Abstract
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the leading cause of liver disease worldwide. Nonalcoholic steatohepatitis (NASH), is a more severe type of NAFLD. Exercise improves NASH, by reversing steatosis, and may arrest fibrosis. However, the mechanisms underlying these interactions are unknown. AMP-activated protein kinase (AMPK) is a fuel-sensing enzyme that is activated by energy stress. Mammalian target of rapamycin in complex 1 (mTORC1) is a nutrient sensor that regulates protein synthesis. In NASH, AMPK activity is low and mTORC1 is high. In healthy persons, exercise activates AMPK and suppresses mTORC1. We examined the effects of exercise on hepatic ribosomal protein S6 phosphorylation, a downstream target of AMPK and mTORC1 in patients with NASH.
Methods
Three subjects with biopsy-proven NASH underwent a structured, 20-week aerobic exercise intervention, five-days a week for 30-min at a moderate intensity (40–55% of VO2max). Immunofluorescence staining for rpS6 phosphorylation in hepatic tissue was quantified by ImageJ software.
Results
Following 20-weeks of aerobic exercise, rpS6 levels were significantly attenuated (3.9 ± 1.9 pre-exercise vs. 1.4 +/0.4 post-exercise, p = 0.04).
Conclusions
These findings suggest exercise modulates the AMPK/mTORC1 pathway in patients with NASH and may guide the design of future studies into the mechanism of how exercise improves NASH and possibly reverses fibrosis.
Keywords: Nonalcoholic fatty liver disease, Fatty liver, Physical activity, Fitness, Mechanism
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the leading cause of liver disease in the United States affecting 40% of adults (100 million persons) and costing $32 billion annually [1]. NAFLD is an umbrella term that comprises a spectrum of liver conditions varying in severity and injury. Nonalcoholic steatohepatitis (NASH), a more severe type of NAFLD, is characterized by inflammation. This activates hepatic stellate cells and, in most cases, leads to hepatic fibrosis. If uncorrected, NASH will progress to cirrhosis, requiring lifesaving liver transplantation. NASH affects 25 million American adults; five million have NASH cirrhosis [2]. NASH is the second leading etiology for liver transplantation in the United States [3]. NASH cirrhosis is expected to become the most common reason for liver transplantation by 2025; it is already the leading indication for liver transplantation in women and simultaneous liver kidney transplantation registration on the transplant waiting list [4, 5].
The development of NAFLD and progression to NASH is in part related to physical inactivity [6]. To date, there are no approved pharmacologic treatments that are effective in either arresting or reversing fibrosis in patients with NASH. In the absence of an effective pharmacologic treatment, lifestyle changes are first-line treatment. As obesity plays a crucial role in the pathophysiology of NASH, lifestyle changes with dietary intervention and increased exercise leading to a goal of 7–10% weight loss are recommended for all patients with NASH [7]. Exercise can improve NASH, by reversing steatosis, and may independently arrest liver fibrosis. However, the exact mechanisms underlying these interactions are unknown. Proposed benefits of exercise leading to NASH improvement involve activation of lipolysis, upregulation of brown adipose tissue-produced uncoupling protein-1 and adipocyte stimulating peroxisome proliferator-activated receptor gamma, and alterations in white adipose-tissue dependent adipocytokines (e.g., adiponectin, tumor necrosis factor-α, and interleukin-6) [8, 9].
Given the uncertainty surrounding the mechanism of exercise-induced improvements in patients with NASH, we aimed to identify a new pathway. AMP-activated protein kinase (AMPK) is a fuel-sensing enzyme that is activated by energy stress [10]. Mammalian target of rapamycin in complex 1 (mTORC1) is a nutrient sensor that regulates the cell cycle and protein synthesis [10, 11]. Activated mTORC1 phosphorylates and activates p70S6kinase which stimulates peptide translation and elongation, in part by phosphorylating ribosomal protein S6 (rpS6) [10, 11]. In NASH, AMPK activity is low and mTORC1 activity is high [12, 13]. Exercise activates AMPK and suppresses mTORC1 in healthy persons without NASH [11]. For these reasons, we hypothesized that exercise in NASH patients downregulates hepatic rpS6 phosphorylation, which is a downstream target of AMPK and mTORC1.
Methods
After informed consent was obtained, subjects aged 18–69 years with biopsy proven NASH and no contraindications to an exercise program were enrolled in a small pilot study at a single tertiary academic medical center in the United States. NASH was defined according to guidelines from the American Association for the Study of Liver Diseases and also a NASH Activity Score (NAS) ≥ 5 [14, 15]. Liver biopsy was required in the six-months preceding study enrollment [16, 17]. Subjects were excluded for any of the following reasons: (1) pregnancy; (2) body mass index < 18 or > 45 kg/m2; (3) uncontrolled diabetes (changes in medication dosing over the previous three months or hemoglobin A1c > 9%); (4) active cardiac symptoms; (5) severe medical comorbidities/psychiatric illness; (6) decompensated cirrhosis (history of bleeding esophageal varices, ascites or hepatic encephalopathy); (7) cancer with life expectancy < 6 months; (8) other liver disease; (9) active substance abuse/smoking; (10) institutionalized/prisoner; (11) inability to walk > 2 blocks or ¼ mile. Baseline demographics, anthropometrics, laboratories and intrahepatic fat content as measured by magnetic resonance imaging (MRI) proton density fat fraction on a 3T Prisma fit, Siemens Medical MRI scanner were obtained. NAS was calculated by an independent liver pathologist. Baseline laboratories including hemoglobin A1c, liver associated enzymes, cholesterol and triglyceride levels were obtained.
Maximal oxygen consumption (VO2max) testing was completed using a Trackmaster Treadmill and Parvomedics Metabolic Measuring System to collect expired gases. Continuous 12-lead electrocardiogram monitoring with Quinton Q-Stress ECG machine was performed as the subject began the Bruce Treadmill test protocol which follows graduated stages: Stage 1 = 1.7 mph at 10% grade; Stage 2 = 2.5 mph at 12% grade; Stage 3 = 3.4 mph at 14% grade; Stage 4 = 4.2 mph at 16% grade; Stage 5 = 5.0 mph at 18% grade; Stage 6 = 5.5 mph at 20% grade; Stage 7 = 6.0 mph at 22% grade; Stage 8 = 6.5 mph at 24% grade; Stage 9 = 7.0 mph at 26% grade. No subject progressed beyond Stage 3. Blood pressure was taken with a manual blood pressure cuff at the end of each stage until the subject began to run. Corresponding Borg Rated of Perceived Exertion scale (range 6–20) was measured at the end of each stage. Subjects were encouraged throughout the test to push their hardest until they were unable to continue. All VO2max tests were symptom limited. VO2max was assessed as the highest oxygen uptake with maximal heart rate within 10 beats per minute of age-predicted heart rate max and/or a respiratory exchange ratio value > 1.05, and or an RPE score of > 18 [18]. Age-predicted VO2max levels were calculated using standard formulas: VO2max (men) = 57.8 - (0.445*age in years); VO2max (women) = 42.3 - (0.356*age in years) [19]. After completion of the VO2max test, a 2–5 min cool down period with continuous heart rhythm monitoring and occasional blood pressure measures was completed. To ensure subject safety, all VO2max testing was supervised by an American College of Sports Medicine certified exercise physiologist and a study physician.
After VO2max testing established corresponding heart rate zones, subjects entered a four week lead-in period where they began at 15 min of moderate intensity (heart rate target corresponding to 45–55% of their VO2max) exercise and progressively increased the duration and frequency until the goal of 30 min of moderate intensity aerobic exercise five times a week was reached. Following the lead-in period, subjects completed 16-weeks of aerobic exercise at the same frequency, intensity and duration. Each session was supervised in-person by an American College of Sports Medicine certified exercise physiologist. Aerobic exercise was completed on either the treadmill, recumbent exercise bike, rowing or the elliptical machine for a duration of 30-min at the prescribed moderate intensity corresponding to 45–55% of subject VO2max. Prior to each session, the subject completed a 10 min warm-up with 5 min of walking on the treadmill at 30–40% of target heart rate followed by five dynamic exercises including knee to chest, 10-yard lateral shuffle, bent over twist, calf sweeps and leg swings. Each exercise session ended with a 5-min cool down on the treadmill at 30–40% of target heart rate. Additional home exercise beyond the in-person sessions were assessed by fitness activity tracker with heart rate monitor (FitBit Charge HR2). If a subject was determined to perform additional exercise beyond that prescribed by the study sessions, corrective feedback and action occurred to prevent the subject from completing extra exercise. Weekly review of activity tracker information occurred following an in-person exercise session. The a priori definition of protocol compliance was attending and completing ≥ 3 exercise sessions each week.
Prior to exercise initiation, subjects also met with a study Registered Dietitian (RD) and individual caloric needs were calculated using the Mifflin St. Jeor Equation and multiplied by an activity factor (AF): males 10(wt. (kg)) + 6.25(ht. (cm)) − 5(age (yr)) + 5 = resting energy expenditure (REE) × AF = total energy expenditure (TEE) and for females 10(wt. (kg)) + 6.25(ht. (cm)) − (age (yr)) – 161 = REE × AF = TEE. AF were as follows: seated work with no option of moving around and little or no strenuous leisure activity (1.4–1.5), seated work with discretion and requirement to move around but little or no strenuous leisure activity (1.6–1.7), standing work such as housework (1.8–1.9), strenuous work or highly active leisure (2.0–2.4). Standard macronutrient distribution was recommended at 50–60% carbohydrate, 15–20% protein, and 20–30% fat with < 10% total calories attributable to saturated fat. A sodium intake of < 2000 mg daily was encouraged. During the study intervention period, directed dietary feedback by a RD was provided based on the weekly food log with the goal of achieving individual caloric, macronutrient and sodium goals as above. Dietary feedback could include education about meal composition, frequency, portion size and food quality.
Following completion of the 20-week exercise protocol, repeat VO2max testing, laboratories and liver biopsy were completed. rpS6 phosphorylation levels in liver tissue was evaluated by immunofluorescence staining. Images were collected using a 20X objective using a Leica SP8 confocal microscope. The fluorescence intensities were quantified using ImageJ software (Version 2, https://www.imagej.nih.gov). The relative fluorescence intensities data were generated from the average of two biopsy images each counting four random sites. Pre- and post-fluorescence intensities were compared with paired t-tests. A p value < 0.05 was considered significant. All statistical analyses were conducted using SAS Version 9.4 (Cary, NC). Institutional review board approval was obtained.
Results
Three subjects (two men, one woman) with mean age 55 ± 3 years were enrolled and completed the exercise protocol. Mean time between liver biopsy and study enrollment was 35 days. No clinically significant changes in baseline demographics were observed between biopsy and study enrollment. Mean NAS was 5.3 ± 0.6 and mean fibrosis stage was 2.3 ± 1.2. Subjects were compliant with the monitored exercise protocol. 100% of subjects completed the a priori definition of adherence by attending at least three exercise sessions each week during the study protocol. Specifically, individual subjects completed 82%, 85% and 99% of the exercise sessions (100 in total). All subjects performed moderate-intensity exercise as monitored by their FitBit and a study exercise physiologist. No subject completed additional exercise as monitored remotely by their Fitbit outside of that prescribed by the study.
Following 20-weeks of aerobic exercise, mean VO2max increased by 3.3 mL/kg/min (21.7 ± 1.3 vs. 25.0 ± 3.3 mL/kg/min, p = 0.18). Intrahepatic fat decreased significantly by 9.3% (19.4 ± 3 vs. 10.1 ± 2.4%, p = 0.04) despite a modest decrease in body weight (4.2 ± 1 5.1 lb, p = 0.79). Phospho-rpS6 levels were significantly attenuated (3.9 ± 1.9 pre-exercise vs. 1.4 +/0.4 post-exercise, p = 0.04) (Fig. 1). Attenuation of Phospho-rpS6 levels was strongly positively correlated with loss of intrahepatic fat (r = 0.98); no correlation was observed between attenuation of Phospho-rpS6 and change in VO2max (r = 0.20) or change in body weight (r = 0.36).
Fig. 1.
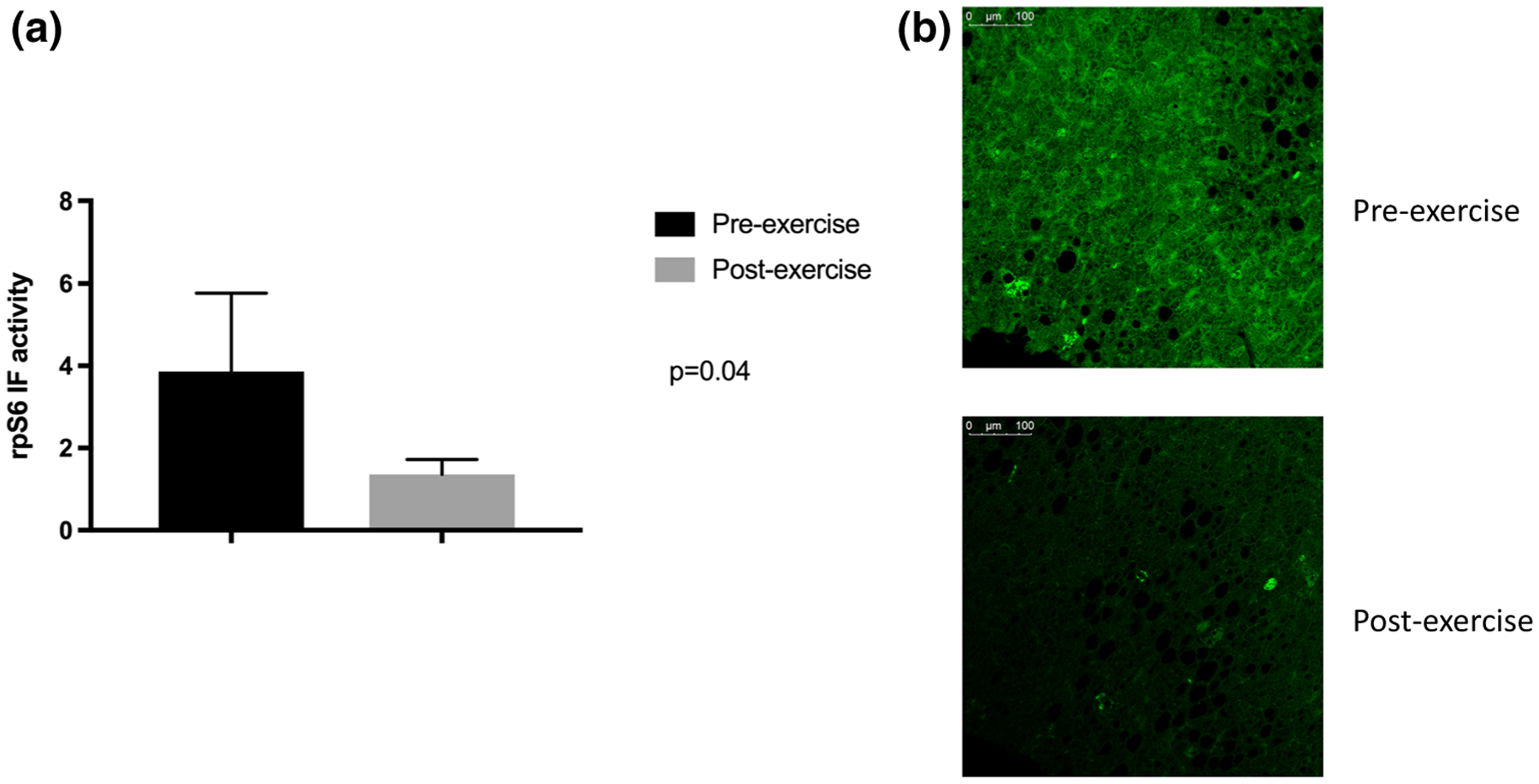
rpS6 activity is attenuated by chronic, repetitive aerobic exercise. a Mean rpS6 activity was significantly decreased following 20-weeks of aerobic exercise training. b Post-exercise hepatic phosphor-rpS6 immunofluorescence decreases with exercise in an individual subject
Tables 1 and 2 list additional salient demographic, laboratory, fitness and histologic measures for each individual subject at baseline and following exercise intervention. In general, subjects lessened their metabolic risk through the exercise intervention.
Table 1.
Baseline characteristics of study subjects
| Subject | Age | Sex | A1c (%) | Weight (#) | Hepatic fat (%) | VO2max (mL/kg/min) | rpS6 activity | ALT (IU/L) | AST (IU/L) | Cholesterol (mg/dL) | Triglyceride (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 54 | M | 6.7 | 268 | 16.3 | 21.5 | 4.29 | 61 | 36 | 135 | 123 |
| 2 | 58 | M | 8.0 | 190 | 22.1 | 20.7 | 1.77 | 81 | 47 | 246 | 246 |
| 3 | 52 | F | 5.7 | 253 | 19.9 | 22.9 | 5.52 | 89 | 101 | 204 | 153 |
A1c hemoglobin A1c; ALT alanine aminotransferase; AST aspartate aminotransferase; Chol total cholesterol; rpS6 ribosomal protein S6; TG triglyceride; VO2max maximal oxygen consumption capacity
Table 2.
Exercise induced changes for each individual subject
| Subject | Age | Sex | End of exercise laboratories | Delta laboratories | Delta weight (#) | Delta hepatic fat (%) | Delta VO2max (mL/kg/min) | Delta rpS6 activity |
|---|---|---|---|---|---|---|---|---|
| 1 | 54 | M | A1c 6.3% | A1c −0.4% | − 10.0 | − 10.1 | + 7.2 | − 2.88 |
| ALT 44 IU/L | ALT −17 IU/L | |||||||
| AST 41 IU/L | AST +5 IU/L | |||||||
| Chol 114 mg/dL | Chol −21 mg/dL | |||||||
| TG 98 mg/dL | TG −25 mg/dL | |||||||
| 2 | 58 | M | A1c 6.8% | A1c −1.2% | − 0.5 | − 7.5 | + 2.8 | − 0.79 |
| ALT 52 iU/L | ALT −29 IU/L | |||||||
| AST 35 iU/L | AST −16 IU/L | |||||||
| Chol 144 mg/dL | Chol −72 mg/dL | |||||||
| TG 199 mg/dL | TG −47 mg/dL | |||||||
| 3 | 52 | F | A1c 5.8% | A1c +0.1% | − 2.1 | − 10.5 | − 0.2 | − 3.83 |
| ALT 47 IU/L | ALT −42 IU/L | |||||||
| AST 52 IU/L | AST −49 IU/L | |||||||
| Chol 182 mg/dL | Chol −21 mg/dL | |||||||
| TG 135 mg/dL | TG −18 mg/dL |
A1c hemoglobin A1c; ALT alanine aminotransferase; AST aspartate aminotransferase; Chol total cholesterol; rpS6 ribosomal protein S6; TG triglyceride; VO2max maximal oxygen consumption capacity
In general, subjects improved their physical fitness while lowering their blood sugars, cholesterol and liver associated enzymes
Discussion
NAFLD/NASH is the leading reason for chronic liver disease globally and is associated with greater all-cause mortality [20, 21]. Consequently, establishing an effective treatment for this common condition is of the utmost importance to global health. While exercise is the only treatment that when coupled with a 7–10% weight loss, can reverse fibrosis in NASH, our understanding of the mechanism behind this benefit is unclear [22]. In this small pilot study, we have identified a novel pathway modified by exercise that may offer new insight into the mechanism of how exercise improves NASH and possibly reverses fibrosis.
Multiple animal models of NAFLD have demonstrated that exercise intervention upregulates AMPK and downregulates mTORC1 independent of weight loss [23, 24]. Our study further extends the literature on the benefit of exercise in NASH, as we demonstrated in human subjects that downstream of the AMPK/mTORC1 pathway, rpS6 phosphorylation is attenuated after completion of a 20-week moderate intensity aerobic exercise program. It has been proposed that AMPK activation may reduce NAFLD by suppression of hepatic de novo lipogenesis leading to lower amounts of intrahepatic lipid accumulation, increased hepatic fatty acid oxidation and promotion of mitochondrial function in adipose tissue [25, 26]. As AMPK/mTORC1 are involved in intrahepatic lipid processing [12, 13] the strong correlation between changes in Phospho-rpS6 and loss of intrahepatic fat are further in support of this possible mechanism, independent of significant total body weight loss. Animal models also demonstrate that exercise in rodents with NAFLD reduces hepatic stellate cell activation which over time may lead to fibrosis reversal [27]. And while the link between the AMPK/mTORC1 pathway, hepatic stellate cell activation and exercise remains unexplored, it is plausible that through changes in AMPK/mTORC1, exercise can lead to fibrosis reversal. This offers and intriguing avenue for future exploratory mechanistic study.
In conclusion, our findings suggest exercise modulates the AMPK/mTORC1 pathway in patients with biopsy proven NASH and may lead to improvement in intrahepatic fat independent of weight loss. Further establishment of the role of exercise in AMPK/mTORC1 signaling may guide the design of future studies into the mechanism of how exercise improves NASH and possibly reverses fibrosis.
Acknowledgments
The authors are thankful to the Penn State Milton S. Hershey Medical Center MRI Core Facility staff for the support on study protocol development/data acquisition/data processing/ data analysis in this study. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Funding This grant was funded in part by NIH grant L30 DK118601. This project is also funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco CURE Funds. The Department specifically disclaims responsibility for any analyses, interpretations or conclusion. The study was supported by NIH/NCATS Grant UL1TR000127 and UL1TR002014.
Abbreviations
- AMPK
AMP-activated protein kinase
- mTOR
Mammalian target of rapamycin
- mTORC1
Mammalian target of rapamycin in complex 1
- MRI
Magnetic resonance imaging
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis
- NAS
NASH Activity Score
- rpS6
Ribosomal protein S6
- VO2max
Maximal oxygen consumption
Footnotes
Conflict of interest The authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest, or non-financial interest in the subject matter or materials discussed in this manuscript. Dr. Stine has received research funding from Target PharmaSolutions, Inc.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. [DOI] [PubMed] [Google Scholar]
- 2.Stine JG, Rinella ME. Editorial: Age and Non-Invasive Markers of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease: Time to Adjust the Clock? Am J Gastroenterol. 2017;112:752–754. [DOI] [PubMed] [Google Scholar]
- 3.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547–555. [DOI] [PubMed] [Google Scholar]
- 4.Noureddin M, Vipani A, Bresee C, et al. NASH Leading Cause of Liver Transplant in Women: Updated Analysis of Indications For Liver Transplant and Ethnic and Gender Variances. Am J Gastroenterol. 2018;113:1649–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singal AK, Hasanin M, Kaif M, Wiesner R, Kuo YF. Nonalcoholic Steatohepatitis is the Most Rapidly Growing Indication for Simultaneous Liver Kidney Transplantation in the United States. Transplantation 2015. [DOI] [PubMed] [Google Scholar]
- 6.Borrelli A, Bonelli P, Tuccillo FM, et al. Role of gut microbiota and oxidative stress in the progression of non-alcoholic fatty liver disease to hepatocarcinoma: Current and innovative therapeutic approaches. Redox Biol. 2018;15:467–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen J, Wong GL, Chan HL, et al. PNPLA3 gene polymorphism and response to lifestyle modification in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol. 2015;30:139–146. [DOI] [PubMed] [Google Scholar]
- 8.Cao H Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014;220:T47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, Takano Y, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J Hepatol 2017;66:142–152. [DOI] [PubMed] [Google Scholar]
- 10.Kimball SR. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc. 2006;38:1958–1964. [DOI] [PubMed] [Google Scholar]
- 11.Richter EA, Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418:261–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Liu J, Yang L, Ling W. AMP-activated protein kinase regulates lipid metabolism and the fibrotic phenotype of hepatic stellate cells through inhibition of autophagy. FEBS Open Bio. 2017;7:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han J, Wang Y. mTORC1 signaling in hepatic lipid metabolism. Protein Cell. 2018;9:145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 16.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vita-min E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394:2184–2196. [DOI] [PubMed] [Google Scholar]
- 18.Argo CK, Stine JG, Henry ZH, Lackner C, Patrie JT, Weltman AL, Caldwell SH. Physical deconditioning is the common denominator in both obese and overweight subjects with nonalcoholic steatohepatitis. Aliment Pharmacol Ther 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. Philadelphia: Wolters Kluwer; 2018. [DOI] [PubMed] [Google Scholar]
- 20.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology (Baltimore, Md.) 2006;44:865–873. [DOI] [PubMed] [Google Scholar]
- 21.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. [DOI] [PubMed] [Google Scholar]
- 22.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015;149:367–378.e365; quiz e314–365. [DOI] [PubMed] [Google Scholar]
- 23.Piguet AC, Saran U, Simillion C, et al. Regular exercise decreases liver tumors development in hepatocyte-specific PTEN-deficient mice independently of steatosis. J Hepatol. 2015;62:1296–1303. [DOI] [PubMed] [Google Scholar]
- 24.Marcinko K, Sikkema SR, Samaan MC, Kemp BE, Fullerton MD, Steinberg GR. High intensity interval training improves liver and adipose tissue insulin sensitivity. Mol Metab. 2015;4:903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 2016;311:E730–e740. [DOI] [PubMed] [Google Scholar]
- 26.Fullerton MD, Galic S, Marcinko K, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. 2013;19:1649–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albano E, Mottaran E, Vidali M, et al. Immune response towards lipid peroxidation products as a predictor of progression of non-alcoholic fatty liver disease to advanced fibrosis. Gut. 2005;54:987–993. [DOI] [PMC free article] [PubMed] [Google Scholar]


