Abstract
Background:
To measure trends in the use of tranexamic acid (TXA) during delivery in the United States and to evaluate demographic data and morbidity outcomes among these patients.
Methods:
This retrospective cohort study includes data from 19 hospitals in the Universal Health Services network. We compared rates of TXA use between January 2015 and June 2019 across geographic sectors. We also evaluated associations of demographic variables and perinatal outcomes of women who received TXA.
Results:
209 cases of TXA use were found from analysis of 101,564 deliveries. TXA use increased over time and rates were higher in the West than in Central and East; the slope of increase over years did not differ between regions. Women who received TXA were more likely to have a history of postpartum hemorrhage (59 (28.2%) vs. 2,290 (2.2%), P<0.0001) but were not more likely to have a chronic disease, including diabetes mellitus, hypertension and heart disease. Women who received TXA were more likely to have estimated blood loss greater than or equal to 1000 mL (adjusted odds ratio (aOR) 15.3; 95% CI 11.1—21.1; P<0.0001). Likelihood of venous thromboembolism was not significantly increased in TXA recipients (aOR 2.0; 95% CI 0.3—14.6; P=0.49).
Conclusion:
Increasing national trends of TXA use in the peripartum period was observed, with variable increases by geographic region. Likelihood of venous thromboembolism was not significantly increased among women who received TXA. Increasing TXA use throughout the country suggests that updated hemorrhage guidelines from national obstetrical organizations can shape clinical practice.
Keywords: tranexamic acid, postpartum hemorrhage, venous thromboembolism, perinatal outcomes
Introduction
Postpartum hemorrhage (PPH), defined as blood loss of over 1000 mL or requiring a blood transfusion at either vaginal or cesarean delivery, continues to pose an important threat to maternal health and survival during the peripartum period worldwide [1]. Although women are at greatest risk of developing PPH up to 24 hours after delivery, PPH can occur as late as twelve weeks after delivery. In recognition of this important threat to maternal health, in 2015 the National Partnership for Maternal Safety created a consensus bundle of guidelines for readiness, prevention, recognition, and prompt response for PPH. This bundle, the first of its kind, called for improved recognition of risk factors and mitigation of outcomes of women who experienced PPH with the goal of increasing standardization for PPH management [2].
In 2016, the World Maternal Antifibrinolytic (WOMAN) trial, which evaluated the efficacy and safety of tranexamic acid (TXA) to treat PPH, found that women who received TXA had significantly reduced rates of both death and hysterectomy from PPH compared to women who did not receive TXA [3]. This promising finding led the American College of Obstetricians and Gynecologists (ACOG) to release a revised practice bulletin in 2017 that endorsed considering TXA to treat PPH when traditional uterotonics fail [4].
In this study, we evaluated demographic data and subsequent outcomes of women who received TXA as a treatment for PPH, including rates of venous thromboembolism, to assess the population most commonly receiving TXA and their subsequent morbidity. We also measured trends in TXA usage over time and by region to find a temporal relationship between the releases of the WOMAN trial and ACOG bulletin and subsequent TXA use. This measure is important to understand how maternal safety recommendations are being utilized by obstetrical providers.
Materials & Methods
In this retrospective cohort study, data from women who delivered at gestational age greater than 24 weeks and less than 43 weeks between January 2015 and June 2019 at nineteen hospitals in the Universal Health Services, Incorporated (UHS) network in the United States were extracted and deidentified from Cerner’s electronic health record (EHR) platform. This network encompasses hospitals of low, medium and high volume of deliveries primarily in community settings [5]. Women were excluded from the analysis if there was no date of delivery or if they delivered earlier than 24 weeks or later than 43 weeks. This dataset was chosen to include a large cohort of deliveries in different types of hospitals within the network. All women, including those with multiple gestations, who delivered a live birth on a labor and delivery unit at a UHS hospital were included (Figure 1). Women who delivered more than one fetus were counted only once. Women were identified if they had TXA in the medications administered in the EHR during admission. The diagnosis of PPH was not required for inclusion in the data set. Peripartum was defined as the time period between the onset of the second stage of labor up to 24 hours following the delivery of the placenta. This study was approved by the George Washington University institutional review board on December 11, 2018.
Fig 1. Materials & Methods inclusion criteria:
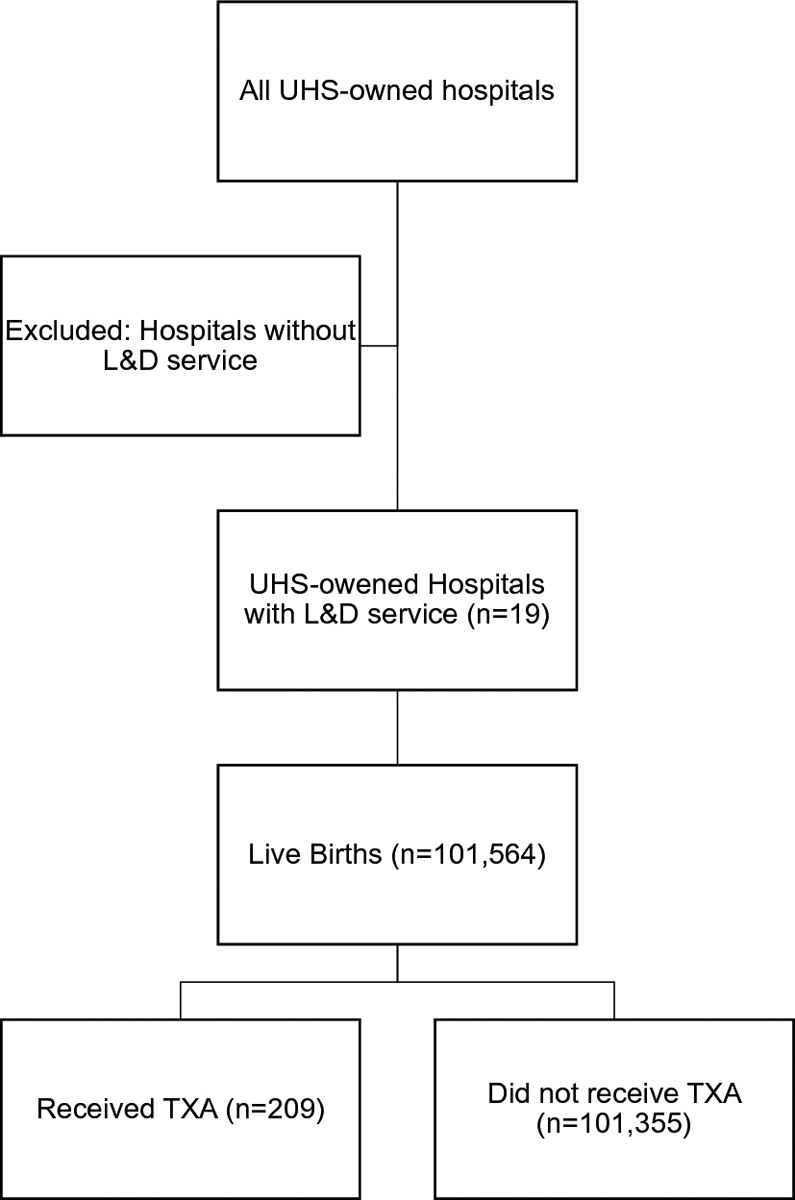
Inclusion criteria for our study included women who delivered at any one of the United Health Services (UHS) hospitals with a labor & delivery unit between January 1, 2015 and June 30, 2019.
The primary outcome of interest was change in rates of TXA use over time and by geographic region. Hospitals with TXA cases were grouped based on both geographic sector (East, Central, and West) and by number of deliveries per year between January 2015 and June 2019. Changes in rates of TXA use over time across all hospitals between January 2015 and June 2019 were evaluated.
Secondary outcomes examined included demographic and patient characteristics of those who received TXA in comparison to those who did not and particularly in cases of diagnosis with PPH. These variables included maternal characteristics (e.g., age, race); maternal medical conditions; pregnancy-related variables (e.g., delivery type, gestational diabetes; history of PPH); and labor-specific variables (e.g., gestational age, antibiotics use). A subgroup of patients with a diagnosis of PPH, defined as estimated blood loss greater than or equal 1000 mL or received blood transfusion, was chosen and demographic and outcome variables were examined between those who received and did not receive TXA.
Finally, we compared perinatal outcomes among these groups. Outcomes including blood transfusion, estimated blood loss (EBL) greater or equal 1000 mL, intensive care unit (ICU) admission, venous thromboembolism (VTE), chorioamnionitis, disseminated intravascular coagulation (DIC), placental abruption, placenta accreta, neonatal intensive care unit (NICU) admission, operative vaginal delivery, eclampsia, hemolysis, elevated liver enzymes and low platelets (HELLP) syndrome, and general anesthesia use.
Trend relations across the different regions were analyzed using a multivariable logistic regression model, with covariates year, region, and year by region interaction. Categorical and continuous independent variables were compared between those who did vs. did not receive TXA using 2-tailed between-group t-tests, or the Wilcoxon rank-sum test for continuous variables, or chi-square or Fisher’s Exact Test for categorical variables. A multivariable logistic regression to determine the independent association of TXA use with outcomes, controlling for any potential confounders. Results are presented as adjusted odds ratio (aOR) with 95% confidence intervals (CI). Potential confounders were determined with clinical relevance and include age, cesarean section, history of postpartum hemorrhage, anemia, placenta previa and use of magnesium sulfate. SAS (version 9.4, Cary, NC) was used for data analysis with P <0.05 considered significant. Our sample size gave us power >0.91 to detect an outcome difference in which incidence was 1% vs. 5% in non-TXA vs. TXA groups.
Results
Nineteen hospitals were grouped based on geographic location into East, Central, and West sectors. Five hospitals were part of the East sector; seven were part of the Central sector; and seven were part of the West sector. In our analysis 31% of hospitals had greater than 15,000 deliveries during the time period and were labeled “high” volume; 32% of hospitals had between 7,500 and 15,000 deliveries during the time period and were categorized as “medium” volume; and 38% of hospitals had fewer than 7,500 deliveries during the time period and were categorized as “low” volume. A total of 101,564 deliveries were analyzed in which there were 209 (0.2%) cases of TXA use and 101,355 (99.8%) cases without TXA use. The East sector had 16 (7.7%) cases of TXA use while the West sector had 168 (80.4%) and the Central sector had 25 (12.0%).
Use of tranexamic acid increased significantly across years when adjusting for sector (OR 3.55, 95% CI 3.02—4.18). The pattern of increase over years did not differ significantly by sector (P=0.18) as shown in Figure 2. Women who received TXA were significantly more likely to have a prior history of PPH (59 (28.2%) vs. 2,290 (2.2%), P<0.0001) or placenta previa (9 (4.3%) vs. 654 (0.6%), P<0.0001) compared to women who did not receive TXA. These patients also were more likely to have hematocrit less than 32%, although this finding was not significant (43 (20.6%) vs. 17,062 (16.8%), P=0.15) (Table 1). Body mass index, rates of pre-gestational diabetes, hypertension, heart disease, asthma, or renal disease did not differ significantly between the two groups. Women who received TXA were significantly more likely to have a cesarean section in comparison to women in the non-TXA group (117 (56%) vs. 36,306 (35.8%), P<0.0001).
Fig 2. Incidence of TXA use by year and by region.
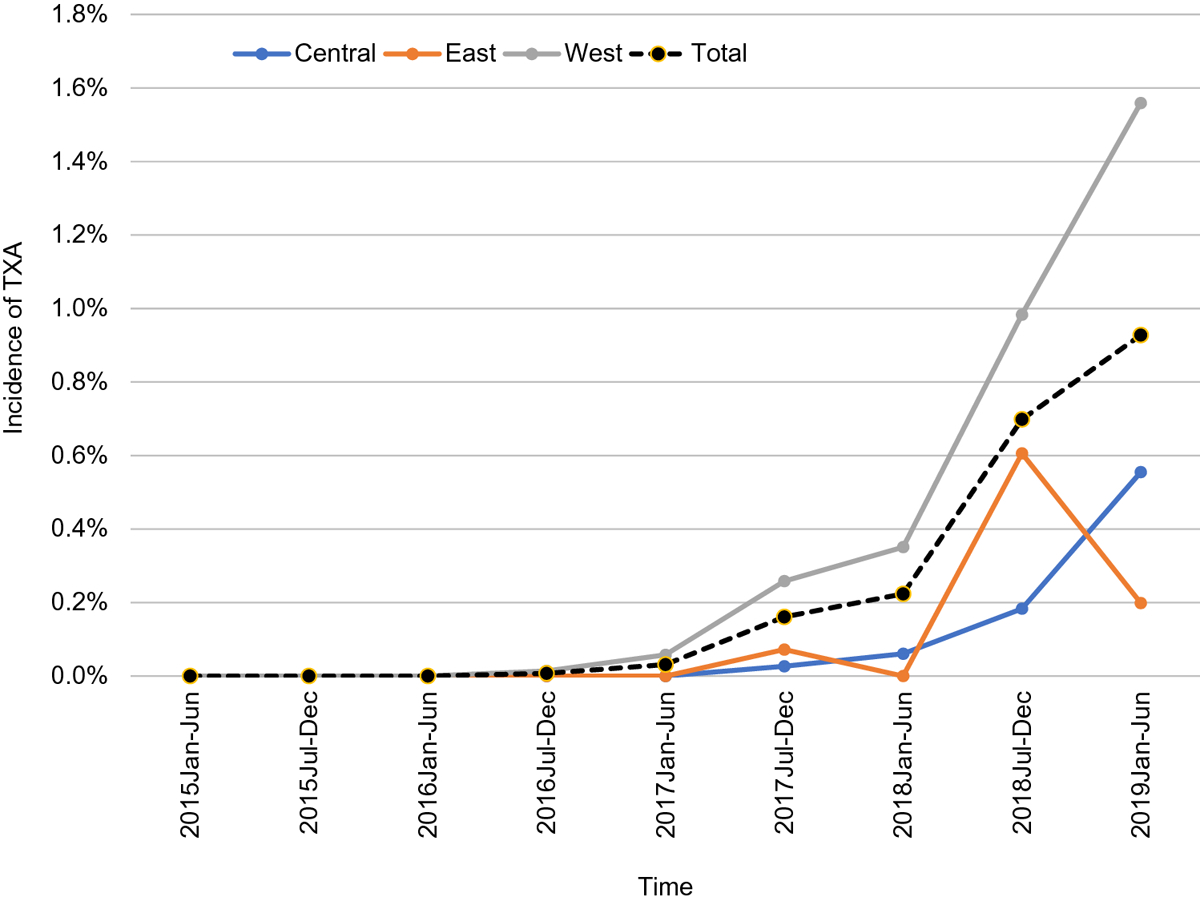
Tranexamic acid use rates increased across all sectors between 2015 and 2019
Table 1.
Patient characteristics in those with TXA and without TXA.
| With TXA (n=209) |
Without TXA (n=101,355) |
P | |
|---|---|---|---|
| Demographic variables | |||
| Age | 28.3 ± 6.1 | 27.9 ± 5.9 | 0.30 |
| Body mass index | 32.3 ± 7.1 | 31.8 ± 6.3 | 0.64 |
| Race | <0.0001 | ||
| White | 85 (40.7) | 54,713 (54.0) | |
| Black | 47 (22.5) | 12,415 (12.2) | |
| Asian | 12 (5.7) | 4,314 (4.3) | |
| Other/Unknown | 65 (31.1) | 1,9913 (29.5) | |
| Sector | <0.0001 | ||
| East | 16 (7.7) | 30,294 (29.9) | |
| West | 168 (80.4) | 18,771 (18.5) | |
| Central | 25 (12.0) | 52,290 (51.6) | |
| Insurance | <0.0001 | ||
| Medicaid | 66 (31.6) | 3,0781 (30.4) | |
| Medicare | 1 (0.5) | 209 (0.2) | |
| Private | 122 (58.4) | 44,785 (44.2) | |
| Uninsured | 5 (2.4) | 2,776 (2.7) | |
| Other/Unknown | 15 (7.2) | 22,681 (22.4) | |
| Maternal medical conditions | |||
| Chronic Hypertension | 4 (1.9) | 1,564 (1.5) | 0.66 |
| Diabetes | 10 (4.8) | 3,316 (3.3) | 0.22 |
| Heart Disease | 0 (0.0) | 15 (<0.1) | 0.86 |
| Asthma | 3 (1.4) | 1,535 (1.5) | 0.92 |
| Renal Disease | 0 (0.0) | 36 (<0.1) | 0.79 |
| Pregnancy-related variables | |||
| Delivery type | <0.0001 | ||
| Vaginal | 91.0 (43.5) | 64,395 (63.5) | |
| Cesarean | 117 (56.0) | 36,306 (35.8) | |
| VBAC | 1 (0.5) | 654 (0.6) | |
| Gestational Diabetes | 17 (8.1) | 6,757 (6.7) | 0.39 |
| History of PPH | 59 (28.2) | 2,290 (2.2) | <0.0001 |
| Preeclampsia | 6 (2.9) | 2665 (2.6) | 0.83 |
| Smoking during pregnancy | 10 (4.8) | 12,756 (12.6) | 0.0007 |
| Placenta previa | 9 (4.3) | 654 (0.6) | <0.0001 |
| Number of previous cesarean deliveries | <0.0001 | ||
| 0 | 183 (87.6) | 92,947 (91.7) | |
| 1 | 10 (4.8) | 4,942 (4.9) | |
| 2 | 8 (3.8) | 2,368 (2.3) | |
| ≥3 | 8 (3.9) | 1,098 (1.0) | |
| Labor-related variables | |||
| Gestational age, stratified | <0.0001 | ||
| 28 – 34 weeks | 15 (7.2) | 1,899 (1.9) | |
| 34 1/7 – 36 6/7 | 43 (20.6) | 15,709 (15.5) | |
| > 37 weeks | 151 (72.2) | 83,747 (82.7) | |
| Antibiotics | 173 (82.8) | 57,862 (57.1) | <0.0001 |
| Platelets <150,000 | 24 (11.5) | 9,908 (9.8) | 0.41 |
| Hematocrit <32% | 43 (20.6) | 17,062 (16.8) | 0.15 |
| Mg for neuroprotection | 27 (12.9) | 4034 (4.0) | <0.0001 |
| Dinoprostone | 18 (8.6) | 8,647 (8.5) | 0.97 |
| Misoprostol | 118 (56.50 | 12,382 (12.2) | <0.0001 |
| Oxytocin | 205 (98.1) | 93,275 (92.0) | 0.0012 |
| Artificial rupture of membrane | 28 (13.4) | 8608 (8.5) | 0.011 |
| Spontaneous rupture of membrane | 7 (3.3) | 3,801 (3.8) | 0.76 |
| Male Baby | 98 (46.9) | 50,336 (49.7) | 0.42 |
VBAC, Vaginal birth after cesarean; PPH, postpartum hemorrhage; Mg, magnesium.
Data are n (%) or mean ± standard deviation
The prevalence of PPH in our cohort was 5.5% (5,641/101,564). Among this PPH subgroup, 106 patients (or 50.7% of 209 total) had received TXA (Table 2). There was no difference in history of placenta previa or a hematocrit level of <32% between patients diagnosed with PPH who received TXA compared to no TXA.
Table 2.
Patient characteristics and outcomes of patients diagnosed with PPH (N=5,641).
| Patient variable | Received TXA (n=106) |
Did not receive TXA (n=5,535) |
P |
|---|---|---|---|
| Sector | <0.0001 | ||
| Central | 15 (14.2) | 1895 (34.2) | |
| East | 7 (6.6) | 1112 (20.1) | |
| West | 84 (79.2) | 2528 (45.7) | |
| Year | <0.0001 | ||
| 2015 | 0 (0.0) | 1001 (18.1) | |
| 2016 | 1 (0.9) | 1121 (20.2) | |
| 2017 | 10 (9.4) | 1406 (25.0) | |
| 2018 | 59 (55.7) | 1394 (25.2) | |
| 2019 | 36 (34.0) | 613 (11.5) | |
| History of PPH | 35 (33) | 368 (7) | <0.0001 |
| History of Placenta previa | 4 (3.7) | 117 (2.1) | 0.24 |
| Hematocrit <32% | 23 (21.7) | 1091 (19.7) | 0.61 |
| Gestational Age < 39 weeks | 55 (52) | 2125 (38) | 0.006 |
| Outcomesa | |||
| Intensive care unit admission | 7 (7) | 47 (0.9) | <.0001 |
| NICU admission | 14 (13) | 307 (6) | 0.003 |
| Transfusion | 3 (3) | 401 (7) | 0.08 |
| EBL ≥ 1000 ml | 106 (100) | 5306 (96) | 0.02 |
| Delivery type | <0.0001 | ||
| CD | 67 (63.2) | 4668 (84.3) | |
| VBAC | 0 (0.0) | 16 (0.3) | |
| Vaginal | 39 (36.8) | 851 (15.4) |
PPH = postpartum hemorrhage; NICU= Neonatal Intensive Care Unit; EBL = Estimated Blood Loss; CD = cesarean delivery; VBAC = vaginal birth after cesarean
Among the perinatal outcomes in patients receiving TXA and after adjusting for variables in the logistic regression, the independent associations with receiving TXA were ICU admission (aOR 12.4, 95% CI 4.9 – 31.7, P<0.0001), estimated blood loss of greater than or equal 1000 mL (aOR 15.3, 95% CI 11.1—21.1, P<0.0001).
Neonates had increased risk of NICU admission (aOR 4.3, CI 2.7—6.9, P<0.0001) if their mothers had received TXA. In addition, risk for venous thromboembolism did not significantly increase in women who received TXA (Table 3; aOR 2.0, 95% CI 0.3—15.9, P=0.49). Specifically, there was one case of VTE in the TXA group (0.5%) versus 202 among women without TXA use (0.2%), P=0.37.
Table 3.
Perinatal outcomes with and without TXA use at delivery.
| Perinatal outcomes | With TXA (n=209) |
Without TXA n=101,355) |
P | aORa (95% CI) | aP |
|---|---|---|---|---|---|
| Blood transfusion | 3 (1.4) | 401 (0.4) | 0.02 | 1.4 (0.4 – 4.7) | 0.56 |
| EBL ≥ 1000 mL | 106 (50.7) | 5,306 (5.2) | <0.0001 | 15.3 (11.1 – 21.1) | <0.0001 |
| ICU admission | 7 (3.3) | 83 (0.1) | <0.0001 | 12.4 (4.9 – 31.7) | <0.0001 |
| VTE | 1 (0.5) | 202 (0.2) | 0.37 | 2.0 (0.3 – 14.6) | 0.49 |
| Chorioamnionitis | 3 (1.4) | 635 (0.6) | 0.14 | 2.0 (0.6 – 6.5) | 0.23 |
| DIC | 2 (1.0) | 12 (<0.1) | <0.0001 | 14.7 (2.4 – 91.1) | 0.004 |
| Placental abruption | 6 (2.9) | 778 (0.8) | 0.0005 | 1.3 (0.5 – 3.5) | 0.58 |
| Placenta accreta | 7 (3.3) | 68 (0.1) | <0.0001 | 13.4 (.1 – 35.2) | <0.0001 |
| NICU admission | 30 (14.4) | 4,343 (4.3) | <0.0001 | 4.3 (2.7 – 6.9) | <0.0001 |
| Vacuum-assisted delivery | 14.0 (6.7) | 6,309 (6.2) | 0.78 | 0.9 (0.5 – 1.6) | 0.79 |
| Forceps-assisted delivery | 2.0 (1.0) | 445 (0.4) | 0.26 | 3.2 (0.8 – 13.0) | 0.11 |
| Eclampsia | 0 (0.0) | 243 (0.2) | 0.48 | - | - |
| HELLP syndrome | 1 (0.5) | 60 (0.1) | 0.013 | 3.2 (0.4 – 25.9) | 0.26 |
| General anesthesia | 5 (9.3) | 657 (0.8) | <0.0001 | 1.5 (0.6 – 4.0) | 0.37 |
CI, confidence interval, EBL, estimated blood loss; ICU, intensive care unit; VTE, venous thromboembolism; DIC, disseminated intravascular coagulation; NICU, neonatal intensive care unit; HELLP, hemolysis, elevated liver enzymes, and low platelets.
Adjusted for age, cesarean section, history of postpartum hemorrhage, anemia, placenta previa, and use of magnesium sulfate.
Discussion
The purpose of this study was to evaluate changes in TXA use by geographical location in the United States and to better understand characteristics and peripartum outcomes of women who received TXA at delivery. The findings of this study suggest that during the study time period TXA use increased across all geographic sectors, with the hospitals in the West having the highest rates of use compared to hospitals in the Central and East sectors. Women who received TXA at the time of delivery were more likely to have risk factors for PPH and had more serious outcomes, possibly due to the relatively recent change to recent guidelines; it is possible that because ACOG recommends TXA be considered only after first-line treatments fail, that the patients receiving TXA at this point in time are more likely to experience severe outcomes because they have failed initial measures and likely have more extensive hemorrhagic disease. Interestingly, about half of women who received TXA were not diagnosed with PPH. This would suggest either that clinicians are using this drug prophylactically for women at high risk for hemorrhage or that they are giving it early enough that the blood loss is near the diagnosis of PPH but does not quite reach it. Notably, we found that TXA did not increase risk of VTE.
To our knowledge, this is the first study that has assessed trends in TXA use among a representative cross-section of US hospitals. Given that the WOMAN trial was published in April 2017 and the ACOG bulletin was published in October 2017, the increased use of TXA may be related to these important publications. Despite several ongoing clinical trials showing benefit of TXA in cases of PPH, the lack of consensus on protocols including dosage and indication (cesarean vs. vaginal) hinders wide spread use by clinicians [6]. Moreover, the unique hypercoagulable status of women during pregnancy has made it difficult to generalize the safety profile of TXA outside of using it for PPH treatment [7]. In fact, much of what is known about TXA has come from trauma and orthopedics settings. In the Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) study, U.S. military researchers showed that TXA significantly decreased mortality and coagulopathy among combat injury victims requiring massive blood transfusion [8]. In addition, a meta-analysis evaluating TXA in cases of total hip replacement found that TXA reduced the proportion of patients who required blood transfusion without increasing rates of VTE or pulmonary embolism [9]. The CRASH-2 trial, one of the largest randomized clinical trials evaluating effects of TXA in acute trauma, found that TXA decreased rates of mortality from massive bleeding without significant increases in rates of thromboembolic events [10]. Following CRASH-2, several professional organizations, including the British Committee for Standards in Hematology, the pan-European, multidisciplinary Task Force for Advanced Bleeding Care in Trauma, and the National Clinical Guideline Centre, recommended the use of TXA to treat patients at risk of hemorrhage in the setting of acute injury [11].
The 2017 WOMAN trial confirmed that TXA decreases rates of mortality from bleeding without additional risk of thromboembolic events [3] and helped to bridge the concept that TXA has a unique application in obstetrics. In fact, the WOMAN trial prompted several international groups to recommend that TXA become a standard of care for PPH. Prior to the release of the WOMAN trial, Shaylor and colleagues [12] evaluated TXA recommendations among several international obstetric and gynecologic societies through systematic review. They found that only the Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG), national guidelines from Germany, Austria, and Switzerland, and an international expert panel of experts in obstetrics, gynecology, hematology, transfusion, and anesthesiology [13] recommended TXA as part of the standard of care. Following the WOMAN trial results, the World Health Organization recommended TXA be used as standard of care among women diagnosed with PPH within 3 hours of delivery [14]. Additionally, ACOG responded to the WOMAN trial findings by updating their own guidelines to encourage providers to consider TXA administration for PPH treatment when more traditional therapies fail [4]. Our research suggests that obstetrical providers in the United States are starting to follow the ACOG guidelines but that we have still have room for improvement to get consistent use with all PPH cases refractory to first line therapies. As more data accumulate surrounding TXA’s effectiveness and safety, it is our hope that professional obstetrical and gynecological societies will continue to update PPH guidelines to include TXA.
There are several limitations of this study. Although TXA use is increasing around the country, we had a limited sample size; a larger sample size might have highlighted additional significant differences that our study might have missed. Our study is retrospective limits collecting important variables from the datasets. Because there are no standard dosing guidelines for TXA to treat PPH, we could not assess a dose response curve. Dosing variability might have affected some of the outcomes we found in the study. Finally, timing of TXA administration could not be assessed; it is possible that some variables such as EBL could have been impacted depending on the early or late administration of the TXA.
Despite the limitations, our data illustrate how national guidelines and large clinical studies can influence clinical practice. Here we highlight an increasing national trend in TXA use at delivery, along with a number of demographic and outcome data points that can be used by providers to better understand patient populations at increased risk of requiring TXA. PPH remains a leading cause of maternal mortality and morbidity the United States during the peripartum period. Particularly in areas around the world where blood products are less available and severe morbidity and mortality during delivery are greater, TXA may provide maximal benefit. Further research is needed to determine the variability in using TXA across the United States regions and how the new recommendations are being received by physicians as they incorporate it into hemorrhage protocols.
Highlights.
Obstetrical tranexamic acid use increased in studied hospitals from 2015 to 2019.
Rates of venous thromboembolism were not increased among TXA recipients.
Obstetrical guidelines on hemorrhage can shape clinical care.
Future studies should evaluate dosing and timing of TXA administration.
Acknowledgements:
Nathan Bible and Megan Beglin for aiding in data extraction; Jamil Kazma for review and editing of the manuscript.
Funding: This project was funded by NHLBI grant (K23HL141640)
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Findings were presented at the Hemostasis and Thrombosis Research Society/North American Society on Thrombosis and Hemostasis Scientific Symposium in New Orleans, LA May 2019.
The authors have no conflict of interest to disclose. All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Homa Ahmadzia, Elaine Hynds, and Richard Amdur. The first draft of the manuscript was written by Elaine Hynds. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- 1.Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. (2014). Global causes of maternal death: A WHO systematic analysis. The Lancet Global Health, 2(6). 10.1016/S2214-109X(14)70227-X [DOI] [PubMed] [Google Scholar]
- 2.Main EK, Goffman BM, Kane LK, Fontaine PL, Gorlin JB, Lagrew DC, & Levy BS (2016). National Partnership for Maternal Safety: Consensus Bundle on Obstetric Hemorrhage. Obstetric Anesthesia Digest, 36(2), 88 10.1097/01.aoa.0000482622.40786.7b [DOI] [Google Scholar]
- 3.Shakur H, Roberts I, Fawole B, Chaudhri R, El-Sheikh M, Akintan A, et al. (2017). Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. The Lancet, 389(10084), 2105–2116. 10.1016/S0140-6736(17)30638-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American College of Obstetrician-Gynecologists. (2017). Practice Bulletin Number 183. Retrieved from https://www.acog.org/Restricted-Access?urlReq=https%3A%2F%2Fwww.acog.org%3A443%2FClinical+Guidance+and+Publications%2FPractice+Bulletins%2FCommittee+on+Practice+Bulletins+Obstetrics%2FPostpartum+Hemorrhage.aspx
- 5.Friedman AM, Ananth C. v, Huang Y, D’Alton ME, & Wright JD (2016). Hospital delivery volume, severe obstetrical morbidity, and failure to rescue. American Journal of Obstetrics and Gynecology, 215(6), 795.e1–795.e14. 10.1016/j.ajog.2016.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmadzia HK, Phillips JM, Katler QS, & James AH (2018). Tranexamic Acid for Prevention and Treatment of Postpartum Hemorrhage: An Update on Management and Clinical Outcomes. Obstetrical and ynecological Survey, 73(10), 587–594. 10.1097/OGX.0000000000000597 [DOI] [PubMed] [Google Scholar]
- 7.Sullivan JT (2017). The expanding role of tranexamic acid in the management of obstetric hemorrhage. Journal of Thoracic Disease, 9(8), 2251–2254. 10.21037/jtd.2017.07.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghazi S, Pierson R, & Torlinski T (2013). Military Application of Tranexamic Acid in Trauma Emergency Resuscitation Study. Media, War and Conflict, 14(1), 86–88. 10.1177/175114371301400118 [DOI] [Google Scholar]
- 9.Sukeik M, Alshryda S, Haddad FS, & Mason JM (2011). Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. The Journal of Bone and Joint Surgery. British Volume, 93-B(1), 39–46. 10.1302/0301-620X.93B1.24984 [DOI] [PubMed] [Google Scholar]
- 10.Williams-Johnson JA, McDonald AH, Strachan GG, & Williams EW (2010). Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2) a randomised, placebo-controlled trial. West Indian Medical Journal, 59(6), 612–624. [PubMed] [Google Scholar]
- 11.Nishida T, Kinoshita T, & Yamakawa K (2017). Tranexamic acid and trauma-induced coagulopathy. Journal of Intensive Care, 5(1). 10.1186/s40560-016-0201-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaylor R, Weiniger CF, Austin N, Tzabazis A, Shander A, Goodnough LT, & Butwick AJ (2017). National and International Guidelines for Patient Blood Management in Obstetrics: A Qualitative Review. Anesthesia and Analgesia, 124(1), 216–232. 10.1213/ANE.0000000000001473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdul-Kadir R, McLintock C, Ducloy A-S, El-Refaey H, England A, Federici AB, et al. (2014). Evaluation and management of postpartum hemorrhage: consensus from an international expert panel. Transfusion, 54(7), 1756–1768. 10.1111/trf.12550 [DOI] [PubMed] [Google Scholar]
- 14.Vogel JP, Oladapo OT, Dowswell T, & Gülmezoglu AM (2018). Updated WHO recommendation on intravenous tranexamic acid for the treatment of post-partum haemorrhage. The Lancet Global Health, 6(1), e18–e19. 10.1016/S2214-109X(17)30428-X [DOI] [PubMed] [Google Scholar]


