Abstract
A role for the adhesion G-protein coupled receptor ADGRE2 (EMR2) in mechanosensing was revealed by the finding of a missense substitution (p.C492Y) associated with familial vibratory urticaria (VU). In these patients, friction of the skin induces mast cell hyper-degranulation through p.C492Y-ADGRE2, causing localized hives, flushing and hypotension. We have now characterized the responses and intracellular signals elicited by mechanical activation in human mast cells expressing p.C492Y-ADGRE2 and attached to dermatan sulfate, a ligand for ADGRE2. The presence of p.C492Y-ADGRE2 reduced the threshold to activation and increased the extent of degranulation along with the percentage of mast cells responding. Vibration caused PLC activation, transient increases in cytosolic calcium, and downstream activation of PI3K and ERK1/2 by Gβγ, Gαq/11 and Gαi/o-independent mechanisms. Degranulation induced by vibration was dependent on PLC pathways, including calcium, PKC and PI3K but not ERK1/2 pathways, along with pertussis toxin (PTX)-sensitive signals. In addition, mechanoactivation of mast cells stimulated the synthesis and release of PGD2, a previously unreported mediator in VU, and ERK1/2 activation was required for this response together with calcium, PKC and, to some extent, PI3K. Our studies thus identify critical molecular events initiated by mechanical forces and potential therapeutic targets for patients with VU.
Keywords: vibratory urticaria, ADGRE2, mast cells, degranulation, calcium, PKC, PGD2
INTRODUCTION
Adhesion G protein-coupled receptors (aGPCRs) represent the second largest class of receptors within the GPCR superfamily. They are involved in cell-to-cell and cell-to-matrix interactions and are thought to play important roles in immunity, tumorigenesis, reproduction and development (Langenhan et al., 2013). ADGRE2, or EMR2 (epidermal growth factor-like module-containing mucin-like hormone receptor-like 2), is an aGPCR that binds dermatan sulfate (DS) (Stacey et al., 2003) and is predominantly expressed in human myeloid cells.
ADGRE2 regulates neutrophil function and survival (Chen et al., 2011, Yona et al., 2008); and induces macrophage differentiation and expression of pro-inflammatory mediators (I et al., 2017, Kwakkenbos et al., 2002). Recently, we found that ADGRE2 is expressed in human mast cells and that a missense variant (p.C492Y) of ADGRE2 identified in patients with autosomal dominant vibratory urticaria (VU) is associated with disease presentation. These patients exhibit mast cell hyperreactivity upon mechanical stimuli resulting in localized hives, increased histamine levels in serum and increased extracellular tryptase staining in the dermis. Vibratory stimulation in vitro causes degranulation of mast cells derived from patients’ progenitors and enforced expression of the p.C492Y-ADGRE2 variant in mast cells reproduces this phenotype. These results provided a link between p.C492Y-ADGRE2 and vibration-induced mast cell degranulation and supported a function for this receptor in sensing physical forces (Boyden et al., 2016), as has been reported for other aGPCR family members (Purcell and Hall, 2018, Scholz et al., 2016). However, the intracellular signals activated by mechanical stimulation of ADGRE2 and other aGPCRs remain elusive.
In the present study, we investigate the mechanisms of p.C492Y-ADGRE2 activation in mast cells with the dual purpose of identifying the signals causing the pathological presentation of VU and gaining insights into vibration-induced signal transduction that may be common to other mechanosensing aGPCRs. We show that ADGRE2 activation by physical stimulation, particularly the p.C492Y variant, assembles functionally distinct signaling events that enhance sensitivity to vibration. Vibration-induced signals include activation of phospholipase C (PLC) and calcium mobilization, which were essential for downstream activation of phosphoinositide 3-kinase (PI3K) and extracellular signal-regulated kinase (ERK1/2) pathways. While calcium and protein kinase C (PKC) were required for degranulation, ERK1/2 pathways were essential for the release of prostaglandin D2 (PGD2) which increased in serum of patients with VU after vibratory challenge. Our data contribute to the understanding of pathways activated by aGPCR-mediated mechanosensing, and identify potential therapeutic targets for treatment of patients with VU.
RESULTS AND DISCUSSION
Characterization of vibration-induced degranulation
We sought to further characterize the responses to physical stimulation using the human mast cell line LAD2 transfected with normal or mutated-ADGRE2 (Figure 1a), which resulted in similar efficiencies of transfection (>70%; Figure S1a and in (Boyden et al., 2016)). We first examined the effect of vibratory stimuli of various strengths in cells attached to dermatan sulfate (DS) on the release of β-hexosaminidase as a read-out for degranulation. Speeds lower than 1,500 rpm caused minor degranulation in cells expressing nonmutated (NM)-ADGRE2, while cells expressing p.C492Y-ADGRE2 readily degranulated at 750 rpm or higher (Figure 1b and c, and Figure S1b, d and e d). Optimal differences in degranulation between the two variants were observed between 750 to 1,500 rpm, while higher stimulus strengths caused substantial degranulation in all cells, diminishing the differences between variants (Figure 1c), a result that was not due to cellular damage as viability was >90% after vibration at 2,000 rpm (Figure S1c). In agreement with clinical observations in VU, the results demonstrate that the p.C492Y-ADGRE2 variant requires lower threshold for activation of mast cells in response to vibration.
Figure 1. Characterization of vibration-induced degranulation.
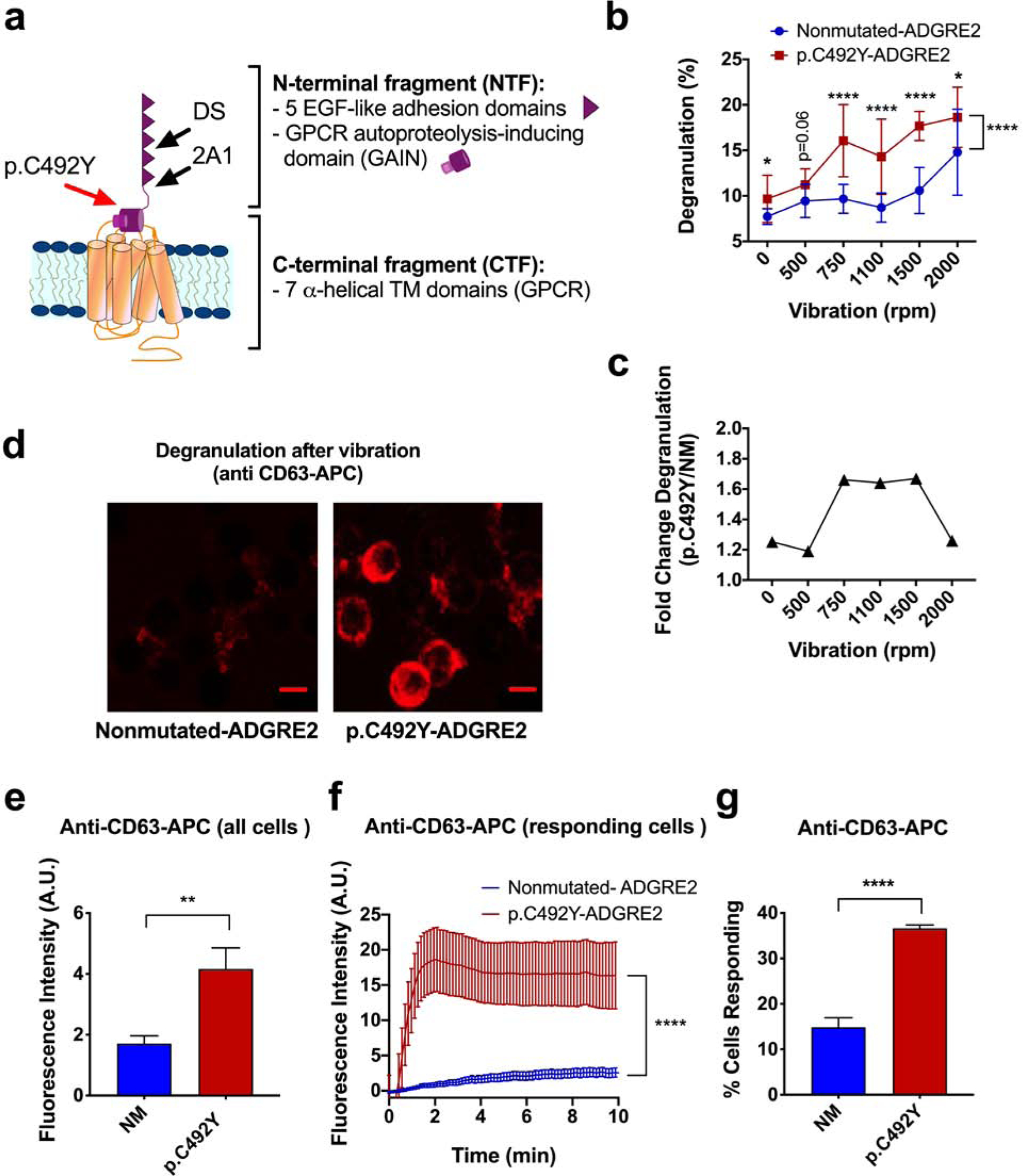
(a) Depiction of ADGRE2. The NTF and CTF, translated as a single polypeptide and self-cleaved in the GAIN domain in the ER, remain non-covalently linked (Kwakkenbos et al., 2002). Arrows indicate the location of p.C492Y and binding sites for DS and the 2A1 antibody. (b) β-hexosaminidase release induced by vibration (20 min) in nonmutated (NM)- or p.C492Y-ADGRE2 cells. Data are mean±SD. Student t-tests were used for the point by point comparisons and a two-way ANOVA for comparison between the curves (side bracket). (c) Fold changes in degranulation (from data in b). (d) Images of CD63 surface expression 5 min after vibration (750 rpm). Scale bar=10 μm. (e) Anti-CD63-APC fluorescent intensity 10 min after vibration; (n≥77). (f) Anti-CD63-APC intensity after vibration in responsive cells; (n=10). (g) Percentage of responsive cells. Data are mean±SEM in e–g. *p<0.05; ** p<0.005; **** p<0.0001.
To gain further insights into the heterogeneity of individual cell degranulation to vibration, we monitored the exposure of the granule marker CD63 onto the cell surface using confocal microscopy. This method efficiently demonstrated increased cell surface expression of CD63 during degranulation in cells stimulated with thapsigargin or with IgE/antigen (Figure S2a and b). Vibration also increased anti-CD63 surface staining, particularly on cells expressing p.C492Y-ADGRE2 (Figure 1d and e), although an increase was also seen in cells with NM-ADGRE2 at higher speeds (Figure S2c). Similar to β-hexosaminidase release (Figure 1b and c), stronger mechanical stimulation reduced the differences in CD63 expression between NM and p.C492Y-ADGRE2 variants (Figure S2c). Analysis of responding cells (i.e. cells showing CD63 surface staining above background), indicated that both the magnitude of degranulation on a per cell basis (Figure 1f) and the percentage of responding cells (Figure 1g) were markedly increased when expressing p.C492Y-ADGRE2.
Degranulation was observed when p.C492Y-ADGRE2 cells were attached to immobilized DS, a reported ligand for ADGRE2 (Stacey et al., 2003) or 2A1, an antibody that recognizes a sequence in the NTF (Figure 1a) and ligates the receptor in macrophages (Huang et al., 2012), but not to chondroitin sulfate A, an analog of DS, polylysine, hyaluronic acid or heparan sulfate (Figure S1d). These and our reported findings (Boyden et al., 2016) suggest that binding of ADGRE2 to immobilized DS or to 2A1 is required, along with mechanical forces, to elicit degranulation in mast cells, as in static conditions no significant degranulation occurred (Figure 1b). Vibration-induced responses in p.C492Y-ADGRE2 cells were similar at concentrations of immobilized DS ranging from 0.1 to 100 μg/mL (Figure S1e and S3c), consistent with the observed maximal adherence of cells to DS within this range (Boyden et al., 2016). In other mechanosensing aGPCRs, activation is also thought to require both ligation and a mechanical load, as ligand binding by itself causes no detectable signaling (Karpus et al., 2013, Langenhan et al., 2013, Scholz et al., 2015) or inhibits signaling (Petersen et al., 2015). A proposed model of activation of aGPCRs suggests that the N-terminal fragment (NTF) normally prevents spontaneous activation and that dissociation of the NTF from the C-terminal fragment (CTF) (Figure 1a) is required for signaling to occur (Purcell and Hall, 2018, Scholz et al., 2016). Consistent with this model, the presence of p.C492Y destabilized the non-covalent interaction between the NTF and CTF in mast cells, rendering the receptor more prone to dissociating the NTF after a vibratory stimulus (Boyden et al., 2016), a hypothesis also consistent with the lower threshold of activation in cells with p.C492Y (Figure 1b and Figure S1b), and recent results showing that proteolytic clipping of the NTF of ADGRE2 by alpha/beta-tryptase heterotetramers makes mast cells susceptible to vibration-triggered degranulation (Le et al., 2019).
Vibration causes calcium mobilization in mast cells
Although other aGPCR members have also been viewed as mechanosensors (Scholz et al., 2016), there is limited information on how such receptors signal. Calcium is viewed as one potential messenger in aGPCR-mediated mechanosensing (Clapham, 2003, Scholz et al., 2015, Scholz et al., 2016) and also a critical signal for mast cell degranulation (Gilfillan and Tkaczyk, 2006). Following this lead, we determined that in mast cells loaded with Fura-2 AM, vibration induced a rise in cytosolic calcium, particularly in p.C492Y-ADGRE2 cells, that was maximal immediately after vibration (~0.85 μM in nonmutated- and ~1.5 μM in p.C492Y-ADGRE2 cells) and declined thereafter (Figure 2a). These transient calcium spikes resembled the typical transient calcium mobilization reported to occur after GPCR activation in mast cells. GPCR-induced calcium responses are associated with a distinct degranulation pattern that causes rapid and brief vascular reactions in vivo (Gaudenzio et al., 2016) which are consistent with the reactions to a vortex challenge in patients with VU (Boyden et al., 2016, Metzger et al., 1976), and contrast with the more sustained calcium responses mediated by IgE receptor stimulation (Figure S2d) that result in compound exocytosis and slower but stronger and more prolonged reactions in mice than those produced by GPCRs (Gaudenzio et al., 2016).
Figure 2. Vibration causes transient calcium mobilization.
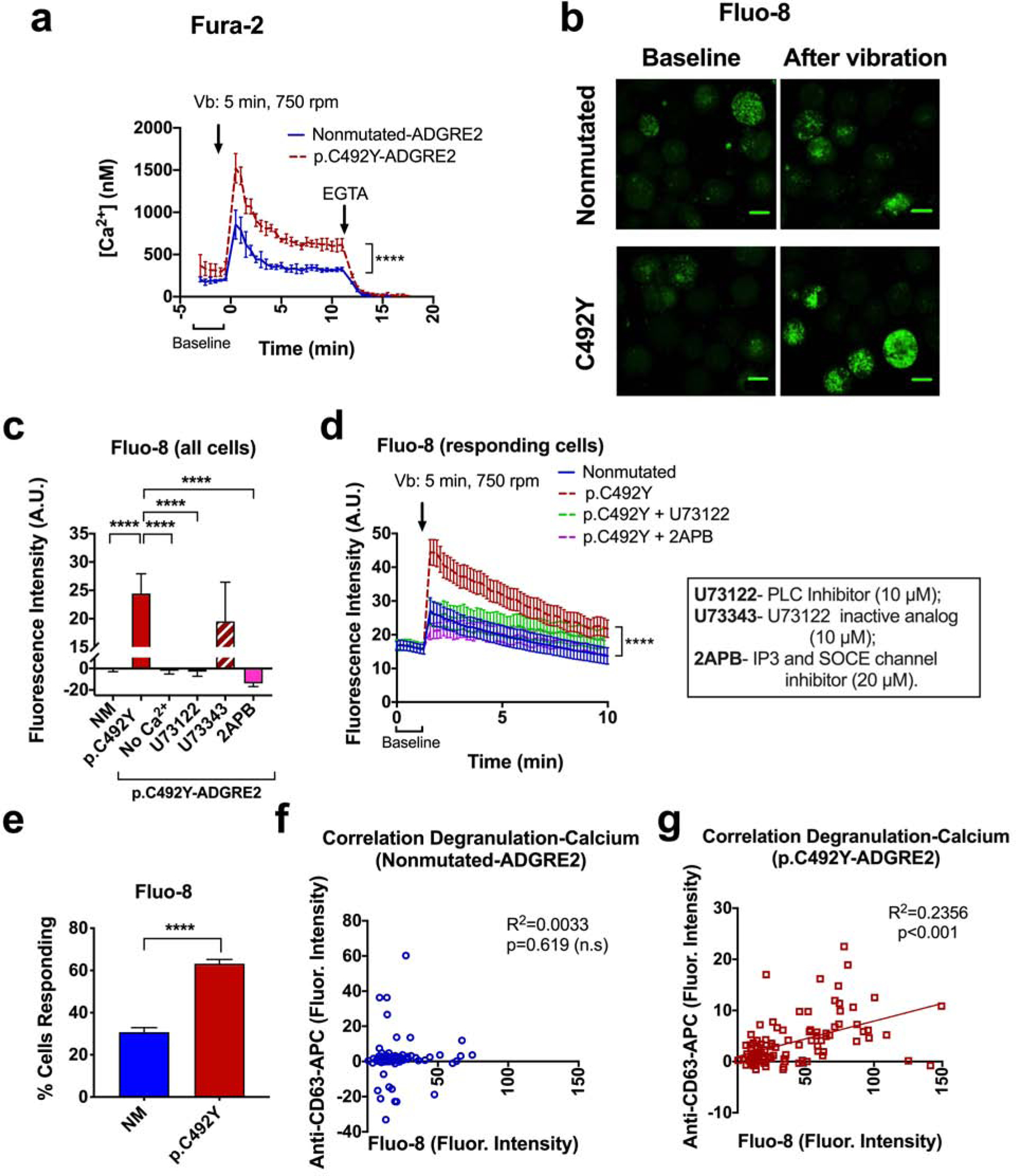
(a) Changes in intracellular calcium measured by Fura-2 in NM- or p.C492Y-ADGRE2 cells. Data are mean±SD of 2 experiments performed in triplicate. (b) Images of changes in intracellular calcium after vibration (Vb) using Fluo-8. Scale bar=10 μm (c, d) Average Fluo-8 fluorescence intensity of all cells 5 min after vibration (c) or in responding cells (with signals above baseline) overtime (d), with or without extracellular calcium, and in cells pretreated with inhibitors, as indicated. Data are mean±SEM (n≥19). (e) Percentage of responsive cells. Data are mean±SEM (n≥77 cells). (f–g) Pearson correlation between calcium changes (Fluo-8 intensity) and degranulation (anti-CD63-APC intensity) from n=4 experiments normalized to average intensities. A.U., arbitrary units. ****p<0.0001. Two-way ANOVA was used in a and d.
Similar to the populational analysis, confocal single-cell measurements using Fluo-8, which readily detected calcium elevations with the expected kinetics after stimulation of the IgE receptor or thapsigargin treatment in LAD2 cells (Figure S2d and e) (Suzuki et al., 2014), showed enhanced calcium responses to vibration in cells with p.C492Y-ADGRE2 (Figure 2b–d), and this increase was abolished in calcium free media (Figure 2c). Since ligation of ADGRE2 by 2A1 activates PLC-β in monocytic cells (I et al., 2017) and PLC activity leads to calcium release via the production of inositol 1,4,5-trisphosphate (IP3), we explored the involvement of this pathway in the calcium responses. Unlike an inactive structural analog (U73343), inhibition of PLC by U73122 or inhibition of IP3 channels by 2-Aminoethoxydiphenyl borate (2APB) blunted the calcium responses in p.C492Y-ADGRE2 cells (Figure 2c and d). Our data thus implicate PLC and mobilization of intracellular calcium in the transients induced by vibration. A contribution of extracellular calcium influx cannot be excluded, since 2APB is also an inhibitor of SOCE and, to a degree, of transient receptor potential (TRP) isoforms (Bootman et al., 2002).
As in degranulation, the expression of p.C492Y-ADGRE2 increased both the magnitude of the calcium responses per cell basis (i.e. in cells with intensities above baseline after vibration) (Figure 2d), and the number of cells with positive responses to vibration (Figure 2e). Of note, only in cells expressing p.C492Y-ADGRE2, did maximal Fluo-8 intensity correlate with the extent of degranulation after vibration at 750 rpm (Figure 2f and g), consistent with the conclusion that vibration normally causes calcium fluxes via ADGRE2 that translate into limited or no degranulation, but both processes are enhanced and linked when the p.C492Y variant is present.
Even though calcium is essential for mast cell degranulation, the strength and/or duration of calcium changes can determine the overall functional consequences. For instance, activation of the IgE receptor by low-affinity antigens induce lower calcium responses and do not render important degranulation as high-affinity antigens do. They, however, transmit different sets of signals with distinct functional outcomes (Suzuki et al., 2014). Overall, the observations support the concept that the structure of ADGRE2 allows mast cells to discriminate the strength of mechanical stimulation and tune the responses accordingly as vibration triggers calcium responses even in cells with the normal variant. Such discrimination of the mechanical strength may be of importance for the immunomodulatory functions of mast cells, much like has been reported for the concentration (Gonzalez-Espinosa et al., 2003) or affinity of antigens (Suzuki et al., 2014) in IgE-receptor mediated activation. However, the molecular and physiological basis for this tuning of the responses, as well as the relevant type of physiological physical stimulus, need further investigation. For instance, polymerization of extracellular matrix components, which occurs during wound healing, may constitute a mechanical stimulus for aGPCRs in vivo, as reported (Petersen et al., 2015); or the crawling of skin parasites that can bind skin glycosaminoglycans (Merida-de-Barros et al., 2018) could mimic a vibration stimulus (stretching or treading). It is tempting to speculate that various physical forces may cause limited but advantageous mast cell responses for the host (i.e. recruitment of immune cells, regulation of wound healing, induction of itch/pain sensing, etc.) without causing a full mast cell response that could be damaging.
Vibration of mast cells with p.C492Y-ADGRE2 induces activation of ERK1/2 and PI3K
We next investigated whether other signaling pathways are activated by vibration. Similar to GPCRs and unlike IgE receptor-mediated activation (Kuehn and Gilfillan, 2007, Mocsai et al., 2003), vibration did not elicit detectable phosphorylation of SRC kinases or spleen tyrosine kinase (SYK) (Figure S3a) in p.C492Y-ADGRE2 mast cells. However, vibration prominently increased pathways commonly associated with agonist-stimulated GPCRs (Gilfillan and Tkaczyk, 2006), such as phosphorylation of protein kinase B (AKT) (Figure 3a and Figure S3b), known to be activated in a PI3K-dependent manner (Manning and Toker, 2017); and phosphorylation of ERK1/2 (Figure 3b and Figure S3c). However, other MAPK pathways, including JNK and p38 (Figure 3b) were not activated. The extent of AKT and ERK1/2 phosphorylation induced by vibration was robust and comparable to that induced by activation of mast cells via the IgE receptor (Figure S3d).
Figure 3. Activation of PI3K and ERK1/2 signaling pathways and their potential crosstalk by mechanoactivation in mast cells with p.C492Y-ADGRE2.
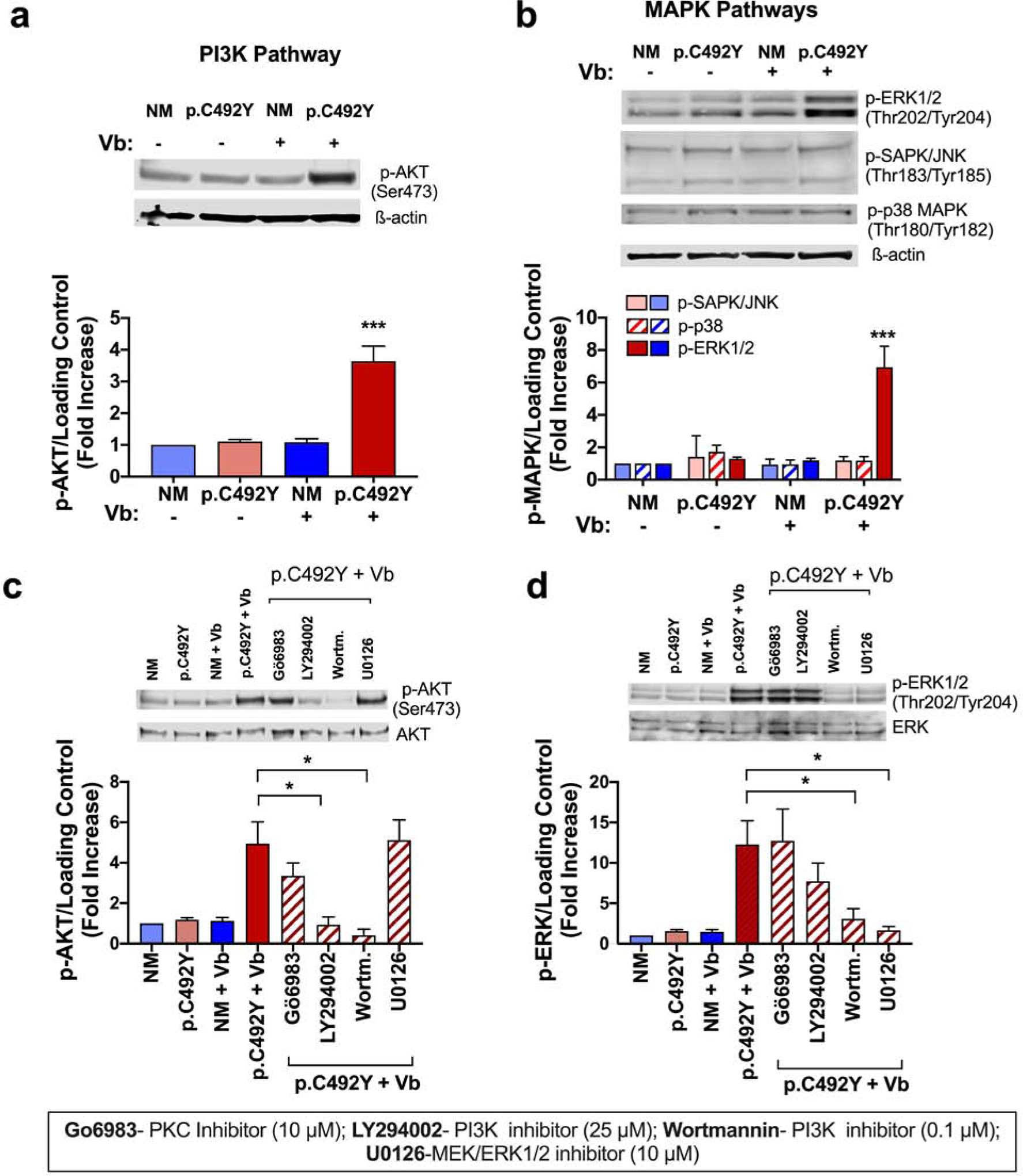
DS-bound cells expressing nonmutated (NM)- or p.C492Y-ADGRE2 were lysed after vibration (+Vb) for 5 min at 750 rpm. (a, b) Phosphorylation of AKT (a), ERK1/2, SAPK/JNK and p38 (b). (c, d) Effects of inhibition of PKC, PI3K, and MEK1/2 on AKT (c) and ERK1/2 (d) phosphorylation. Inhibitors were added 20 min before vibration. The histograms in a–d show the quantification of band intensities (normalized by total AKT/ERK1/2 protein or β-actin) from n≥3 experiments and expressed as fold change compared to non-vibrated, NM-ADGRE2- expressing cells. Data are mean±SEM. * p<0.05; *** p≤0.0001.
PKC stimulation occurs downstream PLC activation. Since PKC can mediate PI3K (Kawakami et al., 2004, Ziemba and Falke, 2018) or MEK/ERK1/2 pathway activation (Mendoza et al., 2011), we examined its involvement in the regulation of these pathways. Pretreatment with a pan-PKC inhibitor, Gö6983, somewhat reduced AKT phosphorylation induced by vibration, albeit the differences did not reach statistical significance (Figure 3c); and did not affect ERK1/2 (Figure 3d) phosphorylation, suggesting that PI3K and EK1/2 pathways are activated by mechanisms largely independent of PKC.
We also examined the potential cross-interactions between PI3K and ERK1/2 pathways. Inhibition of MEK/ERK1/2 did not prevent vibration-induced phosphorylation of AKT (Figure 3c) but blunted ERK1/2 phosphorylation (Figure 3d). In contrast, the pan-inhibitor of PI3K, wortmannin, markedly diminished AKT activation (Figure 3c) and ERK1/2 phosphorylation (Figure 3d). LY294002, a less potent PI3K inhibitor only partially reduced ERK1/2 phosphorylation (Figure 3d). Although we cannot exclude potential off-target effects of wortmannin, the results suggest a directional crosstalk between these pathways where activation of PI3K contributes to ERK1/2 activation, but not vice versa.
PLC activation and calcium mobilization are critical for both PI3K and ERK1/2 activation
We then examined the involvement of PLC stimulation on vibration-induced PI3K and ERK1/2 phosphorylation. An inhibitor of PLC blocked both AKT (Figure 4a) and ERK1/2 (Figure 4b) phosphorylation, while a structural analog of this inhibitor did not. Blockade of calcium fluxes, but not inhibition of PKC, obliterated ERK1/2 and AKT phosphorylation (Figure 4a and b), suggesting that activation of PLC by vibration and consequent calcium mobilization are required for both PI3K and ERK1/2 activation. Given that PI3K may also regulate ERK1/2 phosphorylation (Figure 3d), calcium could activate ERK1/2 partly through PI3K-dependent pathways. Other points of interaction upstream the molecules affected by those inhibitors are possible since MEK/ERK1/2 and PI3K/AKT signaling cascades crosstalk in multiple points (Mendoza et al., 2011) (see summary in Figure 6d).
Figure 4. PLC activation and calcium mobilization are critical for PI3K and ERK1/2 activation independently of βγ, αq/11/14 and αi/o G-protein subunits.
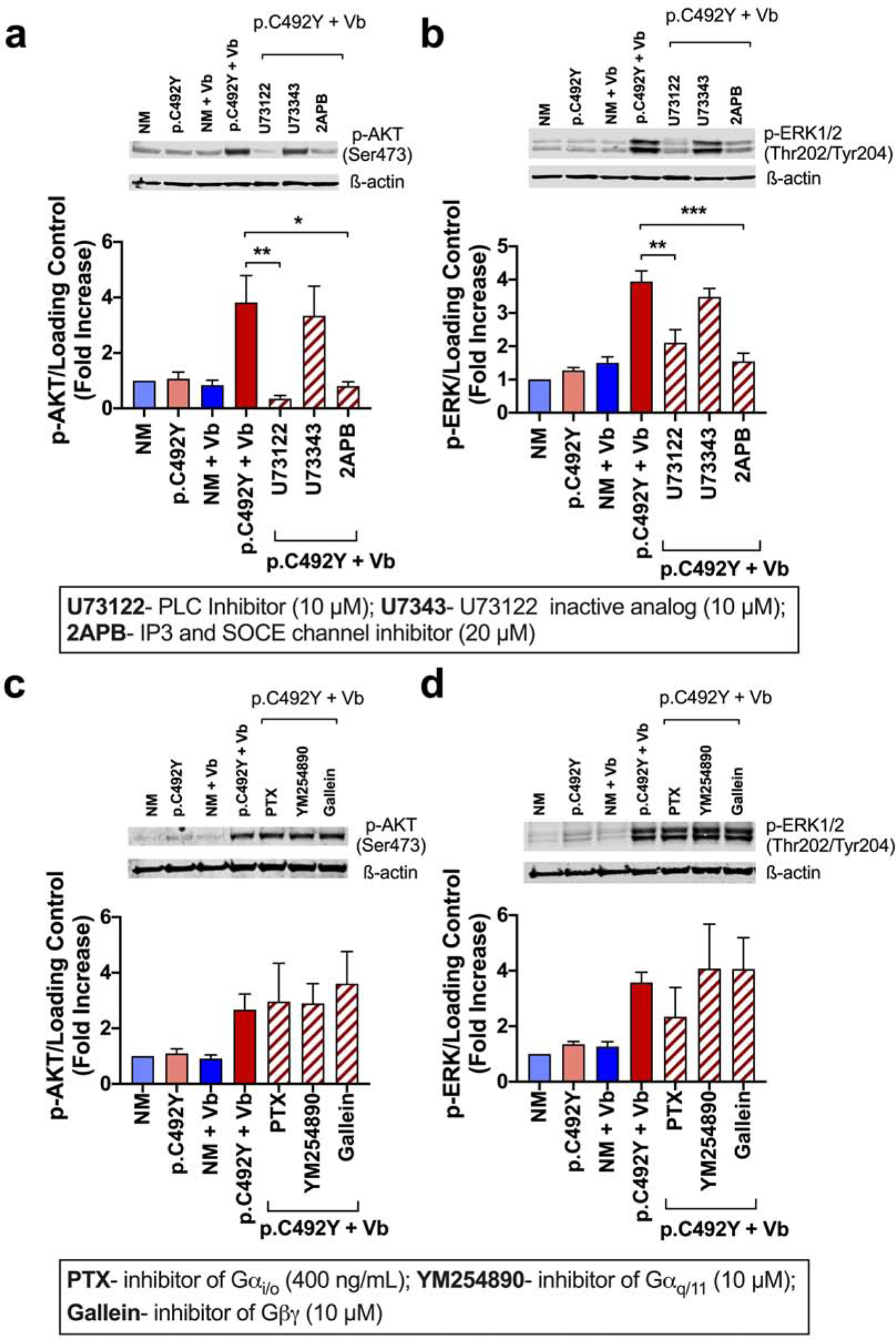
Effects of inhibitors for PLC, IP3 and SOCE channels (a and b), or inhibitors of G-protein subunits βγ, αi/o and αq/11 (c and d), as indicated, on vibration-induced AKT (a, c) and ERK1/2 phosphorylation (b, d). DS-attached LAD2 cells expressing nonmutated (NM)-ADGRE2 or p.C492Y-ADGRE2 were treated with inhibitors for 20 min and vibrated 5 min at 750 rpm. Histograms represent band intensities (normalized by total AKT/ERK1/2 protein or β-actin) from n≥3 experiments and calculated as fold change compared to non-vibrated, NM-ADGRE2 expressing cells. Data are mean±SEM. * p<0.05; ** p<0.01; *** p<0.001.
A similar chain of events for ADGRE2 activation was reported in human monocytic cell lines by crosslinking ADGRE2 with immobilized 2A1 antibody (I et al., 2017) in the absence of mechanical forces. Although it is unclear why crosslinking of ADGRE2 would activate monocytic cells in static conditions while in mast cells vibration is required, both studies reinforce the notion of a consensus chain of signaling events elicited by ADGRE2 activation that are initiated by PLC activation and calcium fluxes, followed by PI3K and MAPK cascades, which in the case of mechanically stimulated mast cells, were restricted to ERK1/2.
Initiation of GPCR signaling involves heterotrimeric G-proteins, although in aGPCRs the specific subtypes have not been well characterized (Hamann et al., 2015). Activation of PLC usually depends on βγ, αq or α11 G-subunits (Boyer et al., 1994). Inhibition of βγ by gallein, αq and α11 subunits by YM254890, or αi/o subunits by pertussis toxin (PTX) did not significantly prevent the activation of AKT or ERK1/2 (Figure 4c and d). The same concentrations of PTX and YM254890 were however effective in reducing Mas-Related G-protein receptor member X2 (MRGPRX2)-mediated degranulation (Figure S4) (Subramanian et al., 2013). Thus, PLC-dependent activation of PI3K and ERK pathways induced by vibration is independent of these subunits, although involvement of other α subtypes such as α16 is possible, as reported (I et al., 2017).
Signaling requirements for vibration-induced mast cell responses
Because of the potential implications for patients with VU, we next investigated which signaling pathways are required for vibration-induced degranulation. Using confocal imaging of CD63 externalization (Figure 5a and b) and/or β-hexosaminidase release (Figure 5c) in the presence of various inhibitors, we determined that PLC activation and consequent calcium mobilization and PKC activation are all necessary for degranulation. Furthermore, downstream of PLC and calcium, PI3K pathways only partly contributed to degranulation (Figure 5b and c) while ERK1/2 was dispensable (Figure 5a–c). Degranulation was also blocked by PTX (Figure 5c), although AKT and ERK1/2 signaling was PTX-independent (Figure 4c and d), suggesting that degranulation depends on both αi/o-sensitive and insensitive pathways (Figure 6d). The specific nature of the αi/o(PTX)-mediated events, as for other GPCRs in mast cells (Subramanian et al., 2013), remains to be elucidated.
Figure 5. PLC/calcium and PKC axis and PTX-sensitive pathways are critical for vibration-induced degranulation, while MEK/ERK1/2 is dispensable.
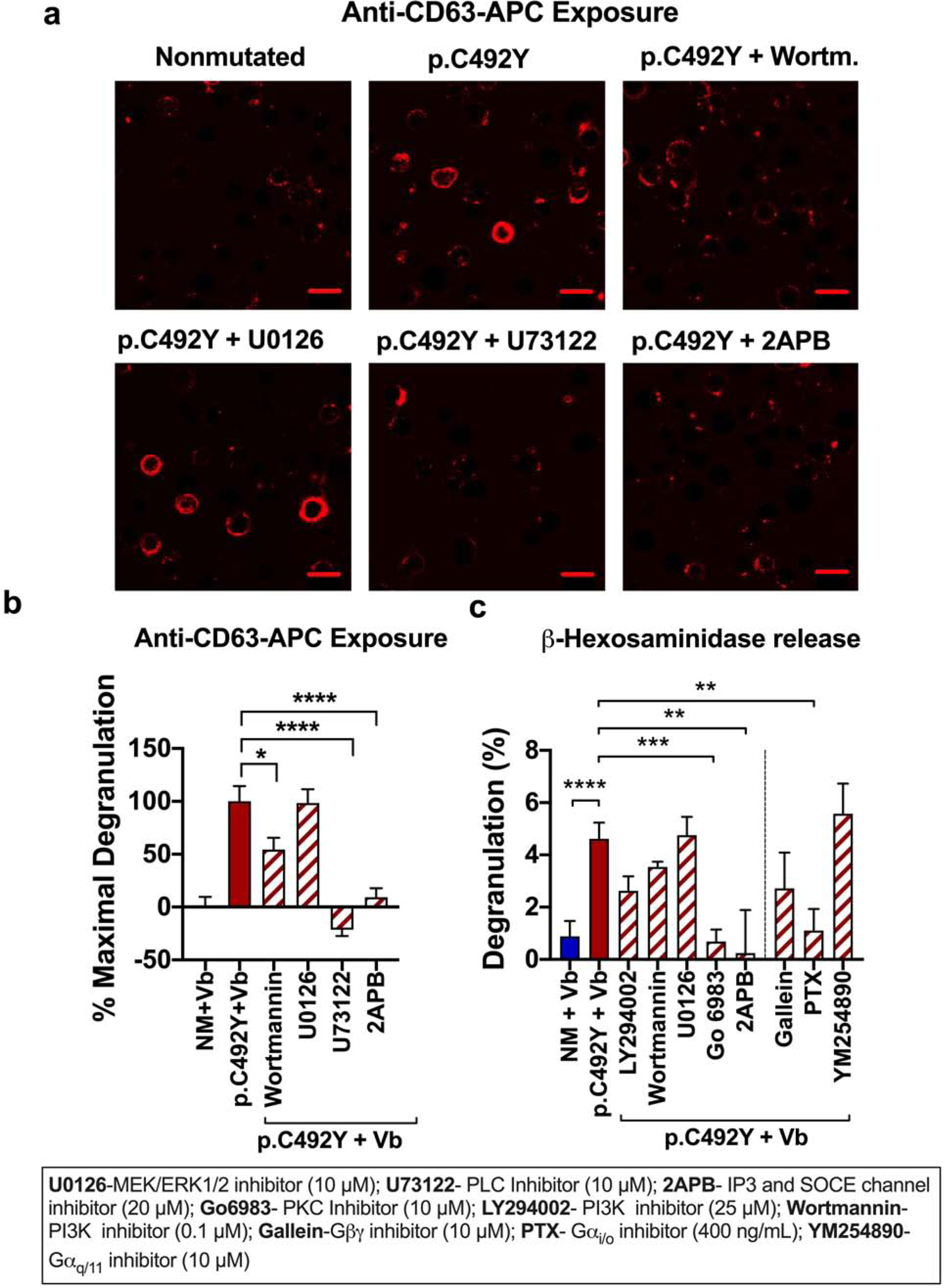
LAD2 cells expressing NM-ADGRE2 or p.C492Y-ADGRE2 plated on DS-coated dishes were treated with the indicated inhibitors for 20 min before vibration. (a) Representative confocal images of anti-CD63-APC on the membrane after vibration for 5 min. Scale bar=20 μm. (b) Quantification of degranulation from data shown in a. Fluorescent intensity of anti-CD63-APC in individual cells (n> 264 cells) was determined, and expressed as % decrement (Δ) of the maximal response (i.e. fluorescent intensity of cells expressing p.C492Y-ADGRE2), being the minimal response fluorescent intensity of anti-CD63-APC in cells with NM-ADGRE2. (c) β-hexosaminidase release after 20 min vibration. Data are mean±SEM, n≥12 *p<0.05; **p<0.005; ***p<0.001; ****p<0.0001.
Figure 6. Vibration-induced PGD2 release is dependent on PLC/calcium, PKC and MEK/ERK1/2; and vortex challenge in patients with VU increases serum PGD2.
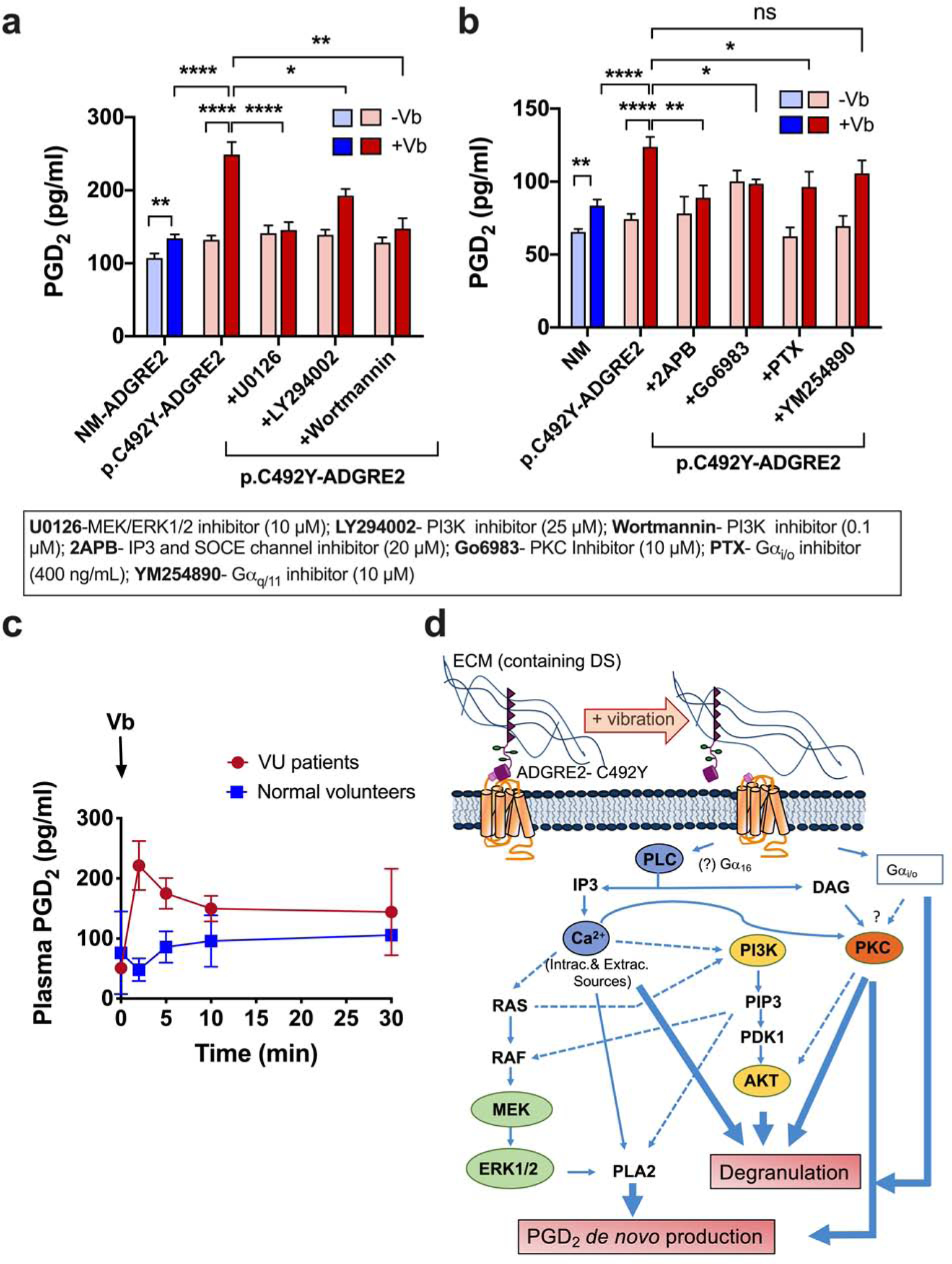
(a, b) PGD2 release induced by vibration in mast cells expressing NM or p.C492Y-ADGRE2 in the presence or absence of the indicated inhibitors. Supernatants of vibrated (750 rpm) and non-vibrated samples after 10 min. Data are mean±SEM; n≥9. *p<0.05; **p<0.005;****p<0.0001. (c) PGD2 in the serum of 2 patients with VU and 2 control subjects after a vibratory challenge. (d) Representation of the proposed signaling pathways involved in mechanical activation of mast cells. Doted lines are potential ways of crosstalk based on our data or the literature (Cullen and Lockyer, 2002, Danciu et al., 2003, Mendoza et al., 2011, Yano et al., 1998).
Since vibration prominently activated ERK1/2 but had no role in degranulation, we interrogated its involvement in other mast cell responses, particularly PGD2 release. PGD2 is one of the most abundant eicosanoid products formed by mast cells as an early de novo mediator (Gilfillan and Tkaczyk, 2006) and ERK1/2 is required for its production via activation of phospholipase A2 (Kuehn et al., 2008). Indeed, vibration induced PGD2 release, particularly in cells with p.C492Y-ADGRE2, was blunted by MEK/ERK1/2 inhibition (Figure 6a). In addition, calcium mobilization and PKC were key to this response, with a partial contribution of PI3K (Figure 6a and b). Blockade of αi/o, but not αq, slightly reduced PGD2 release, suggesting a contributory role for αi/o-dependent signals. However, PGD2 release induced by vibration was especially dependent on PLC-dependent pathways, highlighting the key role for PLC and downstream signals in mechanoactivation of mast cells.
PGD2, an unrecognized mediator in VU
Mirroring the increase in PGD2 production by mast cells after vibration in vitro, we found that in patients with VU, unlike normal subjects, PGD2 was elevated in the venous return of the arm early after vibration. PGD2 remained elevated over baseline 30 min after the vortex challenge (Figure 6c). Although confirmation in larger cohorts is needed, the findings suggest a potential role for PGD2 in the pathology of VU. PGD2 can mediate immediate hypersensitivity processes causing hypotension and flushing but it can also play anti-inflammatory roles (Boyce, 2007, Kulinski et al., 2016), and reduce vascular permeability by tightening the endothelial barrier (Nakamura et al., 2017). Since the increase in PGD2 levels appeared to be sustained longer (Figure 6c) than those of histamine (Boyden et al., 2016, Epstein and Kidd, 1981, Ting et al., 1983), one hypothesis would be that PGD2 downregulates the responses to vibration, contributing to the transient nature of VU pathology. It would be of interest to determine whether PGD2 is not generated in patients with vibratory angioedema (Keahey et al., 1987), who unlike those with in VU, have long-lasting responses.
Concluding Remarks
Herein, we have identified the signaling pathways activated by physical forces in mast cells expressing p.C492Y-ADGRE2, a variant associated with familial VU that renders skin mast cells more susceptible to friction (Boyden et al., 2016). Mechanical signaling encompasses PLC activation as a key early step for mast cell degranulation and PGD2 release; and a parallel PTX-sensitive pathway, also essential for degranulation. The requirement for PLC resonates with the reported gain of PLCγ2 function in a form of inherited cold urticaria where mast cells are also key (Ombrello et al., 2012). Thus, exploration of the role of distinct PLC isoforms and PLC-derived signals in other physical urticarias may be of interest. Downstream of PLC, PKC and calcium were critical for vibration-induced degranulation and eicosanoid production, whereas ERK1/2 was only required for PGD2 release. The distinct requirement for these signals on degranulation and PGD2 release are generally consistent with their roles in IgE receptor- or GPCR-activation (Gilfillan and Tkaczyk, 2006, Kimata et al., 2000, Xing et al., 1997), although their crosstalk appears to be distinct for ADGRE2 (Figure 6d). Our findings not only add to the knowledge of the enigmatic signaling events initiated by mechanical stimulation, their hierarchy and cross-interaction, but also may help design novel therapeutic approaches for VU and give clues into mechanisms for other physical urticarias.
MATERIALS AND METHODS
Further details on the experimental procedures can be found in Supplementary Methods.
Patients
Patients with VU were enrolled and evaluated at the NIH Clinical Center under a protocol approved by the Institutional Review Board of the NIAID (09-I-0126). All subjects provided written informed consent. VU symptoms in a clinical setting were elicited as described (Boyden et al., 2016).
Cell activation
LAD2 cells (Kirshenbaum et al., 2003) transfected with the ADGRE2 constructs by nucleofection (Cruse et al., 2013) were plated overnight on wells coated with 100 μg/mL DS (chondroitin sulfate B, Sigma-Aldrich). After gentle washing, cells were resuspended in 100 μL of pre-warmed HEPES buffer and vibrated at 750 rpm (or as indicated) for 5–20 min on an orbital shaker (Eppendorf ThermoMixer C) at 37 °C. In some experiments, cells were treated with the indicated concentrations of inhibitors or vehicle (0.1% DMSO) 20 min before and during vibration.
Mast cell mediators and signaling
Degranulation was determined as the percentage of β-hexosaminidase released into the media (Kuehn et al., 2010) 20 min after vibration. Alternatively, cells plated in DS-coated imaging chambers (Ibidi #80826), were vibrated for 5 min and anti-CD63-APC was added (1:20). Confocal images were acquired over time using a Leica TCS SP8 microscope and processed using ImageJ, selecting Region of Interest (ROI) Manager to determine anti-CD63-APC mean fluorescent intensity per cell.
PGD2 in cell-free supernatants or serum samples was determined by competitive ELISA (Cayman Chemicals). Phosphorylation of signaling proteins were determined by Western blotting after vibration for 5 min as described (Boyden et al., 2016, Tkaczyk et al., 2002).
Calcium flux measurements
Cells attached to DS-coated chambers were loaded or not with 5 μM Fura-2 AM (Life Technologies) or Fluo-8 AM (Abcam), washed and vibrated at 750 rpm for 5 min. Measurements of Fura-2 fluorescence were done using a Perkin Elmer Wallac 1420 Victor2 microplate reader as described (Cruse et al., 2013, Grynkiewicz et al., 1985) and changes in fluorescence of calcium-bound Fluo-8 by confocal microscopy. Prior to imaging, anti-CD63-APC at 1:20 dilution was added to simultaneously track mast cell degranulation. Mean fluorescent intensity per cell was measured using ROI Manager, and baseline fluorescence was subtracted from each individual cell.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism (version 7.01). Unpaired Student’s t-test was used for most comparisons unless otherwise indicated. A p value <0.05 was considered significant. All experiments were done ≥ 2 independent times and performed at least in triplicate.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGEMENTS
The authors thank the valuable experimental and intellectual contributions of Dr. Michael. A. Beaven, who unfortunately passed away before the completion of this work. They also thank Avanti Desai for valuable technical assistance.
FUNDING: This work was supported by the Division of Intramural Research within the National Institute of Allergy and Infectious Diseases (NIAID) and the National Human Genome Research Institute (NHGRI), at the National Institutes of Health.
ABBREVIATIONS
- 2APB
2-Aminoethoxydiphenyl borate
- ADGRE2
Adhesion G protein-coupled receptor E2
- aGPCR
Adhesion G protein-coupled receptors
- AKT
protein kinase B
- A.U.
arbitrary units
- CTF
C-terminal fragment
- ECM
extracellular matrix
- EMR2
epidermal growth factor (EGF)-like module-containing mucin-like hormone receptor-like 2
- DAG
diacylglycerol
- DS
dermatan sulfate
- ERK1/2
extracellular signal-regulated kinase
- GAIN
GPCR autoproteolysis-inducing domain
- IP3
inositol triphosphate
- NM
nonmutated
- NTF
N-terminal fragment
- PGD2
prostaglandin D2
- PKC
Protein Kinase C
- PLC
phospholipase C
- SA
streptavidin
- SOCE
store operating calcium channels
- SYK
spleen tyrosine kinase
- ROI
region of interest
- Vb
vibration
- VU
vibratory urticaria
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY: The authors declare that all data supporting the findings of this study are available within the manuscript or are available from the corresponding authors upon request.
CONFLICT OF INTEREST: The authors declare that they have no conflicts of interest with the contents of this article.
REFERENCES
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 2002;16(10):1145–50. [DOI] [PubMed] [Google Scholar]
- Boyce JA. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol Rev 2007;217:168–85. [DOI] [PubMed] [Google Scholar]
- Boyden SE, Desai A, Cruse G, Young ML, Bolan HC, Scott LM, et al. Vibratory Urticaria Associated with a Missense Variant in ADGRE2. New England Journal of Medicine 2016;374(7):656–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JL, Graber SG, Waldo GL, Harden TK, Garrison JC. Selective activation of phospholipase C by recombinant G-protein alpha- and beta gamma-subunits. The Journal of biological chemistry 1994;269(4):2814–9. [PubMed] [Google Scholar]
- Chen TY, Hwang TL, Lin CY, Lin TN, Lai HY, Tsai WP, et al. EMR2 receptor ligation modulates cytokine secretion profiles and cell survival of lipopolysaccharide-treated neutrophils. Chang Gung medical journal 2011;34(5):468–77. [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature 2003;426(6966):517–24. [DOI] [PubMed] [Google Scholar]
- Cruse G, Beaven MA, Ashmole I, Bradding P, Gilfillan AM, Metcalfe DD. A truncated splice-variant of the FcepsilonRIbeta receptor subunit is critical for microtubule formation and degranulation in mast cells. Immunity 2013;38(5):906–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ, Lockyer PJ. Integration of calcium and Ras signalling. Nat Rev Mol Cell Biol 2002;3(5):339–48. [DOI] [PubMed] [Google Scholar]
- Danciu TE, Adam RM, Naruse K, Freeman MR, Hauschka PV. Calcium regulates the PI3K-Akt pathway in stretched osteoblasts. FEBS Lett 2003;536(1–3):193–7. [DOI] [PubMed] [Google Scholar]
- Epstein PA, Kidd KK. Dermo-distortive urticaria: an autosomal dominant dermatologic disorder. Am J Med Genet 1981;9(4):307–15. [DOI] [PubMed] [Google Scholar]
- Gaudenzio N, Sibilano R, Marichal T, Starkl P, Reber LL, Cenac N, et al. Different activation signals induce distinct mast cell degranulation strategies. The Journal of Clinical Investigation 2016;126(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol 2006;6(3):218–30. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Espinosa C, Odom S, Olivera A, Hobson JP, Martinez ME, Oliveira-Dos-Santos A, et al. Preferential signaling and induction of allergy-promoting lymphokines upon weak stimulation of the high affinity IgE receptor on mast cells. J Exp Med 2003;197(11):1453–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. The Journal of biological chemistry 1985;260(6):3440–50. [PubMed] [Google Scholar]
- Hamann J, Aust G, Arac D, Engel FB, Formstone C, Fredriksson R, et al. International Union of Basic and Clinical Pharmacology. XCIV. Adhesion G protein-coupled receptors. Pharmacol Rev 2015;67(2):338–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YS, Chiang NY, Hu CH, Hsiao CC, Cheng KF, Tsai WP, et al. Activation of myeloid cell-specific adhesion class G protein-coupled receptor EMR2 via ligation-induced translocation and interaction of receptor subunits in lipid raft microdomains. Mol Cell Biol 2012;32(8):1408–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- I K-Y, Huang Y-S, Hu C-H, Tseng W-Y, Cheng C-H, Stacey M, et al. Activation of Adhesion GPCR EMR2/ADGRE2 Induces Macrophage Differentiation and Inflammatory Responses via Gα16/Akt/MAPK/NF-κB Signaling Pathways. Frontiers in Immunology 2017;8(373). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpus ON, Veninga H, Hoek RM, Flierman D, van Buul JD, Vandenakker CC, et al. Shear stress-dependent downregulation of the adhesion-G protein-coupled receptor CD97 on circulating leukocytes upon contact with its ligand CD55. J Immunol 2013;190(7):3740–8. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Nishimoto H, Kitaura J, Maeda-Yamamoto M, Kato RM, Littman DR, et al. Protein kinase C betaII regulates Akt phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. The Journal of biological chemistry 2004;279(46):47720–5. [DOI] [PubMed] [Google Scholar]
- Keahey TM, Indrisano J, Lavker RM, Kaliner MA. Delayed vibratory angioedema: insights into pathophysiologic mechanisms. J Allergy Clin Immunol 1987;80(6):831–8. [DOI] [PubMed] [Google Scholar]
- Kimata M, Inagaki N, Kato T, Miura T, Serizawa I, Nagai H. Roles of mitogen-activated protein kinase pathways for mediator release from human cultured mast cells. Biochem Pharmacol 2000;60(4):589–94. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AS, Akin C, Wu YL, Rottem M, Goff JP, Beaven MA, et al. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of Fc epsilon RI or Fc gamma RI. Leukemia Research 2003;27(8):677–82. [DOI] [PubMed] [Google Scholar]
- Kuehn HS, Gilfillan AA. G protein-coupled receptors and the modification of Fc epsilon RI-mediated mast cell activation. Immunol Lett 2007;113(2):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn HS, Radinger M, Gilfillan AM. Measuring mast cell mediator release. Current protocols in Immunology 2010; 91(1):7.38.1–7.38.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn HS, Swindle EJ, Kim MS, Beaven MA, Metcalfe DD, Gilfillan AM. The phosphoinositide 3-kinase-dependent activation of Btk is required for optimal eicosanoid production and generation of reactive oxygen species in antigen-stimulated mast cells. J Immunol 2008;181(11):7706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulinski JM, Munoz-Cano R, Olivera A. Sphingosine-1-phosphate and other lipid mediators generated by mast cells as critical players in allergy and mast cell function. Eur J Pharmacol 2016;778:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwakkenbos MJ, Chang GW, Lin HH, Pouwels W, de Jong EC, van Lier RAW, et al. The human EGF-TM7 family member EMR2 is a heterodimeric receptor expressed on myeloid cells. Journal of Leukocyte Biology 2002;71(5):854–62. [PubMed] [Google Scholar]
- Langenhan T, Aust G, Hamann J. Sticky Signaling-Adhesion Class G Protein-Coupled Receptors Take the Stage. Science Signaling 2013;6(276). [DOI] [PubMed] [Google Scholar]
- Le QT, Lyons JJ, Naranjo AN, Olivera A, Lazarus RA, Metcalfe DD, et al. Impact of naturally forming human alpha/beta-tryptase heterotetramers in the pathogenesis of hereditary alpha-tryptasemia. J Exp Med 2019; 216(10):2348–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Toker A. AKT/PKB Signaling: Navigating the Network. Cell 2017;169(3):381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci 2011;36(6):320–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merida-de-Barros DA, Chaves SP, Belmiro CLR, Wanderley JLM. Leishmaniasis and glycosaminoglycans: a future therapeutic strategy? Parasit Vectors 2018;11(1):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger WJ, Kaplan AP, Beaven MA, Irons JS, Patterson R. Hereditary vibratory angioedema: confirmation of histamine release in a type of physical hypersensitivity. J Allergy Clin Immunol 1976;57(6):605–8. [DOI] [PubMed] [Google Scholar]
- Mocsai A, Zhang H, Jakus Z, Kitaura J, Kawakami T, Lowell CA. G-protein-coupled receptor signaling in Syk-deficient neutrophils and mast cells. Blood 2003;101(10):4155–63. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Fukaya T, Uto T, Takagi H, Arimura K, Tono T, et al. Selective depletion of basophils ameliorates immunoglobulin E-mediated anaphylaxis. Biochem Biophys Rep 2017;9:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ombrello MJ, Remmers EF, Sun G, Freeman AF, Datta S, Torabi-Parizi P, et al. Cold urticaria, immunodeficiency, and autoimmunity related to PLCG2 deletions. N Engl J Med 2012;366(4):330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen SC, Luo R, Liebscher I, Giera S, Jeong SJ, Mogha A, et al. The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with laminin-211. Neuron 2015;85(4):755–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell RH, Hall RA. Adhesion G Protein-Coupled Receptors as Drug Targets. Annu Rev Pharmacol Toxicol 2018;58:429–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz N, Gehring J, Guan C, Ljaschenko D, Fischer R, Lakshmanan V, et al. The adhesion GPCR latrophilin/CIRL shapes mechanosensation. Cell Rep 2015;11(6):866–74. [DOI] [PubMed] [Google Scholar]
- Scholz N, Monk KR, Kittel RJ, Langenhan T. Adhesion GPCRs as a Putative Class of Metabotropic Mechanosensors. Handbook of experimental pharmacology 2016;234:221–47. [DOI] [PubMed] [Google Scholar]
- Stacey M, Chang GW, Davies JQ, Kwakkenbos MJ, Sanderson RD, Hamann J, et al. The epidermal growth factor-like domains of the human EMR2 receptor mediate cell attachment through chondroitin sulfate glycosaminoglycans. Blood 2003;102(8):2916–24. [DOI] [PubMed] [Google Scholar]
- Subramanian H, Gupta K, Lee D, Bayir AK, Ahn H, Ali H. beta-Defensins activate human mast cells via Mas-related gene X2. J Immunol 2013;191(1):345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Leach S, Liu W, Ralston E, Scheffel J, Zhang W, et al. Molecular editing of cellular responses by the high-affinity receptor for IgE. Science 2014;343(6174):1021–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting S, Reimann BE, Rauls DO, Mansfield LE. Nonfamilial, vibration-induced angioedema. J Allergy Clin Immunol 1983;71(6):546–51. [DOI] [PubMed] [Google Scholar]
- Tkaczyk C, Metcalfe DD, Gilfillan AM. Determination of protein phosphorylation in Fc epsilon RI-activated human mast cells by immunoblot analysis requires protein extraction under denaturing conditions. Journal of Immunological Methods 2002;268(2):239–43. [DOI] [PubMed] [Google Scholar]
- Xing M, Firestein BL, Shen GH, Insel PA. Dual role of protein kinase C in the regulation of cPLA2-mediated arachidonic acid release by P2U receptors in MDCK-D1 cells: involvement of MAP kinase-dependent and -independent pathways. J Clin Invest 1997;99(4):805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano S, Tokumitsu H, Soderling TR. Calcium promotes cell survival through CaM-K kinase activation of the protein-kinase-B pathway. Nature 1998;396(6711):584–7. [DOI] [PubMed] [Google Scholar]
- Yona S, Lin HH, Dri P, Davies JQ, Hayhoe RPG, Lewis SM, et al. Ligation of the adhesion-GPCR EMR2 regulates human neutrophil function. Faseb Journal 2008;22(3):741–51. [DOI] [PubMed] [Google Scholar]
- Ziemba BP, Falke JJ. A PKC-MARCKS-PI3K regulatory module links Ca2+ and PIP3 signals at the leading edge of polarized macrophages. PLoS One 2018;13(5):e0196678. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


