Abstract
Understanding and targeting of GPCRs remains a critical aspect of airway pharmacology and therapeutics for diseases such as asthma or COPD. Most attention has been on the large Class A GPCRs towards improved bronchodilation and blunting of remodeling. Better known in the central or peripheral nervous system, there is increasing evidence that Class C GPCRs which include metabotropic glutamate and GABA receptors, the calcium sensing receptor, sweet/umami taste receptors and a number of orphan receptors, can contribute to airway structure and function. In this review, we will summarize current state of knowledge regarding the pharmacology of Class C GPCRs, their expression and potential functions in the airways, and the application of pharmacological agents targeting this group in the context of airway diseases.
Keywords: Airway, Asthma, G-protein coupled receptor, calcium, cAMP, contractility, remodeling, Venus fly trap, dimerization
Introduction
G protein–coupled receptors (GPCRs) are one of the largest family of membrane proteins that transduce extracellular signals into a diversity of intracellular pathways to influence every aspect of cellular function. Classical GPCR signaling involves 7-transmembrane peptide chains coupled to G proteins (Gαβγ heterotrimers) that dissociate with agonist binding, with downstream coupling to Gs, Gi/o, Gq/11 vs. G12/13 [1–4]. Recruitment of intracellular heterotrimeric G proteins, and associated β-arrestins or G-protein related kinases control the extent of GPCR activity [4]. GPCRs remain a major aspect of pharmacological targeting across a range of diseases, representing almost a third of FDA approved drugs [5,6]. In this regard, targeting of airway and lung diseases such as asthma, COPD and even pulmonary fibrosis has long-involved modulation of GPCRs or related mechanisms [3,4,7,8], with classical targets being muscarinic and leukotriene receptor blockade and beta2-adrenoceptor activation to induce bronchodilation, and emerging focus on prostaglandin receptor subtypes, adenosine A2B and histamine H4 receptors in obstructive diseases [7]. Conversely, recognizing detrimental effects of some GPCRs (particularly adrenoceptors), there is increasing interest in the use of biased agonists that recruit and maintain beneficial effects of GPCRs [9,10]. In these many efforts, GPCRs on airway smooth muscle (ASM) particularly of the bronchioles, have been the major target. While different airway cell types contribute to overall structure and function, the critical role of ASM in regulating tone and contractility (balance between contractile vs. dilatory processes) cannot be argued, particularly in the context of airway hyperreactivity (AHR). Beyond AHR, the effect of inflammatory mediators, growth factors and local mediators on ASM cell hyperplasia and/or hypertrophy and modification in the extracellular matrix (ECM) are important for airway stiffening and fibrosis (remodeling) [11], and can themselves be influenced by GPCRs.
Much attention has been understandably on the large Class A GPCRs [12] that incorporates small-molecule ligands including biogenic amines, neuropeptides and glycoprotein hormones. The smaller Class B GPCRs involving glucagon and secretin has been less explored although there is evidence that glucagon is a bronchodilator [13]. However, there is limited data on the much larger family of Class C GPCRs that consists of 22 different GPCRs (Figure 1): the calcium sensing receptor (CaSR), two metabotropic GABAB receptors (GABAB1R and GABAB2R), eight metabotropic glutamate (mGluR1–8) receptors, three taste receptors (T1R1–3), the GPRC6, and 7 orphan receptors (GPR156, GPR158, GPR179, GPRC5A-D) [14,15]. Class C receptors respond to nutrients such as a range of amino acids, ions (particularly Ca2+ but also protons), and sugar molecules (in the context of taste) (Table 1), and thus serve important physiological processes across organ systems, making them highly appealing drug targets [16–20]. Here GABABRs, CaSR and mGluRs are particularly well-studied [14,19,20]. These receptors differ substantially from Class A or B in that they share a unique structure composed of a very large extracellular domain and the ability to form constitutive dimers [14] (Figure 2). Indeed, homo-and heterodimers are characteristic features of Class C GPCRs (Table 1) and are obligatory for functionality with extracellular aspects involving a Venus flytrap (VFT) domain and highly conserved nine-cysteine residue rich domain (CRD; except for GABABR that lack CRD) [14,21–24] (Figure 2). Notably, there is no significant amino acid sequence overlap between the 7TM domains of Class C vs. Class A or B GPCRs [14,21–24].
Figure 1:
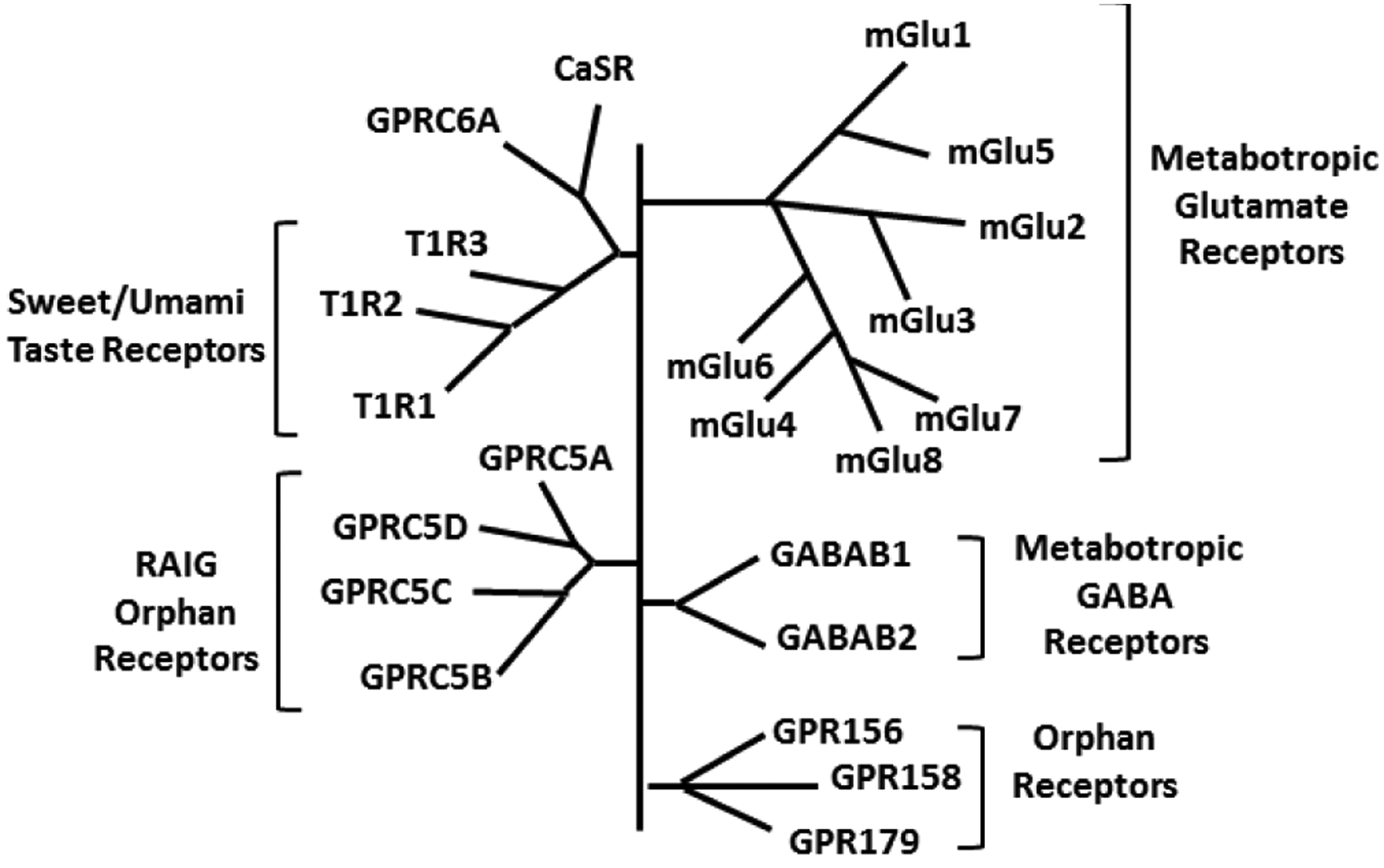
Class C GPCR Family. This class consists of 22 GPCRs including the calcium sensing receptor (CaSR) and the related promiscuous receptor GPRC6, two metabotropic GABAB receptors (GABAB1R and GABAB2R), eight metabotropic glutamate receptors (mGluR1–8), three taste receptors (T1R1–3), and 7 orphan receptors (GPR156, GPR158, GPR179, GPRC5A-D).
Table 1:
Class C GPCR Members
| Member | Pharmacological Modulators | Dimerization | Roles in Lung |
|---|---|---|---|
|
Metabotropic GABA: GABABR1, GABABR2 |
Ligand: GABA Agonist: Baclofen, CGP27492 Antagonist: Phaclofen, Saclofen, CGP36742, CGP35348, CGP55845 PAM: CGP7930 |
Heterodimer | ASM and epithelium ↑ airway contractility ↑ mucus production |
| CaSR |
Ligand: Ca2+, spermine Agonist: Ca2+, Mg2+, polyvalent cations, β-amyloid, aminoglycoside antibiotics PAM: pH, L-α-amino acids, NPS R467, NPS R568, cinacalcet NAM: NPS 2143, Calhex 231 |
Homodimer Heterodimer (mGlu Grp I) | ASM and epithelium ↑ airway and pulmonary vascular contractility ↑ cell proliferation ↑ airway fluid secretion ↑ epithelial inflammation |
| GPRC6A | Ligand: L-α-amino acid | Homodimer | Unkno ‘n |
|
Sweet/Umami Taste: T1R1, T1R2, T1R3 |
Ligand: T1R1/T1R3: L-glutamate; T1R2/T1R3: Sugars | Homodimer Heterodir er (T1R1/T1R3, T1R2/T1R3) | Unknown (potential airway immune modulation) |
|
Metabotropic Glutamate Grp I: mGluR1, mGluR5 |
Ligand: Glutamate, ACPD Agonist: DHPG, CHPG, LY339764 Antagonist: CPPCOEt, BAY36–7620, Ro 674853 |
Homodimer PHetei’dimer (‘rp |’, III) | Unknown |
|
Metabotropic Glutamate Grp II: mGluR2, mGluR3 |
Ligand: Glutamate, ACPD Agonist: LY341495, LY354740 Antagonist: Ro 64–5229, LY487379 |
Homodimer | Unknown |
|
Metabotropic Glutamate Grp III mGluR4, mGluR6, mGluR7, mGluR8 |
Ligand: Glutamate, ACPD Agonist: MAP4, DCPG Antagonist: PHCCC |
Homodimer | Unknown |
| RAIG Orphan: GPRC5A, GPRC5B, GPRC5C, GPRC5D | Unknown | Unknown | Unknown (GPRC5A may be most relevant) |
| Other Orphan: GPR156, GPR158, GPR179 | Unkno n | Unknown | Unknown |
Figure 2:
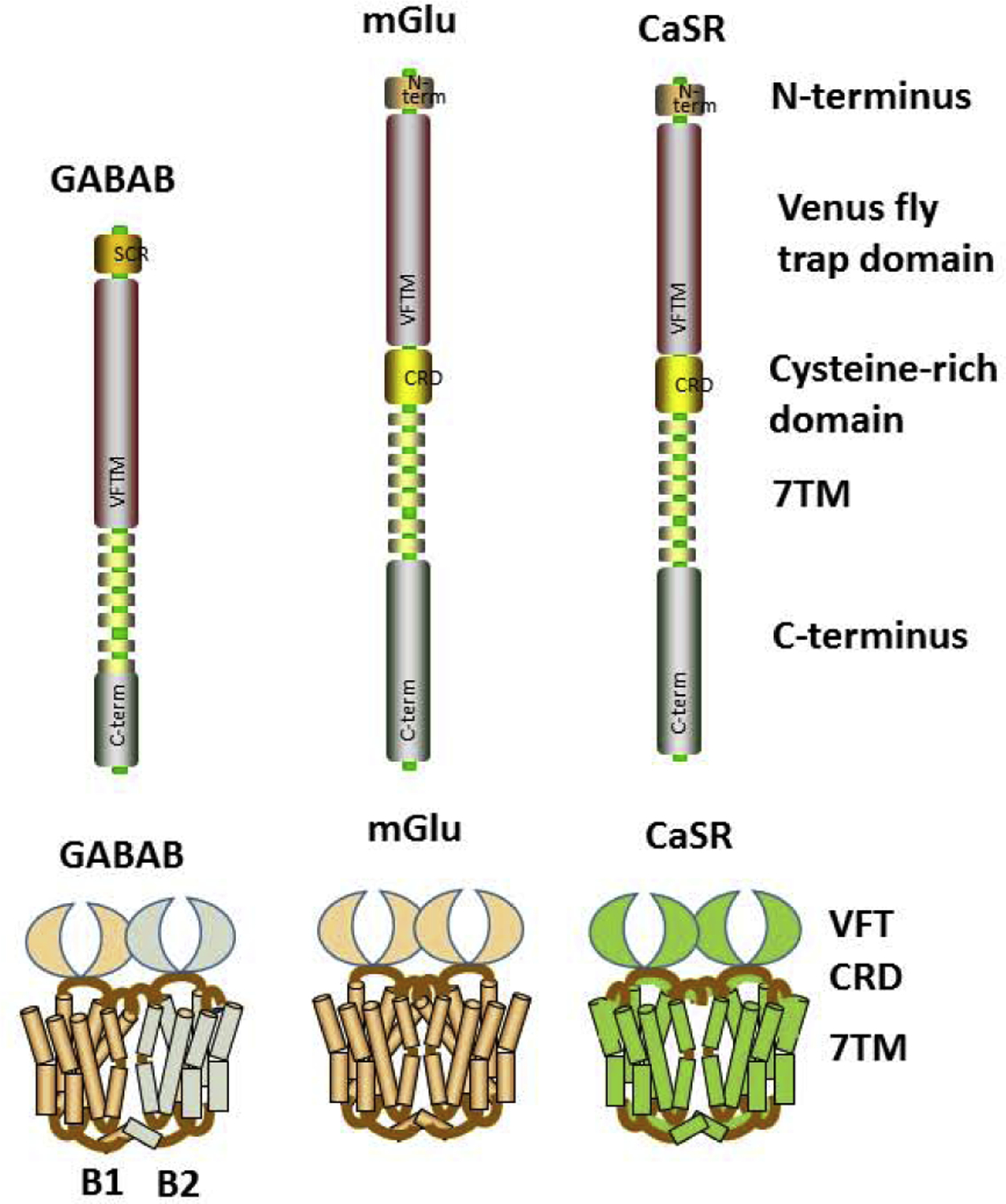
Class C GPCR Structures. Differing substantially from other GPCR classes, members of the Class C GPCR share a unique structure composed of a very large extracellular domain and the ability to form constitutive dimers, with both homo- and heterodimerization depending on the members, and being obligatory for functionality. A unique extracellular aspect is the Venus flytrap (VFT) domain and highly conserved nine-cysteine residue rich domain (CRD; except for GABABR that lack CRD).
There is currently limited information on expression patterns or functionality of Class C GPCRs in the lung, or particularly in airways. The metabotropic GABAB receptors are probably the most explored, with more recent information on the CaSR. The orphan receptors have been barely studied in any cell system, but there are emerging data on specific receptors (see below). In this brief review, we explore current state of knowledge regarding Class C GPCRs particularly in the airways in the context of AHR and remodeling relevant to asthma, and identify potential directions for exploring this class of GPCRs as therapeutic targets.
GABAB Receptors
GABA is well-known as the major inhibitory neurotransmitter in the mammalian central nervous system. As a ligand, GABA is relevant to airways in multiple ways, not the least being its involvement in brainstem circuits that control efferent neuronal output to the airways [25]. Furthermore, GABABR agonists can attenuate parasympathetic-cholinergic responses in the airways, indirectly attenuating cough responses [26,27]. Here, there is interest in novel GABABR agonists such as Lesogaberan in treatment of acute and chronic cough [28]. However beyond neuronal control and airway irritability, there is now evidence that both pulmonary neuroendocrine cells [29] as well as airway epithelium [*30] have the necessary machinery to produce and release GABA, thus creating the potential for local action on neighboring mesenchymal cells including ASM. Here, it is important to distinguish between the two distinct activation pathways by which GABA can act: ligand-gated ionotropic GABAA receptors that are chloride channels and the metabotropic GABAB Class C GPCRs. There is substantial evidence that ASM cells including those in humans express GABAARs and that GABAAR agonists induce bronchodilation [31–33]. Additionally, functional GABABRs in ASM [26,34] and epithelium [30] have been found. However, it should be noted that clinically at least, the GABABR agonist baclofen has been found to actually worsen methacholine responses in asthmatic airways [35] which raises questions as to the mechanisms of action. Interestingly, although acting through Gi and decreasing cAMP (Figure 3), both baclofen and GABA can increase IP3 and [Ca2+]i in airways [34], such that the selective GABAB antagonists CGP35348 or CGP55845 blunt such increases, but yet pertussis toxin which inactivates Gi is also effective. This interesting discrepancy was determined to involve Gβγ mediated effects on PLCβ, and potentiation of Gq signaling (explaining increased IP3) following GABABR activation where the Gβγ inhibitor gallein and PLCβ inhibitor U73122 are effective [35,36]. What is not clear is the upstream GPCR that is linked to the Gq within this pathway. Given the possibility of heterodimerization among Class C GPCRs, one possibility is Gq-coupled entities such as mGluRs or the CaSR: aspects that are currently unexplored. Separately a key aspect of GABABR signaling is that unlike prototypical GPCRs such as β2-adrenoceptors, prolonged agonist exposure does not result in phosphorylation of GPCR kinases, i.e. GRKs, towards termination of GPCR activity and GABABR internalization via recruitment of β-arrestins [37]. GABABR can constitutively undergo clathrin-dependent endocytosis that is not influenced by length of agonist exposure [37]. Conversely, GABABRs can be phosphorylated by cAMP-dependent protein kinase that in fact sustains receptor presence at the plasma membrane and promotes activity [37]. Given the importance of cAMP in ASM and asthma, the potential exists for more complex interactions between GABABR and other GPCRs involved in airway biology.
Figure 3:
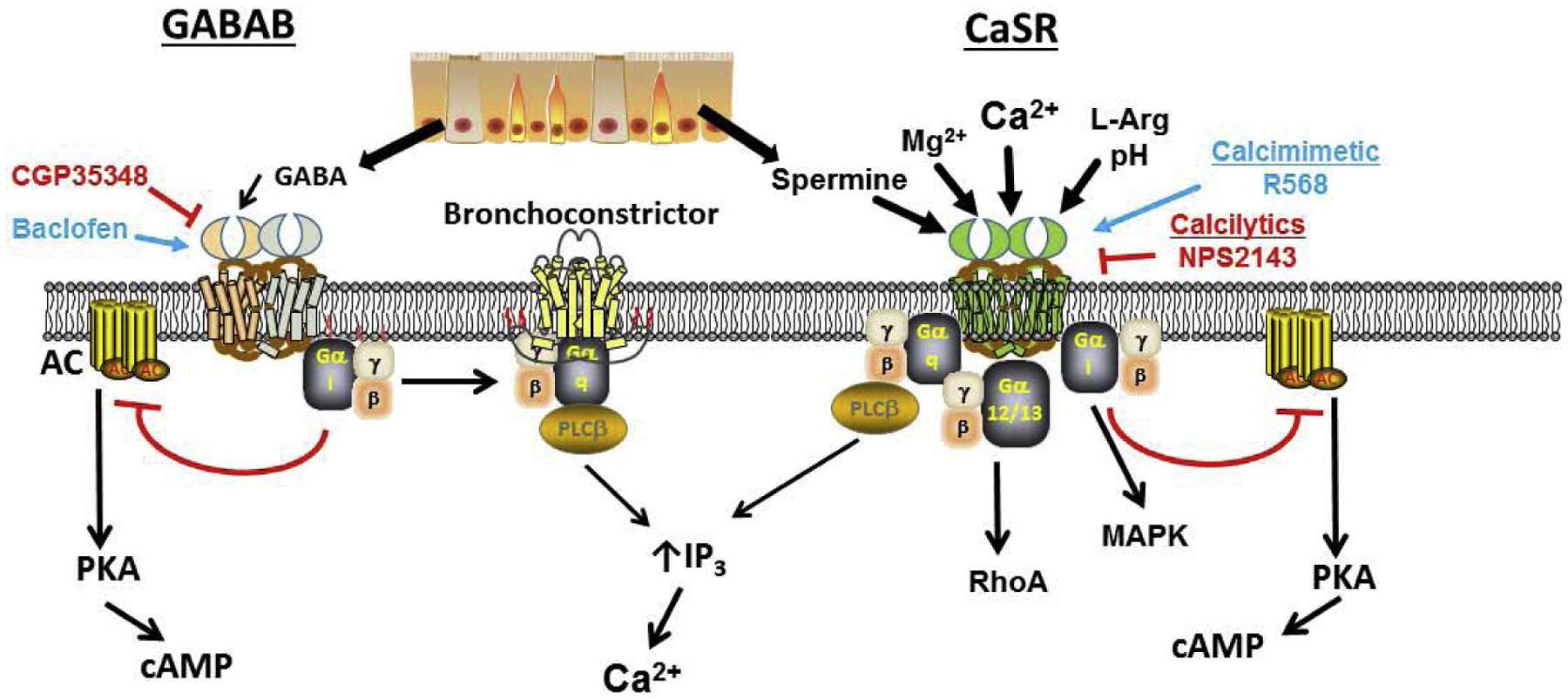
Signaling of GABA and CaSR in airways. While there may be variations in signaling across tissues, the data from human airway smooth muscle (ASM) show that metabotropic GABAB receptors signal through Gi to inhibit cAMP, but interestingly increases [Ca2+]i by cross-activation of Gs (that is typically associated with bronchoconstriction) and through PLC/IP3. In contrast, the CaSR can act through Gq to increase [Ca2+]i, but can also inhibit cAMP via Gi, and furthermore increase RhoA via G12/13.
In addition to ASM, it appears that local GABA signaling also involves epithelium. A recent study found that GABA in lungs of nonhuman primates as well as human and found PNECs to be the major source [29]. Importantly, in an infant primate model of asthma, there is increased GABA secretion and elevated levels GABAA and GABAB receptors in the epithelium that can contribute to increased mucus production and goblet cell proliferation [29]. While there is currently no data on ASM remodeling by GABA, baclofen has been shown to inhibit adenylyl cyclase activity and induce ERK phosphorylation in human ASM [34] which should have downstream influences on cell proliferation or even fibrosis.
CaSR
Originally cloned from bovine parathyroid cells, the CaSR has been a major therapeutic focus given its significant role in regulating calcium homeostasis [18,38–43]. The CaSR senses and maintains extracellular Ca2+ concentration within a tight range via regulation of three calciotropic hormones: secretion of parathyroid hormone (PTH) by the parathyroid gland, calcitonin secretion by thyroid C-cells, and renal conversion of vitamin D to its active from. These hormones then control intestinal absorption and renal and bone resorption of Ca2+. The therapeutic importance of CaSR lies in the fact that it is one of the few GPCRs with genetic mutations linked to >100 clinically important conditions, examples including familial hypocalciuric hypercalcemia, and autosomal dominant hypocalcemia [19].
An important aspect of CaSR function is that is activated by millimolar concentrations of extracellular Ca2+ and Mg2+ (recall that intracellular Ca2+ concentrations do not exceed tens of micromolar). Compared to GABABR, the CaSR is quite promiscuous, and other orthosteric agonists include cations (Ba2+, Gd3+), importantly charged polyvalent molecules including spermine, spermidine and eosinophil cationic peptide, b-amyloid peptides, and aminoglycoside antibiotics can all activate it (Figure 3, Table 1), overall making the CaSR a multi-modal sensor [18,38–41]. Ca2+ is the most potent and a full agonist, with Mg2+ and Ba2+ acting as partial agonists. CaSR sensitivity to Ca2+ is positively modulated by pH and L-amino acids (consistent with its role in the GI system).
From a signaling standpoint, the CaSR is also unique in that it can work through Gq/11, Gi/o, and G12/13 [42,43] such that Gq/11 activation lead to PLCβ, IP3 and DAG towards increasing [Ca2+]I, PKC phosphorylation and MAPK signaling, the latter also resulting from Gi/o activation. CaSR can also activate RhoA/ROCK in the context of cell contractility. In terms of desensitization, there is lack of consensus regarding CaSR internalization, with some studies showing constitutive internalization and recycling to the plasma membrane independent of agonist activation and others showing more classical processes [44,45]. Nonetheless, unlike GABABR that is not a GRK target, CaSR does interact with kinases and β-arrestins towards functional desensitization [45].
Given its critical physiological roles, there has been substantial interest in positive allosteric modulators (calcimimetics such as NPS R-568 and cinacalcet) that enhance CaSR sensitivity to Ca2+, reducing PTH secretion and enhancing calcitonin towards stabilizing systemic Ca2+ levels: relevant to treatment of hyperparathyroidism. Similarly, negative allosteric modulators (calcilytics such as NPS 2143 and Calhex 231) antagonize parathyroid CaSR to enhance PTH secretion, effective for targeting bone growth and osteoporosis [17–20,43].
It is now recognized that multiple non-calciotropic organs including vasculature and the lung also express CaSR [18,19,39,46–49], raising substantial interest in exploring more complex roles for this GPCR: an emerging area of research. Such non-calcitropic roles appear to be tissue-dependent, but include proliferation and apoptosis, differentiation, contractility, and even inflammation. For example, CaSR is expressed in human vascular smooth muscle and endothelial cells and is involved in cell proliferation and blood pressure regulation [48]. In pulmonary artery smooth muscle, CaSR increases [Ca2+]i by promoting plasma membrane influx through receptor-operated channels (particularly TRPC6) and store-operated channels (Orai1) [46]: mechanisms increased in pulmonary hypertension, where CaSR could play an important role. In this regard, the functional relevance of CaSR lies in the fact that in various organs, in addition to extracellular Ca2+ being modulator of CaSR (wherever it is expressed), epithelial and endothelial cells can also produce spermine, the endogenous agonist for CaSR. Polyamines such as spermine and spermidine are a product of the arginase pathway where L-arginine is diverted from the eNOS-mediated NO production pathway towards ornithine and further processing towards putrescine, spermidine and spermine [50,51].
The CaSR is expressed in human fetal lungs, where it is thought to promote luminal fluid secretion towards mediating lung growth and development [39]. One study has reported that epithelial CaSR works in conjunction with chloride channels and the CFTR in regulating fluid secretion [52]. The relevance of CaSR in embryonic lung lies in the fact that during fetal development free ionized [Ca2+]o in the extracellular environment is closer to 2 mM (compared to <1.5 mM in adult) with such relative fetal hypercalcemia being necessary for optimal prenatal fluid secretion. However, the expression or role of CaSR in more proximal airways, and particularly in mesenchymal cells in the context of development has been minimally explored. We recently showed that functional CaSR is expressed in developing human ASM where it responds to endogenous agonist (spermine), enhances [Ca2+]i responses to bronchoconstrictor agonist such as histamine by increasing PLC/IP3 and promoting SOCE: effects inhibited by the calcilytic NPS2143 but enhanced by the calcimimetic R-568 [53]. Furthermore, CaSR activates the MAPK pathway to promote fetal ASM proliferation [53]. In recent studies we further found that insults such as hyperoxia (relevant to oxygen exposure in premature infants requiring ventilator support) increases fetal ASM CaSR expression, and that CaSR mediates hyperoxia enhancement of [Ca2+]i and cell proliferation (Ravix, Roesler et al., unpublished observations). Such findings link CaSR to previous observations that epithelial arginase expression is increased in developing airways by insults such as hyperoxia and impairs bronchodilation [54].
The CaSR is also expressed in adult epithelium and ASM of both humans and mice [49] and is functional, responding to increasing extracellular Ca2+ and to spermine by increasing [Ca2+]i and contractility. Importantly, CaSR expression is increased in airways of asthmatic patients and in mouse models of allergic asthma [49]. Calcilytics blunt CaSR-mediated airway contractility, while nebulized calcilytics significantly suppress AHR and inflammation in mouse models [49]. In these effects, CaSR appears to reduce cAMP, increase IP3, activate RhoA/ROCK and promote pro-proliferative pathways including ERK1/2. An interesting observation is that such effects are largely present in asthmatic airways [49], an advantage in terms of therapeutic targeting, although the mechanisms that promote CaSR expression and functionality (e.g. gene/protein effects vs. trafficking) remain to be explored. The relevance of these findings again lies in the known upregulation of arginase pathways in adult asthma [50,51] and furthermore the potential contribution of polycations such as eosinophil derived cationic and major basic proteins [49]. Spermine has indeed been found to mediate airway contraction in a house dust mite model of asthma [55]. These initial findings highlight the potential for therapeutically targeting the CaSR in conditions such as asthma: a focus of ongoing clinical trials repurposing calcilytics. Other drugs such as quinazoline and amino alcohol calcilytics also appear to be comparably potent in suppressing AHR and inflammation [49,56].The CaSR may also be relevant to COPD given many multivalent cations present in cigarette smoke and environmental pollution [57]. For example, NPS2143 inhibits MUC5AC and proinflammatory mediators in cigarette smoke exposed human airway epithelium [58]. CaSR also appears to drive epithelial inflammation following particulate matter exposure [59]. However, further study is needed in this area.
Taste receptors
Although many compounds recognized by human taste are largely sweet vs. bitter with some overlap, the mechanisms by which such tastants signal are quite different [22,60–65]. Bitter tastants are recognized by a 25-member T2R subfamily of class A GPCRs while sweet and umami tastants involve class C GPCRs (Figure 1, Table 1). Sweet taste is mediated by T1R3, which can form a homodimer or heterodimerize with T1R2, and the receptors are activated by binding of intensely sweet molecules such as artificial sweeteners or high glucose.
There has been considerable focus on bitter tastant T2Rs in the context of non-gustatory effects, particularly in the airways and asthma [60,61,66,67]. T2Rs have been identified in pulmonary chemosensory cells, ciliated epithelial cells, and ASM, and in spite of being linked to the gustducin G protein system that increases IP3/Ca2+ are now known to produce bronchodilation, potentially via indirect mechanisms involving cAMP and mitochondria [67]. There is considerably less information on sweet taste receptors but these have also been identified in extraoral tissues including pancreatic β-cells, adipocytes, cardiomyocytes and vasculature [61,64,65]. However, there is currently no information on T1R expression or function in the airways, particularly in the context of ASM, contractility or remodeling. Given substantial interest in T2Rs, and emerging data on T1R in airway immunity [65], this represents a major novel area for future exploration.
Metabotropic glutamate receptors (mGluR)
As the major excitatory CNS neurotransmitter there is substantial interest in metabotropic glutaminergic signaling pathways that are distinct from the inonotropic AMPA and NMDA receptor channels. mGluRs are divided into three groups with members within a group showing more than 60% sequence identity among each other, and <50% sequence identity between groups [14,15,24,68]. Each group also uses different signal transduction pathways, with group I receptors (R1, R5) activating Gq and thus the PLC/IP3 pathway to increase [Ca2+]I, with additional activation of Gs to stimulate AC and cAMP, while group II (R2, R3) and III (R4, R6, R7, R8) receptors work through Gi/o to inhibit adenylate cyclase and reduce cAMP (Figure 4). All three groups share pharmacological properties such that orthosteric agonists and antagonists can affect the full spectrum of receptors within a group, although there is more therapeutic potential in allosteric modulators with specific activity towards a specific mGluR subtype (Table 1). In terms of GRK and β-arrestins, mGluRs can elegantly modulate their activity where on the one hand, short-term β-arrestin activation can promote internalization while persistent mGluR activity leads to recruitment of MAP kinases that promote binding of β-arrestin and Homer1 adaptor protein to the receptor sustain its activity [69].
Figure 4:
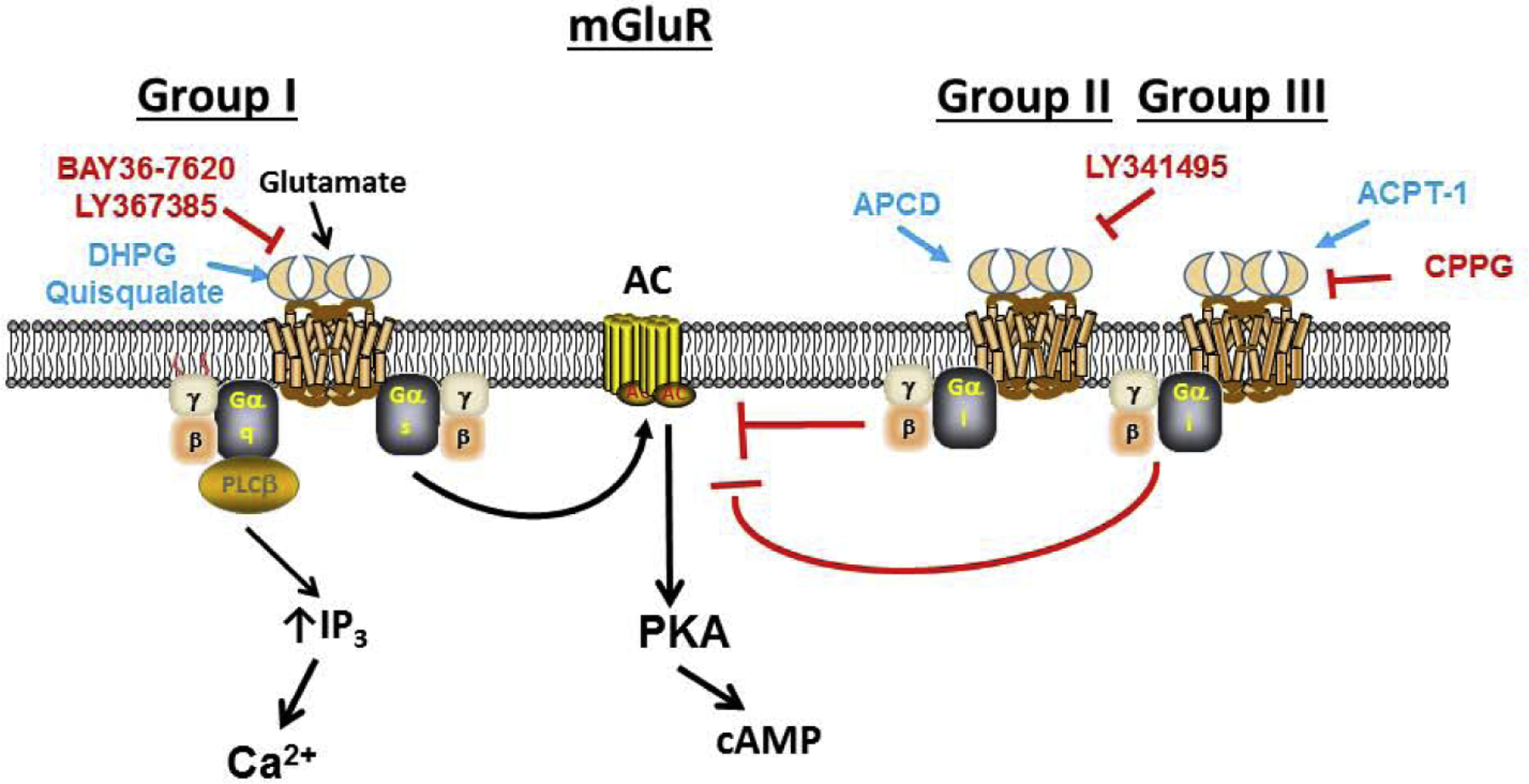
Signaling of mGluRs. Within the three groups of metabotropic glutamate receptors, each group uses different signal transduction pathways, with group I receptors (R1, R5) activating Gq and thus the PLC/IP3 pathway to increase [Ca2+]I, with additional activation of Gs to stimulate AC and cAMP, while group II (R2, R3) and III (R4, R6, R7, R8) receptors work through Gi/o to inhibit AC and reduce cAMP.
mGluRs have been found in a variety of peripheral tissues including bone, heart, GI system etc. with substantial heterogeneity in groups and members expressed within specific cell types [14,15,24,68]. mGluR4 appears most broadly expressed, and thus at least one major role of peripheral mGluRs is reducing cAMP, however, it is important to emphasize that group/member portfolios allow for much more complex effects based on cell and context. There is surprisingly little information on mGluR expression patterns or functionality in the lung, beyond emerging data in lung cancers. However, given ability to act through Gq, Gs or Gi/o, a number of airway-relevant functions could be modulated by mGlu signaling: again a novel and unmet research area.
GPRC5
There is substantial interest, albeit limited information on what are termed the retinoic acid-inducible GPCRs (RAIGs) [14,70]: GPRC5A, GPRC5B, and GPRC5C are induced by retinoic acid in a concentration- and time-dependent manner (while GPRC5D is not) . Unlike other Class C GPCRs whose ligand binding sites are within the large N–terminal domain, for GPRC5 receptors that have short N-terminals, binding occurs in the 7TM domains. However, the endogenous ligand for GPRC5s is unknown. GPRC5A–D are expressed in a highly tissue-specific fashion with GPRC5A being preferentially expressed in lung tissues and GPRC5B in the CNS. Importantly, a number of inflammatory and oncogenic pathways can regulate GPRC5 (particularly GPRC5A) expression, and there increasing evidence of their dysregulation in a variety of cancers including lung adenocarcinoma. Again, there is currently no information on GPRC5 isoform expression in the airways or in ASM and whether or how they contribute to airway structure and function. However, it is important to note that knockout studies show GPRC5A can impact on cAMP signaling, and blunt NFkB and STAT3 signaling, thus suppressing cell proliferation [70]. Conversely, GPRC5A expression is substantially altered by cAMP and inflammatory factors. Thus the potential exists for GPRC5A to play a role in airway inflammation and remodeling.
GPRC6A
GPRC6A has been cloned from multiple species including human and mRNA has been identified in a variety of organs including brain, lung, liver, heart and kidney [14,16,71]. As with the CaSR, to which it is most homologous, GPRC6A is activated by a range of basic and small aliphatic L-α-amino acids particularly L-arginine, L-lysine and L-ornithine. It is directly activated or positively modulated by divalent cations such as Ca2+ but at a much higher, non-physiological level of 5 mM. Recent studies suggest that the peptide osteocalcin and the sex steroid testosterone can act as endogenous GPRC6A agonists [16,71], and that similar to β2-adrenoceptors, GPRC6A is modulated by GRKs and β-arresitins [72]. Thus, GPRC6A could have a range of physiological functions, and studies largely using knockout mice show involvement in regulation of inflammation, metabolism and endocrine function. There is currently no information on GPRC6A expression or function in the airways, but given its similarities to the CaSR and its modulation by factors that may be important in airway structure/function or disease, this promiscuous receptor may also be a novel target to understand.
Regulation of Class C GPCRs
While the above discussion highlights the expression and potential downstream effects of some Class C GPCRs in the airway, there is limited understanding of how their expression is altered in disease. Certainly, upregulation of CaSR or GABABRs in asthma suggest effects of inflammatory mediators, as shown for effects of TNFa and IL-13 for CaSR in ASM for example [49]. Although its function in airway is unknown, GPRC5A expression can be modulated by cAMP or inflammation. However, as the role of Class C GPCRs in airway diseases comes to the forefront, understanding upstream regulatory pathways is another major unmet research avenue. Here, beyond genomic/transcriptomic regulation of protein expression, altered GPCR expression at the plasma membrane and/or sensitization via GRKs and β-arrestins provide another layer of regulation which may be different in disease: a relevant aspect, given increasing understanding and interest in these GPCR regulatory pathways in ASM and asthma in the context of biased agonists [73,74].
Heterogeneity through dimerization
Dimerization is a key aspect of Class C GPCR functionality. mGluRs and CaSR form homodimers, while GABABR1 and GABABR2 heterodimerize and traffic to the plasma surface in order to become functional [14], and taste receptors T1R1 and T1R2 form heterodimers with T1R3 to become functional [61,64]. In vitro FRET studies show that group I mGlu (R1, R5) can heterodimerize with the other two groups [75] but their relevance is unclear. Interestingly, the CaSR can heterodimerize with mGlu1 and mGlu5 [76]. The CaSR is also the closest mammalian homolog of GPRC6A and both receptors are widely expressed with overlapping expression patterns [77]. Thus, depending on expression patterns and context, the potential exists for substantial heterogeneity and complexity in Class C GPCR signaling. Whether such homo/heterodimerization is important in the airways remains totally unknown, but points to novel approaches in targeting this class of GPCRs using combination therapies, particularly when involving different G-proteins.
Conclusions
Several members of the Class C family of GPCRs are demonstrating their potential relevance in airway structure and particularly function in the context of diseases as asthma. While there is substantially more research that is required, data from several other organ systems relating to their structures, dimerization and other aspects of their function, as well as importantly, availability of positive/negative allosteric modulators and inhibitors/activators facilitates exploration of this family of GPCRs in lung diseases.
Supplementary Material
Acknowledgements:
Supported by NIH grants HL056470 (Prakash), HL056470-S1 (Ravix), and HL138402 (Pabelick).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts: The authors have no conflicts of interest.
References
- 1.Billington CK, Penn RB: Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res 2003, 4:2. [PMC free article] [PubMed] [Google Scholar]
- 2.Deshpande DA, Penn RB: Targeting G protein-coupled receptor signaling in asthma. Cell Signal 2006, 18:2105–2120. [DOI] [PubMed] [Google Scholar]
- 3.Johnson EN, Druey KM: Heterotrimeric G protein signaling: role in asthma and allergic inflammation. J Allergy Clin Immunol 2002, 109:592–602. [DOI] [PubMed] [Google Scholar]
- 4.Penn RB, Bond RA, Walker JK: GPCRs and arrestins in airways: implications for asthma. Handb Exp Pharmacol 2014, 219:387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Insel PA, Sriram K, Gorr MW, Wiley SZ, Michkov A, Salmeron C, Chinn AM: GPCRomics: An Approach to Discover GPCR Drug Targets. Trends Pharmacol Sci 2019, 40:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sriram K, Insel PA: G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol Pharmacol 2018, 93:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Wendell SG, Fan H, Zhang C: G Protein-Coupled Receptors in Asthma Therapy: Pharmacology and Drug Action. Pharmacol Rev 2020, 72:1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]; A recent review on GPCRs in asthma that highlights estbalished knowledge and emering areas of interest.
- 8.Wright DB, Tripathi S, Sikarwar A, Santosh KT, Perez-Zoghbi J, Ojo OO, Irechukwu N, Ward JP, Schaafsma D: Regulation of GPCR-mediated smooth muscle contraction: implications for asthma and pulmonary hypertension. Pulm Pharmacol Ther 2013, 26:121–131. [DOI] [PubMed] [Google Scholar]
- 9.Denis C, Saulière A, Galandrin S, Sénard J-M, Galés C: Probing heterotrimeric G protein activation: applications to biased ligands. Current pharmaceutical design 2012, 18:128–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pera T, Penn RB: Bronchoprotection and bronchorelaxation in asthma: New targets, and new ways to target the old ones. Pharmacol Ther 2016, 164:82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *11.Prakash YS: Emerging concepts in smooth muscle contributions to airway structure and function: implications for health and disease. Am J Physiol Lung Cell Mol Physiol 2016, 311:L1113–l1140. [DOI] [PMC free article] [PubMed] [Google Scholar]; A brief summary of novel signaling mechanisms in airway smooth msucle, also highlighting the importance of this cell type in airway diseases.
- 12.Cong X, Topin J, Golebiowski J: Class A GPCRs: Structure, Function, Modeling and Structure-based Ligand Design. Curr Pharm Des 2017, 23:4390–4409. [DOI] [PubMed] [Google Scholar]
- 13.Wilber ST, Wilson JE, Blanda M, Gerson LW, Meerbaum SO, Janas G: The bronchodilator effect of intravenous glucagon in asthma exacerbation: a randomized, controlled trial. Ann Emerg Med 2000, 36:427–431. [DOI] [PubMed] [Google Scholar]
- *14.Brauner-Osborne H, Wellendorph P, Jensen AA: Structure, pharmacology and therapeutic prospects of family C G-protein coupled receptors. Curr Drug Targets 2007, 8:169–184. [DOI] [PubMed] [Google Scholar]; A comprehensive review on Class C GPCRs highlighting the novel aspects of their structure that has driven the search for activators or inhibitors, particularly for clinical use.
- 15.Urwyler S: Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol Rev 2011, 63:59–126. [DOI] [PubMed] [Google Scholar]
- 16.Clemmensen C, Smajilovic S, Wellendorph P, Brauner-Osborne H: The GPCR, class C, group 6, subtype A (GPRC6A) receptor: from cloning to physiological function. Br J Pharmacol 2014, 171:1129–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomsen AR, Smajilovic S, Brauner-Osborne H: Novel strategies in drug discovery of the calcium-sensing receptor based on biased signaling. Curr Drug Targets 2012, 13:1324–1335. [DOI] [PubMed] [Google Scholar]
- *18.Brennan SC, Thiem U, Roth S, Aggarwal A, Fetahu I, Tennakoon S, Gomes AR, Brandi ML, Bruggeman F, Mentaverri R, et al. : Calcium sensing receptor signalling in physiology and cancer. Biochim Biophys Acta 2013, 1833:1732–1744. [DOI] [PubMed] [Google Scholar]; A review on the CaSR that is finding renewed importance and relevance in non-calciotropic organs and conditions
- 19.Hannan FM, Kallay E, Chang W, Brandi ML, Thakker RV: The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases. Nat Rev Endocrinol 2018, 15:33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemeth EF, Shoback D: Calcimimetic and calcilytic drugs for treating bone and mineral-related disorders. Best Pract Res Clin Endocrinol Metab 2013, 27:373–384. [DOI] [PubMed] [Google Scholar]
- 21.Goudet C, Gaven F, Kniazeff J, Vol C, Liu J, Cohen-Gonsaud M, Acher F, Prezeau L, Pin JP: Heptahelical domain of metabotropic glutamate receptor 5 behaves like rhodopsin-like receptors. Proc Natl Acad Sci U S A 2004, 101:378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendy GN, Canaff L, Cole DE: The CASR gene: alternative splicing and transcriptional control, and calcium-sensing receptor (CaSR) protein: structure and ligand binding sites. Best Pract Res Clin Endocrinol Metab 2013, 27:285–301. [DOI] [PubMed] [Google Scholar]
- 23.Zhang F, Klebansky B, Fine RM, Xu H, Pronin A, Liu H, Tachdjian C, Li X: Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci U S A 2008, 105:20930–20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wellendorph P, Brauner-Osborne H: Molecular basis for amino acid sensing by family C G-protein-coupled receptors. Br J Pharmacol 2009, 156:869–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore CT, Wilson CG, Mayer CA, Acquah SS, Massari VJ, Haxhiu MA: A GABAergic inhibitory microcircuit controlling cholinergic outflow to the airways. J Appl Physiol (1985) 2004, 96:260–270. [DOI] [PubMed] [Google Scholar]
- 26.Chapman RW, Hey JA, Rizzo CA, Bolser DC: GABAB receptors in the lung. Trends Pharmacol Sci 1993, 14:26–29. [DOI] [PubMed] [Google Scholar]
- 27.Chung KF: NMDA and GABA receptors as potential targets in cough hypersensitivity syndrome. Curr Opin Pharmacol 2015, 22:29–36. [DOI] [PubMed] [Google Scholar]
- 28.Canning BJ, Mori N, Lehmann A: Antitussive effects of the peripherally restricted GABA B receptor agonist lesogaberan in guinea pigs: comparison to baclofen and other GABA B receptor-selective agonists. Cough 2012, 8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrios J, Kho AT, Aven L, Mitchel JA, Park JA, Randell SH, Miller LA, Tantisira KG, Ai X: Pulmonary Neuroendocrine Cells Secrete gamma-Aminobutyric Acid to Induce Goblet Cell Hyperplasia in Primate Models. Am J Respir Cell Mol Biol 2019, 60:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *30.Mizuta K, Osawa Y, Mizuta F, Xu D, Emala CW: Functional expression of GABAB receptors in airway epithelium. Am J Respir Cell Mol Biol 2008, 39:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]; A nice paper that demonstrates the importance of GABAB receptors in human airways
- 31.Forkuo GS, Nieman AN, Kodali R, Zahn NM, Li G, Rashid Roni MS, Stephen MR, Harris TW, Jahan R, Guthrie ML, et al. : A Novel Orally Available Asthma Drug Candidate That Reduces Smooth Muscle Constriction and Inflammation by Targeting GABAA Receptors in the Lung. Mol Pharm 2018, 15:1766–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallos G, Gleason NR, Zhang Y, Pak SW, Sonett JR, Yang J, Emala CW: Activation of endogenous GABAA channels on airway smooth muscle potentiates isoproterenol-mediated relaxation. Am J Physiol Lung Cell Mol Physiol 2008, 295:L1040–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuta K, Xu D, Pan Y, Comas G, Sonett JR, Zhang Y, Panettieri RA Jr., Yang J, Emala CW Sr.: GABAA receptors are expressed and facilitate relaxation in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2008, 294:L1206–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Osawa Y, Xu D, Sternberg D, Sonett JR, D’Armiento J, Panettieri RA, Emala CW: Functional expression of the GABAB receptor in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 2006, 291:L923–931. [DOI] [PubMed] [Google Scholar]; A nice paper that demonstrates the importance of GABAB receptors in human airway smooth muscle in the context of contractility
- 35.Dicpinigaitis PV: Effect of the GABA-agonist baclofen on bronchial responsiveness in asthmatics. Pulm Pharmacol Ther 1999, 12:257–260. [DOI] [PubMed] [Google Scholar]
- 36.Mizuta K, Mizuta F, Xu D, Masaki E, Panettieri RA Jr., Emala CW: Gi-coupled gamma-aminobutyric acid-B receptors cross-regulate phospholipase C and calcium in airway smooth muscle. Am J Respir Cell Mol Biol 2011, 45:1232–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *37.Terunuma M, Pangalos MN, Moss SJ: Functional modulation of GABAB receptors by protein kinases and receptor trafficking. Adv Pharmacol 2010, 58:113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstration of how GABAB receptors may differ in terms of desensitization compared to more typical GPCRs
- 38.Brennan SC, Davies TS, Schepelmann M, Riccardi D: Emerging roles of the extracellular calcium-sensing receptor in nutrient sensing: control of taste modulation and intestinal hormone secretion. Br J Nutr 2014, 111 Suppl 1:S16–22. [DOI] [PubMed] [Google Scholar]
- *39.Riccardi D, Brennan SC, Chang W: The extracellular calcium-sensing receptor, CaSR, in fetal development. Best Pract Res Clin Endocrinol Metab 2013, 27:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates importance of the CaSR in the context of lung development
- 40.Riccardi D, Brown EM: Physiology and pathophysiology of the calcium-sensing receptor in the kidney. Am J Physiol Renal Physiol 2010, 298:F485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Riccardi D, Kemp PJ: The calcium-sensing receptor beyond extracellular calcium homeostasis: conception, development, adult physiology, and disease. Annu Rev Physiol 2012, 74:271–297. [DOI] [PubMed] [Google Scholar]
- 42.Chavez-Abiega S, Mos I, Centeno PP, Elajnaf T, Schlattl W, Ward DT, Goedhart J, Kallay E: Sensing Extracellular Calcium - An Insight into the Structure and Function of the Calcium-Sensing Receptor (CaSR). Adv Exp Med Biol 2020, 1131:1031–1063. [DOI] [PubMed] [Google Scholar]
- 43.Conigrave AD, Ward DT: Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract Res Clin Endocrinol Metab 2013, 27:315–331. [DOI] [PubMed] [Google Scholar]
- 44.Gorvin CM: Insights into calcium-sensing receptor trafficking and biased signalling by studies of calcium homeostasis. J Mol Endocrinol 2018, 61:R1–R12. [DOI] [PubMed] [Google Scholar]
- 45.Mos I, Jacobsen SE, Foster SR, Brauner-Osborne H: Calcium-Sensing Receptor Internalization Is beta-Arrestin-Dependent and Modulated by Allosteric Ligands. Mol Pharmacol 2019, 96:463–474. [DOI] [PubMed] [Google Scholar]
- 46.Smith KA, Ayon RJ, Tang H, Makino A, Yuan JX: Calcium-Sensing Receptor Regulates Cytosolic [Ca2+] and Plays a Major Role in the Development of Pulmonary Hypertension. Front Physiol 2016, 7:517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz-Soto G, Rocher A, Garcia-Rodriguez C, Nunez L, Villalobos C: The Calcium-Sensing Receptor in Health and Disease. Int Rev Cell Mol Biol 2016, 327:321–369. [DOI] [PubMed] [Google Scholar]
- 48.Schepelmann M, Yarova PL, Lopez-Fernandez I, Davies TS, Brennan SC, Edwards PJ, Aggarwal A, Graca J, Rietdorf K, Matchkov V, et al. : The vascular Ca2+-sensing receptor regulates blood vessel tone and blood pressure. Am J Physiol Cell Physiol 2016, 310:C193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Yarova PL, Stewart AL, Sathish V, Britt RD Jr., Thompson MA, AP PL, Freeman M, Aravamudan B, Kita H, Brennan SC, et al. : Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci Transl Med 2015, 7:284ra260. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates the importance of CaSR in human airways in the context of asthma. Has driven new clinical trials in this area
- 50.Maarsingh H, Pera T, Meurs H: Arginase and pulmonary diseases. Naunyn Schmiedebergs Arch Pharmacol 2008, 378:171–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maarsingh H, Zaagsma J, Meurs H: Arginase: a key enzyme in the pathophysiology of allergic asthma opening novel therapeutic perspectives. Br J Pharmacol 2009, 158:652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brennan SC, Wilkinson WJ, Tseng HE, Finney B, Monk B, Dibble H, Quilliam S, Warburton D, Galietta LJ, Kemp PJ, et al. : The extracellular calcium-sensing receptor regulates human fetal lung development via CFTR. Sci Rep 2016, 6:21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roesler AM, Wicher SA, Ravix J, Britt RD Jr., Manlove L, Teske JJ, Cummings K, Thompson MA, Farver C, MacFarlane P, et al. : Calcium sensing receptor in developing human airway smooth muscle. J Cell Physiol 2019, 234:14187–14197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ali NK, Jafri A, Sopi RB, Prakash YS, Martin RJ, Zaidi SI: Role of arginase in impairing relaxation of lung parenchyma of hyperoxia-exposed neonatal rats. Neonatology 2012, 101:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bourke J, Diao J, Lam M, Maksdi C, Gregory K, Leach K: The calcium-sensing receptor CaSR mediates airway contraction in a house dust mite model of allergic airway disease. European Respiratory Journal 2019, 54:PA3884. [Google Scholar]
- 56.Yarova P, Schepelmann M, Ferla S, Huang P, Telezhkin V, Kidd E, Ford W, Broadley K, Ward J, Corrigan C, et al. : Development of a new calcilytic for the treatment of inflammatory lung disease. European Respiratory Journal 2018, 52:PA1060. [Google Scholar]
- 57.Brown EM, MacLeod RJ: Extracellular calcium sensing and extracellular calcium signaling. Physiological reviews 2001, 81:239–297. [DOI] [PubMed] [Google Scholar]
- 58.Lee JW, Park JW, Kwon OK, Lee HJ, Jeong HG, Kim JH, Oh SR, Ahn KS: NPS2143 Inhibits MUC5AC and Proinflammatory Mediators in Cigarette Smoke Extract (CSE)-Stimulated Human Airway Epithelial Cells. Inflammation 2017, 40:184–194. [DOI] [PubMed] [Google Scholar]
- 59.Mansfield B, Ho T-R, Mudway I, Corrigan C, Ward J, Kemp P, Lewis K, Mur L, Hawrylowicz C, Riccardi D: Calcium-sensing receptor (CaSR) antagonists (calcilytics) prevent urban particulate matter (UPM)-induced dendritic cell activation. European Respiratory Journal 2019, 54:PA2401. [Google Scholar]
- 60.Jaggupilli A, Howard R, Upadhyaya JD, Bhullar RP, Chelikani P: Bitter taste receptors: Novel insights into the biochemistry and pharmacology. Int J Biochem Cell Biol 2016, 77:184–196. [DOI] [PubMed] [Google Scholar]
- *61.Kinnamon SC: Taste receptor signalling - from tongues to lungs. Acta Physiol (Oxf) 2012, 204:158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]; A perspective on how taste receptors could work in non-oral situations
- 62.Leach K, Gregory KJ: Molecular insights into allosteric modulation of Class C G protein-coupled receptors. Pharmacol Res 2017, 116:105–118. [DOI] [PubMed] [Google Scholar]
- 63.Kikut-Ligaj D, Trzcielinska-Lorych J: How taste works: cells, receptors and gustatory perception. Cell Mol Biol Lett 2015, 20:699–716. [DOI] [PubMed] [Google Scholar]
- 64.Kokabu S, Lowery JW, Toyono T, Sato T, Yoda T: On the Emerging Role of the Taste Receptor Type 1 (T1R) Family of Nutrient-Sensors in the Musculoskeletal System. Molecules 2017, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maina IW, Workman AD, Cohen NA: The role of bitter and sweet taste receptors in upper airway innate immunity: Recent advances and future directions. World J Otorhinolaryngol Head Neck Surg 2018, 4:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shaik FA, Singh N, Arakawa M, Duan K, Bhullar RP, Chelikani P: Bitter taste receptors: Extraoral roles in pathophysiology. Int J Biochem Cell Biol 2016, 77:197–204. [DOI] [PubMed] [Google Scholar]
- *67.Nayak AP, Shah SD, Michael JV, Deshpande DA: Bitter Taste Receptors for Asthma Therapeutics. Front Physiol 2019, 10:884. [DOI] [PMC free article] [PubMed] [Google Scholar]; Review of bitter taste receptors in asthma that contrasts with the little knowledge on sweet/umami receptors, setting the stage for identifying an unmet need for research on the latter.
- 68.Julio-Pieper M, Flor PJ, Dinan TG, Cryan JF: Exciting times beyond the brain: metabotropic glutamate receptors in peripheral and non-neural tissues. Pharmacol Rev 2011, 63:35–58. [DOI] [PubMed] [Google Scholar]
- 69.Chung G, Kim SJ: Sustained Activity of Metabotropic Glutamate Receptor: Homer, Arrestin, and Beyond. Neural Plast 2017, 2017:5125624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou H, Rigoutsos I: The emerging roles of GPRC5A in diseases. Oncoscience 2014, 1:765–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jorgensen CV, Brauner-Osborne H: Pharmacology and physiological function of the orphan GPRC6A receptor. Basic Clin Pharmacol Toxicol 2020. [DOI] [PubMed] [Google Scholar]
- 72.Pi M, Oakley RH, Gesty-Palmer D, Cruickshank RD, Spurney RF, Luttrell LM, Quarles LD: Beta-arrestin- and G protein receptor kinase-mediated calcium-sensing receptor desensitization. Mol Endocrinol 2005, 19:1078–1087. [DOI] [PubMed] [Google Scholar]
- 73.Pera T, Hegde A, Deshpande DA, Morgan SJ, Tiegs BC, Theriot BS, Choi YH, Walker JK, Penn RB: Specificity of arrestin subtypes in regulating airway smooth muscle G protein-coupled receptor signaling and function. FASEB J 2015, 29:4227–4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker JK, DeFea KA: Role for beta-arrestin in mediating paradoxical beta2AR and PAR2 signaling in asthma. Curr Opin Pharmacol 2014, 16:142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doumazane E, Scholler P, Zwier JM, Trinquet E, Rondard P, Pin JP: A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J 2011, 25:66–77. [DOI] [PubMed] [Google Scholar]
- 76.Gama L, Wilt SG, Breitwieser GE: Heterodimerization of calcium sensing receptors with metabotropic glutamate receptors in neurons. J Biol Chem 2001, 276:39053–39059. [DOI] [PubMed] [Google Scholar]
- 77.Wellendorph P, Bräuner-Osborne H: Molecular basis for amino acid sensing by family C G-protein-coupled receptors. Br J Pharmacol 2009, 156:869–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


