Abstract
Background:
Genomic assessment previously took months to result and was unable to impact clinical care in the pediatric intensive care unit (PICU). The advent of rapid exome sequencing potentially changes this. We investigated the impact of rapid exome sequencing in a pilot study on pediatric patients admitted to a single PICU with new onset metabolic/neurologic disease.
Methods:
Rapid exome sequencing (7 days to verbal result) was performed on (n=10) PICU patients age <6 years admitted with new onset metabolic/neurologic disease. The primary outcome of interest was inpatient LOS, which served as a proxy for inpatient cost.
Results:
A significant reduction in median LOS was identified when comparing PICU patients who underwent rapid exome sequencing to historical controls. From those patients who underwent rapid sequencing, 5 had likely pathogenic variants. In 3 cases with diagnostic genetic results, there was a modification to clinical care attributable to information provided by exome sequencing.
Conclusions:
This pilot study demonstrates that rapid exome sequencing is feasible to do in the PICU, that genetic results can be returned quickly enough to impact critical care decision-making and management. In a select population of PICU patients, this technology may contribute to a decrease in hospital length of stay.
Introduction:
Given the clinical acuity of pediatric patients admitted to the intensive care unit, effective management relies on quickly obtaining relevant diagnostic information and rapidly delivering a tailored clinical response. Multiple studies have examined how genomic sequencing in critically ill infants may improve the diagnostic yield in caring for these patients. The majority of these investigations have been conducted in neonatal intensive care units (NICUs), but a growing number are now occurring in Pediatric Intensive Care Units (PICUs) as well [1–5]. The clinical value of rapid sequencing was first demonstrated by Saunders et al. in 2012 in 2 neonates who received a genetic diagnosis by undergoing whole genome sequencing within 50 hours [2]. These findings have been confirmed by others in critically ill infants and children, providing a diagnostic yield that ranges from 40% to 57% when genomic sequencing is conducted in a carefully selected pediatric patient population [2, 6].
Apart from the cost of genomic sequencing, which continues to decrease as this technology is more widely utilized in clinical settings, it is the ability to return genetic results in an expedited manner that remains its greatest barrier to clinical application in intensive care units. One study reported that genomic results were clinically useful in 32.6% of diagnosed patients when clinical exome sequencing (CES) was provided with routine turnaround times (median 136 days), but clinically useful in 65–71% of patients diagnosed via rapid genome sequencing (rGS) and rapid clinical exome sequencing (rCES) when results were provided with a median turnaround time of 12–23 days [1,7]. Taken together, these results further highlight that it is the expedited return of results that has greater impact on the clinical utility of genetic testing.
In addition to its clinical utility, rapid genomic sequencing has also been suggested to reduce health care costs [8–10]. A recent study of the application of rCES in pediatric acute illness demonstrated that expedited genetic sequencing is not only feasible in an ICU setting, but also found the technology to be cost-effective and of high diagnostic and clinical utility [11]. Importantly, however, prior studies have failed to link healthcare cost-savings to decreased hospital length of stay (LOS) in patients undergoing expedited genomic sequencing.
To that end, we aimed to investigate the impact of rCES on pediatric patients <6 years old admitted to our PICU with new onset metabolic/neurologic disease in a pilot study. We focused on this narrow subset of PICU patients based on previous observational data from our group suggesting that they might benefit most from a rapid diagnostic approach. Our overarching goal was to better understand the role of rCES in our PICU. We were additionally interested in observing how hospital LOS, as a surrogate for inpatient hospital cost, was impacted by implementation of this technology in our narrowly selected cohort of patients. Here, our hypothesis was that rCES would decrease inpatient hospital LOS in this select group both by providing a diagnosis earlier in the patient’s hospitalization, and completing the standard work-up for these specific diagnoses within a rapid timeframe. This hypothesis was based on previous observational data from our PICU whereby patients meeting these enrollment criteria had long and complicated hospital courses.
Patients and Methods:
Recruitment and consent
rCES was completed prospectively on 10 unrelated PICU patients and their biologic parents. Eligibility criteria were: (1) < 6 years old, (2) admission with a predetermined ICD-10 study inclusion diagnosis code pertaining to new onset metabolic/neurologic disease (Appendix A), (3) anticipated inpatient hospitalization for at least 3 days. Exclusion criteria were: known genetic diagnosis to explain the patient’s clinical phenotype (Figure 1). These eligibility criteria were based on pilot data from patients who received whole exome sequencing in a 12-month period in 2015 and 2016. From 26 patients who had WES performed during this time, 9 of them fulfilled the criteria subsequently established, and all of them had complicated hospital courses. Six of the nine were ultimately found to have a likely disease-causing mutation found only after whole exome sequencing. Notably, patients did not need to have a “suspected” genetic diagnosis or have previously been seen by a geneticist in order to be eligible for enrollment.
Figure 1: rCES proband screening and enrollment algorithm.

Abbreviations: PICU, pediatric intensive care unit; ICD-10: International Classification of Disease 10th revision.
The majority of the patients who underwent rCES were sequenced as trios, but 2/10 were sequenced as duos when both parents were unavailable for genetic testing.
This study was approved by the Columbia University IRB. Participants were recruited between October 2017 and December 2018. Pretest genetic education and counseling was provided for all eligible children by the inpatient pediatric clinical genetics team of medical geneticists at NewYork-Presbyterian Morgan Stanley Children’s Hospital. Parents provided written consent for rCES with the option of receiving secondary medically actionable findings [12–14]. For all consented children, a detailed clinical history and family history were abstracted from the electronic medical record.
Identification of expedited exome sequencing cohort
Following our enrollment algorithm, 1358 unique patients were admitted to the medical-surgical PICU at our institution from Oct 2017-Dec 2018 and screened for the rCES study (Figure 2). 1194 patients did not meet the study criteria on initial screen. The most common reason for exclusion was not possessing an ICD-10 admitting diagnosis meeting the inclusion criteria. Of note, many patients were excluded based upon more than one exclusion criteria. We then manually reviewed the electronic medical record for the remaining 164 patients to determine eligibility. The most common reason for exclusion at this step was that the ICD-10 admitting diagnosis did not accurately reflect the patient’s clinical phenotype—e.g. a patient admitted with diabetic ketoacidosis who had metabolic acidosis listed as their admitting diagnosis. We approached 13 eligible families, and three families declined who did not want genetic testing on their children. For the parents who consented to participate rCES, 8 patients were assessed as trios (patient and biological mother and father) and two patients were assessed as duos (patient and biological mother only). The study was discontinued following enrollment of the tenth patient as funding for this pilot study was available for the first 10 patients who met criteria and consented to enrollment.
Figure 2: Flow Diagram of enrollment of 10 PICU patients for rCES.
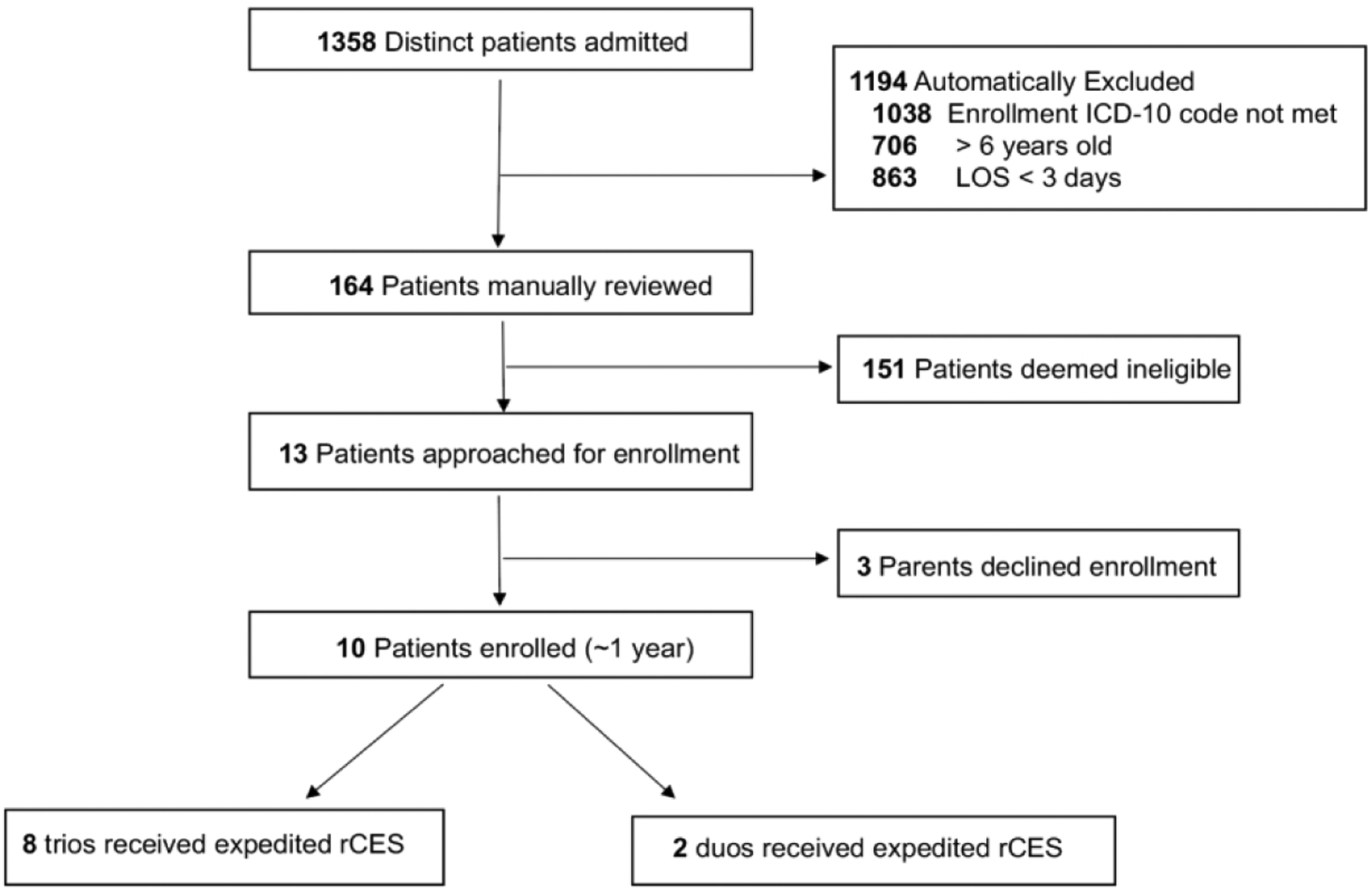
Primary reasons for enrollment exclusion were qualifying ICD-10 admitting diagnosis not met, age at admission greater than 6 years old, LOS less than 72 hours, and pre-existing genetic diagnosis at the time of PICU admission. Of note, multiple patients met multiple exclusion criteria.
Abbreviations: PICU, pediatric intensive care unit; rCES, rapid clinical exome sequencing; LOS, length of stay; ICD-10, International Classification of Disease 10th revision.
Identification of historical controls
All PICU patients admitted to the medical/surgical PICU from 2010–2015 were considered for inclusion as historical controls (n=6967). Historical controls were identified via application of identical study inclusion criteria; historical controls were < 6 years old, admitted with a predefined, corresponding ICD-9 study inclusion code, and admitted to the PICU for at least 72 hours (Figure 3). The most common reason for exclusion from the control cohort was the ICD-9 code. As with the study participants, historical controls were not required to have a suspected genetic diagnosis or been previously seen by a geneticist. Manually review of the remaining 1143 patient charts identified 101 PICU historical controls who met study criteria. This represents 0.88% of all screened PICU admissions from 2010–2015 which is similar to the 0.96% of PICU admissions who met enrollment criteria during the study period.
Figure 3: Flow diagram of identification of 101 PICU historical controls.

All PICU historical controls were admitted to the medical/surgical PICU between 2010–2015. The primary reasons for control exclusion were pre-specified, corresponding ICD-9 admitting diagnosis code not met, age at admission greater than 6 years old, and admission duration less than 72 hours. Of note, multiple patients met multiple exclusion criteria.
Abbreviations: ICU, intensive care unit; ICD-9, International Classification of Disease 9th revision.
Comparison of rCES cohort and historical controls
rCES cohort and historical controls were compared according to gender, race and ethnicity, age, primary admitting diagnosis and median Pediatric Index of Mortality 3 (PIM3) risk of mortality score. Comparisons using Fisher exact test or 2-Tailed t-test were performed, when applicable. PIM3 risk of mortality is a prediction model built using logistic regression that assesses the risk of mortality among children admitted to an ICU [15]. The model accounts for both binary and continuous factors related to a patient’s overall risk of mortality.
Exome sequence analysis
A minimum of 3mL of fresh blood in EDTA was obtained from enrolled PICU patients and their parents. Genetic testing was performed in a CLIA-certified clinical lab (GeneDx, Gaithersburg, MD) with the XomeDxXpress test. Genomic DNA was extracted, and the exonic regions and flanking splice junctions of the genome were captured using a proprietary capture kit developed by GeneDx and sequenced by massively parallel (NextGen) sequencing on an Illumina system with 100bp or greater paired-end reads. Reads were aligned to human genome build GRCh37/UCSC hg19, and analyzed using a custom-developed analysis tool (Xome Analyzer). Capillary sequencing was used to confirm all potentially pathogenic variants identified in the proband and parents. Sequence and copy number alterations were reported according to the Human Genome Variation Society (HGVS) and International System for Human Cytogenetic Nomenclature (ISCN) guidelines, respectively [16].
Following XomeDxXpress analysis, the ordering physician received a verbal summary within 7 days after sample submission if any pathogenic variants were identified that were consistent with the patient’s clinical phenotype. Within 14 days following sample submission, a written report was provided detailing all pathogenic variants identified. Additionally, any variants of uncertain significance were also disclosed at this time.
The reporting of laboratory findings was performed as previously described [17]. Genetic findings were classified as molecularly diagnosed when pathogenic or likely pathogenic variant(s) were detected in a disease gene that was associated with the phenotype in the studied patient. For further clinical assessment, exome sequencing results were additionally reviewed by a multidisciplinary group of physicians including board-certified clinical geneticists, intensivists and neurologists regarding clinical correlation, follow-up evaluation and understanding of the molecular diagnosis.
Results:
Demographic and clinical characteristics
In considering the demographic and clinical characteristics for the 10 study cases in comparison to 101 historical PICU controls, there was no statistically significant difference detected between the two populations with regard to gender, race/ethnicity, age, primary admitting diagnosis or median Pediatric Index of Mortality 3 (PIM3) risk of mortality score (Table 1).
Table 1:
Demographic and Clinical Characteristics of 10 rCES probands and 101 historical controls
| Cases (n=10) | Controls (n=101) | P-valuea,b | ||
|---|---|---|---|---|
| Gender, n (%) | Female | 4 (40%) | 46 (46%) | 1 |
| Male | 6 (60%) | 55 (54%) | ||
| Race and ethnicity, n (%) | European | 3 (30%) | 34 (33%) | 0.075 |
| African/ African American | 1 (10%) | 17 (17%) | ||
| Hispanic/Latino | 5 (50%) | 13 (13%) | ||
| Asian/Native American/Pacific Islander | 0 (0%) | 8 (8%) | ||
| Other | 1 (10%) | 29 (29%) | ||
| Age, m, n (%) | 0–24 | 8 (80%) | 90 (89%) | 0.123 |
| 24–48 | 1 (10%) | 11 (11%) | ||
| 48–72 | 1 (10%) | 0 (0%) | ||
| Primary admitting diagnosis, n (%) | Failure to thrive | 2 (20%) | 24 (24%) | 0.344 |
| Seizures | 3 (30%) | 9 (9%) | ||
| Suspected metabolic disease/ Unexplained acidosis | 2 (20%) | 31 (30%) | ||
| Liver Failure | 0 (0%) | 18 (18%) | ||
| Hypotonia | 0 (0%) | 9 (9%) | ||
| Hypoglycemia | 2 (20%) | 1 (1%) | ||
| Developmental Delay | 0 (0%) | 9 (9%) | ||
| Altered Mental Status | 1 (10%) | 0 (0%) | ||
| PIM3 risk of mortality, median (IQR) | 0.048 (0.004–0.075) | 0.051 (0.014–0.100) | 0.332 |
Abbreviations: m, month; IQR, interquartile range; rCES, rapid clinical exome sequencing.
2-Tailed t test or Fisher exact test, when applicable.
Statistical significance assessed for a p< 0.05 level.
Genetic diagnoses made via rCES
The average turnaround time for verbal return of expedited exome results was 7.7 days, with formal written documentation reported at 14 days. Five of ten patients had pathologic or likely pathologic variants identified. When considering these variants in context of the clinical phenotype for which the rCES was sent, sequencing results demonstrated a 30% diagnostic yield (Table 2).
Table 2:
Rapid exome sequencing results for 10 PICU patients
| ID | Age (m) | Sex | Clinical Indication for rCES | Abnormal Genetic Finding(s) | Variants & Zygosity | Mode of Inheritance | Inherited From | Disease(s) | Lab Variant Classification | Clinical Interpretation | Effect on Clinical Management | LOS (d) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 001 | 52 | M | Seizures | DEPDC5 | c.3562_3563dupTC, Het | AD | Mother | DEPDC5 Related Disorders | Likely pathogenic | Diagnosticb | Trialed everolimus therapy for seizure control, deferred muscle biopsy, redirection of goals of carea | 72 |
| 006 | 9 | F | Seizures | KCNB1 | c.661delGinsAT, Het | AD | De Novo | KCNB1 Related Disorders | Pathogenic | Diagnostic | Etiology of seizures no longer attributed to febrile seizures. Patient discharged on standing AED, with close follow-up with neurology, and referral for early interventiona | 9 |
| 009 | 1 | M | Chronic diarrhea | PIK3CD | c.2564 T>G, Het | AD | De Novo | Immunodeficiency | Likely pathogenic | Diagnostic | Immunology consulted, initiated weekly IVIG infusions. Medical team deferred additional work-up of chronic diarrhea | 56 |
| 012 | 7 | M | Failure to Thrive | SLC45A2 | c. 1532 C>T, Hom | AR | Mother + Father | Oculocutaneous Albinism Type IV | Likely pathogenic | Diagnostic for albinism but not for failure to thrive | Referred to ophthalmology for follow-up | 43 |
| 015 | 8 | M | Altered mental status/ALF | G6PD, AGL, NOTCH2 |
c.202 G>A, Hem c.2390 A>G, Het c. 2647 A>G, Het |
XL, AR, AD |
Mother Mother Unknown |
G6PD Deficiency, Glycogen Storage Disease Type III, NOTCH2 Related Disorders |
Pathogenic, VUS, VUS |
Incidental finding, Not diagnostic Not diagnostic |
Medication selection altered in light of G6PD; deficiency. Patient underwent further work-up for Alagille syndrome and GSD III, which were both negative |
32 |
| 018 | 6 | M | Hypoglycemia | None | 16 | |||||||
| 021 | 36 | F | Suspected metabolic disease/acidosis | None | 13 | |||||||
| 024 | 0 | F | Hypoglycemia | None | 14 | |||||||
| 027 | 3 | M | Seizure | None | 14 | |||||||
| 030 | 2 | F | Suspected metabolic disease/acidosis | None | 8 |
Abbreviations: m, months; rCES, rapid clinical exome sequencing; ALF, acute liver failure; VUS, variant of unclear significance; Het, heterozygous; Hom, homozygous; AD, autosomal dominant; AR, autosomal recessive; AED, antiepileptic drug; IVIG, intravenous immunoglobulin; LOS, hospital length of stay; d, days; GSD III, Glycogen Storage Disease Type III.
Exome results considered by the primary medical team to be clinically impactful to patient’s immediate management in the PICU.
At the time that this patient was admitted to our PICU, the abnormal genetic finding was clinically interpreted by the medical team as diagnostic. Upon later review by a larger multidisciplinary genetics team, however, there was some concern that the findings do not completely capture the patient’s phenotype and that we still do not entirely understand the genetic basis of the patient’s seizure disord
Impact in hospital length of stay
The median length of stay was 15 days for the rCES group compared to 59 days for the historical controls (p< 0.001), and the 75% interquartile range (IQR) for the rCES was 46.2 days compared to 95.5 days for the controls (Table 3).
Table 3:
Significantly reduced hospital length of stay in rCES PICU patients
| rCES PICU Cases | PICU Controls | P-valuea,b | |
|---|---|---|---|
| Number of subjects | 10 | 101 | |
| Average TAT for verbal rCES results, d | 4.97 | ||
| Average TAT for written rCES report, d | 9.83 | ||
| Hospital LOS, d median, (IQR) | 15 (12–46.2) | 59 (41–95.5) | 0.001 |
Abbreviations: TAT, turnaround time; d, days; LOS, length of stay; rCES, rapid clinical exome sequencing; IQR, interquartile range.
P < 0.05.
Mann-Whitney U test conducted given non-parametric distribution of data.
Impact of exome sequencing on clinical management
For the five participants with positive genetic findings, three had genetic diagnoses thought to be clinically actionable with regarding their immediate inpatient management.
Patient 001 was a 4.5-year-old male with new-onset refractory status epilepticus. He had been trialed on multiple anti-epileptic therapies and had medically refractory seizures. He was found to have a likely pathogenic variant in DEPDC5, which is a negative regular of the mTOR complex 1 pathway. Variants at this gene have previously been implicated in autosomal dominant focal epilepsies (OMIM # 604364). Importantly, despite the patient’s positive rCES finding, the pathologic nature of this aberration was questionable to the medical team given that it was found to be inherited from his mother, who did not display the clinical phenotype. Despite this ambiguity, given the refractory nature of the patient’s condition, the medical team opted to act on the results. The team trialed him on an mTOR inhibitor, everolimus, in hopes of controlling his seizures based on his molecular findings. Additionally, a muscle biopsy was cancelled upon receipt of his genetic findings. Ultimately, his seizures were not controlled, and everolimus was discontinued. Anecdotally, the patient’s family, as well as the medical team, felt that having the rCES information returned quickly within his hospitalization was extremely helpful in coordinating the next steps of his care and ultimately determining his disposition.
Patient 006 was a 9-month-old female who was admitted with new onset complex seizures. Testing demonstrated a de novo heterozygous pathogenic variant in KCNB1 that encodes the alpha subunit of a voltage-gated potassium channel highly expressed in the mammalian brain [18]. Heterogeneous de novo variants have been reported in multiple individuals with early-onset epileptic disorders, including infantile spasms and epileptic encephalopathy (OMIM # 616056) [19]. Given her diagnostic genetic findings, the patient was no longer considered to have had a singular “seizure,” but was instead diagnosed to have epilepsy. This diagnosis carries long-term implications regarding the need for intensive neurologic follow-up, tailored medication management, and implementation of early intervention services. Such a comprehensive post-discharge plan may not have been offered without a formal diagnosis of epilepsy if the patient was thought to merely have had an isolated seizure. Furthermore, based on her molecular diagnosis, additional investigation into the etiology of her new-onset complex seizures was not pursued, the patient was maintained on levetiracetam, and 5 days later she was discharged. The medical team caring for the patient felt that her PICU course and management was streamlined with the availability of her genetic diagnosis early in her hospitalization.
Patient 009 was a 2-month-old infant male, initially admitted to the inpatient pediatric service with chronic diarrhea and failure to thrive and transferred to the PICU with worsening metabolic acidosis, hypernatremic dehydration and hypoglycemia. The patient’s rCES demonstrated a de novo, heterozygous likely pathogenic variant in PIK3CD, a member of a family of lipid kinases involved in cell growth, proliferation, development, motility, survival and intracellular trafficking expressed in leukocytes [20]. Heterozygous gain-of-function variants involving PIK3CD have been previously associated with immunodeficiency-14 (OMIM # 615513) [21]. Given the patient’s presenting symptoms, the subspecialist teams felt that his protracted course could be explained by this primary immunodeficiency. Following the initiation of IVIG infusions, coupled with significant modifications to the patient’s diet, his diarrhea improved and he was able to tolerate feedings. Additionally, based on the patient’s molecular diagnosis, the medical team deferred further measures to invasively work-up the etiology of the patient’s failure to thrive. Lastly, the family reported gaining comfort with a genetic diagnosis. They reported that it helped them better understand why the patient’s chronic diarrhea was so refractory, feel comfortable limiting additional clinical investigations and procedures, and helped them to understand the patient’s overall course and prognosis and better understand the implications regarding any future pregnancies.
Discussion:
We studied the impact of rCES on previously undiagnosed metabolic/neurologic patients in our PICU, with a particular focus on hospital length of stay in those select patients who were sequenced. Based on unpublished observational data from our experience with clinical exome sequencing in a more inclusive cohort, we narrowly defined the prospectively identified cohort here based on clinical criteria that we believed would be most impacted by rapid exome sequencing. The frequency of genetic diagnosis observed in our PICU cohort using rCES has been replicated in previous clinical studies conducted in both NICUs and PICUs [22], [6, 23]. Interestingly, we observed a significant decrease in hospital LOS in those patients who underwent rCES in comparison to historical controls, identified via identical inclusion criteria and admitted to our medical/surgical PICU between 2010–2015. While it is impossible to prove from our limited pilot study that this reduction in LOS directly resulted from the use of rCES in these patients, it is a particularly noteworthy observation and one which warrants further exploration and study on a larger scale. Moreover, this study confirms that a pre-established laboratory and clinical pipeline, as well as a multidisciplinary team, are fundamental to not only ensure a rapid turnaround time for genetic results, but also to provide adequate genetic counseling so that the family and the primary medical team understand the results and can meaningfully apply them to clinical decision-making [24, 25].
Our study confirms that the results of exome sequencing need to be rapidly available in order to be clinically impactful in the PICU. Given the variability in published turnaround times for exome sequencing, there is some concern that the 7-day turnaround time provided through this study is still too long to provide clinically actionable information given the nature of PICU management. For all ten of our study probands who underwent rCES, clinicians awaited documented confirmation of the rapid genetic results, and did not act upon verbal results, with regard to disclosing the information to the family and medically acting upon the results. Turnaround times will hopefully continue to decrease as this technology is more broadly adopted in the clinical setting. With regard to its impact on hospital LOS, it would be most useful if rCES was sent at the beginning of the patient’s hospitalization. In the PICU setting, any opportunity to shave time off testing would certainly have an impact of clinical utility and decision-making. The most informative study would provide evidence that rapid return of exome resulted in both cost-savings and also meaningful changes to a patient’s clinical course. Additionally, we utilized a commercial, CLIA-certified clinical laboratory to sequence our rCES cohort. Completion of this project did not depend on having a clinical laboratory capable of expedited exome sequencing on site. Thus, the workflow we present here regarding patient identification and prompt sequencing turnout times with verbal reporting of primary genetic findings could be replicated in any PICU.
Our study focused on PICU patients with the primary admitting diagnoses of: hypotonia, seizure, developmental delay, altered mental status, failure to thrive, metabolic disease, hypoglycemia, or acute liver failure and were chosen because of the reasonable probability of an underlying identifiable genetic condition.
The experience presented here suggests a decrease in inpatient length of stay for those patients who undergo rCES in comparison to historical controls. Similar findings were previously published by Farnaes et al. who demonstrated a decrease in inpatient length of stay in ICU patients secondary to early diagnosis of genetic disease [25]. If this finding continues to hold true in larger studies, the cost of rCES could be ultimately offset by a significant reduction in hospital LOS. Beyond cost, however, incentivizing hospitals to pay for expedited exome sequencing in this unique PICU population, future studies are needed to examine the clinical outcomes and quality of care delivered to patients receiving rCES in the PICU. Additionally, long-term studies are needed to address how exome results are later utilized by the patient and/or family, and how these findings impact family quality of life.
Limitations
The primary limitation of this study is that expedited CES was only completed on 10 PICU patients. Enrollment was limited to 10 PICU patients because this was a pilot study geared towards better understanding the application and feasibility of offering rCES at our institution.
Additionally, this study does not provide cost-effectiveness analysis of exome sequencing in PICU patients compared to other diagnostic strategies. Many challenges exist in performing this analysis, not only in actually establishing how much particular tests and inpatient care costs (and the discrepancies between inpatient charges, costs and reimbursements), but also cost-effectiveness will differ greatly depending on when in a patient’s hospitalization genetic testing is sent. Future studies should strive to not only incorporate cost analysis of rapid versus conventional exome sequencing, but also account for additional modalities of genetic testing (e.g targeted gene panels) often utilized in the PICU.
Conclusions
Our study supports that rapid exome sequencing, in a carefully selected subset of PICU patients, is both a feasible and beneficial test to obtain as genetic results can be returned quickly enough to impact critical care decision-making. Our pilot study observed that genetic diagnoses may alter PICU management with regard to the addition of targeted therapeutics, discontinuing further diagnostic tests/procedures once a diagnosis was established, and decisions regarding surgical interventions. Furthermore, we noted a reduced hospital LOS in our PICU patients who underwent rCES, regardless of identifying a genetic diagnosis, in comparison to historical controls. This observation warrants further clinical study given the implications it may have on hospital throughput and inpatient hospital cost.
IMPACT.
What is the key message of your article?
Ten prospectively enrolled PICU patients with defined clinical criteria and their parents underwent rapid exome sequencing
50% received a genetic diagnosis, and medical management was affected for 60% of those patients
Median hospital LOS was significantly decreased in this selective subset of PICU patients
What does it add to the existing literature?
Genetic disorders and congenital anomalies are a leading cause of pediatric mortality
Genomic assessment previously took weeks to months for results and was therefore unable to acutely impact clinical care in the pediatric intensive care unit (PICU)
The recent advent of rapid exome sequencing changes this in selected patients
What does it add to the existing literature?
Rapid exome sequencing is feasible to do in a PICU
Genetic results can be returned quickly enough to impact critical care decision-making
When done in a carefully selected subset of pediatric patients, rapid exome sequencing can potentially decrease hospital LOS
Statement on informed consent:
This study was approved by the Columbia University IRB. Participants were recruited between October 2017 and December 2018. Pretest genetic education and counseling was provided for all eligible children by the inpatient pediatric clinical genetics team of medical geneticists at NewYork-Presbyterian Morgan Stanley Children’s Hospital. Parents provided written consent for rCES with the option of receiving secondary medically actionable findings [12–14]. For all consented children, a detailed clinical history and family history were abstracted from the electronic medical record.
Acknowledgements
We would like to thank NewYork-Presbyterian Morgan Stanley Children’s Hospital for their financial support of this project as well as Carlos Aguilar Breton and the entire PICU staff for helping to facilitate patient identification, enrollment and disclosures. Additionally, we would like to thank the pediatric genetics team for their assistance in consenting for rCES and for returning results to families. Lastly, we would like to acknowledge that this publication was supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH), through Grant Number UL1TR001873.
Appendix A: Predetermined ICD 9/10 Admission Codes Qualifying for Rapid Exome Sequencing
ICD-9
Hypotonia (p94.2, 781.3)
Seizure (345.9, 780.32, 345.0, 345.4, 345.5, 345.6, 345.8,345.3, 779.0, 780.31, 345.2, 345.7, 345.9, 345.1)
Developmental Delay (315.9, 315.4, 315.8)
Altered mental status (780.97)
Failure to thrive (783.41, 779.34, 783.7)
Metabolic disease (348.31, 276.2, 775.7, 277.9, 277.7, 775.81)
Hypoglycemia (251.1, 775.6, 251.1)
Acute Liver failure (570, 572.2, 573.8, 573.9)
ICD-10
Hypotonia (p94.2, R27.0, R27.8, R27.9)
Seizure (G40.911, G40.919, R56.01, G40.A10, G40.A11, G40.201, G40.209, G40.0, G40.1, G40.2, G40.3, G40.A, G40.8, G40.9, G40.B)
Developmental Delay (F81.9, F89, F82, F88)
Altered Mental Status (R41.82)
Failure to thrive (P92.6, R62.51, R62.7)
Metabolic Disease (G93.41, E87.2, P74.0, E88.9, E88.81, P84)
Hypoglycemia (E16.2, E16.1, P70.4, E16.0)
Acute liver failure (K72.00, K72.90, K72.91, K76.89, K76.9)
Footnotes
Financial Disclosure: All listed authors have indicated they have no financial relationships relevant to this article to disclose.
Conflict of Interest: All authors have indicated they have no potential conflicts of interest to disclose.
Category of Study: Clinical
References
- 1.Willig LK, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med. 2015;3(5):377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saunders CJ, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012;4(154):154ra135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kingsmore SF, et al. Adopting orphans:comprehensive genetic testing of Mendelian diseases of childhood by next-generation sequencing. Expert Rev Mol Diagn. 2011;11:855–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrikin JE, et al. The NSIGHT1-randomized controlled trial: rapid whole-genome sequencing for accelerated etiologic diagnosis in critically ill infants. NPJ Genom Med. 2018; 3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanford EF, et al. Rapid Whole Genome Sequencing Has Clinical Utility in Children in the PICU. Pediatr Crit Care Med. 2019; 20(11):1007–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soden SE, et al. Effectiveness of exome and genome sequencing guided by acuity of illness for diagnosis of neurodevelopmental disorders. Sci Transl Med. 2014;6(265):265ra168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng L, et al. Use of Exome Sequencing for Infants in Intensive Care Units: Ascertainment of Severe Single-Gene Disorders and Effect on Medical Management. JAMA Pediatr. 2017;171(12):e173438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howell KB, et al. A population-based cost-effectiveness study of early genetic testing in severe epilepsies of infancy. Epilepsia. 2018;59(6):1177–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith HS, et al. Clinical Application of Genome and Exome Sequencing as a Diagnostic Tool for Pediatric Patients: a Scoping Review of the Literature. Genet Med. 2019;21(1):3–16. [DOI] [PubMed] [Google Scholar]
- 10.Vissers L, et al. A clinical utility study of exome sequencing versus conventional genetic testing in pediatric neurology. Genet Med. 2017;19(9):1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stark Z, et al. Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genet Med. 2018;20(12):1554–1563. [DOI] [PubMed] [Google Scholar]
- 12.Green RC, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15(7):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grody WW, et al. ACMG position statement on prenatal/preconception expanded carrier screening. Genet Med. 2013;15(6):482–3. [DOI] [PubMed] [Google Scholar]
- 14.Kalia SS,, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med. 2017;19(2):249–255. [DOI] [PubMed] [Google Scholar]
- 15.Straney L, et al. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care*. Pediatr Crit Care Med. 2013;14(7):673–81. [DOI] [PubMed] [Google Scholar]
- 16.Bamshad MJ, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011;12(11):745–55. [DOI] [PubMed] [Google Scholar]
- 17.Retterer K, et al. Assessing copy number from exome sequencing and exome array CGH based on CNV spectrum in a large clinical cohort. Genet Med. 2015;17(8):623–9. [DOI] [PubMed] [Google Scholar]
- 18.de Kovel CGF, et al. Neurodevelopmental Disorders Caused by De Novo Variants in KCNB1 Genotypes and Phenotypes. JAMA Neurol. 2017;74(10):1228–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torkamani A, et al. De novo KCNB1 mutations in epileptic encephalopathy. Ann Neurol. 2014;76(4):529–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jamee M, et al. Clinical, Immunological, and Genetic Features in Patients with Activated PI3Kdelta Syndrome (APDS): a Systematic Review. Clin Rev Allergy Immunol. 2019; 10.1007/s12016-019-08738-9 [DOI] [PubMed] [Google Scholar]
- 21.Nunes-Santos CJ, Uzel G, and Rosenzweig SD. PI3K pathway defects leading to immunodeficiency and immune dysregulation. J Allergy Clin Immunol. 2019;143(5):1676–1687. [DOI] [PubMed] [Google Scholar]
- 22.Daoud H,. Next-generation sequencing for diagnosis of rare diseases in the neonatal intensive care unit. Cmaj. 2016;188(11):E254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stark Z, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18(11):1090–1096. [DOI] [PubMed] [Google Scholar]
- 24.French CE, et al. Whole genome sequencing reveals that genetic conditions are frequent in intensively ill children. Intensive Care Med. 2019;45(5):627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farnaes L, et al. Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genom Med. 2018;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]


