Abstract
Purpose.
Social participation is a key determinant of healthy aging, yet little is known about how people with Parkinson’s disease manage social living. This study describes individual differences in social self-management practices and their association with symptom severity and health quality of life.
Methods.
People with Parkinson’s disease (N = 90) completed measures of healthy routines, activities and relationships, symptom severity and health related quality of life. Cluster analysis identified profiles of social self-management practices. Analysis of variance tested differences between profiles in symptom severity and health quality of life.
Results.
Participants clustered into one of seven groups according to different combinations of three practices: health resources utilization, activities in home and community, and social support relationships. The healthiest cluster engaged equally in all three practices at above sample average degree of engagement. Four clusters that engaged at or above sample average in activities in home and community experienced less health problems than three clusters that engaged below average. Variation in aspects of social lifestyle unrelated to health appeared also to contribute to profile diversity.
Conclusion.
Findings provide insight into similarity and variation in how people with Parkinson’s disease engage with social self-management resources and point to person-centered interventions.
Keywords: Self-management, social ecology, biopsychosocial, social participation, everyday living, person-centered
Introduction
“Central tendency is an abstraction, variation the reality.” Stephen Jay Gould [1, p.48] made this argument about his experience with cancer. Told he had eight months to live, Gould discovered this to be the median length of survival. Fifty percent died within eight months after diagnosis, but the rest lived longer, ranging from eight months to many years. With hope of optimizing his survival, he devoted effort to managing disease while continuing social participation as an influential scholar and teacher of paleontology and evolutionary biology. He lived for 20 years post-diagnosis.
As Gould notes, central tendency measures (e.g., median), although useful, neither describe nor predict particulars of individuals. There is little systematic understanding of how variation in self-managing disease during social daily life relates to health outcomes, especially among individuals living with incurable chronic disease and disability [2]. More information about both centrality and variation is needed to inform person-centered rehabilitation [3,4]. This study investigates heterogeneity of social life among individuals with Parkinson’s disease (PD), and its association with variation in symptom severity and health quality of life.
Aging adults demonstrate heterogeneous health outcomes [5], and considerably so among those with PD, a disease of increasing prevalence [6]. Symptoms typically include impaired speed, flexibility, fluidness, and coordination of movement. Disease progression is unpredictable, symptoms fluctuate, and motor symptoms co-occur with idiosyncratic constellations of non-motor problems (e.g., fatigue, bowel dysfunction, depression, and cognitive impairment) [7].
Heterogeneity extends beyond pathology to performance of daily routines and roles within a societal context [8]. While some people with PD experience a typical trajectory of functional decline and social isolation [9,10], others are exceptions comparable to Gould in his survival of cancer, who experience fuller social participation and healthy outcomes in an atypically slow trajectory [11]. People with PD are living longer, enabling more opportunity to fulfill personal aspirations such as maintaining purposeful activity, autonomous decision-making, meaningful social connection, and wellness [12–17]. The question remains as to whether they are optimally accessing and using these opportunities as part of their health regimen despite social forces that can be isolating. PD symptoms are stigmatizing, which can affect relationships and broader public and formal health care interactions [18–21].
Social self-management practices
This study aimed to close gaps in knowledge about lived experience and naturalistic social practices while living with diseases such as PD. We defined social self-management practices as the social self-care equivalent of World Health Organization’s International Classification of Functioning, Disability and Health codes d570 looking after one’s health and d5702 maintaining one’s health [8]. These codes focus on physical self-care, just as rehabilitation for PD predominantly targets physical motor symptoms and dysfunction [22]. On the other hand, healthy aging evidence suggests daily life practices should include engaging in (1) formal health care with providers and organizations, (2) home and community activities, and (3) socially supportive relationships in one’s informal social network [23–28]. Focusing on social self-care in relation to physical self-care expands the self-care model to the management of biopsychosocial health within the ecology of daily living [29–31]. This study assesses healthy aging social practices within the context of daily living with PD and generates implications for how rehabilitation might help people with PD to proactively manage everyday opportunities to optimize healthy biopsychosocial outcomes.
The healthy aging practice of engaging in formal health care involves accessing, seeking help from, and forming a collaborative therapeutic alliance with providers [32]. Adults who do so are more likely to experience better health outcomes [33,34]. Formal health self-management programs may focus more on biomedical management of a specific health condition than on how to manage person-centered home and community social relationships and roles [24,35]. Nonetheless, many people with neurological conditions report deriving social benefit from peer interaction in these programs [22,36–38].
The healthy aging practice of engaging in home and community activities involves a broad variety of instrumental, leisure, and socializing activities [39]. These activities keep people moving, socially connected, and participating in community, while creating informal social supports in time of need [28]. Valued daily social activities may protect against disability by exercising biopsychosocial capacities as a whole [23]. Older adults who retain more activities have greater functional physical and mental health than those with lower retention [25]. Retaining activities can be challenging for people with PD, in part, because managing symptoms and disability takes considerable skill and time that could be used for other valuable activities [40,41]. However, beginning evidence indicates those who retain old or participate in new activities experience fewer symptoms and improved health quality of life [42–44],
The healthy aging practice of engaging in social support relationships involves receiving and giving instrumental support (e.g., getting things done) and emotional support (e.g., belonging and enjoyment) with members of one’s informal social network [28]. Receiving needed social support from others can contribute to improved health and lower mortality rates [26,27], particularly if the recipient perceives it to be helpful [24,45]. Giving needed support to others may be as important for one’s health outcomes as receiving needed support from others [45]. It may contribute to having a purpose in life, which predicts slower progression of disability among older adults [46], and quicker recovery from severe mobility limitations [47]. However, stigmatizing effects of PD symptoms can interfere with developing and maintaining mutually beneficial relationships [19]. Abnormal movement can create an appearance of physical and mental frailty that prevents ability to reciprocate social support, an appearance that does not map directly onto actual social attributes. Little is known about how people with PD self-manage social support, however, one study found that seeking support from others had little association with their quality of life [48].
Methods
This study is a descriptive and exploratory analysis of cross-sectional baseline data from participants with PD in a mixed-method three-year prospective cohort study: Emergence and Evolution of the Social Self-Management of Parkinson’s Disease [29]. Five other sub-studies have been published [18,20,49–51]. The current study used cluster analysis to differentiate subgroups of individuals who showed similarity in their social self-management practice profiles [52,53]. This approach is tailored to informing person-centered health assessment and intervention by describing heterogeneous patterns of personal attributes within specific diagnostic populations of people with chronic disability [54–57].
Participant recruitment and enrollment
Recruitment occurred through Boston University Medical Center Parkinson’s Disease and Movement Disorders Clinic, regional support groups, older adult service centers, and postings on websites [58]. The neurological medical team (co-authors Saint-Hilaire and Thomas) conducted medical histories and screening. Inclusion criteria were: diagnosis of idiopathic PD utilizing UK Parkinson’s Disease Society Brain Bank clinical diagnostic criteria; disease severity ranging from modified Hoehn and Yahr stage 1 (very mild symptoms with unilateral involvement only) through stage 4 (severe and disabling symptoms, yet still able to walk or stand unassisted) [59]; no dementia (≥ 26 score on the Mini-Mental Status Exam); home within approximately 100 miles of Boston; able to communicate clearly in English; interested in participating and able to provide informed consent. Institutional review boards for Tufts University Social, Behavioral & Educational Research (# 1212038) and Boston University Medical Campus (# H-32114) approved recruitment, consent, and data collection protocol.
Procedures and Measures
Participants were asked to time medications to be “on,” for maximal motor functioning at their in-person assessment, yet four reported being “off” and 12 were unsure. Demographic and basic clinical data (e.g., modified Hoehn & Yahr disease stage [59], duration since diagnosis, Geriatric Depression Scale [60] score, and Montreal Cognitive Assessment [61] score) were collected. Table 1 lists social self-management measures, their scales, and internal consistency coefficients (Cronbach’s α) calculated from participants’ responses [29,33,62–64]. Higher scores indicate more engagement in a social self-management practice.
Table 1.
Example Items and Internal Consistency of Social Self-Management Measures
| Measure | Description | Example Item/s | Scale | Cronbach’s α |
|---|---|---|---|---|
| Health Management Resources Survey [29, 33] | Measure of health support resources utilized | Over the past six months, to what extent… | 1 (not at all) to 5 (a great deal) | |
| Health Care (collaborative management with providers) | has your doctor involved you as an equal partner in making decisions about health management strategies and goals? | .77 | ||
| Personal (self-management) | have you thought about or reviewed how you were doing in accomplishing your health management goals? | .72 | ||
| Organizations (involvement in) | have you attended free or low-cost meetings (for example, PD support groups, church groups, hospital programs) that supported you in managing your health? | .52 | ||
| Neighborhood (healthy activities in) | have you walked or done other exercise activities with neighbors? | .59 | ||
| Family and Friends (healthy activities with) | have family or friends exercised with you? | .49 | ||
| Activity Card Sort (% Retained) [62] | Measure of retained participation in a broad range of activities since a past event or time point | Cooking Dinner/ Going to the Museum/ Exercising/ Visiting with Friends | Never done; Do less often than six months ago; Do same amount as six months ago; Do more often than six months ago; Given up. | .95 |
| Ability to Manage Participation [29]a | One item measure of self-managing participation | How would you rate your overall ability to manage participating in your daily life activities? | 1 (not at all effective) to 5 (highly effective) | |
| Support Relationships [63, 64] | Measure of how much participants receive and give social support. | How often do you… | 1 (not at all) to 5 (a great deal) | |
| Receive Instrumental Support | receive help with daily activities from members of your household / others outside of your household? | .64 | ||
| Receive Emotional Support | receive emotional support from members of your household/ others outside of your household? | .39 | ||
| Give Instrumental Support | give help with daily activities to members of your household/ others outside of your household? | .57 | ||
| Give Emotional Support | give emotional support to members of your household/ others outside of your household? | .49 |
Notes. Health Management Resource Survey is a slightly modified version of Chronic Health Resource Survey: “chronic illness” was changed to “health management”, and in one item “Weight Watchers” was replaced with “Parkinson’s support groups.” For Activity Card Sort, Retained Activities score is percentage of currently retained activities out of total number of retained and given up activities. Current activities entered into this percentage as a weighted function of the degree to which each activity is done more than (1.5), same as (1), or less than (.5) six months ago.
There is no Cronbach’s α because this is a one-item scale.
Symptom severity was measured by Movement Disorder Society’s Unified Parkinson’s Disease Rating Scales (MDS-UPDRS) Total score (possible range = 0 – 260, higher score indicates more severe symptoms) [65]. The examiner rated 65 items from 0 (normal) to 4 (severe) to assess non-motor and motor experiences of daily living, observed motor performance, and motor complications. Our sample’s total score demonstrated high internal consistency (Cronbach’s α = .93).
Health quality of life was measured by Parkinson’s Disease Questionnaire-39 (PDQ-39) Summary Index (possible range = 0 – 100, higher score indicates more severe PD problems) [66]. Respondents self-reported on 39 items how often during the past 30 days they experienced problems due to PD from 0 (never) to 4 (always) in mobility, activities of daily living, emotional well-being, stigma, social support, cognitions, communication, and bodily discomfort. Our sample’s summary index demonstrated high internal consistency (Cronbach’s α = .95).
Data analysis
Data were managed with Research Electronic Data Capture (REDCap) using independent double-entry for accuracy checks [67] and analyzed with Statistical Package for the Social Sciences (IBM SPSS Statistics) version 24. Descriptive analyses demonstrated variables were normally distributed and exerted no outlier effects [68,69].
Principal component analysis with varimax rotation reduced social self-management data into composite scores (regression method) that measured degree of engaging in different management practices [53,69]. These practice engagement scores were entered into two-stage cluster analysis to group together participants with similar profiles of engagement [52,53]. Stage one, Ward’s Method hierarchical cluster analysis agglomerated participants into increasingly smaller numbers of clusters for determining the final number of clusters. Stage two, non-hierarchical k-means cluster analysis, optimized participant assignment to the number of clusters from stage one. Per convention, we compared three different cluster solutions to determine the number of clusters that created statistically distinctive, conceptually meaningful groups of participants [53]. A cluster solution was determined to meet standards of adequate statistical power if the smallest cluster’s sample size exceeded the number of clustering variables (i.e., practice engagement scores derived from principal component analysis) [69, p. 314]. Canonical discriminant analysis cross-validated clusters by calculating rate of accurate classification of participants to their cluster groups. Profile analysis of variance (ANOVA) [69] tested statistical significance and magnitude of differences between clusters repeated (within-cluster) on practice engagement scores.
One-way ANOVA tested differences between clusters’ average symptom severity, health-quality of life scores, and other continuous variables (e.g. age, depression). Cross-tabulations and chi-square analyses tested differences on discrete variables (e.g., marital status. Magnitudes of differences between clusters were calculated with squared effect size coefficients (e.g., partial eta-squared η2) and interpreted using common rules of thumb: with .01 considered a small magnitude of difference, .09 considered medium, and .25 considered large [69–71]. Minimal clinically important differences (MCID) were calculated for health outcomes: MCID threshold of 6.30 points for MDS-UPDRS Total score [72] and 4.22 points for PDQ-39 Summary Index [73].
Results
Participant characteristics
The sample included 90 people (34 women) with PD. Gender and race percentages were within typical PD diagnosis and research recruitment rates [58,74] (Table 2). Generally, participants were non-Hispanic white men, in their 60s, educated beyond high school, with income similar to median income of Boston area [75], currently married, with children and grandchildren, and lived with others. Hoehn and Yahr stage of disease, generally was stage 2 (mild disease severity with bilateral involvement and no impairment of balance) and ranged from stage 1 through 4. Sample mean Geriatric Depression Scale-15 score fell below clinical level of depression (< 5), although 13 participants (14.4%) showed potential depression [76]. Compared to participants’ Mini-Mental Status Exam scores falling within normal range, Montreal Cognitive Assessment scores were more sensitive to variation in cognitive impairment, with 36 participants (40%) showing mild cognitive impairment (< 26) [77].
Table 2.
Demographic and Clinical Characteristics of Study Participants (N = 90)
| Variable | n (%) | m (SD) | median (min - max) |
|---|---|---|---|
| Age (years) | 65.47 (9.72) | 65.69 (28.56 – 89.26) | |
| < 50 | 5 (5.6%) | ||
| 50–59 | 17 (18.9%) | ||
| 60–69 | 44 (48.9%) | ||
| 70+ | 24 (26.7% | ||
| Education (Highest Achieved) | |||
| High School Graduate or GED | 9 (10.0%) | ||
| Some College/Associate Degree | 19 (21.1%) | ||
| Bachelors | 22 (24.4%) | ||
| Masters | 31 (34.4%) | ||
| Doctorate | 9 (10.0%) | ||
| Household Income | |||
| $12,000 – $15,999 | 4 (4.4%) | ||
| $16,000 – $24,999 | 3 (3.3%) | ||
| $25,000 – $34,999 | 3 (3.3%) | ||
| $35,000 – $49,999 | 4 (4.4%) | ||
| $50,000 – $74,999 | 24 (26.7%) | ||
| $75,000 – $99,999 | 9 (10.0 %) | ||
| $100,000 and Greater | 25 (27.8%) | ||
| Unsure/Unspecified | 18 (20.0%) | ||
| Occupation Status | |||
| Part- or Full-Time Work | 31 (34.4%) | ||
| Retired | 49 (54.4%) | ||
| Other/Unemployed | 10 (11.1%) | ||
| Marital Status and Family | |||
| Single (Never Married) | 7 (7.8%) | ||
| Married | 65 (72.2%) | ||
| Divorced/Separated | 10 (11.1%) | ||
| Widowed | 6 (6.7%) | ||
| Other Marital Status | 2 (2.2%) | ||
| Number of Children | 2.24 (1.28) | 2.0 (0 – 8) | |
| Number of Grandchildren | 2.01 (2.81) | 1.0 (0 – 13) | |
| Lives with Others | 76 (84.4%) | ||
| Hoehn & Yahr Stage of Disease | 2.21 (0.68) | 2.0 (1 – 4) | |
| 1 | 6 (6.7%) | ||
| 2 | 66 (73.3%) | ||
| 3 | 11 (12.2%) | ||
| 4 | 7 (7.8%) | ||
| Duration of Diagnosis (Years) | 7.34 (7.06) | 5.0 (.17 – 34) | |
| Geriatric Depression Scale | 2.43 (2.62) | 2.0 (0 – 13) | |
| Montreal Cognitive Assessment | 25.71 (3.43) | 26.5 (14 – 30) |
Principal components: three social self-management practice engagement scores
A three-component solution from principal component analysis accounted for 55.5% of the variance in social self-management measures (Table 3). Three standardized composite scores per participant (total sample M = 0, SD = 1) indicated degree of engagement in each of three practices: Activities in Home and Community (Activities), Social Support Relationships (Support), and Health Resources Utilization (Health). Positive scores indicated more engagement and negative scores indicated less engagement than total sample average.
Table 3.
Component Loadings from Principal Components Analysis with Varimax Rotation for Social Self-management Measures
| Activities in Home and Community | Social Support | Health Resource Utilization | |
|---|---|---|---|
| HMRS Neighborhood | 0.76 | 0.06 | 0.14 |
| HMRS Family and Friends | 0.75 | 0.06 | −0.01 |
| ACS Global Activities (% Retained) | 0.70 | −0.01 | 0.02 |
| Ability to Manage Participation | 0.69 | 0.12 | 0.19 |
|
| |||
| Receive Emotional Support | 0.22 | 0.79 | −0.08 |
| Give Emotional Support | 0.28 | 0.66 | 0.28 |
| Receive Instrumental Support | −0.35 | 0.64 | −0.04 |
| Give Instrumental Support | 0.01 | 0.46 | 0.65 |
|
| |||
| HMRS Health Care | −0.09 | 0.08 | 0.73 |
| HMRS Personal | 0.32 | −0.16 | 0.55 |
| HMRS Organizations | 0.45 | −0.09 | 0.54 |
Notes. HMRS = Health Management Resources Survey; ACS = Activity Card Sort. Component loadings ≥ .40 are bolded. For conceptual coherence, Giving Instrumental Support is included as a Support practice, although it also loaded on Health practice. Involvement in Organizations was included as a Health practice, although it also loaded on Activities practice.
Cluster profiles: seven social self-management profiles
Stage one cluster analysis indicated there to be at least six distinctive clusters of participants. At stage two, a seven-cluster solution produced the most comprehensive, conceptually meaningful social self-management profiles: maximizing heterogeneity of practice profiles and forming cohesive groups of individuals sharing similar profiles. Cluster sizes ranged from 10 to 16 participants (Median, Mode = 13). Adequate statistical power to detect significant differences between seven clusters was demonstrated by smallest cluster size (n =10) being larger than 3, the number of clustering variables [69, p.317]. Canonical discriminant analysis cross-validated the cluster solution: three discriminant functions significantly differentiated the cluster profiles from one another (p < .0001) and correctly classified 88 of the 90 (97.8%) participants to clusters.
For consistency, we describe practices in order of Health, Activities, and Support. Profile ANOVA (between seven clusters by three within-cluster practices) also validated the cluster solution. It showed statistically significant large differences among clusters on the combined average of the three practice scores (F (6, 83) = 28.56, p < .0001, partial η2 = .67). Furthermore, interaction of cluster with type of practice (Health, Activities, Support) was statistically significant and of large magnitude (F (12, 166) = 40.04, p < .0001, partial η2 = .74). This finding and canonical discriminant analysis finding reject the null hypothesis of no differences in profiles across the clusters, validating that the clusters represent different profiles of practice. These statistically significant findings also corroborate adequate statistical power to detect differences between clusters that were practically and clinically meaningful [68,69].
Naming clusters by degree of engagement in health, activities and support practices
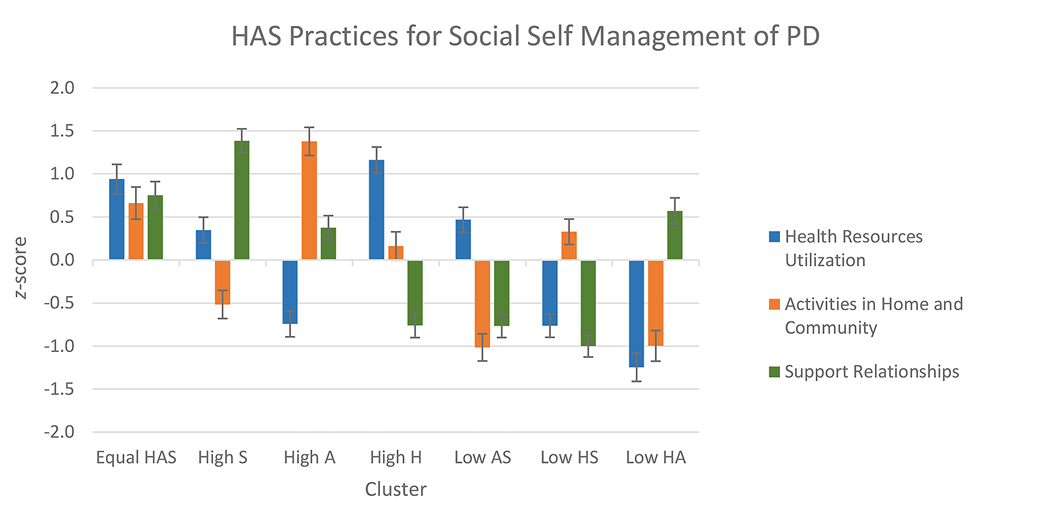
To name clusters, we ordered clusters from most to least combined average of their three practice scores (Table 4, Figure 1). The first four clusters (Equal Health-Activities-Support; High Support; High Activities; and High Health) demonstrated combined engagement above total sample average. These clusters are named by Health, Activities or Support practices in which members, on average, had high engagement relative to total sample average (≥ 0.50 SD above 0) [78]. The three remaining clusters (Low Activities-Support; Low Health-Support; and Low Health-Activities) had a combined engagement in the three practices below sample average. They are named by practices in which members, on average, had low engagement relative to the total sample (≤ − 0.50 SD). A cluster’s average practice engagement score between − 0.50 and + 0.50 is considered average relative to total sample.
Table 4.
Cluster Names and Overall Engagement in Social Self-management Practices
| Cluster | Practice Engagement | n | Overall Engagement Mean | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|---|
| Health | Activities | Support | Lower | Upper | |||
| a. Equal HAS | High | High | High | 10 | 0.78 | 0.57 | 1.00 |
| b. High S | Average | Low | High | 13 | 0.40 | 0.22 | 0.59 |
| c. High A | Low | High | Average | 13 | 0.34 | 0.15 | 0.53 |
| d. High H | High | Average | Low | 13 | 0.19 | 0.00 | 0.38 |
| e. Low AS | Average | Low | Low | 14 | −0.44 | −0.62 | −0.26 |
| f. Low HS | Low | Average | Low | 16 | −0.48 | −0.65 | −0.31 |
| g. Low HA | Low | Low | High | 11 | −0.56 | −0.76 | −0.35 |
Notes. H = Health practice. A = Activities practice. S = Support practice. Level of practice engagement is relative to the total sample’s average engagement (M = 0, SD = 1.00). Overall engagement is the combined average of the H, A, S engagement scores.
Figure 1.
Social self-management practice component score means by cluster. H = Health practice. A = Activities practice. S = Support practice. Standard errors are represented by error bars attached to each column. A higher score indicates more engagement in the practice. Clusters are ordered left to right according to their overall degree of engagement across the three practices, as shown in Table 4.
Addition of individual measures of social self-management to cluster description
Cluster profiles on individual measures (listed in Table 1) describe differences in how Health, Activities, and Support practices were implemented across clusters. Each measure’s raw score was standardized (z-transformed M = 0, SD = 1, on total sample) to facilitate comparison.
All four Activities measures (middle panel, Figure 2) consistently tracked together in directionality and magnitude across clusters. Health measures (top panel) showed less consistency: only two (Personal self-management and involvement in Organizations) of three measures tracked together across clusters. In contrast, the four Support measures showed inconsistent tracking across clusters (bottom panel). Their profiles suggest five different types of support lifestyles: (1) high receiving and giving emotional and instrumental support (Equal Health-Activities-Support and High Support clusters); (2) higher receiving and giving emotional than instrumental support (High Activities); (3) higher giving than receiving support, primarily instrumental (High Health); (4) low receiving and giving emotional and instrumental support (Low Activities-Support and Low Health-Support); and (5) higher receiving than giving support, primarily instrumental (Low Health-Activities).
Figure 2.
Social self-management strategy practice individual measure score means by cluster. H = Health practice. A = Activities practice. S = Support practice. Standard errors are represented by error bars attached to each column. A higher score indicates more engagement in the practice.
Severity of symptoms and health quality of life differs by social self-management profile
Cluster differences were statistically significant and approached large magnitude for severity of symptoms (F (6, 83) = 4.48, p = .001, η2 = .25) and health quality of life (F (6, 83) = 4.00, p = .001, η2 = .22) (Figure 3). Post-hoc linear contrast analyses demonstrated that as severity of symptoms and problematic health quality of life increased between clusters (Table 5, with clusters reordered by degree of symptom severity), the clusters’ degree of engagement in Activities practice decreased, a statistically significant finding at a medium to large magnitude for symptom severity (F (1, 83) = 23.68, p < .0001, η2 = .22) and quality of life (F (1, 83) = 21.07, p < .0001, η2 = .20). While Activities practice had a primary association with cluster differences in severity of symptoms, Health practice had a secondary association. Contrast analyses demonstrated that as symptom severity increased between clusters, the clusters’ engagement in Health practice decreased (F (1, 83) = 5.71, p < .02, η2 = .15). However, this pattern was non-significant and of small magnitude for quality of life (F (1, 83) = 2.74, p < .11, η2 = .03). Support practice had non-significant and negligible association with cluster differences in symptom severity (F (1, 83) = 0.07, p = .80, η2 = .00) and quality of life (F (1, 83) = 0.49, p = .49, η2 = .01).
Figure 3.
MDS-UPDRS Total and PDQ-39 Summary Index by cluster. H = Health practice. A = Activities practice. S = Support practice. Standard errors are represented by error bars attached to each column. A higher score indicates more problematic symptoms and health quality of life.
Table 5.
Severity of Symptoms and Health Quality of Life by Cluster Ordered by Low to High Rank of MDS-UPDRS Total Score
| Cluster | Practice Engagement | MDS-UPDRS Total | PDQ-39 Summary Index | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Score | 95% Confidence Interval | Score | 95% Confidence Interval | ||||||||
| Health | Activities | Support | Rank | Mean (SD) | Lower | Upper | Rank | Mean (SD) | Lower | Upper | |
| a. Equal HAS | High | High | High | 1 | 40.60 (24.95) | 22.75 | 58.45 | 1 | 19.25 (7.53) | 13.87 | 24.63 |
| c. High A | Low | High | Average | 2 | 47.77 (16.17) | 38.00 | 57.54 | 2 | 19.85 (11.12) | 13.13 | 26.57 |
| d. High H | High | Average | Low | 3 | 52.08 (11.95) | 44.85 | 59.3 | 4 | 23.09 (11.97) | 15.86 | 30.33 |
| f. Low HS | Low | Average | Low | 4 | 55.75 (15.52) | 47.48 | 64.02 | 3 | 21.94 (7.69) | 17.84 | 26.04 |
| b. High S | Average | Low | High | 5 | 66.08 (21.66) | 52.99 | 79.16 | 5 | 31.82 (13.17) | 23.87 | 39.78 |
| e. Low AS | Average | Low | Low | 6 | 71.36 (30.06) | 54.00 | 88.71 | 6 | 34.46 (18.44) | 23.81 | 45.11 |
| g. Low HA | Low | Low | High | 7 | 74.64 (18.43) | 62.26 | 87.02 | 7 | 37.08 (17.20) | 25.53 | 48.64 |
Notes. MDRS-UPDRS = Movement Disorder Society Unified Parkinson’s Disease Scales. PDQ-39 = Parkinson’s Disease Questionnaire - 39. H = Health practice. A = Activities practice. S = Support practice. Higher scores for MDS-UPDRS total score and PDQ-39 summary index indicate more severe health problems. Cluster prefix a. through g. is retained to facilitate comparison to Table 4‘s ordering of clusters according to their overall degree of engagement across the three practices. Prefixes alphabetically closer to a. indicate higher overall engagement in these practices.
Two clinically different symptom severity cluster sets
Although clusters statistically differed in social self-management practice profiles, comparisons of clusters on MDS-UPDRS Total showed that four clusters fell together into a Less Severe Symptom cluster set, and the remaining into a More Severe Symptom set (Table 6). One-way ANOVA validated Less Severe clusters formed a statistically homogenous set on symptom severity (F (3, 48) = 1.74, p = .17, η2 = .10), as did the More Severe clusters (F (2, 35) = 0.38, p = 0.67, η2 = .02). One-way ANOVA with contrast analysis validated the grouping into two cluster sets: Less Severe clusters had less severe symptoms compared to the More Severe clusters (M Less = 49.92, M More = 70.50; F (1, 83) = 24.07, p < .0001, η2 = .23). All pairwise differences between Less versus More Severe Symptom clusters met MCID criterion for MDS-UPDRS total score (lower left quadrant, Table 6).
Table 6.
Clinical and Statistical Differences between Clusters on Severity of Symptoms
| Pairwise Comparison Difference Scores between Clusters | ||||||||
|---|---|---|---|---|---|---|---|---|
| MDS-UPDRS Total Score |
||||||||
| Less Severe Symptom Clusters | More Severe Symptom Clusters | |||||||
|
|
||||||||
| Cluster Severity Set | Cluster | a. Equal HAS | c. High A | d. High H | f. Low HS | b. High S | e. Low AS | g. Low HA |
| Less Severe | a. Equal HAS | |||||||
| c. High A | 7.17 | |||||||
| d. High H | 11.48 | 4.31 | ||||||
| f. Low HS | 15.15 | 7.98 | 3.67 | |||||
|
| ||||||||
| More Severe | b. High S | 25.48 * | 18.31 * | 14.00 | 10.33 | |||
| e. Low AS | 30.76 * | 23.59 * | 19.28 * | 15.61 * | 5.28 | |||
| g. Low HA | 34.04 * | 26.87 * | 22.56 * | 18.89 * | 8.56 | 3.28 | ||
Notes. MDRS-UPDRS = Movement Disorder Society Unified Parkinson’s Disease Scales. H = Health practice. A = Activities practice. S = Support practice. Clusters are arranged top to bottom and left to right according to their rank from low (1) to high (7) on the MDS-UPDRS total score, as shown on Table 5. Their alphabetical prefixes indicate their order in overall engagement across the three practices. Difference score = higher ranked cluster score minus lower ranked cluster score. A positive score indicates that the higher MDS-UPDRS ranked cluster had more severe symptoms. Bolded differences meet the minimal clinically important difference (MCID) criterion for MDS-UPDRS of +6.30.
p < .05, for paired comparison difference scores
Clusters’ PDQ-39 Summary Index findings were consistent with MDS-UPDRS comparisons (Table 7). Less Severe clusters formed a statistically homogeneous set on health quality of life (F (3, 48) = 0.41, p = 0.75, η2 = .03), as did More Severe clusters (F (2, 35) = 0.31, p = .74, η2 = .02). Less Severe clusters showed less problematic quality of life than More Severe clusters (M Less = 21.19, M More = 34.32; F (1, 83) = 22.94, p < .0001, η2 = .22). All pairwise differences between Less versus More Severe Symptom clusters met MCID criterion for PDQ-39 summary index (lower left quadrant, Table 7).
Table 7.
Clinical and Statistical Differences between Clusters on Severity of Health Quality of Life Outcomes
| Pairwise Comparison Difference Scores between Clusters | ||||||||
|---|---|---|---|---|---|---|---|---|
| PDQ-39 Summary Index |
||||||||
| Less Severe Symptom Clusters | More Severe Symptom Clusters | |||||||
|
|
||||||||
| Cluster Severity Set | Cluster | a. Equal HAS | c. High A | d. High H | f. Low HS | b. High S | e. Low AS | g. Low HA |
| Less Severe | a. Equal HAS | |||||||
| c. High A | 0.60 | |||||||
| d. High H | 3.84 | 3.24 | ||||||
| f. Low HS | 2.69 | 2.09 | −1.15 | |||||
|
| ||||||||
| More Severe | b. High S | 12.57 * | 11.97 * | 8.73 | 9.88 * | |||
| e. Low AS | 15.21 * | 14.61 * | 11.37 * | 12.52 * | 2.64 | |||
| g. Low HA | 17.83 * | 17.23 * | 13.99 * | 15.14 * | 5.26 | 2.62 | ||
Notes. PDQ-39 = Parkinson’s Disease Questionnaire - 39. H = Health practice. A = Activities practice. S = Support practice. Clusters are arranged top to bottom and left to right according to their rank from low (1) to high (7) on the Movement Disorder Society Unified Parkinson’s Disease Scales (MDS-UPDRS) total score, as shown on Table 5. Their alphabetical prefixes indicate their order in overall engagement across the three practices. Difference score = higher ranked cluster score minus lower ranked cluster score. A positive score indicates that the higher MDS-UPDRS ranked cluster had more problematic health quality of life. Bolded differences meet the minimal clinically important difference (MCID) criterion for PDQ-39 of +4.22.
p < .05, for paired comparison difference scores
Participant characteristics as related to social self-management profile
One-way ANOVA found no statistically significant differences between clusters on demographic characteristics (p > .05). However, consistent with MDS-UPDRS and PDQ-39 findings, the seven clusters were differentiated by Hoehn & Yahr disease stage (F (6, 83) = 3.97, p = .002, η2 = .22) and depression (F (6, 83) = 3.19, p = .007, η2 = .19). The Less Severe cluster set included participants at earlier disease stages with less depression than did the More Severe set (Disease stage: M Less = 1.96, M More = 2.55; F (1, 83) = 21.02, p < .0001, η2 = .20. Depression: M Less = 1.56, M More = 3.63; F (1, 83) = 16.73, p < .0001, η2 = .17).
Discussion
Our findings suggest that people with PD participate, to varying degrees, in overarching social practices similar to those documented in healthy aging literature. In our sample, degree of engaging in Health, Activities and Support practices yielded seven profiles that were associated with, but not completely explained by, disease severity. Four different profiles of practices were found among participants with less severe symptoms, while three additional different profiles were found among participants with more severe symptoms. That there are different profiles within each of the Less and More Severe Symptom cluster sets points to the contribution of personal attributes, beyond that of disease severity, playing a role in diversity of social self-management profiles.
The primary difference between profiles of clusters with less severe versus those with more severe symptoms was the degree to which their members engaged in Activities. The more cluster members exercised and ate healthy foods with others, retained previous activities in home and community, and reported ability to manage activity participation, the less severe their disease symptoms and problematic health quality of life. This finding is consistent with emerging evidence about potency of Activities for biopsychosocial health outcomes among aging adults in general [11,23–25,28], and emerging evidence for adults with PD in particular [43–44]. Activities protect and extend activity capacity [42,43], provide opportunities for achieving life goals [15], and support continuity of a sense of purpose and self-identity across the lifespan [14,46]. Within and across the two symptom severity cluster sets, people with PD reported different patterns of Health and Support practices, suggesting that Activities alone, though important for understanding health, are insufficient for describing heterogeneity in social self-management.
Health practice had a robust association with symptom severity, secondary to that of Activities. After considering a cluster’s profile of high, moderate, or low level of engagement in Activities, the more that cluster members collaborated with health care providers, actively self-managed health, and participated in formally organized health events, the less severe their symptoms. In contrast to Activities, and consistent with implications from other studies [24,35], Health did not contribute to the explanation of cluster differences in health quality of life, implying it manages symptoms more than it manages quality of life.
Support practice, while contributing robustly with the other two practices to differentiating clusters of participants, was not robustly associated with health outcomes. It appears to identify person-centered variation not clearly related to health outcomes, which corroborates some findings in older adult and PD literature [24,28,48]. Social support may have complex and multi-factorial associations with health [24–27,48,63]. Our study assessed self-perceived degree of giving and receiving support, and not the degree to which this support was perceived as helpful, warranted, or desired [24,45], which may contribute to predicting health. Nonetheless, it is important to note that clusters whose profiles included high levels of Support were the healthiest clusters in their symptom severity subset. Equal Health-Activities-Support ranked as the healthiest cluster among the less severe clusters, while High Support ranked as the healthiest cluster among the more severe clusters. This finding suggests Support has an additive contribution to health beyond differentiating individual variants of social lifestyle.
Rehabilitation Implications
Our findings contribute a new profile-based typology that makes explicit how centrality and variation in healthy aging social practices describe naturalistic social living with PD. This typology, derived through a series of hierarchical analytical steps, compiled individuals into larger and larger subsets, moving from individually-based self-management attributes into centrality-based evidence about individuals who followed similar self-management practices, and finally, into a higher level of centrality-based evidence by grouping clusters into two different health outcome sets.
Stephen J. Gould’s case provides an example of how a combined centrality-variation perspective can guide personalization of self-management [1]. Gould started his self-management journey with awareness of centrality evidence about typical survival post diagnosis. It provided him a benchmark against which to compare his trajectory. Next, his awareness of variation in survival provided him hope and inspiration to survive beyond the typical period. This awareness guided him toward choosing self-management practices that fit his circumstances. This drilling down to his particulars began with social comparison to similar others. He went on to create feasible health goals, form an action plan, and make reasoned choices; all components of cognitive-behavioral models of health self-management programming [30–31,37–39,79,80].
We suggest three steps for how a rehabilitation provider might drill down from our study’s highest-level centrality evidence to relevant person-centered evidence in order to promote healthy self-management of PD:
Step 1—Assess client symptom severity and health quality of life, then facilitate health awareness through engaging client and family in self-reflecting about client symptom severity and quality of life and comparing their MDS-UPDRS and PDQ scores to others with PD using Table 5. Objective—to promote self-awareness of current health status compared to others with PD and recognize potential for health improvement.
Step 2—Assess client Health-Activities-Support self-management profile (Table 1), then facilitate social self-management awareness through engaging client and family in self-reflecting about client degree of engaging in the three practices (low, average, and high) in comparison to client, family, and peer expectations in their social network. Since few external gold standards exist in PD population literature for measuring low, average or high engagement, the provider uses best practice expertise and reasoning in combination with client and family perceptions. Tables 6 and 7 offer social comparison information for helping clients identify whether they experience less versus more symptom severity relative to study participants, and, and how their own practices compares to those of each cluster symptom set. The cluster profiles demonstrate the variability in how people similar to the client engage in daily social practices. Objective—to promote self-awareness of current social practices compared to others with PD and recognize opportunities for optimizing engagement in healthy social practices.
Step 3— Implement cognitive-behavioral strategies for facilitating client mastery of self-selected healthy practices toward achievement of feasible health goals [22,37,80]. Since retention of Activities is a potent indicator of health outcomes, along with a balanced high engagement in all three self-management practices (as in our healthiest cluster), rehabilitation might begin with the provider and client creating short- and long-term goals and action plans for blending and balancing Activities with Health and Support. For example, the three practices can overlap in the same event, as in Parkinson’s community events (e.g., dancing, singing, fitness, support groups, walks) that engage biopsychosocial capacities all at once. Or practices can occur separately in real-time, scheduled in a proactively balanced manner across day, week, or month. Time-management, habit and skill training are essential for client coordination of medication with practice engagement. Client capacity training includes how to make compensatory adaptations for full participation (e.g. grading activity complexity and effort, selecting adaptive devices, maximizing available resources) [22]. Practicing self-monitoring of goal achievement and how to resolve potential barriers facilitate efficacy and mastery of self-management [79]. Objective—to promote client mastery in accessing opportunities and making choices that maximize fit between (a) personal functional capacities, skills, and life goals (b) the capacity and skill demands of social and physical environments, and (c) the capacity and skill demands of personally valued activities [15,22].
Study Limitations and Future Studies
Findings should be interpreted in accordance with this study’s (a) cross-sectional design, (b) homogeneity of participant demographics, (c) operationalization of social self-management, and (d) cluster group sizes. First, the cross-sectional design is a snapshot of current patterns of participants’ social life practices and health status. Our findings build evidence supporting that life practices offer protection of health; health status contributes to the form of these practices; and practices and health are mutually interactive. Future analyses of the larger study’s three-year longitudinal social and health trajectories will inform causal implications and prioritization of intervention strategies [29].
Second, the sample was relatively homogenous demographically despite recruitment targeting diversity [58]. The study’s seven profiles demonstrate that engagement in the three practices vary considerably even in people who share similar demographic characteristics. Our profile approach serves as an example of how to operationalize person-centered assessment of social self-management that is sensitive to variation in health. This approach should be replicated with samples that represent more demographic diversity [13,63].
Third, the specification and measurement of the relatively novel concept of social self-management is in its infancy, consistent with the healthcare system’s emerging understanding of the biopsychosocial ramifications of the daily lived experience of disabling conditions [17,31,37]. This study focused on actionable and naturalistic healthy aging practices of Health, Activities, and Support because of their documented research and clinical validity, and their utility for cognitive-behavioral approaches to self-management rehabilitation. Conceptual specification and measurement require continued development, especially with respect to Support.
Fourth, the cluster sample sizes (a median of 13) met the standards of statistical power for developing multi-variate cluster profiles [68,69], and resulted in statistically significant and clinically meaningful results. Yet, for continuing validation and development it is important to validate these profiles with larger samples of the population living with PD.
Conclusion
This study contributes to expanding self-management of PD beyond physical management to biopsychosocial self-management that is informed by naturalistic lived experience and personal circumstances. It provides novel evidence that social life is a component or determinant of health for people with PD, just as is social life for their aging peers without neurodegenerative disorders. Gould’s case demonstrates that centrality evidence is insufficient for informing the particulars of individuals, and yet can be used by individuals to become aware of and fine-tune their self-management practices toward placing themselves in the healthy end of their potential health outcomes [1]. An informed use of both centrality and variation evidence supports person-centered rehabilitation. Profiles of social self-management can be used to this end.
Implications for Rehabilitation.
Social self-management is a biopsychosocial construct to identify and describe self-care practices that engage one’s social resources for managing healthful daily living.
People with Parkinson’s disease vary in their profiles of engaging in social self-management practices in daily living, and this variability relates to severity of symptoms and health quality of life.
Learning how to identify health-centered social self-management practices may help people with Parkinson’s disease to focus on the healthfulness of their own practices.
Learning how to strategically engage one’s social resources as part of self-care may help people with Parkinson’s disease to master managing their health and well-being in daily life.
Acknowledgements
Our gratitude to research assistants, staff, and visiting scholars in the Health Quality of Life Lab at Tufts University for invaluable contributions to this study. Research reported here was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number R01NR013522, and the National Center for Advancing Translational Sciences, National Institutes of Health, Award Number UL1TR002544. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- 1.Gould SJ. Full house: the spread of excellence from Plato to Darwin. New York: Three Rivers Press; 1996. [Google Scholar]
- 2.Hammel J, Magasi S, Heinemann A, et al. What does participation mean? an insider perspective from people with disabilities. Disabil Rehabil. 2008;30:1445–1460. [DOI] [PubMed] [Google Scholar]
- 3.Glasziou P, Guyatt GH, Dans AL, et al. Editorial: applying the results of trials and systematic reviews to individual patients. ACP J Club. 1998;129:A15–A16. [PubMed] [Google Scholar]
- 4.Tickle-Degnen L, Bedell G. Heterarchy and hierarchy: a critical appraisal of the “levels of evidence” as a tool for clinical decision making. Am J Occup Ther. 2003;57:234–237. [DOI] [PubMed] [Google Scholar]
- 5.Lowsky DJ, Olshansky SJ, Bhattacharya J, et al. Heterogeneity in healthy aging. J Gerontol A Biol Sci Med Sci. 2014;69(6):640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willis AW, Evanoff BA, Lian M, et al. Geographic and ethnic variation in Parkinson disease: a population-based study of US Medicare beneficiaries. Neuroepidemiology. 2010;34(3):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Mov Disord. 2016;31(8):1095–1102. [DOI] [PubMed] [Google Scholar]
- 8.ICF Browser [internet]. Geneva. World Health Organization; 2001. International Classification of Functioning, Disability and Health [cited 2019 Feb 20]; [about 3 p.]. Available from: http://apps.who.int/classifications/icfbrowser/
- 9.Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. Predictors and course of health-related quality of life in Parkinson’s disease. Mov Disord. 2008;23(10):1420–1427. [DOI] [PubMed] [Google Scholar]
- 10.Karlsen KH, Tandberg E, Årsland D, et al. Health related quality of life in Parkinson’s disease: a prospective longitudinal study. J Neurol Neurosurg Psychiatry. 2000;69(5):584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassan A, Wu SS, Schmidt P, et al. The profile of long-term Parkinson’s disease survivors with 20 years of disease duration and beyond. J Parkinsons Dis. 2015;5(2):313–319. [DOI] [PubMed] [Google Scholar]
- 12.Rajput AH, Uitti RJ, Rajput AH, et al. Timely Levodopa (LD) administration prolongs survival in Parkinson’s disease. Parkinsonism Relat Disord. 1997;3(3):159–165. [DOI] [PubMed] [Google Scholar]
- 13.de Meideros K, Jackson SJ, Perkinson MA. Successful aging. In: Barney K, Perkinson MA, editors. Occupational therapy with aging adults. St. Louis, MO: Elsevier; 2016. p. 300–314. [Google Scholar]
- 14.Habermann B. Continuity challenges of Parkinson’s disease in middle life. J Neurosci Nurs. 1999;31(4):200–207. [DOI] [PubMed] [Google Scholar]
- 15.McNamara P, Durso R, Harris E. Life goals of patients with Parkinson’s disease: a pilot study on correlations with mood and cognitive functions. Clin Rehabil. 2006;20:818–826. [DOI] [PubMed] [Google Scholar]
- 16.McQuillen AD, Licht MH, Licht BG. Contributions of disease severity and perceptions of primary and secondary control to the prediction of psychosocial adjustment to Parkinson’s disease. Health Psychol. 2003;22(5):504–512. [DOI] [PubMed] [Google Scholar]
- 17.Molton IR, Yorkston KM, Growing older with physical disability: a special application of the successful aging paradigm. J Gerontol B Psychol Sci Soc Sci. 2017;72(2):290–299. [DOI] [PubMed] [Google Scholar]
- 18.Gunnery SD, Habermann B, Saint-Hilaire M, et al. The relationship between the experience of hypomimia and social wellbeing in people with Parkinson’s disease and their care partners. J Parkinsons Dis. 2016;6:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemmesch AR. The detrimental effects of atypical nonverbal behavior on older adults’ first impressions of individuals with Parkinson’s disease. Psychol Aging. 2014;29(3):521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma HI, Saint-Hilaire M., Thomas CA, et al. Stigma as a key determinant of health-related quality of life in Parkinson’s disease. Qual Life Res. 2016;25:3037–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tickle-Degnen L, Zebrowitz LA, Ma H. Culture, gender, and health care stigma: practitioner’s response to facial masking experienced by people with Parkinson’s disease. Soc Sci Med. 2011;73(1):95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tickle-Degnen L, Ellis T, Saint-Hilaire M, et al. Self-management rehabilitation and health-related quality of life in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2010;25(2):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buchman AS, Boyle PA, Wilson RS, et al. Association between late-life social activities and motor decline in older adults. Arch Intern Med. 2009;169(12):1139–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen S. Social relationships and health. Am Psychol. 2004;59(8):676–684. [DOI] [PubMed] [Google Scholar]
- 25.Everard KM, Lach HW, Fisher EB, et al. Relationship of activity and social support to the functional health of older adults. J Gerontol B Sci Soc Sci. 2000;55(4):S208–S212. [DOI] [PubMed] [Google Scholar]
- 26.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7(7):1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinquart M, Sörensen S. Influences of socioeconomic status, social network, and competence on subjective well-being in later life: a meta-analysis. Psychol Aging. 2000;15(2):187–224. [DOI] [PubMed] [Google Scholar]
- 28.Thoits PA. Mechanisms linking social ties and support to physical and mental health. J Health Soc Behav. 2011;52(2):145–161. [DOI] [PubMed] [Google Scholar]
- 29.Tickle-Degnen L, Saint-Hilaire M, Thomas CA, et al. Emergence and evolution of social self-management of Parkinson’s disease: study protocol for a 3-year prospective cohort study. BMC Neurol. 2014;14(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Latkin CA, Knowlton AR. Social network assessments and interventions for health behavior change: a critical review. Behav Med. 2015;41(3):90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wade DT, Halligan PW. The biopsychosocial model of illness: a model whose time has come. Clin Rehabil. 2017;31(8):995–1004. [DOI] [PubMed] [Google Scholar]
- 32.Von Korff M, Gruman J, Schaefer J, et al. Collaborative management of chronic illness. Ann Int Med. 1997;127(12):1097–1102. [DOI] [PubMed] [Google Scholar]
- 33.Glasgow RE, Strycker LA, Toobert DJ, et al. The Chronic Illness Resources Survey: a social-ecologic approach to assessing support for disease self-management. J Behav Med. 2000;23(6):559–583. [DOI] [PubMed] [Google Scholar]
- 34.Martin DJ, Garske JP, Davis MK. Relation of the therapeutic alliance with outcome and other variables: a meta-analytic review. J Consult Clin Psychol. 2000;68(3):438. [PubMed] [Google Scholar]
- 35.Hughes S, Lewis S, Willis K, et al. The experience of facilitators and participants of long term condition self-management group programmes: a qualitative synthesis. Patient Educ Couns. 2017;100(12):2244–2254. [DOI] [PubMed] [Google Scholar]
- 36.Hellqvist C, Dizdar N, Hagell P, et al. Improving self-management for persons with Parkinson’s disease through education focusing on management of daily life: patients’ and relatives’ experience of the Swedish National Parkinson School. J Clin Nurs. 2018;27(19–20):3719–3728. [DOI] [PubMed] [Google Scholar]
- 37.Audulv Å, Ghahari S, Kephart G, et al. The taxonomy of everyday self-management strategies (TEDSS): a framework derived from the literature and refined using empirical data. Patient Educ Couns. 2019;102(2):367–375. [DOI] [PubMed] [Google Scholar]
- 38.Pappa K, Doty T, Taff SD, et al. Self-management program participation and social support in Parkinson’s disease: mixed methods evaluation. Phys Occup Ther Geriatr. 2017;35(2):81–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levasseur M, Richard L, Gauvin L, et al. Inventory and analysis of definitions of social participation found in the aging literature: proposed taxonomy of social activities. Soc Sci Med. 2010;71(12):2141–2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson G. ‘Signposts on the journey’: medication adherence and the lived body in men with Parkinson’s disease. Soc Sci Med. 2016;152:27–34. [DOI] [PubMed] [Google Scholar]
- 41.Devins GM, Styra R, O’Connor P, et al. Psychosocial impact of illness intrusiveness moderated by age in multiple sclerosis. Psychol Health Med. 1996;1(2):179–191. [Google Scholar]
- 42.Foster ER, Golden L, Duncan RP, et al. Community-based Argentine tango dance program is associated with increased activity participation among individuals with Parkinson’s disease. Arch Phys Med Rehabil. 2013;94(2):240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabari JS, Ortiz D, Pallatto K, et al. Activity engagement and health quality of life in people with Parkinson’s disease. Disabil Rehabil. 2015;37:1411–1415. [DOI] [PubMed] [Google Scholar]
- 44.Duncan RP, Earhart GM. Measuring participation in individuals with Parkinson disease: relationships with disease severity, quality of life, and mobility. Disabil Rehabil. 2011;33:1440–1446. [DOI] [PubMed] [Google Scholar]
- 45.Mars GM, Kempen GI, Mesters I, et al. Characteristics of social participation as defined by older adults with a chronic physical illness. Disabil Rehabil. 2008;30(17):1298–1308. [DOI] [PubMed] [Google Scholar]
- 46.Boyle PA, Buchman AS, Bennett DA. Purpose in life is associated with a reduced risk of incident disability among community-dwelling older persons. Am J Geriatr Psychiatry. 2012;18(12):1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Latham K, Clarke PJ, Pavela G. Social relationships, gender, and recovery from mobility limitation among older Americans. J Gerontol B Psychol Sci Soc Sci. 2015;70(5):769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreurs KM, De Ridder DT, Bensing JM. A one year study of coping, social support and quality of life in Parkinson’s disease. Psychol Health. 2000;15(1):109–121. [Google Scholar]
- 49.Berger S, Chen T, Eldridge J, et al. The self-management balancing act of spousal care partners in the case of Parkinson’s disease. Disabil Rehabil. Epub 2017. December 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gunnery SD, Naumova EN, Saint-Hilaire M, et al. Mapping spontaneous facial expression in people with Parkinson’s disease: a multiple case study design. Cogent Psychol. Epub 2017. September 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma HI, Gunnery SD, Stevenson MT, et al. Experienced facial masking indirectly compromises quality of life through stigmatization of women and men with Parkinson’s disease. Stigma Health. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Everitt BS, Landau S, Leese M, et al. Cluster Analysis. 5th ed. Chichester, West Sussex, U.K.: John Wiley & Sons, Ltd; 2011. [Google Scholar]
- 53.Hair JF, Black WC. Cluster analysis. In: Grimm LG, Yarnold PR, editors. Reading and understanding more multivariate statistics. Washington, D.C.: American Psychological Association; 2000. p. 147–205. [Google Scholar]
- 54.Chan RCK, Lee PWH, Lieh-Mak F. The pattern of coping in persons with spinal cord injuries. Disabil Rehabil. 2000;22(11):501–507. [DOI] [PubMed] [Google Scholar]
- 55.LaBelle DR, Walsh RR, Banks SJ. Latent cognitive phenotypes in de novo Parkinson’s disease: a person-centered approach. J Int Neuropsychol Soc. 2017;23(7):551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sjöquist ES, Almqvist L, Åsenlöf P, et al. Physical-activity coaching and health status in rheumatoid arthritis: a person-oriented approach. Disabil Rehabil. 2012;32(10):816–825. [DOI] [PubMed] [Google Scholar]
- 57.Van Balkom TD, Vriend C, Berendse HW, et al. Profiling cognitive and neuropsychiatric heterogeneity in Parkinson’s disease. Parkinsonism Relat Disord. 2016;28:130–136. [DOI] [PubMed] [Google Scholar]
- 58.Sprague-Martinez L, Thomas CA, Saint-Hilaire M, et al. Using a macro social work strategy to improve outreach in Parkinson’s disease research. Soc Work. 2018;63:265–268. [DOI] [PubMed] [Google Scholar]
- 59.Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations the Movement Disorder Society Task Force on rating scales for Parkinson’s disease. Mov Disord. 2004;19(9):1020–1028. [DOI] [PubMed] [Google Scholar]
- 60.Sheikh J, Yesavage J. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, editor. Clinical gerontology: a guide to assessment and intervention. New York: Haworth Press;1986. p. 165–173. [Google Scholar]
- 61.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 62.Baum CM, Edwards D. Activity Card Sort. 2nd ed. Bethesda, MD: AOTA Press; 2008. [Google Scholar]
- 63.Litwin H. Social networks and well-being: a comparison of older people in Mediterranean and non-Mediterranean countries. J Gerontol B Psychol Sci Soc Sci. 2009;65B(5):599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen S, Underwood LG, Gottlieb BH. Social support measurement and intervention: a guide for health and social scientists. New York: Oxford University Press; 2000. [Google Scholar]
- 65.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15): 2129–2170. [DOI] [PubMed] [Google Scholar]
- 66.Peto V, Jenkinson C, Fitzpatrick R, et al. The development and validation of a short measure of functioning and well being for individuals with Parkinson’s disease. Qual Life Res. 1995;4(3):241–248. [DOI] [PubMed] [Google Scholar]
- 67.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Field A. Discovering Statistics Using IBM SPSS Statistics. 4th ed. London: Sage; 2013. [Google Scholar]
- 69.Tabachnick BG, Fidell LS. Using multivariate statistics. 6th ed. Boston, MA: Pearson; 2013. [Google Scholar]
- 70.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Mahwah, NJ: Erlbaum; 1988. [Google Scholar]
- 71.Watson P. Rules of thumb on magnitudes of effect sizes [internet]. University of Cambridge: MRC Cognition and Brain Sciences Unit; 2018. [cited 2019 Feb 20]. Available from: http://imaging.mrc-cbu.cam.ac.uk/statswiki/FAQ/effectSize. [Google Scholar]
- 72.Makkos A, Kovács M, Aschermann Z, et al. Are the MDS-UPDRS–based composite scores clinically applicable? Mov Disord. 2018;33(5):835–839. [DOI] [PubMed] [Google Scholar]
- 73.Horváth K, Aschermann Z, Kovács M, et al. Changes in quality of life in Parkinson’s disease: how large must they be to be relevant? Neuroepidemiology. 2017;48:1–8. [DOI] [PubMed] [Google Scholar]
- 74.Branson CO, Ferree A, Hohler AD, et al. Racial disparities in Parkinson disease: a systematic review of the literature. Advances in Parkinson’s Disease. 2016;5:87–96. [Google Scholar]
- 75.Income in the past 12 months (in 2015 inflation-adjusted dollars), 2011–2015 American Community Survey 5-year estimates [Internet]. Suitland (MD): United States Census Bureau. 2015. [cited 2019 Feb 20]. Available from: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=CF [Google Scholar]
- 76.Weintraub D, Oehlberg KA, Katz IR, et al. Test characteristics of the 15-item geriatric depression scale and Hamilton depression rating scale in Parkinson disease. Am J Geriatr Psychiatry. 2006;14:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nazem S, Siderowf AD, Duda JE, et al. Montreal Cognitive Assessment performance in patients with Parkinson’s disease with “normal” global cognitive according to Mini-Mental State Examination score. J Am Geriatr Soc. 2009;57(2):304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care. 2003;4(5):582–592. [DOI] [PubMed] [Google Scholar]
- 79.Bandura A. Self-efficacy: the exercise of control. New York: WH Freeman; 1997. [Google Scholar]
- 80.Michie S, Ashford S, Sniehotta FF, et al. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALORE taxonomy. Psychol Health. 2011:2v6(11):1479–1498. [DOI] [PubMed] [Google Scholar]