol 2013, 25:414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Renzis S, Sonnichsen B, Zerial M: Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat Cell Biol 2002, 4:124–133. [DOI] [PubMed] [Google Scholar]
- 8.Liu O, Grant BD: Basolateral endocytic recycling requires RAB-10 and AMPH-1 mediated recruitment of RAB-5 GAP TBC-2 to endosomes. PLoS Genet 2015, 11, e1005514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo M, Raposo G, Thery C: Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 2014, 30:255–289. [DOI] [PubMed] [Google Scholar]
- 10.**.Cullen PJ, Steinberg F: To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat Rev Mol Cell Biol 2018, 19:679–696.A comprehensive review focused on active sorting mechanisms in endocytic recycling, revising the view that most recycling follows a default bulk flow pathway
- 11.Henne WM, Buchkovich NJ, Emr SD: The ESCRT pathway. Dev Cell 2011, 21:77–91. [DOI] [PubMed] [Google Scholar]
- 12.Gatta AT, Carlton JG: The ESCRT-machinery: closing holes and expanding roles. Curr Opin Cell Biol 2019, 59:121–132. [DOI] [PubMed] [Google Scholar]
- 13.Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, Taunton J, Weiner OD, Parton RG, von Zastrow M: Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 2010, 143:761–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.**.MacDonald E, Brown L, Selvais A, Liu H, Waring T, Newman D, Bithell J, Grimes D, Urbé S, Clague MJ, Zech T: HRS – WASH axis governs actin-mediated endosomal recycling and cell invasion. J Cell Biol 2018, 217:2549–2564.This paper shows a requirement for ESCRT-0 component Hrs for recruitment of actin promoting factor and recycling mediator WASH in the adjacent microdomain. Moreover, the authors demonstrate that forced actin binding can direct a ubiquitinated cargo to recycle, suggesting an instructive role of actin in recycling cargo sorting.
- 15.Anitei M, Hoflack B: Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat Cell Biol 2012, 14:11–19. [DOI] [PubMed] [Google Scholar]
- 16.**.Simonetti B, Cullen PJ: Actin-dependent endosomal receptor recycling. Curr Opin Cell Biol 2019, 56:22–33.An important comprehensive review on the role of actin, actin binding proteins, and actin regulators on endosomes, with an especially cogent discussion of endosomal actin as a potential diffusion barrier that retains cargo for recycling.
- 17.**.Chen D, Yang C, Liu S, Hang W, Wang X, Chen J, Shi A: SAC-1 ensures epithelial endocytic recycling by restricting ARF-6 activity. J Cell Biol 2018, 217:2121–2139.This work demonstrates a non-enzymatic role for SAC1 in PI(4,5)P2 phosphoinositide metabolism and productive recycling on recycling endosomes.
- 18.*.Gong T, Yan Y, Zhang J, Liu S, Liu H, Gao J, Zhou X, Chen J, Shi A: PTRN-1/CAMSAP promotes CYK-1/formin-dependent actin polymerization during endocytic recycling. EMBO J 2018, 37, 10.15252/embj.201798556. Epub 2018 Mar 22.This paper demonstrates a role for formins, and presumably linear F-actin, in mediating endocytic recycling.
- 19.Tanabe K, Ohashi E, Henmi Y, Takei K: Receptor sorting and actin dynamics at early endosomes. CommunIntegrBiol 2011, 4:742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhiyong Bai, Grant Barth D: A TOCA/CDC-42/PAR/WAVE functional module required for retrograde endocytic recycling. Proc Natl Acad Sci USA 2015, 112:E1443–E1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anbing Shi, Ou Liu, Sabine Koenig, Riju Banerjee, Chen Carlos Chih-Hsiung Eimer Stefan, Grant Barth D: RAB-10-GTPase–mediated regulation of endosomal phosphatidylinositol-4,5-bisphosphate. Proc Natl Acad Sci USA 2012, 109: 13892–13893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.**.Norris A, Tammineni P, Wang S, Gerdes J, Murr A, Kwan KY, Cai Q, Grant BD: SNX-1 and RME-8 oppose the assembly of HGRS-1/ESCRT-0 degradative microdomains on endosomes. Proc Natl Acad Sci USA 2017, 114:E307–E316.This paper develops a system to study microdomains in an intact organism using a cell type with naturally enormous endosomes, greatly facilitating microdomain analysis by light microscopy. This study also demonstrates a requirement for recycling microdomain components SNX-1 and RME-8 in cross-regulating ESCRT-0 in the degradative microdomain to prevent microdomain mixing.
- 23.Popoff V, Mardones GA, Bai SK, Chambon V, Tenza D, Burgos PV, Shi A, Benaroch P, Urbe S, Lamaze C, et al. : Analysis of articulation between clathrin and retromer in retrograde sorting on early endosomes. Traffic 2009, 10: 1868–1880. [DOI] [PubMed] [Google Scholar]
- 24.**.Zhang J, Liu J, Norris A, Grant BD, Wang X: A novel requirement for ubiquitin-conjugating enzyme UBC-13 in retrograde recycling of MIG-14/Wntless and Wnt signaling. Mol Biol Cell 2018, 29:2098–2112.This paper documents a requirement for ubiquitin-ligase UBC-13 in retromer-based recycling, and further indicates feedback between degradative and recycling microdomains. Results suggest that ubiquitinated cargo bound for degradation is important for separation of degradative and retrograde recycling microdomains and proper retrograde cargo recycling.
- 25.**.Kvainickas A, Nägele H, Qi W, Dokládal L, Jimenez-Orgaz A, Stehl L, Gangurde D, Zhao Q, Hu Z, Dengjel J, et al. : Retromer and TBC1D5 maintain late endosomal RAB7 domains to enable amino acid-induced mTORC1 signaling. J Cell Biol 2019, 218:3019–3038.This is a key paper linking mTORC1 signaling to microdomains on late endosomes and lysosomes, providing evidence for regulation between opposing microdomains as an important mechanism for the cell to react to changing nutrient status. The results demonstrate that Retromer limits RAB-7-GTP accumulation to balance adjacent mTORC1/Ragulator and Rab7 microdomains on late endosomes and lysosomes.
- 26.Shi A, Sun L, Banerjee R, Tobin M, Zhang Y, Grant BD: Regulation of endosomal clathrin and retromer-mediated endosome to Golgi retrograde transport by the J-domain protein RME-8. EMBO J 2009, 28:3290–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen KE, Healy MD, Collins BM: Towards a molecular understanding of endosomal trafficking by Retromer and Retriever. Traffic 2019, 20:465–478. [DOI] [PubMed] [Google Scholar]
- 28.Temkin P, Lauffer B, Jager S, Cimermancic P, Krogan NJ, von Zastrow M: SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol 2011, 13:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seaman MN, McCaffery JM, Emr SD: A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol 1998, 142:665–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallon M, Cullen PJ: Retromer and sorting nexins in endosomal sorting. Biochem Soc Trans 2015, 43:33–47. [DOI] [PubMed] [Google Scholar]
- 31.van Weering JR, Sessions RB, Traer CJ, Kloer DP, Bhatia VK, Stamou D, Carlsson SR, Hurley JH, Cullen PJ: Molecular basis for SNX-BAR-mediated assembly of distinct endosomal sorting tubules. EMBO J 2012, 31:4466–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kovtun O, Leneva N, Bykov YS, Ariotti N, Teasdale RD, Schaffer M, Engel BD, Owen DJ, Briggs JAG, Collins BM: Structure of the membrane-assembled retromer coat determined by cryo electron tomography. Nature 2018, 561: 561–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas M, Gershlick DC, Vidaurrazaga A, Rojas AL, Bonifacino JS, Hierro A: Structural mechanism for cargo recognition by the retromer complex. Cell 2016, 167:1623–1635. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rojas R, Kametaka S, Haft CR, Bonifacino JS: Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol Cell Biol 2007, 27:1112–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bonifacino JS, Hurley JH: Retromer. Curr Opin Cell Biol 2008, 20:427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seaman MN, Harbour ME, Tattersall D, Read E, Bright N: Membrane recruitment of the cargo-selective retromer subcomplex is catalysed by the small GTPase Rab7 and inhibited by the Rab-GAP TBC1D5. J Cell Sci 2009, 122: 2371–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonetti B, Danson CM, Heesom KJ, Cullen PJ: Sequence-dependent cargo recognition by SNX-BARs mediates retromer-independent transport of CI-MPR. J Cell Biol 2017, 216:3695–3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kvainickas A, Jimenez-Orgaz A, Nägele H, Hu Z, Dengjel J, Steinberg F: Cargo-selective SNX-BAR proteins mediate retromer trimer independent retrograde transport. J Cell Biol 2017, 216:3677–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harterink M, Port F, Lorenowicz MJ, Mcgough IJ, Silhankova M, Betist MC, van Weering JRT, van Heesbeen RGHP, Middelkoop TC, Basler K, et al. : A SNX3-dependent retromer pathway mediates retrograde transport of the Wnt sorting receptor Wntless and is required for Wnt secretion. Nat Cell Biol 2011, 13:914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.*.McNally KE, Faulkner R, Steinberg F, Gallon M, Ghai R, Pim D, Langton P, Pearson N, Danson CM, Nagele H, et al. : Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat Cell Biol 2017, 19:1214–1225.This paper describes a new Retromer-like complex called Retriever that is important for recycling cargo not recognized by Retromer. Like Retromer, Retriever interacts with WASH and is part of the recycling microdomain adjacent to ESCRT-0 degradative domains.
- 41.Rowland A, Chitwood P, Phillips M, Voeltz G: ER contact sites define the position and timing of endosome fission. Cell 2014, 159:1027–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoyer MJ, Chitwood PJ, Ebmeier CC, Striepen JF, Qi RZ, Old WM, Voeltz GK: A novel class of ER membrane proteins regulates ER-associated endosome fission. Cell 2018, 175: 254–265. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong R, Saheki Y, Swarup S, Lucast L, Harper JW, De Camilli P: Endosome-ER contacts control actin nucleation and retromer function through VAP-dependent regulation of PI4P. Cell 2016, 166:408–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.*.Zewe JP, Wills RC, Sangappa S, Goulden BD, Hammond GR: SAC1 degrades its lipid substrate PtdIns4P in the endoplasmic reticulum to maintain a steep chemical gradient with donor membranes. Elife 2018, 7, 10.7554/eLife.35588.This paper provides evidence that a PI(4)P gradient at ER/organelle contacts provides a motive force for exchange of other lipids.
- 45.Henne WM, Stenmark H, Emr SD: Molecular mechanisms of the membrane sculpting ESCRT pathway. Cold Spring Harb Perspect Biol 2013, 5:a016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.*.Adell MAY, Migliano SM, Upadhyayula S, Bykov YS, Sprenger S, Pakdel M, Vogel GF, Jih G, Skillern W, Behrouzi R, et al. : Recruitment dynamics of ESCRT-III and Vps4 to endosomes and implications for reverse membrane budding. Elife 2017, 6, 10.7554/eLife.31652.An in-depth analysis of ESCRT-III and Vps4 dynamics on yeast endosomes that provided important evidence that Vps4 is likely an active partner of ESCRT-III during ILV budding
- 47.*.Frankel EB, Audhya A: ESCRT-dependent cargo sorting at multivesicular endosomes. Semin Cell Dev Biol 2018, 74:4–10.This recent review on ESCRT function that provides an excellent discussion of ESCRT function, and extends the hypothesis that ESCRT-III spirals limit loss of deubiquitinated cargo by diffusion.
- 48.**.Quinney KB, Frankel EB, Shankar R, Kasberg W, Luong P, Audhya A: Growth factor stimulation promotes multivesicular endosome biogenesis by prolonging recruitment of the late-acting ESCRT machinery. Proc Natl Acad Sci USA 2019, 116: 6858–6867.Using endogenously tagged mammalian ESCRT components this elegant paper defined the characteristic residence times of ESCRT-0, ESCRT-I, and Vps4 on endosomes, and nicely showed that EGF growth factor signaling strongly affects residence time, with differential effects for different components.
- 49.White IJ, Bailey LM, Aghakhani MR, Moss SE, Futter CE: EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J 2006, 25: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Razi M, Futter CE: Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol Biol Cell 2006, 17:3469–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.**.Wenzel EM, Schultz SW, Schink KO, Pedersen NM, Nähse V, Carlson A, Brech A, Stenmark H, Raiborg C: Concerted ESCRT and clathrin recruitment waves define the timing and morphology of intraluminal vesicle formation. Nat Commun 2018, 9:2932–3018.Simultaneously following labeled EGF and multiple labeled ESCRT subunits, this work clearly shows the concerted recruitment of multiple ESCRT subcomplexes to endosomes in recurring waves, with one wave likely giving rise to one ILV. This work also provides new insight into the connect of clathrin to ESCRT-0.
- 52.Mayers JR, Fyfe I, Schuh AL, Chapman ER, Edwardson JM, Audhya A: ESCRT-0 assembles as a heterotetrameric complex on membranes and binds multiple ubiquitinylated cargoes simultaneously. J Biol Chem 2011, 286:9636–9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raiborg C, Wesche J, Malerod L, Stenmark H: Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J Cell Sci 2006, 119:2414–2424. [DOI] [PubMed] [Google Scholar]
- 54.**.Frankel EB, Shankar R, Moresco JJ, Yates 3, John R, Volkmann N, Audhya A: Ist1 regulates ESCRT-III assembly and function during multivesicular endosome biogenesis in caenorhabditis elegans embryos. Nat Commun 2017, 8: 1439–1513.This paper creates a novel system to study de novo biogenesis of MVBs in an intact organism. The authors, demonstrate that, like plants, multiple tissues in C. elegans produce concatenated ILV’s, suggesting multiple rounds of budding from a stable microdomain. Moreover, results indicate a larger role for Ist1 in membrane bending than previously appreciated.
- 55.*.Buono RA, Leier A, Paez-Valencia J, Pennington J, Goodman K, Miller N, Ahlquist P, Marquez-Lago TT, Otegui MS: ESCRT-mediated vesicle concatenation in plant endosomes. J Cell Biol 2017, 216:2167–2177.A visually stunning paper demonstrating concatenated ILV’s in MVB formation. The authors also present mathematical models that propose the curvature of the ILV is not sufficient to explain the retention of cargo, and suggest that ESCRT components may provide the needed barrier to restrict deubiqutinated cargo in the ILV.
- 56.MacDonald C, Payne J, Aboian M, Smith W, Katzmann D, Piper R: A family of tetraspans organizes cargo for sorting into multivesicular bodies. Dev Cell 2015, 33:328–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsuo H, Chevallier J, Mayran N, Le Blanc I, Ferguson C, Faure J, Blanc NS, Matile S, Dubochet J, Sadoul R, et al. : Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 2004, 303:531–534. [DOI] [PubMed] [Google Scholar]
- 58.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M: Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319:1244–1247. [DOI] [PubMed] [Google Scholar]
- 59.MacDonald C, Stamnes MA, Katzmann DJ, Piper RC: Tetraspan cargo adaptors usher GPI-anchored proteins into multivesicular bodies. Cell Cycle 2015, 14:3673–3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Odintsova E, van Niel G, Conjeaud H, Raposo G, Iwamoto R, Mekada E, Berditchevski F: Metastasis suppressor tetraspanin CD82/KAI1 regulates ubiquitylation of epidermal growth factor receptor. J Biol Chem 2013, 288:26323–26334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Niel G, Charrin S, Simoes S, Romao M, Rochin L, Saftig P, Marks MS, Rubinstein E, Raposo G: The tetraspanin CD63 regulates ESCRT-independent and -dependent endosomal sorting during melanogenesis. Dev Cell 2011, 21:708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGough IJ, Cullen PJ: Clathrin is not required for SNX-BAR-retromer-mediated carrier formation. J Cell Sci 2013, 126: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xhabija B, Taylor GS, Fujibayashi A, Sekiguchi K, Vacratsis PO: Receptor mediated endocytosis 8 is a novel PI(3)P binding protein regulated by myotubularin-related 2. fEbS Lett 2011, 585:1722–1728. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Grant B, Hirsh D: RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans. Mol Biol Cell 2001, 12:2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Silver PA, Way JC: Eukaryotic DnaJ homologs and the specificity of Hsp70 activity. Cell 1993, 74:5–6. [DOI] [PubMed] [Google Scholar]
- 66.Jiang RF, Greener T, Barouch W, Greene L, Eisenberg E: Interaction of auxilin with the molecular chaperone, Hsc70. J Biol Chem 1997, 272:6141–6145. [DOI] [PubMed] [Google Scholar]
- 67.Walsh P, Bursac D, Law YC, Cyr D, Lithgow T: The J-protein family: modulating protein assembly, disassembly and translocation. EMBO Rep 2004, 5:567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chang HC, Hull M, Mellman I: The J-domain protein Rme-8 interacts with Hsc70 to control clathrin-dependent endocytosis in Drosophila. J Cell Biol 2004, 164:1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Girard M, Poupon V, Blondeau F, McPherson PS: The DnaJ-domain protein RME-8 functions in endosomal trafficking. J Biol Chem 2005, 280:40135–40143. [DOI] [PubMed] [Google Scholar]
- 70.Freeman CL, Hesketh G, Seaman MNJ: RME-8 coordinates the activity of the WASH complex with the function of the retromer SNX dimer to control endosomal tubulation. J Cell Sci 2014, 127:2053–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sato M, Konuma R, Sato K, Tomura K, Sato K: Fertilization-induced K63-linked ubiquitylation mediates clearance of maternal membrane proteins. Development 2014, 141: 1324–1331. [DOI] [PubMed] [Google Scholar]
- 72.Duncan LM, Piper S, Dodd RB, Saville MK, Sanderson CM, Luzio JP, Lehner PJ: Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J 2006, 25:1635–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.MacDonald C, Buchkovich NJ, Stringer DK, Emr SD, Piper RC: Cargo ubiquitination is essential for multivesicular body intralumenal vesicle formation. EMBO Rep 2012, 13:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erpapazoglou Z, Dhaoui M, Pantazopoulou M, Giordano F, Mari M, Leon S, Raposo G, Reggiori F, Haguenauer-Tsapis R: A dual role for K63-linked ubiquitin chains in multivesicular body biogenesis and cargo sorting. Mol Biol Cell 2012, 23: 2170–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Row PE, Clague MJ, Urbe S: Growth factors induce differential phosphorylation profiles of the Hrs-STAM complex: a common node in signalling networks with signal-specific properties. Biochem J 2005, 389:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.**.Jimenez-Orgaz A, Kvainickas A, Nagele H, Denner J, Eimer S, Dengjel J, Steinberg F: Control of RAB7 activity and localization through the retromer-TBC1D5 complex enables RAB7-dependent mitophagy. EMBO J 2018, 37:235–254.This paper demonstrates that RAB-7 shifts from a broad membrane localization to late endosomes and lysosomes upon GTP exchange, and importantly, this activation status of RAB-7 is dependent on an interaction with TBC1D5-retromer.
- 77.Seaman MNJ, Mukadam AS, Breusegem SY: Inhibition of TBC1D5 activates Rab7a and can enhance the function of the retromer cargo-selective complex. J Cell Sci 2018, 131, jcs217398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.*.Curnock R, Calcagni A, Ballabio A, Cullen PJ: TFEB controls retromer expression in response to nutrient availability. J Cell Biol 2019, 218:3954–3966.This work demonstrates a feedback loop between mTORC and retromer, as TFEB controls transcription of retromer genes.
- 79.*.Chandra M, Chin YK, Mas C, Feathers JR, Paul B, Datta S, Chen K, Jia X, Yang Z, Norwood SJ, et al. : Classification of the human phox homology (PX) domains based on their phosphoinositide binding specificities. Nat Commun 2019, 10: 1528–1614.This is a comprehensive study of the phospholipid binding specificity of human PX domains. Importantly, it provides a mechanism for how the PX domain can have specificity for more than one species of phospholipid, with implications for how even transient and/minor phospholipid species can influence membrane trafficking.
- 80.*.Dickson EJ, Hille B: Understanding phosphoinositides: rare, dynamic, and essential membrane phospholipids. Biochem J 2019, 476:1–23.This is an important review of the burgeoning field of phosphotidylinositol phosphates as key regulators of membrane trafficking.
- 81.Yoshida A, Hayashi H, Tanabe K, Fujita A: Segregation of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate into distinct microdomains on the endosome membrane. BBA - Biomembranes 2017, 1859:1880–1890. [DOI] [PubMed] [Google Scholar]
- 82.Hong NH, Qi A, Weaver AM: PI(3,5)P2 controls endosomal branched actin dynamics by regulating cortactin-actin interactions. J Cell Biol 2015, 210:753–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gillooly DJ, Raiborg C, Stenmark H: Phosphatidylinositol 3-phosphate is found in microdomains of early endosomes. Histochem Cell Biol 2003, 120:445–453. [DOI] [PubMed] [Google Scholar]
- 84.Cheng S, Wang K, Zou W, Miao R, Huang Y, Wang H, Wang X: PtdIns(4,5)P2 and PtdIns3P coordinate to regulate phagosomal sealing for apoptotic cell clearance. J Cell Biol 2015, 210:485–502. [DOI] [PMC free article] [PubMed] [Google Scholar]


