Abstract
Background & Aims:
Idiopathic chronic pancreatitis (ICP) is the second most common subtype of CP. In 1994, researchers reported the bimodal age at onset of ICP symptoms: early-onset ICP (EO-ICP; median age, 19.2 years) and late-onset ICP (LO-ICP; median age, 56.2 years). Ages of onset and clinical features of ICP differed from those of alcohol-related CP (ACP). However, variants in PRSS1 had not yet been associated with ICP. We reexamined ages of onset of ICP in a large, North American cohort of patients, and investigated the effects of genetic factors and alcohol use in patients with EO-ICP, LO-ICP, or ACP.
Methods:
We performed a cross-sectional analysis of patients with CP of European ancestry enrolled in the North American Pancreatitis Study 2 studies, a prospective study of 1195 patients with CP from 26 centers in the United States from August 2000 through December 2014. We compared age at onset of symptoms for 130 patients with CP who were lifetime abstainers from alcohol (61 patients with early onset and 69 patients with late onset), 308 light to moderate alcohol drinkers with CP, and 225 patients with ACP and heavy to very heavy alcohol use. DNA from available patients was analyzed for variants associated with CP in SPINK1, CFTR, and CTRC. The Kruskal-Wallis test was used to compare continuous variables across groups and based on genetic variants.
Results:
Median ages at onset of symptoms were 20 years for patients with EO-ICP and no alcohol use, 58 years for patients with LO-ICP and no alcohol use, 47 years for light to moderate alcohol drinkers with CP, and 44 years for patients with ACP. A higher proportion of patients with EO-ICP had constant pain (65%) than patients with LO-ICP (31%) (P=.04). A higher proportion of patients with ACP had pseudocysts (43%) than patients with EO-ICP (11%) (P=.001). A higher proportion of patients with EO-ICP had pathogenic variants in SPINK1, CFTR, or CTRC (49%) than patients with LO-ICP (23%), light to moderate alcohol drinking with CP (26%), or ACP (23%) (P=.001). Among patients with variants in SPINK1, those with EO-ICP had onset of symptoms at a median age of 12 years, and light to moderate alcohol drinkers with CP had an age at onset of 24 years. Among patients with variants in CFTR, light to moderate alcohol drinkers had an age at onset of symptoms of 41 years, but this variant did not affect age at onset of EO-ICP or ACP.
Conclusions:
We confirmed previously reported ages at onset of symptoms for EO-ICP and LOICP in a North American cohort. We found differences in clinical features among patients with EO-ICP, LO-ICP, and ACP. Almost half of patients with EO-ICP have genetic variants associated with CP, compared to about one-quarter of patients with LO-CP or ACP. Genetic variants affect ages at onset of symptoms in some groups.
Keywords: risk factor, prognostic, disease progression, mutation
Introduction
Chronic pancreatitis (CP), a syndrome of pancreatic inflammation and dysfunction, is a worldwide problem. While earlier studies suggested that the vast majority of patients were heavy alcohol users and nearly all men,1–4 there is growing recognition of other risk factors, such as genetic susceptibility, smoking, and autoimmunity.5,6 Idiopathic CP (ICP) is the second most common form of CP.
In 1994, Layer et al7 reported a retrospective analysis of ICP patients seen at Mayo Clinic between 1976 and 1982, with prospective follow-up through 1985. They identified 66 patients who were abstinent from alcohol and had no other identifiable causes of CP. They reported a bimodal age at onset of disease, early-onset ICP (EO-ICP) and late-onset ICP (LO-ICP), with the distinction set as an age at onset of 35 years or younger or older than 35 years, and compared these patient groups to 249 patients with alcohol-related CP (ACP). ACP was defined by consumption of 50 g or more of alcohol per day (≥4 drinks/day). Patients with alcohol consumption of less than 50 g per day were excluded from the study. In the 2 ICP groups, the median age at onset was 19.2 and 56.2 years, respectively, while the median age at onset of ACP was 43.9 years. This study suggested an equal distribution among the sexes in both EO-ICP and LO-ICP, while ACP patients were more frequently men. Patients with EO-ICP had more pain, but slower onset of calcifications and pancreatic dysfunction than LO-ICP. Conversely, nearly half the patients with LO-ICP were without pain. In a follow-up evaluation, the authors reported that intake of alcohol, even smaller amounts (ie, low to moderate alcohol consumption group) resulted in earlier onset of disease, more frequent pain, and calcifications.8 To our knowledge, these results have not been validated in the United States.
Since 1994, the etiologic spectrum of CP has markedly expanded. In 1996, we reported mutations in the cationic trypsinogen gene (PRSS1) to be the cause of hereditary pancreatitis,9 opening the door to a broader view of pancreatitis to include genetic risk and etiologies.10–15 The study by Layer et al7 was limited to a “family history of pancreatitis” as an exclusion factor for inherited disease, and therefore, was not able to separate patients with PRSS1 mutations without suggestive family history or complex genetic disorders associated with sequence variants in CFTR, SPINK1, and CTRC in their ICP group. Furthermore, smoking is now recognized as an important risk factor for pancreatitis with important interactions with alcohol use,5,6 and autoimmune pancreatitis is recognized as a form of CP.16,17
The North American Pancreatitis Study 2 (NAPS2) provides an ideal cohort to reexamine the findings by Layer et al.7 Given our current understanding, we hypothesized that major pathogenic genetic variants would be more prevalent in patients with EO-ICP when compared with LO-ICP and ACP, and may explain a bimodal distribution of ICP.
Materials and Methods
North American Pancreatitis Studies
NAPS2 is a set of 3 studies (original NAPS2, NAPS2 continuation and validation study, and NAPS2 Ancillary Study) that prospectively enrolled 1,195 patients with CP from 26 centers in the United States from August 2000 to December 2014.18–20 The diagnosis of CP was based on definitive changes on abdominal imaging studies, specifically endoscopic retrograde cholangiopancreatography, contrast-enhanced computed tomography, magnetic resonance imaging, endoscopic ultrasound, or histology as previously described. On endoscopic ultrasound, at least 5 parenchymal or ductal changes or the presence of calcifications were required. Detailed information was collected from patients and their enrolling physician using 2 case report forms. All patients provided a written informed consent prior to any study procedures. Study patients also provided a blood sample for research purposes. The studies were approved by the institutional review board at each participating institution. Detailed methodology of NAPS2 studies has been published previously.18–20 All authors had access to the study data and have reviewed and approved the final manuscript.
Study Cohort
To make the study cohort comparable to the Layer et al study,7 of the 1,195 CP patients in the NAPS2 cohort, we sequentially excluded patients as shown in Figure 1. Initially, we excluded those who self-identified themselves as non-European ancestry and those for whom ancestry was unknown. Among CP patients of European ancestry, we excluded those with PRSS1 variants associated with hereditary pancreatitis (ie, p.N29I, p.R122H) on post hoc genetic testing (done for research after enrollment) and those for whom results were unknown. We further excluded patients for whom the enrolling physician identified another etiology for CP, such as autoimmune, obstructive, or hyperlipidemia, those who reported a family history of hereditary pancreatitis, those with incomplete drinking history, and those in whom genetic testing for CFTR, SPINK1, and CTRC was incomplete or unsuccessful.
Figure 1:
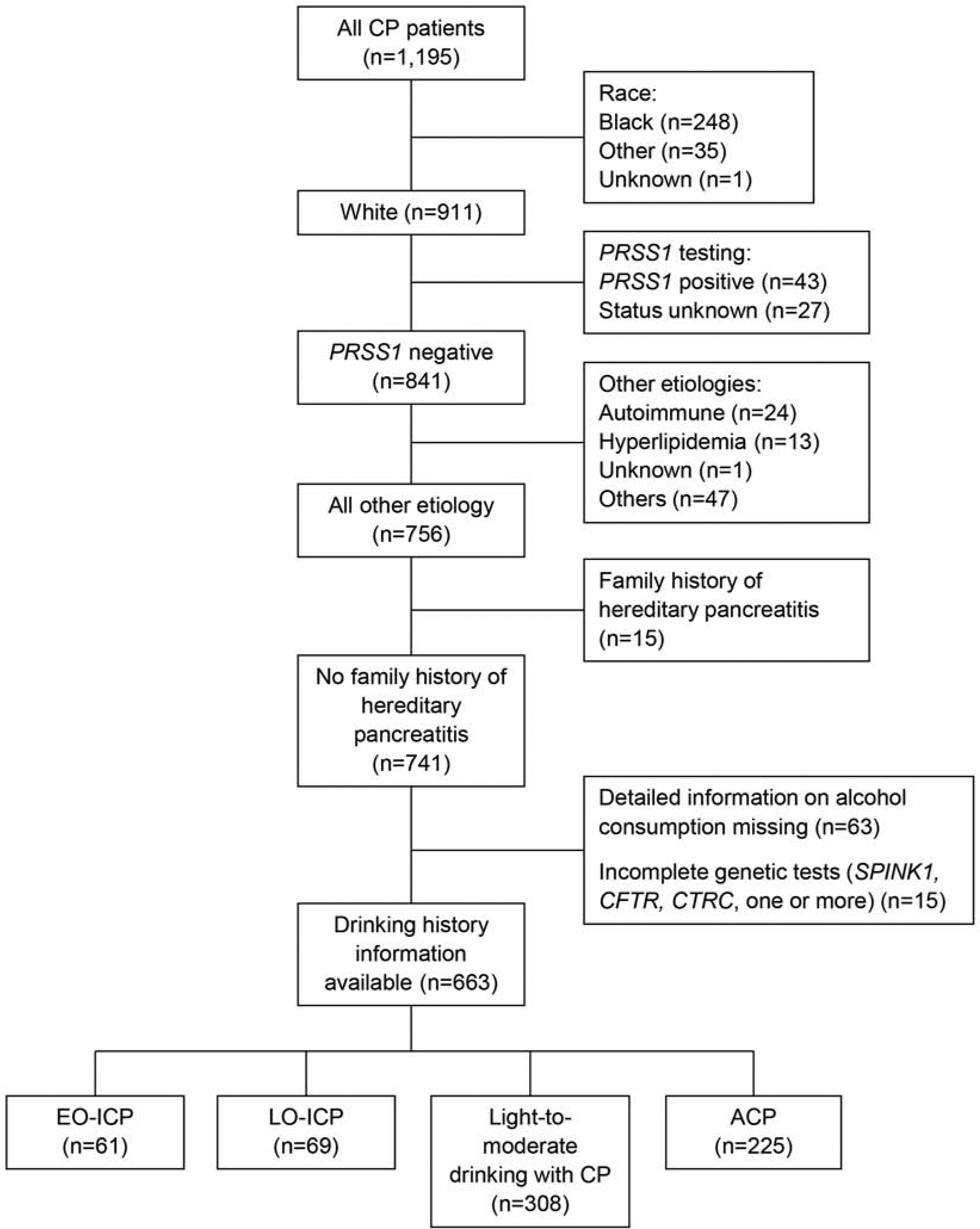
Study Population Flow Chart. ACP indicates alcohol-related chronic pancreatitis; CP, chronic pancreatitis; EO-ICP, early-onset idiopathic chronic pancreatitis; LO-ICP, late-onset idiopathic chronic pancreatitis.
Patient Classification
Patients who self-reported to be lifetime abstainers of alcohol and had onset of pancreatitis symptoms at 35 years of age or younger were classified as having EO-ICP, while those with onset of pancreatitis symptoms after 35 were classified as having LO-ICP, as in the Layer et al study.7 For self-reported ever drinkers, we used information on drinking during the maximum lifetime drinking period to classify patients into those with alcohol consumption of 4 drinks or fewer per day (light to moderate drinking with CP) and more than 4 drinks per day (ACP), similar to the study by Layer et al.7
Genetic Testing
Post hoc analysis for known genetic mutations in PRSS1, CFTR, SPINK1, and CTRC genes was performed in all NAPS2 patients for whom an adequate amount of DNA sample was available. Detailed methods have been described previously.21–24
Clinical Data
Relevant data for this study were obtained from case report forms completed by the patients with assistance from the site coordinator and the enrolling physician as needed. This information included demographics, self-reported alcohol and smoking history, physician-defined etiology, age at first symptoms of pancreatitis, history of acute pancreatitis (AP), pain symptoms and disability, exocrine insufficiency, diabetes mellitus, morphologic changes in the pancreas, and medical, endoscopic, and surgical treatments received prior to enrollment. Data on pain and narcotic use were limited to the NAPS2 continuation and validation study as this study specifically asked patients to report presence of pain in the year preceding enrollment and physicians to report on patients’ patterns of pain medication use. Smoking status was classified as never, past, and current, and the amount was quantified as fewer than 1 or 1 or more packs per day.
Statistical Analysis
For discrete variables, the counts and proportions within each etiology group are presented as descriptive statistics. For continuous variables, the median and interquartile range (IQR) are presented as descriptive statistics for each etiology group. The Pearson χ2 test with continuity correction was used to compare discrete variables across all 4 etiology groups and between the EO-ICP and LO-ICP groups. The Kruskal-Wallis test was used to compare continuous variables across all 4 etiology groups, between the EO-ICP and LO-ICP groups, and within groups based on presence of genetic mutations.
Results
Study Cohort
The final sample size for this study was 663 patients with CP. Of these, 130 (19.6%) were lifetime alcohol abstainers placed in 1 of the 2 ICP groups, 308 (46.5%) were light to moderate alcohol drinkers with CP, and 225 (33.9%) were heavy to very heavy alcohol drinkers placed in the ACP group (Figure 1). Among the 130 patients with ICP, 61 (46.9%) had EO-ICP, and 69 (53.1%) had LO-ICP.
Demographics, History of Smoking, and Age at Onset of Symptoms
The age at onset of pancreatitis symptoms and pancreatitis diagnosis in patients with ICP followed a bimodal distribution (Table 1, Figure 2). Median (IQR) age at onset was 20 (12–27) years and 58 (46–67) years in patients with EO-ICP and LO-ICP, respectively. In light to moderate alcohol drinkers with CP, the median (IQR) age at onset of symptoms was 47 (35–61) years and in those with ACP it was 44 (36–52) years (Table 1).
Table 1.
Characteristics of Patients with EO-ICP, LO-ICP, Light to Moderate Drinking with CP, and ACP
| Light to Moderate | ||||||
|---|---|---|---|---|---|---|
| Characteristic | EO-ICP (n=61) | LO-ICP (n=69) | Drinking with CP (n=308) | ACP (n=225) | P Value (Overall) | P Value (EO-ICP vs LO-ICP) |
| Sex, male , No. (%) | 23 (38) | 13(19) | 164(53) | 165 (73) | <.001 | .02 |
| Age at symptom onset, median, (IQR) | 20 (12–27) | 58 (46–67) | 47 (35–61) | 44 (36–52) | <.001 | <.001 |
| Age at diagnosis, median (IQR) | 26.5 (18–37) | 60 (48–68) | 52 (40–63) | 47 (41–55) | <.001 | <.001 |
| Age at enrollment, median (IQR) | 30.1 (20.8–40.0) | 64.3 (52.1–70.9) | 54.4 (42.4–65.6) | 50.8 (44.0–57.4) | <.001 | <.001 |
| BMI, current, median (IQR) | 25.0 (20.6–28.7) | 25.8 (22.4–29.1) | 24.2 (21.4–27.4) | 23.4 (20.6–26.2) | .003 | .19 |
| BMI, maximum, median (IQR) | 29.5 (25.1–36.5) | 31.0 (27.8–33.5) | 28.4 (25.5–32.7) | 29.1 (25.0–32.8) | .05 | .23 |
| History of smoking, No. (%)a | <.001 | .23 | ||||
| Never | 40/60 (67) | 39 (57) | 75/307 (24) | 15 (7) | ||
| Past | 4/60 (7) | 11 (16) | 106/307 (35) | 44 (20) | ||
| Current | 16/60 (27) | 19 (28) | 126/307 (41) | 166 (74) | ||
| Smoked ≥1 pack/day, No. (%) | 7/57 (12) | 9/62 (15) | 126/302 (42) | 127/221 (57) | <.001 | .82 |
| History of AP, No. (%)b | 48/61 (79) | 37/66 (56) | 192/295 (65) | 141/207 (68) | .04 | .02 |
| Disability due to CP, No. (%) | 18 (30) | 11 (16) | 51 (17) | 60 (27) | .02 | .10 |
| Calcifications, No. (%) | 28 (46) | 35 (51) | 165 (54) | 140 (62) | .09 | .71 |
| Pseudocysts, No. (%) | 7 (11) | 19 (28) | 73 (24) | 96 (43) | <.001 | .04 |
| Endocrine insufficiency, No. (%) | 18 (30) | 19 (28) | 80 (26) | 67 (30) | .85 | .96 |
| Exocrine insufficiency, No. (%) | 18 (30) | 25 (36) | 108 (35) | 82 (36) | .86 | .53 |
| PERT, No. (%) | 52 (85) | 46 (67) | 205 (67) | 148 (66) | .04 | .02 |
| Antioxidant vitamins, No. (%) | 21 (34) | 20 (29) | 109/307 (36) | 94 (42) | .27 | .63 |
| Pancreatic sphincterotomy, No. (%)c | 14/26 (54) | 10/26 (38) | 58/154 (38) | 32/133 (24) | .02 | .40 |
| Pancreatic duct stent, No. (%) | 31 (51) | 24 (35) | 100 (32) | 63 (28) | .016 | .10 |
| Pancreatic duct stone removal, No. (%) | 6 (10) | 4 (6) | 41 (13) | 30 (13) | .45 | .59 |
| Drainage operations, No. (%) | 4 (7) | 5 (7) | 22 (7) | 17 (8) | ≥99 | ≥99 |
| Partial or complete pancreatectomy, No. (%) | 10 (16) | 15 (22) | 53 (17) | 41 (18) | .91 | .58 |
Abbreviations: ACP, alcohol-related chronic pancreatitis; AP, acute pancreatitis; BMI, body mass index; CP, chronic pancreatitis; EO-ICP, early-onset idiopathic chronic pancreatitis; IQR, interquartile range; LO-ICP, late-onset idiopathic chronic pancreatitis; PERT, pancreatic enzyme replacement therapy; Q1, first quartile; Q3, third quartile.
Smoking data not reported for all patients.
AP status was never or unclear in remaining patient.
Only includes the North American Pancreatitis Studies continuation and validation (NAPS2-CV) study participants.
Figure 2.
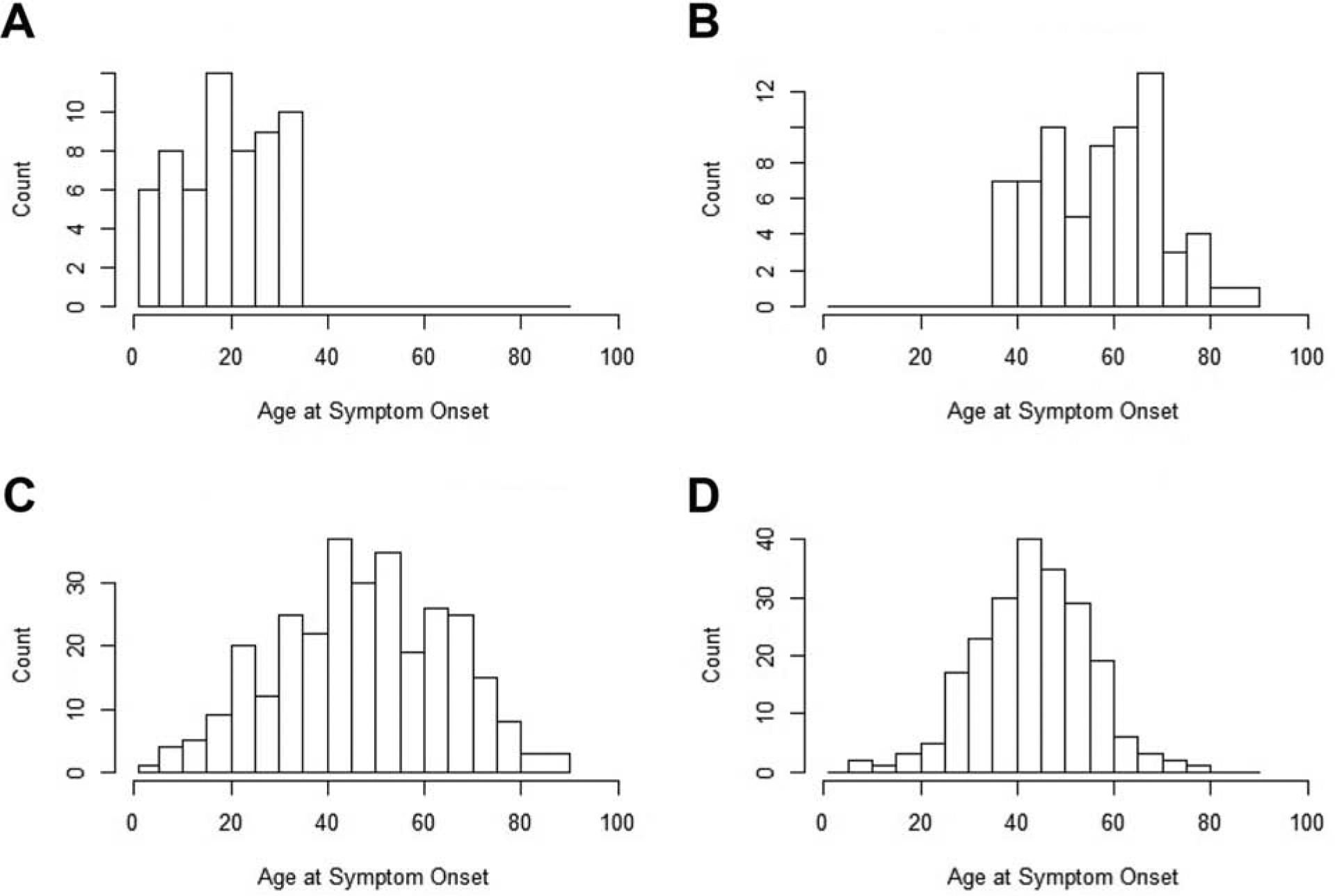
Distribution of Age at Onset of Pancreatitis Symptoms. A, Patients with early-onset idiopathic chronic pancreatitis. B, Patients with late-onset idiopathic chronic pancreatitis. C, Light to moderate alcohol drinkers with chronic pancreatitis. D, Patients with alcohol-related chronic pancreatitis.
Table 1 shows the distribution of sex significantly differed between patient groups. Patients with EO-ICP or LO-ICP were predominantly women (62% and 81%, respectively), light to moderate alcohol drinkers with CP were evenly split (53% men), and those with ACP were predominantly men (73%). Patients with LO-ICP were significantly more likely to be women when compared with EO-ICP (Table 1). Almost three-quarters of patients with ACP and 41% of light to moderate alcohol drinkers with CP were current smokers, which was significantly higher than those with ICP. Similarly, over half of those with ACP and 42% of light to moderate alcohol drinkers with CP smoked 1 or more packs per day, while patients with ICP smoked significantly fewer packs per day. Smoking habits were similar among patients with EO-ICP and LO-ICP. There was no difference in body mass index between the ICP groups.
AP, Pain Frequency and Severity, and Disability
Over 50% of patients in each of the groups had at least 1 attack of AP during their clinical course. The highest prevalence of AP was in the EO-ICP group, which was significantly higher than in the LO-ICP group (Table 1).
Patient pain characteristics, including temporal nature and pain severity, are shown in Table 2. Almost all patients with EO-ICP reported pain (96%), which was significantly more than patients with LO-ICP (69%; P=.04). The majority of light to moderate alcohol drinkers with CP and those with ACP also reported pain, though at a lower frequency than patients with EOICP. The temporal nature and severity of pain was mostly similar among patients with EO-ICP, with description of constant and severe pain being more frequent than in patients with LO-ICP.
Table 2.
Pain Patterns and Pain Medication Use in Patients with EO-ICP, LO-ICP, Light to Moderate Drinking with CP, and ACPa
| Light to Moderate | ||||||
|---|---|---|---|---|---|---|
| Pain Characteristic | EO-ICP (n=26) | LO-ICP (n=26) | Drinking with CP (n=154) | ACP (n=133) | P Value (Overall) | P Value (EO-ICP vs LO-ICP) |
| Pain pattern, No. (%) | ||||||
| None | 1 (4) | 8 (31) | 27 (18) | 19 (14) | .10 | .04 |
| Intermittent | 8 (31) | 10 (38) | 53 (34) | 34 (26) | ||
| Constant | 17 (65) | 8 (31) | 74 (48) | 80 (60) | ||
| Pain severity, No. (%) | ||||||
| None | 1 (4) | 8 (31) | 27 (18) | 19 (14) | .07 | .08 |
| Moderate | 5 (19) | 5 (19) | 31 (20) | 13 (10) | ||
| Severe | 20 (77) | 13 (50) | 96 (62) | 101 (76) | ||
| Pain medication, No. (%) | ||||||
| None | 5 (19) | 11 (42) | 65 (42) | 33 (25) | .01 | .27 |
| Nonnarcotics | 0 (0) | 2 (8) | 13 (8) | 7 (5) | ||
| Intermittent narcotics | 8 (31) | 7 (27) | 37 (24) | 29 (22) | ||
| Constant narcotics | 13 (50) | 6 (23) | 39 (25) | 64 (48) | ||
Abbreviations: ACP, alcohol-related chronic pancreatitis; CP, chronic pancreatitis; EO-ICP, early-onset idiopathic chronic pancreatitis; LO-ICP, late-onset idiopathic chronic pancreatitis.
Data shown only from the North American Pancreatitis Studies continuation and validation (NAPS2-CV) study.
Many patients in all 4 groups reported narcotic use to treat pain, either intermittently or on a constant basis (Table 2). While approximately 50% of patients with EO-ICP or ACP were on constant narcotics, only about 25% of patients with LO-ICP or light to moderate alcohol drinkers with CP required constant narcotic use. Overall, about 1 in 5 patients reported disability due to CP (140/663 [21.1%], Table 1). The prevalence of disability was the highest among patients with EO-ICP when compared to other groups.
Sequelae of CP and Treatment
The sequelae of CP and treatments are presented in Table 1. No major differences were detected in the rates of endocrine and exocrine insufficiency between groups. Although presence of pancreatic calcifications was not statistically different between groups, calcifications were most frequent in patients with ACP (62%) and least frequent in those with EO-ICP (46%). Prevalence of pseudocysts was significantly higher in patients with ACP compared to the other groups (P<.001). Medical treatment with pancreatic enzyme replacement therapy was more common in the EO-ICP group (85%) when compared with the other 3 groups, where it was used in about two-thirds of patients. Just over 50% of patients with EO-ICP underwent pancreatic sphincterotomy or pancreatic duct stent placement, which was more than the other 3 groups. There was no difference between performances of surgical interventions between the groups.
Genetic Analysis
We evaluated the prevalence of pathogenic variants in CFTR, SPINK1, and CTRC genes previously associated with pancreatitis in the 4 patient groups and their effect on age at symptom onset (Tables 3 and 4). Nearly 50% of patients with EO-ICP carried a pathogenic variant associated with at least 1 of these 3 genes, which was significantly greater than the other 3 groups, where variants were observed in about 25% of patients each. Interestingly, presence of mutations accelerated the clinical presentation with earlier onset of symptoms, especially in light to moderate alcohol drinkers with CP (Table 4). In subgroup analyses, the effects of having a SPINK1 mutation significantly accelerated the onset of symptoms in the EO-ICP group from 22 years to 12 years (P=.004) and in the light to moderate alcohol drinkers with CP group from 50 years to 24 years (P<.001). Having a CFTR mutation accelerated the age at onset of symptoms for light to moderate alcohol drinkers with CP from 50 years to 41 years (P=.03), but did not affect age at onset in the other 3 groups.
Table 3.
Genetic Mutations in Patients with EO-ICP, LO-ICP, Light to Moderate Drinking with CP, and ACP
| Light to Moderate | ||||||
|---|---|---|---|---|---|---|
| Mutation | EO-ICP (n=61) | LO-ICP (n=69) | Drinker with CP (n=308) | ACP (n=225) | P Value (Overall) | P Value (EO-ICP vs LO-ICP) |
| Any, No. (%) | 30 (49) | 16 (23) | 79 (26) | 51 (23) | <.001 | .004 |
| SPINK1, No. (%) | 15 (24) | 6 (9) | 22 (7) | 21 (9) | <.003 | .03 |
| CTRC, No. (%) | 2 (3) | 2 (3) | 3 (1) | 3 (1) | .83 | ≥99 |
| CFTR, No. (%) | 22 (36) | 10 (15) | 59 (19) | 30 (13) | .001 | .008 |
Abbreviations: ACP, alcohol-related chronic pancreatitis; CP, chronic pancreatitis; EO-ICP, early-onset idiopathic chronic pancreatitis; LO-ICP, late-onset idiopathic chronic pancreatitis.
Table 4.
Age at Onset of Pancreatitis By Mutation Status in Patients with EO-ICP, LO-ICP, Light to Moderate Drinking with CP, and ACP
| Mutation | EO-ICP (n=61) | LO-ICP (n=69) | Light to Moderate Drinking with CP (n=308) | ACP (n=225) |
|---|---|---|---|---|
| Any, y, median (IQR) | ||||
| Yes | 19.5 (8–23) | 54.5 (45.5–67) | 39 (25–52)a | 44 (36–49) |
| No | 20 (16–29) | 58 (47–67) | 51 (40–63) | 44 (36–53) |
| SPINK1, y, median (IQR) | ||||
| Yes | 12 (8–20) | 54 (45–67) | 24 (20–36)a | 39 (28–44) |
| No | 22 (16.5–29)b | 58 (46–67) | 50 (38–62) | 45 (36–52) |
| CTRC, y, median (IQR) | ||||
| Yes | 15.5 (4–27) | 51.5 (42–61) | 42 (39–45) | 40 (39–48) |
| No | 20 (12–27) | 58 (46–67) | 47(35–61) | 44 (36–52) |
| CFTR, y, median (IQR) | ||||
| Yes | 21 (8–27) | 47 (45–67) | 41 (28–55)c | 46.5 (41–51) |
| No | 19 (13–27) | 59 (47–67) | 50 (37–62) | 43.5 (35.5–52) |
Abbreviations: ACP, alcohol-related chronic pancreatitis; CP, chronic pancreatitis; EO-ICP, early-onset idiopathic chronic pancreatitis; LO-ICP, late-onset idiopathic chronic pancreatitis; Q1, first quartile; Q3, third quartile.
P<.001.
P=.004.
P=.03.
Discussion
The NAPS2 cohort allows for the reevaluation of prior descriptive studies of cohorts of CP in light of newer insights into etiopathogenesis, including genetics. We confirmed the existence of 2 types of ICP, EO-ICP and LO-ICP, with a nearly identical bimodal age distribution to the previously published series.7 We also confirmed several other clinical features that characterize patients with EO-ICP and LO-ICP, suggesting that they represent 2 distinct populations of patients with overlapping features. We found that almost half of patients with EOICP had underlying genetic variations, suggesting a potential genetic etiology for these previously described ICP cases. Furthermore, we highlighted the possibility of a gene-environment interaction between the presence of genetic variations and alcohol consumption.
Layer et al7 recognized the existence of rare patients with hereditary pancreatitis, but had to rely on a strong family history. In 1996, PRSS1 p.R122H and p.N29I were discovered as causes of hereditary pancreatitis, allowing for genetic testing of individual patients.9 Since then, numerous genetic factors associated with pancreatitis have been discovered and divided into pathogenic variants (ie, sufficient to cause a Mendelian disorder) and risk factors (ie, part of multi-risk factor complexes).15 In the present study, we focused on pathogenic variants in PRSS1, CFTR, SPINK1, and CTRC (excluding the CTRC p.G60G risk haplotype). Our findings highlight the significance of genetic variations on clinical presentation, especially in patients with EO-ICP, and in their interaction with alcohol use, most notably lowering the age at disease onset in light to moderate alcohol drinkers with CP. It is likely that patients with EO-ICP in whom no mutations were identified may harbor currently unknown mutations or complex risk factor signatures in multiple genes currently under investigation.
Patients with EO-ICP were more likely to have had episodes of AP than those with LOICP, and had far more constant abdominal pain. These results are strikingly similar to Layer et al,7 where 100% of patients with EO-ICP reported pain. As in their LO-ICP group, we found that LO-ICP was a painless disease in approximately 30% of patients.7 Pain was substantial enough in all groups to necessitate narcotic medication use and lead to disability. In our study, EO-ICP and LO-ICP had similar rates of endocrine and exocrine insufficiency, in agreement with the previous study.7 Calcifications were more frequently found in patients with ACP than with EOICP, but not with statistical significance found by Layer et al.7 Calcifications were seen in almost half of the EO-ICP group (46%) in our study; this prevalence is different from the findings reported by Layer et al,7 where none of the 25 patients with EO-ICP had calcifications at presentation. The reason for a higher prevalence in our study is likely related to recording this observation at the time of study enrollment rather than at the time of symptom onset; most patients were enrolled several years after symptom onset. It may also be related to improvements in imaging technology now compared to over 2 decades ago. It is also notable that over 50% of patients with EO-ICP underwent sphincterotomies and pancreatic duct stent placements, much higher than in the other 3 groups. If genetic variants are likely to contribute to these younger patients having CP, the effectiveness of anatomic-based, endoscopic treatments deserves further study.
Our classification of ICP mimics that of Layer et al7 in that all equivocal or uncertain diagnoses were excluded; however, important differences exist. We now recognize smoking as a common risk factor for CP and its notable interactions with alcohol use.5,6 In addition, we now recognize genetic etiologies as common causes of CP in younger patients.20 Although uncommon, patients suspected of having autoimmune pancreatitis were excluded from the current study and may have been included in the Layer et al study7 as it was not defined until 2001.16
Although EO-ICP and LO-ICP were defined more than 2 decades ago,7 this observation has not been formally replicated. A strength of NAPS2 is that it is a multicenter study providing a sampling of pancreatitis cases from referral centers throughout the United States. It also has the advantage of a much larger number of patients, so that sampling bias and random chance are less likely reasons for these observations. In addition, the NAPS2 patients were prospectively enrolled using improved diagnostic equipment that was not available in the 1980s.
One limitation of our study was that it was a cross-sectional cohort study, not allowing for longitudinal follow-up of patients. Thus, important long-term clinical outcomes, such as endocrine and exocrine insufficiency and changes in abdominal pain, could not be determined.
In summary, our results confirm a bimodal distribution of ICP and demonstrate that a subset of these is likely related to an underlying genetic variation. Future studies using more advanced genetic analysis may further reduce the number of patients with truly idiopathic disease and better characterize the long-term course of these patients.
What You Need to Know.
Background:
Studies are needed to reexamine ages at onset of idiopathic chronic pancreatitis (ICP) in a modern, North American cohort of patients, as well as the effects of genetic factors and alcohol use in patients with early-onset, late-onset, and alcohol-related CP.
Findings:
We confirmed previously reported median at onset of symptoms for early-onset (20 years) and late-onset (58 years) ICP in a North American cohort. We found differences in clinical and genetic features among patients with early-onset, late-onset, and alcohol-related CP.
Implications for patient care:
Early-onset, late-onset, and alcohol-related CP have distinct clinical and genetic features; these groups of patients should be managed differently.
Acknowledgments:
The authors acknowledge contributions from Kimberly Stello and Danielle Dwyer for laboratory management, Michael O’Connell, PhD, for the Epidemiology Data Center, and Lisa Kennard, PhD, Tian Ye, and Stephen R. Wisniewski, PhD, for data management.
Grant support: This research was partly supported by the National Institutes of Health (NIH) DK061451 (D.C.W.), DK077906 (D.Y.), U01 DK108306 (D.C.W., D.Y.), UO1DK108320 (C.E.F.). This publication was made possible in part by grants UL1 RR024153 and UL1TR000005 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research (University of Pittsburgh. PI: Steven E. Reis, MD). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at Digestive Disease Week, Chicago, Illinois, May 5–9, 2017
Conflict of interest: DCW serves as a consultant for AbbVie, Regeneron, Ariel Precision Medicine, is a cofounder of Ariel Precision Medicine and may have equity. The other authors have no conflicts of interest to report.
References
- 1.Ammann RW, Akovbiantz A, Largiader F, et al. Course and outcome of chronic pancreatitis. Longitudinal study of a mixed medical-surgical series of 245 patients. Gastroenterology 1984;86:820–828. [PubMed] [Google Scholar]
- 2.Marks IN, Bank S, Louw JH. Chronic pancreatitis in the Western Cape. Digestion 1973;9:447–453. [DOI] [PubMed] [Google Scholar]
- 3.Sarles H, Cros RC, Bidart JM. A multicenter inquiry into the etiology of pancreatic diseases. Digestion 1979;19:110–125. [DOI] [PubMed] [Google Scholar]
- 4.Strum WB, Spiro HM. Chronic pancreatitis. Ann Intern Med 1971;74:264–277. [DOI] [PubMed] [Google Scholar]
- 5.Tolstrup JS, Kristiansen L, Becker U, et al. Smoking and risk of acute and chronic pancreatitis among women and men: a population-based cohort study. Arch Intern Med 2009;169:603–609. [DOI] [PubMed] [Google Scholar]
- 6.Yadav D, Hawes RH, Brand RE, et al. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med 2009;169:1035–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Layer P, Yamamoto H, Kalthoff L, et al. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology 1994;107:1481–1487. [DOI] [PubMed] [Google Scholar]
- 8.Lankisch MR, Imoto M, Layer P, et al. The effect of small amounts of alcohol on the clinical course of chronic pancreatitis. Mayo Clin Proc 2001;76:242–251. [DOI] [PubMed] [Google Scholar]
- 9.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet 1996;14:141–145. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed Ali U, Issa Y, Hagenaars JC, et al. Risk of Recurrent Pancreatitis and Progression to Chronic Pancreatitis After a First Episode of Acute Pancreatitis. Clin Gastroenterol Hepatol 2016;14:738–746. [DOI] [PubMed] [Google Scholar]
- 11.Cohn JA, Friedman KJ, Noone PG, et al. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med 1998;339:653–658. [DOI] [PubMed] [Google Scholar]
- 12.Rosendahl J, Witt H, Szmola R, et al. Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat Genet 2008;40:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitcomb DC, LaRusch J, Krasinskas AM, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet 2012;44:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet 2000;25:213–216. [DOI] [PubMed] [Google Scholar]
- 15.Zator Z, Whitcomb DC. Insights into the genetic risk factors for the development of pancreatic disease. Therap Adv Gastroenterol 2017;10:323–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamano H, Kawa S, Horiuchi A, et al. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med 2001;344:732–738. [DOI] [PubMed] [Google Scholar]
- 17.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas 2011;40:352–358. [DOI] [PubMed] [Google Scholar]
- 18.Whitcomb DC, Yadav D, Adam S, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2). Pancreatology 2008;8:520–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilcox CM, Sandhu BS, Singh V, et al. Racial Differences in the Clinical Profile, Causes, and Outcome of Chronic Pancreatitis. Am J Gastroenterol 2016;111:1488–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilcox CM, Yadav D, Ye T, et al. Chronic pancreatitis pain pattern and severity are independent of abdominal imaging findings. Clin Gastroenterol Hepatol 2015;13:552–560; quiz e528–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaRusch J, Jung J, General IJ, et al. Mechanisms of CFTR functional variants that impair regulated bicarbonate permeation and increase risk for pancreatitis but not for cystic fibrosis. PLoS Genet 2014;10:e1004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaRusch J, Lozano-Leon A, Stello K, et al. The Common Chymotrypsinogen C (CTRC) Variant G60G (C.180T) Increases Risk of Chronic Pancreatitis But Not Recurrent Acute Pancreatitis in a North American Population. Clin Transl Gastroenterol 2015;6:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phillips AE, LaRusch J, Greer P, et al. Known genetic susceptibility factors for chronic pancreatitis in patients of European ancestry are rare in patients of African ancestry. Pancreatology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider A, Larusch J, Sun X, et al. Combined bicarbonate conductance-impairing variants in CFTR and SPINK1 variants are associated with chronic pancreatitis in patients without cystic fibrosis. Gastroenterology 2011;140:162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]


