Abstract
The next phase of clinical trials in neonatal encephalopathy (NE) focuses on hypothermia adjuvant therapies targeting alternative recovery mechanisms during the process of hypoxic brain injury. Identifying infants eligible for neuroprotective therapies begins with the clinical detection of brain injury and classification of severity. Combining a variety of biomarkers (serum, clinical exam, EEG, movement patterns) with innovative clinical trial design and analyses will help target infants with the most appropriate and timely treatments. The timing of magnetic resonance imaging (MRI) and MR spectroscopy after NE both assists in identifying the acute perinatal nature of the injury (days 3–7) and evaluates the full extent and evolution of the injury (days 10–21). Early, intermediate outcome of neuroprotective interventions may be best defined by the 21-day neuroimaging, with recognition that the full neurodevelopmental trajectory is not yet defined. An initial evaluation of each new therapy at this time point may allow higher-throughput selection of promising therapies for more extensive investigation. Functional recovery can be assessed using a trajectory of neurodevelopmental evaluations targeted to a pre-specified and mechanistically-derived hypothesis of drug action. As precision medicine revolutionizes healthcare, it should also include the redesign of NE clinical trials to allow safe, efficient and targeted therapeutics.
INTRODUCTION
Neonatal encephalopathy (NE), defined as disturbed neurologic function in the earliest postnatal days, manifests with a subnormal level of consciousness or seizures, difficulty maintaining respiration and depressed tone and reflexes.(1) Neonatal encephalopathy is complex and dynamic, and clinical examinations may evolve from lethargy to hyperexcitability to stupor over the first few hours and days of life.(2) The design of NE therapeutic strategies began with understanding the sequence of mechanisms involved in brain injury, where oxidative stress, excitotoxicity and inflammation contribute to accelerated cell death over the first hours, days and weeks of life.(3) Therapeutic hypothermia (TH), in infants with moderate to severe NE, reduces death or major neurodevelopmental disability and increases rates of survival with normal neurologic function.(4–6) Although rates of death and disability in infants receiving hypothermia are lower than in early trials(4,7), they still range between 16% and 29% in recent studies(8–10). Improved outcomes have been attributed to translation of TH into routine care, wider use of passive cooling after resuscitation and treatment of infants with milder NE.(8–10) Because adverse outcomes are less frequent, designing future trials of therapeutics has become more challenging: eligibility criteria for those at highest risk of poor outcomes may have changed, and trials may require larger sample sizes when using traditional neurodevelopmental measures.(8,10) Changes in the timing of the insult and multiplicity of underlying mechanisms (hypoxic, inflammatory, infectious, metabolic) leading to the clinical and biochemical presentation of NE may also contribute to recent outcomes variability.(11,12) However, while research in novel treatments continues and the landscape of NE is changing, the design of clinical trials to test safety and efficacy of novel agents has not changed in the past twenty years. The same designs, assessment tools and analyses are repeated in trial after trial, in contrast to research in fields such infectious diseases or genomics where high throughput systems and new models are revolutionizing clinical testing.
In response to this challenge, an expert panel met at the “Hypoxic-Ischemic Encephalopathy Symposium: Developing the Future” in Norfolk, Virginia to discuss current and future treatment of NE. The goal was to recognize hurdles and opportunities in clinical trial design, assessment modalities, neurodevelopmental testing, biomarker use and biostatistical analyses of NE clinical trials. The experts had as their mandate to envision the future of NE clinical trials, by critically examining the past and avoiding preconceptions. A combination of the Delphi method and nominal group technique(13) was utilized to establish consensus. The organizing committee put together a broad range of current topics and questions around NE and invited a panel of experts to answer these questions. The experts convened and presented their summaries to an audience ranging from academics to industry partners. Following this, audience and organizers questioned the experts during round table gatherings. Comments and clarifying points were recorded and incorporated into a summary which was circulated back to the expert panel to reach a consensus. Finally, the draft of the summary was sent to the Society for Pediatric Research (SPR) which provided an outside perspective from the greater research community and requested clarifications and additions. The final consensus is presented in this manuscript.
SUMMARY OF CURRENT AND POTENTIAL FUTURE THERAPIES FOR NE
The next phase of NE clinical trials focuses on adjuvant therapies to hypothermia to further improve neurodevelopmental outcomes. Of interest are therapies to promote neuronal regeneration, functional and structural recovery from injury, neurovascular remodeling, optimal healing and ongoing development.(14) While TH primarily works in the latent and secondary phases of NE, new therapies also target the tertiary phase.(14) Examples of ongoing trials and potential future therapies for NE are presented in Table 1.(15,16,25,17–24)
TABLE 1.
Current and Potential Future Therapies for NE
| Current NE Clinical Trials | ||
|---|---|---|
| Agent | Highlighted results | Ongoing studies |
| Erythropoietin | NEATO (phase II) trial with decreased brain injury on MRI and improved short-term motor outcomes.(15,16) | HEAL (phase III): multicenter, double-blind, placebo-controlled study of high-dose erythropoietin.(17) |
| Cord blood/stem cells | Phase-I open-label study of term neonates with NE demonstrated safety and feasibility.(18) | Phase II randomized, double-blind, placebo-controlled, multi-site trial of autologous cord blood cells for NE.(19) |
| Melatonin | Neuroprotective in preclinical studies(20,21) | Phase I, open-label, dose-escalation trial of melatonin in neonates with NE undergoing TH.(22) |
| Anti-epileptics | Prophylactic barbiturate therapy for NE not recommended.(23) Topiramate adjuvant to TH is safe, did not reduce combined frequency of mortality and severe neurologic disability.(24) | Randomized, double-blind, placebo- controlled trial of topiramate as adjuvant to TH.(25) |
NEATO, Neonatal Erythropoietin and Therapeutic Hypothermia Outcomes; NE, neonatal encephalopathy; MRI,magnetic resonance imaging; HEAL,High-Dose Erythropoietin for Asphyxia and Encephalopathy; TH,therapeutic hypothermia.
TOOLS FOR CLASSIFICATION AND PROGNOSIS
Neurological Examination
Clinical detection of brain injury and classification of severity leverages clinical neurological examination scoring systems that allow classification of the degree of encephalopathy. However, many (Sarnat, Thompson) were developed for use in the first week of life, for the purpose of prediction rather than classification.(26) The National Institute of Child Health and Human Development Neonatal Research Network (NICHD NRN) scoring system and Score of the Iberoamerican Society of Neonatology (SIBEN) Neurologic Score were developed to define severity of NE to assist in selection of appropriate patients for TH and have been widely used in large clinical trials.(5,6,9,26,27) Regardless of the neurological classification system used, past and future trials must critically examine the rigorous nature of training and establishment of examiner reliability to ensure reproducibility of trial results.(5,6,9,27,28) The neurological examination within the first 6 hours of life is complicated by provider subjectivity, maternal or neonatal anesthesia/analgesia, dynamic evolution of the neurologic injury and compensatory responses, and timing of initiation of TH and neuroprotective therapies. The neurological examination performed in the first 6 hours has moderate predictive value for long-term outcomes.(8,29,30) However, repeated exams throughout hospitalization and discharge can improve predictive value.(31)
Serum Biomarkers
Serial measurements of serum neuronal and inflammatory biomarkers may classify the severity of NE, determine the timing, pathophysiology and extent of brain injury, identify infants for appropriate adjuvant neuroprotective therapies and assist in predicting neurodevelopmental outcomes. Biomarkers have the potential to advance the current landscape of NE clinical trials by monitoring real-time response to treatment and predict functional outcome rather than waiting 5–7 years to evaluate the success of a new therapy.(32)
For neuronal injury, multiple protein-derived markers have potential for biomarkers from neurons (neuron specific enolase [NSE] and ubiquitin carboxy-terminal hydrolase-L1 [UCH-L1]), glial cells (glial fibrillary acidic protein [GFAP] and S100B protein), Tau protein; inflammatory cytokines and chemokines (interleukins [IL], interferon gamma [IFN-γ], tumor necrosis factor alpha [TNF-α], brain-derived neurotrophic factor [BDNF], monocyte chemotactic protein-1 [MCP-1] and vascular endothelial growth factor [VEGF]) are also promising.(32–34) For example, Chalak et al(35), demonstrated a concentration-dependent relationship between serum GFAP and UCH-L1 at birth and severity of encephalopathy, with GFAP predictive of later abnormal outcomes; Massaro et al(15) proposed BDNF as a marker of neuronal repair with higher levels at day 5 associated with reduced brain injury on magnetic resonance imaging (MRI) and improved motor performance at 1 year.
Neonatal Electroencephalogram
When infants experience neural insults, the electroencephalogram (EEG) commonly reverts back to primitive and premature patterns.(36) EEG can display background abnormalities of amplitude, continuity, frequency, symmetry, interhemispheric synchrony, sleep states, and maturation.(36)
Inter-rater agreement in neonatal EEG background scoring is highest for voltage, seizure presence, continuity, burst voltage, suppressed background presence and overall impression but poor or inconsistent for other patterns, highlighting the importance of using major neonatal EEG background categories for interpretation.(37) EEG background activity at 24, 36 and 48 hours after birth is highly predictive of severity of brain injury on MRI and later neurodevelopmental outcomes in infants with NE undergoing TH.(38–40) Finally, amplitude-integrated EEG (aEEG) background at 6 hours in infants undergoing TH has some limited positive predictive value.(41) However, the progression of aEEG patterns in the setting of hypothermia (particularly, time to normalization of the background and the time of onset of sleep-wake cycling) have important prognostic value over time; an aEEG that remains abnormal at 48 hours is predictive of an adverse outcome.(41–43) Prognostic power of early aEEG improves substantially when combined with a standardized clinical neurological examination.(44,45)
Near-Infrared Spectroscopy
Dysfunctional vasoreactivity and autoregulation in NE may contribute to secondary brain injury and poor neurologic outcomes.(46–48) Impaired cerebral autoregulation, measured by near-infrared spectroscopy (NIRS) parameters correlates with neurologic injury on MRI(47,49–53) and 2-year neurodevelopmental outcomes in NE.(54) NIRS can determine an individual neonates’ ideal mean arterial pressure to optimize autoregulatory vasoreactivity.(46) Maintenance of mean arterial pressure within this individualized optimal range, correlates with neurologic injury on MRI and later neurodevelopmental outcomes.(46,52–54) NIRS, blood pressure and EEG metrics have been combined into a novel, wavelet neurovascular bundle analytical system to allow real-time, non-invasive quantification of cerebral autoregulation and neurovascular coupling at the bedside.(51,55,56) While still being validated in larger cohorts, this bundle may facilitate a precision medicine approach to patients with NE, facilitating individualized risk-stratification and addition of adjunct neuroprotective therapies.(47,55,56)
Neuroimaging
MRI with proton MR spectroscopy (MRS) in neonates with NE is an important prognostication tool as it provides the best anatomical delineation of brain structure and injury with multiple complementary sequences available.(57) However, challenges with MRI in neonates include transportation and distance of tertiary care centers, potential need for sedation, and requirement of expert interpretation. Typical MR imaging protocols in NE include structural sequences (T1, T2), diffusion weighted sequences (diffusion tensor imaging [DTI], diffusion weighted imaging [DWI], apparent diffusion coefficient [ADC]), susceptibility weighted imaging (SWI) and spectroscopy (MRS).(58)
Due to the pathophysiology of perinatal asphyxia with initial ischemia and subsequent reperfusion, diffusion imaging injury patterns evolve over time (Figure 1).(59,60) Existing challenges include the ability to obtain high quality, high resolution imaging without motion, and the effects of variability of timing and nature of injury on diffusion patterns, as well as the subjective nature of interpretation. Recently, progress has substantially improved standardization of diffusion imaging interpretation through publicly available, normative, age-specific ADC atlases.(61)
Figure 1. Evolution of Injury on MRI.
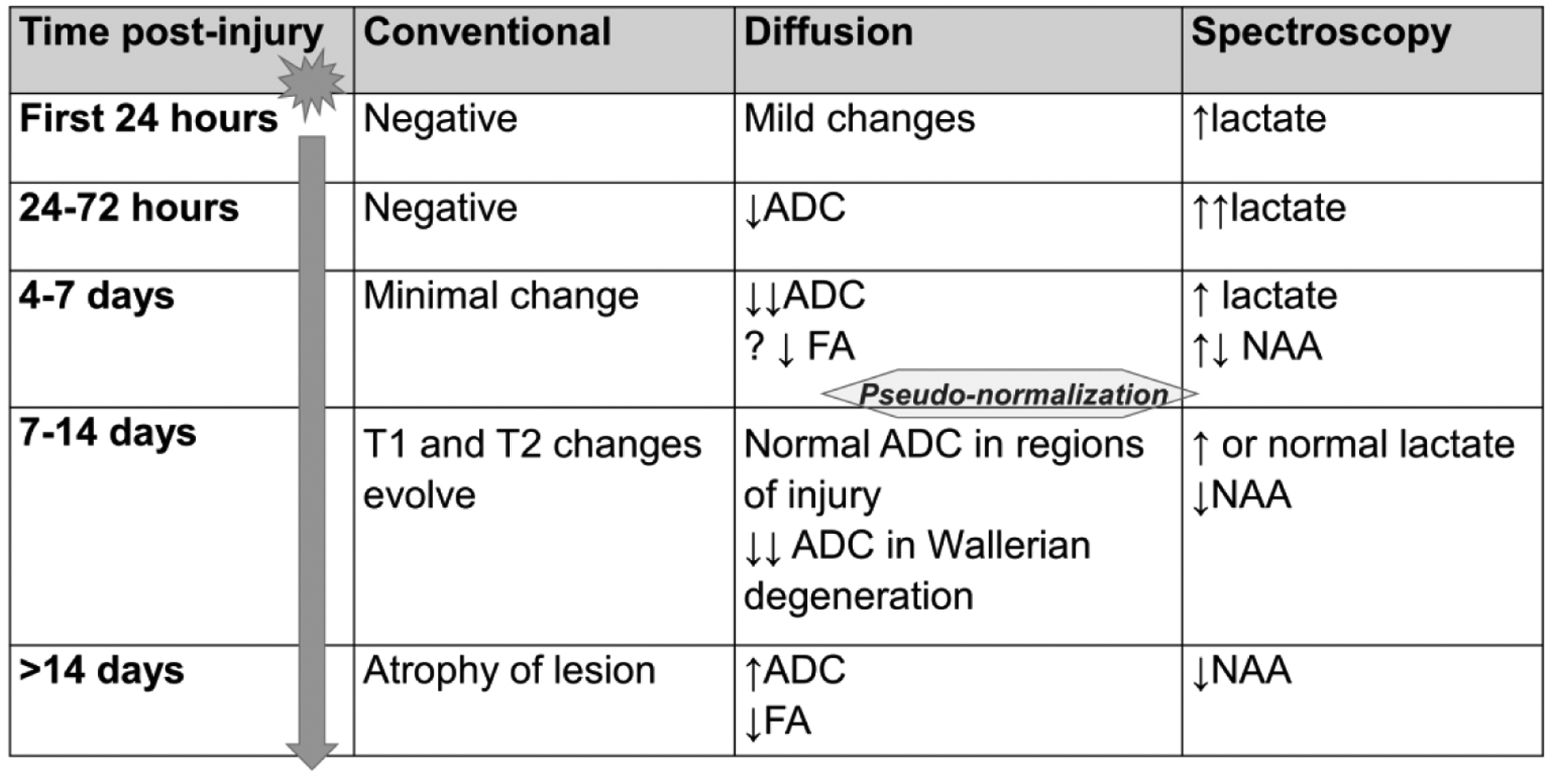
The evolution of injury patterns seen on MRI (conventional, diffusion and spectroscopy sequences) from 24 hours post-injury to more than 2 weeks post-injury. Diffusion abnormalities normalize over time with pseudo-normalization occurring after the first week of life.(59,60) The interval between initial insult and pseudo-normalization presents an opportunity for recovery using additional neuroprotective strategies.
MRI, magnetic resonance imaging; ADC, apparent diffusion coefficient; FA, fractional anisotropy; NAA, N-acetyl aspartate.
The search for optimal MR biomarkers has recently focused on MR spectroscopy. A meta-analysis of prognostic accuracy of cerebral MR biomarkers in infants with NE demonstrated that in the first 30 days, MRS measures, particularly the deep gray matter lactate/N-acetyl-aspartate (NAA) ratio, most accurately predicted an adverse neurodevelopmental outcome.(62,63) However, the biggest roadblock in widespread use of MRS as a biomarker in NE has been the difficulty of achieving harmonization across multiple centers and models of MR scanners.(8,63) In the MARBLE study, a large prospective, multicenter cohort study of MRS biomarkers, harmonization of robust thalamic proton MRS biomarkers across several centers was achieved using 3.0 Tesla MR systems from three different manufacturers. MR spectroscopy thalamic NAA concentration alone, accurately predicted an adverse neurodevelopmental outcome at 2 years (AUC 0.99, sensitivity 100%, specificity 97%).(8) Thalamic NAA concentration outperformed other current predictive methods such as the NICHD neurological exam (within 6 hours of birth and at discharge), aEEG background (within 6 hours of birth) and other MR sequences. The MRS protocol developed from this study is being validated in a trial of TH in low and middle income countries.(64) Ongoing research will assess whether adjunctive neuroprotective interventions affect MRS metrics and whether changes in these biomarkers reflect later neurodevelopmental outcome.(17,64) MRS biomarkers could improve the efficiency of neonatal neuroprotective trials by functioning as surrogate outcome measures in phase 2 studies(65) or by allowing optimizing of protocols prior to phase 3 trials.(8,10)
NEONATAL ENCEPHALOPATHY CLINICAL TRIAL OVERSIGHT
Multicenter parallel design randomized controlled trials (RCT) have been the mainstay of NE intervention testing. Most have leveraged large academic networks and professional centers specializing in data coordination(66), while others have added additional study management knowledge gained from industry-sponsored trials.(17) Lessons learned from the multicenter High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL) trial can help rethink trial management processes and infrastructure to design the next generation of NE neuroprotection RCTs, with particular focus on process building, efficiency, and customer service.
NE trials frequently enroll subjects within hours of birth, while addressing the challenges of shocked families facing a critically ill neonate and often separation from transfer to a tertiary hospital. In trials involving administration of study drug, clinical pharmacies may be called upon to prepare medication at off-hours when investigational pharmacies are closed; laboratory and informed consent procedures are often required at precise times, outside of standard work hours. These challenges require unique solutions and well-organized study management.
Study Design and Startup
When designing any RCT, it is critical that all study decisions be made with study end-goals and primary endpoints in mind. Primary endpoints should be clearly articulated, and must address the study goals, whether they be regulatory approval, change in clinical practice, data collection for future studies, or reimbursement. Prior to creating data collection instruments, it is more efficient to create initial shell tables for future publications, with consort diagrams as integrated components.(67) Ensuring all essential data are collected in an appropriate and consistent manner is critical to study success and collection points can be viewed as process flow measures.
Then, as study processes are designed, pre-specified study endpoints should be referred to frequently in developing flows, lest a change to a study procedure inadvertently compromise crucial study data.
Because NE trials often involve enrollments at multiple sites, site-by-site processes may differ. Through collaborative central coordination, awareness of site practice differences, and use of flexible process templates created by a central study team, consistency across sites can be achieved. Efficient NE trial processes may necessitate having a rotating call schedule for evening and weekend enrollments. In a trial involving the investigational drug service (IDS) division of pharmacy, individual meetings with each IDS director are important to establish logistics of potential off-hours enrollment. Study teams must also develop primary and contingency plans for biological samples processing (personnel, equipment, and long-term storage). If neuroimaging endpoints are used, harmonization across multiple neuroimaging platforms, and processes for central image archiving and review should be considered.(17) NE trials often include neurodevelopmental follow-up extending into childhood. Participating sites should therefore have the proven capacity not just to enroll subjects, but to have high long-term follow-up rates. The study should also consider how standardization of neurodevelopmental scales will occur, as clinical exams are often not performed with the consistency and level of rigor that a clinical trial outcome measure demands. For all of these processes, clear and consistent methods should be communicated to participating sites in a collaborative manner. This approach is common in industry where Lean Six Sigma and other efficiency tools are standard.(68)
Study Management and Oversight
After study startup, trial leadership should make themselves personally accessible to answer time-sensitive study questions, ensuring consistency amongst enrollments and continued engagement. Frequent communication of enrollment and deliverable achievement is a motivational technique that also updates collaborators on trial progress. Collaborative regular team meetings may include smaller leadership groups, and separately, the larger study team. Communication of pre-specified metrics helps clarify expectations and ensure a common goal. Suggested metrics include overall and site-specific study enrollment, follow-up rates, and compliance with data entry and data quality expectations. For many NE studies, enrollment and consent processes may be complex and a study monitoring plan is necessary. On-site monitoring visits may be required in addition to remote conferencing to ensure regulatory compliance, and provide an opportunity to review source data for critical study data fields.
BIOSTATISTICAL CHALLENGES AND OPPORTUNITIES IN TRIAL DESIGN AND ANALYSIS
Challenges to statistical design and analyses plans in NE clinical trials include reproducibility, efficient data use, limited eligible subject pool (especially in single-center designs), and long-term follow-up. Using pre-specified, innovative biostatistical analyses can help overcome many of these challenges.
Bayesian Analyses
Bayesian analysis integrates prior information with newly collected data to obtain a posterior estimate of the effect of difference.(69,70) It is a natural way to incorporate prior evidence into the analysis of current data in the setting of a limited sample size. The late cooling trial (JAMA 2017) used a pre-specified Bayesian analysis given the anticipated limited sample size.(27) This analysis can produce clinically meaningful measures, such as the probability of treatment benefit.
Criticism of this method lies in the fact that using prior information is a form of assumption. To overcome this, Laptook et al(27) represented the prior information with several different options-a neutral, skeptical and enthusiastic prior, generating different final summaries of the evidence.(70)
Adaptive Clinical Trials
Adaptive clinical trials allow planned modifications to one or more aspects of the design, based on data collected from the study’s subjects while the trial is still ongoing. This type of design may allow patients to access the most significant benefit of a treatment by allocating more subjects to the most clinically efficacious arm or dose, after an interim analysis. Adaptive clinical trials allow investigators to use information not available at the start of the trial to improve efficiency and provide a greater chance of detecting the true effect of an intervention, often with a smaller sample size or in a shorter timeframe. This can reduce the number of patients exposed to unnecessary risks or ineffective investigational treatments; it can even increase the willingness of parents to enroll their children in these types of trials as they increase the probability that subjects will be assigned to the most effective treatments.(71,72)
Biostatistical Opportunities in Clinical Trial Design
Investigators can utilize internet resources to improve standard surveys, experiments, and health reporting. Incorporating modern statistical methodology (and Bayesian methods) can increase efficient use of all available information. Complex designs benefit from early statistical involvement with carefully considered and pre-specified analysis plans.
INTERMEDIATE AND LONG-TERM NEUROPHYSIOLOGY AND DEVELOPMENT
Monitoring of Neurodevelopmental Trajectories
Neurodevelopment is a complex continuum, occurring in the context of maturation, genetics and experiences.(73) Additionally, trajectories can change with experience and environmental factors, infants can form new neuronal connections and develop new functionality. This neuroplasticity, which peaks in the first 3 years of life is however also an area of vulnerability. Evidence-based interventions, focused on cognition, feeding, language, motor, pain, parenting, spasticity management, sleep, or vision, many of which start in infancy, take advantage of this plasticity after perinatal brain injury. Previous and current NE clinical trials focus on neurodevelopmental trajectories that include only term-equivalent age (TEA) and 18–26 month time points. However, this “trajectory” is poorly predictive of school age and later outcomes as it does not account for the complexity of factors occurring between these two time points, some confounded by treatment allocation.(74–76) While the 2-year Bayley does not accurately predict outcomes from childhood to adolescence, using the Bayley at multiple time points, to obtain a true developmental slope may be more predictive. An ideal early neurodevelopmental trajectory as illustrated in Figure 2, would include a variety of time points with targeted assessments from TEA to 2 years. However, not every outcome may be of interest for every NE treatment.
Figure 2. A True Neurodevelopmental Trajectory.
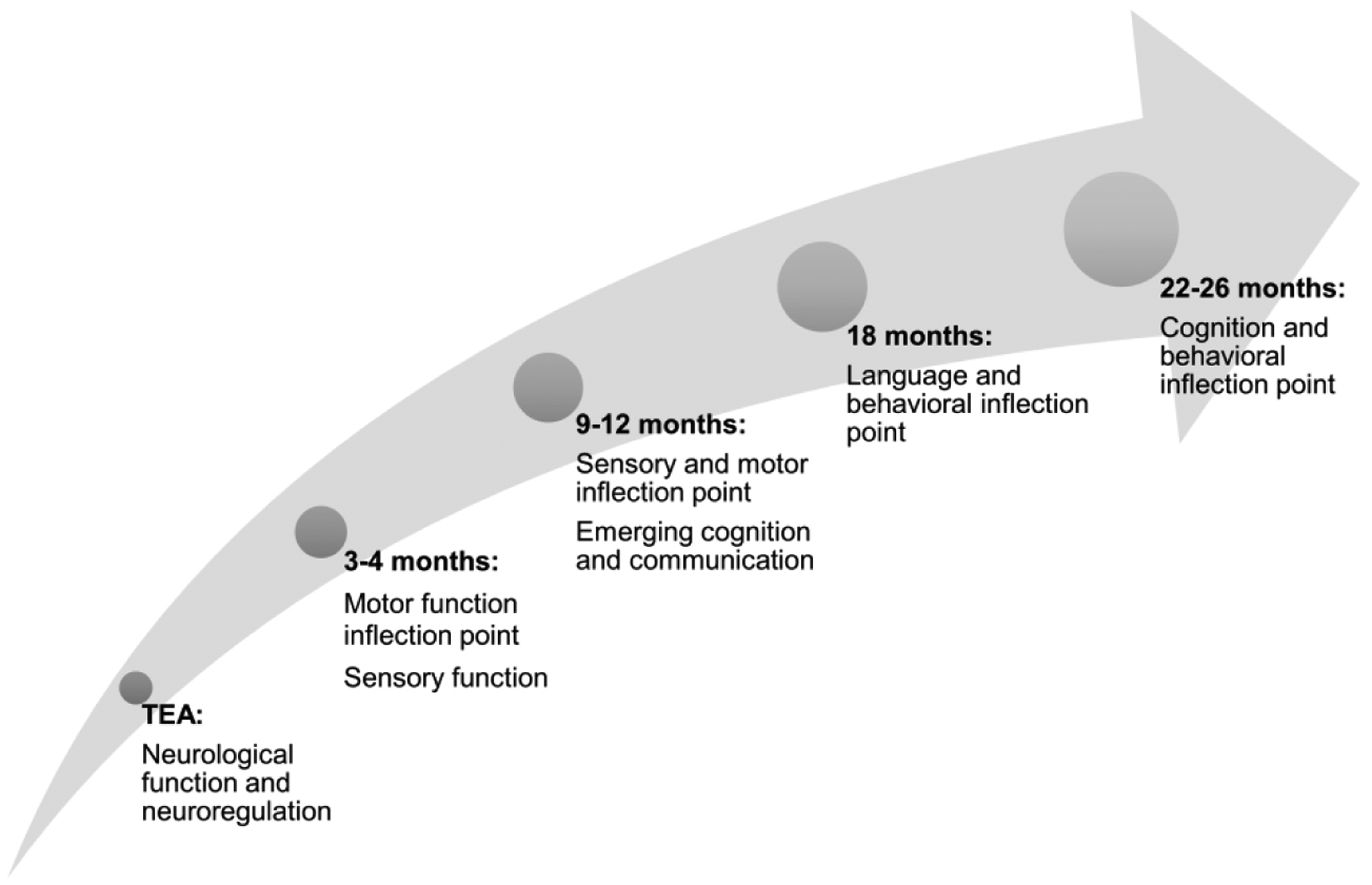
An ideal early neurodevelopmental trajectory includes a variety of time points, each of which represent a specific developmental domain inflection point with targeted assessments from TEA to 2 years.
TEA, term-equivalent age.
Tests of Neurophysiological Responses
Tests of neurophysiological responses including standardized scorable neurological exams and functional neuroelectrical imaging commonly allow longitudinal evaluation; however these tests may be less sensitive to interventions in the short-term.
Standardized scorable neurological exams
While the modified Sarnat has been used widely to quickly categorize stages of NE, it was never intended to be a tool to determine patient eligibility for randomized controlled trials.(26) It is therefore intrinsically limited and may need revisions for use in newer clinical trials. Other exams may provide a more precise characterization of neurological condition, but with increasing complexity and more validation of inter- and intra-rater reliability to ensure rigor and reproducibility. Such an assessment, the Hammersmith Neonatal Neurological Examination (HNNE) is reliable as early as the first few hours of life(77) throughout the newborn period to TEA, allowing for longitudinal evaluation with a single, quantitative test.(78) Age-specific optimality scores allow comparisons with neurophysiologic and imaging findings, with good correspondence between HNNE scores and neuroimaging.(78,79) The Hammersmith Infant Neurological Examination (HINE), for infants aged 2–24 months demonstrates good correspondence between MRI findings and HINE scores in infants with NE.(80) Sequential use of the HNNE in the newborn period and the HINE through 2 years of age, offers a continuum for assessment of neurologic function and allows identification of early signs of cerebral palsy (CP) and other neurological disorders.
Functional neuroelectrical imaging
At its simplest, functional neuroelectrical imaging (time-locked EEG/event-related potential [ERP]) can test the cortical processing of a single stimulus using somatosensory (SEP) or visual evoked potentials (VEP). Combining somatosensory or visual evoked potentials with MRI and EEG improves prediction of later global neurodevelopment. SEP and VEP are especially important prognostic tools in infants with a normal MRI after NE and TH.(81) Advanced analysis techniques leveraging topographic analyses and inverse-source localization can allow measurement of more involved functions such as speech sound discrimination, touch processing or multisensory integration to accurately predict more complex, functional processing later in life.(82–84)
Behavioral Tests of Systemic Function
Behavioral tests of systemic function can be divided into those that evaluate a single major domain or those that test multiple domains. Each test has strengths and drawbacks; some allow for longitudinal evaluation (Bayley Scales of Infant and Toddler Development, 3rd and 4th editions [Bayley-III and Bayley-IV], Infant/Toddler Sensory Profile [ITSP], Child Behavior Checklist [CBCL]); some are very sensitive to interventions (Peabody Developmental Motor Scales[PDMS-2]); many have difficulties adapting for children with neurosensory or motor impairments.(85) Predictive validity for school-age outcomes differs with each individual test, but overall increases with age at testing. Systematic reviews exist for many of the individual domain tests and are detailed in Table 2.(85–94)
TABLE 2.
Examples of Single Domain Assessments
| Domain | Tests | Systematic Reviews |
|---|---|---|
| Motor | AIMS, Bayley-III, BOT-2, MABC-2, MAND, Mullen, NSMDA, PDMS-2 | Griffiths 2018(86) Kjolbye 2018(87) |
| Sensory Processing | TSFI, SRS, ITSP | Eeles 2013(88) |
| Communication | Bayley-III, CAT/CLAMS, CSBS-DP, Mullen, REEL-3, PLS-5 | Nelson 2006(89) |
| Adaptive Behavior/Social Emotional | ABAS-II, ADEC, CARS-2, CBCL, ITSEA, Vineland SEEC | Hanratty 2015(90) Halle 2016(91) McCrae 2018(92) |
| Cognitive | Bayley-III, DAS-II, FTII, GMDS, SB-5, WJ-III, WPPSI, Mullen, MMFC | Brydges 2018(93) Morgan 2019(85) Wong 2016(94) |
ABAS-II,Adaptive Behavior Assessment System; ADEC,Autism Detection in Early Childhood; AIMS, Alberta Infant Motor Scale, Bayley-III, Bayley Scale of Infant and Toddler Development 3rd edition; BOT-2, Bruininks-Oseretsky Test of Motor Proficiency; CARS-2, Childhood Autism Rating Scale second edition; CBCL,Child Behavior Checklist; CAT/CLAMS, Clinical Adaptive Test/Clinical Linguistic Auditory Milestone Scale; CSBS-DP, Communication and Symbolic Behavior Scales-Developmental Profile; DAS-II, Differential Ability Scales; FTII,Fagan Test of Infant Intelligence; GMDS, Griffiths Mental Development Scales; ITSEA, Infant Toddler Social Emotional Assessment; MABC-2, Movement Assessment Battery for Children; MAND,McCarron Assessment of Neuromuscular Development; MMFC,Mayes Motor-Free Compilation; Mullen,Mullen Scales of Early Learning; NSMDA,Neurological Sensory Motor Developmental Assessment; PDMS-2, Peabody Developmental Motor Scales; SB-5,Stanford-Binet Intelligence Scales; TSFI,Test of Sensory Function in Infants; SRS,Sensory Rating Scale for Infants and Young Children; ITSP,Infant/Toddler Sensory Profile; Vineland SEEC, Vineland Social-Emotional Early Childhood Scales; WJ-III,Woodcock-Johnson III Tests of Cognitive Abilities; WPPSI-III, Wechsler Preschool and Primary Scale of Intelligence
While single domain tests can isolate the area of concern and may be quicker to perform, the feasibility of training personnel for testing, analyzing and scoring each can be problematic. Advantages of multi-domain assessments include efficiency, a global assessment of the child’s strengths and weaknesses at a single time point, with value for longitudinal assessments. Conversely, multi-domain assessments can be time-consuming, costly, require extensive reliability monitoring, and have mixed predictive value into adulthood when performed at 2 years.(95,96)
Biomarkers of neurologic function
Neurodevelopmental biomarkers include movement patterns (Prechtl’s General Movements Assessment [GMA]), HNNE/HINE optimality and cutoff scores and scoring/classification systems (Gross Motor Function Classification System [GMFCS]). These biomarkers are used at a single time point evaluation, may not respond to interventions and were developed for their diagnostic, classification or prognostic value. The GMA evaluates spontaneous movements originating in the brainstem that begin in fetal life and function to build neural connectivity between motor and sensory systems.(97) Movement patterns such as cramped-synchronized movements at TEA and absent fidgety movements at 3–5 months are predictive of CP and other developmental problems.(98) The HINE optimality score is based on the frequency distribution of the scores in the normal population.(99) Specific HINE cut-off scores, with a high sensitivity and specificity for prediction of CP during the first year of age are reported in both preterm and term-born high-risk infants (99) For children with CP, classification systems exist for multiple functional domains (fine and gross motor, communication) which often predict late childhood or adult function, when performed after 2 years of age.(100,101)
PERSPECTIVES FROM THE SPR
The next phase of NE clinical trials focuses on hypothermia adjuvant therapies.(15–19,22,24,25) Many adjuvant therapies target alternative recovery mechanisms or multiple phases during the progression of hypoxic brain injury.(14) Future clinical trial designs could incorporate new paradigms to address common challenges, considering various phases of injury and recovery, and no longer a pseudo-normalization of 2-year test-scores (see Table 3 for a summary of recommendations).(8,10,56–60,63,64,71,73,74,15,75,77–79,81–84,98,102,17,103–111,27,30,32,35,47,55)
TABLE 3:
Future of Neuroprotection Trials for NE: Recommendations and Future Directions
| Identification of appropriate neonates for TH and for adjunctive neuroprotective therapies |
|
| Predicting response to TH |
|
| Methodology for improved trial consistency |
|
| Timing of neuroimaging | Two MRI scans: |
| Surrogate endpoints |
|
| Long-term neurodevelopmental follow-up | |
| TH and trial design in LMIC |
|
aEEG,amplitude-integrated electroencephalogram; GMA, Prechtl’s General Movements Assessment; HELIX,hypothermia for encephalopathy in low and middle-income countries; LMIC,low- and middle-income countries; MRI,magnetic-resonance imaging; MRS, proton magnetic-resonance spectroscopy; NAA,N-acetyl-aspartate; NE,neonatal encephalopathy; NIRS,near-infrared spectroscopy; TH,therapeutic hypothermia
Identification and classification of trial participants: Solutions to optimize the predictive value of the neurologic exam include performing consistently-timed, serial exams, standardization through extensive practice in other newborn populations, video recording of the exam, and use of standardized, scorable and predictive exams such as the HNNE.(77–79) A panel of multiple inflammatory and neuronal biomarkers may also advance the current landscape of NE clinical trials and individualize care by characterizing degree of initial injury, risk stratification, and acute assessment of treatment efficacy. For example, metabolomic profiling could characterize mechanisms of injury that differentiate mild from more severe NE and need for specific adjuvants.(112) Finally, use of early aEEG augmented by new machine-learning algorithms holds promise in classifying the presence of neural insults even when overt signs may not be clear or in settings where other approaches are challenging.(102)
Timing of evaluation after NE is critical. Two recommended MRI time points assist in identifying the acute perinatal nature of the injury (days 3–7) as well as the full extent and evolution of the injury (days 10–21).(58,113) The later in this window follow-up imaging occurs, the more likely it is to reflect the full evolution of injury and a possible intermediate endpoint. However, return to the hospital soon after discharge can be challenging for families, often necessitating an earlier follow-up MRI (day 10) while injury is still evolving. Finally, MRS biomarkers obtained between 4 and 14 days of life can provide added value for prediction of adverse neurodevelopmental outcomes in cooled neonates with NE with recognition that the full neurodevelopmental trajectory is not completely defined at this time point.(8) An initial evaluation of each new therapy at this time point, with neuroimaging and other measures (Figure 3) may allow higher-throughput selection of promising therapies for more extensive investigation. Ultimately, this approach would circumvent the impact of concurrent clinical trials of post-discharge interventions and rehabilitative therapies that may mask global effects traditionally measured at 2 years.
Rethinking trial management processes and infrastructure with attention to aspects of process building, efficiency and customer service, along with leveraging hospital quality improvement policies, can help focus study design and management. The following strategies can be useful: making study metrics regularly available to site teams (through automation or standardized processes); augmenting on-site visits with remote, database review allowing sites to correct anomalous data in near-real time(114); and videoconference check-ins after the first 3 patients enrolled to support process flow review. Additionally, using pre-specified, innovative biostatistical analyses, such as Bayesian analyses or adaptive clinical trials, can help overcome challenges to statistical designs and analysis plans, decreasing wasted time and resources in vulnerable and rare populations.(27,70,71)
A developmental trajectory: Rather than reducing the complex human brain and nervous system to a single poorly predictive primary outcome at 2 years, targeting a trajectory of multiple developmental assessments, exams and biomarkers used for evaluation at critical inflection points allows detection of intervention-specific outcomes (Figure 2). As innovative therapies evolve from “shotgun” approaches to precision medicine, so should developmental testing. Feasibility and predictive value increase as the specificity of the outcomes desired increase, whether they be improved parent-infant interactions or motor function. However, behavioral, emotional and executive function outcomes at school-age remains the ultimate safety metric of all neonatal trials.
Figure 3. Measurements in the Continuum of Injury and Recovery in NE.
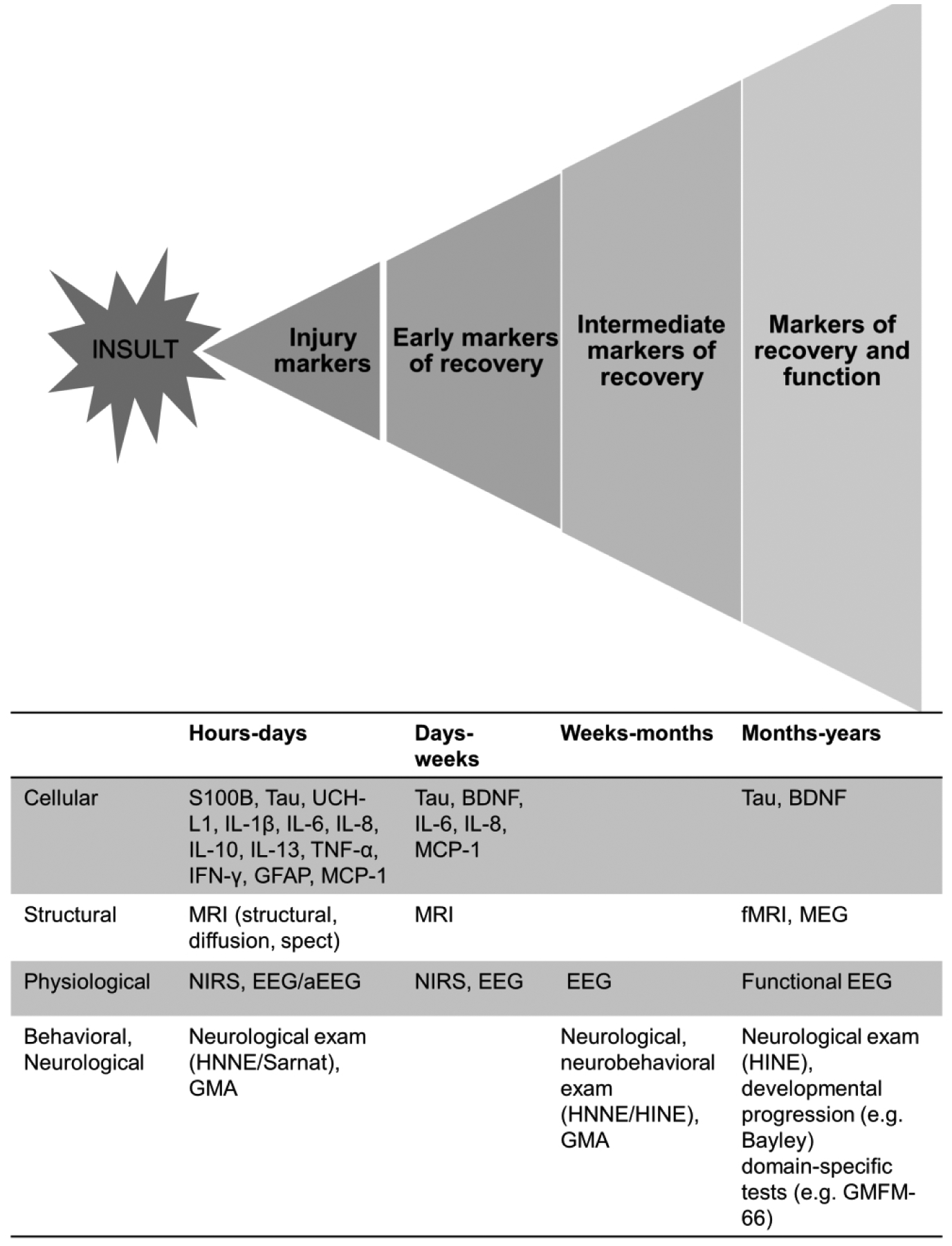
Markers of injury and recovery after neonatal NE as measured at the cellular, structural, physiologic, behavioral and neurological level. Recovery after NE occurs on a continuum, first with recovery at the cellular and microstructural level and leading to functional recovery with each step dependent and building on the previous. As time from injury increases, the imprecision of prediction provided by the assessment also increases as reflected in the pyramidal shape in the figure.
aEEG, amplitude-integrated electroencephalogram; Bayley, Bayley Scales of Infant Development; BDNF, brain-derived neurotrophic factor; EEG, electroencephalogram; fMRI, functional magnetic resonance imaging; GFAP, glial fibrillary acidic protein; GMA, Prectl’s General Movements Assessment; GMFM-66, Gross Motor Function Measure; HINE, Hammersmith Infant Neurological Examination; HNNE, Hammersmith Neonatal Neurological Examination IFN-γ, interferon gamma; IL, interleukin-1β, IL-6, IL-8, IL-10, IL-13; MCP-1, monocyte chemotactic protein-1; MEG, magnetoencephalography; MRI, magnetic resonance imaging; NE, neonatal encephalopathy; NIRS, near-infared spectroscopy; TNF-α, tumor necrosis factor alpha; UCHL1, ubiquitin carboxy-terminal hydrolase-L1
CONCLUSION
While new neuroprotective therapies appear promising, the design of clinical trials to test the safety and efficacy of these agents has hardly changed in the past twenty years. Combining a variety of biomarkers with innovative and carefully planned clinical trial design and analysis will help target infants with the most appropriate and timely treatments (Table 3 and Figure 3). The combination of serial, standardized neurological examinations, serum biomarkers, EEG background patterns and neuroimaging may give the best indication of early and intermediate recovery from injury.(38,39) Functional recovery can be assessed using a trajectory of neurodevelopmental evaluations targeted to a pre-specified and mechanistically-derived hypothesis of drug action. Ultimately, recovery after NE occurs on a continuum, first at a cellular and microstructural level, and eventually at functional levels with each step dependent and building on the previous (Figure 3). As precision medicine revolutionizes healthcare, it should also include the redesign of NE clinical trials to allow safe, efficient and targeted therapeutics.
Impact.
What is the key message of your article?
As precision medicine revolutionizes healthcare, it should also include the redesign of NE clinical trials to allow faster development of safe, effective and targeted therapeutics.
What does it add to the existing literature?
This article provides a multidisciplinary perspective on the future of clinical trials in NE; novel trial design, study management and oversight, biostatistical methods and a combination of serum, imaging and neurodevelopmental biomarkers can advance the field and improve outcomes for infants affected by NE.
What is the impact?
Innovative clinical trial designs, new intermediate trial end points and a trajectory of neurodevelopmental evaluations targeted to a pre-specified and mechanistically-derived hypothesis of drug action can help address common challenges in NE clinical trials, allow for faster selection and validation of promising therapies for more extensive investigation.
ACKNOWLEDGEMENTS
Thank you to Kenji M. Cunnion and Neel K. Krishna for hosting the 2019 HIE Symposium: Developing the Future.
Statement of financial support: The symposium was made possible through support from ReAlta Life Sciences, Children’s Specialty Group, Eastern Virginia Medical School and the Children’s Hospital of The King’s Daughters. This work was supported by 1R01HD081120-01A1 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to N.L.M. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding organizations.
Footnotes
Disclosure statement: Amy M. Goodman is a consultant for Radial Medical, Autonomic Technologies, and Element Science. All other authors have disclosed that they do not have any relevant relationships with commercial companies.
REFERENCES
- 1.AAP and ACOG Task Force on Neonatal Encephalopathy. Neonatal Encephalopathy and Neurologic Outcome, Second Edition 2014;123:896–901. [DOI] [PubMed] [Google Scholar]
- 2.Ferriero DM. Neonatal Brain Injury. N Engl J Med 2004;351:1985–95. [DOI] [PubMed] [Google Scholar]
- 3.Gunn AJ, Bennet L, Gunn AJ. Fetal Hypoxia Insults and Patterns of Brain Injury : Insights from Animal Models. Clin Perinatol [Internet] 2009. [cited 2019 Feb 22];36:579–93. Available from: https://www.clinicalkey.com/service/content/pdf/watermarked/1-s2.0-S0095510809000505.pdf?locale=en_US [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagin MA, Woolcott CG, Vincer MJ, Whyte RK, Stinson DA. Hypothermia for neonatal hypoxic ischemic encephalopathy: An updated systematic review and meta-analysis. Arch Pediatr Adolesc Med 2012;166:558–66. [DOI] [PubMed] [Google Scholar]
- 5.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-Body Hypothermia for Neonates with Hypoxic–Ischemic Encephalopathy. N Engl J Med 2005;353:1574–84. [DOI] [PubMed] [Google Scholar]
- 6.Shankaran S, Pappas A, McDonald SA, et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med 2012;366:2085–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacobs SE, Berg M, Hunt R, Tarnow-Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy [Internet]. Cochrane Database Syst. Rev 2013. [cited 2019 Jan 22];2013 Available from: http://doi.wiley.com/10.1002/14651858.CD003311.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lally PJ, Montaldo P, Oliveira V, et al. Magnetic resonance spectroscopy assessment of brain injury after moderate hypothermia in neonatal encephalopathy: a prospective multicentre cohort study. Lancet Neurol 2019;18:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shankaran S, Laptook AR, Pappas A, et al. Effect of depth and duration of cooling on death or disability at age 18 months among neonates with hypoxic-ischemic encephalopathy a randomized clinical trial. JAMA 2017;318:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunn AJ, Battin M. Towards faster studies of neonatal encephalopathy. Lancet Neurol [Internet] 2019;18:21–2. Available from: 10.1016/S1474-4422(18)30370-3 [DOI] [PubMed] [Google Scholar]
- 11.Chalak L, Ferriero DM, Gressens P, Molloy E, Bearer C. A 20 years conundrum of neonatal encephalopathy and hypoxic ischemic encephalopathy: are we closer to a consensus guideline? Pediatr Res [Internet] 2019;86:548–9. Available from: 10.1038/s41390-019-0547-9 [DOI] [PubMed] [Google Scholar]
- 12.Molloy EJ, Bearer C. Neonatal encephalopathy versus Hypoxic-Ischemic Encephalopathy. Pediatr Res [Internet] 2018;84:574 Available from: 10.1038/s41390-018-0169-7 [DOI] [PubMed] [Google Scholar]
- 13.Jones J, Hunter D. Consensus methods for medical and health services research. BMJ 1995;311:376–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson JO, Wassink G, van den Heuij LG, Bennet L, Gunn AJ. Therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy - Where to from here? Front Neurol 2015;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massaro AN, Wu YW, Bammler TK, et al. Plasma Biomarkers of Brain Injury in Neonatal Hypoxic-Ischemic Encephalopathy. J Pediatr [Internet] 2018;194:67–75.e1. Available from: 10.1016/j.jpeds.2017.10.060 [DOI] [PubMed] [Google Scholar]
- 16.Wu YW, Mathur AM, Chang T, et al. High-Dose Erythropoietin and Hypothermia for Hypoxic-Ischemic Encephalopathy: A Phase II Trial. Pediatrics [Internet] 2016;137:e20160191–e20160191. Available from: http://pediatrics.aappublications.org/cgi/doi/10.1542/peds.2016-0191 [DOI] [PubMed] [Google Scholar]
- 17.Juul SE, Comstock BA, Heagerty PJ, et al. High-Dose Erythropoietin for Asphyxia and Encephalopathy (HEAL): A Randomized Controlled Trial-Background, Aims, and Study Protocol. Neonatology [Internet] 2018. [cited 2019 Jan 24];113:331–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29514165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cotten CM, Murtha AP, Goldberg RN, et al. Feasibility of Autologous Cord Blood Cells for Infants with Hypoxic-Ischemic Encephalopathy. J Pediatr [Internet] 2014. [cited 2019 Feb 13];164:973–979.e1. Available from: 10.1016/j.jpeds.2013.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotten CM. A Multi-site Study of Autologous Cord Blood Cells for Hypoxic Ischemic Encephalopathy [Internet]. [cited 2019 Feb 13];Available from: https://clinicaltrials.gov/ct2/show/NCT02612155?cond=Hypoxic-Ischemic+Encephalopathy&rank=38
- 20.Robertson NJ, Martinello K, Lingam I, et al. Melatonin as an adjunct to therapeutic hypothermia in a piglet model of neonatal encephalopathy: A translational study. Neurobiol Dis [Internet] 2019;121:240–51. Available from: 10.1016/j.nbd.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 21.Robertson NJ, Faulkner S, Fleiss B, et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 2013;136:90–105. [DOI] [PubMed] [Google Scholar]
- 22.Weiss MD. Melatonin as a Neuroprotective Therapy in Neonates With HIE Undergoing Hypothermia - Full Text View - ClinicalTrials.gov [Internet] [cited 2019 Feb 13];Available from: https://clinicaltrials.gov/ct2/show/NCT02621944
- 23.Young L, Berg M, Soll R. Prophylactic barbiturate use for the prevention of morbidity and mortality following perinatal asphyxia. Cochrane Database Syst Rev 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filippi L, Fiorini P, Catarzi S, et al. Safety and efficacy of topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia (NeoNATI): a feasibility study. J Matern Neonatal Med [Internet] 2018. [cited 2019 Feb 14];31:973–80. Available from: https://www.tandfonline.com/doi/full/10.1080/14767058.2017.1304536 [DOI] [PubMed] [Google Scholar]
- 25.Hoffman KR. Topiramate in Neonates Receiving Whole Body Cooling for Hypoxic Ischemic Encephalopathy - Full Text View - ClinicalTrials.gov [Internet] [cited 2019 Feb 14];Available from: https://clinicaltrials.gov/ct2/show/NCT01765218?cond=Hypoxic-Ischemic+Encephalopathy&rank=5
- 26.Inder TE, Volpe JJ. Hypoxic-Ischemic Injury in the Term Infant: Clinical-Neurological Features, Diagnosis, Imaging, Prognosis, Therapy. Clinical-Neurological Features, Diagnosis, Imaging, Prognosis, Therapy. [Internet] In: Volpe’s Neurology of the Newborn: Elsevier Inc.; 2018. p. 510–563.e15.Available from: 10.1016/B978-0-323-42876-7.00020-X [DOI] [Google Scholar]
- 27.Laptook AR, Shankaran S, Tyson JE, et al. Effect of therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with hypoxic-ischemic encephalopathy a randomized clinical trial. JAMA 2017;318:1550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natarajan G, Laptook A, Shankaran S. Therapeutic Hypothermia: How Can We Optimize This Therapy to Further Improve Outcomes? Clin Perinatol [Internet] 2018;45:241–55. Available from: 10.1016/j.clp.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Laerhoven H, De Haan TR, Offringa M, Post B, Van Der Lee JH. Prognostic tests in term neonates with hypoxic-ischemic encephalopathy: A systematic review. Pediatrics 2013;131:88–98. [DOI] [PubMed] [Google Scholar]
- 30.Merchant N, Azzopardi D. Early predictors of outcome in infants treated with hypothermia for hypoxic-ischaemic encephalopathy. Dev Med Child Neurol [Internet] 2015. [cited 2019 Apr 9];57:8–16. Available from: http://doi.wiley.com/10.1111/dmcn.12726 [DOI] [PubMed] [Google Scholar]
- 31.Shankaran S, Laptook AR, Tyson JE, et al. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr [Internet] 2012. [cited 2019 Feb 22];160:567–572.e3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22050871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graham EM, Burd I, Everett AD, Northington FJ. Blood biomarkers for evaluation of perinatal encephalopathy. Front Pharmacol 2016;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins DD, Rollins LG, Perkel JK, et al. Serum cytokines in a clinical trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J Cereb Blood Flow Metab 2012;32:1888–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orrock JE, Panchapakesan K, Vezina G, et al. Association of brain injury and neonatal cytokine response during therapeutic hypothermia in newborns with hypoxic-ischemic encephalopathy. Pediatr Res 2016;79:742–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chalak LF, Sánchez PJ, Adams-Huet B, Laptook AR, Heyne RJ, Rosenfeld CR. Biomarkers for severity of neonatal hypoxic-ischemic encephalopathy and outcomes in newborns receiving hypothermia therapy. J Pediatr 2014;164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mizrahi EM, Hrachovy RA. Atlas of neonatal electroencephalography. Fourth Edition Demos Medical Publishing; 2016. [Google Scholar]
- 37.Massey SL, Shou H, Clancy R, et al. Interrater and Intrarater Agreement in Neonatal Electroencephalogram Background Scoring. J Clin Neurophysiol [Internet] 2019;36:1 Available from: http://insights.ovid.com/crossref?an=00004691-900000000-99510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weeke LC, Boylan GB, Pressler RM, et al. Role of EEG background activity, seizure burden and MRI in predicting neurodevelopmental outcome in full-term infants with hypoxic-ischaemic encephalopathy in the era of therapeutic hypothermia. Eur J Paediatr Neurol [Internet] 2016;20:855–64. Available from: 10.1016/j.ejpn.2016.06.003 [DOI] [PubMed] [Google Scholar]
- 39.Murray DM, O’Connor CM, Anthony Ryan C, Korotchikova I, Boylan GB. Early EEG Grade and Outcome at 5 Years After Mild Neonatal Hypoxic Ischemic Encephalopathy. Pediatrics 2016;138. [DOI] [PubMed] [Google Scholar]
- 40.Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology 2011;76:556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chandrasekaran M, Chaban B, Montaldo P, Thayyil S. Predictive value of amplitude-integrated EEG (aEEG) after rescue hypothermic neuroprotection for hypoxic ischemic encephalopathy: A meta-analysis. J Perinatol [Internet] 2017;37:684–9. Available from: 10.1038/jp.2017.14 [DOI] [PubMed] [Google Scholar]
- 42.Ter Horst HJ, Sommer C, Bergman KA, Fock JM, Van Weerden TW, Bos AF. Prognostic significance of amplitude-integrated EEG during the first 72 hours after birth in severely asphyxiated neonates. Pediatr Res 2004;55:1026–33. [DOI] [PubMed] [Google Scholar]
- 43.Thoresen M, Hellström-Westas L, Liu X, De Vries LS. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics 2010;126:131–41. [DOI] [PubMed] [Google Scholar]
- 44.Shalak LF LA. Amplitude-Integrated Electroencephalography Coupled With an Early Persistent Encephalopathy. Pediatrics 2003;111:351–7. [DOI] [PubMed] [Google Scholar]
- 45.Skranes JH, Løhaugen G, Schumacher EM, et al. Amplitude-Integrated Electroencephalography Improves the Identification of Infants with Encephalopathy for Therapeutic Hypothermia and Predicts Neurodevelopmental Outcomes at 2 Years of Age. J Pediatr [Internet] 2017;187:34–42. Available from: 10.1016/j.jpeds.2017.04.041 [DOI] [PubMed] [Google Scholar]
- 46.Carrasco M, Perin J, Jennings JM, et al. Cerebral Autoregulation and Conventional and Diffusion Tensor Imaging Magnetic Resonance Imaging in Neonatal Hypoxic-Ischemic Encephalopathy. Pediatr Neurol [Internet] 2018;82:36–43. Available from: 10.1016/j.pediatrneurol.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massaro AN, Govindan RB, Vezina G, et al. Impaired cerebral autoregulation and brain injury in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. J Neurophysiol 2015;114:818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chalak LF, Tarumi T, Zhang R. The “neurovascular unit approach” to evaluate mechanisms of dysfunctional autoregulation in asphyxiated newborns in the era of hypothermia therapy. Early Hum Dev [Internet] 2014;90:687–94. Available from: 10.1016/j.earlhumdev.2014.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thewissen L, Caicedo A, Lemmers P, Van Bel FV, Van Huffel SV, Naulaers G. Measuring Near-Infrared Spectroscopy Derived Cerebral Autoregulation in Neonates: From Research Tool Toward Bedside Multimodal Monitoring. Front Pediatr 2018;6:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee JK, Poretti A, Perin J, et al. Optimizing Cerebral Autoregulation May Decrease Neonatal Regional Hypoxic-Ischemic Brain Injury. Dev Neurosci 2017;39:248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian F, Tarumi T, Liu H, Zhang R, Chalak L. Wavelet coherence analysis of dynamic cerebral autoregulation in neonatal hypoxic-ischemic encephalopathy. NeuroImage Clin [Internet] 2016;11:124–32. Available from: 10.1016/j.nicl.2016.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tekes A, Poretti A, Scheurkogel MM, et al. Apparent Diffusion Coefficient Scalars Correlate with Near-Infrared Spectroscopy Markers of Cerebrovascular Autoregulation in Neonates Cooled for Perinatal Hypoxic-Ischemic Injury. Am J Neuroradiol 2015;36:188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Howlett JA, Northington FJ, Gilmore MM, et al. Cerebrovascular autoregulation and neurologic injury in neonatal hypoxic-ischemic encephalopathy. Pediatr Res 2013;74:525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burton VJ, Gerner G, Cristofalo E, et al. A pilot cohort study of cerebral autoregulation and 2-year neurodevelopmental outcomes in neonates with hypoxic-ischemic encephalopathy who received therapeutic hypothermia. BMC Neurol [Internet] 2015;15:1–13. Available from: 10.1186/s12883-015-0464-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chalak LF, Tian F, Adams-Huet B, et al. Novel Wavelet Real Time Analysis of Neurovascular Coupling in Neonatal Encephalopathy. Sci Rep [Internet] 2017. [cited 2019 Jun 28];7 Available from: www.nature.com/scientificreports [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chalak LF, Zhang R. New Wavelet Neurovascular Bundle for Bedside Evaluation of Cerebral Autoregulation and Neurovascular Coupling in Newborns with Hypoxic-Ischemic Encephalopathy. Dev Neurosci [Internet] 2017;39:89–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28355608%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5519424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Groenendaal F, de Vries LS. Fifty years of brain imaging in neonatal encephalopathy following perinatal asphyxia. Pediatr Res [Internet] 2017. [cited 2018 May 25];81:150–5. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27673422 [DOI] [PubMed] [Google Scholar]
- 58.Weeke LC, Groenendaal F, Mudigonda K, et al. A Novel Magnetic Resonance Imaging Score Predicts Neurodevelopmental Outcome After Perinatal Asphyxia and Therapeutic Hypothermia. J Pediatr [Internet] 2018;192:33–40.e2. Available from: 10.1016/j.jpeds.2017.09.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McKinstry R, Miller J, Snyder A, et al. A prospective, longitudinal diffusion tensor imaging study of brain injury in newborns. Neurology 2002;59:824. [DOI] [PubMed] [Google Scholar]
- 60.Barkovich AJ, Miller SP, Bartha A, et al. MR Imaging, MR Spectroscopy, and Diffusion Tensor Imaging of Sequential Studies in Neonates with Encephalopathy. AJNR 2006;27:533–47. [PMC free article] [PubMed] [Google Scholar]
- 61.Ou Y, Zöllei L, Retzepi K, et al. Using clinically acquired MRI to construct age-specific ADC atlases: Quantifying spatiotemporal ADC changes from birth to 6-year old. Hum Brain Mapp 2017;38:3052–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Robertson NJ, Thayyil S, B. Cady E, Raivich G. Magnetic Resonance Spectroscopy Biomarkers in Term Perinatal Asphyxial Encephalopathy: From Neuropathological Correlates to Future Clinical Applications. Curr Pediatr Rev 2014;10:37–47. [DOI] [PubMed] [Google Scholar]
- 63.Thayyil S, Chandrasekaran M, Taylor A, et al. Cerebral magnetic resonance biomarkers in neonatal encephalopathy: A meta-analysis. Pediatrics 2010;125. [DOI] [PubMed] [Google Scholar]
- 64.Thayyil S, Oliveira V, Lally PJ, et al. Hypothermia for encephalopathy in low and middle-income countries (HELIX): Study protocol for a randomised controlled trial. Trials 2017;18:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Azzopardi D, Robertson NJ, Bainbridge A, et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): A proof-of-concept, open-label, randomised controlled trial. Lancet Neurol 2016;15:145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.NICHD Neonatal Research Network [Internet]. [cited 2019 Apr 9];Available from: https://neonatal.rti.org/
- 67.Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ [Internet] 2010. [cited 2019 May 7];340:c332 Available from: http://www.ncbi.nlm.nih.gov/pubmed/20332509 [Google Scholar]
- 68.Lean Six Sigma Institute [Internet]. [cited 2019 Apr 9];Available from: https://www.leansixsigmainstitute.org/
- 69.Lilford RJ, Thornton JG, Braunholtz D. Clinical trials and rare diseases: A way out of a conundrum. BMJ 1995;311:1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quintana M, Viele K, Lewis R. Bayesian Analysis: Using Prior Information to Interpret the Results of Clinical Trials. JAMA 2017;318:1605–6. [DOI] [PubMed] [Google Scholar]
- 71.FDA guidance. Adaptive Designs for Clinical Trials of Drugs and Biologics Guidance for Industry. 2018;Available from: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
- 72.Chow S-C. Adaptive Clinical Trial Design. Annu Rev Med [Internet] 2014. [cited 2019 Feb 21];65:405–15. Available from: www.annualreviews.org [DOI] [PubMed] [Google Scholar]
- 73.Maitre NL. Neurorehabilitation after neonatal intensive care: Evidence and challenges. Arch Dis Child Fetal Neonatal Ed 2015;100:F534–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anderson PJ, Luca CR De, Hutchinson E, Roberts G, Doyle LW. Underestimation of Developmental Delay by the New Bayley-III Scale. Arch Pediatr Adolesc Med [Internet] 2010. [cited 2019 Feb 18];164:352 Available from: http://archpedi.jamanetwork.com/article.aspx?doi=10.1001/archpediatrics.2010.20 [DOI] [PubMed] [Google Scholar]
- 75.Chalak LF, Dupont TL, Sánchez PJ, et al. Neurodevelopmental outcomes after hypothermia therapy in the era of Bayley-III. J Perinatol [Internet] 2014. [cited 2019 Feb 18];34:629–33. Available from: www.nature.com/jp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hack M, Taylor HG, Drotar D, et al. Poor predictive validity of the Bayley Scales of infant development for cognitive function of extremely low birth weight children at school age. Pediatrics [Internet] 2005. [cited 2019 Feb 18];116:333–41. Available from: www.aappublications.org/news [DOI] [PubMed] [Google Scholar]
- 77.Romeo DM, Bompard S, Cocca C, et al. Neonatal neurological examination during the first 6 h after birth. Early Hum Dev [Internet] 2017;108:41–4. Available from: 10.1016/j.earlhumdev.2017.03.013 [DOI] [PubMed] [Google Scholar]
- 78.Dubowitz L, Mercuri E, Dubowitz V. An optimality score for the neurologic examination of the term newborn. J Pediatr 1999;133:406–16. [DOI] [PubMed] [Google Scholar]
- 79.George JM, Fiori S, Fripp J, et al. Relationship between very early brain structure and neuromotor, neurological and neurobehavioral function in infants born <31 weeks gestational age. Early Hum Dev [Internet] 2018;117:74–82. Available from: 10.1016/j.earlhumdev.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 80.Haataja L, Mercuri E, Guzzetta A, et al. Neurologic examination in infants with hypoxicischemic encephalopathy at age 9 to 14 months: Use of optimality scores and correlation with magnetic resonance imaging finding. J Pediatr 2001;138:332–7. [DOI] [PubMed] [Google Scholar]
- 81.Cainelli E, Trevisanuto D, Cavallin F, Manara R, Suppiej A. Evoked potentials predict psychomotor development in neonates with normal MRI after hypothermia for hypoxic-ischemic encephalopathy. Clin Neurophysiol [Internet] 2018;129:1300–6. Available from: 10.1016/j.clinph.2018.03.043 [DOI] [PubMed] [Google Scholar]
- 82.Key APF, Lambert EW, Aschner JL, Maitre NL. Influence of gestational age and postnatal age on speech sound processing in NICU infants. Psychophysiology 2012;49:720–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Papadelis C, Butler EE, Rubenstein M, et al. Reorganization of the somatosensory cortex in hemiplegic cerebral palsy associated with impaired sensory tracts. NeuroImage Clin [Internet] 2018. [cited 2019 Apr 9];17:198–212. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29159037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murray MM, Brunet D, Michel CM. Topographic ERP analyses: A step-by-step tutorial review [Internet]. Brain Topogr. 2008. [cited 2019 Apr 9];20:249–64. Available from: http://link.springer.com/10.1007/s10548-008-0054-5 [DOI] [PubMed] [Google Scholar]
- 85.Morgan C, Honan I, Allsop A, Novak I, Badawi N. Psychometric properties of assessments of cognition in infants with cerebral palsy or motor impairment: A systematic review. J Pediatr Psychol [Internet] 2019. [cited 2019 Feb 20];44:238–52. Available from: https://academic.oup.com/jpepsy/article-abstract/44/2/238/5095906 [DOI] [PubMed] [Google Scholar]
- 86.Griffiths A, Toovey R, Morgan PE, Spittle AJ. Psychometric properties of gross motor assessment tools for children: a systematic review. BMJ Open 2018;8:e021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kjølbye CB, Bo Drivsholm T, Ertmann RK, Lykke K, Køster-Rasmussen R. Motor function tests for 0–2-year-old children – A systematic review. Dan Med J 2018;65:1–8. [PubMed] [Google Scholar]
- 88.Eeles AL, Spittle AJ, Anderson PJ, et al. Assessments of sensory processing in infants: A systematic review. Dev Med Child Neurol 2013;55:314–26. [DOI] [PubMed] [Google Scholar]
- 89.Nelson HD, Nygren P, Walker M, Panoscha R. Screening for speech and language delay in preschool children: systematic evidence review for the US Preventive Services Task Force. Pediatrics [Internet] 2006. [cited 2019 Feb 20];117:e298–319. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2643801 [DOI] [PubMed] [Google Scholar]
- 90.Hanratty J, Livingstone N, Robalino S, et al. Systematic Review of the Measurement Properties of Tools Used to Measure Behaviour Problems in Young Children with Autism. PLoS One [Internet] 2015. [cited 2019 Feb 20];10:1–21. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4689504/pdf/pone.0144649.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Halle TG, Darling-Churchill KE. Review of measures of social and emotional development. J Appl Dev Psychol [Internet] 2016. [cited 2019 Feb 20];45:8–18. Available from: 10.1016/j.appdev.2016.02.003 [DOI] [Google Scholar]
- 92.McCrae JS, Brown SM. Systematic Review of Social–Emotional Screening Instruments for Young Children in Child Welfare. Res Soc Work Pract [Internet] 2018. [cited 2019 Feb 20];28:767–88. Available from: https://journals.sagepub.com/doi/pdf/10.1177/1049731516686691?casa_token=uC4dESwJiuEAAAAA:aG0QPANjrpa68vngPc8ZYuoDT83pCtDeFpw9WArp7Ob_5EbCXO_5hOKPCtJcT4lrs3AECY1lYrde [Google Scholar]
- 93.Brydges CR, Landes JK, Reid CL, Campbell C, French N, Anderson M. Cognitive outcomes in children and adolescents born very preterm: a meta-analysis. Dev Med Child Neurol [Internet] 2018. [cited 2019 Feb 20];60:452–68. Available from: http://doi.wiley.com/10.1111/dmcn.13685 [DOI] [PubMed] [Google Scholar]
- 94.Wong HS, Santhakumaran S, Cowan FM, Modi N. Developmental assessments in preterm children: A meta-analysis. Pediatrics 2016;138. [DOI] [PubMed] [Google Scholar]
- 95.Hack M Poor Predictive Validity of the Bayley Scales of Infant Development for Cognitive Function of Extremely Low Birth Weight Children at School Age. Pediatrics [Internet] 2005;116:333–41. Available from: http://pediatrics.aappublications.org/cgi/doi/10.1542/peds.2005-0173 [DOI] [PubMed] [Google Scholar]
- 96.Maitre NL, Slaughter JC, Aschner JL. Early prediction of cerebral palsy after neonatal intensive care using motor development trajectories in infancy. Early Hum Dev [Internet] 2013;89:781–6. Available from: 10.1016/j.earlhumdev.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Einspieler C, Prechtl HF, Bos A, Ferrari F, Cioni G. Prechtl’s method on the qualitative assessment of general movements in preterm, term and young infants [Internet]. First edition Mac Keith Press; 2008. [cited 2019 Feb 21]. Available from: http://www.mackeith.co.uk/shop/prechtls-method-on-the-qualitative-assessment-of-general-movements-in-preterm-term-and-young-infants/ [Google Scholar]
- 98.Kwong AKL, Fitzgerald TL, Doyle LW, Cheong JLY, Spittle AJ. Predictive validity of spontaneous early infant movement for later cerebral palsy: a systematic review. Dev Med Child Neurol 2018;60:480–9. [DOI] [PubMed] [Google Scholar]
- 99.Romeo DM, Ricci D, Brogna C, Mercuri E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: A critical review of the literature. Dev Med Child Neurol 2016;58:240–5. [DOI] [PubMed] [Google Scholar]
- 100.Palisano RJ, Avery L, Gorter JW, Galuppi B, Mccoy SW. Stability of the Gross Motor Function Classification System, Manual Ability Classification System, and Communication Function Classification System. Dev Med Child Neurol [Internet] 2018. [cited 2019 Feb 21];60:1026–32. Available from: https://journals.ohiolink.edu/pg_99?207338413130677::NO::P99_ENTITY_ID,P99_ENTITY_TYPE:276730985,MAIN_FILE&cs=3d5IpzV28ljr0OlGCW7HBqJMfHhQVtcR2r-xCIP9GVCX9cyTx60WlmA4tAvvLNFo1KE2-MDs0uyzzRiu-OiJ8UA [DOI] [PubMed] [Google Scholar]
- 101.Gorter JW, Ketelaar M, Rosenbaum P, Helders PJM, Palisano R. Use of the GMFCS in infants with CP: The need for reclassification at age 2 years or older. Dev Med Child Neurol 2009;51:46–52. [DOI] [PubMed] [Google Scholar]
- 102.Abbasi H, Unsworth CP. Applications of advanced signal processing and machine learning in the neonatal hypoxic-ischemic electroencephalography. Neural Regen Res 2020;15:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jackson TC, Kotermanski SE, Kochanek PM. Infants uniquely express high levels of RBM3 and other cold-adaptive neuroprotectant proteins in the human brain. Dev Neurosci 2018;40:325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jackson TC, Kochanek PM. A New Vision for Therapeutic Hypothermia in the Era of Targeted Temperature Management: A Speculative Synthesis. Ther Hypothermia Temp Manag 2019;9:13–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jenkins DD, Lee T, Chiuzan C, et al. Altered circulating leukocytes and their chemokines in a clinical trial of therapeutic hypothermia for neonatal hypoxic ischemic encephalopathy. Pediatr Crit Care Med 2013;14:786–95. [DOI] [PubMed] [Google Scholar]
- 106.Vik SD, Torp H, Follestad T, Støen R, Nyrnes SA. NeoDoppler: New ultrasound technology for continous cerebral circulation monitoring in neonates. Pediatr Res 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Smyser CD, Wheelock MD, Limbrick DD, Neil JJ. Neonatal brain injury and aberrant connectivity. Neuroimage. 2019;185:609–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.De Vis JB, Hendrikse J, Petersen ET, et al. Arterial spin-labelling perfusion MRI and outcome in neonates with hypoxic-ischemic encephalopathy. Eur Radiol 2015;25:113–21. [DOI] [PubMed] [Google Scholar]
- 109.Mohammadi-Nejad A-R, Mahmoudzadeh M, Hassanpour MS, et al. Neonatal brain resting-state functional connectivity imaging modalities. Photoacoustics [Internet] 2018. [cited 2019 Apr 9];10:1 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5832677/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li HX, Yu M, Zheng A Bin, et al. Resting-state network complexity and magnitude changes in neonates with severe hypoxic ischemic encephalopathy. Neural Regen Res 2019;14:642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.The Nobel Prize in Economic Sciences Committee, Banerjee A, Duflo E, Kremer M. Understanding Development and Poverty Alleviation. 2019.
- 112.Denihan NM, Boylan GB, Murray DM. Metabolomic Profiling in Perinatal Asphyxia: A Promising New Field. 2015. [cited 2019 Feb 22];Available from: 10.1155/2015/254076 [DOI] [PMC free article] [PubMed]
- 113.AAP and ACOG Task Force on Neonatal Encephalopathy. Neonatal Encephalopathy and Neurologic Outcome, Second Edition Pediatrics [Internet] 2014;133:e1482–8. Available from: http://pediatrics.aappublications.org/cgi/doi/10.1542/peds.2014-0724 [DOI] [PubMed] [Google Scholar]
- 114.U.S. Department of Health and Human Services Food and Drug Administration. Oversight of Clinical Investigations — A Risk-Based Approach to Monitoring [Internet]. 2013. [cited 2019 May 7]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/oversight-clinical-investigations-risk-based-approach-monitoring


