Abstract
Many drugs of abuse are mixed with other psychoactive substances (e.g., caffeine) prior to their sale or use. Synthetic cathinones (e.g., 3,4-methylenedioxypyrovalerone [MDPV]) are commonly mixed with caffeine or other cathinones (e.g., 3,4-methylenedioxy-N-methylcathinone [methylone]), and these “bath salts” mixtures (e.g., MDPV+caffeine) can exhibit supra-additive interactions with regard to their reinforcing and discriminative stimulus properties. However, little is known about relapse-related effects of drug mixtures. In these studies, male Sprague-Dawley rats self-administered 0.032 mg/kg/inf MDPV or a mixture of MDPV+caffeine (0.029 + 0.66 mg/kg/inf, respectively), and then underwent multiple rounds of extinction and reinstatement testing to evaluate the influence of reinforcement history and drug-associated stimuli on the effectiveness of saline (drug-paired stimuli alone), MDPV (0.032–1.0 mg/kg), caffeine (1.0–32 mg/kg), and mixtures of MDPV:caffeine (in 3:1, 1:1, and 1:3 ratios, relative to each drug’s ED50) to reinstate responding. Dose-addition analyses were used to determine the nature of the drug-drug interaction for each mixture. MDPV and caffeine dose-dependently reinstated responding and were equally effective, regardless of reinforcement history. Most fixed ratio mixtures of MDPV+caffeine exhibited supra-additive interactions, reinstating responding to levels greater than was observed with caffeine and/or MDPV alone. Drug-associated stimuli also played a key role in reinstating responding, especially for caffeine. Together, these results demonstrate that the composition of drug mixtures can impact relapse-related effects of drug mixtures, and “bath salts” mixtures (MDPV+caffeine) may be more effective at promoting relapse-related behaviors than the constituents alone. Further research is needed to determine how other polysubstance reinforcement histories can impact relapse-related behaviors.
Keywords: Bath salts, Caffeine, Drug mixtures, MDPV, Reinstatement
1). Introduction
Many drugs of abuse (e.g., heroin, cocaine, synthetic cathinones) are mixed with other psychoactive drugs (e.g., fentanyl, caffeine) prior to their sale and use. Although much is known about the pharmacology of single drug entities, relatively little is known about how interactions among psychoactive constituents of abused drug preparations impact their abuse-related and toxic effects. Previous work suggests caffeine can synergistically enhance the discriminative stimuluse.g.,1–3 and reinforcing effectse.g.,4,5 of stimulants, such as cocaine and synthetic cathinones. Synthetic cathinones have stimulant properties and act as inhibitors (e.g., 3,4-methylenedioxypyrovalerone [MDPV]) or substrates (e.g., 3,4-methylenedioxy-N-methylcathinone [methylone]) at monoamine transporters. Synthetic cathinones (a.k.a. “bath salts”) produce a wide range of effects in humans, including euphoria, tachycardia, craving, and paranoia, with many users reporting the effects of “bath salts” as more intense than cocaine or methamphetamine.6–9 The magnitude of these effects may be due to inherent differences in the pharmacology of synthetic cathinones and cocaine or methamphetamine, or because “bath salts” preparations often contain multiple synthetic cathinones and/or multiple psychoactive substances (e.g., caffeine) in a single preparation.
Pyrrolidine-containing cathinones (e.g., MDPV, α-PVP) represent one of the more popular and well-studied sub-families of synthetic cathinones, and function as cocaine-like inhibitors of monoamine transporters, with varying degrees of selectivity for the dopamine and serotonin transporters.10 For example, MDPV is ~800-fold selective for the dopamine and norepinephrine relative to serotonin transporters.11 MDPV is also a highly effective reinforcer, maintaining ~3-fold more responding than cocaine under progressive ratio schedules of reinforcement.10,12 In contrast, caffeine, an adenosine receptor antagonist, is a relatively weak reinforcer, maintaining responding at near saline-like levels.4,13,14 Yet, addition of caffeine to cocaine appears to enhance the reinforcing effects of cocaine,15–17 and mixing caffeine with MDPV or methylone produces supra-additive enhancements in reinforcing potency and effectiveness, respectively.4,5
Though the reinforcing effects of caffeine are much weaker than cocaine or MDPV, the discriminative stimulus properties of caffeine overlap with those of cocaine, and caffeine typically produces full generalization to the discriminative stimulus effects of cocaine or methamphetamine.1,3,18 Unsurprising, MDPV shares discriminative stimulus effects with methamphetamine and cocaine.1,19–22 Mixtures of MPDV and caffeine exhibit supra-additive interactions in rats trained to discriminate cocaine from saline,1 suggesting caffeine can enhance not only the reinforcing,4,5 but also discriminative stimulus effects of MDPV.
Despite numerous studies describing the reinforcing and discriminative stimulus effects of MDPV,e.g.,4,5,12,19–22 little is known about relapse-related effects of MDPV23,24, and nothing is known about how these effects might be altered by common “bath salts” constituents, such as caffeine. Although MDPV (1 mg/kg) can reinstate responding in the presence or absence of infusion-paired stimuli,23 other factors (e.g., priming dose, self-administration history) that mediate the magnitude of the reinstatement response have yet to be explored. Caffeine can reinstate responding in rats that self-administered cocaine, although it is generally less effective than cocaine.25–27 Although studies have evaluated the capacity of mixtures of cocaine and caffeine to reinstate responding,26 they have not investigated the importance of the composition of the self-administered drug/drug mixture to the magnitude of the reinstatement response.
Mixtures of MDPV and caffeine can exhibit supra-additive interactions with regard to reinforcing and discriminative stimulus effects;1,4,5 however, similar supra-additive interactions between MDPV and caffeine have not been established with regard to their potency/effectiveness to promote relapse-related behaviors. Accordingly, the current study used two groups of rats, self-administering either MDPV alone, or a mixture of MDPV+caffeine to test the hypotheses that: 1) caffeine is more effective at reinstating responding in rats with a history of MDPV+caffeine self-administration than in rats that self-administered MDPV alone; and 2) supra-additive interactions will be observed between MDPV and caffeine, with mixtures of MDPV+caffeine being more potent and/or effective at reinstating responding than either drug alone.
2). Materials and methods
2.1). Subjects
Forty male Sprague Dawley rats (275–300g; Envigo, Indianapolis, IN) were singly housed in a temperature- and light-controlled vivarium (24°C; 14/10-hour light/dark cycle) with ad libitum access to food and water. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals28 and the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
2.2). Apparatus
Experimental sessions were conducted in standard operant conditioning chambers (Med Associates, St. Albans, VT) located within ventilated, sound-attenuating enclosures. The right wall had 2 response levers, with a set of green, yellow, and red LEDs above each lever. A white house light was located on the opposite wall. A variable speed syringe driver delivered infusions through Tygon tubing connected to a stainless steel fluid swivel and spring tether held by a counterbalanced arm.
2.3). Surgical procedure
Rats were anesthetized using 2% isoflurane and surgically prepared with an indwelling catheters in the left femoral vein and a vascular access port, which was secured mid-scapulae, as previously described.4,5,10,12 Following surgery, Penicillin G (60,000 U/rat) was administered subcutaneously to prevent infection. Catheters were flushed daily with 0.5 ml heparinized saline during the 5-day recovery period.
2.4). Acquisition of responding
Rats initially responded under a fixed ratio (FR) 1 schedule of reinforcement for MDPV (0.032 mg/kg/inf; n=20), or a mixture of MDPV+caffeine (0.029 and 0.66 mg/kg/inf, respectively; n=20) during daily 90-min sessions. The doses for the MDPV+caffeine mixture were based on a previous study4 so that the unit dose of caffeine would contribute ~10% of the total effect to the self-administered mixture (i.e., reduced the MDPV dose by 10% effectiveness), and to allow for a sufficiently large intake of caffeine (~20–50 mg/kg/session) without also decreasing the number of infusions (drug-CS pairings).1–5,18 Illumination of a yellow LED above the active lever (counterbalanced left or right) signaled drug availability (i.e., the discriminative stimulus; SD), and completion of the response requirement resulted in an infusion (0.1 ml/kg over ~1-sec) paired with a 5-sec illumination of the 3 LEDs above the active lever and the houselight (i.e., infusion-paired stimuli; SR), during which no additional infusions could be earned (i.e., a 5-sec timeout [TO]). Together, the SD and SR comprise the conditioned stimuli (CS). Responses during TOs and on the inactive lever were recorded but had no scheduled consequence. The FR1:TO 5-sec schedule was in place for a minimum of 10 sessions, and until acquisition criteria were met (≥20 infusions, and ≥80% responding on the active lever for 2 consecutive days). Response requirement was subsequently increased to FR5 for the remainder of the experiment.
2.5). Self-administration – Extinction – Reinstatement test cycles
Following acquisition, rats underwent repeated cycles of reinstatement testing, with each cycle consisting of 3 conditions: self-administration (10–15 sessions); extinction (7–9 sessions); and reinstatement testing (7–19 sessions for 3–7 test conditions). Experiments consisted of ≥5 cycles. Briefly, rats were allowed to self-administer their assigned drug(s) under an FR5:TO 5-sec schedule for a minimum of 10 sessions, and until stability criteria were met (±20% of the mean of 3 consecutive sessions; no increasing or decreasing trend), or a maximum of 15 sessions. Subsequently, rats transitioned to extinction conditions, during which the SD and SR were never presented;e.g.,29,30 however, completion of the response requirement resulted in a saline infusion. Rats underwent at least 7 extinction sessions (maximum of 9 sessions) and transitioned to reinstatement testing once active lever responses were ≤15% of baseline (i.e., mean active lever responses during the 3 self-administration sessions that immediately preceded extinction). Reinstatement tests were conducted under 3 different conditions: CS tests; CS+drug tests; and drug tests. CS reinstatement tests were identical to self-administration conditions with the exceptions that: 1) rats received an intravenous pretreatment of saline (CS test) or drug (CS+drug test) 5-min before the session; and 2) saline rather than drug was delivered once response requirements were met. e.g.,29,30 Each block of reinstatement testing consisted of 3–7 reinstatement tests, with CS tests always occurring first, followed by 2–6 additional CS+drug tests. Each reinstatement test was separated by at least 2 extinction sessions, with additional extinction sessions conducted until extinction criterion was met. To evaluate the importance of the CS to the reinstatement response, tests were also conducted in the absence of the CS (drug test). During drug tests, pretreatments were administered 5-min before the session, however, the SD was never presented, and saline infusions were delivered without the SR.
2.6). Experiment 1 – Drugs alone
Experiment 1 evaluated the potency and effectiveness of MDPV (0.032–1 mg/kg), caffeine (1–32 mg/kg), and heroin (0.1–0.32 mg/kg) to reinstate responding in rats with a history of MDPV (n=10), or MDPV+caffeine (n=10) self-administration. CS tests (saline pretreatment) were followed by 2–4 doses of a test drug (CS+drug test) evaluated in random order. Dose-response curves for MDPV and caffeine were doubly determined, with the order of testing counterbalanced across rats (MDPV-caffeine-MDPV-caffeine, or caffeine-MDPV-caffeine-MDPV). Heroin was included as a negative control and was always tested during the fifth cycle. A subset of rats failed to complete the second determination of the caffeine, (MDPV+caffeine: n=1), MDPV (MDPV+caffeine: n=2), and heroin (MDPV: n=1; MDPV+caffeine: n=3) dose response curves due to technical issues.
2.7). Experiment 2 – Drug mixtures
Experiment 2 evaluated the potency and effectiveness of MDPV+caffeine mixtures to reinstate responding in rats with a history of self-administering MDPV (n=10), or a mixture of MDPV+caffeine (n=10). During test cycles 1–3, mixtures of MDPV:caffeine were evaluated at 3:1, 1:1, and 1:3 ratios of the doses of each drug that were estimated to produce a 50% effect (i.e., the group mean ED50s; determined in Experiment 1). Briefly, the mean dose-response curve for each rat was normalized to the CS test (saline; 0% effect level), with the dose that reinstated the most responding serving as the 100% effect level (i.e., Emax). Normalized dose-response curves were fit using a linear regression of the data spanning the 20–80% effect levels, from which the ED50, slope, and y-intercept were obtained for individual subjects. To fully evaluate the capacity of the mixtures to reinstate responding, each mixture (3:1, 1:1 and 1:3 MDPV:caffeine) was tested at 4 unique dose pairs (Table S1) spanning the 0–100% predicted effect levels (see below), with the order of testing counterbalanced across rats. The 4th and 5th cycle tested the most effective doses of MDPV and caffeine (4th) and each of the fixed dose mixtures (5th) with and without CS presentation. Due to technical issues, a subset of rats failed to complete the 3:1 (MDPV+caffeine: n=2) and 1:1 MDPV:caffeine (MDPV+caffeine: n=2) dose response curves, and the experiments examining the impact of CS presentation (MDPV: n=1; MDPV+caffeine: n=4).
2.8). Drugs
MDPV HCl was synthesized by Drs. Sulima and Rice (Bethesda, MD). Caffeine was purchased from Sigma-Aldrich (St. Louis, MO). Heroin was provided by the NIDA Drug Supply Program (Bethesda, MD). All drugs were dissolved in sterile saline and solutions were administered in a volume of 0.1 ml/kg for self-administration and 1 ml/kg for reinstatement tests, when feasible. Solubility limits required doses of caffeine >14 mg/kg to be administered in volumes of 3.2 ml/kg during reinstatement tests.
2.9). Statistical analyses
Predicted effect levels for each drug pair were calculated based on the concepts of dose equivalence and dose addition.1,4 Briefly, the dose of caffeine (DoseB) was converted to an equivalent dose of MDPV (DoseBeqA) using the following equation:4
| (1) |
where SlopeA and SlopeB are the slope parameters and intA and intB are the y-intercepts for MDPV and caffeine, respectively, and derived from the normalized dose-response curves described above. The sum of DoseA and DoseBeqA (DoseeqA) represents the total equivalent dose for a dose pair, expressed in terms of drug A (MDPV). DoseeqA is then used to calculate the predicted effect level for an additive interaction using the following equation:
| (2) |
The number of responses made during self-administration and extinction sessions were analyzed by two-way (test cycle and day) repeated-measures (RM) ANOVA with post-hoc Tukey’s tests. Two-way (CS condition and test cycle) RM ANOVAs with post-hoc Sidak’s tests were used to determine whether responding during CS tests was significantly different from responding during the last day of extinction (i.e., no-CS conditions). Dose-response curves for MDPV, caffeine, and mixtures of MDPV+caffeine were analyzed by two-way (self-administration history and drug dose) RM ANOVA with post hoc Tukey’s tests. To obtain measures of potency and effectiveness, individual subject dose-response curves were analyzed by linear regression. Emax (±SEM) values for each drug and fixed ratio mixture of MDPV+caffeine were compared both within and between groups using two-way ANOVA with post-hoc Tukey’s tests. Non-overlapping 95% confidence intervals (CIs) served as in indicator of statistical significance for comparisons of ED50 values. To evaluate the nature of the interaction for mixtures of MDPV+caffeine, experimentally determined ED50 (95% CIs) and Emax values for each fixed ratio mixture (e.g., 3:1 MDPV:caffeine) were compared to the ED50 (95% CIs) or Emax derived from the dose-response curve predicted for an additive interaction. When 95% CIs overlapped, the interaction was considered to be strictly additive; however, when 95% CIs did not overlap the interaction was considered to either supra-additive (mean ED50 was smaller or Emax was larger than predicted) or sub-additive (mean ED50 was larger or Emax was smaller than predicted). A two-way (CS and drug pretreatment) RM ANOVA with post-hoc Tukey’s tests was used to determine if the CS interacted with the direct effects of the drug(s) to reinstate responding.
3). Results
3.1). Self-administration
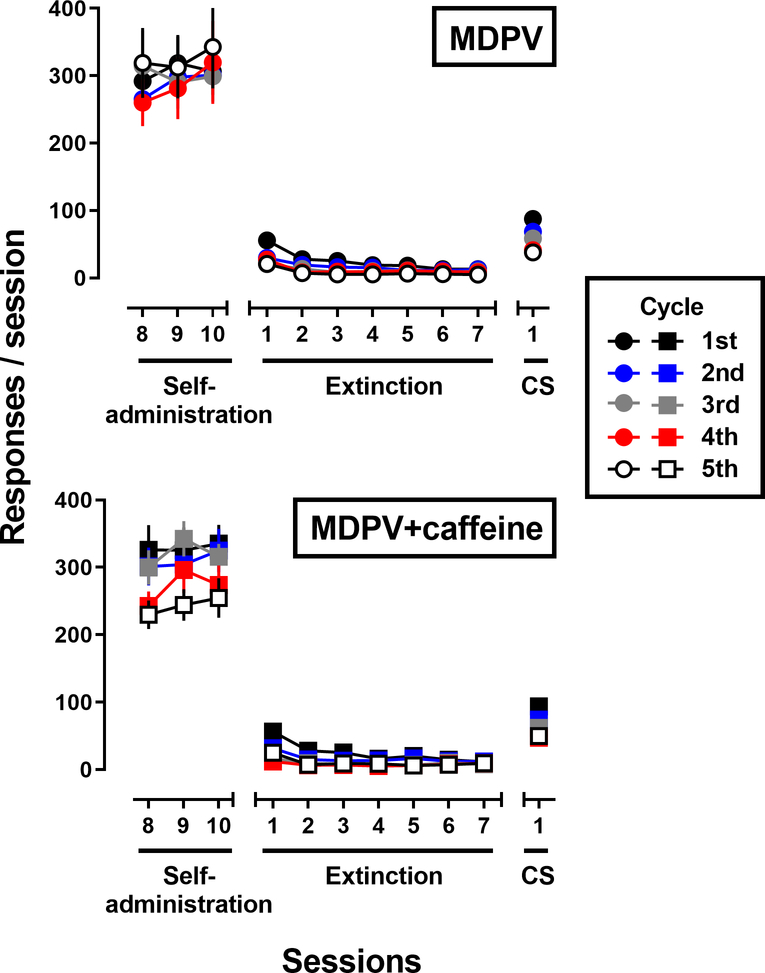
Nearly all rats (95% for MDPV and 100% for MDPV+caffeine) met acquisition criteria within the first 10 days of self-administration under the FR1:TO 5-sec schedule of reinforcement (data not shown). There were no differences in mean number of days to meet criteria between the MDPV (4.5 ± 0.5 sessions) and MDPV+caffeine (4.8 ± 0.4 sessions) conditions (p=0.6), or the level of responding maintained by MDPV (55.0 ± 13.6 responses) or MDPV+caffeine (43.3 ± 2.4 responses) at the end of the 10 day period (p=0.5). Figure 1 shows the number of responses made during the 3 self-administration sessions that immediately preceded extinction conditions for rats that self-administered MDPV (top panel) or MDPV+caffeine (bottom panel). In the MDPV+caffeine group, there was a significant effect of day (F[2,154] = 4.7; p<0.01) where post-hoc analyses revealed responding was significantly lower on days 9 and 10 of self-administration compared to day 8 (p<0.05 for all). There was no main effect of cycle for either group (MDPV – F[4,94]=0.14, p>0.05; MDPV+caffeine – F[4,77]=1.57, p>0.05), indicating that the level of self-administration did not vary across test cycle. Additionally, the level of responding did not differ as a function of the drug(s) that were available for self-administration (MDPV – 301.2 ± 11.0 responses; MDPV+caffeine – 299.5 ± 7.6 responses).
Figure 1.
Active lever responses across repeated cycles of self-administration, extinction, and reinstatement testing in male Sprague-Dawley rats that self-administered MDPV (upper panel; n=18–20) or a mixture of MDPV+caffeine (lower panel; n=15–20). Abscissa: the last 3 days of self-administration, the first 7 days of extinction, and the CS test (saline pretreatment) Ordinate: total responses on the active lever during the 90-min session. Data represent the mean (± 1 S.E.M.) for 5 cycles of the procedure.
3.2). Extinction
Figure 1 also shows the number of responses made during the 7 extinction sessions that followed MDPV (top panel) or MDPV+caffeine (bottom panel) self-administration. A two-way, RM ANOVA (by day) found a main effect of day (MDPV – F[6,564]=51.8, p<0.0001; MDPV+caffeine – F[6,468]=43.3, p<0.0001), a main effect of cycle (MDPV – F[4,94]=18.3, p<0.0001; MDPV+caffeine – F[4,78]=15.5, p<0.0001), and a day x cycle interaction (MDPV – F[24,564]=4.0, p<0.0001; MDPV+caffeine – F[24,468] =7.2, p<0.0001). Post-hoc analyses indicated that the number of responses made on day 1 of extinction was significantly greater during cycle 1, compared to all other cycles (p<0.05 for all). Additionally, rats responded more on day 1 of extinction compared to the last day, for all cycles (p<0.05 for all); however, the level of responding on day 7 of extinction was not different across cycles (p>0.05 for all). A one-way ANOVA found a main effect of cycle on the number of days to meet extinction criterion (MDPV – F[4,94]=6.0, p<0.001; MDPV+caffeine – F[4,77]=9.5, p<0.0001), with post-hoc analyses indicating that extinction occurred more slowly during cycle 1 compared to the remaining cycles (p<0.05 for all); however, the time to extinction during cycles 2–5 did not differ for either group.
3.3). Reinstatement: CS tests
The number of responses made during the 5 CS tests are show in Figure 1. A one-way ANOVA indicated a significant effect of cycle for both groups (MDPV – F[4,94]=9.5, p<0.0001; MDPV+caffeine – F[4,78]=6.9, p<0.0001), with post-hoc tests revealing that the effectiveness of the CS to reinstate responding was significantly reduced by cycle 2 for the MDPV group, and by cycle 3 for the MDPV+caffeine group. Despite this effect of cycle, reintroduction of the CS reinstated significant levels of responding (compared to the last day of extinction) during all 5 test cycles (p<0.05 for all).
3.4). Reinstatement: CS+Drug tests - MDPV and caffeine alone
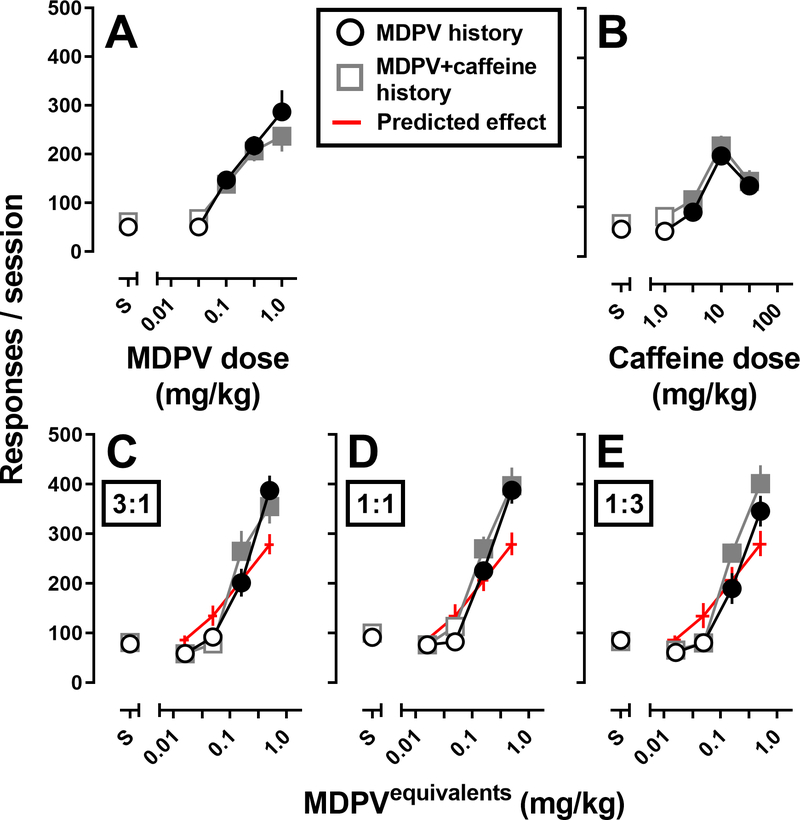
Reinstatement dose-response curves for MDPV (Figure 2A) and caffeine (Figure 2B) were doubly determined. When tested alone, MDPV and caffeine reinstated similar levels of responding (F[1,62]=0.96, p=0.3). In addition, the effects of MDPV and caffeine did not differ as a function of self-administration history (F[1,62]=0.48, p=0.5), and did not differ across repeated determinations (MDPV – F[1,9]=0.19, p=0.7; MDPV+caffeine – F[1,16]=0.4, p=0.6). To confirm 1.0 mg/kg MDPV was the most effective dose of MDPV to reinstate responding, a larger dose of MDPV (3.2 mg/kg) was tested in a subset of rats from Experiment 2 (n=7) and resulted in a mean of 6.4 (± 3.2) reinstatement response (data not shown). Unlike MDPV and caffeine, doses of 0.1 and 0.32 mg/kg heroin failed to reinstate responding (36.3 ± 4.6 and 36.7 ± 5.8 responses, respectively; data not shown).
Figure 2.
Dose-response curves for the number of responses made during CS+drug reinstatement tests in rats with a history of self-administering MDPV (black open circles; n=10) or a mixture of MDPV+caffeine (gray open squares; n=8–10). Experimentally determined dose-response curves (circle or square symbols) for MDPV (panel A), caffeine (panel B) alone, as well as mixtures of MDPV+caffeine at 3 fixed-dose ratios, (panel C [3:1], panel D [1:1], and panel E [1:3]) represent the mean (± 1 S.E.M.) number of responses, whereas the predicted dose-response curves (red lines, no symbols) represent the mean (± 1 S.E.M.) number of responses calculated for an additive interaction. Abscissa: S represents saline pretreatment (CS test), whereas the numbers refer to doses of MDPV (panel A), caffeine (panel B), or MDPV equivalents (panels C-E) administered intravenously 5-min before the CS or CS+drug test session. Ordinates: total number of responses made on the active lever during the 90-min session. Filled symbols represent responding significantly greater than saline (CS test).
3.5). Reinstatement: CS+Drug tests - MDPV+caffeine mixtures
Figure 2C–E show the dose-response curves predicted for an additive interaction (dashed lines) and experimentally-determined dose-response curves for the 3:1, 1:1, and 1:3 fixed ratio mixtures of MDPV:caffeine during CS+drug tests. All 3 mixtures of MDPV+caffeine reinstated responding in a dose-dependent manner (3:1 – F[1.7,27.5]=99.6, p<0.0001; 1:1 – F[1.8,29.0]=120.6, p<0.0001; 1:3 – F[2.1,36.0], p<0.0001); effects did not differ as a function of self-administration history (3:1 – F[1,16]=0.02, p=0.9; 1:1 – F[1,16]=1.1, p=0.3; 1:3 – F[1,16]=1.9, p=0.2). Although all 3 mixtures of MDPV+caffeine exhibited additive interactions with regard to potency to reinstate responding (Table 1), significant departures from additivity were observed with regard to effectiveness to reinstate responding. Importantly, these interactions were observed in rats that self-administered either MDPV (3:1 and 1:1 mixtures), or a mixture of MDPV+caffeine (1:1 and 1:3 mixtures). In addition, the largest dose pair of each ratio (3:1, 1:1, and 1:3) was significantly more effective at reinstating responding than the dose-independent maximal reinstatement response (Emax) produced by either caffeine or MDPV in Experiment 1 (Table 1).
Table 1.
Measures of potency (expressed as mg/kg) and effectiveness (expressed as responses / session) in CS+drug reinstatement.
| MDPV history | MDPV+caffeine history | |||
|---|---|---|---|---|
| ED50 | Emax | ED50 | Emax | |
| Mean (95% CI) | Mean ± SEM | Mean (95% CI) | Mean ± SEM | |
| MDPV | 0.15 (0.11, 0.20) | 308.9 ± 37.1 | 0.14 (0.10, 0.19) | 260.1 ± 27.2 |
| Caffeine | 4.30 (3.54, 5.22) | 204.7 ± 14.6 | 3.83 (3.22, 4.55) | 229.7 ± 19.7 |
| 3:1 MDPV:caffeine | 0.18 (0.15, 0.21) | 387.4 ± 30.1 # | 0.13 (0.11, 0.16) | 368.9 ± 43.4 # |
| 1:1 MDPV:caffeine | 0.16 (0.15, 0.17) | 387.8 ± 26.7 # | 0.14 (0.10, 0.18) | 406.4 ± 32.0 *# |
| 1:3 MDPV:caffeine | 0.15 (0.13, 0.18) | 345.8 ± 31.4 # | 0.14 (0.13, 0.16) | 409.5 ± 28.0 *# |
p<0.05 indicates a significant difference from the MDPV Emax for the same self-administration history
p<0.05 indicates a significant difference from the caffeine Emax for the same self-administration history.
3.6). Reinstatement: Drug tests (no CS)
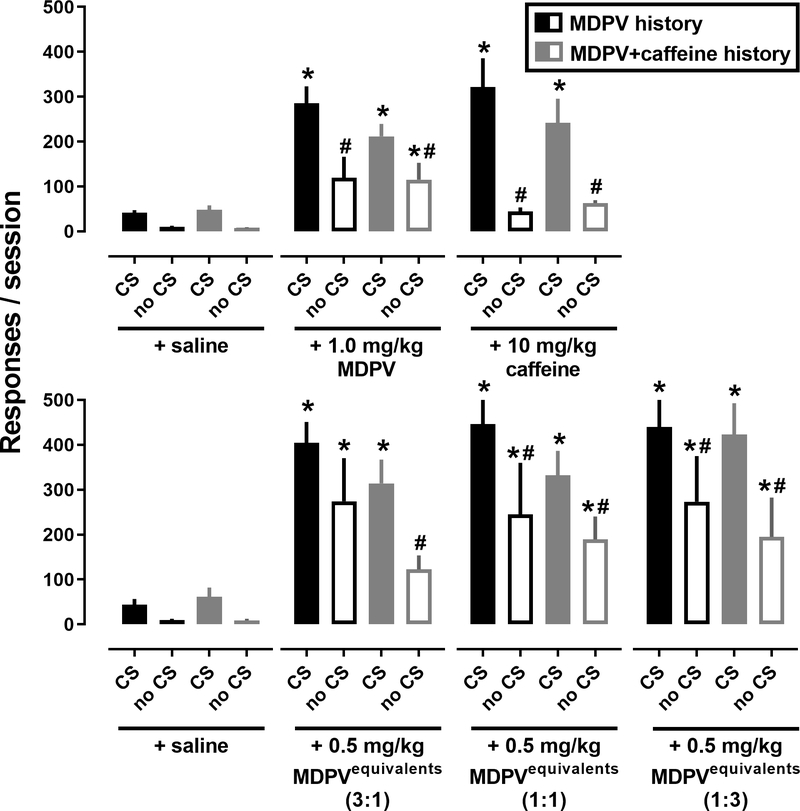
Reinstatement tests were conducted with maximally effective doses of MDPV, caffeine, and their mixtures under both CS and no CS conditions (Figure 3). There were main effects of both CS condition and drug pretreatment as well as a CS condition x drug pretreatment interaction in rats that self-administered either MDPV (CS – F[1,8]=43.0, p<0.001; Drug – F[6,48]=22.2, p<0.0001; Interaction – F[6,48]=2.6, p<0.05), or MDPV+caffeine (CS – F[1,5]=12.1, p<0.05; Drug – F[6,30]=14.2, p<0.0001; Interaction – F[6,30]=4.2, p<0.01). When tested in the absence of the CS (drug test), 1.0 mg/kg MDPV reinstated significantly less responding than when the CS was presented (CS+drug test), regardless of self-administration history (p<0.05 for both; Figure 3, top panels). Similarly, caffeine (10 mg/kg) was significantly less effective at reinstating responding during the drug test compared to the CS+drug test in both the MDPV (p<0.001) and MDPV+caffeine groups (p<0.01).
Figure 3.
Influence of CS presentation during reinstatement tests in rats with a history of self-administering MDPV (black bars; n=9) or a mixture of MDPV+caffeine (gray bars; n=6). Filled bars represent CS+drug tests whereas open bars represent drug tests (no CS). Abscissa: CS and no CS refer to the presence (CS+drug test) and absence (drug test) of CS presentation, respectively. The drug and dose (or saline) indicate the pretreatment administered intravenously 5-min before the reinstatement session. Ordinate: total number of responses made on the active lever during the 90-min session. *p<0.05 indicates a significant difference from saline for the same self-administration history and CS condition. #p<0.05 indicates a significant from the CS conditions for the same pretreatment and self-administration history.
Similar to effects observed with MDPV and caffeine, CS+drug tests generally engendered more responding than drug tests; however, mixtures of MDPV+caffeine (Figure 3, bottom panels) generally retained their effectiveness to reinstate responding during drug tests (no CS), an effect that was rarely observed with either MDPV or caffeine alone. Unlike when MDPV and caffeine were tested in the absence of CS (i.e., drug tests), the reinstatement responses elicited by mixtures of MDPV+caffeine were more variable, with a subset of rats responding at similarly high levels under both CS and no CS conditions.
3.7). Intra-session Patterns of Reinstatement Responding
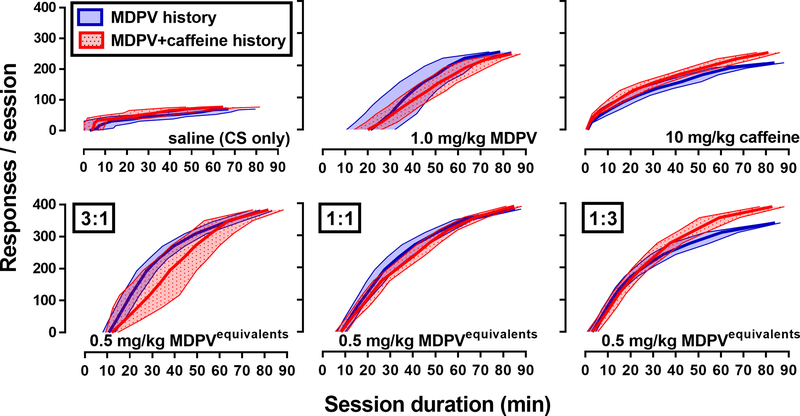
Post-hoc analyses of cumulative records from tests of the maximally effective doses of MDPV, caffeine, and the 3:1, 1:1, and 1:3 mixtures of MDPV:caffeine were performed to determine if patterns of responding differed between the drugs/drug mixtures during CS+drug tests (Figure 4). Unlike with saline (CS test), when the majority of responding occurred early in the session, pretreatment with MDPV (1 mg/kg) resulted in few responses being made during the first 20 min of the test, followed by high rates of responding over ~40 min. Although MDPV and caffeine (10 mg/kg) reinstated similar levels of responding, caffeine reinstated responding sooner (within the first minute), with low rates of responding observed over a prolonged period of time (~80 min). To determine if the delayed effect of MDPV was due to a competing behavioral effect (e.g., stereotypy), 1.0 mg/kg of MDPV was also administered as a 25-min pretreatment (n=8). Under these tests, a similar lag in responding was observed, and the magnitude of the reinstatement response was similar (253.4 ± 63.4 responses) to when MDPV was administered the 5 min before the start of the session (285.2 ± 38.3 responses; data not shown).
Figure 4.
Mean response pattern during the CS+drug tests for saline (upper left) and the most effective dose of MDPV (upper center), caffeine (upper right), and each fixed-dose mixture [3:1 (lower left), 1:1 (lower center), and 1:3 (lower right)]. Solid line represents the mean and shaded region represents the 95% confidence intervals for the response pattern in rats with a history of self-administering MDPV (blue; n=10) or a mixture of MDPV+caffeine (red; n=10). Abscissa: the session duration (min). Ordinates: total number of responses made on the active lever during the 90-min session.
The patterns of responding reinstated by mixtures of MDPV+caffeine appeared to have aspects of both constituent drugs. For instance, for mixtures where MDPV predominated (3:1 MDPV:caffeine), there was a noticeable lag (~10 min) in the initiation of responding, followed by high rates of responding that extended beyond that observed with MDPV alone. Conversely, for mixtures where caffeine predominated (1:3 MDPV:caffeine), high rates of responding initiated were early in the session and persisted for a longer period of time than when caffeine was tested alone. Patterns of responding did not appear to vary as a function of self-administered drug(s).
4). Discussion
Synthetic cathinones, such as MDPV, are often used in preparations that contain multiple psychoactive constituents (e.g., “bath salts”). Numerous studies have described the reinforcing and discriminative stimulus effects of synthetic cathinones; however, relapse-related behaviors associated with the self-administration of synthetic cathinones and/or “bath salts” mixtures have been understudied. This study used multiple cycles of reinstatement testing to characterize the potency and effectiveness of MDPV and caffeine to reinstate responding in rats that self-administered either MDPV or a mixture of MDPV+caffeine, and then applied dose addition analyses to determine if “bath salts” mixtures of MDPV+caffeine were more effective at reinstating responding than either MDPV or caffeine alone. The first central finding was that MDPV and caffeine dose-dependently reinstated responding, and that the magnitude of this response was not impacted by the drug(s) that were self-administered. The second main finding was that when administered as mixtures, MDPV and caffeine exhibited supra-additive interactions with regard to their effectiveness to reinstate responding, regardless of whether rats had previously self-administered MDPV or a mixture of MDPV+caffeine. Finally, these studies found that, though drug-associated conditioned stimuli (CS) appeared to play a prominent role in enhancing the effectiveness of both MDPV and caffeine to reinstate responding, the importance of the CS to the reinstatement response was reduced when MDPV+caffeine were administered as mixtures. Taken together, these findings suggest that even though histories of self-administering MDPV or a mixture of MDPV+caffeine did not differentially affect the reinstating effects of MDPV or caffeine, when mixed, the reinstating effects of MDPV and caffeine were supra-additive, suggesting that the composition of drug primes and the presence of drug-associated stimuli can impact the promotion of relapse-related behavioral responses.
The levels of self-administration maintained by MDPV and a mixture of MDPV+caffeine did not differ; however, it is important to note that the MDPV+caffeine group had a history of response-contingent caffeine delivery whereas the MDPV group did not. This key difference allowed for testing hypotheses regarding interactions between the composition of the self-administered drug(s) and the relative potency and effectiveness of constituent drugs to reinstate responding. Specifically, that MDPV would be equi-effective in both groups, but that caffeine would be more effective at reinstating responding in rats that self-administered a mixture of MDPV+caffeine compared to rats that only ever self-administered MDPV. Although MDPV reinstated equivalent amounts of responding in both groups, contrary to predictions, caffeine also reinstated high levels of responding in rats with a history of MDPV or MDPV+caffeine self-administration. Numerous studies suggest caffeine can reinstate low levels of responding in rats that previously self-administered cocaine;e.g.,25–27 so it was somewhat surprising that caffeine was as effective as MDPV at reinstating responding. Though the factors that underlie discrepancies between present and previous reports of the effectiveness of caffeine to reinstate responding are unclear, pharmacokinetic differences between MDPV and caffeine cannot be ruled out. For instance, with MDPV, reinstatement of responding was delayed, but ultimately occurred at high rates over a relatively short period of time. However, with caffeine, reinstatement of responding was more immediate, but occurred at lower rates over a relatively longer period of time. Thus, if slope/rate is used as a proxy for the magnitude/strength of the reinstatement response, then MDPV would be considered more effective at reinstating responding than caffeine. Nevertheless, that the effects of caffeine did not differ as a function of self-administration history suggests that, at least for mixtures of MDPV and caffeine, the composition of a self-administered drug mixture did not impact the effectiveness of the constituent drugs to reinstate responding.
Regardless of self-administration history, MDPV+caffeine mixtures were more effective at reinstating responding than would be predicted based on the effects of the constituents alone, suggesting mixtures may be more likely to promote relapse-related behaviors in abstinent individuals. These findings are consistent with previous studies that have identified supra-additive interactions between the discriminative stimulus1 and reinforcing effects4,5 of MDPV and caffeine in rats. Interestingly, unlike prior studies which described supra-additive enhancements in potency for mixtures of MDPV+caffeine to serve as discriminative stimuli or reinforce responding,1,4 the present findings suggest that mixtures of MDPV+caffeine were just as potent, but significantly more effective at reinstating responding than would be expected based on the effects of MDPV and caffeine alone. Although speculative, these differences may be due to procedural differences, such as the fact that MDPV produced near exclusive choice of the drug-appropriate lever in drug discrimination assays,1 and maintained unusually large final ratios (>1000 responses) when available for self-administration under a PR schedule of reinforcement,4 thus making it difficult, if not impossible, for the addition of caffeine to increase the maximal effect.
In addition to frequent inclusion in “bath salts” preparations, caffeine is also a common adulterant of other illicit stimulants, including cocaine.e.g.,9,31–33 While caffeine is likely added to provide bulk (i.e., to reduce the amount of illicit drug per gram of powder), it is also the most widely consumed psychoactive compound,34 and numerous studies suggest that caffeine can enhance a variety of abuse-related effects of stimulants.e.g.,1,2,4,5,18 This is important because the 10 mg/kg dose of caffeine that produced a robust reinstatement of responding in the present study has been shown to produce blood levels of caffeine (~10 0μ/ml; R. W. Seaman Jr, et al., unpublished data, January 2020) comparable to those observed in humans following oral administration of ~300–400 mg of caffeine,35 a dose roughly equivalent to 1–2 cups of coffee or an energy drink.34,36 Thus, consumption of highly caffeinated beverages may promote relapse-related behaviors in otherwise abstinent people, especially if consumed in the presence of other drug-associated cues (e.g., environmental or paraphernalia). Though a rigorous evaluation of the mechanism that accounts for the supra-additive interactions between MDPV and caffeine was outside the scope of the current study, a growing body of literature suggests that dopamine D2 and adenosine A2A receptors form heteromers in the striatum and tonic activation of A2A by adenosine serves to dampen signaling through dopamine D2 receptors.37,38 Indeed, adenosine A2A receptor agonists can inhibit the reinstatement responding for cocaine by dopamine D2 receptor agonists,39–41 and dopamine D2 receptor antagonists can interfere with the reinstatement of responding for cocaine by adenosine A2A receptor antagonists, such as caffeine.39 Thus, convergent evidence suggests the reinstating effects of caffeine, as well as the supra-additive enhancements in the reinstating effects of mixtures of caffeine and MDPV, may be mediated by caffeine’s capacity to effectively remove the tonic inhibition of dopamine D2 receptors, thereby enhancing the dopamine D2 receptor signaling pathways that underlie relapse-related behaviors.
The importance of the CS to the magnitude of response may further implicate dopamine D2 receptors, as previous reports have demonstrated that dopamine D2 receptor antagonists can attenuate the CS reinstatement effects of relatively weak reinforcers such as nicotine,42 and maintenance or reinstatement of responding by dopamine D2-like receptor agonists is mediated by enhancement of the conditioned properties of cocaine-associated stimuli.39,43–45 While these findings provide a potential explanation as to why the effectiveness of caffeine to reinstate responding was so highly dependent on the presence of the CS, previous studies have clearly established that drug-associated stimuli are not necessary for all drugs to reinstate an extinguished drug-taking response.e.g.,16,25,26,46 Indeed, the present study also clearly showed that the reinstating effects of mixtures of MDPV+caffeine were less reliant on response contingent CS presentations, suggesting that the supra-additive interactions observed between the reinstating effects of MDPV and caffeine may be influenced by additional factors, such as overlapping discriminative stimulus effects.
Although previous studies have shown that the cocaine-like discriminative stimulus properties of dopamine D1-like receptor agonists are not sufficient to reinstate extinguished responding for cocaine,26,46–50 one cannot rule out the possibility that the overlap, and interactions between, the discriminative stimulus properties of caffeine and MDPV contributed to effectiveness of mixtures of MDPV+caffeine to reinstate responding under both CS and no CS conditions, especially when considering the inability of heroin, which lacks MDPV-like discriminative stimulus properties, to reinstate responding. Indeed, given that supra-additive interactions have been reported between the discriminative stimulus effects of MDPV and caffeine,1 it is possible that synergism between the discriminative stimulus effects of MDPV and caffeine is sufficient to reinstate responding even in the absence of the CS. Thus, even though the similarities between the discriminative stimulus effects of the drugs that comprised our drug mixture may have confounded attempts to address hypotheses regarding the relative effectiveness of the individual constituents of drug mixtures to reinstate responding, studies with mixtures of drugs with non-overlapping discriminative stimulus effects (e.g., stimulants and opioids) could provide a better understanding of the factors that underlie relapse-related behaviors following more complex histories of polysubstance abuse.
In summary, MDPV, caffeine, and mixtures of MDPV+caffeine were all highly effective at promoting relapse-related behavior in rats, and the interactions between MDPV and caffeine, two common “bath salts” constituents, were supra-additive. Despite predictions about the importance of self-administration history (e.g., MDPV, or MDPV+caffeine) to relapse-related behaviors, the potency and effectiveness of MDPV, caffeine, or mixtures of MDPV+caffeine to reinstate responding did not differ as a function of reinforcement history. Though the present study was unable to identify history-dependent effects of MDPV, caffeine, and mixtures of MDPV+caffeine on relapse-related behaviors, the fact that illicit drug preparations commonly contain multiple psychoactive substances, and that polysubstance abuse is common, highlights the need for continued research into how complex reinforcement histories, and interactions among commonly used drugs can impact relapse-related behaviors.
Supplementary Material
Acknowledgements
The authors would also like to thank Kayla Galindo and Melson Mesmin for their technical assistance in the completion of these studies. This research was supported by National Institutes of Health and National Institute on Drug Abuse (R01DA039146 [GTC]), the jointly-sponsored National Institutes of Health Predoctoral Training Program in the Neurosciences (T32NS082145 [MRD]), and the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute of Alcohol Abuse and Alcoholism (KCR).
References
- 1.Collins GT, Abbott M, Galindo K, Rush EL, Rice KC, France CP. Discriminative stimulus effects of binary drug mixtures: studies with cocaine, MDPV, and caffeine. J Pharmacol Exp Ther. 2016;359(1):1–10. doi: 10.1124/jpet.116.234252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munzar P, Justinova Z, Kutkat SW, Ferré S, Goldberg SR. Adenosinergic modulation of the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology (Berl). 2002;161(4):348–355. doi: 10.1007/s00213-002-1075-5 [DOI] [PubMed] [Google Scholar]
- 3.Justinova Z, Ferre S, Segal PN, et al. Involvement of adenosine A1 and A2A receptors in the adenosinergic modulation of the discriminative-stimulus effects of cocaine and methamphetamine in rats. J Pharmacol Exp Ther. 2003;307(3):977–986. doi: 10.1124/jpet.103.056762 [DOI] [PubMed] [Google Scholar]
- 4.Gannon BM, Galindo KI, Mesmin MP, Rice KC, Collins GT. Reinforcing effects of binary mixtures of common bath salt constituents: studies with 3,4-methylenedioxypyrovalerone (MDPV), 3,4-methylenedioxymethcathinone (methylone), and caffeine in rats. Neuropsychopharmacology. 2018;43(4):761–769. doi: 10.1038/npp.2017.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gannon BM, Mesmin MP, Sulima A, Rice KC, Collins GT. Behavioral economic analysis of the reinforcing effects of “bath salts” mixtures: studies with MDPV, methylone, and caffeine in male Sprague-Dawley rats. Psychopharmacology (Berl). 2019;236(3):1031–1041. doi: 10.1007/s00213-018-5046-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forrester MB. Synthetic cathinone exposures reported to Texas poison centers. Am J Drug Alcohol Abuse. 2012;38(6):609–615. doi: 10.3109/00952990.2012.677890 [DOI] [PubMed] [Google Scholar]
- 7.Johnson PS, Johnson MW. Investigation of “bath salts” use patterns within an online sample of users in the United States. J Psychoactive Drugs. 2014;46(5):369–378. doi: 10.1080/02791072.2014.962717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA. Psychoactive “bath salts” intoxication with methylenedioxypyrovalerone. Am J Med. 2012;125(9):854–858. doi: 10.1016/j.amjmed.2012.02.019 [DOI] [PubMed] [Google Scholar]
- 9.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49(6):499–505. doi: 10.3109/15563650.2011.590812 [DOI] [PubMed] [Google Scholar]
- 10.Gannon BM, Baumann MH, Walther D, et al. The abuse-related effects of pyrrolidine-containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology. 2018;43(12):2399–2407. doi: 10.1038/s41386-018-0209-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumann MH, Partilla JS, Lehner KR, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology. 2013;38(4):552–562. doi: 10.1038/npp.2012.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gannon BM, Galindo KI, Rice KC, Collins GT. Individual differences in the relative reinforcing effects of 3, 4-methylenedioxypyrovalerone under fixed and progressive ratio schedules of reinforcement in rats. J Pharmacol Exp Ther. 2017;361(1):181–189. doi: 10.1124/jpet.116.239376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briscoe RJ, Vanecek SA, Vallett M, Baird TJ, Holloway FA, Gauvin D V. Reinforcing effects of caffeine, ephedrine, and their binary combination in rats. Pharmacol Biochem Behav. 1998;60(3):685–693. doi: 10.1016/S0091-3057(98)00041-0 [DOI] [PubMed] [Google Scholar]
- 14.Collins RJ, Weeks JR, Cooper MM, Good PI, Russell RR. Prediction of abuse liability of drugs using IV self-administration by rats. Psychopharmacology (Berl). 1983;82(1–2):6–13. doi: 10.1007/BF00426372 [DOI] [PubMed] [Google Scholar]
- 15.Comer SD, Carroll ME. Oral caffeine pretreatment produced modest increases in smoked cocaine self-administration in rhesus monkeys. Psychopharmacology (Berl). 1996;126(4):281–285. doi: 10.1007/BF02247378 [DOI] [PubMed] [Google Scholar]
- 16.Horger BA, Wellman PJ, Morien A, Davies BT, Schenk S. Caffeine exposure sensitizes rats to the reinforcing effects of cocaine. Neuroreport. 1991;2(1):53–56. doi: 10.1097/00001756-199101000-00013 [DOI] [PubMed] [Google Scholar]
- 17.Schenk S, Valadez A, Horger BA, Snow S, Wellman PJ. Interactions between caffeine and cocaine in tests of self-administration. Behav Pharmacol. 1994;5(2):153–158. doi: 10.1097/00008877-199404000-00006 [DOI] [PubMed] [Google Scholar]
- 18.Justinova Z, Ferré S, Barnes C, et al. Effects of chronic caffeine exposure on adenosinergic modulation of the discriminative-stimulus effects of nicotine, methamphetamine, and cocaine in rats. Psychopharmacology (Berl). 2009;203(2):355–367. doi: 10.1007/s00213-008-1270-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused “bath salt” constituent 3,4- methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38(4):563–573. doi: 10.1038/npp.2012.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berquist MD, Baker LE. Characterization of the discriminative stimulus effects of 3,4-methylenedioxypyrovalerone in male Sprague-Dawley rats. Behav Pharmacol. 2017;28(5):394–400. doi: 10.1097/FBP.0000000000000310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE. Stereoselective effects of abused “bath salt” constituent 3,4-methylenedioxypyrovalerone in mice: drug discrimination, locomotor activity, and thermoregulation. J Pharmacol Exp Ther. 2016;356(3):615–623. doi: 10.1124/jpet.115.229500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of “bath salt” cathinones. Behav Pharmacol. 2013;24(5–6):437–447. doi: 10.1097/FBP.0b013e328364166d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks C, Huang P, Ramos L, et al. Dopamine D1-like receptor agonist and D2-like receptor antagonist (−)-stepholidine reduces reinstatement of drug-seeking behavior for 3,4-methylenedioxypyrovalerone (MDPV) in rats. ACS Chem Neurosci. 2018;9(6):1327–1337. doi: 10.1021/acschemneuro.7b00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duart-Castells L, Blanco-Gandía MC, Ferrer-Pérez C, et al. Cross-reinstatement between 3,4-methylenedioxypyrovalerone (MDPV) and cocaine using conditioned place preference. Prog Neuro-Psychopharmacology Biol Psychiatry. 2020;100. doi: 10.1016/j.pnpbp.2020.109876 [DOI] [PubMed] [Google Scholar]
- 25.Worley CM, Valadez A, Schenk S. Reinstatement of extinguished cocaine-taking behavior by cocaine and caffeine. Pharmacol Biochem Behav. 1994;48(1):217–221. doi: 10.1016/0091-3057(94)90519-3 [DOI] [PubMed] [Google Scholar]
- 26.Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists. Science. 1996;271(5255):1586–1589. doi: 10.1126/science.271.5255.1586 [DOI] [PubMed] [Google Scholar]
- 27.Regier PS, Claxton AB, Zlebnik NE, Carroll ME. Cocaine-, caffeine-, and stress-evoked cocaine reinstatement in high vs. low impulsive rats: treatment with allopregnanolone. Drug Alcohol Depend. 2014;143(1):58–64. doi: 10.1016/j.drugalcdep.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Research Council. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academy Press; 2011. [Google Scholar]
- 29.Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc Natl Acad Sci U S A. 2000;97(8):4321–4326. doi: 10.1073/pnas.97.8.4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerak LR, Collins GT, France CP. Effects of lorcaserin on cocaine and methamphetamine self-administration and reinstatement of responding previously maintained by cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2016;359(3):383–391. doi: 10.1124/jpet.116.236307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cole C, Jones L, McVeigh J, Kicman A, Syed Q, Bellis M. Adulterants in illicit drugs: a review of empirical evidence. Drug Test Anal. 2011;3:89–96. doi: 10.1002/dta.220 [DOI] [PubMed] [Google Scholar]
- 32.Seely KA, Patton AL, Moran CL, et al. Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int. 2013;233(1–3):416–422. doi: 10.1016/j.forsciint.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 33.Bernardo NP, Siqueira MEPB, De Paiva MJN, Maia PP. Caffeine and other adulterants in seizures of street cocaine in Brazil. Int J Drug Policy. 2003;14(4):331–334. doi: 10.1016/S0955-3959(03)00083-5 [DOI] [Google Scholar]
- 34.Mitchell DC, Knight CA, Hockenberry J, Teplansky R, Hartman TJ. Beverage caffeine intakes in the U.S. Food Chem Toxicol. 2014;63:136–142. doi: 10.1016/j.fct.2013.10.042 [DOI] [PubMed] [Google Scholar]
- 35.Kaplan GB, Greenblatt DJ, Ehrenberg BL, et al. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol. 1997;37(8):693–703. doi: 10.1002/j.1552-4604.1997.tb04356.x [DOI] [PubMed] [Google Scholar]
- 36.Cappelletti S, Daria P, Sani G, Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr Neuropharmacol. 2014;13(1):71–88. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20(10):482–487. doi: 10.1016/S0166-2236(97)01096-5 [DOI] [PubMed] [Google Scholar]
- 38.Ferré S Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology (Berl). 2016;233(10):1963–1979. doi: 10.1007/s00213-016-4212-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wydra K, Suder A, Borroto-Escuela DO, Filip M, Fuxe K. On the role of A2A and D2 receptors in control of cocaine and food-seeking behaviors in rats. Psychopharmacology (Berl). 2015;232(10):1767–1778. doi: 10.1007/s00213-014-3818-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Neill CE, Letendre ML, Bachtell RK. Adenosine A2A receptors in the nucleus accumbens bi-directionally alter cocaine seeking in rats. Neuropsychopharmacology. 2012;37(5):1245–1256. doi: 10.1038/npp.2011.312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bachtell RK, Self DW. Effects of adenosine A2A receptor stimulation on cocaine-seeking behavior in rats. Psychopharmacology (Berl). 2009;206(3):469–478. doi: 10.1007/s00213-009-1624-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Jernigen C, Gharib M, Booth S, Caggiula AR, Sved AF. Effects of dopamine antagonists on drug cue-induced reinstatement of nicotine-seeking behavior in rats. Behav Pharmacol. 2010;21(2):153–160. doi: 10.1097/FBP.0b013e328337be95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Collins GT, Woods JH. Drug and reinforcement history as determinants of the response-maintaining effects of quinpirole in the rat. J Pharmacol Exp Ther. 2007;323(2):599–605. doi: 10.1124/jpet.107.123042 [DOI] [PubMed] [Google Scholar]
- 44.Collins GT, Woods JH. Influence of conditioned reinforcement on the response-maintaining effects of quinpirole in rats. Behav Pharmacol. 2009;20(5–6):492–504. doi: 10.1097/FBP.0b013e328330ad9b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins GT, Cunningham AR, Chen J, Wang S, Newman AH, Woods JH. Effects of pramipexole on the reinforcing effectiveness of stimuli that were previously paired with cocaine reinforcement in rats. Psychopharmacology (Berl). 2012. doi: 10.1007/s00213-011-2382-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Vries TJ, Schoffelmeer ANM, Binnekade R, Vanderschuren LJMJ. Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long-term withdrawal of IV drug self-administration. Psychopharmacology (Berl). 1999;143(3):254–260. doi: 10.1007/s002130050944 [DOI] [PubMed] [Google Scholar]
- 47.Callahan PM, Appel JB, Cunningham KA. Dopamine D1 and D2 mediation of the discriminative stimulus properties of d-amphetamine and cocaine. Psychopharmacology (Berl). 1991;103(1):50–55. doi: 10.1007/BF02244073 [DOI] [PubMed] [Google Scholar]
- 48.Spealman RD, Bergman J, Madras BK, Melia KF. Discriminative stimulus effects of cocaine in squirrel monkeys: Involvement of dopamine receptor subtypes. J Pharmacol Exp Ther. 1991;258(3):945–953. [PubMed] [Google Scholar]
- 49.Witkin JM, Nichols DE, Terry P, Katz JL. Behavioral effects of selective dopaminergic compounds in rats discriminating cocaine injections. J Pharmacol Exp Ther. 1991;257(2):706–713. [PubMed] [Google Scholar]
- 50.Khroyan TV, Barrett-Larimore RL, Rowlett JK, Spealman RD. Dopamine D1- and D2-like receptor mechanisms in relapse to cocaine-seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther. 2000;294(2):680–687. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.