Abstract
Objective:
Necrotizing enterocolitis (NEC) develops through exaggerated toll-like receptor 4 (TLR4) signaling in the intestinal epithelium. Breast milk is rich in non-digestible oligosaccharides and prevents NEC through unclear mechanisms. We now hypothesize that the human milk oligosaccharides 2’-Fucosyllactose (2’-FL) and 6’-Sialyllactose (6’-SL), can reduce NEC through inhibition of TLR4 signaling.
Design:
NEC was induced in newborn mice and premature piglets and infant formula was supplemented with 2’-FL, 6’-SL or lactose. Intestinal tissue was obtained at surgical resection. HMO inhibition of TLR4 was assessed in IEC-6 enterocytes, mice, human tissue explants, and via in silico modeling.
Results:
Supplementation of infant formula with either 2’-FL and/or 6’-SL, but not the parent sugar lactose, reduced NEC in mice and piglets via reduced apoptosis, inflammation, weight loss, and histological appearance. Mechanistically, both 2’-FL and 6’-SL, but not lactose, reduced TLR4-mediated nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) inflammatory signaling in the mouse and human intestine. Strikingly, in silico modeling revealed 2’-FL and 6’-SL, but not lactose, to dock into the binding pocket of the TLR4-MD2 complex, explaining their ability to inhibit TLR4 signaling.
Conclusion:
2’-FL and 6’-SL, but not lactose, prevent NEC in mice and piglet models, and attenuate NEC-inflammation in human ileum, in part through TLR4 inhibition.
INTRODUCTION
Necrotizing enterocolitis (NEC) is the leading cause of death from gastrointestinal disease in premature infants, and is characterized by sudden necrosis of the small intestine, leading to overwhelming sepsis and death in many cases (1). The overall survival for patients with NEC has not improved since the disease was first described (2), and with the steady rise in the overall number of premature births worldwide (3), there exists a greater urgency than ever to understand its origins, and to develop novel prevention strategies. We (4–6) and others (7) have identified a molecular explanation for NEC development, in which NEC results from exaggerated signaling in the intestinal epithelium in response to activation of the lipopolysaccharide receptor toll-like receptor 4 (TLR4), which is higher in the premature as compared with the full-term intestine (8). TLR4 activation by luminal LPS leads to a disruption of the intestinal mucosa (9), resulting in bacterial translocation into the blood stream (10), where the activation of TLR4 on the endothelium results in vasoconstriction and the intestinal ischemia that characterizes NEC (11). Given the lack of specific treatments for NEC, the focus has shifted to prevention strategies, especially in those infants at greatest risk for disease development.
Of all the strategies that are associated with NEC protection in infants, the administration of breast milk is decidedly the most effective. In seeking to understand the molecular components of breast milk that could be responsible for its protective benefits, we (12) and others (13,14) have shown that human milk oligosaccharides (HMOs), a family of non-digestible carbohydrates present in breast milk, can prevent NEC in experimental models, through mechanisms that are unknown. Moreover, there remains controversy regarding the role of HMOs early in life, as previous authors have shown that certain HMOs may not prevent NEC in piglets early in life (13). We now hypothesize that HMOs prevent NEC in part through direct inhibition of TLR4 signaling in the premature intestine, which we seek to test using mouse, piglet and human tissue models of this disease.
METHODS
Chemical reagents
RNeasy® kit (catalogue no. 74106; Qiagen) and QuantiTect® Reverse Transcription (catalogue no. 205313; Qiagen), TUNEL kit (In Situ Cell Death Detection Kit, Fluorescein, Catalogue no. 11684795910; Roche), 3’-Nitrotyrosine antibody (Catalogue no. ab61392; Abcam), NF-kBp65(F-6) antibody (catalogue no. sc-8008; Santa Cruz Biotechnology), 4’,6-diamidino-2-phenylindole, dihydrochloride (DAPI, catalogue no. D9542; Sigma), LPS, Lipopolysaccharide from Escherichia coli 0127:B8 (Sigma catalogue no. L3129). Forward and reverse primers were custom designed using NCBI Primer-BLAST online program and ordered from Integrated DNA Technologies, and appear in Table. 1 The small intestinal epithelial cell IEC-6 was obtained from American Type Culture Collection (ATCC, Manassas, VA). TUNEL was from Roche Life sciences. Quantitative RT-PCR was performed using primers in Table. 1 as described(15). All human intestine was obtained via a waiver of consent from the Office of Human Subjects Research, Johns Hopkins University (IRB00094036) and was collected in a deidentified manner. GOS (galacto-oligosaccharides) was from Biosynth Carbosynth (Newbury, Berkshire, UK).
Table 1.
Primer sequences
| Gene | Species | Forward sequence | Reverse sequence | Amplicon (bp) |
|---|---|---|---|---|
| IL-1β | Mouse | AGTGTGGATCCCAAGCAATACCCA | TGTCCTGACCACTGTTGTTTCCCA | 175 |
| Pig | CCGTCTTCCTGGGAAACTCC | GTCAGCTTCGGGGTTCTTCA | 121 | |
| iNOS | Mouse Rat | CTGCTGGTGGTGACAAGCACATTT | ATGTCATGAGCAAAGGCGCAGAAC | 167 |
| TLR4 | Human | AAGCCGAAAGGTGATTGTTG | CTGAGCAGGGTCTTCTCCAC | 153 |
| Mouse | TTTATTCAGAGCCGTTGGTG | CAGAGGATTGTCCTCCCATT | 186 | |
| Pig | TGCGTCAGTTCTCACCTTCC | TCTTCGTCCTGGCTGGAGTA | 153 | |
| Rat | ACTGGGTGAGAAACGAGCTGGTA | AAGCCTTCCTGGATGATGTTGGCA | 124 | |
| TNF-α | Mouse | TTCCGAATTCACTGGAGCCTCGAA | TGCACCTCAGGGAAGAATCTGGAA | 144 |
| Human | GGCGTGGAGCTGAGAGATAAC | GGTGTGGGTGAGGAGCACAT | 120 | |
| Pig | GCCCTTCCACCAACGTTTTC | TCTGGCAAGGGCTCTTGATG | 102 | |
| Rplp0 | Human | GGCGACCTGGAAGTCCAACT | CCATCAGCACCACAGCCTTC | 143 |
Animal studies.
All experiments involving mice were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and are approved by Animal Care and Use Committee of The Johns Hopkins University (Protocol numbers MO17M304 and SW18M206), according to The ARRIVE Guidelines of the ‘NC3R’(16). Experimental NEC was induced in 7–8 day old (approximately 3g body weight) neonatal mice pups as previously described and validated (17). NEC was induced after the oral gavage of formula which is described in our recent paper (18), and is composed of Similac Advance: Esbilac Canine Milk Replacer in equal concentrations, supplemented with bacterial stock that had been cultured from the stool of an infant with severe NEC (12.5μl of stool slurry in 1ml of formula). Neonatal pups were gavage-fed (50μl/g) five times per day with formula supplemented with bacterial using a 24-French angiocatheter placed into the mouse esophagus. Mice were exposed to hypoxia (5% O2, 95% N2) for 10 min in a chamber (Billups-Rothenberg Inc.), at 7AM and 1PM, immediately after feed for 4 days. NEC formula was supplemented with either 2’-FL, 6’-SL, or lactose or GOS where indicated. In parallel, 3-week old C57/BL6 mice were randomly divided into five groups, i) saline-control, ii) LPS, iii) LPS+2’-FL, iv) LPS+6’-SL, v) LPS+2’-FL+6’-SL, vi) LPS+lactose. Where indicated, HMO, GOS or lactose was administered orally at 10mg/kg in 200uL/mice) 1h before injection of LPS (5mg/kg, intraperitonially). All mice were harvested 6h later and samples of the small intestine were harvested.
NEC was induced in piglets to determine the effects 2’-FL and 6’-SL as we have described(19). Timed-pregnant White Yorkshire (Yorkshire × Landrace) sows were obtained from Oak Hill Genetics (Ewing, IL), and piglets were delivered prematurely via cesarean section at ~90% gestation, and administered either 2’-FL or 6’-SL (10mg/ml, each individually or 5mg/ml in combination). Piglets that were not induced to develop NEC were euthanized at birth and used as controls.
Evaluation of molecular docking of 2’-FL, 6’-SL and lactose (LAT) with TLR4-MD2.
Molecular docking was used to analyze the binding efficiency of 2’-FL, 6’-SL, and a negative standard, lactose (LAT), to the LPS binding pocket of the TLR4-MD2 complex. Spartan 18 (Wavefunction, Inc., Irvine CA) was used to remove the antagonist Eritoran from PDB Code 2Z65, the crystal structure of the ternary eritoran-TLR4-MD2 complex(20) and to prepare the resulting TLR4-MD2 apoprotein complex for molecular docking. Biologically relevant conformations of 2’-FL and 6’SL, extracted from PDB Code 5DUX(21) and 4EN9(22), respectively, were then used for the docking experiment to TLR4-MD2. Autodock Vina™ combined with PyRx™ yielded optimized docked complexes with docking scores of −5.6 kcal/mol for 2’-FL, and − 6.7 kcal/mol for 6’-SL. In contrast, docking of the negative control LAT provided a lower docking score of −4.8 kcal/mol. Following the standard docking procedure, ligands were docked by defining a grid box of 20 Å~ 20 Å~ 20 Å around the cup-sized LPS recognition pocket of the TLR4-MD2 complex. PyMOL™ (The PyMOL Molecular Graphics System, Version 2.3.1 Schrödinger, LLC) was used for visualizations.
RESULTS
Supplementation of infant formula with 2’-FL and 6’-SL prevents NEC in mice in a dose-dependent manner.
We first sought to evaluate whether the most abundant neutral HMO, namely 2’-FL, and the most abundant acidic HMO, namely 6’-SL could prevent NEC, either alone or in combination, in well-established models of NEC. As shown in Fig. 1, the intestine of mice that were induced to develop NEC show extensive edema, thickening and air within the bowel wall (also called pneumatosis intestinalis, a signature finding in human NEC), and which is not seen in dam-fed control mice, where the intestine is pink and healthy appearing (Fig. 1ai–ii). Histological examination of NEC intestine revealed extensive villous sloughing, separation of submucosa, and edema in the submucosal and muscular layers (Fig. 1bi–ii) NEC induction also resulted in significant inflammation in the intestinal mucosa, as indicated by expression of the pro-inflammatory cytokine TNF-α and high NEC severity score as assessed using our well validated scoring system(23), in comparison to breast fed controls, in whom proinflammatory cytokine induction and disease severity scores were both minimal (Fig. 1e–f). Since both 2’-FL and 6’-SL are derived from the milk carbohydrate lactose, we evaluated lactose as a negative control, given that it is an oligosaccharide that can be enzymatically digested, unlike the HMOs 2’-FL and 6’-SL, and because it serves as the molecular backbone of these HMOs (24). As shown in Fig. 1, the administration of infant formula that was supplemented with lactose resulted in no protective benefit against NEC, as measured by gross appearance of the intestine (Fig. 1aiii), intestinal histology (Fig. biii), cytokine induction (Fig. 1e), NEC severity (Fig. 1f) body weight (Fig. 1g). By contrast, supplementation of the infant formula with either 2’-FL or 6’-SL significantly protected against NEC development, as manifest by improved gross appearance of the intestine (Fig. 1aiv, ci), intestinal histology (Fig. 1biv, di), cytokine induction (Fig. 1e), NEC severity (Fig. 1f) and body weight (Fig. 1g). The combination of 2’-FL and 6’-SL together, and at the same total HMO dosage as either carbohydrate alone yielded no additional protection (Fig. 1e, f). It is noteworthy that lower doses of either 2’-FL or 6’-SL had no effect on NEC severity, especially at the shorter time points (Supplemental Fig. s1).
Fig. 1.
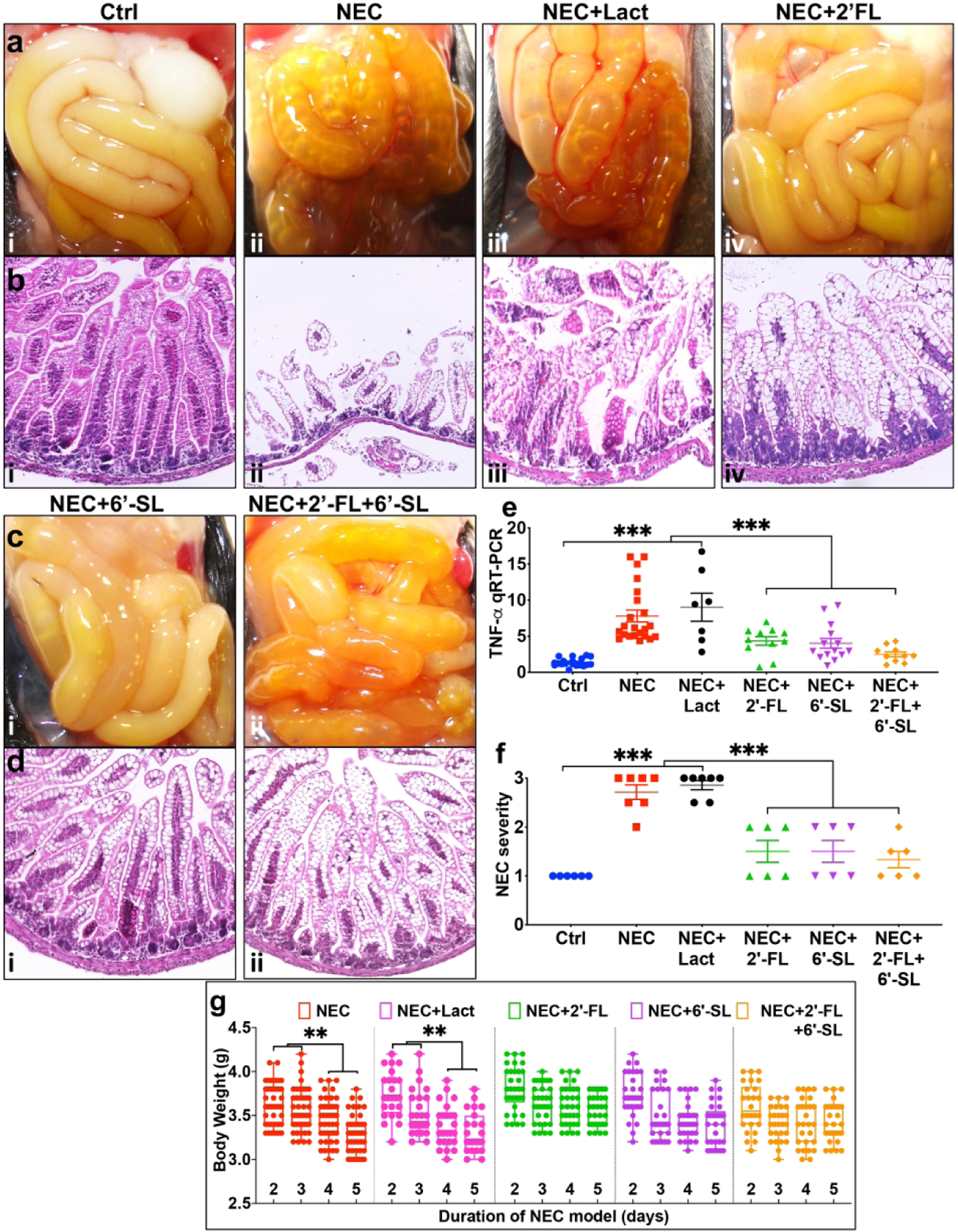
Supplementation of nutritional formula with 2’-FL and 6’-SL protects from disease development in neonatal mice subjected to an experimental model of necrotizing enterocolitis (NEC). ai-iv, ci-ii photomicrographs showing gross morphology, bi-iv, di-ii Haematoxylin-eosin (H&E)-stained images showing histology of small intestine (ileum), e quantitative real-time PCR (qRT-PCR) of pro-inflammatory cytokine tumor necrosis factor-alpha (TNF-α), f NEC severity scores, g nested graph showing body weight changes, in neonatal mice subjected to no treatment (Ctrl, control–breast-fed) or experimental NEC treatments without or with supplementation with lactose (10mg/ml), 2’-FL (10mg/ml/), 6’-SL (10mg/ml), or 2’-FL+6’-SL (5mg/ml, each). **p<0·01, *** p<0·001, each dot in dot-graphs represents data from an individual mouse.
To further evaluate the extent to which 2’-FL and 6’-SL could prevent NEC, two additional measurements which are integral into the pathogenesis of NEC were undertaken: reactive oxygen species-mediated mucosal damage, as revealed by the expression 3’-nitrotyrosine (3’-NT) in the intestinal mucosa (25), and enterocyte apoptosis, as measured by TUNEL staining. As shown in Fig. 2, 3’-NT and TUNEL staining were both strongly expressed within the intestinal epithelium of mice with NEC (Fig. 2aii-ii’, bii-ii’), while breast fed-control mice showed minimal expression (Fig. 2ai-i’, bi-i’), consistent with our prior reports (23,26). Importantly, both 3’-NT and TUNEL staining in the intestinal mucosa were significantly reduced in mice with NEC in which the infant formula was supplemented with either 2’-FL or 6’-SL (Fig. 2aiii–iv, aiii’-iv’, biii–iv, biii’-iv’), or their combination (Fig. 2av-v’, bv-v’), see quantification in Fig. 2 c–d. Taken together, these findings illustrate that the administration of 2’-FL and 6’-SL, alone or in combination, protect against NEC in a mouse model. To evaluate the potential translational significance of these findings, we next evaluated a well-validated piglet model of the disease(19).
Fig. 2.
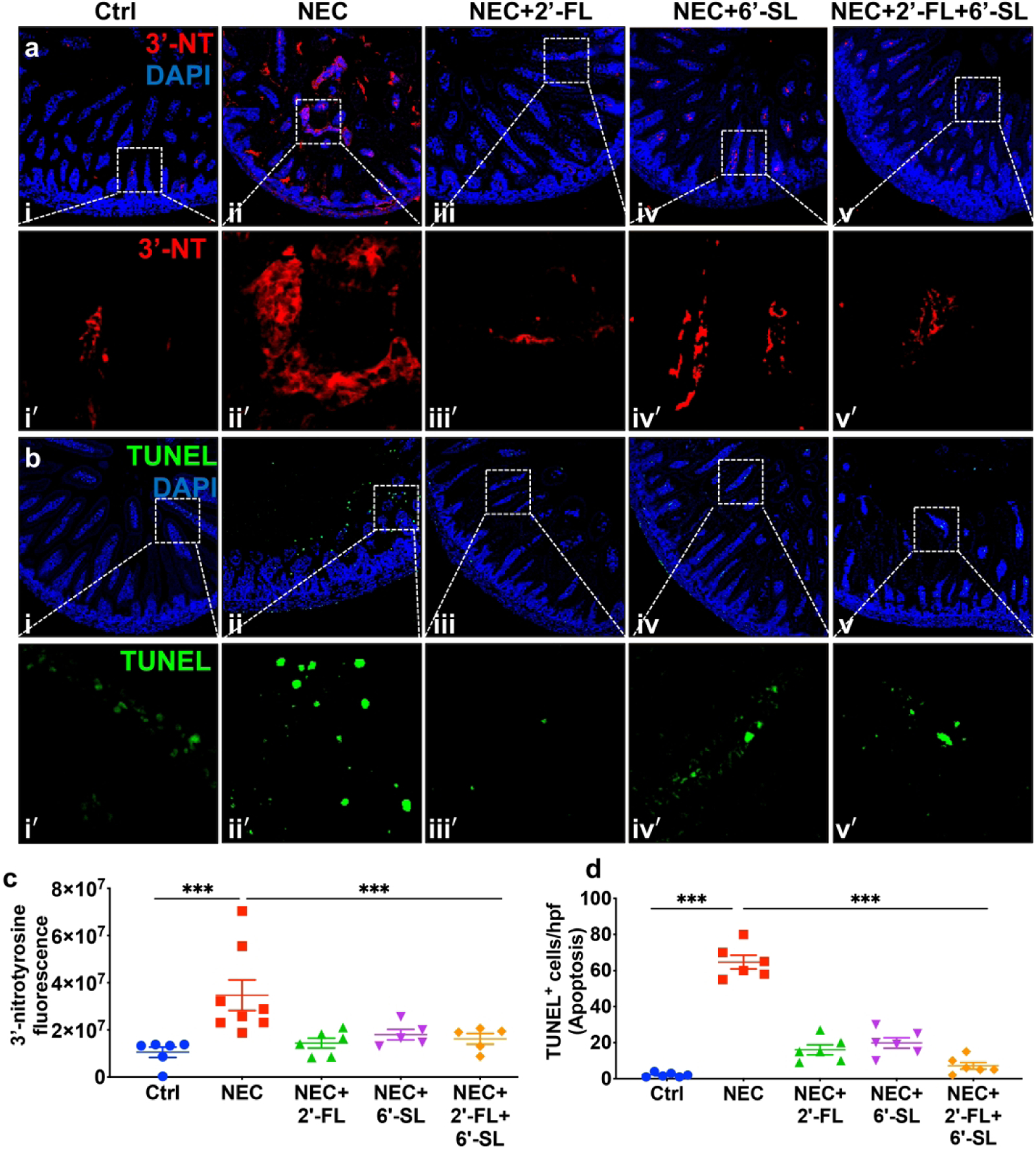
2’-FL and 6’-SL supplementations in nutritional formula prevents small intestinal mucosal injury in mice induced to develop necrotizing enterocolitis (NEC). ai-v, i’-v’ immunofluorescence images of 3’-Nitrotryosine (3’-NT, green fluorescence) as an indicator of oxidative injury, bi-v, i’-v’ immunofluorescence images of TUNEL (green fluorescence) as an indicator of apoptosis, in ileal sections of neonatal mice subjected to no treatment (Ctrl, control–breast-fed) or experimental NEC without or with supplementation with 2’-FL (10mg/ml/), 6’-SL (10mg/ml), or 2’-FL+6’-SL (5mg/ml, each), c quantification of fluorescent intensity of 3’-NT staining, d quantification of fluorescent intensity of TUNEL staining, measured using ImageJ software, *** P<0·001. Each dot in dot-graphs represents data from an individual mouse.
Supplementation of nutritional formula with 2’-FL and 6’-SL protects against NEC development in a piglet model of NEC.
As compared to piglets administered control formula without the addition of HMOs, the supplementation of formula with either 2’-FL (10 mg/kg) or 6’-SL (10mg/kg) or their combination at 10mg/kg each, significantly reduced NEC severity (Fig. 3), as revealed by improved gross morphological appearance (Fig. 3a), intact histological architecture (Fig. 3b), normal levels of pro-inflammatory cytokines TNF-α and IL-1 (Fig. 3c), and significantly lower average NEC scores (Fig. 3d). Moreover, while 3’-NT expression and mucosal epithelial apoptosis were both abundant in the intestinal mucosa of piglets with NEC (Fig. 3e–f), these findings were both significantly reduced to the levels of expression in the control piglets (Fig. 3ei-i’, fi-i’), in NEC piglets supplemented with 2’-FL alone (Fig. 3eiii-iii’, fiii-iii’), 6’-SL alone (Fig. 3eiv-iv’, fiv-iv’), or both HMOs combined (Fig. 3ev-v’, Fv-v’, fluorescence quantification of 3’-NT and TUNEL staining is shown in Fig. 3g and h). Taken together, these findings illustrate that 2’-FL and 6’-SL protect against NEC development in both small and large animal models. We next sought to investigate the mechanisms involved, and so next determined whether these HMOs could inhibit TLR4 signaling.
Fig. 3.
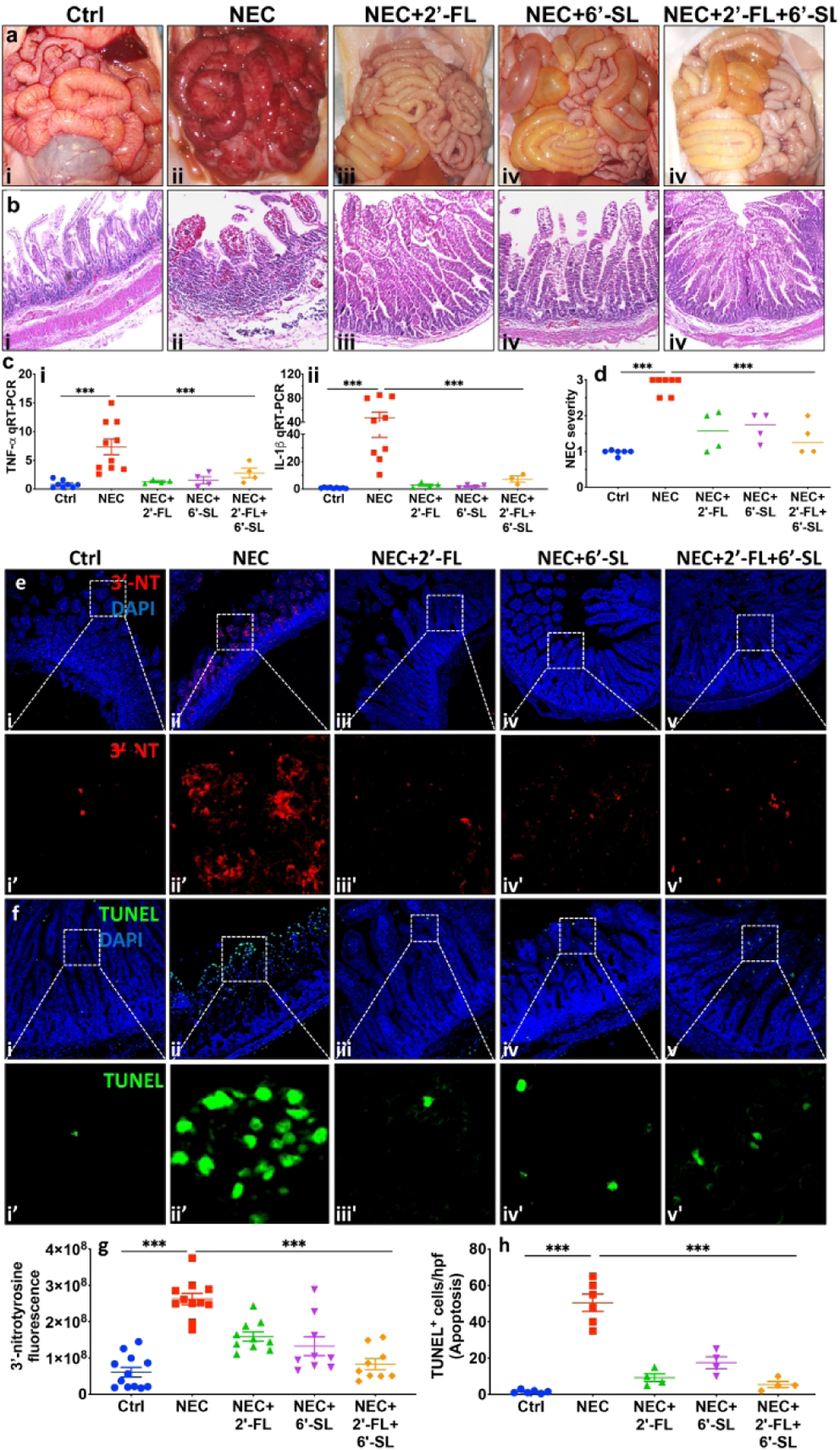
Supplementation of nutritional formula with 2’-FL and 6’-SL protects from disease development in a piglet model of necrotizing enterocolitis (NEC). ai-v representative photomicrographs of gross morphology, bi–v Hematoxylin–eosin (H&E)-stained images of premature piglets that were either untreated and euthanized at birth (controls) or induced to develop experimental NEC in the absence or presence of 2’-FL, 6’-SL or 2’-FL+6’-SL, 5μm paraffin imbedded sections of small intestine (ileum), ci-ii quantitative real-time PCR (qRT-PCR) of pro-inflammatory cytokines Tumor necrosis factor-alpha (TNF-α) and Interleukin-beta (IL-1β), d NEC severity scores. *** P<0·001, each dot in dot-graphs represents data from an individual piglet. e-h: 2’-FL) and 6’-SL supplementation prevents small intestinal mucosal damage in a piglet model of necrotizing enterocolitis (NEC). ei- v, i’-v’ immunofluorescence images of 3’-Nitrotryosine (3’-NT, green fluorescence) as an indicator of oxidative mucosal injury, fi- v, i’-v’ immunofluorescence images of TUNEL (green fluorescence) as an indicator of apoptosis injury, in small intestine (ileum) sections of premature piglets subjected to no treatment (Ctrl, control–breast-fed) or experimental NEC treatments without or with supplementation with 2’-FL (10mg/ml/), 6’-SL (10mg/ml), or 2’-FL+6’-SL (5mg/ml, each), g quantification of fluorescent intensity of 3’-NT staining, h quantification of fluorescent intensity of TUNEL staining, measured using ImageJ software, *** P<0·001. Each dot in dot-graphs represents data from an individual piglet.
2’-FL) and 6’-SL inhibit TLR4 signaling in IEC-6 enterocytes.
Given the critical importance of TLR signaling in the pathogenesis of NEC (4,5,8), we next sought to determine whether 2’-FL and 6’-SL could protect against NEC development through TLR4 inhibition. To determine whether 2’-FL or 6’-SL could block TLR4 signaling, we first measured the effects of these HMOs on LPS-induced NF-kB translocation from the cytoplasm into the nucleus (Fig. 4) and on TLR4-induced enterocyte apoptosis, pathways which are central to the mucosal inflammation that leads to NEC induction. These studies were performed using the model cell line IEC-6, which is a non-transformed crypt cell line that expresses TLR4 abundantly and responds to LPS(27), and thus allows for the direct study of HMO-TLR4 interactions without the potential influence of circulating factors. As shown in Fig. 4, as compared with control cells in which the p65 subunit of NFkB is localized in the cytoplasm (Fig. 4ai-i’), LPS induced its translocation to the nucleus (Fig. 4aii-ii’, quantification in Fig. 4b). Although pre-treatment with lactose had no effect on the degree of LPS-induced NF-kB translocation (Fig. 4aiii-iii’, b), treatment of IEC with 2’-FL or 6’-SL, either alone or in combination significantly blocked LPS-induced NF-kB signaling (Fig. 4aiv–vi, iv’–vi’, b). In parallel, while LPS induced significant apoptosis (Fig. 4cii-ii’, d) of IEC-6 cells, the degree of apoptosis was not reduced by lactose (Fig. 4ciii-iii’, d), but was markedly reduced by both 2’-FL and 6’-SL, either alone or in combination (Fig. 4civ–vi, iv’–vi’). Taken in aggregate, these findings illustrate that 2’-FL and 6’-SL prevent TLR4 signaling in vitro, providing a mechanism by which they reduce NEC severity. We next sought to determine the impact on TLR4 signaling in human and mouse explants.
Fig. 4.
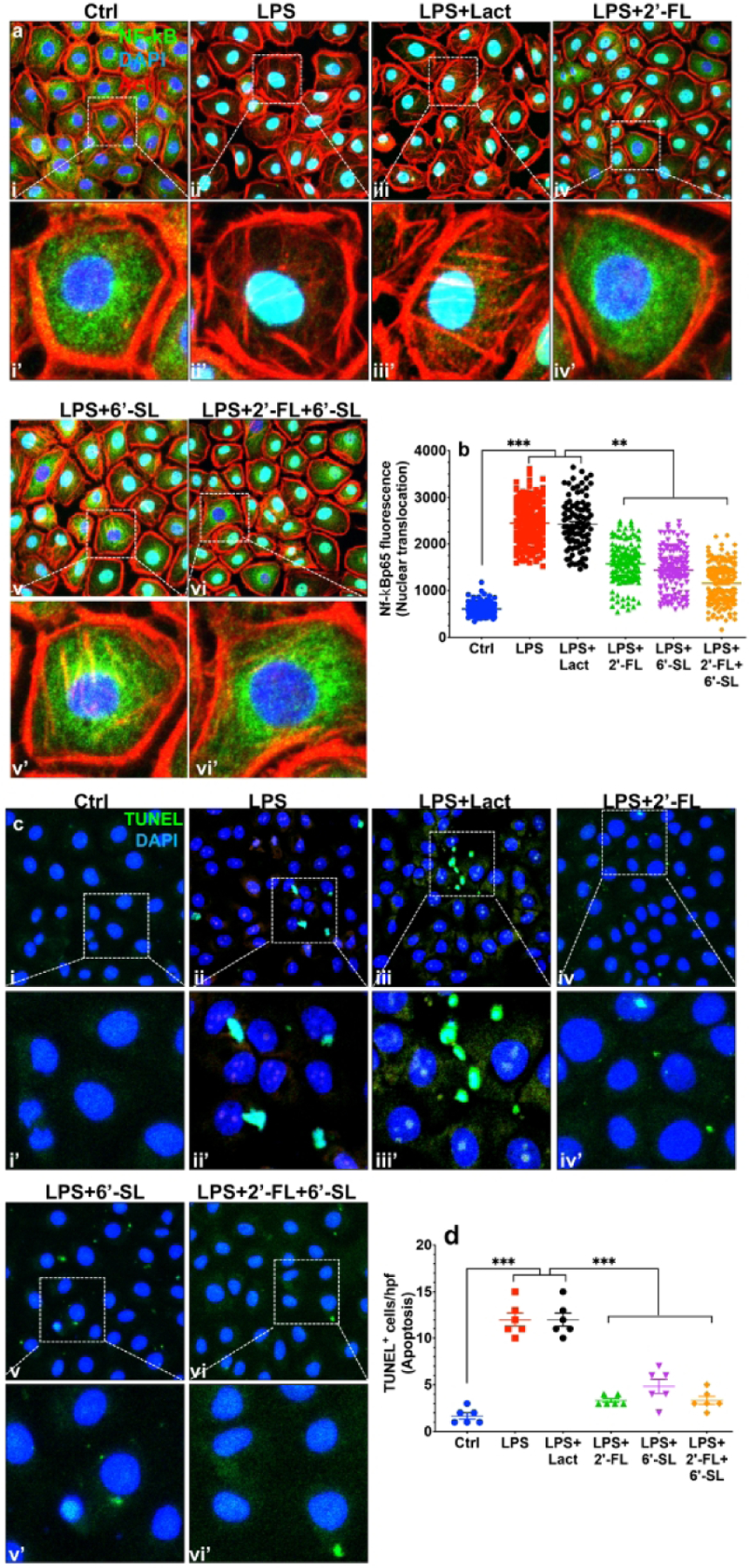
2’-FL and 6’-SL but not lactose prevents LPS induced NF-kB translocation in cultured intestinal epithelial (IEC6) cells. ai-vi, ai’-vi’ photomicrographs of IEC6 cells immunostained for NF-kB p65, b quantification of fluorescence intensity measured using ImageJ, *** P<0·001. Each dot in dot-graphs represents data from an individual cell. C–d: 2’-FL and 6’-SL but not lactose prevents LPS induced apoptosis in cultured intestinal epithelial (IEC6) cells. ci-vi, i’-vi’ photomicrographs of IEC6 cells immuno-stained with in Situ death staining kit, TUNEL, d quantification of fluorescent intensity measured using ImageJ software, *** P<0·001. Each dot represents data from an individual cell.
2’-FL and 6’-SL inhibit TLR4 signaling in NEC and in human and mouse intestinal explants
We next evaluated the potential effects of 2’-FL and 6’-SL on LPS-TLR4 signaling in vivo. As shown in Fig. 5, the induction of NEC in mice and piglets led to an increase in the expression of TLR4 in the intestine as compared to controls – consistent with increased TLR4 signaling – and was reduced after 2’-FL and 6’-SL administration (Fig. 5ai–ii). To directly evaluate whether 2’-FL or 6’-SL could inhibit LPS-TLR4 signaling in vivo, newborn mice were administered a single dose of either 2’-FL or 6’-SL by oral gavage one hour before the intraperitoneal injection of LPS, and TLR4 expression and TLR4-induced cytokine expression were assessed in the intestinal epithelium. As shown in Fig. 5b, the injection of LPS significantly upregulated both TLR4 and TNF-α (Fig. 5bi–ii), and these were both reduced by either 2’-FL, 6’-SL, or their combination (Fig. 5bi–ii). Further, the treatment of intestinal explants from either embryonic mice or premature human infants (obtained during stoma closure surgery) with LPS similarly led to increased expression of both TLR4 and TNF, which were both reduced by treatment with 2’-FL or 6’-SL (Fig. 5ci–ii, di–ii). In additional negative control experiments, we tested the effects of GOS on TLR4 signaling in vitro and in vivo, given that Autran et al have shown that GOS do not impact NEC severity (14). As shown in Supplemental Fig. s2, GOS supplementation of formula did not reduce NEC as reflected by gross morphological and the histological appearance of the small intestine which were still typical of NEC. GOS treatment also failed to reduce LPS signaling in the intestinal mucosa of 7d old mice or in IEC-6 enterocytes (Supplemental Fig. s3), and did not reduce LPS-induced NFkB translocation (Supplemental Fig. s4i–iii, vii) or apoptosis (Supplemental Fig. 4 siv–vi, viii) in IEC-6 cells. Taken together, these findings illustrate that the HMOs 2’-FL and 6’-SL, can inhibit TLR4 signaling, both in vitro and in vivo. We next sought to assess the underlying mechanisms involved, and focused on the possibility that 2’-FL and 6’-SL could directly bind to TLR4 and thus interfere with LPS signaling, as assessed below.
Fig. 5.
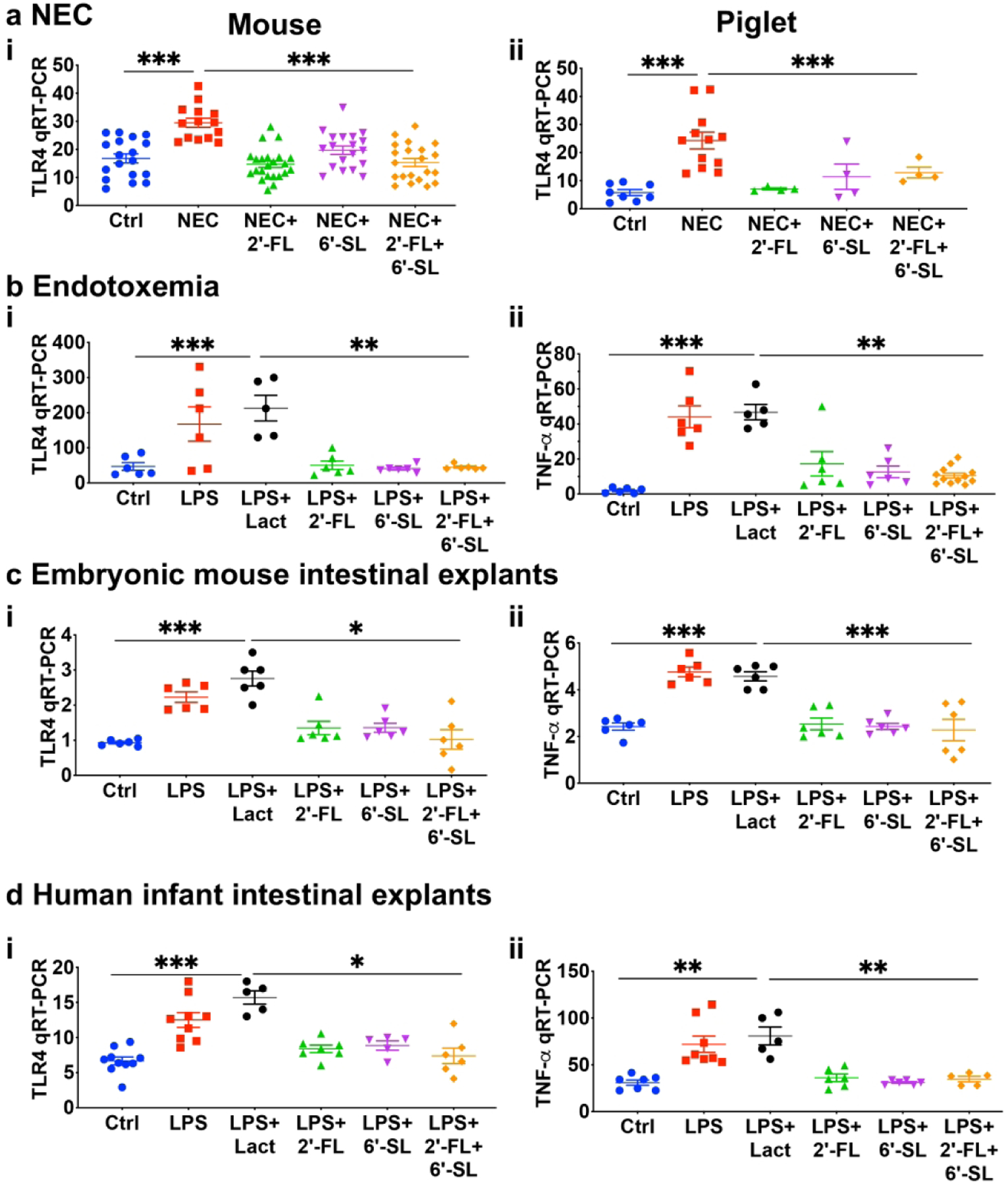
2’-FL and 6’-SL but not lactose inhibits TLR4 signaling. ai-ii TLR4 expression in mouse and piglet models of necrotizing enterocolitis, bi-ii TLR4 and pro-inflammatory cytokine TNF-α levels in mouse model of LPS-endotoxemia alone or 1h pre-treated (oral gavage) with 2’-FL and 6’-SL, ci-ii, TLR4 expression and pro-inflammatory cytokine TNF-α expression in embryonic (embryonic day 18.5, e18.5) intestinal tissue ex-vivo, di-ii TLR4 and pro-inflammatory cytokine TNF-α levels in resected human intestinal tissue obtained at time of NEC stoma closure surgery, treated with LPS, lactose, 2’-FL, and 6’-SL HMOs. **P<0·01, *** P<0·001, each dot in dot-graphs represents data from each individual sample.
2’-FL and 6’-SL but not lactose dock into the LPS binding pocket of TLR4.
In the final series of studies, we considered whether 2’-FL and 6’-SL could dock into the LPS binding pocket of TLR4 as an explanation of their ability to inhibit TLR4 signaling and prevent NEC. To assess this possibility directly, we performed molecular modeling studies of binding using Autodock Vina™ in PyRx™. As shown in Fig. 8, both 2’-FL and 6’-SL were found to dock with relative affinities of −5.6 and −6.7 kcal/mol., respectively, into the narrow, deep pocket of the TLR4-MD2 complex (Fig. 6a–b). This pocket is thought to bind LPS through a combination of internal hydrophobic residues and positively charged residues lining the rim of the cavity. Importantly, and in contrast, lactose was found to be a poorer fit (Fig. 6c), and its docking score into the same cavity is only −4.8 kcal/mol, which is consistent with the lack of protective activity of lactose against TLR4 signaling or NEC in Fig. 1. Taken in aggregate, these findings reveal that 2’-FL and 6’-SL inhibit TLR4 signaling through direct binding, providing a direct mechanism to explain their protection against the development of NEC in mice and piglets.
Fig. 6. Docking poses of 2’-FL and 6’-SL, and Lactose (LAT) into the eritoran/LPS binding pocket of the TLR4-MD2 complex.
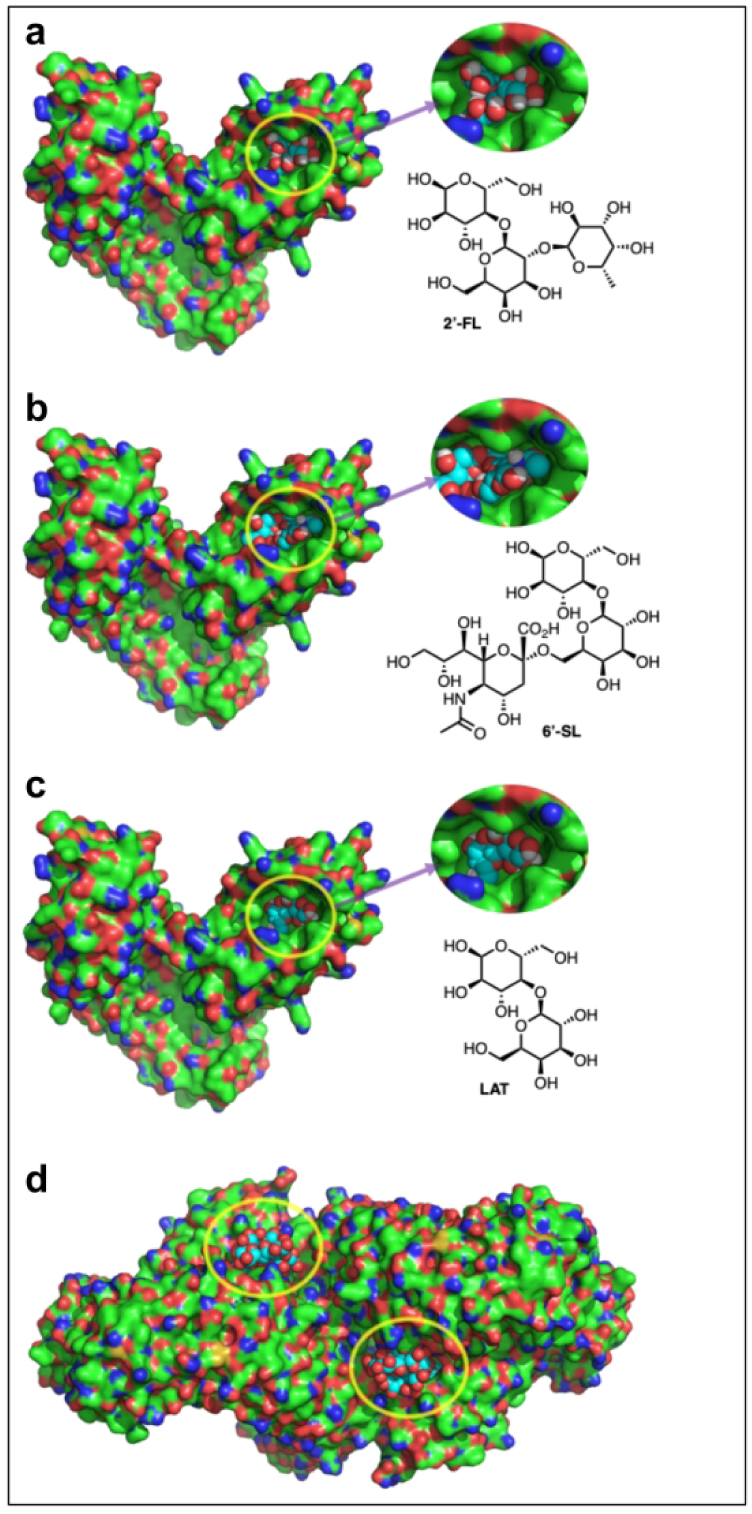
a CPK model of 2’-FL (cyan carbons) docked to into the x-ray structure of the TLR4-MD2 complex 2Z65, b CPK model of 6’-SL (cyan carbons) docked to into the x-ray structure of the TLR4-MD2 complex 2Z65, c CPK model of Lactose (cyan carbons) docked to into the x-ray structure of the TLR4-MD2 complex 2Z65, d structure of the TLR4-MD2-LPS symmetrical dimer complex 3FXI. The dimerization interface is triggered by LPS (cyan carbons) insertion into the MD2 hydrophobic binding pocket.
DISCUSSION.
We now demonstrate that the human milk oligosaccharides 2’-FL and 6’-SL play important roles in preventing the development of NEC in both mice and piglet models. In defining their mechanisms of action, we show that 2’-FL and 6’-SL, but not the control lactose backbone, inhibit TLR4 signaling in cultured enterocytes, as well as in intestinal explants from mice and humans, an effect that was revealed through computational analysis to involve direct binding to the LPS docking pocket. Given the critical role of TLR4 in the pathogenesis of NEC (4), these findings provide an explanation as to how HMOs can prevent NEC, and also explain their protective effects on intestinal epithelial apoptosis and mucosal barrier injury. Perhaps most importantly, these findings suggest the possibility that HMOs may soon be part of the armamentarium of tools which could be administered to neonates at risk of NEC, with the hope of either preventing or treating this devastating disease. Further, ours is the first study to evaluate both 2’-FL and 6’-SL alone or in combination, to evaluate their roles in mouse, piglet and human tissue, and to link HMO effects to the inhibition of TLR4 signaling.
While we now show that HMOs 2’-FL and 6’-SL exert their NEC protective effects through TLR4 inhibition, other protective mechanisms have been described, and are almost certain to play contributing roles. Specifically, prior authors have shown that 2’-FL and DSLNT act as prebiotics with putative effects on the ability of bacteria to bind to the colonic epithelium (28). In addition, HMOs are thought to encourage the growth of Bifidobacterium that are associated with a non-inflammatory state within the intestinal mucosa, suggesting a possible protective mechanism (29). While certainly plausible, it is not clear how these various oligosaccharides would interface with the rich oligosaccharides containing mucus layer which itself has important effects on the microflora, and which would seemingly be endowed with oligosaccharides at much greater concentrations than that of any exogenously provided molecules. More recently, Pierro and colleagues have revealed that HMOs can modify the transcriptional profile of the intestinal epithelium, resulting in a shift towards goblet cells, with subsequent protective effects on the intestinal mucosa and protection from barrier injury (30). Our group has shown using cultured endothelial cells that the addition of HMOs can lead to an induction of the vasodilatory enzyme eNOS, resulting in the observation that mesenteric perfusion is significantly elevated in HMO fed mice, and that the eNOS inhibitor can reverse the proactive effects of HMO for NEC (11). Given the various differences in proposed and also demonstrated mechanisms of action of individual HMOs, additional studies will be required in order to evaluate their roles as preventive strategies specifically for infants at risk for the development of NEC.
In summary, we have now shown that 2’-FL and 6’-SL – two HMOs – act to prevent NEC in mice and piglet models, and that our docking studies predict these molecules interact with the LPS binding site of TLR4, providing a mechanism of action. Taken together, we propose that the addition of 2’-FL and 6’-SL, either alone or in combination, can exert anti NEC effects, and may offer new approaches for the prevention of this devastating disease.
Supplementary Material
Impact.
What is the key message of the article:
Necrotizing enterocolitis (NEC) is a major cause of morbidity and mortality in premature infants which occurs in the setting of bacterial colonization of the gut and administration of formula feeds, and activation by the innate immune receptor toll like receptor 4 (TLR4). Breast milk prevents NEC through unclear mechanisms. We now show that breast milk enriched human milk oligosaccharides (HMOs) that are derived from lactose prevent NEC through inhibition of TLR4.
What does it add to the existing literature:
The human milk oligosaccharides 2’-FL and 6’-SL, but not the backbone sugar lactose, prevent NEC in mice and piglets.
2’-FL and 6’-SL but not lactose inhibited TLR4 signaling in cultured enterocytes, in enteroids derived from mouse intestine, and in human intestinal explants obtained at the time of surgical resection for patients with NEC.
In seeking the mechanisms involved, 2’-FL and 6’-SL but not lactose were found to directly bind to TLR4, explaining the inhibition and protection against NEC.
What is the impact?
These findings may impact clinical practice by suggesting that administration of HMOs could serve as a preventive strategy for premature infants at risk for NEC development.
Statement of Financial support:
DJH is supported by R01GM078238 and R01DK083752 from the National Institutes of Health and this research was funded in part by a Sponsored Research Grant from Abbott Nutrition.
Disclosure statement: This work was supported in part by a sponsored research grant from Abbott Nutrition to DJH. No author has any financial relationship or conflict of interest with the current work.
Footnotes
Statement of Consent: Samples were obtained de-identified under IRB 00094036 and consent was not required.
References
- 1.Stoll BJ, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA [Internet] 2015. [cited 2019 Jun 9];314:1039 Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2015.10244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzgibbons SC, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg [Internet] 2009. [cited 2019 Jun 9];44:1072–6. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0022346809001602 [DOI] [PubMed] [Google Scholar]
- 3.Martin JA, Osterman MJK. Describing the Increase in Preterm Births in the United States, 2014–2016. NCHS Data Brief [Internet] 2018. [cited 2019 Jun 9];1–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30044213 [PubMed] [Google Scholar]
- 4.Leaphart CL, et al. A critical for TLR4 in the pathogenesis of necrotizing enterocolitis by modulating intestinal injury and repair. J Immunol [Internet] 2007. [cited 2019 Jun 9];179:4808–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17878380 [DOI] [PubMed] [Google Scholar]
- 5.Sodhi CP, et al. Intestinal Epithelial Toll-Like Receptor 4 Regulates Goblet Cell Development and Is Required for Necrotizing Enterocolitis in Mice. Gastroenterology [Internet] 2012. [cited 2019 Jun 6];143:708–718.e5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0016508512009535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gribar SC, Richardson WM, Sodhi CP, Hackam DJ. No longer an innocent bystander: epithelial toll-like receptor signaling in the development of mucosal inflammation. Mol Med [Internet] 2008;14:645–59. Available from: http://www.molmed.org/content/pdfstore/645_659.Gribar.00035.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jilling T, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol [Internet] 2006. [cited 2019 Jun 9];177:3273–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16920968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gribar SC, et al. Reciprocal expression and signaling of TLR4 and TLR9 in the pathogenesis and treatment of necrotizing enterocolitis. J Immunol [Internet] 2009. [cited 2019 Jun 9];182:636–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19109197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hackam DJ, Good M, Sodhi CP. Mechanisms of gut barrier failure in the pathogenesis of necrotizing enterocolitis: Toll-like receptors throw the switch. Semin Pediatr Surg [Internet] 2013. [cited 2019 Jun 9];22:76–82. Available from: 10.1053/j.sempedsurg.2013.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dheer RS et al. Intestinal Epithelial Toll-Like Receptor 4 Signaling Affects Epithelial Function and Colonic Microbiota and Promotes a Risk for Transmissible Colitis. Infect Immun [Internet] 2016. [cited 2019 Jun 9];84:798–810. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26755160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yazji I, et al. Endothelial TLR4 activation impairs intestinal microcirculatory perfusion in necrotizing enterocolitis via eNOS-NO-nitrite signaling. Proc Natl Acad Sci U S A [Internet] 2013. [cited 2019 Jun 9];110:9451–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23650378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Good M, et al. The human milk oligosaccharide 2′-fucosyllactose attenuates the severity of experimental necrotising enterocolitis by enhancing mesenteric perfusion in the neonatal intestine. Br J Nutr 2016;116:1175–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bering SB. Human milk oligosaccharides to prevent gut dysfunction and necrotizing enterocolitis in preterm neonates [Internet]. Nutrients. 2018. [cited 2019 Jun 9];10:1461 Available from: http://www.ncbi.nlm.nih.gov/pubmed/30297668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Autran CA, Schoterman MHC, Jantscher-Krenn E, Kamerling JP, Bode L. Sialylated galacto-oligosaccharides and 2′-fucosyllactose reduce necrotising enterocolitis in neonatal rats. Br J Nutr [Internet] 2016. [cited 2019 Jun 9];116:294–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27212112 [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods [Internet] 2001. [cited 2019 Jun 6];25:402–8. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1046202301912629 [DOI] [PubMed] [Google Scholar]
- 16.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol 2010;8:e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niño DF, et al. Cognitive impairments induced by necrotizing enterocolitis can be prevented by inhibiting microglial activation in mouse brain. Sci Transl Med [Internet] 2018. [cited 2018 Dec 26];10:eaan0237 Available from: http://stm.sciencemag.org/lookup/doi/10.1126/scitranslmed.aan0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sodhi CP, et al. Fat composition in infant formula contributes to the severity of necrotising enterocolitis. Br J Nutr 2018;120:665–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Good M, et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: evidence in mice for a role of TLR9. Am J Physiol Gastrointest Liver Physiol 2014;306:G1021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HM, et al. Crystal structure of the TLR4-MD-2 complex with bound endotoxin antagonist Eritoran. Cell 2007;130:906–17. [DOI] [PubMed] [Google Scholar]
- 21.Bum-Erdene K, Leffler H, Nilsson UJ, Blanchard H. Structural characterisation of human galectin-4 N-terminal carbohydrate recognition domain in complex with glycerol, lactose, 3’-sulfo-lactose, and 2’-fucosyllactose. Sci Rep 2016;6:20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamashita S, et al. Carbohydrate recognition mechanism of HA70 from Clostridium botulinum deduced from X-ray structures in complexes with sialylated oligosaccharides. FEBS Lett 2012;586:2404–10. [DOI] [PubMed] [Google Scholar]
- 23.Egan CE, et al. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest [Internet] 2016;126:495–508. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26690704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudloff S, et al. Incorporation of orally applied 13C-galactose into milk lactose and oligosaccharides. Glycobiology [Internet] 2006. [cited 2020 Jan 2];16:477–87. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16495330 [DOI] [PubMed] [Google Scholar]
- 25.Ahsan H. 3-Nitrotyrosine: A biomarker of nitrogen free radical species modified proteins in systemic autoimmunogenic conditions. Hum Immunol [Internet] 2013. [cited 2019 Jun 9];74:1392–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23777924 [DOI] [PubMed] [Google Scholar]
- 26.Afrazi A, et al. Intracellular heat shock protein-70 negatively regulates TLR4 signaling in the newborn intestinal epithelium. J Immunol [Internet] 2012;188:4543–57. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22461698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cetin S, et al. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J Biol Chem [Internet] 2004;279:24592–600. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15169791 [DOI] [PubMed] [Google Scholar]
- 28.Moukarzel S, Bode L. Human Milk Oligosaccharides and the Preterm Infant: A Journey in Sickness and in Health. Clin Perinatol [Internet] 2017. [cited 2019 Jun 9];44:193–207. Available from: https://www.sciencedirect.com/science/article/pii/S0095510816301129?via%3Dihub [DOI] [PubMed] [Google Scholar]
- 29.Musilova S, Rada V, Vlkova E, Bunesova V. Beneficial effects of human milk oligosaccharides on gut microbiota. Benef Microbes [Internet] 2014. [cited 2019 Jun 10];5:273–83. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24913838 [DOI] [PubMed] [Google Scholar]
- 30.Wu RY, et al. Human Milk Oligosaccharides Increase Mucin Expression in Experimental Necrotizing Enterocolitis. Mol Nutr Food Res [Internet] 2019. [cited 2019 Jun 10];63:e1800658 Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/mnfr.201800658 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


