Abstract
Intracellular trafficking requires extensive changes in membrane morphology. Cells utilize several distinct molecular factors and physical cues to remodel membranes. Here we highlight recent advances in identifying the biophysical mechanisms of membrane curvature generation. In particular, we focus on the cooperation of molecular and physical drivers of membrane bending during three stages of vesiculation: budding, cargo selection, and scission. Taken together, the studies reviewed here emphasize that, rather than a single dominant mechanism, several mechanisms typically work in parallel during each step of membrane remodeling. Important challenges for the future of this field are to understand how multiple mechanisms work together synergistically and how a series of stochastic events can be combined to achieve a deterministic result – assembly of the trafficking vesicle.
Introduction
Trafficking routes throughout the cell are highly diverse. Each route involves distinct cargos, coats, membrane compositions, and carrier morphologies. Nonetheless, assembly of each type of trafficking vesicle requires a drastic increase in the curvature of cellular membranes [1]. How does the cell use diverse sets of proteins to drive membrane remodeling in each trafficking pathway? Much of our understanding of these events has emerged from structural biology, which has focused on the independent contributions of individual curved or wedge-like proteins to membrane surfaces [2, 3]. However, it has become increasingly apparent that multiple proteins and curvature driving domains are involved at each step of vesicle biogenesis [4, 5]. Therefore, individual proteins rarely need to act exclusively, and in many cases cannot individually account for the membrane bending requirements. More broadly, many curvature-driving mechanisms appear to contribute additively toward the membrane bending requirements at each stage of vesicular trafficking. Here we highlight recent advances in identifying some of the physical mechanisms at work during three stages of trafficking: vesicle budding, cargo selection, and scission. In particular, we address how seemingly disparate mechanisms could work together at each step.
Physical mechanisms of vesicle budding
Clathrin as a driver vs. organizer
While the cage-like structure of clathrin was first observed more than 40 years ago [6], the last few years have seen a renewal of the debate around clathrin’s role in membrane remodeling. A 2015 paper presented data from correlative light and electron microscopy (CLEM), which suggested that a flat clathrin lattice assembles at the plasma membrane of mammalian cells and then abruptly takes on a high curvature in a process that largely conserves the area of the lattice [7]. Further the lattice appeared highly dynamic in FRAP experiments. A recent follow up paper from the same groups suggested that in yeast, clathrin does not regulate the shape or the elongation of endocytic invaginations [8]. These findings suggest that clathrin lacks a direct mechanical role in membrane remodeling. This conclusion is supported by a recent paper, also based on CLEM, showing that about 70% of the coat is recruited prior to the onset of membrane curvature [9]. These findings are complementary to AFM-based data showing that the clathrin lattice relies on AP2 for much of its stiffness [10], and the finding that uncoating proteins promote dynamic exchange of clathrin triskelia during coated pit growth, helping to explain how the lattice could rearrange to achieve a sudden change in curvature (Figure 1a) [11].
Figure 1: Mechanisms involving membrane curvature that contribute to vesicle biogenesis.
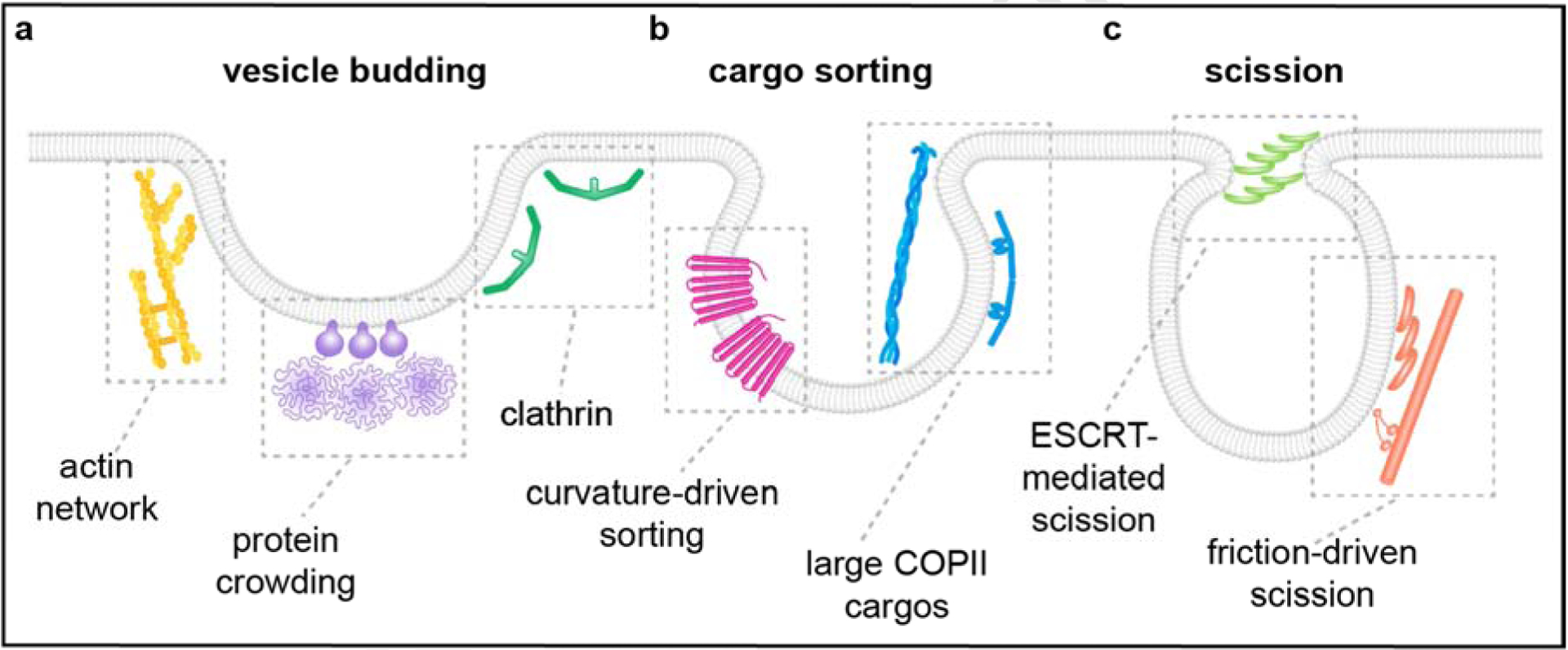
Each stage of vesicle formation likely employs multiple mechanisms that raise the spontaneous curvature of the membrane or that take advantage of this membrane curvature. (a) As the vesicle buds from the membrane, curvature may be generated by the assembly of clathrin, a branched actin network, or crowded disordered proteins. These mechanisms are best understood in the context of endocytic vesicle budding, but likely act at other membrane trafficking routes as well. (b) As cargo is sorted into the nascent vesicle, the shape of transmembrane cargo can result in stronger partitioning into the curved vesicular membrane structure. Cargo size has also been shown to contribute to the morphology of membrane carriers, as is the case for large cargos that drive the formation of tubular COPII vesicles. (c) Finally, scission of membrane trafficking vesicles can involve the polymerization of proteins with a preference of curved membranes, including ESCRT subunits and BAR-domain containing proteins. These protein assemblies can work together with cargo, cytoskeletal networks, and motors to generate the force necessary to drive neck constriction and scission.
However, CLEM does not resolve the changes in morphology experienced by individual coated pits, making it challenging to determine the precise sequence of events. Toward addressing this gap, recent work by the Kural lab, posted on biorxiv [12], suggests that the projected area of the clathrin coat on the membrane surface does not undergo the pronounced reduction that would be associated with an abrupt flat to curved transition. However, the optical diffraction limit makes it difficult to precisely resolve the shape of the curved membrane, requiring a model-based interpretation of the data. These conflicting results highlight the difficulty of precisely monitoring the nanometer-scale changes in membrane shape that occur within seconds during coated vesicle biogenesis.
Actin network-driven curvature
The role of the actin network in membrane remodeling appears to differ significantly across different organisms. Specifically, actin plays a major driving role in yeast endocytosis as compared to most mammalian cell types. The contrasting physical requirements for deforming the yeast versus mammalian plasma membranes, which have vastly different turgor pressures [13], may point to an explanation for these differences in the contributions of actin [14].
Recent work in budding yeast has examined the role of the branched actin network at sequential stages during endocytosis (Figure 1a). Here a stiff, crosslinked actin network was required to initiate and maintain membrane curvature [15, 16]. The force requirement then decreased, such that additional actin network nucleation, but not crosslinking, was sufficient to promote further membrane invagination [15]. Finally, it was proposed that the forces required for the final stages of endocytosis are generated by the viscoelastic properties of the actin network as actin crosslinkers are released and the network relaxes [13, 15]. These studies highlight the ability of the actin network to meet variable force requirements throughout endocytosis.
The stage-specific contributions of actin in mammalian endocytosis were also recently elucidated through investigations of FCHSD2, a protein that is recruited to the flat rims of endocytic pits after initial membrane bending. FCHSD2 promoted actin polymerization as the vesicle matured [17]. In contrast, loss of FCHSD2 hindered the transition from intermediate, budded pits to fully formed vesicles. Collectively, these reports suggest that actin provides a readily available and potent source of membrane bending in intracellular trafficking.
Crowding and intrinsic disorder
Many proteins involved in membrane remodeling contain large, intrinsically disordered domains of 100–1500 amino acids, including many components of clathrin and COPII coated vesicles [18, 19]. However, the role of these domains in membrane remodeling is little understood. A key physical attribute of disordered domains is that they have large hydrodynamic radii in comparison to structured domains of equal molecular weight. Recent work has suggested that when disordered domains become highly crowded at endocytic sites, they can generate local steric pressure that promotes membrane bending (Figure 1a) [20], substantially increasing membrane remodeling in comparison to structured domains alone [21]. Further, disordered domains of can generate sufficient pressure to drive membrane fission [22], an effect which is strengthened when combined with the action of structured scaffolds [23]. Interestingly, disordered proteins are also capable of sensing membrane curvature through at least two distinct physical mechanisms. First, binding to the membrane surface substantially restricts the conformation entropy of a disordered domain, leading to a preference for membrane surfaces of high convex curvature [24]. A second mechanism arises from electrostatic interactions between disordered domains and membrane surfaces, both of which typically carry a net negative charge [25]. In line with these findings, it was recently reported that the disordered C-terminal domain of alpha synuclein can sense and drive membrane curvature [26], building on earlier work [27]. More broadly, crowding among membrane-bound cargo proteins has recently been suggested as a possible explanation for membrane budding during biogenesis of multi-vesicular bodies [28] and lipid droplets [29].
In addition to their high conformational entropy, intrinsically disordered domains also drive the assembly of extended multi-valent protein networks. Specifically, short peptide motifs within disordered regions frequently bind to structured domains within multiple other endocytic proteins [30]. Multi-valent networks of intrinsically disordered proteins throughout the cell have recently been associated with liquid-liquid protein phase separation [31]. Interestingly, two recent manuscripts posted on biorxiv have reported that early initiator proteins of clathrin-mediated endocytosis, Eps15 (Ede1 in yeast) and Fcho1, assemble into liquid-like networks that help to catalyze the assembly of endocytic vesicles [32, 33]. Similar multi-valent, disordered protein networks are found throughout membrane traffic, inspiring future work aimed at decoding the functional relationship between fluid protein droplets and structured vesicular coats.
Physical mechanisms of cargo selection
Curvature-driven sorting of membrane proteins
Membrane trafficking carriers must coordinate vesiculation with loading of the appropriate transmembrane protein cargo. One way to accomplish this task is through protein-protein or protein-lipid interactions that localize cargo at sites of vesicle assembly [34, 35]. This mechanism requires a specific molecular code or signal sequence to be present in every cargo protein. However, recent studies have emphasized the utility of a more general mechanism based on the physical properties of membrane proteins. Specifically, recent work showed that the shape of a transmembrane protein can influence its partitioning into curved membrane structures [36]. Specifically, G protein-coupled receptors (GPCRs) whose structures possess high intrinsic curvature were sorted into curved membrane regions (Figure 1b). Thermodynamic modeling indicated that matching the curvature of proteins and membranes was energetically favorable despite the loss of entropy associated with uneven partitioning of the GPCR.
Curvature-driven sorting could assist in capture of the GPCR cargo at several stages: as the newly synthesized GPCR travels through the biosynthetic pathway, during internalization from the cell surface, and when recycling from endosomes. To drive localization specificity, it is likely that membrane curvature is not the sole mechanism responsible for sorting GPCRs. Indeed, several binding partners for GPCRs also influence their localization [37]. It has been shown for other membrane-associated proteins that the oligomeric state of the protein can also enhance its sorting to highly curved membranes by amplifying the intrinsic curvature [38, 39]. Thus, oligomerization may also strengthen the preference of some GPCRs for curved membranes, suggesting that curvature-driven sorting and protein-protein interactions work together to drive cargo specificity.
Control of COPII vesicle morphology by large cargos
The COPII coat must be able to accommodate large cargos exiting the ER, including the 300 nm long rod-like procollagen 1 (Figure 1b) [40]. Therefore, it has been shown that COPII can form both spherical vesicles of 60–100 nm diameter, and long, tubular carriers, which are promoted by cargo receptors including TANGO1 and cTAGE5 [41]. However, the mechanism by which the COPII coat meshwork assembles to drive different membrane curvatures has remained unclear. Recent cryo-tomography data have demonstrated that the interaction between the inner COPII coat with either the outer coat or cargo receptors can influence the membrane curvature of the nascent COPII vesicle [42]. These interactions modulate the extent of Sar1 GTPase helical insertion into the membrane, either permitting random orientation to drive spherical vesicles or promoting ordered parallel helical insertion, which could drive the anisotropic curvature required for formation of tubules.
How does the COPII coat provide sufficient flexibility to generate carriers of vastly different morphologies while also providing the rigidity needed to drive membrane curvature? Structural studies indicate that the COPII outer coat possesses an architecture of rigid rods connected by flexible hinges [43]. Recent work has identified an additional hinge region in the Sec31 subunit of the outer coat that limits this flexibility to promote rigidity [44]. Binding of cargo to Sec31 could sequester this domain to permit the enhanced flexibility necessary to adapt the vesicle morphology to different cargo requirements.
Physical mechanisms of vesicle scission
Scission in endocytosis
During endocytosis, dynamin, BAR domain-containing proteins, cytoskeletal networks, and motor proteins work together to generate the forces required for scission. Dynamin is thought to be the major driver of scission during clathrin-mediated endocytosis. Nonetheless, dynamin is often aided by BAR proteins [45], actin, and myosin, particularly in contexts where scission must overcome a highly rigid or taut membrane, such as the apical surface of polarized cells [46].
In clathrin-independent endocytosis, a process termed friction-driven scission, which involves BAR proteins and cytoskeletal motors, was recently described (Figure 1c) [47]. Here, a BAR protein scaffold forms on a membrane tubule as it is extended by motors. The protein scaffold is thought to stabilize the tubule while generating friction with the underlying lipids. This friction hinders lipid diffusion. If the rate of lipid diffusion into the tubule fails to keep up with tubule extension, increased tension can drive pore formation in the membrane, resulting in scission of the tubule. The diversity of BAR and motor proteins in membrane trafficking pathways suggests that this mechanism could extend to other cell types and endomembrane contexts, including endosomal tubules [48].
ESCRT-mediated scission
The formation of intraluminal vesicles during multivesicular body (MVB) biogenesis represents a topologically distinct membrane budding process from most membrane trafficking events. As a result, it has been challenging to fully elucidate the mechanism of ESCRT-driven scission. Recent reconstitution experiments have provided a way to measure the force exerted on membranes by ESCRT assembly. The axial force applied by ESCRT-III and Vps4 was shown to be sufficient to drive membrane scission [49], confirming previous functional predictions [50, 51]. In addition, several recent studies have provided new structural evidence of how the molecular geometry and timing of the late ESCRT proteins lead to productive scission.
A recent study examined how ESCRT-III subunits interact to form a three-dimensional helical assembly at the MVB neck (Figure 1c) [52]. ESCRT-III must assemble within the neck with enough freedom to slide past other subunits, yet tightly enough to constrict the membrane. Here it was shown that an electrostatic interface on the sides of each subunit in the helical assembly permitted ESCRT-III proteins to slide past each other as the coil constricted. What then provides the driving force for helical constriction? Theoretical modeling suggests that the shape and directionality of helical assembly are important for understanding how membrane neck constriction proceeds [53]. Further experimental data will be required to generate a better structural understanding of MVB neck geometry, and in turn, a more complete mechanistic understanding.
Another study has recently provided insight into the dynamics of ESCRT-III and the ATPase Vps4 [28]. Through lattice light-sheet microscopy, researchers examined the timing and stoichiometry of ESCRT subunit assembly. Surprisingly, they found that continuous exchange of subunits occurred during ESCRT-III polymerization, simultaneously with Vps4 recruitment. Vps4 activity resulted in tighter ratcheting of the ESCRT-III helix, which in turn crowded together membrane cargo in the MVB. This crowding was postulated to produce the directional membrane buckling needed for invagination.
Perspective
Membrane bending is critical for the vesicular transport of diverse cargo and signals throughout the cell. It is therefore not surprising that cells employ multiple distinct mechanisms to overcome the variable obstacles to membrane bending across biology. Importantly, the force required to generate membrane curvature can vary substantially across different cell types and subcellular locations. For example, rigid membranes and membranes under high tension require higher energetic inputs during membrane vesiculation. Therefore, when considering the relative contributions of each membrane remodeling mechanism, it is important to consider membranes in their native cellular contexts. Interestingly, recent work on these mechanisms suggests that they are rarely exclusive. Therefore, rather than attempting to determine which mechanism dominates each step of trafficking vesicle formation, it is likely more useful to consider how several mechanisms can be coordinated to work together synergistically.
Funding
This work was supported by the National Institutes of Health through grants R01GM120549 to J.C.S. and F32GM133138 to K.J.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Papers of particular interest, published within the period of review, have been highlighted as:
● of special interest
●● of outstanding interest
- 1.McMahon HT and Gallop JL, Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature, 2005. 438(7068): p. 590–6. [DOI] [PubMed] [Google Scholar]
- 2.Campelo F, McMahon HT, and Kozlov MM, The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys J, 2008. 95(5): p. 2325–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itoh T and De Camilli P, BAR, F-BAR (EFC) and ENTH/ANTH domains in the regulation of membrane-cytosol interfaces and membrane curvature. Biochim Biophys Acta, 2006. 1761(8): p. 897–912. [DOI] [PubMed] [Google Scholar]
- 4.Lacy MM, et al. , Molecular mechanisms of force production in clathrin-mediated endocytosis. FEBS letters, 2018. 592(21): p. 3586–3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarsch IK, Daste F, and Gallop JL, Membrane curvature in cell biology: An integration of molecular mechanisms. J Cell Biol, 2016. 214(4): p. 375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson MS, Forty Years of Clathrin-coated Vesicles. Traffic, 2015. 16(12): p. 1210–38. [DOI] [PubMed] [Google Scholar]
- 7.Avinoam O, et al. , ENDOCYTOSIS. Endocytic sites mature by continuous bending and remodeling of the clathrin coat. Science, 2015. 348(6241): p. 1369–72. [DOI] [PubMed] [Google Scholar]
- 8.Kukulski W, et al. , Clathrin modulates vesicle scission, but not invagination shape, in yeast endocytosis. Elife, 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.●.Bucher D, et al. , Clathrin-adaptor ratio and membrane tension regulate the flat-to-curved transition of the clathrin coat during endocytosis. Nat Commun, 2018. 9(1): p. 1109. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using correlative light and electron microscopy, this paper provided evidence for a flat-to-curved transition during clathrin coat assembly.
- 10.Lherbette M, et al. , The AP2 adaptor enhances clathrin coat stiffness. FEBS J, 2019. 286(20): p. 4074–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, et al. , Dynamic instability of clathrin assembly provides proofreading control for endocytosis. J Cell Biol, 2019. 218(10): p. 3200–3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willy NM, et al. , Endocytic Clathrin Coats Develop Curvature at Early Stages of Their Formation. biorxiv, 2019. 10.1101/715219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma R and Berro J, Crosslinking actin networks produces compressive force. Cytoskeleton (Hoboken, N.J.), 2019. 76(5): p. 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulant S, et al. , Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol, 2011. 13(9): p. 1124–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.●●.Picco A, et al. , The contributions of the actin machinery to endocytic membrane bending and vesicle formation. Molecular Biology of the Cell, 2018. 29(11): p. 1346–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrated a role for actin network at multiple stages during endocytosis, suggesting that the actin network dynamically adjusts to provide the force required to carry out each stage.
- 16.Planade J, et al. , Mechanical stiffness of reconstituted actin patches correlates tightly with endocytosis efficiency. PLoS biology, 2019. 17(10): p. e3000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.●.Almeida-Souza L, et al. , A Flat BAR Protein Promotes Actin Polymerization at the Base of Clathrin-Coated Pits. Cell, 2018. 174(2): p. 325–337.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper described a generalizable mechanism by which BAR protein assembly recruited and activated the actin network in order to generate the force needed to promote endocytic pit maturation.
- 18.Dafforn TR and Smith CJ, Natively unfolded domains in endocytosis: hooks, lines and linkers. EMBO reports, 2004. 5(11): p. 1046–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietrosemoli N, Pancsa R, and Tompa P, Structural disorder provides increased adaptability for vesicle trafficking pathways. PLoS computational biology, 2013. 9(7): p. e1003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Busch DJ, et al. , Intrinsically disordered proteins drive membrane curvature. Nature Communications, 2015. 6: p. 7875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snead WT, et al. , Membrane fission by protein crowding. Proc Natl Acad Sci U S A, 2017. 114(16): p. E3258–E3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snead WT, et al. , BAR scaffolds drive membrane fission by crowding disordered domains. J Cell Biol, 2019. 218(2): p. 664–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boucrot E, et al. , Membrane fission is promoted by insertion of amphipathic helices and is restricted by crescent BAR domains. Cell, 2012. 149(1): p. 124–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.●.Zeno WF, et al. , Synergy between intrinsically disordered domains and structured proteins amplifies membrane curvature sensing. Nat Commun, 2018. 9(1): p. 4152. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrated that the disordered and structured domains present in many endocytic proteins can collaborate to drive potent membrane curvature sensing.
- 25.Zeno WF, et al. , Molecular Mechanisms of Membrane Curvature Sensing by a Disordered Protein. J Am Chem Soc, 2019. 141(26): p. 10361–10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fakhree MAA, et al. , Cooperation of Helix Insertion and Lateral Pressure to Remodel Membranes. Biomacromolecules, 2019. 20(3): p. 1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Z, de Messieres M, and Lee JC, Membrane remodeling by alpha-synuclein and effects on amyloid formation. Journal of the American Chemical Society, 2013. 135(43): p. 15970–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.●●.Adell MAY, et al. , Recruitment dynamics of ESCRT-III and Vps4 to endosomes and implications for reverse membrane budding. Elife, 2017. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using quantitative microscopy, this paper identified the stoichiometry and timing of ESCRT-III and Vps4 recruitment during ILV formation. The authors found that dynamic ESCRT subunit exchange and cargo crowding promoted invagination and scission.
- 29.Kory N, et al. , Protein Crowding Is a Determinant of Lipid Droplet Protein Composition. Developmental Cell, 2015. 34(3): p. 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen DJ, Collins BM, and Evans PR, Adaptors for clathrin coats: structure and function. Annual review of cell and developmental biology, 2004. 20: p. 153–91. [DOI] [PubMed] [Google Scholar]
- 31.Shin Y and Brangwynne CP, Liquid phase condensation in cell physiology and disease. Science, 2017. 357(6357). [DOI] [PubMed] [Google Scholar]
- 32.Day KJ, et al. , Liquid-like protein interactions catalyze assembly of endocytic vesicles. biorxiv, 2019. https://www.biorxiv.org/content/10.1101/860684v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kozak M and Kaksonen M, Phase separation of Ede1 promotes the initiation of endocytic events. biorxiv, 2019. 10.1101/861203 [DOI] [Google Scholar]
- 34.Bonifacino JS and Traub LM, Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem, 2003. 72: p. 395–447. [DOI] [PubMed] [Google Scholar]
- 35.Guo Y, Sirkis DW, and Schekman R, Protein sorting at the trans-Golgi network. Annu Rev Cell Dev Biol, 2014. 30: p. 169–206. [DOI] [PubMed] [Google Scholar]
- 36.●●.Rosholm KR, et al. , Membrane curvature regulates ligand-specific membrane sorting of GPCRs in living cells. Nature Chemical Biology, 2017. 13(7): p. 724–729. [DOI] [PubMed] [Google Scholar]; This paper showed that transmembrane proteins with an intrinsic curvature preferentially partitioned into curved membrane structures. These findings indicated that protein shape and ligand-induced shape changes may have a role in cargo sorting during trafficking vesicle formation.
- 37.Ritter SL and Hall RA, Fine-tuning of GPCR activity by receptor-interacting proteins. Nat Rev Mol Cell Biol, 2009. 10(12): p. 819–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno-Pescador G, et al. , Curvature- and Phase-Induced Protein Sorting Quantified in Transfected Cell-Derived Giant Vesicles. ACS Nano, 2019. 13(6): p. 6689–6701. [DOI] [PubMed] [Google Scholar]
- 39.Saaki TNV, Strahl H, and Hamoen LW, Membrane Curvature and the Tol-Pal Complex Determine Polar Localization of the Chemoreceptor Tar in Escherichia coli. J Bacteriol, 2018. 200(9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zanetti G, et al. , COPII and the regulation of protein sorting in mammals. Nat Cell Biol, 2011. 14(1): p. 20–8. [DOI] [PubMed] [Google Scholar]
- 41.Ma W and Goldberg J, TANGO1/cTAGE5 receptor as a polyvalent template for assembly of large COPII coats. Proc Natl Acad Sci U S A, 2016. 113(36): p. 10061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.●●.Hutchings J, et al. , Subtomogram averaging of COPII assemblies reveals how coat organization dictates membrane shape. Nature Communications, 2018. 9(1): p. 4154. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using cryo-tomography, this paper revealed the structure of the COPII coat with improved resolution and identified a mechanism describing how tubular vs. spherical vesicles may be generated through the orientation of amphipathic helix insertion coupled to inner and outer COPII coat assembly.
- 43.Stagg SM, et al. , Structure of the Sec13/31 COPII coat cage. Nature, 2006. 439(7073): p. 234–8. [DOI] [PubMed] [Google Scholar]
- 44.Paraan M, et al. , Flexibility of the Sec13/31 cage is influenced by the Sec31 C-terminal disordered domain. J Struct Biol, 2018. 204(2): p. 250–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meinecke M, et al. , Cooperative recruitment of dynamin and BIN/amphiphysin/Rvs (BAR) domain-containing proteins leads to GTP-dependent membrane scission. J Biol Chem, 2013. 288(9): p. 6651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Biancospino M, et al. , Clathrin light chain A drives selective myosin VI recruitment to clathrin-coated pits under membrane tension. Nature Communications, 2019. 10(1): p. 4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.●●.Simunovic M, et al. , Friction Mediates Scission of Tubular Membranes Scaffolded by BAR Proteins. Cell, 2017. 170(1): p. 172–184.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper revealed an exciting mechanism for vesicle scission by which BAR protein assembly generated friction with the underlying lipids, ultimately resulting in membrane severing as vesicles were elongated by motor proteins.
- 48.Ripoll L, et al. , Myosin VI and branched actin filaments mediate membrane constriction and fission of melanosomal tubule carriers. J Cell Biol, 2018. 217(8): p. 2709–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.●●.Schoneberg J, et al. , ATP-dependent force generation and membrane scission by ESCRT-III and Vps4. Science, 2018. 362(6421): p. 1423–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using in vitro reconstitution in nanotubes, the authors provided the first direct evidence that ESCRT-III and Vps4 exert force on membranes sufficient to drive scission.
- 50.Mierzwa BE, et al. , Dynamic subunit turnover in ESCRT-III assemblies is regulated by Vps4 to mediate membrane remodelling during cytokinesis. Nat Cell Biol, 2017. 19(7): p. 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elia N, et al. , Computational model of cytokinetic abscission driven by ESCRT-III polymerization and remodeling. Biophys J, 2012. 102(10): p. 2309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banjade S, et al. , Electrostatic lateral interactions drive ESCRT-III heteropolymer assembly. Elife, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Agudo-Canalejo J and Lipowsky R, Domes and cones: Adhesion-induced fission of membranes by ESCRT proteins. PLoS Comput Biol, 2018. 14(8): p. e1006422. [DOI] [PMC free article] [PubMed] [Google Scholar]


