Abstract
Background
The prognosis of patients with renal cell carcinoma (RCC) depends greatly on the presence of extra-renal metastases.
Purpose
To investigate the value of total tumor volume (TTV) and enhancing tumor volume (ETV) as three-dimensional (3D) quantitative imaging biomarkers for disease aggressiveness in patients with RCC.
Material and Methods
Retrospective, HIPAA-compliant, IRB-approved study including 37 patients with RCC treated with image-guided thermal ablation during 2007–2015. TNM stage, RENAL Nephrometry Score, largest tumor diameter, TTV, and ETV were assessed on cross-sectional imaging at baseline and correlated with outcome measurements. The primary outcome was time-to-occurrence of extra-renal metastases and the secondary outcome was progression-free survival (PFS). Correlation was assessed using a Cox regression model and differences in outcomes were shown by Kaplan–Meier plots with significance and odds ratios (OR) calculated by Log-rank test/generalized Wilcoxon and continuity-corrected Woolf logit method.
Results
Patients with a TTV or ETV > 5 cm3 were more likely to develop distant metastases compared to patients with TTV (OR 6.69, 95% confidence interval [CI] 0.33–134.4, P=0.022) or ETV (OR 8.48, 95% CI 0.42–170.1, P=0.016) < 5 cm3. Additionally, PFS was significantly worse in patients with larger ETV (P = 0.039; median PFS 51.87 months vs. 69.97 months). In contrast, stratification by median value of the established, caliper-based measurements showed no significant correlation with outcome parameters.
Conclusion
ETV, as surrogate of lesion vascularity, is a sensitive imaging biomarker for occurrence of extra-renal metastatic disease and PFS in patients with RCC.
Keywords: Renal cell carcinoma, prognosis, biomarkers, tumor volume, metastases
Introduction
Renal cell carcinoma (RCC) is a growing health problem with global incidence rising about 2% each year (1). The rise in incidence rates of early stage disease is mostly attributed to greater availability of high-resolution abdominal imaging, thus enabling incidental detection of small and asymptomatic RCC cases in almost 50% of patients in Western countries (2). Using ultrasound, the administration of contrast agent shows very promising results for differentiating benign from malign renal masses and even helps distinguishing different RCC subtypes (3,4).
However, the equally rising incidence rates of advanced-stage disease cannot be explained by more widely available imaging (5,6). As such, RCC etiology appears to be connected with lifestyle risk factors, such as obesity, hypertension, and smoking, in addition to genetic susceptibility syndromes such as Von-Hippel–Lindau disease (VHL) (7,8).
Despite rising incidence, mortality related to RCC has steadily declined in Europe and the United States during the last decade. This can be explained by earlier diagnosis and widely available curative therapies such as resection and image-guided ablation, as well as recent advances in drug therapy (2,9).
Despite the falling mortality rate, 25% of all RCC patients present with metastatic disease at diagnosis, which is connected with a dramatically reduced survival (10,11). No reliable predictors exist to estimate the risk of developing metastatic disease in the remaining 75% of patients who initially present with local disease only.
Tumor angiogenesis is essential for local and metastatic tumor growth. Studies of RCC imaging features repeatedly demonstrate that some radiological characteristics, such as contrast enhancement, can be used as surrogates for tumor angiogenesis because they show a strong correlation with vascular endothelial growth factor (VEGF) expression in the tumor (12). Recent advances in software-assisted image analysis techniques allow for the quantification of tumor enhancement as surrogate marker for tumor viability in a variety of cancers, including RCC metastases to the liver, thus enabling a more precise assessment of prognosis as well as tumor response to targeted therapies (13). Histopathological evaluation of the tumor alone is limited in prognostic value: biopsies are subject to sampling error, especially in RCC, which is known to exhibit varying degrees of histological alteration within the same tumor (14).
The aim of the present study was to investigate the value of total tumor volume (TTV) and enhancing tumor volume (ETV) as 3D quantitative imaging biomarkers for disease aggressiveness in patients with RCC.
Material and Methods
Study design and cohort
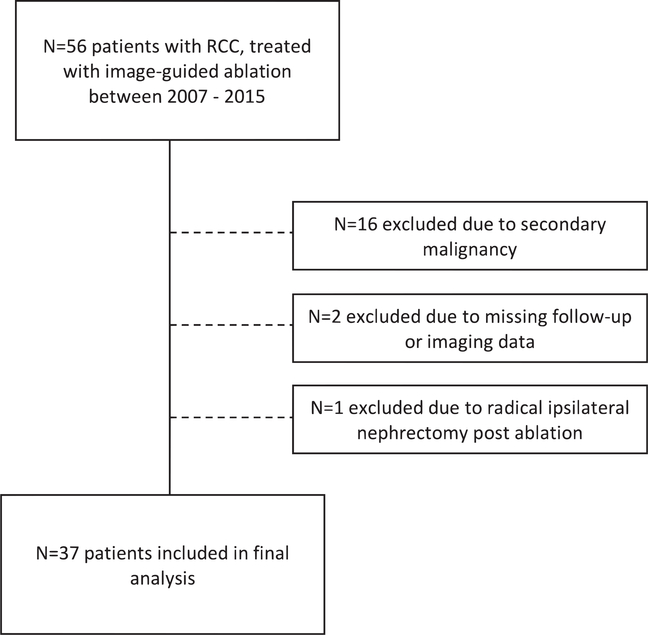
The present study obtained IRB approval from the Yale University Institutional Review Board and the need for informed consent was waived (HIC/HSC protocol no. 1510016734). This retrospective study was conducted in compliance with the Health Insurance Portability and Accountability Act (HIPAA). The manuscript was prepared according to STROBE guidelines (15). A total of 56 patients with non-metastatic RCC treated with image-guided ablation during 2007–2015 were considered for the analysis. The final analysis included 37 patients with RCC who underwent image-guided ablation. One patient was treated for two RCCs simultaneously, both at the upper pole of the right kidney. Fig. 1 illustrates the process of patient selection.
Fig. 1.
Flow chart of patient selection and exclusion criteria.
The baseline characteristics of the included patients are summarized in Table 1.
Table 1.
Baseline characteristics of study cohort.
| Baseline characteristics | |
|---|---|
| Demographics | |
| Patients (n) | 37 |
| Gender (male) | 24 (64.9) |
| Age (years) | 65.7 (41–86) |
| VHL disease | 2 (5.4) |
| Tumor characteristics | |
| Tumors (n) | 1.03 (1–2) |
| Tumor diameter (cm) | 2.2±0.75 |
| TTV (cm3) | 5.4±9.3 |
| ETV (cm3) | 5.3±8.2 |
| Histology (n=21) | |
| Clear cell | 15 (71.4) |
| Papillary | 4 (19.1) |
| Chromophobe | 2 (9.5) |
| TNM stage | |
| 1 | 25 (67.6) |
| 3 | 7 (18.9) |
| 4 | 5 (13.5) |
| RENAL score | 6.5 (4–10) |
| Fuhrman grade | 1.9 (1–3) |
| Treatment characteristics | |
| Ablation | |
| Percutaneous cryoablation | 25 (67.6) |
| Laparoscopic cryoablation | 4 (10.8) |
| Radiofrequency ablation | 8 (21.6) |
| Pre-ablative embolization | 2 (5.4) |
| Previous treatments | |
| Contralateral radical nephrectomy | 4 (10.8) |
| Contralateral partial nephrectomy | 2 (5.4) |
| Ipsilateral partial nephrectomy | 5 (13.5) |
| Imaging modality | |
| Contrast-enhanced MRI | 19 (51.4) |
| Contrast-enhanced CT | 18 (48.6) |
Values are given as n (%), mean (range), or median±SD.
CT, computed tomography; ETV, enhancing tumor volume; MRI, magnetic resonance imaging; TTV, total tumor volume.
Treatment and imaging protocols
All included patients were treated either percutaneously with computed tomography (CT)-guided radiofrequency ablation (n = 8) or cryoablation (n = 25), or laparoscopically with ultrasound-guided cryoablation (n = 4). All lesions treated in this study were treatment-naïve and did not undergo additional surgical treatment. Before ablation, all patients underwent contrast-enhanced CT with an iodine-based contrast agent or contrast-enhanced magnetic resonance imaging (MRI) with a gadolinium-based contrast agent. For MRI, the institutional renal mass protocol was applied, which included axial and coronal T2-weighted (T2W) single-shot fast spin-echo images, in- and opposed-phase images, and axial diffusion-weighted images. T1-weighted (T1W) 3D spoiled gradient-echo images were acquired in axial and sagittal planes before contrast, as well as in the axial plane only during the corticomedullary, nephrographic, and excretory phases (30 s, 100 s, and 3 min after the administration of contrast agent, respectively). Patients who underwent CT imaging were examined using native and contrast-enhanced images in the corticomedullary and nephrographic phases, with excretory phase images added in some cases. Additionally, biopsies were obtained in 21 (37.5%) patients, especially when imaging was not absolutely distinct.
Qualitative and quantitative image analysis
Lesions were assessed using the TNM staging system, RENAL Nephrometry Score, and largest tumor diameter, manually measured on axial imaging using the caliper tool (16, 17). The radiological readings and classifications were performed on anonymized images by a board-certified radiologist with 10 years of experience in body imaging (AA).
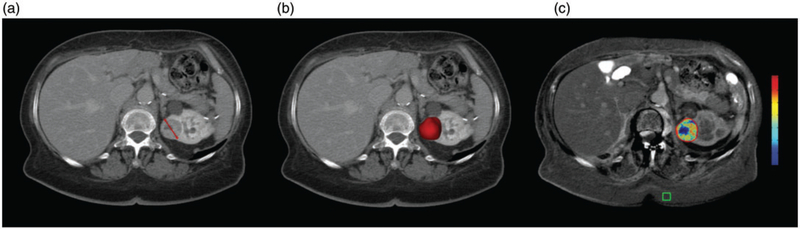
The 3D analysis was performed by a radiological reader with one year of experience in the usage of the 3D tool (BB). The tumor contours as well as the placement of the region of interest (ROI) were then confirmed by an experienced reader (five years, CC). A 3D mask of the tumor was created on contrast-enhanced CT or contrast-enhanced MRI using a semi-automated software for volumetric tumor segmentation (IntelliSpace Portal V8, Philips) as described in detail elsewhere (18,19). The TTV was derived from the 3D tumor mask after segmentation. The native images were then subtracted from the contrast-enhanced images to reduce background enhancement. The 3D tumor mask was transferred onto the subtracted image set and a ROI (1 cm3) was placed in homogeneously enhancing areas of subcutaneous fat tissue. The ETV was defined as the tumor volume in which the enhancement exceeded that of the reference ROI by >2 SD. Fig. 2 provides an overview of the different image assessment techniques.
Fig. 2.
Image assessment techniques: (a) measurement of axial tumor diameter; (b) three-dimensional (3D) measurement of total tumor volume based on the segmentation mask; (c) 3D measurement of enhancing tumor volume on subtracted images relative to a region of interest (green cube). Red represents maximum enhancement and blue represents no enhancement.
Endpoints and statistical analysis
The primary endpoint was time-to-occurrence of extra-renal metastases, measured starting the first day after ablation. Patients who did not develop extra-renal metastases at the time of last follow-up (30 September 2016) were censored. The secondary endpoint was progression-free survival (PFS), defined as either time from treatment to disease progression on follow-up imaging or death. Patients who were alive at the end-of-observation date and did not demonstrate disease progression were censored.
Baseline characteristics were reported using descriptive statistics. Adverse events (AEs) were reported according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) and the Society of Interventional Radiology (SIR) grading system (20,21).
Univariate analysis of baseline parameters and time-to-occurrence of extra-renal metastases was computed using a Cox regression model. Parameters that achieved significance were taken into multivariate analysis together with the conventional staging scores (TNM, RENAL Nephrometry Score, largest tumor diameter, TTV, ETV) independently of their respective significance in the univariate analysis. Individual Kaplan–Meier curves were plotted to visualize both time-to-occurrence of extra-renal metastases and PFS based on either conventional staging scores, TTV or ETV. The cohort was stratified at the median value of each of these measures. Odds ratios (OR) were calculated with continuity correction using the Woolf logit method and significance was determined using Log-rank test and generalized Wilcoxon with a P value < 0.05 considered statistically significant. We calculated the statistical power of the analysis using a general linear model. The statistical analysis was performed using commercially available statistical software (SPSS version 23.0, IBM and Prism Version 7, GraphPad Software).
Results
Procedural outcome and follow-up
All interventions were technically successful with complete tumor coverage on intra-procedural imaging. Two (5.4%) patients received trans-arterial embolization one day before ablation to reduce the risk of bleeding during the procedure. No patient died within 30 days after the intervention. No major AEs occurred according to CTCAE or SIR criteria. Minor AEs are summarized in Table 2 (20,22).
Table 2.
Adverse events (AEs).
| Patients | CTCAE grade | SIR grade | |
|---|---|---|---|
| Major AEs | None | n/a | n/a |
| Minor AEs | |||
| Small peri-renal hematoma* | 10 (27.0) | 1 | A |
| Ablation of adjacent liver tissue* | 2 (5.4) | 1 | A |
| Hematuria for two days* | 1 (2.7) | 1 | A |
Values are given as n (%).
Overview of AEs that occurred during the course of the study. Major AEs comprise AEs of grades C-F according to the SIR grading system, while minor AEs are those in categories A and B.
No therapeutic intervention required.
Follow-up imaging demonstrated progression of the ablated lesion in 4 (10.8%) cases and 1 (2.7%) of these patients underwent re-ablation of the target lesion. Three (8.1%) patients developed new renal lesions, two of which were ablated. Three patients received additional systemic therapies (Interleukin-2, Bevacizumab, and anti-PD-1). Four (10.8%) patients developed extra-renal metastases during follow-up after a median time of 17.8 months (range = 7.6–50.7 months) and 2 (5.4%) died due to multi-metastatic progressive disease within 5.43 and 5.97 months. Overall, 7 (18.9%) patients died before the end-of-observation date (30 September 2016).
Time-to-occurrence of extra-renal metastases
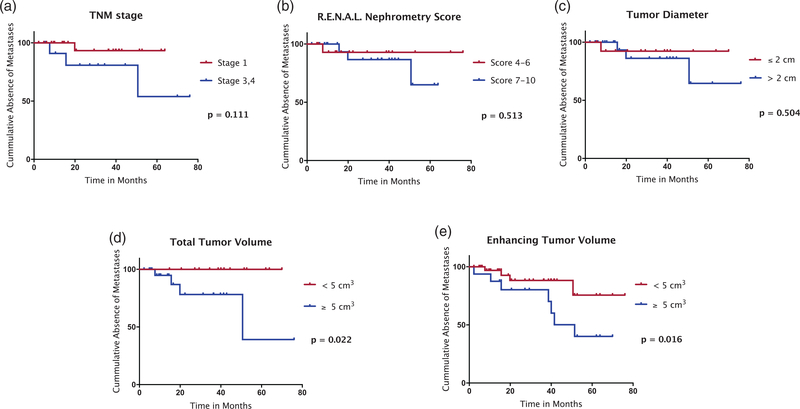
The univariate as well as the multivariate analysis of the patients’ baseline characteristics including tumor diameter, TNM stage, and RENAL Nephrometry Score revealed no significant correlation with time-to-occurrence of extra-renal metastases (Table 3). The statistical power was 73.3% based on an alpha level of 0.05. Kaplan–Meier analyses of the cohort stratified according to the median values of TNM stage, RENAL Nephrometry Score, tumor diameter, TTV, or ETV showed that time-to-occurrence of extra-renal metastases was significantly longer in patients with TTV < 5cm3 (P = 0.022) and ETV < 5 cm3 (P = 0.016) compared to patients with larger total and enhancing tumor volumes. The continuity-corrected OR was 6.69 (95% confidence interval [CI] = 0.33–134.4) for TTV ≥ 5 cm3 and 8.48 (95% CI = 0.42–170.1) for ETV ≥ 5 cm3. TNM, RENAL Nephrometry Score, and tumor diameter did not achieve significant separation of the compared cohorts (Fig. 3).
Table 3.
Time-to-occurrence of extra-renal metastases: uni- and multivariate analysis.
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Method | HR | 95% CI | P value | HR | 95% CI | P value |
| Tumor diameter (cm) | 2.12 | 0.29–15.52 | 0.504 | 1.97 | 0.27–14.42 | 0.227 |
| <2 vs. ≥2 | ||||||
| TNM stage | 5.24 | 0.68–40.21 | 0.111 | 5.17 | 0.68–39.43 | 0.167 |
| 1 vs. 3, 4 | ||||||
| RENAL Nephrometry Score | 2.09 | 0.29–15.34 | 0.513 | 1.95 | 0.27–14.28 | 0.284 |
| 4–6 vs. 7–10 | ||||||
| TTV (cm3) | 6.69 | 0.33–134.4 | 0.022* | 2.56 | 0.67–8.46 | 0.009* |
| <5 vs. ≥5 | ||||||
| ETV (cm3) | 8.48 | 0.42–170.1 | 0.016* | 3.01 | 0.88–10.27 | 0.007* |
| <5 vs. ≥5 | ||||||
Results of the uni - and multivariate analysis for Time-to-Occurrence of Extra-renal Metastases according to the examined
Imaging Biomarkers calculated by Cox regression.
Statistical significance.
CI, confidence interval; ETV, enhancing tumor volume; HR, hazard ratio; TTV, total tumor volume.
Fig. 3.
Kaplan–Meier analysis of time-to-occurrence of extra-renal metastases according to TNM stage (a), RENAL Nephrometry Score (b), and tumor diameter (c) as well as TTV (d) and ETV (e) as grouped by the median of the respective measurement being set as cut-off value. No patient was classified as TNM stage 2. Only TTV and ETV provided a significant separation of the curves using the log-rank test. ETV, enhancing tumor volume; TTV, total tumor volume.
Progression-free survival
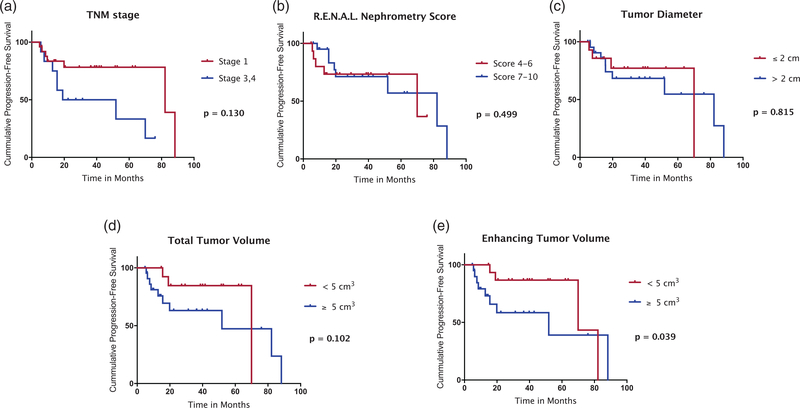
The median PFS was 69.97 months (95% CI = 45.9–94.0 months) for the entire cohort. Neither tumor diameter, TNM stage, RENAL Nephrometry Score, or TTV resulted in a statistically significant separation of the cohort using Kaplan–Meier analysis. However, patients with an ETV < 5 cm3 demonstrated significantly longer median PFS (69.97 months; 95% CI = 0.0–141.2 months) compared to patients with an ETV ≥ 5 cm3 (51.87 months; 95% CI = 0.0–109.6 months; P = 0.039) (Fig. 4). The OR for the occurrence of progressive disease or death was 2.25 (95% CI = 0.54–9.35) for patients with ETV ≥ 5 cm3. Table 4 demonstrates the differences in median time-to-occurrence of extra-renal metastases and median PFS of the cohort when stratified by ETV at 5cm3.
Fig. 4.
Kaplan–Meier analysis of PFS of the cohort stratified according to the median value of TNM stage (a), RENAL Nephrometry Score (b), tumor diameter (c), TTV (d), and ETV (e). The PFS was significantly longer in patients with an ETV < 5 cm3 compared to patients with an ETV ≥ 5 cm3 (P = 0.039 using the generalized Wilcoxon test). ETV, enhancing tumor volume; PFS, progression-free survival; TTV, total tumor volume.
Table 4.
Survival: stratification by ETV.
| ETV<5 cm3 | ETV ≥ 5 cm3 | P value | OR | 95% CI | |
|---|---|---|---|---|---|
| Median time-to-occurrence of extra-renal metastases (months) | n/a* | 50.67 | 0.016† | 8.48 | 0.42–170.1 |
| Median PFS (months) | 69.97 | 51.87 | 0.039† | 2.25 | 0.54–9.35 |
Median time-to-occurrence of extra-renal metastases and median PFS for the cohort according to ETV larger or smaller than 5 cm3, respectively.
No extra-renal metastases occurred in patients with an ETV<5 cm3.
Statistical significance.
CI, confidence interval; ETV, enhancing tumor volume; OR, odds ratio; PFS, progression-free survival.
Discussion
In the present study, we introduce ETV as a new imaging biomarker to predict PFS and the probability of metastatic progression in patients with unresectable advanced RCC scheduled for loco-regional ablation therapy. Extra-renal metastases represent an important prognostic factor in this group of patients; we accordingly observed a mortality rate of 50% when metastases were present compared to 15.2% for patients with local disease only.
Patients with ETV ≥ 5 cm3 at baseline had a significantly higher risk of developing extra-renal metastases. ETV was also significantly correlated with PFS, which suggests tumor enhancement on baseline imaging is an unfavorable prognostic factor. Therefore, patients with ETV ≥ 5 cm3 may benefit from more extensive radiological surveillance or even adjuvant therapy. A large TTV also correlated with a higher probability of developing extra-renal metastases but failed to predict PFS. This is consistent with the observation that tumor size alone does not sufficiently represent the extent of the disease and that metrics of tumor viability are needed to assess the real tumor burden.
Our findings are concordant with a study recently published by Yin et al. (12), which found a strong correlation between imaging patterns such as enhancement and pathologically determined micro-vessel density (MVD), a commonly accepted metric of tumor angiogenesis crucial for metastatic tumor spread. Likewise, Chapiro et al. (19) reported a strong correlation between decreased enhancement and tumor necrosis during pathologic examination for patients with hepatocellular carcinoma after transarterial chemoembolization. In another study, Chapiro et al. demonstrated that enhancement-based measurements can predict overall survival in patients with colorectal liver metastases (23). The literature is not entirely consistent, as Coy et al. (24) found enhancement to be inversely correlated with Fuhrman grade. However, these analyses were limited to clear-cell RCC and no clinical outcomes were reported. This makes it difficult to assess whether tumors with low enhancement were also clinically more aggressive. Furthermore, instead of an absolute measure for the patients’ enhancing tumor mass (e.g. in cm3), Coy et al. calculated the mean degree of enhancement of the tumor relative to the native phase (in Hounsfield units). This method might be disproportionally influenced by portions of necrotic tissue within the tumor which skews the mean downward because of its disproportionally darker appearance.
One-dimensional and 3D measurements such as tumor diameter alone do not sufficiently represent the extent of disease and thus, their prognostic value remains limited as reported in previous studies (25). As such, Klatte et al. (26) stated that prognosis as well as the risk of developing synchronous metastases are independent of tumor diameter in patients with RCC.
Furthermore, ETV is derived from a quantitative 3D assessment of the whole lesion. Since RCC is known to exhibit irregular vascularity patterns inside the tumor, ETV allows highly accurate volumetric measurement of enhancing portions of the tumor. In contrast, biopsy-derived analysis only provides a random sample that is not necessarily representative of the whole tumor (27,28).
According to the current clinical practice guidelines for kidney cancer, all patients receive cross-sectional imaging using CT or MRI at the time of first diagnosis (1,5,29). The availability of diagnostic imaging in nearly every patient supports the role of imaging-derived parameters for prognostication. In this setting, the semi-automatic assessment of ETV allows for high reproducibility and fast measurement, with segmentation and enhancement calculations taking approximately 1 min or less (19,30). The lack of sampling error and fast, non-invasive measurements illustrate the advantage of radiological tumor analysis compared to histopathological staging scores such as the ISUP grading system (31). With new molecular therapies evolving, the field of radio-genomics will play an important role in allocating the best treatment to an individual patient. For instance, Jamshidi et al. (32) showed that a radiological risk score could be used to stratify the outcome of patients after pre-surgical bevacizumab therapy in mRCC.
The present study has some limitations. First, the number of included patients and patients with extra-renal metastases was relatively small, leading to a large 95% CI and limited statistical power. The limitation to a single institution has to be considered as potential selection bias with regards to ablation technique and patient care. For instance, not all patients received biopsy as in patients with radiologically distinct RCC, we often refrained from biopsy in the clinical management of the patients. The relatively high local progression rate (10.8%) may be attributed to the fact that approximately one-third (32.4%) of the patients were TNM/AJCC stage 3 or 4, while most other studies examine local tumor control in cohorts with small renal masses (stage 1 disease) (33–35).
In conclusion, the present study demonstrates a potential prognostic value for the 3D quantitative imaging biomarkers TTV and ETV with respect to predicting metastatic spread in patients with RCC. Contrary to TTV, ETV has also proven to be predictive of PFS. Prospective trials with larger cohorts are needed for independent confirmation of these findings so they can be incorporated into existing staging scores and translated into clinical practice.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: BRT received a scholarship from the Rolf W. Guenther Foundation of Radiological Sciences; LJS has a Leopoldina postdoctoral fellowship and received a scholarship from the Rolf W. Guenther Foundation of Radiological Sciences and a research grant from the National Institutes of Health (NIH/NCI R01 CA206180) and from the Society of Interventional Oncology; MDL is an employee of Philips Research North America and has a research grant from the National Institutes of Health (NIH/NCI R01 CA206180); JC received a research grant from the National Institutes of Health (NIH/NCI R01 CA206180) Koninklijke Philips NV and the Society of Interventional Oncology.
Footnotes
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MDL is an employee of Visage Imaging, Inc. and former employee of Philips Research North America; BG is a consultant for PAREXEL, BAYER, and ICON. BG received lecture fees from Sirtex Medical, Pfizer, ROCHE, BAYER, BMS Medical, Siemens, and Philips, a travel grant from AngioDynamics; JC received a research grant from Guerbet, and Boston Scientific.
References
- 1.Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 2015;67:913–924. [DOI] [PubMed] [Google Scholar]
- 2.Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519–530. [DOI] [PubMed] [Google Scholar]
- 3.Rubenthaler J, Negrao de Figueiredo G, Mueller-Peltzer K, et al. Evaluation of renal lesions using contrast-enhanced ultrasound (CEUS); a 10-year retrospective European single-centre analysis. Eur Radiol 2018;28:4542–4549. [DOI] [PubMed] [Google Scholar]
- 4.Xue LY, Lu Q, Huang BJ, et al. Differentiation of subtypes of renal cell carcinoma with contrast-enhanced ultrasonography. Clin Hemorheol Microcirc 2016;63:361–371. [DOI] [PubMed] [Google Scholar]
- 5.Escudier B, Porta C, Schmidinger M, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27: v58–v68. [DOI] [PubMed] [Google Scholar]
- 6.Tyson MD, Humphreys MR, Parker AS, et al. Age-period-cohort analysis of renal cell carcinoma in United States adults. Urology 2013;82:43–47. [DOI] [PubMed] [Google Scholar]
- 7.Chow WH, Dong LM, Devesa SS. Epidemiology and risk factors for kidney cancer. Nat Rev Urol 2010;7: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adeniran AJ, Shuch B, Humphrey PA. Hereditary renal cell carcinoma syndromes: clinical, pathologic, and genetic features. Am J Surg Pathol 2015;39:e1–e18. [DOI] [PubMed] [Google Scholar]
- 9.Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers 2017;3:17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel HD, Gorin MA, Gupta N, et al. Mortality trends and the impact of lymphadenectomy on survival for renal cell carcinoma patients with distant metastasis. Can Urol Assoc J 2016;10:389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj GV, Thompson RH, Leibovich BC, et al. Preoperative nomogram predicting 12-year probability of metastatic renal cancer. J Urol 2008;179:2146–2151; discussion 2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yin Q, Hung SC, Wang L, et al. Associations between tumor vascularity, vascular endothelial growth factor expression and PET/MRI radiomic signatures in primary clear-cell-renal-cell-carcinoma: proof-of-concept study. Sci Rep 2017;7:43356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleckenstein FN, Schernthaner RE, Duran R, et al. Renal cell carcinoma metastatic to the liver: early response assessment after intraarterial therapy using 3D quantitative tumor enhancement analysis. Transl Oncol 2016;9:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delahunt B, Egevad L, Samaratunga H, et al. Gleason and Fuhrman no longer make the grade. Histopathology 2016;68:475–481. [DOI] [PubMed] [Google Scholar]
- 15.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 16.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometrys core: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009;182:844–853. [DOI] [PubMed] [Google Scholar]
- 17.Pichler M, Hutterer GC, Chromecki TF, et al. Comparison of the 2002 and 2010 TNM classification systems regarding outcome prediction in clear cell and papillary renal cell carcinoma. Histopathology 2013;62: 237–246. [DOI] [PubMed] [Google Scholar]
- 18.Chapiro J, Lin M, Duran R, et al. Assessing tumor response after loco-regional liver cancer therapies: the role of 3D MRI. Expert Rev Anticancer Ther 2015;15:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chapiro J, Wood LD, Lin M, et al. Radiologic-pathologic analysis of contrast-enhanced and diffusion-weighted MR imaging in patients with HCC after TACE: diagnostic accuracy of 3D quantitative image analysis. Radiology 2014;273:746–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.03. Washington, DC: NIH, 2010. [Google Scholar]
- 21.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. Radiology 2014;273:241–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacks D, McClenny TE, Cardella JF, et al. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 2003;14:S199–202. [DOI] [PubMed] [Google Scholar]
- 23.Chapiro J, Duran R, Lin M, et al. Early survival prediction after intra-arterial therapies: a 3D quantitative MRI assessment of tumour response after TACE or radioembolization of colorectal cancer metastases to the liver. Eur Radiol 2015;25:1993–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coy H, Young JR, Douek ML, et al. Association of qualitative and quantitative imaging features on multiphasic multidetector CT with tumor grade in clear cell renal cell carcinoma. Abdom Radiol (NY) 2019;44:180–189. [DOI] [PubMed] [Google Scholar]
- 25.Chapiro J, Duran R, Lin M, et al. Identifying staging markers for hepatocellular carcinoma before transarterial chemoembolization: comparison of three-dimensional quantitative versus non-three-dimensional imaging markers. Radiology 2015;275:438–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klatte T, Patard JJ, de Martino M, et al. Tumor size does not predict risk of metastatic disease or prognosis of small renal cell carcinomas. J Urol 2008;179:1719–1726. [DOI] [PubMed] [Google Scholar]
- 27.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multi-region sequencing. N Engl J Med 2012;366:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan Q, Kapur P, Zhang Y, et al. Intratumor heterogeneity of perfusion and diffusion in clear-cell renal cell carcinoma: correlation with tumor cellularity. Clin Genitourin Cancer 2016;14:e585–e594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell SC, Novick AC, Belldegrun A, et al. Guideline for management of the clinical T1 renal mass. J Urol 2009;182:1271–1279. [DOI] [PubMed] [Google Scholar]
- 30.Lin M, Pellerin O, Bhagat N, et al. Quantitative and volumetric European Association for the study of the liver and response evaluation criteria in solid tumors measurements: feasibility of a semiautomated software method to assess tumor response after transcatheter arterial chemoembolization. J Vasc Interv Radiol 2012;23:1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delahunt B, Eble JN, Egevad L, et al. Grading of renal cell carcinoma. Histopathology 2019;74:4–17. [DOI] [PubMed] [Google Scholar]
- 32.Jamshidi N, Jonasch E, Zapala M, et al. The radiogenomic risk score stratifies outcomes in a renal cell cancer phase 2 clinical trial. Eur Radiol 2016;26:2798–2807. [DOI] [PubMed] [Google Scholar]
- 33.Psutka SP, Feldman AS, McDougal WS, et al. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol 2013;63:486–492. [DOI] [PubMed] [Google Scholar]
- 34.Thompson RH, Atwell T, Schmit G, et al. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol 2015;67:252–259. [DOI] [PubMed] [Google Scholar]
- 35.Pantelidou M, Challacombe B, McGrath A, et al. Percutaneous radiofrequency ablation versus robotic-assisted partial nephrectomy for the treatment of small renal cell carcinoma. Cardiovasc Intervent Radiol 2016;39:1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]