Abstract
Introduction:
Neuropsychiatric symptoms (NPS) are common in Alzheimer’s disease (AD). NPS contribute to patients’ distress, caregiver burden, and institutionalization. White matter hyperintensities (WMH) appear on MRI, usually indicative of cerebrovascular disease. WMH have been associated with certain NPS. We aimed to assess the relationship between WMH and NPS severity in MCI due to AD (MCI-AD) and AD and to assess the ability of WMH to predict NPS progression. Data was obtained from NACC.
Methods:
252 participants (114MCI-AD/138AD) were used in this study. Baseline WMH were quantified using an automated segmentation technique. NPS were measured using the Neuropsychiatric Inventory. Mixed-effect models and correlations were used to determine the relationship between WMH and NPS.
Results:
Longitudinal mixed-effect models revealed a significant relationship between increase in NPI total scores and baseline WMH (p=0.014). There was a significant relationship between baseline WMH and an increase in delusions (p=0.023), hallucinations (p=0.040), agitation (p=0.093), depression (p=0.017), and irritability (p= 0.002). Correlation plot analysis showed that baseline whole brain WMH predicted change in future NPI total scores (r=0.169, p=0.008) and predicted change in future agitation severity scores (r= 0.165, p= 0.009). WMH in the temporal (r=0.169, p=0.008) and frontal (r=0.153, p=0.016) lobes contributed most to this change.
Conclusion:
Depression, irritability, and agitation are common NPS and very distressful to patients and caregivers. Our findings of increased NPS severity over time in MCI-AD and AD with increased WMH have important implications for treatment, arguing for aggressive treatment of vascular risk factors in patients with MCI-AD and AD.
Keywords: Alzheimer’s disease, mild cognitive impairment, neuropsychiatric symptoms, white matter hyperintensities, cerebrovascular disease, neuroimaging
1. INTRODUCTION
Neuropsychiatric symptoms (NPS) are common in patients with dementia and are a major source of patients’ distress, caregiver burden, and can lead to institutionalization (1, 2). Moreover, while psychotropic drugs may temporarily alleviate certain NPS, some have severe and harmful effects (3). As such, there is a need to better understand NPS and their underlying pathology. In patients with Alzheimer’s disease (AD), the most commonly reported NPS are apathy, agitation (4, 5), irritability (4), and depression (5, 6). Some studies have reported the presence and severity of depressive symptoms as predictive of MCI progression to AD (7, 8), but this has not been observed in all studies (9, 10).
The cause of NPS in dementia is unknown, but there is some evidence that focal white matter hyperintensities (WMH) are associated with NPS (11, 12). WMH are areas of increased signal relative to the surrounding white matter regions, as seen on magnetic resonance imaging (MRI) T2-weighted and fluid attenuated inversion recovery (FLAIR) sequences (13). WMH can be seen in cognitively normal individuals and are known to increase with age. The pathological correlates of WMH are heterogeneous including edema, inflammation or infection, but are most commonly indicative of cerebral small vessel disease (SVD) (12). Cerebral SVD is associated with vascular risk factors, such as hypertension, hypercholesterolemia, and diabetes. Some studies have shown an association between increased WMH and AD (14, 15, 16). In a recent systematic review, Liu and colleagues (2018) found that cerebral SVD markers, such as WMHs and cerebral micro-bleeds, were associated with increased risk of clinically diagnosed AD (18).
WMH have been associated with specific NPS in patients with dementia. In AD, depression (18), aberrant motor behaviours (19, 20, 21), anxiety (19), night-time behaviours (19), and apathy (22) have been reported in association with increased prevalence of WMH. Some studies have related periventricular WMH volumes, especially in the frontal lobes, to hallucinations, depression, and anxiety in patients with AD and Vascular dementia (VaD) (23, 24, 25). Focal frontal WMH have also been associated with depression in patients with dementia with Lewy bodies (26) apathy in vascular cognitive impairment (11), verbal aggressiveness in amnestic MCI and AD (27) and delusions in patients with AD (28, 29, 30). Parieto-occipital white matter changes have been shown to contribute to the development of delusional misidentification in patients with AD (31). However, there are also reports of no significant relationship between NPS and WMH in patients with AD (32, 33). Given the inconclusiveness of the current literature, there is a need to better understand the relationship between WMH and NPS in patients with MCI or AD.
The present study explores the relationship between WMH and NPS in patients with MCI-AD and AD from a large cohort of patients collected by the National Alzheimer’s Coordinating Center (NACC). Our study aims were to determine the relationship between NPS and WMH burden in patients with MCI-AD or AD and to assess the relationship between WMH volume and development of specific NPS in a longitudinal cohort of these patients. A clear relationship between NPS and WMH may justify more aggressive treatment of vascular risk factors given the implication of NPS in patients’ distress, caregiver burden, and institutionalization.
2. MATERIALS AND METHODS
2.1. PARTICIPANTS
We used data collected by the National Alzheimer’s Coordinating Center’s Uniform Data Set (NACC), n=753. NACC developed a database of standardized clinical research data obtained from past and present NIA-funded Alzheimer’s Disease Research Centers across the United States. NACC data collection has been previously described elsewhere (34). Participants included in the database have a range of cognitive statuses: normal cognition, mild cognitive impairment (MCI), and demented due to various clinical diagnoses.
Our dataset was created using data from September 2005 through March 2017. Participants in our study had to have a diagnosis of MCI due to AD (MCI-AD) or AD, which refers to those with dementia and Alzheimer’s disease presumptive etiology in accordance with both NINCDS-ADRDA criteria (36) and NIA-AA criteria, aged 50 or older, a Neuropsychiatric Inventory (NPI) and a baseline MRI. We removed participants whose clinical data and MR imaging data were not within three months of each other and those who did not have at least one follow up clinic visit (N=447). Other exclusion criteria included: history of seizures, psychiatric disorders, traumatic brain injury, stroke, other cognitive disorders, and primary etiologic diagnoses other than AD. The final study cohort consisted of 252 participants (N=114 with MCI-AD, N=138 with AD).
2.2. VARIABLES
The following NACC data was used for this study: diagnosis, MRI data, age, education, Clinical Dementia Rating® (CDR®) Dementia Staging Instrument sum of boxes scores, MMSE sub-domain and total scores, presence/absence of vascular risk factors, presence/absence and severity of NPS obtained from the NPI-Q, and the use of psychotropic and/or vascular risk factor-controlling medications. A vascular risk factor included: history of/or currently smoking, heart attack/cardiac arrest, atrial fibrillation, angioplasty/ endarterectomy/ stent, bypass surgery, pacemaker, congestive heart failure, other cardiovascular disease, TIAs, diabetes, hypertension, hypercholesterolemia, and whether the subject was currently taking any vascular medications. Medication to control vascular risk factors included the use of the following categories: anti-adrenergic, anticoagulant/antiplatelet, ACE inhibitor, antihypertensive or blood pressure medication, angiotensin II inhibitor, beta blocker, calcium channel blocking agent, diabetes medication, diuretic, antihypertensive combination, lower lipid levels, and the use of a vasodilator.
The NPI-Q was used to evaluate NPS. The NPI is based on scripted questions administered to the participants’ caregivers or an informant familiar with the participant (36). It is used to evaluate the presence, severity, and frequency of twelve commonly encountered NPS in dementia: delusions, hallucinations, agitation, depression, anxiety, elation, apathy or indifference, disinhibition, irritability, aberrant motor behaviour, sleep disturbances, and appetite (36). The Neuropsychiatric Inventory – Questionnaire (NPI-Q) used in the UDS was developed and cross-validated with the standard NPI to provide a brief assessment of neuropsychiatric symptomatology in routine clinical practice settings (37). The NPI-Q is a commonly used scale to assess patients with dementias and other neurological disorders with acceptable validity and reliability in outpatient settings (37). We focused on the presence or absence and the severity of each symptom. The severity of each symptom is scored on a 3-point scale (1=mild, 2=moderate, 3=severe).
Since it is well known that medications can have an impact on NPS, we took psychotropic medication usage into account. Data on psychotropic medication use was provided by NACC, specifically the use of antipsychotics, antidepressants, and anxiolytics.
Antipsychotics, antidepressants, and anxiolytics were coded as absence/presence (0/1). We added the variable absence/presence (0/1) of sleep medications. A coding of 1 was given if any of the following sleep medications were listed: Ambien, melatonin, Zolpidem, and/or Zopiclone. The presence/absence of these psychotropic medications were included in all statistical analyses.
2.3. MRI PROCESSING
NACC participants diagnosed with either MCI-AD or AD, with a baseline MRI that included a FLAIR sequence within 3 months of clinical assessment were used for analysis in our study. Only baseline MRIs were used for this study, as we did not have enough follow up scans to match follow up NPI scores. The MRI parameters have been previously described elsewhere (38). All MRI scans were pre-processed in three steps: i) denoising (39), ii) intensity inhomogeneity correction (40), and iii) intensity normalization (41). FLAIR scans were rigidly co-registered to the T1W scans (42). The T1W scans were registered to the ADNI template. Concatenating the two transformations, the FLAIR scans were also registered to the ADNI template (43). Using a previously validated automatic WMH segmentation technique and a library of manual segmentations from the NACC dataset, the WMHs were segmented automatically (38, 44, 45). The quality of the segmentations was assessed and verified by an expert (MD). The total volumes of the WMHs were then calculated (in mm3) and normalized for head size and log-transformed to achieve normal distribution.
2.4. STATISTICAL ANALYSIS
Cross sectional analysis was performed in order to assess the association between baseline total NPS severity scores and baseline whole brain WMH load. WMH load, age at baseline, and education were used as continuous predictors. Sex was used as a categorical predictor. In addition, the cross sectional analysis was repeated for the twelve individual NPI severity scores. FDR was used to correct for multiple comparisons.
Longitudinal mixed-effects models were used to assess the association of WMH load at baseline with the longitudinal changes in NPI symptoms. WMH load, age, education, as well as the baseline NPI scores were used as continuous predictors. For these longitudinal mixed-effect models, age was divided into two variables: (1) Age at baseline and (2) Time (between baseline and clinical exam date). An interaction term between WMH load and time was also included. Sex was used as a categorical predictor. This analysis was repeated for the twelve individual NPI sub-scores, but using the respective baseline sub-score as a continuous predictor. FDR was used to correct for multiple comparisons.
Correlation plots were used to determine if baseline whole brain WMH load could predict change in future NPI severity scores. Subjects were considered as categorical random effects in all the mixed effects models. All continuous variables were z-scored prior to the analysis. Mixed effects models were fitted using fitlme in MATLAB version R2017b. P-values reported are not corrected for multiple comparisons.
For any sub-score correlations that are statistically significant, we completed a secondary analysis to investigate which lobe contributed to the correlation. For all of these statistical models, use of psychotropic medications were included. Additionally, baseline WMH volumes were log-transformed to achieve normal distribution consistently to all analyses stated above.
3. RESULTS
The final cohort consisted of 252 participants (138 AD, 114 MCI-AD), 134 were male, 118 were female. The average number of years of education was 15.65±8.35 (Table 1).
Table 1.
Basic demographics at baseline visit and absence/presence (N/Y) of various medications.
| Diagnosis | MCI-AD | AD |
|---|---|---|
| N | 114 | 138 |
| Age (mean ± SD) | 76.1 ± 7.12 | 75.8 ± 8.62 |
| Sex (M/F) | 68/46 | 66/72 |
| Education (mean ±SD) | 16.4 ± 8.54 | 15.0 ± 8.16 |
| CDR sum of boxes | 1.88 ± 1.65 | 5.42 ± 3.41 |
| VRF Medications (0/1) | 13/101 | 27/111 |
| Antipsychotics (0/1) | 114/0 | 134/4 |
| Antidepressants (0/1) | 98/16 | 90/48 |
| Anxiolytics (0/1) | 102/12 | 117/21 |
| Sleep Medications (0/1) | 110/4 | 132/6 |
Cognitive score domains are provided as well (see Supplementary Table 1). There was an average of 22.8 days in between the baseline NPI and the baseline MRI scan. The usage of psychotropic medications and medications to control vascular risk factors were reported at baseline (Table 1). Each of the clinic visits thereafter, which included NPI scores, were approximately one year after the baseline MRI scan (see Supplementary Table 2). Whole brain and lobar WMH volumes were calculated for the baseline MRI scans (Table 2). At baseline, all twelve NPS were present (see Supplementary Table 3). All twelve NPS were also present at each follow-up visit (Table 3). The cross-sectional analysis at baseline revealed no significant association between any of the NPS scores (either total or sub-scores) and WMH (see Supplementary Table 3).
Table 2.
Regional WMH volumes (mm3) for baseline MRI scan
| MCI-AD | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Left- frontal lobe |
Right- frontal lobe |
Left- temporal lobe |
Right- temporal lobe |
Left- parietal lobe |
Right- parietal lobe |
Left- occipital lobe |
Right- occipital lobe |
Whole brain |
|
| Mean | 3.99 | 4.46 | 1.14 | 1.36 | 2.52 | 2.48 | 1.36 | 1.35 | 18.78 |
| SD | 3.88 | 4.08 | 0.88 | 1.00 | 2.74 | 2.89 | 1.10 | 1.17 | 16.01 |
| AD | |||||||||
| Left- frontal lobe |
Right- frontal lobe |
Left- temporal lobe |
Right- temporal lobe |
Left- parietal lobe |
Right- parietal lobe |
Left- occipital lobe |
Right- occipital lobe |
Whole brain |
|
| Mean | 4.80 | 5.10 | 1.11 | 1.31 | 2.59 | 2.48 | 1.17 | 1.17 | 19.89 |
| SD | 5.59 | 5.62 | 1.12 | 1.26 | 3.35 | 3.03 | 1.16 | 1.10 | 20.86 |
Table 3.
Prevalence (%) of NPS sub-score at each clinical visit
| NPS (%) | ||||||
|---|---|---|---|---|---|---|
| Visit Number | Visit 1 (N=252) |
Visit 2 (N=247) |
Visit 3 (N=184) |
Visit 4 (N=117) |
Visit 5 (N=55) |
Visit 6 (N=30) |
| Delusions | 7.1 | 12.6 | 13.6 | 15.4 | 10.9 | 20.0 |
| Hallucinations | 2.0 | 6.9 | 7.6 | 8.5 | 7.3 | 6.7 |
| Agitation | 27.0 | 32.0 | 38.0 | 23.9 | 40.0 | 16.7 |
| Depression | 29.8 | 34.8 | 34.8 | 27.4 | 21.8 | 33.3 |
| Anxiety | 30.2 | 32.0 | 35.9 | 40.2 | 29.1 | 36.7 |
| Elation | 2.8 | 6.5 | 4.3 | 6.0 | 1.8 | 3.3 |
| Apathy | 31.0 | 34.4 | 41.8 | 44.4 | 45.5 | 46.7 |
| Disinhibition | 17.5 | 19.8 | 15.8 | 20.5 | 27.3 | 26.7 |
| Irritability | 36.1 | 36.0 | 31.5 | 36.8 | 21.8 | 40.0 |
| Aberrant Motor behaviour |
14.7 | 20.2 | 25.5 | 23.1 | 16.4 | 33.3 |
| Night-time Behaviour |
27.0 | 30.4 | 28.3 | 33.3 | 36.4 | 30.0 |
| Appetite | 17.1 | 27.1 | 26.6 | 27.4 | 30.9 | 33.3 |
With regards to the longitudinal mixed effect model analyses, there was a statistically significant relationship (after correction for multiple comparisons) between change in NPI total scores and baseline whole brain WMH load (p= 0.014) (Table 4). For the longitudinal mixed effect model analyses of the NPS sub-scores, baseline WMH load was associated with significantly worse future NPI total scores (p=0.014), delusions severity sub-scores (p=0.023), hallucinations severity sub-score (p=0.040), agitation severity sub-score (p=0.093), depression severity sub-score (p=0.017), and irritability severity sub-score (p= 0.002). No other NPS severity scores were significantly associated with baseline WMH load (Supplementary Table 4).
Table 4.
Longitudinal Mixed-Effect Models of annualized change in NPI total score explained by whole brain WMH load, age, sex, education and baseline total NPI scores.
| Estimate | SE | tStat | pValue | |
|---|---|---|---|---|
| Intercept | −0.017 | 0.044 | −0.394 | 0.693 |
| Age | −0.002 | 0.035 | −0.069 | 0.945 |
| Sex | 0.069 | 0.066 | 1.039 | 0.299 |
| Education | 0.022 | 0.031 | 0.705 | 0.481 |
| WMH | 0.085 | 0.035 | 2.454 | 0.014** |
| NPI total Baseline | 0.629 | 0.033 | 19.204 | 9.8252E-68 |
denotes statistical significance, ◇Denotes a trend; one-tailed test p<0.1
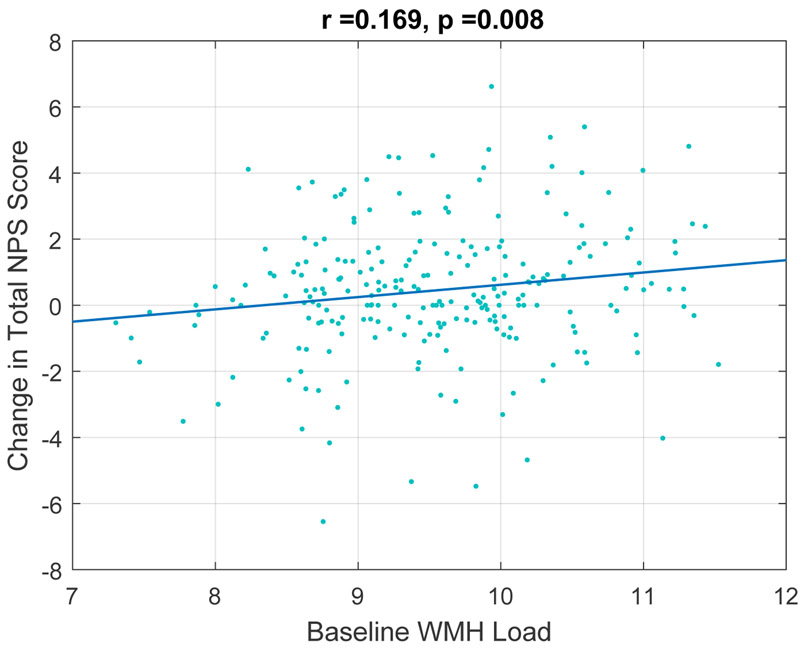
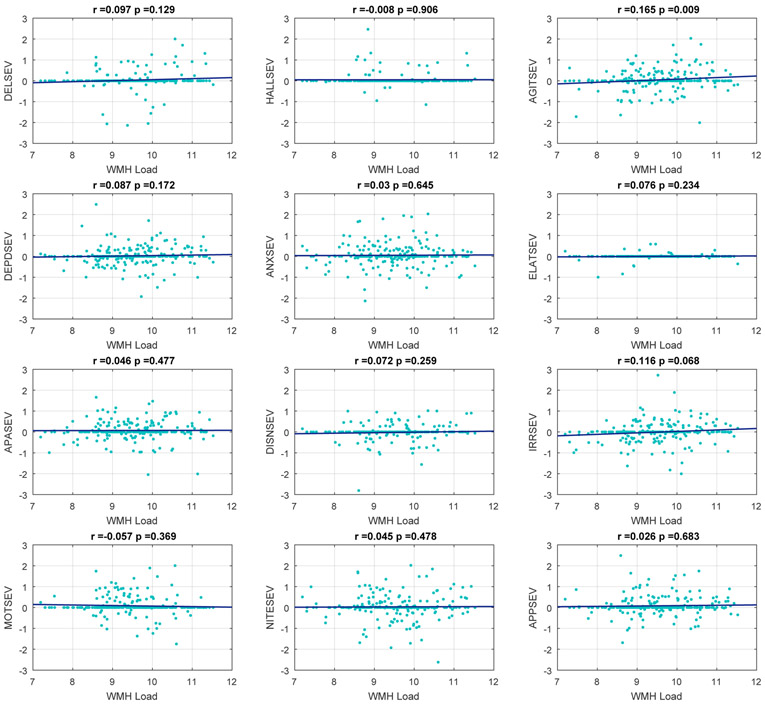
In the correlation plots, baseline whole brain WMH load predicted the change in total NPI scores at a future visit (r=0.169, p=0.008) (Figure 1). Whole brain WMH load also predicted the change in some NPI severity sub-scores at a future visit. More specifically, there was a significant correlation between baseline WMH load and severity of agitation (r= 0.165, p= .009) (Figure 2). This relationship withstood correction for multiple comparisons and controlling for age, sex, and education. There was also a correlation between baseline whole brain WMH load and severity of irritability that although statistically significant, did not withstand correction for multiple comparisons (r=0.116, p= 0.068). Inclusion of the use of psychotropic medications (antipsychotics, anxiolytics, antidepressants, and sleep medications) into the model did not significantly alter these results. Baseline whole brain WMH load did not predict any other NPS severity scores.
Figure 1.
Correlation plot of baseline whole brain WMH load to predict the change in future NPI total scores over time
Figure 2.
Correlation plots of baseline whole brain WMH load to predict the annualized change in future NPI severity scores over time, correcting for age, sex and education (without meds). From left to right: DELSEV = delusion severity score, HALLSEV = hallucination severity score, AGITSEV = agitation severity score, DEPD = depression severity score, ANXSEV = anxiety severity score, ELATSEV = elation severity score, APASEV = apathy severity score, DISNSEV = disinhibition severity score, IRRSEV = irritability severity score, MOTSEV = aberrant motor behaviour severity score, NITESEV = Night-time behaviour severity score, APPSEV = appetite severity score
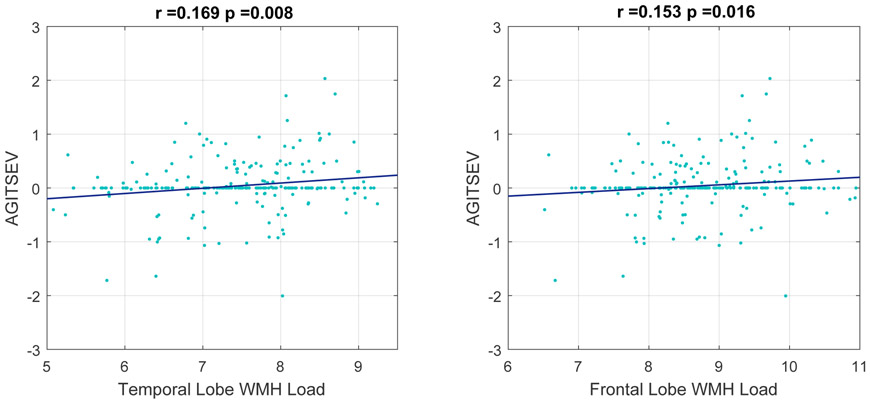
As a secondary analysis, we determined which lobar WMH loads contributed most to the significant correlation between baseline whole brain WMH load and severity of agitation. The temporal lobe WMH (r=0.169, p=0.008) and frontal lobe WMH load contributed most to this finding (r=0.153, p=0.016) (Figure 3).
Figure 3.
Correlation plots of baseline temporal and frontal lobe WMH load to predict the change in future agitation severity scores over time. AGITSEV = agitation severity score
4. DISCUSSION
4.1. DISCUSSION
The current literature regarding the relationship between WMH and NPS in patients with MCI or AD is inconclusive. As such in this study, we sought to assess the association between WMH load and NPS and found a significant relationship between baseline WMH and changes in the severity of NPS when conducting a longitudinal analysis in patients with MCI-AD and AD. Overall, our findings suggest that WMH load in MCI-AD and AD is associated with the progression or worsening of certain NPS.
Like others, our cross sectional results revealed no association between WMH and NPS (32, 33, 46, 47). The lack of correlation in our study at baseline may be partially explained by the heterogeneity that can exist in any MCI and AD cohort without pathological or biomarker confirmation. Another explanation is the low amount of WMH in the NACC dataset.
One previous study had divided WMH severity into four categories based on neuroradiology reports in an AD cohort and no association was found between WMH and NPS (32). In another study, WMH were rated using the Wahlund scale and again no association between WMH and NPS was found in AD (33). In a review on neuroimaging studies of agitation in AD (46) only one cross sectional study examined the association between WMH load and NPS (47) and they also found no association between WMH and NPS in AD. However, the WMH were not quantified but categorized according to the Fazekas scale. In all of these aforementioned studies where no association was found between WMH and NPS, WMH were not quantified but instead rated using visual scales. In contrast, a cross-sectional study using regional WMH volumes revealed that higher right frontal lobe WMH volume was associated with the presence of delusions in AD (28) and Ogama and colleagues (27) also reported correlations between WMH and certain NPS in MCI-AD and AD.
In our current study, through post-hoc correlation analysis, we also found both baseline frontal WMH load and baseline temporal WMH load were correlated with the agitation severity score. The frontal and temporal lobes are often implicated in NPS (6) and so it is not surprising that WMH load in these areas would be an important contributor to NPS. Although the regional (frontal lobe r=0.153, temporal lobe r=0.169) and whole brain (r=0.165) WMH loads effects on agitation are not large, it remains an important finding as WMH load often reflects cerebrovascular disease, which is often due to modifiable risk factors such as hypertension and type 2 diabetes. In a recent study, pre-diabetes and diabetes have been shown to accelerate cognitive decline and predict micro-vascular lesions in healthy older adults (48). However, the effects of type 2 diabetes and other vascular risk factors mentioned above, can be significantly reduced by lifestyle changes. Modifications in diet and increased physical activity can help control vascular risk factors and reduce the risk of cardiac events (49), which can contribute to the development of certain NPS.
There are other possible explanations for the associations between increased WMH load and longitudinal NPS that need to be considered. Firstly, WMH are not necessarily a reflection of only small vessel disease. Therefore, there may be heterogeneity in pathology with differing destructive effects so that in some cases WMH may reflect more serious injury than others. Secondly, although the NPI is the gold standard for assessing NPS in dementia, it is an informant based measure and so there is a significant amount of subjectivity in the variables obtained from this questionnaire.
In the longitudinal mixed effect model, baseline WMH load was associated with significantly worse future NPI total scores (p=0.014), NPI delusions severity sub-scores (p=0.023), NPI hallucinations severity sub-score (p=0.040), NPI agitation severity sub-score (p=0.093), NPI depression severity sub-score (p=0.017), and NPI irritability severity sub-score (p= 0.002). Our finding that the baseline whole brain WMH load was associated with increased future irritability severity scores supports previous findings (6, 27, 50). One study found focal frontal WMH to be associated with verbal aggressiveness in amnestic MCI and AD (27), which supports our current finding of the association between frontal lobe WMH load and worsening agitation and irritability. Additionally, this finding echoes the results of Misquitta and colleagues (50), wherein they found WMH load to be a significant contributor to the NPI sub-syndrome of hyperactivity, which includes the following: agitation, euphoria, disinhibition, irritability, and aberrant motor behaviour. The current study suggests that irritability and agitation are the NPS within the sub-syndrome of hyperactivity that is associated with WMH load. These results are significant as both studies utilized large and established datasets: Alzheimer’s Disease Neuroimaging Initiative (ADNI) for Misquitta and colleagues (50) and NACC here.
Furthermore, our findings with regards to the association between baseline WMH volume and increased hallucinations supports a previous finding (51) as does our finding of increased delusions (28, 29, 30, 31). Of the studies that analyzed delusion severity and WMH load, two of them did not localize the WMH volumes (29, 30) whereas the other two studies did localize WMH volumes (28, 31). One found parieto-occipital WMH volume in AD patients was associated with delusions (31), while the other found frontal lobe WMH volume was associated with delusions (28). Delusions are complex phenomenon with likely multiple regions contributing to their manifestation and severity.
Our finding of increased depression severity sub-scores also supports previous findings (18, 25, 24, 29). Of the studies listed, significantly larger right parietal WMH volumes were associated with increased symptoms of depression (25). These WMH volumes were rated visually and so this data warrants validation with quantification to confirm the results, as depression is one of the most common NPS found in patients with MCI and AD (1).
Our study has a number of limitations. Although our sample was collected as part of a large standardized clinical data set, our final cohort was significantly decreased when excluding patients whose clinical data and MR imaging data were not within three months of each other, who did not have at least one follow up clinic visit, and who had a history of cognitive disorders other than AD. Next, some factors with a potential impact on NPI scores, such as basic demographic variables and dementia severity scores were not included in the statistical models. This is because the main question for this paper was whether WMH as surrogate markers of cerebrovascular disease were related to NPS and their progression. As such, we were underpowered to consider all the variables listed above. In addition, because the participants’ MRIs were collected with different MRI machines, this affected the quality of the images and increased the heterogeneity of images in our study. However, we implemented a previously validated automated WMH segmentation technique (38) and utilized a library of manual segmentations based on the NACC dataset, verified by an expert (MD) to mitigate this issue. Therefore, the quality of the segmentations is reliable. Next, only baseline MRIs were used for this study, as we did not have enough follow up scans to match follow up NPI scores. Therefore, future studies investigating the relationship between NPS and WMH load in AD may benefit from having follow up MRI scans available for each participant in order to track simultaneously the change in WMH load and NPS. Finally, the baseline WMH are correlated with NPS sub-scores with small r-values, which only explains part of the variability. Other factors that could explain the variability are the effects of underlying AD pathology that lead to underlying structural and functional changes.
The contribution that WMH load has on NPS is likely a synergistic result of the underlying interactions between cerebrovascular and AD pathologies. More specifically, neurofibrillary tangles and amyloid-β are hallmarks of underlying AD pathology. These neurofibrillary tangles are found everywhere in grey matter and commonly found in subcortical regions, such as the hypothalamic nuclei, which is known to be involved in the regulation of certain NPS (6). Therefore, the presence of WMH within these regions could cause further damage to that caused by the neurofibrillary tangles. One study also has postulated that SVD potentially could be involved earlier on in the AD process by hastening the accumulation of amyloid deposition due to improper perivascular drainage of amyloid-β (52). Most recently, a study has shown an association between amyloid pathology and a greater rate of development of new NPS in MCI (53). Agitation, irritability, and apathy were shown to be significant predictors for conversion from MCI to AD (53). Therefore, the interactions between cerebrovascular and AD neuropathologies, in addition to the significant role that genetics and environmental factors, may cumulatively contribute to NPS development and expression.
4.2. CONCLUSIONS
Overall, our study has shown a significant association between baseline WMH load and worsening of delusions, hallucinations, depression, irritability, and agitation over time. Given how common and distressful both NPS are to patients and their care providers and given that NPS can be clinical indicators of preclinical dementia syndromes, our findings have potentially important implications for treatment, arguing for aggressive treatment of cerebrovascular disease, even in patients with MCI-AD and mild AD. By controlling and better managing vascular risk factors, it could reduce the burden of cerebrovascular disease and may also decrease the development of NPS in patients with MCI-AD and AD as a result.
Supplementary Material
5. ACKNOWLEDGMENTS
The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA-funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P50 AG047266 (PI Todd Golde, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Thomas Wisniewski, MD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P50 AG047366 (PI Victor Henderson, MD, MS), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG005131 (PI James Brewer, MD, PhD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD), P30 AG053760 (PI Henry Paulson, MD, PhD), P30 AG010124 (PI John Trojanowski, MD, PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg, MD), P30 AG049638 (PI Suzanne Craft, PhD), P50 AG005136 (PI Thomas Grabowski, MD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), P50 AG005681 (PI John Morris, MD), P50 AG047270 (PI Stephen Strittmatter, MD, PhD).
List of abbreviations:
- AD
Alzheimer’s disease
- DLB
Dementia with Lewy Bodies
- FTD
Frontotemporal dementia
- MCI
Mild cognitive impairment
- MRI
magnetic resonance imaging
- NACC
National Alzheimer’s Coordinating Center
- NPI
Neuropsychiatric Inventory
- NPS
neuropsychiatric symptoms
- VaD
Vascular dementia
- WMH
white matter hyperintensities
Footnotes
FINANCIAL DISCLOSURES: All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. REFERENCES
- 1.Huang SS, Lee MC, Liao YC, Wang WF, Lai TJ, 2012. Caregiver burden associated with behavioral and psychological symptoms of dementia (BPSD) in Taiwanese elderly. Arch. Gerontol. Geriatr 10.1016/j.archger.2011.04.009 [DOI] [PubMed] [Google Scholar]
- 2.Steele C, Rovner B, Chase GA, Folstein M, 1990. Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am. J. Psychiatry 10.1176/ajp.147.8.1049 [DOI] [PubMed] [Google Scholar]
- 3.I. M, J. C, 2005. Behavioural changes and psychological symptoms in dementia disorders. Lancet Neurol. [DOI] [PubMed]
- 4.Koenig AM, Arnold SE, Streim JE, 2016. Agitation and Irritability in Alzheimer’s Disease: Evidenced-Based Treatments and the Black-Box Warning. Curr. Psychiatry Rep 10.1007/s11920-015-0640-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Q.-F. Z, H.-F. W, T. J, M.-S. T, L. T, W. X, J.-Q. L, et al. 2016. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J. Affect. Disord [DOI] [PubMed]
- 6.Boublay N, Schott AM, Krolak-Salmon P, 2016. Neuroimaging correlates of neuropsychiatric symptoms in Alzheimer’s disease: a review of 20 years of research. Eur. J. Neurol 10.1111/ene.13076 [DOI] [PubMed] [Google Scholar]
- 7.M. HH, B.V., W.H. C, 2008. A predictive depression pattern in mild cognitive impairment. Int. J. Geriatr. Psychiatry [DOI] [PubMed] [Google Scholar]
- 8.Van Der Mussele S, Fransen E, Struyfs H, Luyckx J, Marien P, Saerens J, et al. 2014. Depression in mild cognitive impairment is associated with progression to alzheimer’s disease: A longitudinal study. J. Alzheimer’s Dis 10.3233/JAD-140405 [DOI] [PubMed] [Google Scholar]
- 9.Palmer K, Berger AK, Monastero R, Winblad B, Bäckman L, Fratiglioni L, 2007. Predictors of progression from mild cognitive impairment to Alzheimer disease. Neurology. 10.1212/01.wnl.0000260968.92345.3f [DOI] [PubMed] [Google Scholar]
- 10.Palmer K, Di Iulio F, Varsi AE, Gianni W, Sancesario G, Caltagirone C, Spalletta G, 2010. Neuropsychiatric predictors of progression from amnestic - Mild cognitive impairment to alzheimer’s disease: The role of depression and apathy. J. Alzheimer’s Dis 10.3233/JAD-2010-1352 [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Kang SJ, Kim C, Kim GH, Jeon S, Lee JM, et al. 2013. The effects of small vessel disease and amyloid burden on neuropsychiatric symptoms: A study among patients with subcortical vascular cognitive impairments. Neurobiol. Aging 10.1016/j.neurobiolaging.2013.01.002 [DOI] [PubMed] [Google Scholar]
- 12.Pantoni L, 2010. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 10.1016/S1474-4422(10)70104-6 [DOI] [PubMed] [Google Scholar]
- 13.M. F, A. E, R. MA, F. M, M. M, C. PG 2018. Cardiovascular disease and brain health: Focus on white matter hyperintensities. IJC Hear. Vasc 10.1016/j.ijcha.2018.04.006 LK - http://limo.libis.be/resolver?&sid=EMBASE&issn=23529067&id=doi:10.1016%2Fj.ijcha.2018.04.006&atitle=Cardiovascular+disease+and+brain+health%3A+Focus+on+white+matter+hyperintensities&stitle=nC+Heart+Vascul.&title=IJC+Heart+and+Vasculature&volume=19&issue=&spage=63&epage=69&aulast=Moroni&aufirst=Francesco&auinit=F.&aufull=Moroni+F.&coden=&isbn=&pages=63-69&date=2018&auinit1=F&auinitm= [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erkinjuntti T, Gao F, Lee DH, Eliasziw M, Merskey H, Hachinski VC, 1994. Lack of Difference in Brain Hyperintensities Between Patients With Early Alzheimer’s Disease and Control Subjects. Arch. Neurol 10.1001/archneur.1994.00540150054016 [DOI] [PubMed] [Google Scholar]
- 15.Leys D, Soetaert G, Petit H, Fauquette A, Pruvo JP, Steinling M, 1990. Periventricular and White Matter Magnetic Resonance Imaging Hyperintensities do not Differ Between Alzheimer’s Disease and Normal Aging. Arch. Neurol 10.1001/archneur.1990.00530050040010 [DOI] [PubMed] [Google Scholar]
- 16.Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas, et al. 2006. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 10.1212/01.wnl.0000249119.95747.1f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.L. Y, B. N, P. A, C. DKY, S. P 2018. Cerebral small vessel disease and the risk of Alzheimer’s disease: A systematic review. Ageing Res. Rev [DOI] [PubMed] [Google Scholar]
- 18.Clark LM, McDonald WM, Welsh-Bohmer KA, Siegler IC, Dawson DV, Tupler LA, Krishnan KRR, 1998. Magnetic resonance imaging correlates of depression in early- and late- onset Alzheimer’s disease. Biol. Psychiatry 10.1016/S0006-3223(98)00106-1 [DOI] [PubMed] [Google Scholar]
- 19.Berlow YA, Wells WM, Ellison JM, Sung YH, Renshaw PF, Harper DG, 2010. Neuropsychiatric correlates of white matter hyperintensities in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 10.1002/gps.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirono N, Kitagaki H, Kazui H, Hashimoto M, Mori E, 2000. Impact of white matter changes on clinical manifestation of Alzheimer’s disease: A quantitative study. Stroke. 10.1161/01.STR.31.9.2182 [DOI] [PubMed] [Google Scholar]
- 21.Hirono Nobutsugu, Yasuda M, Tanimukai S, Kitagaki H, Mori E, 2000. Effect of the apolipoprotein E ε4 allele on white matter hyperintensities in dementia. Stroke. 10.1161/01.STR.31.6.1263 [DOI] [PubMed] [Google Scholar]
- 22.Starkstein SE, Sabe L, Vázquez S, Di Lorenzo G, Martinez A, Petracca G, Tesón A, et al. 1997. Neuropsychological, psychiatric, and cerebral perfusion correlates of leukoaraiosis in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 10.1136/jnnp.63.L66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park KH, Lee JY, Na DL, Kim SY, Cheong HK, Moon SY, et al. 2011. Different associations of periventricular and deep white matter lesions with cognition, neuropsychiatric symptoms, and daily activities in dementia. J. Geriatr. Psychiatry Neurol 10.1177/0891988711402351 [DOI] [PubMed] [Google Scholar]
- 24.Soennesyn H, Oppedal K, Greve OJ, Fritze F, Auestad BH, Nore SP, et al. 2012. White Matter Hyperintensities and the Course of Depressive Symptoms in Elderly People with Mild Dementia. Dement. Geriatr. Cogn. Dis. Extra 10.1159/000335497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starkstein SE, Mizrahi R, Capizzano AA, Acion L, Brockman S, Power BD, 2009. Neuroimaging correlates of apathy and depression in Alzheimer’s disease. J. Neuropsychiatry Clin. Neurosci 10.1176/jnp.2009.2L3.259 [DOI] [PubMed] [Google Scholar]
- 26.Barber R, Scheltens P, Gholkar A, Ballard C, McKeith I, Ince P, et al. 1999. White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer’s disease, vascular dementia, and normal aging. J. Neurol. Neurosurg. Psychiatry 10.1136/jnnp.67.L66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogama N, Sakurai T, Saji N, Nakai T, Niida S, Toba K, et al. 2018. Frontal White Matter Hyperintensity Is Associated with Verbal Aggressiveness in Elderly Women with Alzheimer Disease and Amnestic Mild Cognitive Impairment. Dement. Geriatr. Cogn. Dis. Extra 10.1159/000486826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anor CJ, O’Connor S, Saund A, Tang-Wai DF, Keren R, Tartaglia MC, 2017. Neuropsychiatric Symptoms in Alzheimer Disease, Vascular Dementia, and Mixed Dementia. Neurodegener. Dis 17 10.1159/000455127 [DOI] [PubMed] [Google Scholar]
- 29.M. H, M. I 2015. White matter lesion and Alzheimer’s disease: The association between small vessel disease and neuropsychiatric symptoms in Alzheimer’s disease. Brain and Nerve. [DOI] [PubMed]
- 30.Ogawa Y, Hashimoto M, Yatabe Y, Kaneda K, Honda K, Yuuki S, et al. 2013. Association of cerebral small vessel disease with delusions in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 10.1002/gps.3781 [DOI] [PubMed] [Google Scholar]
- 31.Lee DY, Choo ILH, Kim KW, Jhoo JH, Youn JC, Lee UY, Woo JI, 2006. White matter changes associated with psychotic symptoms in Alzheimer’s disease patients. J. Neuropsychiatry Clin. Neurosci 10.1176/jnp.2006.18.2.191 [DOI] [PubMed] [Google Scholar]
- 32.Klugman A, Marshall J, Tabet N, 2009. Impact of cerebrovascular pathology on behavioural and neuropsychiatric symptoms in patients with Alzheimer’s dementia: Findings from a retrospective, naturalistic study. Int. J. Clin. Pract 10.1111/j.1742-1241.2009.02079.x [DOI] [PubMed] [Google Scholar]
- 33.Modrego PJ, Rios C, Prez Trullen JM, Errea JM, Garca-Gmara MJ, Sanchez S, 2008. The cerebrovascular pathology in Alzheimer’s disease and its influence on clinical variables. Am. J. Alzheimers. Dis. Other Demen 10.1177/1533317507309274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris JC, Weintraub S, Chui HC, Cummings J, DeCarli C, Ferris S, et al. 2006. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer disease centers. Alzheimer Dis. Assoc. Disord 10.1097/01.wad.0000213865.09806.92 [DOI] [PubMed] [Google Scholar]
- 35.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM, 1984. Clinical diagnosis of alzheimer’s disease: Report of the NINCDS-ADRDA work group⋆ under the auspices of department of health and human services task force on alzheimer’s disease. Neurology. [DOI] [PubMed]
- 36.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J, 1994. The neuropsychiatric inventory: Comprehensive assessment of psychopathology in dementia. Neurology. [DOI] [PubMed]
- 37.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. 2000. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 12:233–239 [DOI] [PubMed] [Google Scholar]
- 38.Dadar M, Pascoal TA, Manitsirikul S, Misquitta K, Fonov VS, Tartaglia MC, et al. 2017. Validation of a Regression Technique for Segmentation of White Matter Hyperintensities in Alzheimer’s Disease. IEEE Trans. Med. Imaging. 10.1109/TMI.2017.2693978 [DOI] [PubMed] [Google Scholar]
- 39.Manjón JV, Coupé P, Martí-Bonmatí L, Collins DL, Robles M, 2010. Adaptive non-local means denoising of MR images with spatially varying noise levels. J. Magn. Reson. Imaging, 10.1002/jmri.22003 [DOI] [PubMed] [Google Scholar]
- 40.Zijdenbos AP, Evans AC, 1998. Sled JG, Zijdenbos AP, Evans ACA non-parametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging 87–97. 10.1109/42.668698 [DOI] [PubMed] [Google Scholar]
- 41.Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL, 2011. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 10.1016/j.neuroimage.2010.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins DL, Neelin P, Peters TM, Evans AC, 1994. Automatic 3d intersubject registration of mr volumetric data in standardized talairach space. J. Comput. Assist. Tomogr 10.1097/00004728-199403000-00005 [DOI] [PubMed] [Google Scholar]
- 43.Collins DL, Evans AC, 1997. Animal: Validation and application of nonlinear registration-based segmentation. Int. J. Pattern Recognit. Artif. Intell
- 44.Dadar M, Maranzano J, Misquitta K, Anor CJ, Fonov VS, Tartaglia MC, et al. 2017. Performance comparison of 10 different classification techniques in segmenting white matter hyperintensities in aging. Neuroimage 157 10.1016/j.neuroimage.2017.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dadar M, Maranzano J, Ducharme S, Carmichael OT, Decarli C, Collins DL, 2018. Validation of T1w-based segmentations of white matter hyperintensity volumes in large-scale datasets of aging. Hum. Brain Mapp 10.1002/hbm.23894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenberg PB, Nowrangi MA, Lyketsos CG, 2015. Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits? Mol. Aspects Med https://doi.Org/10.1016/j.mam.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staekenborg SS, Gillissen F, Romkes R, Pijnenburg YAL, Barkhof F, et al. 2008. Behavioural and psychological symptoms are not related to white matter hyperintensities and medial temporal lobeatrophy in alzheimer’s disease. Int. J. Geriatr. Psychiatry 10.1002/gps.1891 [DOI] [PubMed] [Google Scholar]
- 48.Marseglia A, Fratiglioni L, Kalpouzos G, Wang R, Bäckman L, Xu W, 2018. Prediabetes and diabetes accelerate cognitive decline and predict microvascular lesions. Alzheimer’s Dement, 10.1016/jjalz.2018.06.1283 [DOI] [PubMed] [Google Scholar]
- 49.Aggarwal M, Bozkurt B, Panjrath G, Aggarwal B, Ostfeld RJ, Barnard ND, et al. 2018. Lifestyle Modifications for Preventing and Treating Heart Failure. J. Am. Coll. Cardiol 10.1016/j.jacc.2018.08.2160 [DOI] [PubMed] [Google Scholar]
- 50.Misquitta K, Dadar M, Collins DL, Tartaglia MC Manuscript under review.
- 51.Lin SH, Yu CY, Pai MC, 2006. The occipital white matter lesions in Alzheimer’s disease patients with visual hallucinations. Clin Imaging. 30(6):388–93. [DOI] [PubMed] [Google Scholar]
- 52.Ehrenberg AJ, Suemoto CK, Frana Resende E, de P, Petersen C, Leite REP, et al. 2018. Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J. Alzheimers. Dis 10.3233/JAD-180688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goukasian N, Hwang KS, Romero T, Grotts J, Do TM, Bateman DR, Apostolova LG, 2019. Association of brain amyloidosis with the incidence and frequency of neuropsychiatric symptoms in ADNI: a multisite observational cohort study. BMJ Open. doi: 10.1136/bmjopen-2019-031947 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.