Abstract
Food-associated Lactiplantibacillus plantarum (Lpb. plantarum) strains, previously classified as Lactobacillus plantarum, are a promising strategy to face intestinal inflammatory diseases. Our study was aimed at clarifying the protective role of food-borne Lpb. plantarum against inflammatory damage by testing the scavenging microbial ability both in selected strains and in co-incubation with normal mucosa intestinal cells (NCM460). Here, we show that Lpb. plantarum endure high levels of induced oxidative stress through partially neutralizing reactive oxygen species (ROS), whereas they elicit their production when co-cultured with NCM460. Moreover, pre-treatment with food-borne Lpb. plantarum significantly reduce pro-inflammatory cytokines IL-17F and IL-23 levels in inflamed NCM460 cells. Our results suggest that food-vehicled Lpb. plantarum strains might reduce inflammatory response in intestinal cells by directly modulating local ROS production and by triggering the IL-23/IL-17 axis with future perspectives on health benefits in the gut derived by the consumption of functional foods enriched with selected strains.
Subject terms: Cell biology, Microbiology
Introduction
Over the past decades, with the rapid economic development and improvements in quality of life, our lifestyle and dietary habits have significantly changed, leading to an increasing occurrence of chronic gut inflammation and/or anomalous immune response. The human gut hosts a complex ecosystem generated by the integrity and stable cooperation between immune cells, resident microbiota and the gastrointestinal (GI) epithelium1, which is in charge of both organ specific and immune functions and represents one of the major sites for generation of pro-oxidants, due to the presence of food components, microbes and direct interaction with the immune system2.
Oxidative stress, caused by an overproduction and accumulation of reactive oxygen species (ROS), can upregulate the expression of genes involved in adaptive and innate immune responses in the GI tract, leading to the alterations of intestinal morphology and contributing to enhance gut inflammation3. Currently, intervention with natural antioxidants, mainly from food sources4, nutrients5 and other bioactive components including probiotics6,7, has received much attention from scientists as dietary strategies to counteract oxidative stress, inflammation and some related chronic disorders2,8. Similarly to other natural antioxidants (i.e. plant extracts), the antioxidant role of lactic acid bacteria (LAB) has been associated with up- and down- regulation of antioxidant host functions as well as modulation of host signalling pathways9.
Among LAB, Lactobacillus plantarum (recently reclassified as Lactiplantibacillus (Lpb.) plantarum10) is a flexible and versatile species that can be found as a dominant microbiota not only in several foods, but also in the human GI tract as a natural inhabitant11. Beside human-derived probiotics, food-associated microbes, especially related to fermented foods, have recently recovered scientific interest for their potential health-promoting effects12. Previous in vitro studies have shown that Lactobacillus strains can modulate both oxidative stress and pro- and anti-inflammatory cytokines release13,14. In this context, we should recall that a redox protective effect has been recently ascribed to Lacticaseibacillus casei Shirota on the cellular damages induced by an oxidative stressor in an in vitro model of enterocytes14.
Moreover, it has been found that probiotic bacteria can reduce or even block inflammatory signalling via ROS modulation13,15, and some in vivo studies indicate that ingestion of probiotic LAB strains significantly modulates the oxidative stress and related inflammatory damage7,16,17, even though the mechanisms behind these beneficial effects are not entirely understood.
However, due to the transient condition in the GI tract, the strength of probiotics as well as of food-ingested microbes resides in sharing genes and specific metabolites, and directly interacting with epithelial and immune cells rather than in affecting persistently the microbiota composition18. Indeed, traditional probiotics, such as Lactobacillus or Bifidobacterium, and next-generation beneficial microbes, including Akkermansia muciniphila, have been reported to influence the gut barrier function19 and to control the secretion of different gut peptides involved in the regulation of energy metabolism20 via the production of short-chain fatty acids as well as to modulate gene transcription21.
Experimental and human studies showed that the Interleukin (IL)-23 and the downstream cytokines IL-17A and IL-17F could play a crucial role in the pathogenesis of chronic intestinal inflammation processes22. Experimental colitis mice models revealed IL-23 as a key cytokine that drives the intestinal inflammation23, whereas human studies carried out in Inflammatory Bowel Disease (IBD) patients have identified single nucleotide polymorphisms in many genes encoding for proteins involved in the IL-23/IL-17 pathway24 as well as increased levels of IL-23 and IL-17 cytokines23. Based on that, IL-23/IL-17 inflammation pathway has been proposed as a novel therapeutic target in IBD and other gastrointestinal disorders25 and interestingly, IL-17/IL-23 axis inhibition by Lactobacillus commensal bacteria showed an amelioration of DSS-induced colitis symptoms26,27, suggesting putative alternative treatment strategy.
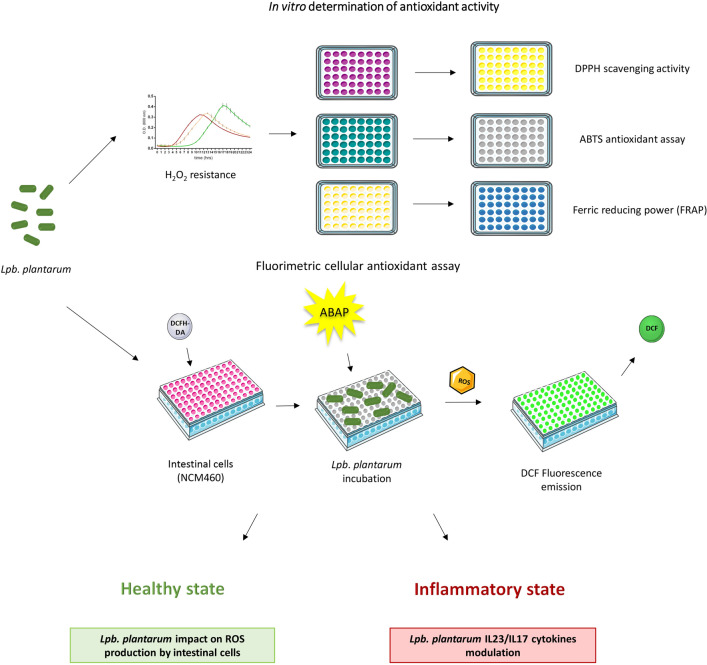
These findings prompted us to elucidate the protective role of previously selected food-associated Lpb. plantarum strains, already characterized for several properties28–31, to face oxidative stress and related inflammatory damage at intestinal level. For this purpose, food-associated and human Lpb. plantarum strains (Table 1) were examined for their in vitro capacity to tolerate oxidative stress as well as for their antioxidant potential by three different microplate chemical assays (DPPH (1,1-diphenyl-2-picrylhydrazyl), 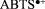 (2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and FRAP (ferric reducing antioxidant power)). In addition, the specific ability of each strain to modulate ROS levels in response to either oxidative or inflammatory stress and to reduce IL-17A, IL-17F and IL-23 release in an inflamed intestinal cell model was examined. Figure 1 shows the workflow for in vitro determination of Lpb. plantarum antioxidant activity and Lpb. plantarum differential impact on intestinal cells reported in the study.
(2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and FRAP (ferric reducing antioxidant power)). In addition, the specific ability of each strain to modulate ROS levels in response to either oxidative or inflammatory stress and to reduce IL-17A, IL-17F and IL-23 release in an inflamed intestinal cell model was examined. Figure 1 shows the workflow for in vitro determination of Lpb. plantarum antioxidant activity and Lpb. plantarum differential impact on intestinal cells reported in the study.
Table 1.
Lpb. plantarum strains used in the study.
| Strain | Origin | Source |
|---|---|---|
| WCFS1 | Human saliva | Reference strain, UNITE collection |
| IMC513 | Human gut | Probiotic strain, Synbiotec srl |
| O13, C9O4 | Table olives | UNITE collection |
| LT52, LT100 | Raw-milk cheeses | UNITE collection |
Figure 1.
Graphical scheme showing the workflow for in vitro determination of Lpb. plantarum antioxidant activity and Lpb. plantarum differential impact on intestinal cells, reported in the study. Graphical illustrations were created by using some graphical elements from Servier Medical Art by Servier, available on https://smart.servier.com/ under a Creative Commons Attribution 3.0 Unported License.
Results
Antioxidant activity of Lpb. plantarum strains
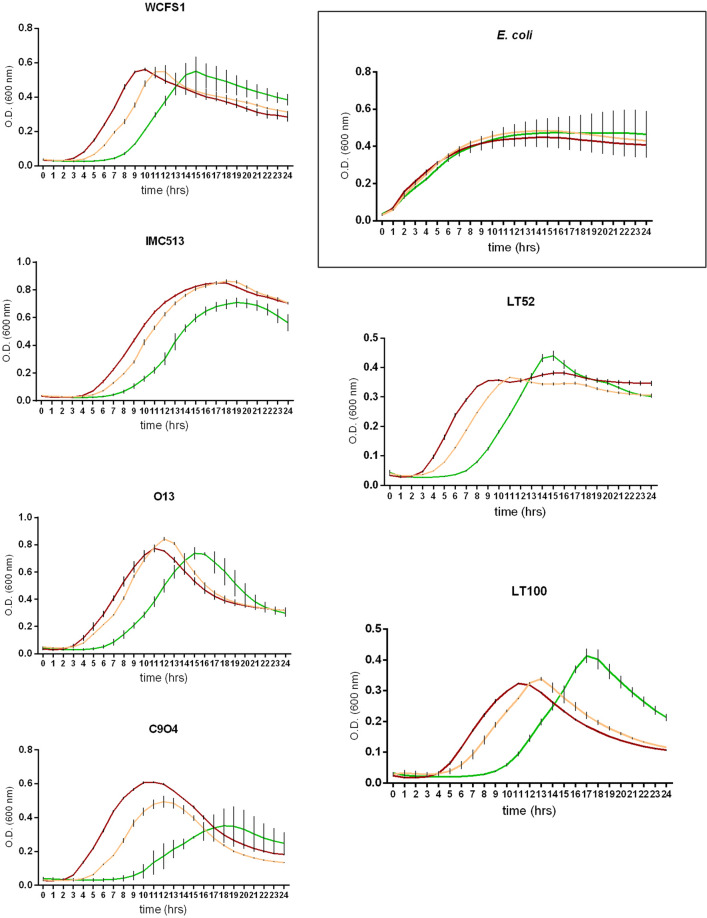
The ability of food-associated and human-derived Lpb. plantarum strains to tolerate oxidative stress was assessed in the presence of hydrogen peroxide by monitoring microbial growth for 24 h. As shown in Fig. 2, Lpb. plantarum growth was not significantly affected in presence of low concentration of hydrogen peroxide (with the exception of C9O4), whereas the higher concentration highlighted a strain-dependent behaviour. In all Lpb. plantarum strains, 10 mM hydrogen peroxide influenced the microbial growth by causing an extension of the lag phase, whereas, among all the strains tested, a higher cell density was showed by the food-associated strains LT52 and LT100 at the end of the exponential phase. Therefore, all Lpb. plantarum strains were able to endure levels of induced oxidative stress much higher than the levels usually tested in the well-known semi quantitative method of Buchmeier and co-workers32. In this context, it should be noted that Escherichia coli, used as catalase positive reference strain, showed no appreciable growth inhibition in presence of both hydrogen peroxide concentrations (Fig. 2).
Figure 2.
Lactiplantibacillus plantarum survival in presence of hydrogen peroxide (red line means MRS broth, yellow line means MRS broth with 5.0 mM hydrogen peroxide, green line means MRS with 10 mM hydrogen peroxide). Escherichia coli was used as catalase positive reference strain.
The potential antioxidant activity of food-borne Lpb. plantarum strains was tested by three different in vitro assays (ABTS, DPPH and FRAP), all of them optimized and adapted to a microplate format. These methods were chosen since, as they differ in several aspects such as the mechanism of action (ABTS, DPPH are based on radical reactions while FRAP on a redox one) and the environmental conditions (solvent polarity and pH), they can provide an insight into the in vitro antioxidant capacity of the strains under investigation. As shown in Table 2, Lpb. plantarum cells showed an overall higher ability to scavenge the 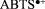 radical cation compared to the DPPH method, in which the inhibition percentage was around two-fold lower, for the majority of strains. In particular, Lpb. plantarum LT100 (isolated from raw-milk cheeses) displayed a high value of antioxidant activity (48.9 ± 1.74) in term of
radical cation compared to the DPPH method, in which the inhibition percentage was around two-fold lower, for the majority of strains. In particular, Lpb. plantarum LT100 (isolated from raw-milk cheeses) displayed a high value of antioxidant activity (48.9 ± 1.74) in term of 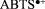 inhibition percentage, similar to that of IMC513 (from human source), as reported in Table 2. Regarding the reducing power of Lpb. plantarum, the table olives-associated O13 strain showed the highest FRAP value (209.6 ± 4.70 mmol Fe2+/ml), higher than the strains WCFS1 (163.3 ± 11.08 mmol Fe2+/ml) and IMC513 (134.9 ± 6.43 mmol Fe2+/ml). Therefore, the combinations of all these results suggested the potential ability of some Lpb. plantarum strains to partially neutralize free radicals by different mechanisms with variations in a strain-dependent manner.
inhibition percentage, similar to that of IMC513 (from human source), as reported in Table 2. Regarding the reducing power of Lpb. plantarum, the table olives-associated O13 strain showed the highest FRAP value (209.6 ± 4.70 mmol Fe2+/ml), higher than the strains WCFS1 (163.3 ± 11.08 mmol Fe2+/ml) and IMC513 (134.9 ± 6.43 mmol Fe2+/ml). Therefore, the combinations of all these results suggested the potential ability of some Lpb. plantarum strains to partially neutralize free radicals by different mechanisms with variations in a strain-dependent manner.
Table 2.
Determination of antioxidant activity of Lpb. plantarum by ABTS, DPPH and FRAP methods.
| Strains |
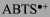 (%)
(%) |
DPPH (%) | FRAP (mmol Fe2+/ml) |
|---|---|---|---|
| WCFS1 | 59.4 ± 0.43a | 20.1 ± 1.21a | 163.3 ± 11.08d |
| IMC513 | 41.6 ± 1.93b | 20.1 ± 0.98a | 134.9 ± 6.43c |
| O13 | 30.2 ± 2.91c | 14.4 ± 2.58b | 209.6 ± 4.70a |
| C9O4 | 29.3 ± 1.36c | 24.6 ± 0.85a | 97.26 ± 1.80b |
| LT52 | 32.4 ± 1.49c | 17.3 ± 0.59a | 131.7 ± 3.34c |
| LT100 | 48.9 ± 1.74b | 19.5 ± 3.07a | 101.8 ± 2.09b |
a–cMean values with the same superscript are not different (p > 0.05) by ANOVA Bonferroni’s test.
ROS modulation by Lpb. plantarum
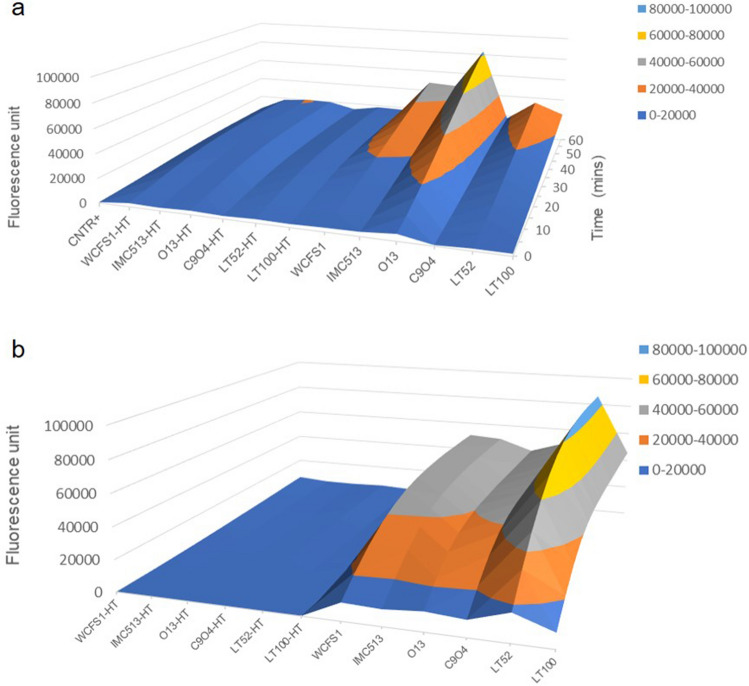
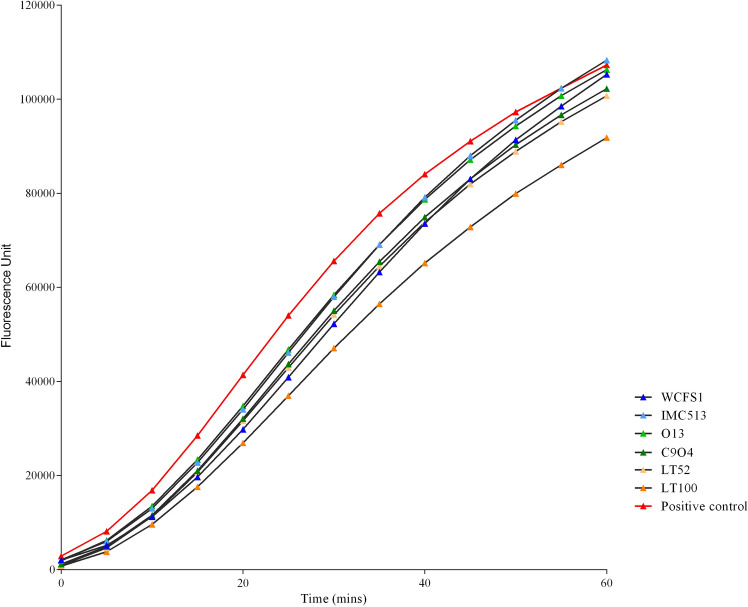
Based on the above described ability to either endure induced oxidative stress or partially neutralize ROS, we tested the potential protective impact of four food-associated Lpb. plantarum, besides the two human-derived strains WCFS1 and IMC513, on both normal and inflamed NCM460 cells. In order to confirm ROS production in response to the oxidative treatment with 2,2'-azobis (2-aminidopropane) dihydrochloride solution (ABAP) and to evaluate the impact of Lpb. plantarum strains on cellular ROS levels, the non fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA), that in presence of ROS inside the cells is oxidized into the highly fluorescent dichlorofluorescein (DCF), was used to determine ROS generation in NCM460 preliminarily co-incubated with live and heat-treated (HT) bacterial cells. Interestingly, intestinal cells pre-treated with live Lpb. plantarum cells showed increased ROS levels in response to induced oxidative stress over 1 h exposure to ABAP, while inactivated HT bacterial cells did not display any effect on ROS production, as clearly shown by the surface chart in Fig. 3a. In particular, Lpb. plantarum O13 isolated from table olives showed the highest ROS generation over time (up to 2.5-fold compared to the control). To confirm the different behaviour of live and heat-inactivated Lpb. plantarum cells on NCM460 cells, their response to the fluorescence DCF probe was also tested in the absence of intestinal cells (Fig. 3b). The surface chart in Fig. 3b displays a similar response compared to Fig. 3a, confirming that live cells are needed to exert a potential effect on human cells. Moreover, in order to evaluate whether Lpb. plantarum strains can reduce inflammatory signalling via ROS modulation, we investigated the Lpb. plantarum impact on ROS production in an in vitro inflammation model29. Compared to the positive control (inflamed intestinal cells) pre-treatment with all food-associated Lpb. plantarum strains resulted to be effective in the reduction of ROS levels, generated by intestinal cells after 24 h of exposure to the inflammatory stimulus. They also showed a similar ability of human strains WCFS1 and IMC513 to modulate human cells anti-inflammatory responses (Fig. 4).
Figure 3.
ROS modulation by Lactiplantibacillus plantarum strains. (a) Peroxyl radical-induced oxidation of DCFH to DCF in NCM460 cells by live and heat-treated (HT) Lpb. plantarum strains over time. (b) Lpb. plantarum live and heat-treated (HT) cells response to the fluorescence probe DCFH-DA (25 µM) over time.
Figure 4.
ROS production by inflamed NCM460 cells over time, after pre-treatment with live Lpb. plantarum strains. Data of one representative experiment are reported in the graph.
Protective impact on cytokine release in inflamed intestinal cells by Lpb. plantarum
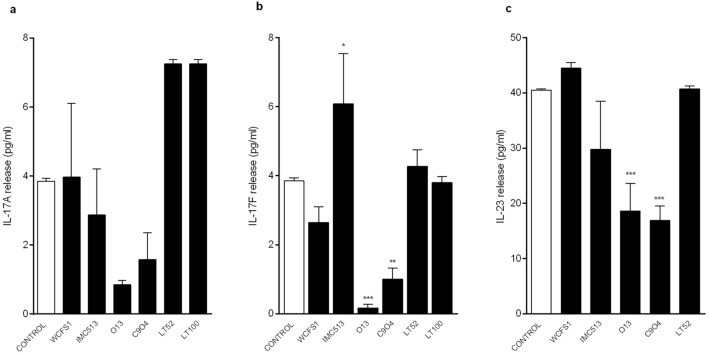
Figure 5 shows the overall ability of Lpb. plantarum to modulate pro-inflammatory cytokines IL-17A, IL-17F and IL-23 levels in our inflamed cell model. Among all the tested strains, Lpb. plantarum O13 and C9O4 significantly reduced IL-17F (0.17 pg/ml and 1.00 pg/ml, respectively) and IL-23 (18.6 pg/ml and 16.9 pg/ml, respectively) levels compared to the control (3.85 pg/ml for IL-17F and 40.5 pg/ml for IL-23), and a similar, but not significant trend, was also observed in IL-17A reduction (Fig. 5). Overall, these findings suggest that our Lpb. plantarum might affect in a strain-dependent manner the potential key role of IL-23/IL-17 inflammation axis in driving the intestinal inflammation.
Figure 5.
Lpb. plantarum modulation of (a) IL-17A, (b) IL-17F and (c) IL-23 release on inflamed NCM460 cells. ***p < 0.0002.
Discussion
Modulation of host immunity and stimulation of host defence systems through anti-inflammatory and antioxidant responses are the most claimed beneficial effects of both commensal and probiotic bacteria mutualistic interactions with the human host33. In the last years, Lpb. plantarum strains have been studied not only for their functional traits, but also for their demonstrated health-promoting properties11,34. However, there is a lack of investigation on evaluating the impact of food-ingested Lpb. plantarum strains, which are likely to be consumed at high concentrations in fermented foods such as table olives 35. Indeed, in fermented foods they are one of the most predominant species, depending on their innate capability to overcome spontaneous developing microbiota or as a consequence of a deliberately addition to confer new functionality to fermented foods34,36. In view of their promising potential properties, here we investigated four selected food-borne and two human derived Lpb. plantarum strains, to test both their in vitro antioxidant activities and their ability to reduce the inflammatory response via ROS modulation in a recently reported in vitro cell model that mimics inflammatory conditions29.
The molecular mechanisms of Lpb. plantarum antioxidative response is still not entirely understood, and it has been previously shown that some lactobacilli counteract induced oxidative stress in different manners37,38. For this reason, we applied a combined approach of in vitro techniques to determine antioxidant activity in terms of direct free radicals neutralization via hydrogen or electron-transfer, ferric reducing power and resistance to hydrogen peroxide (H2O2). Firstly, growth curves in the presence of 5 mM and 10 mM H2O2 were performed in order to assess the ability of Lpb. plantarum strains to endure induced oxidative stress. It emerged that all of the Lpb. plantarum strains tested remained viable in the presence of 10 mM H2O2 with a clear strain-dependent behaviour. This is in contrast to the study of Tang and co-workers who showed that a concentration of 2.5 mM of H2O2 completely inhibited the growth of the Lpb. plantarum strain MA239. However, as it has been previously reported39, the oxidative environment does markedly influence the growth of the strains, causing an extension of the lag phase without any killing effect, likely as a result of the initial stress conditions. Moreover, during the exponential phase two food-associated strains (LT52 and LT100) showed a strong recovery of the growth rate, with optical density (OD) values much higher than those of the control (without H2O2), revealing a potential inducible repair system. Even though Lpb. plantarum does not have a complex regulation system to defend against oxidation as eukaryotic cells, the presence of some enzymes (such as NADH-dependent enzymes and superoxide dismutase), the production of antioxidant metabolites (folate and glutathione) and exopolysaccharides are regarded as important defence mechanisms to face oxidative stress among lactobacilli9,40,41.
Overall results from the in vitro assays DPPH, ABTS and FRAP, showed that Lpb. plantarum strains displayed strong and strain-dependent antioxidant activity, mainly characterized by their relevant ferric reducing power (Table 2). This potent ability of chelating metal ions such as Fe2+ has been previously described for other lactobacilli strains42. Moreover, results of direct free radicals neutralization showed that food-associated Lpb. plantarum strains displayed strong antioxidant activity, with an overall higher ability to scavenge the 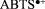 radical cation compared to the DPPH method, in which the inhibition percentage was around two-fold lower for the majority of strains (Table 2). Regardless of this, the observed DPPH free radical scavenging activity of Lpb. plantarum strains in this study are comparable to the levels obtained by Li and co-workers42. Indeed, they found the DPPH free radical scavenging activity of selected Lpb. plantarum strains, when measured at 109 CFU/ml, are comparable to levels obtained for other lactobacilli38 and to our results, with values ranging from 15–20% (Table 2). In general, for those strains with higher antioxidant activity, we can ascribe a correlation among the different in vitro test performed. This is the case of the food-associated Lpb. plantarum LT100 strain, that shows the highest value of antioxidant activity in term of
radical cation compared to the DPPH method, in which the inhibition percentage was around two-fold lower for the majority of strains (Table 2). Regardless of this, the observed DPPH free radical scavenging activity of Lpb. plantarum strains in this study are comparable to the levels obtained by Li and co-workers42. Indeed, they found the DPPH free radical scavenging activity of selected Lpb. plantarum strains, when measured at 109 CFU/ml, are comparable to levels obtained for other lactobacilli38 and to our results, with values ranging from 15–20% (Table 2). In general, for those strains with higher antioxidant activity, we can ascribe a correlation among the different in vitro test performed. This is the case of the food-associated Lpb. plantarum LT100 strain, that shows the highest value of antioxidant activity in term of 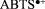 inhibition percentage, and is also one of the most resistant strains to hydrogen peroxide-induced oxidative stress, as well as the strains WCFS1 and IMC513.
inhibition percentage, and is also one of the most resistant strains to hydrogen peroxide-induced oxidative stress, as well as the strains WCFS1 and IMC513.
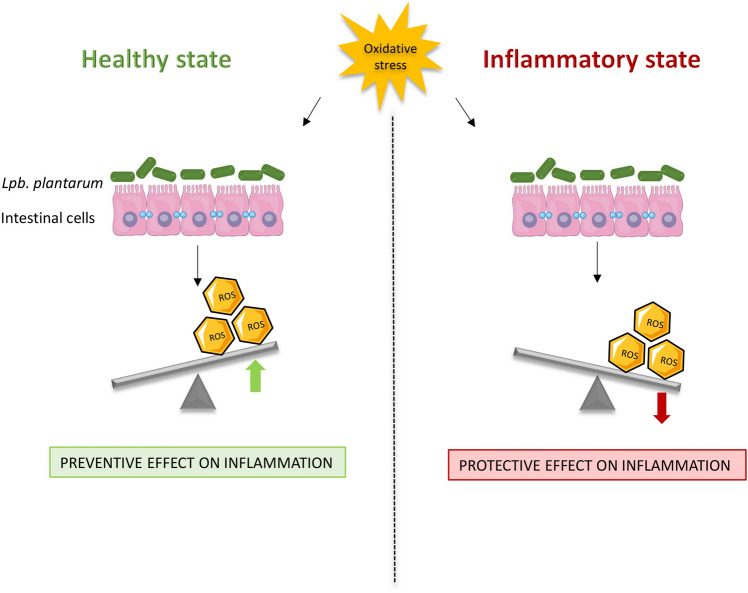
Although these chemical assays are widely applied methods for testing antioxidant activity, they may not reflect the actual biological activity of bacteria inside human cells43. Therefore, we tested the antioxidant activity of Lpb. plantarum strains through the cellular DCFH-DA assay, a more biologically representative method, largely applied to assess microbial ROS modulation in different cell lines14,43–45. Interestingly, the results showed that ROS modulation by Lpb. plantarum strains is markedly influenced by the health status of the intestinal cells. Whilst a potential preventive role of Lpb. plantarum was observed with a healthy intestinal cell model by increasing ROS production (Fig. 3), the data obtained with the inflamed intestinal cells indicate a potential protective role in ameliorating inflammation conditions by decreasing ROS release (Fig. 4). Our results are in agreement with several reports showing that the administration of probiotics promotes the development of some cellular antioxidant defence mechanisms in different pathological and inflamed enterocytes-like cell models37,43,44. This relationship between inflammatory status of the cells and oxidative stress has been previously documented by other investigators46–49. In view of the pivotal role of cytokines in modulating oxidative stress and the potential of probiotic bacteria to reduce or even block inflammatory signalling via ROS modulation, we investigated the ability of our strains to trigger the IL-17/IL-23 axis in the inflamed intestinal cell model. In accordance with other studies, in which it has been demonstrated that the down-regulation of the IL23/Th17 pathway could ameliorate chronic inflammatory symptoms14,15, we observed that two strains isolated from table olives, O13 and C9O4, in addition to reducing ROS production in inflamed cells, significantly decreased IL-17F and IL-23 levels compared to the control, whereas a similar, but not significant trend, was also observed in IL-17A reduction (Fig. 5).
The interaction of microorganisms with the host, together with their anti-oxidative and anti-inflammatory potential role, can occur through different mechanisms of action, depending on a wide range of factors, such as physiological and/or pathological conditions as well as individual strain activity. Although the precise determination of the complex microbe-host relationship is still a hard-scientific challenge, this in vitro study suggests a differential impact of Lpb. plantarum on ROS production by healthy and inflamed intestinal cells upon oxidative stress (Fig. 6), opening a promising scenario for further investigations.
Figure 6.
Schematic representation of the speculated effects of Lpb. plantarum on ROS production by healthy and inflamed intestinal cells upon oxidative stress. Graphical illustrations were created by using some graphical elements from Servier Medical Art by Servier, available on https://smart.servier.com/ under a Creative Commons Attribution 3.0 Unported License.
In conclusion, this study evidences that our Lpb. plantarum strains are able to endure levels of induced oxidative stress through the modulation of ROS and IL-23/IL-17 axis, suggesting a promising environmental fitness for their potential use as a personalized probiotic supplement tailored for the benefit of patients affected by GI disorders. Further in vivo experimental animal studies are needed to clarify and validate the beneficial contribution of our Lpb. plantarum strains to overcome the limits due to an in vitro approach. However, we must recall in this context the warning of the United States Environmental Protection Agency to stop the studies on mammals by 2035, reinforcing thus the use of innovative in vitro models to translate the health benefits observed during research into real-life outcomes50. Additionally, since these strains can be commonly ingested with foods, such as table olives with a recognized antioxidant capability due to high polyphenols content35 they could beneficially affect the consumer by providing another dietary source of natural antioxidants or by exerting a potential protective role in the GI tract to counteract gut inflammation and oxidative disorders.
Methods
Bacterial strains
Lpb. plantarum strains investigated in this study were selected among our laboratory collection at the University of Teramo (Table 1). All Lpb. plantarum strains were previously isolated from different sources as well as characterized for several properties including their potential ability to survive and interact in an in vitro cellular model28–31. Lpb. plantarum WCFS1 and a commercial probiotic strain, Lpb. plantarum IMC513 (kindly provided by Synbiotec s.r.l., Camerino, MC, Italy), were included in the study as human-derived reference strains (Table 1). All the strains were routinely grown under microaerophilic conditions using de Man, Rogosa and Sharp (MRS) medium (Oxoid Ltd, Basingstoke, United Kingdom) at 37 °C. Lpb. plantarum strains were grown in MRS broth at 37 °C for 8 h. Subsequently, bacterial cells in exponential growth phase were harvested by centrifugation (14,000 rpm, 10 min, 4 °C), washed twice with sterile phosphate buffer saline (PBS) and resuspended in sterile PBS at 109 CFU/ml, before each assay. In order to assess Lpb. plantarum impact on ROS production by intestinal cells, experiments were carried out using both live and heat treated (HT) cells at 100 °C for 30 minutes51.
Intestinal cell culture
Normal human colon mucosal epithelial (NCM460) cells (INCELL Corporation, LLC, Sant’Antonio, TX, USA) were grown in INCELL’s enriched M3Base medium supplemented with 1% (v/v) Penicillin/Streptomycin 100 × (Corning, NY, United States), 1% (v/v) Non-Essential Amino Acids 100 × solution (Corning, NY, United States), and 10% (v/v) heated inactivated Fetal Bovine Serum (FBS; Corning, NY, United States). Cells were grown in culture dishes at 37 °C in a 5% CO2 atmosphere, and seeded at 60–70% confluence (105 cells/well in 96-well plate) for 24 h prior to co-incubation with the bacterial strains.
In vitro determination of antioxidant activity of Lpb. plantarum strains (chemical assays)
Resistance to hydrogen peroxide
Microbial survival rate under oxidative stress was assessed for each strain by monitoring growth in presence of hydrogen peroxide (Sigma Aldrich, St Louis, USA). Briefly, Lpb. plantarum strains were incubated in MRS broth containing 5 mM and 10 mM of hydrogen peroxide (30% wt. solution, Sigma Aldrich, St Louis, USA) at 37 °C for 24 h. Bacterial cell growth was monitored at 600 nm using an EnSpire multimode plate reader (PerkinElmer, Waltham, MA, United States). The plate reader was run in discontinuous mode with absorbance readings performed in 60 min intervals before 30-s shaking at medium speed. Cultures were grown in three biologically independent replicates and the resulting growth data were expressed as the mean of these replicates. Escherichia coli, used as catalase positive reference strain, was prepared and tested as Lpb. plantarum strains by incubation in Nutrient broth (Oxoid Ltd, Basingstoke, United Kingdom).
Antioxidant capacity using ABTS method
A microplate format of the 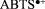 [2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] radical cation method52 was optimized and used to assess the antioxidant activity of Lpb. plantarum strains. ABTS stock solution (7 mM) was mixed with 2.45 mM potassium persulfate to produce ABTS radical cation (
[2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] radical cation method52 was optimized and used to assess the antioxidant activity of Lpb. plantarum strains. ABTS stock solution (7 mM) was mixed with 2.45 mM potassium persulfate to produce ABTS radical cation (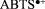 ) and the mixture was stored in the dark at room temperature for 12-16 h. Before use,
) and the mixture was stored in the dark at room temperature for 12-16 h. Before use, 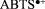 working solution was prepared by diluting
working solution was prepared by diluting 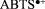 solution in PBS to adjust the absorbance at 734 nm to 0.9 ± 0.0.02. The assay was performed by adding 0.25 ml of either each strain suspension or PBS (used as control) in 1.0 ml of
solution in PBS to adjust the absorbance at 734 nm to 0.9 ± 0.0.02. The assay was performed by adding 0.25 ml of either each strain suspension or PBS (used as control) in 1.0 ml of 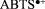 working solution. After 5 min at room temperature, each sample was harvested by centrifugation (14,000 rpm, 5 min, 4 °C) to remove bacterial cells. A volume of 0.2 ml/well was used for each sample, blank (0.2 ml PBS) and control, thus absorbance readings from three independent biological replicates were carried out by using an EnSpire multimode plate reader (PerkinElmer, Waltham, MA, United States). Antioxidant activity of each strain is expressed as:
working solution. After 5 min at room temperature, each sample was harvested by centrifugation (14,000 rpm, 5 min, 4 °C) to remove bacterial cells. A volume of 0.2 ml/well was used for each sample, blank (0.2 ml PBS) and control, thus absorbance readings from three independent biological replicates were carried out by using an EnSpire multimode plate reader (PerkinElmer, Waltham, MA, United States). Antioxidant activity of each strain is expressed as:
where Ac is the absorbance of the control, As is the absorbance of 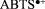 after co-incubation with each Lpb. plantarum strain.
after co-incubation with each Lpb. plantarum strain.
Scavenging ability on DPPH (1,1-diphenyl-2-picrylhydrazyl) free radical
The DPPH radical-scavenging capacity of Lpb. plantarum strains was determined by optimizing the method described by Wang and co-workers53 to a microplate format. Briefly, 0.5 ml of each Lpb. plantarum suspension was mixed with 1.0 ml of 0.2 mM DPPH ethanolic solution and was allowed to stand in the dark for 30 min at room temperature. Subsequently, each sample was harvested by centrifugation (14,000 rpm, 5 min, 4 °C) to remove bacterial cells. Equally, a same proportion (0.5 ml) of PBS was added to the DPPH solution and use as control. A volume of 0.2 ml/well was used for each sample, blank (0.2 ml absolute ethanol) and control, then absorbance readings at 517 nm from three independent biological replicates were recorded by using an EnSpire multimode plate reader (PerkinElmer, Waltham, MA, United States). Antioxidant activity of each strains is expressed as:
where Ac is the absorbance of the control, As is the absorbance of DPPH after co-incubation with each Lpb. plantarum strain.
Ferric reducing antioxidant power (FRAP)
The ferric reducing antioxidant power (FRAP) was assessed by a microplate format of FRAP assay54. FRAP working-solution was prepared daily by mixing 10-volumes of acetate buffer (300 mM, pH 3.6) with 1-volume of 2,4,6-Tripyridyl-s-Triazine (TPTZ, 10 mM dissolved with 40 mM HCl) and 1-volume of ferric chloride (20 mM in water). The assay was performed by adding 0.2 ml of Lpb. plantarum cultures (or PBS as blank) to 0.8 ml FRAP working-solution, pre-warmed at 37 °C. The mixtures were incubated in the dark at 37 °C for 30 min. After removing bacterial cells by centrifugation (14,000 rpm, 5 min, 4 °C), absorbance readings from three independent biological replicates were recorded at 593 nm by using an EnSpire multimode plate reader (PerkinElmer, Waltham, MA, United States). FeSO4·7H2O solutions, in the range 100-1000 µmol/liter, were used to graph a calibration plot, and the reducing activity of each strain was expressed as mmol/ml of Fe2+.
Assessment of potential protection of Lpb. plantarum strains on intestinal cell model
Lpb. plantarum impact on ROS production by intestinal cells
The Lpb. plantarum modulation of ROS levels in both normal and inflamed intestinal cell model was investigated by a fluorimetric microplate dichlorofluorescein diacetate (DCFH-DA) assay43. Briefly, normal NCM460 cells were incubated with 25 μM DCF-DA dissolved in Hanks’ Balance Salt Solution (HBSS) for 1 h at 37 °C, then washed twice with HBSS and incubated with live and heat-treated (100 °C, 30 min) Lpb. plantarum strains for 1.5 h at 37 °C. Subsequently, 0.1 ml of 600 µM of 2,2′-azobis (2-amidinopropane) dihydrochloride solution (ABAP) was added as free radical generator. 2′,7′ dichlorofluorescein (DCF) fluorescence was monitored every 5 min for 1 h by using an EnSpire Multimode Plate Reader (PerkinElmer, Waltham, MA, USA) at excitation and emission wavelengths of 485 and 535 nm, respectively. DCFH-DA assay was also carried out to assess the Lpb. plantarum live and HT cells response to the fluorescence probe DCFH-DA over time. For each single experiment, HBSS fluorescence values were used as blank, whereas cells treated with DCFH-DA and ABAP were used as positive control, and results are expressed as fluorescence unit over time.
Lpb. plantarum cytokines modulation
To evaluate Lpb. plantarum cytokines modulation on inflamed NCM460 cells, IL-17F and IL-23 cytokine production were detected through an extremely sensitive high-throughput method for multiplex protein analysis (LUNARIS Technology, AYOXXA Biosystem GmbH). NCM460 cells were incubated with Lpb. plantarum strains for 4 h, then they were treated for 24 h with human cytokines mix (IL-1β, TNF-α, and INF-γ) to induce the inflammation29. Finally, the supernatants were collected and analysed. Briefly, 50 µl of supernatants were placed on the planar surface of LUNARIS BioChip wells, harboring thousands of microbeads coated with different antibodies, for the simultaneous determination of multiple cytokines. A calibration curve for each cytokines was constructed and used to compute samples concentrations. Inflamed NCM460 cells without bacterial pre-treatment were included in the study as control.
Data analysis
Results were expressed as mean ± SEM of triplicate experiments. Data were analyzed by means of Prism 7.0 program (GraphPad Software Inc., La Jolla, CA, United States) using the one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc analysis. A level of p < 0.05 was considered statistically significant. Cytokines data were assessed by Student’s t-test, with a level of p < 0.05 considered statistically significant.
Acknowledgements
A.C., N.B. and R.P. were in part of the Italian Ministry of University and Research (PRIN Project 20152LFKAT) and the work has received financial support from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant Agreement 713714 ESR 07 (to N.G.G.). The authors gratefully acknowledge Synbiotec s.r.l. (Camerino, Italy) to kindly provide us Lpb. plantarum IMC513.
Author contributions
N.B and A.C. conceived the experiments, supported manuscript writing and supervised the overall study. R.P. performed the experiments and discussed the data with N.B., A.C. and C.D.M. R.P. and N.G.G. drafted the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McCracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001;3:1–11. doi: 10.1046/j.1462-5822.2001.00090.x. [DOI] [PubMed] [Google Scholar]
- 2.Moura FA, de Andrade KQ, Dos Santos JCF, Araújo ORP, Goulart MOF. Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol. 2015;6:617–639. doi: 10.1016/j.redox.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian T, Ziling W, Jinhua Z. Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid. Med. Cell Longev. 2017 doi: 10.1155/2017/4535194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biasi F, Astegiano M, Maina M, Leonarduzzi G, Poli G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr. Med. Chem. 2011;18:4851–4865. doi: 10.2174/092986711797535263. [DOI] [PubMed] [Google Scholar]
- 5.Oliveras-López M, Berná G, Jurado-Ruiz E, de la Serrana H, Martín FLG. Consumption of extra-virgin olive oil rich in phenolic compounds has beneficial antioxidant effects in healthy human adults. J. Funct. Foods. 2014;10:475–484. doi: 10.1016/j.jff.2014.07.013. [DOI] [Google Scholar]
- 6.Amaretti A, et al. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl. Microbiol. Biotechnol. 2013;97:809–817. doi: 10.1007/s00253-012-4241-7. [DOI] [PubMed] [Google Scholar]
- 7.Zaylaa M, et al. Probiotics in IBD: combining in vitro and in vivo models for selecting strains with both anti-inflammatory potential as well as a capacity to restore the gut epithelial barrier. J. Funct. Foods. 2018;47:304–315. doi: 10.1016/j.jff.2018.05.029. [DOI] [Google Scholar]
- 8.Mishra V, et al. Probiotics as potential antioxidants: a systematic review. J. Agric. Food Chem. 2015;63:3615–3626. doi: 10.1021/jf506326t. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, et al. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9:521. doi: 10.3390/nu9050521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng J, et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 11.Seddik HA, et al. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob. Proteins. 2017;9:111–122. doi: 10.1007/s12602-017-9264-z. [DOI] [PubMed] [Google Scholar]
- 12.Marco ML, et al. Health benefits of fermented foods: microbiota and beyond. Curr. Opin. Biotechnol. 2017;44:94–102. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Seenappanahalli Nanjundaiah Y, et al. Lactobacillus rhamnosus GG conditioned media modulates acute reactive oxygen species and nitric oxide in J774 murine macrophages. Biochem. Biophys. Rep. 2016;6:68–75. doi: 10.1016/j.bbrep.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Finamore A, et al. Redox role of Lactobacillus casei Shirota against the cellular damage induced by 2,2′-azobis (2-amidinopropane) dihydrochloride-induced oxidative and inflammatory stress in enterocytes-like epithelial cells. Front. Immunol. 2018;9:1131. doi: 10.3389/fimmu.2018.01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin PW, et al. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic. Biol. Med. 2009;47:1205–1211. doi: 10.1016/j.freeradbiomed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka A, et al. Oral administration of Lactobacillusplantarum 06CC2 prevents experimental colitis in mice via an anti-inflammatory response. Mol. Med. Rep. 2020;21:1181–1191. doi: 10.3892/mmr.2020.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao J, et al. Lactobacillus plantarum CCFM10 alleviating oxidative stress and restoring the gut microbiota in d-galactose induced aging mice. Food Funct. 2018;9:917–924. doi: 10.1039/c7fo01574g. [DOI] [PubMed] [Google Scholar]
- 18.Wieërs G, et al. How probiotics affect the microbiota. Front. Cell Infect. Microbiol. 2019;9:454. doi: 10.3389/fcimb.2019.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly CJ, et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial hif augments tissue barrier function. Cell. Host Microbe. 2015;17:662–671. doi: 10.1016/j.chom.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneeberger M, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015;5:16643. doi: 10.1038/srep16643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salazar N, et al. Functional effects of EPS-producing Bifidobacterium administration on energy metabolic alterations of diet-induced obese mice. Front. Microbiol. 2019;10:1809. doi: 10.3389/fmicb.2019.01809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaffen SL, Jain R, Garg AV, Cua DJ. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neurath MF. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2019;45:1–8. doi: 10.1016/j.cytogfr.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Omrane I, et al. Significant association between interleukin-17A polymorphism and colorectal cancer. Tumour Biol. 2014;35:6627–6632. doi: 10.1007/s13277-014-1890-4. [DOI] [PubMed] [Google Scholar]
- 25.Allocca M, et al. Can IL-23 be a good target for ulcerative colitis? Best Pract. Res. Clin. Gastroenterol. 2018;32–33:95–102. doi: 10.1016/j.bpg.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, et al. Lactobacillus acidophilus suppresses colitis-associated activation of the IL-23/Th17 axis. J. Immunol. Res. 2015;2015:909514. doi: 10.1155/2015/909514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang C, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat. Immunol. 2018;19:755–765. doi: 10.1038/s41590-018-0134-y. [DOI] [PubMed] [Google Scholar]
- 28.Prete R, et al. Food-associated Lactobacillus plantarum and yeasts inhibit the genotoxic effect of 4-nitroquinoline-1-oxide. Front. Microbiol. 2017;8:2349. doi: 10.3389/fmicb.2017.02349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Gonzalez N, Prete R, Battista N, Corsetti A. Adhesion properties of food-associated Lactobacillus plantarum strains on human intestinal epithelial cells and modulation of IL-8 release. Front. Microbiol. 2018;9:2392. doi: 10.3389/fmicb.2018.02392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prete R, et al. Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci. Rep. 2020;10:1165. doi: 10.1038/s41598-020-58069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prete R, Long SL, Joyce SA, Corsetti A. Genotypic and phenotypic characterization of food-associated Lactobacillus plantarum isolates for potential probiotic activities. FEMS Microbiol. Lett. 2020;367:076. doi: 10.1093/femsle/fnaa076. [DOI] [PubMed] [Google Scholar]
- 32.Buchmeier N, et al. SlyA, a transcriptional regulator of Salmonella typhimurium, is required for resistance to oxidative stress and is expressed in the intracellular environment of macrophages. Infect. Immun. 1997;65:3725–3730. doi: 10.1128/IAI.65.9.3725-3730.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butel MJ. Probiotics, gut microbiota and health. Med. Mal. Infect. 2014;44:1–8. doi: 10.1016/j.medmal.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Behera SS, Ray RC, Zdolec N. Lactobacillus plantarum with functional properties: an approach to increase safety and shelf-life of fermented foods. Biomed. Res. Int. 2018;2018:9361614. doi: 10.1155/2018/9361614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perpetuini G, Prete R, Garcia-Gonzalez N, Khairul Alam M, Corsetti A. Table olives more than a fermented food. Foods. 2020;9:178. doi: 10.3390/foods9020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rezac S, Kok CR, Heermann M, Hutkins R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018;9:1785. doi: 10.3389/fmicb.2018.01785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mu G, et al. Assessing and comparing antioxidant activities of lactobacilli strains by using different chemical and cellular antioxidant methods. J Dairy Sci. 2018;101:10792–10806. doi: 10.3168/jds.2018-14989. [DOI] [PubMed] [Google Scholar]
- 38.Chooruk A, Piwat S, Teanpaisan R. Antioxidant activity of various oral Lactobacillus strains. J. Appl. Microbiol. 2017;123:271–279. doi: 10.1111/jam.13482. [DOI] [PubMed] [Google Scholar]
- 39.Tang W, Xing Z, Li C, Wang J, Wang Y. Molecular mechanisms and in vitro antioxidant effects of Lactobacillus plantarum MA2. Food Chem. 2017;221:1642–1649. doi: 10.1016/j.foodchem.2016.10.124. [DOI] [PubMed] [Google Scholar]
- 40.Watanabe M, van der Veen S, Nakajima H, Abee T. Effect of respiration and manganese on oxidative stress resistance of Lactobacillus plantarum WCFS1. Microbiology. 2012;158:293–300. doi: 10.1099/mic.0.051250-0. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, et al. Optimization, partial characterization and antioxidant activity of an exopolysaccharide from Lactobacillus plantarum KX041. Int. J. Biol. Macromol. 2017;103:1173–1184. doi: 10.1016/j.ijbiomac.2017.05.118. [DOI] [PubMed] [Google Scholar]
- 42.Li S, et al. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem. 2012;135:1914–1919. doi: 10.1016/j.foodchem.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 43.Xing J, et al. Determining antioxidant activities of lactobacilli by cellular antioxidant assay in mammal cells. J. Funct. Foods. 2015;19:554–562. doi: 10.1016/j.jff.2015.09.017. [DOI] [Google Scholar]
- 44.Xing J, et al. Cellular model to assess the antioxidant activity of lactobacilli. RSC Adv. 2015;5:37626–37634. doi: 10.1039/c5ra02215k. [DOI] [Google Scholar]
- 45.Tang W, et al. Probiotic properties and cellular antioxidant activity of Lactobacillus plantarum MA2 isolated from Tibetan kefir grains. Probiotics Antimicrob. Proteins. 2018;10:523–533. doi: 10.1007/s12602-017-9349-8. [DOI] [PubMed] [Google Scholar]
- 46.Hussain T, et al. Oxidative stress and inflammation: what polyphenols can do for us? Oxid. Med. Cell Longev. 2016;2016:7432797. doi: 10.1155/2016/7432797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dandekar A, Mendez R, Zhang K. Cross talk between ER stress, oxidative stress, and inflammation in health and disease. Methods Mol. Biol. 2015;1292:205–214. doi: 10.1007/978-1-4939-2522-3_15. [DOI] [PubMed] [Google Scholar]
- 48.Lauridsen C. From oxidative stress to inflammation: redox balance and immune system. Poult. Sci. 2019;98:4240–4246. doi: 10.3382/ps/pey407. [DOI] [PubMed] [Google Scholar]
- 49.Sottero B, Rossin D, Poli G, Biasi F. Lipid oxidation products in the pathogenesis of inflammation-related gut diseases. Curr. Med. Chem. 2018;25:1311–1326. doi: 10.2174/0929867324666170619104105. [DOI] [PubMed] [Google Scholar]
- 50.Spacova I, et al. Future of probiotics and prebiotics and the implications for early career researchers. Front. Microbiol. 2020;11:1400. doi: 10.3389/fmicb.2020.01400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lim LH, et al. The effects of heat-killed wild-type Lactobacillus casei Shirota on allergic immune responses in an allergy mouse model. Int. Arch. Allergy Immunol. 2009;148:297–304. doi: 10.1159/000170383. [DOI] [PubMed] [Google Scholar]
- 52.Re R, et al. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 53.Wang AN, Yi XW, Yu HF, Dong B, Qiao SY. Free radical scavenging activity of Lactobacillus fermentum in vitro and its antioxidative effect on growing-finishing pigs. J. Appl. Microbiol. 2009;107:1140–1148. doi: 10.1111/j.1365-2672.2009.04294.x. [DOI] [PubMed] [Google Scholar]
- 54.Benzie IF, Choi SW. Antioxidants in food: content, measurement, significance, action, cautions, caveats, and research needs. Adv. Food. Nutr. Res. 2014;71:1–53. doi: 10.1016/B978-0-12-800270-4.00001-8. [DOI] [PubMed] [Google Scholar]