Abstract
Background:
Although supervised exercise therapy (SET) is effective in improving walking distance among adults with symptomatic peripheral artery disease (PAD), some research suggests that individuals with comorbid PAD and type 2 diabetes mellitus (T2DM) may experience a blunted response to SET. It is unknown whether free-living sedentary time changes during SET, and if increases in sedentary time could, in part, explain poor response to SET.
Objectives:
The purposes of this pilot study were to: 1) determine if older adults with PAD (with and without T2DM) engaging in SET change their sedentary behavior; and 2) examine the relationship between changes in sedentary behavior and SET outcomes. We hypothesized that decreased sedentary time during SET would be associated with greater improvements in six-minute walk test (6MWT) total distance and other key SET outcomes.
Methods:
Participants (n = 44) initiating a 12-week SET program completed the six-minute walk test (6MWT), Short Physical Performance Battery, Walking Impairment Questionnaire, and accelerometer-assessed sedentary behavior at SET initiation, 6 weeks, and 12 weeks.
Results:
Participants’ mean age was 72.3 (7.1) years, mean ankle-brachial index was 0.71 (0.25), and 47.7% were female. On average, sedentary time did not change following SET, although there was substantial variability (−40% to +38% change in minutes of sedentary time/day). Participants with T2DM experienced greater improvements in claudication onset distance when compared to participants without T2DM (mean 35 m, p = .044, 95% CI 1.6 to 115.4 meters). Neither changes in sedentary time from baseline to 6 weeks (p = .419) nor T2DM (p = .154) predicted changes in 6MWT total distance from baseline to 12 weeks.
Conclusions:
As SET availability increases, further examination of factors that may influence SET outcomes will help maximize benefits of this proven therapy.
Keywords: sedentary lifestyle, exercise therapy, type 2 diabetes mellitus, peripheral artery disease
1. INTRODUCTION
Peripheral artery disease (PAD) is a progressive condition characterized by diminished blood flow to the lower extremities. The hallmark of PAD is exercise-induced limb ischemia, or claudication, which can cause severe pain, walking impairment, and reduced quality of life.1 PAD is associated with increased cardiovascular morbidity and mortality and older adults with claudication have a 2 to 3.5-fold greater risk of all-cause mortality compared to healthy older adults.2 The cornerstone of treatment to improve physical function, mobility, and quality of life of individuals with symptomatic PAD is supervised exercise therapy (SET).3,4
One of the primary risk factors for PAD is diabetes mellitus. Epidemiologic studies indicate 20-30% of adults with diabetes have PAD.5–8 The combination of PAD and diabetes puts individuals at a greater risk of poor health outcomes, compared to either condition alone.9 While SET has been shown to be an effective therapy for improving pain-free and peak walking distance among adults with PAD,10,11 it has been reported that some individuals, particularly those with PAD and diabetes, may experience a blunted response to exercise therapy.12 It is unclear why such a discrepancy in exercise response may exist, but it has been suggested that diabetes contributes to reduced blood volume expansion and impaired skeletal muscle oxygenation during exercise.13,14 Additionally, dehydration, of particular concern among older adults,15 may also affect PAD symptoms.16 Together, these factors could influence the potential benefits gained from exercise among adults with PAD and comorbid diabetes.
Two systematic reviews17,18 have directly examined the issue of blunted response among individuals with diabetes, however they had conflicting conclusions. In the first, Lyu et al.18 conducted a meta-analysis stratifying the results of exercise studies in PAD based on the percentage of the study sample with diabetes (<25% vs. 25-50%) and concluded that studies that had a higher percentage of patients with diabetes had poorer outcomes. In the second, in which Hageman et al.17 included only studies that directly examined the effect of diabetes on exercise response (n = 3 studies), the authors concluded that while the available data do not suggest a differential response in individuals with and without diabetes, the evidence is insufficient to draw significant conclusions. Additionally, it is unclear how diabetes type (type 1 vs. type 2) may influence the clinical course of PAD and the response to exercise and other interventions.19 It has been proposed that the longer average duration of diabetes among individuals with type 1 diabetes (when compared to individuals with type 2 diabetes [T2DM]) leads to PAD onset at a younger age and thus the potential for greater disease severity in this population.20 However, distribution by diabetes type was not explicitly reported in the published articles that examined outcomes of exercise for PAD in individuals with diabetes.12,21,22 Therefore, there is a need for research to evaluate potential reasons for a blunted response to exercise interventions and strategies to improve response in a defined sample of individuals with PAD and T2DM.
One behavioral factor that may also contribute to a blunted response is sedentary behavior. Research has demonstrated consistent associations between sedentary behavior and risk of cardiovascular disease morbidity and mortality that are distinct from those of regular exercise23–25 and physical activity26. Individuals with PAD tend to be highly sedentary and have little variation in their activity patterns throughout the course of the day27 and individuals with PAD and comorbid T2DM are particularly sedentary.28 Importantly, greater sedentary time (defined as self-reported hours sitting per day) has been linked to a faster decline in peak walking distance over about 4 years.29
It has been reported that SET does not result in significant changes in average number of steps or time spent in ambulatory activities per day,30 however the authors noted large variations between patients, with some showing a decline in physical activity level, and others exhibiting no change or an increase in physical activity level. This concept of behavioral compensation – in which an individual may reduce their non-exercise physical activity in response to increased structured exercise – has been well documented in the weight loss literature,31–33 but has yet to be adequately examined in the context of SET for the management of symptomatic PAD.
Given the potentially poorer outcomes of SET among older adults with PAD and T2DM and the detrimental health effects of sedentary behavior, it could be important to consider changes in sedentary behavior when evaluating response to SET. This may be particularly true for older adults with PAD and comorbid T2DM who may not experience the same improvements in walking distance as a result of SET when compared to peers with PAD alone.12,21 Therefore, the purposes of the present study were to 1) quantify the changes in sedentary time that occur during participation in SET for the treatment of symptomatic PAD, and 2) examine the relationship between changes in sedentary time and outcomes of SET among older adults with PAD, with and without comorbid T2DM. We hypothesized that decreased sedentary time during SET would be associated with greater improvements in 6MWT total distance and other key SET outcomes. A conceptual model of the proposed relationship between sedentary time, T2DM, and outcomes of SET examined in this study is shown in Figure 1.
Figure 1.
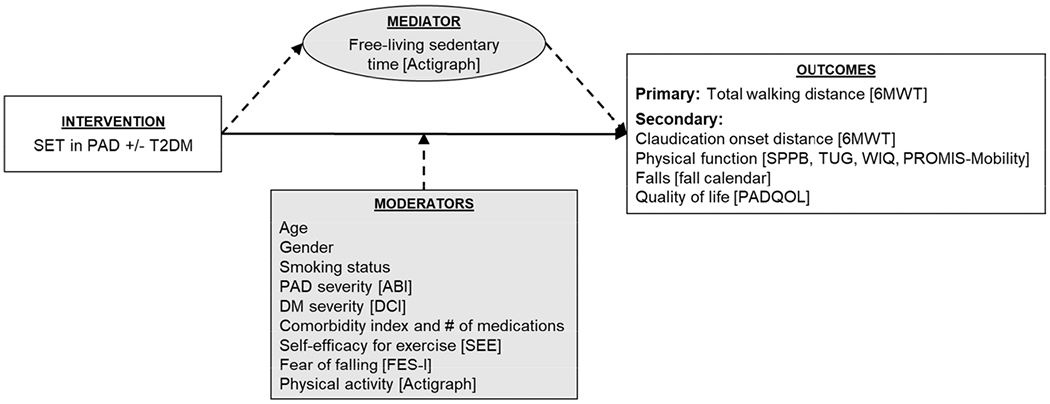
Conceptual model of proposed relationship between sedentary behavior, mediating and moderating variables, and outcomes of SET among older adults with PAD with and without T2DM.
6MWT, six-minute walk test; ABI, ankle-brachial index; DCI, Diabetes Complications Index; FES-I, Falls Efficacy Scale-International; PAD, peripheral artery disease; PADQOL, Peripheral Artery Disease Quality of Life questionnaire; PROMIS-Mobility, Patient Reported Outcomes Measurement Information System Physical Function Mobility item bank; SEE, Self-Efficacy for Exercise; SET, supervised exercise therapy; SPPB, Short Physical Performance Battery; T2DM, type 2 diabetes mellitus; TUG, Timed Up and Go; WIQ, Walking Impairment Questionnaire
2. METHOD
2.1. Study Design and Population
This pilot study used a single group, repeated measures design of participants newly enrolled in SET programs offered in the Twin Cities metropolitan area. Eligibility criteria included: being 60 years or older, having a diagnosis of PAD, being referred by a health care provider for SET for PAD, and able to speak and read English. Given the lower prevalence of type 1 diabetes among older adults34 and the resulting lack of adequate sample sizes to enable comparison of outcomes among patients with PAD with type 1 and type 2 diabetes, participants with type 1 diabetes were excluded from the present study. Participants were also excluded if they had current non-healing wounds.
2.2. Setting
SET was provided through five outpatient cardiac and pulmonary rehabilitation programs of a Midwestern hospital system, all within 60 miles of the Twin Cities metropolitan area. This hospital system has substantial experience in the implementation of SET, having offered SET through its phase three cardiac rehabilitation program since 2015. Briefly, patients completed two to three SET sessions per week for 12 weeks consisting primarily of repeated bouts of treadmill walking exercise. Sessions broadly followed an established protocol for patients with PAD but were individualized according to patient needs.4 Exercise intensity was gradually increased over 12 weeks. Although treadmill walking was the primary exercise, participants were also instructed in the use of arm and leg ergometry, total body recumbent stepping, and overground walking, depending on their abilities, therapy goals, and treatment plan developed in collaboration with the exercise therapists. For the purpose of analysis, participants were classified as using combination therapy if ≥20% of their exercise bouts were completed using a non-treadmill modality.
2.3. Ethical Considerations
This study was reviewed and approved by the University of Minnesota Institutional Review Board (IRB# 1701P03681) and all participants provided written informed consent. Participants received $20 for each data collection visit and $30 per month (for 3 months) to offset the costs of transportation and parking at SET sites (total $150).
2.4. Data Collection
Participants wore an accelerometer for objective measurement of sedentary time and physical activity and completed assessments of physical function and fall risk at three time points: baseline, 6 weeks, and 12 weeks. Each data collection session was approximately 1-1.5 hours in length and occurred at each participant’s typical exercise site.
2.5. Measures
2.5.1. Sedentary time and physical activity.
Sedentary behavior was defined as any waking behavior characterized by an energy expenditure ≥1.5 metabolic equivalents of task (METs) and occurring in a sitting, reclining, or lying posture.35 Sedentary time and physical activity were assessed using a wrist-worn Actigraph wGTX3-BT accelerometer. Participants were instructed to wear the monitor on their nondominant wrist for 24 hours a day for 2 weeks at each time point. Data were collected at a sampling frequency of 30Hz. The Actigraph wGTX3-BT has been validated for measurement of sedentary time in older adults36 and was used to assess average minutes of sedentary time per day, average minutes per day in sedentary bouts, and number and length of bouts and breaks in sedentary time. Although waist-worn accelerometers have been more commonly used to assess sedentary time and physical activity, wrist-worn accelerometers were specifically chosen to maximize wear compliance and identification of sleep periods.37,38
2.5.2. Physical function.
Physical function was assessed objectively using the six-minute walk test (6MWT), Timed Up and Go (TUG), and Short Physical Performance Battery (SPPB). The 6MWT is a performance-based functional measure in which the participant is asked to walk as far as possible in 6 minutes.39 The standard 6MWT protocol was followed,39 with the exception that participants were also instructed to notify the assessor during the test as soon as they experienced any leg symptoms. The total distance covered in 6 minutes and the distance at which the participant first reported claudication symptoms (claudication onset distance) were recorded. The TUG measures functional mobility in older adults40 by assessing the time (seconds) required for the participant to rise from a chair, walk 3 meters, turn around, walk back to the chair, and sit down. The SPPB consists of three measures: standing balance, 4-meter walking velocity, and repeated chair rises.41 Total scores range from 0-12, with higher scores indicating better function. The SPPB has been used extensively with older adults, including individuals with PAD, to evaluate functional status and risk of falling.42,43 All three measures have demonstrated high test-retest reliability.44–46
Physical function was also evaluated using two self-report measures, the Walking Impairment Questionnaire (WIQ) and the Patient Reported Outcomes Measurement Information System (PROMIS) Physical Function-Mobility (PROMIS-Mobility) item bank. The WIQ was developed specifically for patients with PAD47 and consists of three subscales: walking distance, speed, and stairs. Scores range from 0 to 100, with 100 representing no impairment and 0 representing complete impairment. The PROMIS-Mobility item bank contains 15 items evaluating an individual’s difficulty performing tasks such as standing unsupported for 30 minutes, jumping up and down, and standing on tiptoes.48 Scores range from 0-100 with higher scores indicating better function.
2.5.3. Moderating variables.
To address variables that may moderate the relationship between SET and the primary and secondary outcomes (Figure 1), participants also completed measures of comorbidity, disease severity, and self-efficacy at enrollment and 12 weeks. To quantify PAD severity, all participants had an ABI performed using a standard protocol.49 T2DM severity was assessed via the Diabetes Complications Index (DCI), a 17-item questionnaire designed to identify diagnoses and symptoms of 6 common complications of diabetes (coronary artery disease, cerebrovascular disease, peripheral neuropathy, autonomic neuropathy, foot ulceration, and retinopathy).50 An index of multiple chronic conditions was also used to summarize the presence of conditions in 8 categories: cardiovascular disease, cancer, arthritis, diabetes, stroke, chronic lung disease, depression, and obesity or metabolic syndrome51–54 and the number of prescribed medications. Fear of falling was assessed using the Falls Efficacy Scale – International (FES-I), a 16-item scale that asks how concerned a person is about falling in a variety of situations such as going up and down stairs and when visiting a friend or relative.55,56 Scores on the FES-I range from 16 to 64; higher scores indicate greater concern about falling. Finally, in order to consider the role of motivation in outcomes of SET, participants completed the Self-Efficacy for Exercise (SEE) questionnaire, a 9-item tool developed for use with older adults.57 Higher scores indicate greater self-efficacy for exercise. The duration, intensity, and mode of exercise for each bout in an exercise session was abstracted from the medical record in order to address the potential role of exercise adherence in outcomes. Demographic data including age, sex, race, ethnicity, height, weight, marital status, education, and employment status were also collected.
2.6. Statistical Analysis
Activity data were processed using ActiLife (Version 9.0.0, Actigraph Corp., Pensacola, FL). Files were reintegrated from 10-second to 60-second epochs to enable sleep detection and exclusion of sleep periods from wear time. Sleep periods were detected using the ActiLife+Sleep software to enable batched autoscoring of time in bed and time out of bed using the Tudor-Locke 2014 sleep detection algorithm.58 Non-wear time was identified using Choi 2011 criteria.59 A valid day for inclusion was defined as at least 10 hours of non-sleep wear time. Files were required to have a minimum of five valid days in order to be included in all analyses. Keadle Women’s Health 2014 cut points,60 which were established in a large cohort study of older women (mean age 71.6), were used to define sedentary (0-99 counts per minute [CPM]), light (100-1951 CPM), and moderate (≥1952 CPM) activity. Sedentary bouts were defined as periods of ≥10 minutes with less than 99 counts per minute. The ActiLife “worn on wrist” option was used to scale count calculations from wrist to hip.
Descriptive statistics were used to summarize demographic characteristics at baseline and to compare baseline and 12-week outcomes for the entire sample. Change scores from baseline for average minutes sedentary per day, time in bouts of sedentary behavior, and number and length of bouts of sedentary behavior and breaks in sedentary time were computed at 6 weeks and 12 weeks. We estimated the variability and calculated 95% confidence intervals (CI) for changes within the PAD only and PAD with comorbid T2DM groups and compared changes between these groups using two-sample t-tests. Given that a reduction of sedentary time of approximately 30 minutes per day is considered to be clinically meaningful,61 changes in average minutes of sedentary time per day were also dichotomized into two groups according to whether participants decreased their mean sedentary time per day by ≥30 minutes or ≤29 minutes. Chi-square tests were used to determine if the number of participants in each sedentary time group differed by the presence of T2DM. Linear regression was used to determine the strength of the association between T2D, changes in sedentary time, and change in 6MWT total distance (primary outcome) while adjusting for age, gender, smoking status, wear time, and other key confounding variables, including adherence to SET, defined a priori as completing at least 24 (67%) sessions within a 12-week period. Analyses were conducted using SPSS Statistics (Version 25, IBM Corp., Armonk, NY) and R (Version 3.5.2, Vienna, Austria). Statistical significance was set at p < .05.
3. RESULTS
3.1. Study Sample
Study screening, enrollment, data collection, and analysis are outlined in Figure 2. Between May 2017 and November 2018, 101 patients were screened for eligibility and invited to participate in the study; of these, 53 enrolled, 23 patients declined participation, and 25 were ineligible due to age <60, type 1 diabetes, or the decision to forgo implementation of SET (due to significant co-payments, transportation concerns, or plans to initiate a home-based exercise program). Eighty-five percent of participants (n = 44) completed the study.
Figure 2.
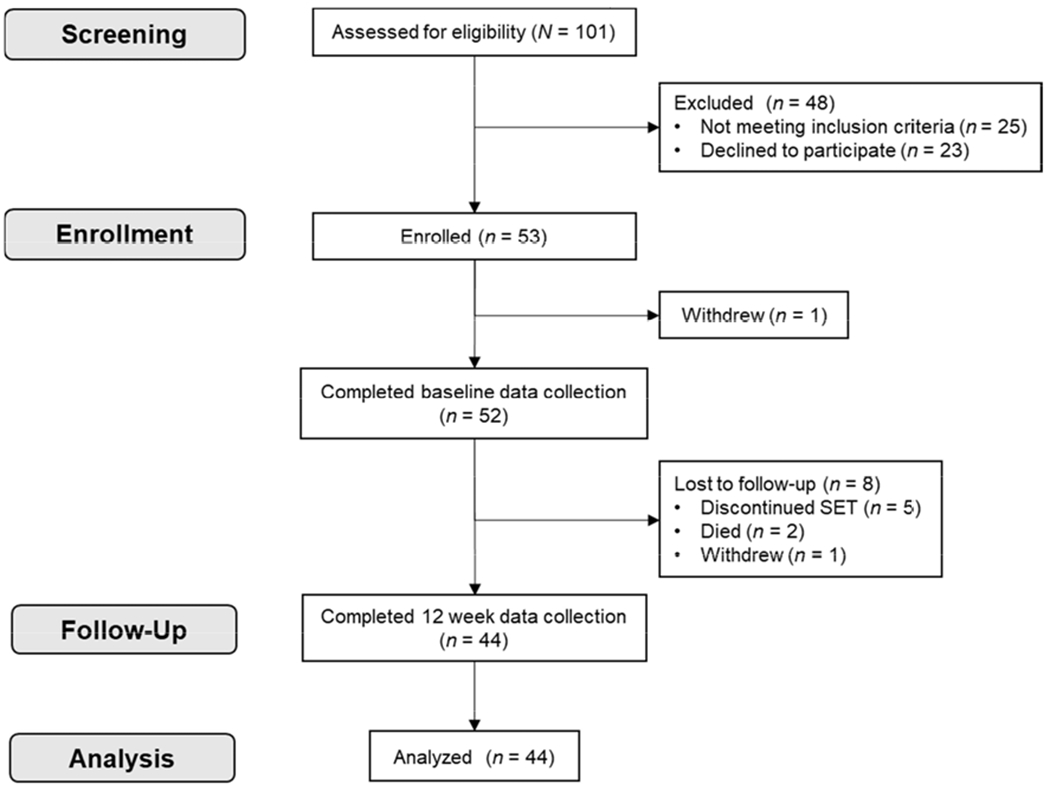
Flow diagram of study enrollment, data collection, and analysis.
SET, supervised exercise therapy
Most participants (90.4%) were non-Hispanic white, 50% were female, 43.2% had T2DM, and the mean age was 71.6 (6.9) years. Participants who did not complete the study (n = 7) were more likely to be divorced (37.5% vs. 7.0%, p = .014), less likely to be widowed (0% vs. 20.9%), less likely to be retired (50.0% vs. 81.8%, p = .049), less likely to have completed graduate school (0% vs. 13.6%), less likely to have hypercholesterolemia (57.1% vs. 95.5%, p = .002), and less likely to have osteoarthritis (14.3% vs. 63.6%, p = .014). Only one participant who did not complete the study had T2DM.
A total of 52 valid accelerometer files were available for analysis at baseline, which decreased to 46 valid files at 6 weeks and 44 valid files at 12 weeks due to participants lost to follow-up (Figure 2). On average, participants had 13.9 (1.5), 14.0 (1.1), and 13.4 (2.4) days meeting wear criteria at baseline, 6 weeks, and 12 weeks, respectively, with an average of 16.5 (1.8), 16.5 (1.7), 16.5 (1.8) valid hours per day at each time point. There were no significant differences in intra-individual minutes of valid wear time between baseline, 6 weeks, and 12 weeks (all p > .10).
Demographic and health characteristics of participants who completed the study (n = 44), overall and categorized by T2DM status, are summarized in Table 1. On average, participants with T2DM had a higher body mass index (BMI) (estimated mean difference 3.42 kg/m2, p =.05, 95% CI 0.005 to 6.83 kg/m2) and a greater number of chronic conditions (estimated mean difference 1.3, p = .001, 95% CI 0.6 to 2.1). There were no other differences between individuals with and without T2DM with respect to measured demographic and health characteristics.
Table 1.
Demographic and health characteristics of participants (n = 44) by type 2 diabetes (T2DM) status.
| Mean (SD) or n [%] | ||||
|---|---|---|---|---|
| Characteristic | Overall (n = 44) | PAD+T2DM (n = 19) | PAD (n = 25) | p+ |
| Age (years) | 72.3 (7.1) | 72.1 (8.8) | 72.5 (5.7) | .846 |
| Sex (female) | 21 [47.7] | 7 [36.8] | 14 [56.0] | .208 |
| Non-Hispanic white | 40 [90.9] | 17 [89.5] | 23 [92.0] | .585† |
| BMI (kg/m2) | 28.6 (5.8) | 30.5 (6.3) | 27.1 (5.0) | .050 |
| Marital Status | .471^ | |||
| Single | 3 [7.0] | 2 [10.5] | 1 [4.2] | |
| Married/living with partner | 28 [65.1] | 10 [52.6] | 18 [75.0] | |
| Divorced | 3 [7.0] | 2 [10.5] | 1 [4.2] | |
| Widowed | 9 [20.9] | 5 [26.3] | 4 [16.7] | |
| Education | .643^ | |||
| High school diploma or less | 10 [22.7] | 3 [15.8] | 7 [28.0] | |
| Some college | 23 [52.3] | 11 [57.9] | 12 [48.0] | |
| 4-year college degree | 5 [11.4] | 3 [15.8] | 2 [8.0] | |
| Graduate school | 6 [13.6] | 2 [10.5] | 4 [16.0] | |
| Employment status | .912^ | |||
| Full-time | 6 [13.6] | 3 [15.8] | 3 [12.0] | |
| Part-time | 2 [4.5] | 1 [5.3] | 1 [4.0] | |
| Retired | 36 [81.8] | 15 [78.9] | 21 [84.0] | |
| ABI (lower leg) | 0.71 (0.25) | 0.68 (0.27) | 0.73 (0.24) | .534 |
| Lower extremity revascularization (any) | 18 [40.9] | 8 [42.1] | 10 [40.0] | .888 |
| Percutaneous | 17 [38.6] | 7 [36.8] | 10 [40.0] | .831 |
| Bypass | 7 [15.9] | 3 [15.8] | 4 [16.0] | .657† |
| Amputation | 1 [2.3] | 1 [5.3] | 0 [0] | .432† |
| Smoking status | ||||
| Never | 8 [18.2] | 4 [21.1] | 4 [16.0] | 1.000 |
| Current | 4 [9.1] | 1 [5.3] | 3 [12.0] | .415† |
| Former | 32 [72.7] | 14 [73.7] | 18 [72.0] | .622† |
| Pack years | 45.3 (28.7) | 46.5 (34.0) | 44.5 (25.2) | .850 |
| Comorbid Conditions | ||||
| Hypertension | 41 [93.2] | 18 [94.7] | 23 [92.0] | .604† |
| Hypercholesterolemia | 42 [95.5] | 17 [89.5] | 25 [100] | .181† |
| Coronary artery disease/MI | 24 [54.5] | 11 [57.9] | 13 [52.0] | .697 |
| Carotid endarterectomy | 6 [13.6] | 2 [10.5] | 4 [16.0] | .684† |
| Stroke/TIA | 5 [11.4] | 3 [15.8] | 2 [8.0] | .638† |
| COPD/Emphysema | 12 [27.3] | 7 [36.8] | 5 [20.0] | .214 |
| Osteoarthritis | 28 [63.6] | 12 [63.2] | 16 [64.0] | .954 |
| Comorbidity index (out of 8) | 3.6 (1.4) | 4.4 (1.2) | 3.0 (1.2) | .001 |
| Medication use | ||||
| Number of medications | 13.0 (5.4) | 14.0 (5.2) | 12.3 (5.6) | .331 |
| Current use of cilostazol/pentoxifylline | 9 [20.5] | 3 [15.8] | 6 [24.0] | .710† |
| Current use of metformin | 11 [25.0] | 11 [57.9] | 0 [0] | N/A |
Note.
t-test or Pearson chi-square as appropriate for continuous or categorical data, respectively.
Fisher’s Exact Test (expected cell counts less than 5).
Likelihood ratio.
ABI, ankle-brachial index; BMI, body mass index; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; PAD, peripheral artery disease; PAD+T2DM, peripheral artery disease and comorbid type 2 diabetes mellitus; T2DM, type 2 diabetes mellitus; TIA, transient ischemic attack
3.2. Overall SET Completion and Outcomes
The 44 participants who completed the study attended an average of 27.7 (5.8) sessions (range 16-42 in the 12-week period), for an average of 2.3 sessions per week. Thirty-four participants (77.3%) completed at least 24 exercise sessions. The average time between baseline and follow-up assessments was 79.5 (7.5) days. There were no differences in length of follow-up or session attendance between participants with and without T2DM.
Treadmill walking was the most common exercise modality, although 11.4% of participants (n = 5) used a combination of exercise modalities, completing 20% or greater of their exercise bouts using an alternative mode of exercise (mean 46.2% of bouts, range 19.5-100%). The most commonly used alternative mode of exercise was total body recumbent stepping (n = 4). Participants who used combination therapy had significantly lower 6MWT total distances at baseline (mean [SD] of 194.6 [74.7] m vs. 331.0 [85.7] m, p = .002, 95% CI 55.1 to 217.5), but there were no differences in change in 6MWT total distance from baseline to 12 weeks between participants who completed treadmill walking and those who used combination therapy (p = .637). There were no differences in number of exercise sessions completed, time between baseline and follow-up, and adherence between participants who used the treadmill only and those who used combination therapy and the prevalence of the use of combination therapy was similar in participants with and without T2DM.
After 12 weeks of SET, participants had statistically significant improvements in SPPB total score, 6MWT claudication onset distance, 6MWT total distance, and treadmill workload (Table 2). Additionally, improvements were noted in the distance and speed domains of the WIQ as well as PROMIS-Mobility total scores (Table 2).
Table 2.
Overall changes in objective and self-reported physical function outcomes after 12 weeks of supervised exercise therapy (SET) for the treatment of symptomatic peripheral artery disease (PAD) (n = 44).
| Mean (SD) |
t | p | 95% CI | |||
|---|---|---|---|---|---|---|
| Characteristic | Baseline | 12 Weeks | Lower | Upper | ||
| Objective measures | ||||||
| SPPB total score | 9.6 (2.3) | 10.4 (2.0) | 4.15 | <.001 | 0.4 | 1.3 |
| Six-minute walk test | ||||||
| Claudication onset distance (m) | 111.8 (54.9) | 153.9 (82.5) | 3.32 | .002 | 16.4 | 67.8 |
| Total distance (m) | 315.5 (94.4) | 344.5 (85.1) | 3.32 | .002 | 11.4 | 46.6 |
| TUG (sec) | 9.7 (1.8) | 9.6 (2.0) | −0.36 | .720 | −0.5 | 0.4 |
| Treadmill METs | 2.8 (0.8) | 4.1 (1.5) | 8.65 | <.001 | 1.0 | 1.6 |
| Subjective measures | ||||||
| WIQ | ||||||
| Distance | 30.4 (25.7) | 38.8 (27.7) | 2.77 | .008 | 2.3 | 14.5 |
| Speed | 31.7 (23.1) | 42.2 (21.9) | 3.80 | <.001 | 4.9 | 16.1 |
| Stairs | 39.0 (27.4) | 42.5 (29.1) | 0.98 | .332 | −3.7 | 10.7 |
| PROMIS-Mobility | 52.9 (12.3) | 55.8 (12.0) | 2.74 | .009 | 0.8 | 5.1 |
MET, metabolic equivalent of task; PROMIS-Mobility, Patient Reported Outcomes Measurement Information System Physical Function Mobility item bank; SPPB, Short Physical Performance Battery; TUG, Timed Up and Go; WIQ, Walking Impairment Questionnaire
3.3. Overall Changes in Sedentary Behavior
Physical activity and sedentary behavior at each time point and changes over time are summarized in Table 3. On average, participants had a 2.8% increase in the average minutes of sedentary time per day from baseline to 12 weeks, although there was substantial variability, ranging from a 40% decrease to a 38% increase in average minutes of sedentary time per day. There were no statistically significant changes in any of the sedentary behavior variables or percent time in sedentary, light, or moderate activities from baseline to 6 weeks or from baseline to 12 weeks.
Table 3.
Physical activity level and sedentary time at each time point and changes during supervised exercise therapy (SET) for the treatment of symptomatic peripheral artery disease (PAD) (n = 44).
| Mean (SD) | 6W – Baseline | 12W – Baseline | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | Baseline | 6W | 12W | Mean (SD) | Range | Mean (SD) | Range |
| Activity level (percent time) | |||||||
| Sedentary+ | 44.9 (9.9) | 45.3 (9.8) | 45.8 (10.6) | 0.4 (4.9) | −12.4, 12.4 | 0.8 (6.0) | −13.6, 11.3 |
| Light | 46.9 (8.2) | 46.7 (7.8) | 46.3 (8.0) | −0.2 (4.4) | −12.4, 7.3 | −0.6 (5.0) | −11.1, 13.5 |
| Moderate | 8.1 (4.4) | 8.0 (4.6) | 7.9 (5.3) | −0.1 (2.2) | −4.7, 8.6 | −0.2 (3.6) | −7.0, 18.3 |
| Average time sedentary per day (min)+ | 444.2 (101.8) | 444.7 (96.6) | 451.2 (104.7) | 0.5 (64.3) | −140.3, 140.1 | 7.1 (74.1) | −158.7, 140.9 |
| Percent change in average sedentary time per day (min)+ | 1.3 (15.6) | −31.0, 38.0 | 2.8 (17.9) | −40.0, 38.0 | |||
| Sedentary bouts | |||||||
| Average time in bouts per day (min)+ | 173.2 (74.3) | 174.2 (68.6) | 185.0 (79.8) | 1.1 (43.2) | −83.8, 83.0 | 11.8 (46.6) | −97.4, 116.4 |
| Average number per day | 8.3 (3.4) | 8.2 (3.1) | 8.8 (3.5) | −0.03 (1.9) | −3.9, 3.6 | 0.5 (2.1) | −4.6, 4.1 |
| Average length (min)+ | 21.3 (3.6) | 21.3 (3.9) | 20.9 (2.8) | 0.1 (4.2) | −14.2, 18.8 | −0.4 (3.6) | −17.3, 5.1 |
| Sedentary breaks | |||||||
| Average number per day | 8.2 (3.4) | 8.2 (3.1) | 8.7 (3.5) | −0.03 (1.9) | −3.94, 3.64 | 0.5 (2.1) | −4.6, 4.2 |
| Average length (min)† | 172.9 (77.0) | 176.4 (113.3) | 161.7 (71.0) | 3.6 (79.3) | −178.3, 369.8 | −11.1 (64.9) | −163.6, 127.4 |
Negative values represent a “desirable” response – decrease in that metric from baseline to follow-up.
One participant excluded due to erroneous data on this metric.
6W, 6 weeks; 12W, 12 weeks
Fourteen participants (31.8%) decreased their average sedentary time per day by ≥30 minutes, which is considered clinically meaningful,61 at 12 weeks. Among participants who achieved this reduction in average sedentary time per day, the mean relative decrease was 77.8 (35.4) minutes. Thirty participants (68.2%) did not attain a 30-minute or more decrease at 12 weeks and their mean increase in average sedentary time per day was 46.7 (49.9) minutes. At 12 weeks, there was a trend for participants with T2DM to be more likely to achieve a “clinically meaningful” reduction in mean sedentary time per day when compared to participants without T2DM, with 47.4% of participants with T2DM and 20.0% of participants without T2DM achieving this reduction (χ2 = 3.727, p = .054).
3.4. Role of T2DM in Changes in Sedentary Behavior
T2DM was not a significant independent predictor of changes from baseline to 6 weeks in any of the measured sedentary behavior variables. T2DM was a significant predictor of changes in average sedentary time per day, percent change in average sedentary time per day, and average time in sedentary bouts per day from baseline to 12 weeks after adjusting for relevant covariates (Table 4). Participants with T2DM had an average reduction in sedentary time per day of 18.2 (46.7) minutes, while participants without T2DM increased their average sedentary time per day by 32.5 (29.4) minutes (p = .018). Similarly, the percent reduction in average sedentary time per day and average time in sedentary bouts per day was greater in participants with T2DM when compared to those without. Although not statistically significant, having T2DM was associated with an increase in the average length of breaks in sedentary behavior, compared to those without T2DM who decreased their time in breaks between sedentary bouts (p = .091). Current smoking was a significant predictor of change in the average length of sedentary bouts, with current smokers having a greater decrease in the average length of sedentary bouts from baseline to 12 weeks when compared to nonsmokers (mean [SD] of −4.5 [0.9] min vs. −0.03 ([1.1] min, p = .020, 95% CI −0.9 to −9.8). None of the other included variables were significant predictors in the models.
Table 4.
Association between type 2 diabetes (T2DM) and changes in sedentary behavior patterns after completing 12 weeks of supervised exercise therapy (SET) for the treatment of symptomatic peripheral artery disease (PAD) (n = 44).
| Effect of T2DM | t | p | 95% CI | Model R2 | Marginal Predicted Mean (SD) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | PAD+T2DM | PAD | |||||||
| B | SE | Lower | Upper | (n = 19) | (n = 25) | ||||
| Average time sedentary per day (min)+ | −53.2 | 21.3 | −2.50 | .018 | −96.5 | −9.9 | 0.394 | −18.2 (46.7) | 32.5 (29.4) |
| Percent change in average sedentary time per day (min)+ | −12.9 | 5.3 | −2.43 | .021 | −23.6 | −2.1 | 0.379 | −3.7 (10.1) | 8.8 (8.2) |
| Sedentary bouts | |||||||||
| Average time in bouts per day (min)+ | −34.4 | 13.6 | −2.52 | .017 | −62.1 | −6.6 | 0.329 | −4.5 (18.4) | 29.3 (19.3) |
| Average number per day | −1.0 | 0.7 | −1.56 | .129 | −2.4 | 0.3 | 0.239 | 0.1 (0.9) | 1.1 (0.8) |
| Average length (min)+ | −0.5 | 1.2 | −0.45 | .659 | −3.0 | 1.9 | 0.224 | −0.8 (1.5) | −0.2 (1.9) |
| Sedentary breaks | |||||||||
| Average number per day | −1.0 | 0.7 | −1.55 | .131 | −2.4 | 0.3 | 0.238 | 0.1 (0.9) | 1.1 (0.8) |
| Average length (min) | 37.0 | 21.2 | 1.75 | .091 | −6.2 | 80.3 | 0.248 | 8.8 (27.7) | −31.4 (20.4) |
Each row represents an individual multiple linear regression model with the listed variable as the dependent variable and the listed B, SE, t, p, and 95% CI for the effect of T2DM adjusted for age, sex, BMI, ABI, current smoking, history of any peripheral revascularization procedure, number of exercise sessions completed, and minutes of valid wear time at baseline and 12 weeks.
Negative values represent a “desirable” response – decrease in that metric from baseline to follow-up.
ABI, ankle-brachial index; BMI, body mass index; PAD, peripheral artery disease; PAD+T2DM, peripheral artery disease and comorbid type 2 diabetes mellitus
3.5. Role of T2DM in SET Outcomes
There were no significant differences in 6MWT total distance and claudication onset distance between patients with and without T2DM at baseline (all p > 0.17) (Table 5). T2DM was a significant predictor of change in claudication onset distance. Participants with T2DM had a predicted improvement in claudication onset distance of approximately 35 meters when compared to participants without T2DM. Unadjusted changes in 6MWT total and claudication onset distance over time among participants with and without T2DM are shown in Figure 3. None of the other included variables were significant predictors in the models, with the exception of age being a significant predictor of improvement on the stairs domain of the WIQ (p = .040).
Table 5.
Association of type 2 diabetes (T2DM) with changes in functional outcomes after completing 12 weeks of supervised exercise therapy (SET) for the treatment of symptomatic peripheral artery disease (PAD) (n = 44).
| Effect of T2DM | t | p | 95% CI | Model R2 | Marginal Predicted Mean (SD) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | PAD+T2DM | PAD | |||||||
| B | SE | Lower | Upper | (n = 19) | (n = 25) | ||||
| SPPB total score | 0.43 | 0.46 | 0.93 | .358 | −0.50 | 1.36 | .148 | 1.16 (0.53) | 0.63 (0.39) |
| Six-minute walk test | |||||||||
| Claudication onset distance (m) | 58.5 | 27.7 | 2.11 | .044 | 1.6 | 115.4 | .255 | 59.6 (36.5) | 24.3 (30.7) |
| Total distance (m) | −14.0 | 20.6 | −0.68 | .500 | −55.8 | 27.8 | .078 | 18.0 (14.0) | 38.0 (12.3) |
| Percent change in total distance | −20.8 | 17.2 | −1.21 | .236 | −55.7 | 14.2 | .181 | −3.7 (6.9) | 8.8 (7.0) |
| TUG (sec)+ | −0.1 | 0.5 | −0.18 | .857 | −1.1 | 1.0 | −.074 | −0.2 (0.5) | 0.1 (0.5) |
| Treadmill METs | −0.07 | 0.30 | −0.23 | .822 | −0.68 | 0.54 | .179 | 1.14 (0.39) | 1.26 (0.36) |
| WIQ | |||||||||
| Distance | 0.03 | 6.63 | 0.01 | .996 | −13.45 | 13.51 | .130 | 7.48 (8.08) | 7.52 (6.22) |
| Speed | 0.86 | 6.26 | 0.14 | .892 | −11.86 | 13.58 | .145 | 10.87 (7.47) | 10.69 (6.87) |
| Stairs | −1.63 | 7.08 | −0.23 | .820 | −16.01 | 12.76 | .268 | −0.22 (13.95) | 4.34 (9.44) |
| PROMIS-Mobility | −1.6 | 2.3 | −0.12 | .499 | −6.3 | 3.2 | .088 | 1.5 (1.7) | 3.4 (1.8) |
Each row represents an individual multiple linear regression model with the listed variable as the dependent variable and the listed B, SE, t, p, and 95% CI for the effect of T2DM adjusted for age, sex, BMI, ABI, current smoking, history of any peripheral revascularization procedure, and number of exercise sessions completed.
Negative values represent a “desirable” response – decrease in that metric from baseline to follow-up.
ABI, ankle-brachial index; BMI, body mass index; CI, confidence interval; MET, metabolic equivalent of task; PAD+T2DM, peripheral artery disease and comorbid type 2 diabetes mellitus; PROMIS-Mobility, Patient Reported Outcomes Measurement Information System Physical Function Mobility item bank; SPPB, Short Physical Performance Battery; TUG, Timed Up and Go; WIQ, Walking Impairment Questionnaire
Figure 3.
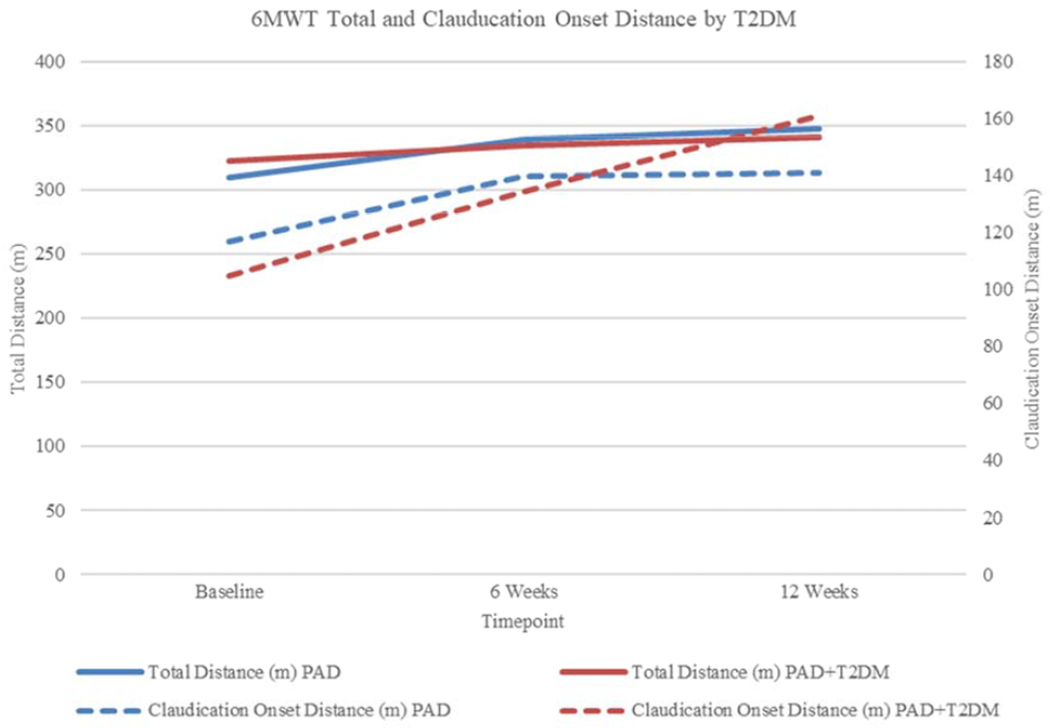
Changes in 6MWT total and claudication onset distance during 12 weeks of SET by T2DM diagnosis.
SET, supervised exercise therapy; T2DM, type 2 diabetes mellitus
In multiple linear regression, neither T2DM nor changes in average minutes of sedentary time per day from baseline to 6 weeks were significant predictors of changes in 6MWT total distance from baseline to 12 weeks (Table 6). Self-efficacy and number of T2DM-related complications at baseline were the only significant predictors of change in 6MWT total distance (p = .039 and p = .021, respectively). The overall model explained 56% of the variance in changes in 6MWT total distance.
Table 6.
Multiple linear regression model examining the relationship between changes in sedentary time from baseline to 6 weeks and changes in 6MWT total distance from baseline to 12 weeks adjusting for baseline characteristics and changes in moderate activity (n = 44).
| Unstandardized Coefficients |
|||
|---|---|---|---|
| Estimate | SE | p | |
| Baseline characteristics | |||
| Age (years) | 1.3 | 1.6 | .448 |
| Sex (female) | 22.8 | 18.3 | .223 |
| BMI (kg/m2) | 0.4 | 1.8 | .828 |
| Current smoker | 14.1 | 30.4 | .647 |
| ABI (lower leg) | 34.4 | 40.8 | .407 |
| Previous LE revascularization (any) | −4.5 | 19.0 | .816 |
| T2DM | −14.2 | 17.3 | .419 |
| SEE total | −1.3 | 0.6 | .039 |
| DCI total | 19.7 | 8.0 | .021 |
| FES-I total | −3.1 | 1.6 | .064 |
| 6MWT total distance (m) | −0.2 | 0.1 | .092 |
| Accelerometer variables | |||
| Minutes of valid wear time (Baseline) | −0.01 | 0.01 | .072 |
| Minutes of valid wear time (6 weeks) | 0.01 | 0.01 | .219 |
| Number of exercise sessions completed | 2.6 | 1.6 | .121 |
| Change in average minutes in moderate activity per day from baseline to 6 weeks | −1.1 | 0.6 | .088 |
| Change in average minutes in sedentary time per day from baseline to 6 weeks | −0.3 | 0.2 | .154 |
Multiple R2 = .562, Adjusted R2 = .293, F = 2.086 on 16 and 26 degrees of freedom, p = .046. 6MWT, six-minute walk test; ABI, ankle-brachial index; BMI, body mass index; DCI, Diabetes Complications Index; FES-I, Falls Efficacy Scale-International; LE, lower extremity; T2DM, type 2 diabetes mellitus
4. DISCUSSION
In this sample of older adults with PAD participating in SET, we found that on average, sedentary time did not change after 12 weeks of SET, but that there was substantial interindividual variability, with changes in average minutes of sedentary time per day ranging from 40% less to 38% more at 12 weeks when compared to baseline. We also found that, on average, participants with T2DM tended to reduce their sedentary time more than individuals without T2DM. Participants with T2DM also experienced greater improvements in claudication onset distance, an average of 35 meters, compared to participants without T2DM. However, in regression analyses, neither changes in sedentary time in the first six weeks of SET nor T2DM were significant predictors of changes in 6MWT total distance from baseline to 12 weeks.
With respect to the lack of overall change observed in physical activity and sedentary time, the results of the present study are similar to those reported by Fokkenrood et al.30 Although Fokkenrood and colleagues reported that a higher number of participants met the 2007 American College of Sports Medicine and American Heart Association minimum recommendation for physical activity after three months of SET (30 minutes of moderate activity 5 days per week or 20 minutes of vigorous activity 3 days per week),62 there were no overall changes in the total number of steps or time spent in physical activity.30 Therefore, it appears that on average, participants in SET may compensate for the additional activity introduced by participating in SET by reducing their free-living physical activity, as has been observed in studies of individuals without PAD.33 However, as was observed in our study, there were large variations between participants, with some participants showing a decline in physical activity, while others did not change or increased their physical activity.
This study also adds to the literature with respect to differential responses to SET among individuals with T2DM and potential sources of variability in response to SET programs. This pilot study is one of only a few that was specifically designed to examine differences in outcomes between patients with and without T2DM, and the first to examine these differences in a clinically-available, Centers for Medicare and Medicaid Services (CMS)-reimbursed SET program.63 We found that T2DM was not associated with poorer response to SET. Conversely, individuals with T2DM exhibited greater improvements in claudication onset distance, as measured with the 6MWT, when compared with individuals without T2DM. Our findings are similar to those of Ubels et al.64 who reported that patients with T2DM attained greater relative gains in maximal walking distance as measured by a graded treadmill test when compared to patients without T2DM. However, in a study of home-based walking with only participants with PAD with comorbid T2DM, Collins et al. did not observe a significant improvement in claudication onset distance.65
It is notable that the studies that have evaluated the impact of T2DM on response to date have used graded treadmill tests to examine outcomes, while we assessed claudication onset distance and total distance using the 6MWT, which may be why our results are not fully consistent with the available literature.22 However, it has been argued that the 6MWT is perhaps a better functional outcome test than a graded treadmill test in individuals with PAD as it is more representative of walking in daily life66 and thus is important to evaluate. Additionally, graded treadmill testing to obtain maximal walking time and pain-free walking time are not standard outcome measures of SET as implemented in the health system in which the study was conducted. This study is unique in that it focused on the population of patients referred by a healthcare provider for SET following the initiation of the CMS-reimbursed SET program.63 As the availability of SET programs increases, it is important to examine how factors such as T2DM influence real-world outcomes of this first-line therapy.
Although we did find that current smoking was related to greater reductions in the length of sedentary bouts over 12 weeks, a very small sample size (n = 4) of individuals who were currently smoking limits potential conclusions regarding the role of smoking in changes in sedentary bout length. Rather than an indication that smoking is a positive factor, this reduction in bout length may have been due to individuals getting up to smoke more frequently, and thus shortening their sedentary bout length. It is unclear what effect engaging in SET may have on overall smoking habits.
An additional finding of our study was that individuals with T2DM were more likely to achieve a clinically meaningful reduction in average minutes sedentary per day of ≥30 minutes61 than participants without T2DM. Reasons for these differences in changes in sedentary time are unclear, but it is possible that participants with T2DM experienced positive metabolic changes with respect to glycemic control as a result of the aerobic exercise that led to improved energy and well-being, and as a result, greater activity outside of SET. We did not observe any direct evidence that sedentary behavior changes are associated with changes in key outcomes of SET after adjusting for other key variables, however, the substantial variability observed may have hindered our ability to detect any differences, if present. Future research should investigate reasons for the observed variability in activity patterns.
4.2. Study Strengths and Limitations
This study has several important strengths. First is the use of accelerometry for the objective assessment of multiple characteristics of sedentary behavior throughout SET. This is the first study to examine how patterns of sedentary behavior are impacted by SET participation, particularly with respect to the length and number of sedentary bouts and breaks in sedentary behavior. This study provides preliminary data for a larger-scale investigation of what factors lead to reductions or increases in sedentary behavior during SET and, if those are modifiable, how they could be addressed to promote reduction in sedentary behavior. Second, we also had high and consistent adherence to wearing the accelerometer, which resulted in no missing or invalid accelerometer files for the 44 participants who completed the study. Third, this study provides unique insight into characteristics, outcomes, and behavior patterns of patients enrolled in a clinically-available, CMS-reimbursed SET program. The CMS decision in mid-201763 provides an opportunity to examine SET outcomes among patients who, on average, have a greater number of cardiovascular, pulmonary, and orthopedic comorbid conditions compared to patients often enrolled in large randomized controlled trials.67 Finally, we were able to address a weakness noted by previous studies – the lack of even distribution of patients by gender. Women are frequently underrepresented in PAD clinical research68 and our even distribution by gender adds to our understanding of SET outcomes in both men and women with and without T2DM.
Limitations of the present study include limited generalizability of the study findings due to the racial and ethnic makeup of the study sample, although it was typical of the locations in which these rehabilitation sites are located within the Twin Cities metropolitan area. Second, our relatively small sample size and substantial variability between participants may have limited our power to detect changes in sedentary behavior and differences between participants with and without T2DM. Larger studies are needed to more fully examine these potential relationships. Third, due to differences in accelerometer data processing and scoring algorithms selected, along with using the wrist wear adjustment in ActiLife, the absolute values time spent in various activities are not comparable across studies or individuals. However, since we examined changes within each individual, the potential bias of the device and that introduced by the scoring procedure selected was minimized. Fourth, we limited our analyses to sedentary break and bout lengths, but future studies should consider more detailed analysis of day-to-day physical activity and sedentary behavior patterns due to emerging research about the unique impact of behavior patterns.69 Fifth, although we did assess T2DM complications, we did not collect data on duration of T2DM. Therefore, it is unknown how disease duration or complications of which the participants were unaware may have influenced the observed relationships. Sixth, during the performance of the 6MWT participants were instructed to notify the assessor of claudication onset, but to comply with the standard 6MWT protocol,39 participants were not asked about the presence of claudication repeatedly. This may have resulted in an overestimation of claudication onset distance or led to some missing data on claudication onset distance for some participants. Finally, since the present study was embedded into an existing SET program, we did not include a control group, and thus could not account for natural changes in activity patterns or physical function over time.
5. CONCLUSION
This study adds to our understanding of changes in free-living sedentary time of older adults with PAD while participating in a clinically available SET program. It quantified changes that occurred in sedentary time during the course of a 12-week SET program and explored the relationships between changes in free-living sedentary time and physical function in this population. Our work adds additional data to support the conclusion that patients with PAD and comorbid T2DM do not experience a blunted response to SET and may actually experience greater relative improvements in distance to symptom onset. As SET becomes more readily available, it is important to examine other factors or characteristics that may influence SET outcomes. Additional exploration of nonresponse is also needed through evaluation of other relevant outcomes (e.g., gait speed, self-reported walking difficulty, and quality of life) to examine individual outcome patterns and to determine if patients respond similarly across different outcomes. This will enable a clearer understanding of individual response to SET in individuals with PAD and inform future work to couple different approaches to make exercise interventions both more effective and more widely applicable to individuals with PAD.
Acknowledgements
Dr. Whipple was a 2015-2017 National Hartford Center of Gerontological Nursing Excellence (NHCGNE) Patricia G. Archbold Scholar. The Patricia G. Archbold Scholar program is supported by a grant to the Gerontological Society of America (GSA)/NHCGNE from the John A. Hartford Foundation. This publication was made possible by a Dissertation Research Grant from the Midwest Nursing Research Society, by the National Institute of Nursing Research (NINR), NIH under a Ruth L. Kirschstein National Research Service Award (F31NR016614, PI Whipple), by the National Institute of Aging (NIA), NIH (T32AG000279, PI Schwartz), and by the National Center for Advancing Translational Sciences (NCATS), NIH (UL1TR002494, PI Blazar). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Oka RK, Sanders MG. The impact of type 2 diabetes and peripheral arterial disease on quality of life. J Vasc Nurs 2005; 23: 61–66. [DOI] [PubMed] [Google Scholar]
- 2.Mueller T, Hinterreiter F, Luft C, et al. Mortality rates and mortality predictors in patients with symptomatic peripheral artery disease stratified according to age and diabetes. J Vasc Surg 2014; 59: 1291–1299. [DOI] [PubMed] [Google Scholar]
- 3.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2017; 135: e726–e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treat-Jacobson D, McDermott MM, Bronas UG, et al. Optimal exercise programs for patients with peripheral artery disease. Circulation 2019; 139: e10–e33. [DOI] [PubMed] [Google Scholar]
- 5.Beks PJ, Mackaay AJ, de Neeling JN, et al. Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: The Hoorn study. Diabetologia 1995; 38: 86–96. [DOI] [PubMed] [Google Scholar]
- 6.Elhadd TA, Robb R, Jung RT, et al. Pilot study of prevalence of asymptomatic peripheral arterial occlusive disease in patients with diabetes attending a hospital clinic. Pract Diabetes Int 1999; 16: 163–166. [Google Scholar]
- 7.Hirsch AT, Criqui MH, Treat-Jacobson DJ, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA 2001; 286: 1317–1324. [DOI] [PubMed] [Google Scholar]
- 8.Marso SP, Hiatt WR. Peripheral arterial disease in patients with diabetes. J Am Coll Cardiol 2006; 47: 921–929. [DOI] [PubMed] [Google Scholar]
- 9.Vinik AI, Vinik EJ, Colberg SR, et al. Falls risk in older adults with type 2 diabetes. Clin Geriatr Med 2015; 31: 89–99, viii. [DOI] [PubMed] [Google Scholar]
- 10.Lane R, Ellis B, Watson L, et al. Exercise for intermittent claudication. Cochrane database Syst Rev 2014; 7: CD000990. [DOI] [PubMed] [Google Scholar]
- 11.Parmenter BJ, Raymond J, Dinnen P, et al. A systematic review of randomized controlled trials: Walking versus alternative exercise prescription as treatment for intermittent claudication. Atherosclerosis 2011; 218: 1–12. [DOI] [PubMed] [Google Scholar]
- 12.Gardner AW, Parker DE, Montgomery PS, et al. Diabetic women are poor responders to exercise rehabilitation in the treatment of claudication. J Vasc Surg 2014; 59: 1036–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohler ER, Lech G, Supple GE, et al. Impaired exercise-induced blood volume in type 2 diabetes with or without peripheral arterial disease measured by continuous-wave near-infrared spectroscopy. Diabetes Care 2006; 29: 1856–1859. [DOI] [PubMed] [Google Scholar]
- 14.Mason McClatchey P, Bauer TA, Regensteiner JG, et al. Dissociation of local and global skeletal muscle oxygen transport metrics in type 2 diabetes. J Diabetes Complications 2017; 31: 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sesti G, Antonelli Incalzi R, Bonora E, et al. Management of diabetes in older adults. Nutr Metab Cardiovasc Dis 2018; 28: 206–218. [DOI] [PubMed] [Google Scholar]
- 16.Fernández S, Parodi JC, Moscovich F, et al. Reversal of lower-extremity intermittent claudication and rest pain by hydration. Ann Vasc Surg 2018; 49: 1–7. [DOI] [PubMed] [Google Scholar]
- 17.Hageman D, Gommans LN, Scheltinga MR, et al. Effect of diabetes mellitus on walking distance parameters after supervised exercise therapy for intermittent claudication: A systematic review. Vasc Med 2017; 22: 21–27. [DOI] [PubMed] [Google Scholar]
- 18.Lyu X, Li S, Peng S, et al. Intensive walking exercise for lower extremity peripheral arterial disease: A systematic review and meta-analysis. J Diabetes 2016; 8: 363–377. [DOI] [PubMed] [Google Scholar]
- 19.Edmonds M Vascular disease in the lower limb in type 1 diabetes. Cardiovasc Endocrinol Metab 2019; 8: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zander E, Heinke P, Reindel J, et al. Peripheral arterial disease in diabetes mellitus type 1 and type 2: Are there different risk factors? Eur J Vasc Med 2002; 31: 249–254. [DOI] [PubMed] [Google Scholar]
- 21.Allen JD, Stabler T, Kenjale AA, et al. Diabetes status differentiates endothelial function and plasma nitrite response to exercise stress in peripheral arterial disease following supervised training. J Diabetes Complications 2014; 28: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Pul KM, Kruidenier LM, Nicolai SP, et al. Effect of supervised exercise therapy for intermittent claudication in patients with diabetes mellitus. Ann Vasc Surg 2012; 26: 957–963. [DOI] [PubMed] [Google Scholar]
- 23.Stamatakis E, Hamer M, Dunstan DW. Screen-based entertainment time, all-cause mortality, and cardiovascular events: population-based study with ongoing mortality and hospital events follow-up. J Am Coll Cardiol 2011; 57: 292–299. [DOI] [PubMed] [Google Scholar]
- 24.Thorp AA, Healy GN, Owen N, et al. Deleterious associations of sitting time and television viewing time with cardiometabolic risk biomarkers: Australian Diabetes, Obesity and Lifestyle (AusDiab) study 2004-2005. Diabetes Care 2010; 33: 327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijndaele K, Healy GN, Dunstan DW, et al. Increased cardiometabolic risk is associated with increased TV viewing time. Med Sci Sports Exerc 2010; 42: 1511–1518. [DOI] [PubMed] [Google Scholar]
- 26.Patel AV, Bernstein L, Deka A, et al. Leisure time spent sitting in relation to total mortality in a prospective cohort of US adults. Am J Epidemiol 2010; 172: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernandez H, Myers SA, Schieber M, et al. Quantification of daily physical activity and sedentary behavior of claudicating patients. Ann Vasc Surg 2019; 55: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loprinzi PD, Abbott K. Association of diabetic peripheral arterial disease and objectively-measured physical activity: NHANES 2003-2004. J Diabetes Metab Disord 2014; 13: 63-6581-13-63. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott MM, Liu K, Ferrucci L, et al. Greater sedentary hours and slower walking speed outside the home predict faster declines in functioning and adverse calf muscle changes in peripheral arterial disease. J Am Coll Cardiol 2011; 57: 2356–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fokkenrood HJ, Lauret GJ, Verhofstad N, et al. The effect of supervised exercise therapy on physical activity and ambulatory activities in patients with intermittent claudication. Eur J Vasc Endovasc Surg 2015; 49: 184–191. [DOI] [PubMed] [Google Scholar]
- 31.Herrmann SD, Willis EA, Honas JJ, et al. Energy intake, nonexercise physical activity, and weight loss in responders and nonresponders: The Midwest Exercise Trial 2. Obesity (Silver Spring) 2015; 23: 1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozey-Keadle S, Staudenmayer J, Libertine A, et al. Changes in sedentary time and physical activity in response to an exercise training and/or lifestyle intervention. J Phys Act Health 2014; 11: 1324–1333. [DOI] [PubMed] [Google Scholar]
- 33.Melanson EL, Keadle SK, Donnelly JE, et al. Resistance to exercise-induced weight loss: Compensatory behavioral adaptations. Med Sci Sports Exerc 2013; 45: 1600–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu G, Liu B, Sun Y, et al. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: Population based study. BMJ 2018; 361: k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tremblay MS, Aubert S, Barnes JD, et al. Sedentary Behavior Research Network (SBRN) – Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act 2017; 14: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aguilar-Farias N, Brown WJ, Peeters GM. ActiGraph GT3X+ cut-points for identifying sedentary behaviour in older adults in free-living environments. J Sci Med Sport 2014; 17:293–299. [DOI] [PubMed] [Google Scholar]
- 37.Troiano RP, Mcclain JJ, Chen KY. Evolution of accelerometer methods for physical activity research. Br J Sports Med 2014; 48: 1019–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerr J, Marinac CR, Ellis K, et al. Comparison of accelerometry methods for estimating physical activity. Med Sci Sport Exerc 2017; 49: 617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 40.Podsiadlo D, Richardson S. The Timed ‘Up & Go’: A test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 41.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994; 49: M85–94. [DOI] [PubMed] [Google Scholar]
- 42.McDermott MM, Liu K, Ferrucci L, et al. Vitamin D status, functional decline, and mortality in peripheral artery disease. Vasc Med 2014; 19: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDermott MM, Guralnik JM, Criqui MH, et al. Unsupervised exercise and mobility loss in peripheral artery disease: A randomized controlled trial. J Am Heart Assoc 2015; 4: e001659–e001659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication. JAMA 2009; 301: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc 1998; 46: 706–711. [DOI] [PubMed] [Google Scholar]
- 46.Schaubert KL, Bohannon RW. Reliability and validity of three strength measures obtained from community-dwelling elderly persons. J Strength Cond Res 2005; 19: 717–720. [DOI] [PubMed] [Google Scholar]
- 47.Regensteiner JG, Steiner JF, Panzer RJ, et al. Evaluation of walking impairment by questionnaire in patients with peripheral arterial disease. J Vasc Med Biol 1990; 2: 142–152. [Google Scholar]
- 48.Hays RD, Spritzer KL, Amtmann D, et al. Upper-extremity and mobility subdomains from the Patient-Reported Outcomes Measurement Information System (PROMIS) adult physical functioning item bank. Arch Phys Med Rehabil 2013; 94: 2291–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the Ankle-Brachial Index: A scientific statement from the American Heart Association. Circulation 2012; 126: 2890–2909. [DOI] [PubMed] [Google Scholar]
- 50.Fincke BG, Clark JA, Linzer M, et al. Assessment of long-term complications due to type 2 diabetes using patient self-report: The Diabetes Complications Index. J Ambul Care Manage 2005; 28: 262–273. [DOI] [PubMed] [Google Scholar]
- 51.Centers for Disease Control and Prevention. Chronic diseases and health promotion, http://www.cdc.gov/chronicdisease/overview/index.htm (2014).
- 52.Gorina Y, Kramarow EA. Identifying chronic conditions in medicare claims data: Evaluating the chronic condition data warehouse algorithm. Health Serv Res 2011; 46: 1610–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.National Quality Forum. Multiple Chronic Conditions Measurement Framework. Washington, D.C., 2012. [Google Scholar]
- 54.Schneider KM, O’Donnell BE, Dean D. Prevalence of multiple chronic conditions in the United States’ Medicare population. Health Qual Life Outcomes 2009; 7: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yardley L, Beyer N, Hauer K, et al. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005; 34: 614–619. [DOI] [PubMed] [Google Scholar]
- 56.Hauer K, Yardley L, Beyer N, et al. Validation of the Falls Efficacy Scale and Falls Efficacy Scale International in geriatric patients with and without cognitive impairment: results of self-report and interview-based questionnaires. Gerontology 2010; 56: 190–199. [DOI] [PubMed] [Google Scholar]
- 57.Resnick B, Jenkins LS. Testing the reliability and validity of the Self-Efficacy for Exercise Scale. Nurs Res 2000; 49: 154–159. [DOI] [PubMed] [Google Scholar]
- 58.Tudor-Locke C, Barreira T V, Schuna JM, et al. Fully automated waist-worn accelerometer algorithm for detecting children’s sleep-period time separate from 24-h physical activity or sedentary behaviors. Appl Physiol NutrMetab 2014; 39: 53–57. [DOI] [PubMed] [Google Scholar]
- 59.Choi L, Liu Z, Matthews CE, et al. Validation of accelerometer wear and nonwear time. Med Sci Sport Exerc 2011; 43: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Keadle SK, Shiroma EJ, Freedson PS, et al. Impact of accelerometer data processing decisions on the sample size, wear time and physical activity level of a large cohort study. BMC Public Health 2014; 14: 1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buman MP, Winkler EA, Kurka JM, et al. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005-2006. Am J Epidemiol 2014; 179: 323–334. [DOI] [PubMed] [Google Scholar]
- 62.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007; 39: 1435–1445. [DOI] [PubMed] [Google Scholar]
- 63.Centers for Medicare and Medicaid Services. Decision memo for supervised exercise therapy (SET) for symptomatic peripheral artery disease (PAD) (CAG-00449N), https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=287 (2017).
- 64.Ubels FL, Links TP, Sluiter WJ, et al. Walking training for intermittent claudication in diabetes. Diabetes Care 1999; 22: 198–201. [DOI] [PubMed] [Google Scholar]
- 65.Collins TC, Lunos S, Carlson T, et al. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: A randomized controlled trial. Diabetes Care 2011; 34: 2174–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McDermott MM, Guralnik JM, Criqui MH, et al. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014; 130: 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dörenkamp S, Mesters EPE, Sanden MWGN Der, et al. How well do randomized controlled trials reflect standard care: A comparison between scientific research data and standard care data in patients with intermittent claudication undergoing supervised exercise therapy. PLoS One 2016; 11: e0157921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fakhry F, van de Luijtgaarden KM, Bax L, et al. Supervised walking therapy in patients with intermittent claudication. J Vasc Surg 2012; 56: 1132–1142. [DOI] [PubMed] [Google Scholar]
- 69.Bellettiere J, Winkler EAH, Chastin SFM, et al. Associations of sitting accumulation patterns with cardio-metabolic risk biomarkers in Australian adults. PLoS One 2017; 12:e0180119. [DOI] [PMC free article] [PubMed] [Google Scholar]


