Abstract
Background
Standard guidelines recommend selective serotonin reuptake inhibitors (SSRIs) as first-line antidepressants for adults with Major Depressive Disorder (MDD), but success is limited and patients who fail to benefit are often switched to non-SSRI agents. This study investigated whether brain and behavior-based markers of reward processing might be associated with response to bupropion after sertraline nonresponse.
Methods
In a two-stage, double-blinded clinical trial (Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care), 296 participants were randomized to receive 8 weeks of sertraline or placebo in Stage 1. Individuals who responded continued on another 8-week course of the same intervention in Stage 2, while sertraline and placebo non-responders crossed-over to bupropion and sertraline, respectively. Data from 241 participants were analyzed. The Stage 2 sample comprised 87 MDD patients who switched medication and 38 healthy controls. 116 MDD participants treated with sertraline in Stage 1 served as an independent replication sample. The probabilistic reward task and resting-state functional magnetic resonance imaging were administered at baseline.
Results
Greater pretreatment reward sensitivity, as well as higher resting-state functional connectivity between bilateral nucleus accumbens and rostral anterior cingulate cortex, were associated with positive response to bupropion, but not sertraline. Null findings for sertraline were replicated in the Stage 1 sample.
Conclusion
Pretreatment reward sensitivity and frontostriatal connectivity may identify patients likely to benefit from bupropion following SSRI failures. Results call for a prospective replication based on these biomarkers to advance clinical care.
Trial registration
clinicaltrials.gov Identifier: NCT01407094
Keywords: Biomarkers, antidepressant response, sertraline, bupropion, reward sensitivity, frontostriatal connectivity
Introduction
Major depressive disorder (MDD) is a debilitating and recurrent condition associated with substantial personal socioeconomic costs (1,2). Despite significant efforts, treatment of MDD remains imprecise and involves trial-and-error to determine the most effective approach. Findings from the STAR*D trial revealed that only about half of individuals with MDD responded (i.e., exhibited ≥50% reduction in depressive symptoms) to the selective serotonin reuptake inhibitor (SSRI) citalopram (3), and over one-third failed to respond to two or more antidepressants (4,5). The situation is even worse in primary care, where only ~30% respond to first-line antidepressants (6). To exacerbate these issues, it takes at least four weeks to evaluate the efficacy of an antidepressant. This can lead to lengthy treatment trials that are insufficient and unnecessary, thereby increasing patient morbidity, drop-outs and suicide risk.
This limited success partially stems from the fact that treatment selection is not based on identification of the underlying biomarker abnormality that reflects pathophysiology (7,8). Hence, some depressed individuals may benefit from SSRIs while others might be better suited to other classes of medication. Identifying objective markers that reliably predict responses to different classes of antidepressants would critically help clinicians decide whether a particular medication might be suitable for the patient.
Functional magnetic resonance imaging (fMRI) studies have reported that pretreatment activation to emotional stimuli in the anterior cingulate cortex (9) and amygdala (10), as well as to non-emotional stimuli in frontocingulate (11–13) and parietal regions (14), were associated with greater improvements in depressive symptoms on SSRIs (15). Moreover, a recent study found that connectivity within the cognitive control network during a response inhibition task differentially predicts response to sertraline and venlafaxine (16). Converging evidence from resting-state studies also suggests that increased pretreatment activity in the rostral anterior cingulate cortex (rACC) predicts treatment response across a variety of interventions, including multiple antidepressants (17,18). Additionally, executive dysfunction, psychomotor slowing and impaired memory at baseline have been linked to poor clinical outcome on various medications (19–31), although lack of replications exists (32–34). Finally, higher pretreatment levels of C-reactive protein (35), interleukin-17 (36) and platelet derived growth factor (37) were associated with better improvement in depressive severity when treated with a combination of bupropion plus escitalopram.
Despite these promising findings, two important gaps exist in prior literature. First, to the best of our knowledge, no study has examined brain-behavior factors associated with response to second-line antidepressants, especially after failing a full course of SSRI. Current guidelines recommend SSRIs as first-line antidepressant treatments (38), but response rates are modest and depressed patients who fail to benefit are often switched to non-SSRI agents (38–41). Previous studies have never explored whether pretreatment biological and behavioral markers can differentiate between responders to a second antidepressant, after failure on a pharmacologically distinct class of medication, and non-responders resistant to both arms of treatment.
Second, alterations in the reward processing circuitry – modulated by dopamine and centered on the ventral striatum (VS) and medial prefrontal cortex (mPFC) – have been implicated in MDD (15,17,42–53). Emerging research also suggests that an impaired ability to respond to rewards is associated with anhedonia, a core feature of MDD (45,54,55). However, few studies have examined the degree to which markers of reward processing predict antidepressant response. A small open-label study in adolescents showed that pretreatment VS activity during reward anticipation was not linked to the severity of depressive symptoms after cognitive behavioral therapy (CBT) or CBT plus SSRI (56). The placebo-controlled Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care (EMBARC) trial in unmedicated MDD individuals also reported that pretreatment reward responsiveness did not associate with treatment outcome to the SSRI sertraline (57); however, better response to sertraline was linked to more abnormal VS temporal dynamics during a reward task (58). Given the key role of dopamine in reward processing (59,60), these previous findings beg the question of whether, and if so, which reward markers might be associated with response to dopaminergic (but not serotonergic-based) antidepressants.
The present study sought to address the two aforementioned gaps in the context of the two-stage, double-blinded EMBARC study (61). A probabilistic reward task (PRT) was a priori selected to investigate response to bupropion, a noradrenaline/dopamine reuptake inhibitor. PRT reward responsiveness and resting-state fMRI data were collected at baseline of an 8-week clinical trial, where outpatients with recurrent and non-psychotic MDD were randomized to receive sertraline or placebo (Stage 1). Participants who achieved satisfactory response at the end of Stage 1 continued on another 8-week course of the same intervention, while non-responders were crossed over under double-blinded conditions. Thus, sertraline non-responders received bupropion, and placebo non-responders received sertraline in Stage 2. For comparison, baseline PRT and resting-state fMRI data were also collected from healthy controls.
Our goal was to examine whether neural and behavioral markers of reward processing were associated with response to secondary treatment by bupropion (after non-response to sertraline) and sertraline (after non-response to placebo). Based on the premise that dopaminergic blunting plays an important role in anhedonic phenotypes (62,63), we hypothesized that patients with more impaired reward responsiveness and resting-state functional connectivity within the reward circuit would disproportionally benefit from a dopaminergic antidepressant (bupropion), after failure to respond to an SSRI (sertraline), and distinguish them from non-responders who were resistant to both classes of medication. Also, we did not expect these reward markers to differentiate response to sertraline.
Methods and Materials
Participants
The EMBARC trial recruited MDD outpatients and healthy volunteers from Columbia University (New York), Massachusetts General Hospital (Boston), University of Texas Southwestern Medical Center (Dallas) and University of Michigan (Ann Arbor) between July 29, 2011, and December 15, 2015, after approval by the institutional review board of each site. All enrolled participants provided written informed consent and were aged between 18–65 years old. Details of the study design and list of inclusion/exclusion criteria can be found in (61)
Probabilistic Reward Task
The probabilistic reward task (PRT) assessed the ability to modulate behavior based on rewards received (55). On every trial, participants viewed one of two briefly presented (100ms) and perceptually similar stimuli (11.5mm vs. 13.0mm lines). Participants had to indicate which stimulus was shown via a button-press. Importantly, and unbeknownst to participants, a 3:1 reinforcement ratio was adopted such that correct responses to one stimulus were rewarded three times more frequently than the other – a manipulation that induces a response bias (i.e., preference for the more frequently rewarded stimulus). Performance was analyzed in terms of response bias (objective measure of reward responsiveness) and discriminability (ability to distinguish between the stimuli). See Supplemental Methods for details.
Computational modelling
To dissociate the influence of reward sensitivity (i.e., immediate behavioral impact of rewards) and learning rate (i.e., ability to accumulate and learn from rewards over time) on PRT performance, four different models were fitted to participants’ trial-by-trial data (64). Following previously established procedures, we used expectation-maximization to derive group priors and individual Laplace approximation of posterior distributions for parameter estimations for each participant. Models were compared using integrated group-level Bayesian Information Criterion factors. See Supplemental Methods for details.
Region of Interest
Analyses focused on voxelwise resting-state functional connectivity (RSFC) of a seed region of bilateral nucleus accumbens (NACC), defined using the AAL atlas (65). The NACC was selected because significant evidence has implicated this region as a key area in different aspects of reward processing (60), including reinforcement learning and reward anticipation (66–71), as well as acquisition and development of reward- based behavior (72–74). Moreover, the ventral striatum (which includes the NACC) contains widespread afferent connections to cortical regions that mediate reward processes, such as the ventromedial prefrontal, orbitofrontal and anterior cingulate cortices (60,75). Pharmacological challenge studies provide further support, showing that the administration of drugs that enhance ventrostriatal signaling improves reward learning while disrupting phasic dopamine release causes an impairment (50,52). Collectively, these findings motivated us to focus on the NACC ROI in the RSFC analyses.
Magnetic Resonance Imaging Acquisition and Analyses
Acquisition, preprocessing, head motion and artifact detection, and denoising
See Supplemental Methods.
First-level analysis
Fisher’s z-transformed Pearson’s correlation coefficient was computed between timecourse of the NACC seed and that of all other voxels. For each participant, this yielded a beta map containing, at each voxel, an estimate of the correlation in activity between the NACC seed and that voxel over the scan duration.
Group-level analyses
Group level analyses were performed by entering first-level maps into a whole-brain analysis to test for an interaction between medication type (sertraline vs. bupropion) and response status (responders vs. non-responders) in voxelwise NACC. The contrast was sertraline responder (−1), sertraline non-responder (+1), bupropion responder (+1), bupropion non-responder (−1). Scanner site and motion variables were included as covariates, but the inclusion of these covariates did not affect the significance of RSFC effects. Group-level effects were considered significant if they exceeded a peak amplitude of p<0.001 (two-sided), cluster corrected to false discovery rate (FDR) of p<0.05.
Post-hoc RSFC analyses
To interrogate the nature of group differences underlying significant interaction effects, RSFC estimates were extracted from clusters identified by voxelwise analysis using REX (https://www.nitrc.org/proiects/rex/) (76). Then, RSFC in clusters of effect was compared between sertraline responders vs. non-responders, and between bupropion responders vs. non-responders, using independent t-tests and effect sizes comparison. Additionally, post-hoc voxelwise analyses were performed comparing bilateral NACC RSFC of responders vs. non-responders within each medication group.
Clinical Measure
17-item Hamilton Rating Scale for Depression (HAMD) (77)
The HAMD was administered at baseline, Stage 1 (weeks 1, 2, 3, 4, 6 and 8) and Stage 2 (weeks 9, 10, 12 and 16). Here, patients were defined as responders for each stage if they completed at least 4 weeks of treatment and showed a decrease in HAMD score of ≥50% at the last observation compared to when treatment started.
Statistical Analysis
We included participants who passed the PRT quality control criteria, were non-responders to sertraline or placebo in Stage 1 and completed ≥4 weeks of Stage 2 treatment on bupropion (after switching from sertraline) or sertraline (after switching from placebo). Independent samples t-tests assessed whether responders and non-responders to bupropion or sertraline differed in baseline HAMD, week 8 HAMD and change in HAMD from baseline to week 8. Next, separate two-way Treatment (sertraline vs. bupropion) × Response (responder vs. non-responder) ANOVAs were run to evaluate pretreatment differences in response bias, discriminability, reward sensitivity and learning rate. Significant Treatment × Response interactions were followed up with simple effects analyses comparing responders and non-responders to each treatment. P<0.05 was taken to be statistically significant unless otherwise stated. Bayesian statistical analyses were also conducted using JASP (78) to complement classical statistics. The Bayes Factor (BF10) quantifies the amount of evidence in favor of the alternative hypothesis (H1) and generally (79): 1<BF10<3 indicates anecdotal evidence, 3<BF10<10 indicates substantial evidence, 10< BF10<30 indicates strong evidence, 30<BF10<100 indicates very strong evidence and BF10>100 indicates extreme evidence for H1.
Results
Participant characteristics
Data from 241 participants were analyzed. 87 patients had valid PRT data (of which 84 had valid MR data) and completed ≥4 weeks of Stage 2 medication (Supplemental Fig. 1). Thirty-eight were non-responders to sertraline in Stage 1 and took bupropion in Stage 2, while 49 were placebo non-responders who switched to sertraline. Thirty-eight healthy controls were also analyzed. The clinical and demographic characteristics are reported in Table 1. In addition, we included a replication sample of 116 MDD patients who had valid PRT data (of which 112 had valid MR data) and completed ≥4 weeks of sertraline treatment in Stage 1 (Supplemental Table S2). These participants served as an independent group to verify our Stage 2 sertraline findings.
Table 1.
Clinical and demographic characteristics of Stage 2 sample
| Variable | Healthy controls | MDD patients | BUP | SER | ||||
|---|---|---|---|---|---|---|---|---|
| Resp | Non-resp | p | Resp | Non-resp | p | |||
| N | 38 | 87 | 16 | 22 | 25 | 24 | ||
| Age, mean (SD), years | 37.4 (14.9) | 39.9 (13.8) | 37.0 (14.6) | 39.4 (15.1) | 0.63a | 42.1 (11.9) | 40.0 (14.5) | 0.57a |
| Women, No. (%) | 23 (60.5) | 56 (64.4) | 10 (62.5) | 17 (77.3) | 0.32b | 16 (64.0) | 13 (54.2) | 0.48b |
| Education, mean (SD), years | 15.6 (4.5) | 15.2 (2.6) | 15.6 (2.0) | 14.6 (3.0) | 0.28a | 15.4 (2.6) | 15.4 (2.5) | 0.93a |
| Age at MDD onset, mean (SD), years | - | 16.1 (5.5) | 14.1 (3.6) | 16.3 (6.8) | 0.26a | 16.4 (5.5) | 17.0 (5.2) | 0.70a |
| Length of current MDE, median, months | - | 24 | 27 | 36 | - | 18 | 27 | - |
| No. of prior MDEs, median | - | 5 | 5 | 6.5 | - | 6 | 3.5 | - |
| Baseline HAMD score, mean (SD) | 0.7 (0.8) | 18.7 (4.1) | 18.5 (4.0) | 19.2 (4.6) | 0.62a | 18.3 (4.6) | 18.7 (3.2) | 0.74a |
| †Week 4–8 HAMD score, mean (SD) | - | 16.7 (4.9) | 17.1 (5.1) | 16.7 (5.0) | 0.79a | 16.9 (5.3) | 16.2 (4.2) | 0.61a |
| †Week 12–16 HAMD score, mean (SD) | - | 10.1 (6.2) | 5.9 (3.3) | 13.9 (4.5) | <.001a | 5.8 (3.6) | 13.9 (6.6) | <.001a |
| Baseline QIDS score, mean (SD) | 1.4 (1.3) | 18.3 (2.9) | 19.6 (3.2) | 18.3 (3.1) | 0.22a | 17.7 (2.5) | 18.1 (3.0) | 0.61a |
Note: p-values are comparisons between responders and non-responders via at-tests or bchi-square tests.
If patients completed at least 4 weeks of treatment but not the full 8-week course, we considered their last HAMD observation as the outcome of the treatment.
Pretreatment response bias differentiated responders to bupropion after failing sertraline from non-responders resistant to both classes of medication
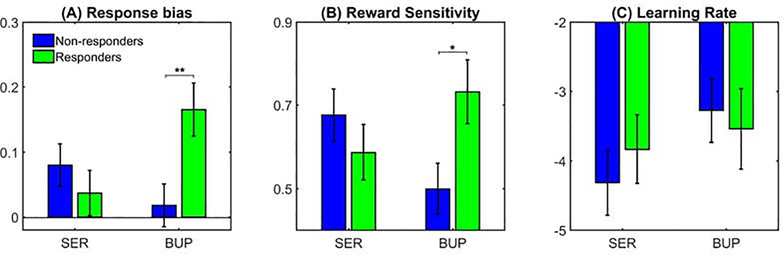
To investigate whether PRT response bias could differentiate between response to bupropion (after switching from sertraline) and sertraline (after previous non-response to placebo) in Stage 2, we conducted a Treatment (sertraline vs. bupropion) × Response (responders vs. non-responders) ANOVA. Notably, the only significant effect to emerge was the Treatment × Response interaction (F(1,83)=7.21, p<0.01, ηp2=0.080, BF10=5.27) (Fig. 1A). Follow-up simple effects tests revealed that eventual Stage 2 bupropion responders had larger (rather than lower, as originally hypothesized) pretreatment response bias than non-responders (p<0.01, d=0.90, BF10=15.57). Conversely, there was no difference between sertraline responders and non-responders (p>0.05, d=0.26, BF10=0.38). We conducted a separate analysis including site as a covariate and obtained similar results. Control analyses using discriminability also showed no significant interaction or main effects, suggesting that findings were specific to response bias (Supplemental Results). Moreover, bupropion responders exhibited comparable response bias scores as healthy controls, (t(52)=1.17, p>0.05, d=0.35, BF10=0.51) but non-responders had significantly lower response bias than healthy counterparts (t(58)=−2.77, p<0.01, d=0.74, BF10=5.90). This suggests that individuals who eventually responded favorably to bupropion had normal reward responsiveness, whereas non-responders did not.
Figure 1. Comparison of (A) response bias, (B) reward sensitivity and (C) learning rate for the probabilistic reward task at baseline.
Bupropion responders in Phase 2 have significantly greater baseline (pretreatment) response bias and reward sensitivity, but not learning rate, compared to non-responders (*p<0.05, **p<0.01). On the other hand, there was no difference on these metrics between responders and non-responders to sertraline. Note that the reward sensitivity and learning rate parameters have been transformed to prevent issues with non-Gaussianity.
Importantly, for each treatment, responders and non-responders to bupropion or sertraline did not differ in HAMD at baseline (BUP: t(36)=0.51, p>0.05, d=0.17, BF10=0.35; SER: t(47)=0.34, p>0.05, d=0.10, BF10=0.30) and Week 8 (BUP: t(36)=−0.27, p>0.05, d=0.09, BF10=0.33; SER: t(47)=−0.52, p>0.05, d=0.15, BF10=0.32), and change in HAMD from baseline to Week 8 (BUP: t(36)=−0.41, p>0.05, d=0.13, BF10=0.34; SER: t(47)=−0.63, p>0.05, d=0.18, BF10=0.34, Table 1). Thus, PRT findings were not influenced by differences in symptom severity at baseline or during Stage 1, and baseline response bias distinguished Stage 2 responders and non-responders 1216 weeks later.
Computational modelling revealed that bupropion responders had greater reward sensitivity, but not learning rate, than non-responders
An ANOVA revealed a significant Treatment × Response interaction for reward sensitivity (F(1,83)=7.12, p<0.05, ηp2=0.079, BF10=5.15, Fig. 1B). Follow-up tests showed that eventual bupropion responders were more sensitive to rewards at the pretreatment session than non-responders (p<0.05, d=0.87, BF10=7.48), whereas Stage 2 sertraline responders and non-responders did not differ (p>0.05, d=0.29, BF10=0.36). We also found that reward sensitivity for bupropion responders was similar to healthy volunteers (t(52)=0.82, p>0.05, d=0.26, BF10=0.39), but that for non-responders was significantly lower than controls (t(58)=−2.14, p<0.05, d=0.59, BF10=1.75). This suggests that patients who responded better to bupropion showed normative reward sensitivity. When considering learning rate, the Treatment × Response effect was not significant (F(1,83)=0.55, p>0.05, ηp2=0.007, BF10=0.38, Fig. 1C). Results remained significant when including site as a covariate (Supplemental Results). Thus, the difference in response bias between bupropion responders and non-responders was likely driven by variations in reward sensitivity, rather than learning rate.
Higher resting-state functional connectivity between nucleus accumbens and rostral anterior cingulate cortex was associated with better response to bupropion
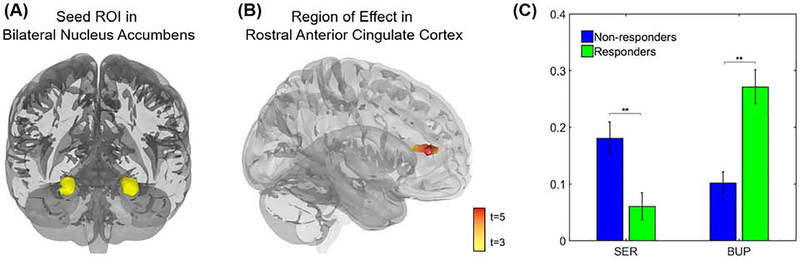
Whole-brain analyses showed a significant interaction between medication type and medication response in RSFC between bilateral NACC and a region of rostral anterior cingulate cortex (rACC; cluster peak at MNI coordinates −6, 30, 12, maximum t=5.76, k=170 voxels, clustering threshold p<0.001 FDR p<0.05, Fig. 2). Post-hoc analyses indicated that among those assigned to bupropion, patients with higher NACC-rACC RSFC showed better treatment response than those with lower NACC-rACC RSFC (t(34)=4.48, p<0.01, d=1.21, BF10>100). There was also a significant positive correlation between reward sensitivity and NACC-rACC RSFC (r=0.22, p<0.05), indicating that individuals with greater frontostriatal connectivity were more sensitive to rewards.
Figure 2. Baseline resting-state functional connectivity (RSFC) of bilateral nucleus accumbens (NACC) is associated with differential response to bupropion (BUP) compared with sertraline (SER).
(A) Shown is the seed region of interest (ROI) in bilateral nucleus accumbens, anatomically defined using the AAL atlas. (B) The interaction between antidepressant type and response to treatment was associated with RSFC (Fisher’s z-transformed Pearson’s correlations across the full duration of the resting scan) between bilateral nucleus accumbens and a region of rostral anterior cingulate cortex (rACC). (C) Patients randomized to bupropion for Stage 2 who responded to treatment showed higher NACC-rACC RSFC before the onset of Stage 1 than patients who failed to respond to bupropion, and this pattern also emerged in separate voxelwise analysis within the bupropion group (Fig. 3). Patients randomized to sertraline who responded to treatment showed lower NACC-rACC RSFC than sertraline non-responders, but this effect failed to emerge in separate voxelwise analyses within the sertraline group (Fig. 3). Note: Voxelwise analyses thresholded at peak p<0.001, two-sided, FDR corrected p<0.05.
Compared to healthy controls, bupropion responders had significantly larger NACC-rACC RSFC (t(51)=3.64, p<0.001, d=1.05, BF10=44.25) while that for non-responders were lower than controls at a trend level (t(55)=−1.84, p=0.07, d=0.51, BF10=1.10). This suggests that patients who responded better to bupropion exhibited elevated NACC-rACC RSFC. Conversely, among individuals randomized to sertraline, patients with higher NACC-rACC RSFC showed poorer treatment response than those with lower NACC-rACC RSFC (t(46)=4.48, p<0.01, d=0.93, BF10=37.47). Sertraline responders also had lower NACC-rACC RSFC than healthy controls (t(60)=−3.70, p<0.001, d=0.97, BF10=58.92), but there was no difference between non-responders and controls (t(58)=0.83, p>0.05, d=0.21, BF10=0.36).
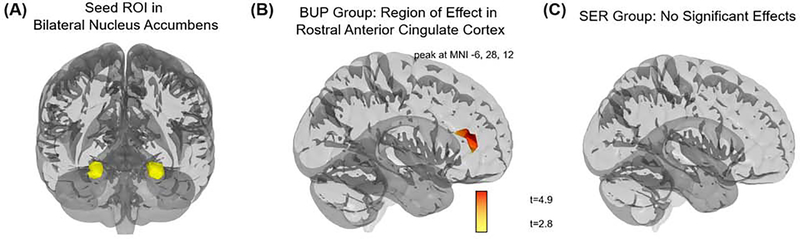
Of note, separate voxelwise analyses performed within each medication group converged with the full-group results, and suggested that NACC-rACC RSFC was especially related to treatment response in the bupropion group. Within the bupropion group, those who responded to treatment showed higher NACC-rACC RSFC, and no other significant effects were observed across the brain; however, within the sertraline group, there were no significant differences in NACC RSFC across the brain (Fig. 3).
Figure 3. Voxelwise resting-state functional connectivity (RSFC) of bilateral nucleus accumbens (NACC) of responders versus non-responders, within treatment groups.
(A) Shown is the seed region of interest (ROI) in bilateral nucleus accumbens, anatomically defined using the AAL atlas. (B) Patients randomized to bupropion who responded to treatment showed higher NACC-rACC RSFC than patients who failed to respond to bupropion. (C) Among patients randomized to sertraline (SER), there was no difference in NACC RSFC between those who responded, or failed to respond, to treatment. Note: Voxelwise static analyses thresholded at peak p<0.005, two-sided, FDR corrected p<0.05.
Findings for sertraline were replicated in an independent sample
Unique individuals were treated with sertraline in Stages 1 vs. 2. Hence, patients randomized to sertraline in Stage 1 could serve as an independent sample to replicate results. Consistent with Stage 2 findings, responders and non-responders to sertraline in Stage 1 did not differ in PRT response bias (t(114)=0.24, p>0.05, d=0.04, BF10=0.23), reward sensitivity (t(114)=−0.15, p>0.05, d=0.03, BF10=0.20) or learning rate (t(114)=−0.58, p>0.05, d=0.11, BF10=0.27). There was also no statistical difference in NACC-rACC RSFC between Stage 1 responders and non-responders to sertraline (t(110)=1.53, p>0.05, d=0.29, BF10=0.57).
No difference in dosage of sertraline received in Stage 1 by eventual bupropion responders and non-responders
The mechanism of action of bupropion is postulated to be primarily related to the inhibition of the reuptake of both dopamine and norepinephrine (80). Conversely, sertraline typically inhibits the neuronal reuptake of serotonin – although it also shows relatively high affinity for the dopamine transporter. As such, it has been suggested that sertraline might inhibit the reuptake of dopamine, particularly at high doses of 200mg and above (63). When evaluating sertraline doses in Stage 1 by patients who went on to receive bupropion in Stage 2, we found that the average dose was well below 200mg (mean=118.3mg, SD=26.7, range=57.1–155.2). Hence, it is difficult to disentangle the contributions of dopamine and norepinephrine to the efficacy of bupropion.
Discussion
Treatment for MDD is challenging and often proceeds with SSRIs as first-line antidepressants (38). Unfortunately, treatment selection is not informed by biomarkers, response rates are modest, and depressed patients who do not benefit from an adequate trial of SSRI are typically switched to non-SSRI agents (38–41). To the best of our knowledge, this is the first study to investigate behavioral and neural factors associated with response to the atypical antidepressant bupropion (which is assumed to increase dopaminergic and noradrenergic transmission), following a failure to respond to the serotonergic-based antidepressant, sertraline.
Notably, we found that greater reward sensitivity and higher RSFC between the NACC and rACC distinguished bupropion responders, who previously failed to respond to sertraline, from non-responders resistant to both classes of medication. Moreover, patients who responded better to bupropion had comparable reward sensitivity and potentiated NACC-rACC RSFC relative to healthy controls. In contrast, both reward sensitivity and NACC-rACC connectivity in bupropion non-responders were lower than healthy volunteers. Our results cannot provide a mechanistic explanation, but we speculate that these might reflect compensatory mechanisms in depression, in which elevated frontostriatal network functional connectivity is needed to respond normatively to reward. Future studies are needed to test this hypothesis. Our findings also suggest that depressed individuals with more normative reward behavior and potentiated brain reward system responded better to bupropion after failing an 8-week treatment with sertraline. In contrast, we found that these reward markers were not associated with response to sertraline in Stage 2 (after previous non-response to placebo), and replicated this null finding in an independent sample of patients randomized to sertraline in Stage 1. These findings contrast with our original hypotheses, which were originally derived from the assumptions that (1) SSRIs poorly address anhedonic phenotypes (81) and (2) patients with behavioral and neural markers indexing blunted reward processing would disproportionally benefit from pharmacological treatment assumed to increase dopaminergic (and noradrenergic) transmission (62,63,82).
Although unexpected, our results are in line with earlier suggestions that patients with a subtype of depression characterized by preserved reward sensitivity may preferentially improve with dopaminergic pharmacotherapy (83) and recent reports that MDD patients with more normative reward-related brain responses benefitted the most from Behavioral Activation treatment (84,85). Moreover, a recent study found that depressed individuals with higher baseline response bias responded more favorably to treatment by pramipexole, a selective dopamine agonist (86,87). However, this latter study did not include placebo or non-dopaminergic control. The current study demonstrated that better reward sensitivity and more positive RSFC among regions putatively involved in reward processing were associated with superior response to treatment by bupropion, one of the few antidepressants that prevent the reuptake of dopamine. In contrast, these effects were not found for the common serotonin reuptake inhibitor sertraline.
Current results might have significant clinical implications. Although extant guidelines recommend SSRIs when starting treatment for MDD (38) – with sertraline being the most widely prescribed antidepressant in the United States (88) and Japan (89) – only 50% of patients benefit from them. A failure to respond to first-line antidepressants requires consideration of various second-line treatments, which includes switching to a different medication, augmenting with a non-antidepressant drug, dose escalation or combination with a different antidepressant (38). However, there is no clear evidence for a particular strategy being superior (40,41,90–101), and secondary treatment guidelines are needed (102). Although further scrutiny is required, our results suggest that laboratory-based paradigms, such as the PRT, and/or imaging might be useful in informing whether NDRIs could be prescribed if first-line SSRIs are not beneficial. Individuals likely to be resistant to NDRIs could be recommended alternative strategies, including augmentation, psychotherapy, or neurostimulation. Hence, a prospective replication based on these biomarkers could advance clinical care.
Limitations of this work should be acknowledged. First, although the sample size for Stage 1 was large (N=296), that for Stage 2 was more modest with N=38 bupropion (16 responders vs. 22 non-responders) and N=49 sertraline (25 responders vs. 24 non-responders) patients. Nevertheless, this is the first study to examine reward biomarkers of second-line antidepressant response and, thus, will be valuable in guiding future studies. Second, the EMBARC trial adopted relatively strict inclusion criteria to minimize clinical heterogeneity. Hence, it is unclear whether findings will generalize to other depressed samples, such as those with psychotic features or comorbid substance abuse. Third, our results are not sufficient to provide any mechanistic explanation for why patients with intact, rather than impaired, reward processing systems respond more favorably to bupropion. Future, more mechanistic studies should investigate this.
Fourth, we have shown that reward sensitivity and frontostriatal connectivity distinguished between responders to bupropion, after failing to benefit from sertraline, and non-responders resistant to both classes of medication. However, it remains to be investigated whether these reward markers might also differentiate responders to secondary treatment by placebo, since non-responders to sertraline in Stage 1 of the EMBARC trial were all given bupropion, rather than being randomized to bupropion or placebo. In other words, due to the lack of placebo controls for the active treatments in Stage 2, the specific secondary treatment effect of bupropion cannot be determined. This should be noted when interpreting our findings because of the considerable placebo response rate observed in Stage 1. Nevertheless, the results of our study might still be useful in informing choice of second-line antidepressant when primary SSRI treatments fail since placebos are not prescribed in practice. Fifth, patients who received bupropion in Stage 2 took sertraline in Stage 1 while those in the sertraline group had previously been given placebo. While we confirmed that responders and non-responders to secondary treatment with bupropion or sertraline did not differ in depressive symptomatology at baseline, as well as during and after Stage 1, it is still possible that the baseline states prior to Stages 1 and 2 may have been different. Sixth, unlike previous investigations such as the International Study to Predict Optimized Treatment in Depression (iSPOT-D) (103), measures in EMBARC were not collected post-treatment. Hence, it is unknown whether reward sensitivity and frontostriatal connectivity will change with treatment to bupropion as a function of response.
Conclusion
Using a multimodal approach, the current study showed that behavioral and neural markers of reward processing-specifically, computationally derived reward sensitivity and NACC-rACC connectivity–distinguished depressed individuals likely to benefit from a dopaminergic medication, following failure on SSRIs, and patients expected to be resistant to both classes of antidepressants. With further scrutiny, these findings could have important implications for clinical care.
Supplementary Material
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Biological Sample | EMBARC Dataset (MDD Datients and controls) | NIMH Data Archive | Collection ID: 2199 | URL: https://nda.nih.gov/edit_collection.html?id=2199 |
| Software; Algorithm | MATLAB R2017a | MathWorks | RRID: SCR_001622 | |
| Software; Algorithm | JASP v0.11.1 | JASP Team | RRID:SCR_015823 | |
| Software; Algorithm | SPSS v22.0 | IBM | RRID:SCR_002865 | |
Acknowledgements
The EMBARC study was supported by the National Institute of Mental Health under award numbers U01MH092221 (Trivedi, M.H.) and U01MH092250 (McGrath, P.J., Parsey, R.V., Weissman, M.M.). YSA and DAP were supported by the A*STAR National Science Scholarship and R37 MH068376, respectively. This work was supported by the EMBARC National Coordinating Center at UT Southwestern Medical Center, Madhukar H. Trivedi, M.D., Coordinating PI, and the Data Center at Columbia and Stony Brook Universities. The funder has no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors are grateful to Daniel G. Dillon, Ph.D., for his helpful comments on the manuscript.
Disclosures
In the last three years, the authors report the following financial disclosures, for activities unrelated to the current research:
Dr. Trivedi: Dr. Trivedi reports the following lifetime disclosures: research support from the Agency for Healthcare Research and Quality, Cyberonics Inc., National Alliance for Research in Schizophrenia and Depression, National Institute of Mental Health, National Institute on Drug Abuse, National Institute of Diabetes and Digestive and Kidney Diseases, Johnson & Johnson, and consulting and speaker fees from Abbott Laboratories Inc., Akzo (Organon Pharmaceuticals Inc.), Allergan Sales LLC, Alkermes, AstraZeneca, Axon Advisors, Brintellix, Bristol-Myers Squibb Company, Cephalon Inc., Cerecor, Eli Lilly & Company, Evotec, Fabre Kramer Pharmaceuticals Inc., Forest Pharmaceuticals, GlaxoSmithKline, Health Research Associates, Johnson & Johnson, Lundbeck, MedAvante Medscape, Medtronic, Merck, Mitsubishi Tanabe Pharma Development America Inc., MSI Methylation Sciences Inc., Nestle Health Science-PamLab Inc., Naurex, Neuronetics, One Carbon Therapeutics Ltd., Otsuka Pharmaceuticals, Pamlab, Parke-Davis Pharmaceuticals Inc., Pfizer Inc., PgxHealth, Phoenix Marketing Solutions, Rexahn Pharmaceuticals, Ridge Diagnostics, Roche Products Ltd., Sepracor, SHIRE Development, Sierra, SK Life and Science, Sunovion, Takeda, Tal Medical/Puretech Venture, Targacept, Transcept, VantagePoint, Vivus, and Wyeth-Ayerst Laboratories.
Dr. Fava: Dr. Fava reports the following lifetime disclosures: http://mghcme.org/faculty/faculty-detail/maurizio_fava
Dr. Phillips: consulting fees from Sunovion Pharmaceuticals.
Dr. Weissman: funding from NIMH, the National Alliance for Research on Schizophrenia and Depression (NARSAD), the Sackler Foundation, and the Templeton Foundation; royalties from the Oxford University Press, Perseus Press, the American Psychiatric Association Press, and MultiHealth Systems.
Dr. Oquendo: funding from NIMH; royalties for the commercial use of the Columbia-Suicide Severity Rating Scale. Her family owns stock in Bristol Myers Squibb.
Dr. McInnis: funding from NIMH; consulting fees from Janssen and Otsuka Pharmaceuticals.
Dr. Deckersbach: funding from NIH, NIMH, PCORI, NARSAD, TSA, IOCDF, Tufts University, DBDAT, Otsuka Pharmaceuticals, Cogito, Inc, Sunovion. and Assurex Pharmaceuticals; honoraria, consultation fees and/or royalties from the MGH Psychiatry Academy, BrainCells Inc., Clintara, LLC., Systems Research and Applications Corporation, Boston University, the Catalan Agency for Health Technology Assessment and Research, the National Association of Social Workers Massachusetts, the Massachusetts Medical Society, Tufts University, NIDA, NIMH, Oxford University Press, and Guilford Press. Dr. Deckersbach has also participated in research funded by Assurex, DARPA, NIH, NIMH, NIA, AHRQ, PCORI, Janssen Pharmaceuticals, The Forest Research Institute, Shire Development Inc., Medtronic, Cyberonics, Northstar, Takeda, and Sunovion.
Dr. Pizzagalli: funding from NIMH, Brain and Behavior Research Foundation, the Dana Foundation, and Millennium Pharmaceuticals; consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehreinger Ingelheim, Compass Pathway, Posit Science, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; one honorarium from Alkermes. Dr. Pizzagalli has a financial interest in BlackThorn Therapeutics, which has licensed the copyright to the Probabilistic Reward Task through Harvard University. Dr. Pizzagalli’s interests were reviewed and are managed by McLean Hospital and Partners Healthcare in accordance with their conflict of interest policies.
All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. (2003): The Epidemiology of Major Depressive Disorder: Results From the National Comorbidity Survey Replication (NCS-R). JAMA 289: 3095. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg PE, Fournier A-A, Sisitsky T, Pike CT, Kessler RC (2015): The Economic Burden of Adults With Major Depressive Disorder in the United States (2005 and 2010). J Clin Psychiatry 76: 155–162. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. (2006): Evaluation of Outcomes With Citalopram for Depression Using Measurement-Based Care in STAR*D: Implications for Clinical Practice. Am J Psychiatry 163: 28–40. [DOI] [PubMed] [Google Scholar]
- 4.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. (2006): Acute and Longer-Term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. Am J Psychiatry 163: 1905–1917. [DOI] [PubMed] [Google Scholar]
- 5.Souery D, Papakostas GI, Trivedi MH (2006): Treatment-resistant depression. J Clin Psychiatry 67 Suppl 6: 16–22. [PubMed] [Google Scholar]
- 6.Katon W, Robinson P, Von Korff M, Lin E, Bush T, Ludman E, et al. (1996): A multifaceted intervention to improve treatment of depression in primary care. Arch Gen Psychiatry 53: 924–932. [DOI] [PubMed] [Google Scholar]
- 7.Hasler G, Drevets WC, Manji HK, Charney DS (2004): Discovering endophenotypes for major depression. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 29: 1765–1781. [DOI] [PubMed] [Google Scholar]
- 8.Pizzagalli DA (2014): Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu Rev Clin Psychol 10: 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen C-H, Ridler K, Suckling J, Williams S, Fu CHY, Merlo-Pich E, Bullmore E (2007): Brain Imaging Correlates of Depressive Symptom Severity and Predictors of Symptom Improvement After Antidepressant Treatment. Biol Psychiatry 62: 407–414. [DOI] [PubMed] [Google Scholar]
- 10.Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein-Piekarski A, et al. (2015): Amygdala Reactivity to Emotional Faces in the Prediction of General and Medication-Specific Responses to Antidepressant Treatment in the Randomized iSPOT-D Trial. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 40: 2398–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, et al. (2007): Frontal and Limbic Activation During Inhibitory Control Predicts Treatment Response in Major Depressive Disorder. Biol Psychiatry 62: 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy M, Harvey P-O, Berlim MT, Mamdani F, Beaulieu M-M, Turecki G, Lepage M (2010): Medial prefrontal cortex activity during memory encoding of pictures and its relation to symptomatic improvement after citalopram treatment in patients with major depression. J Psychiatry Neurosci JPN 35: 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh ND, Williams SCR, Brammer MJ, Bullmore ET, Kim J, Suckling J, et al. (2007): A longitudinal functional magnetic resonance imaging study of verbal working memory in depression after antidepressant therapy. Biol Psychiatry 62: 1236–1243. [DOI] [PubMed] [Google Scholar]
- 14.Gyurak A, Patenaude B, Korgaonkar MS, Grieve SM, Williams LM, Etkin A (2016): Frontoparietal Activation During Response Inhibition Predicts Remission to Antidepressants in Patients With Major Depression. Biol Psychiatry 79: 274–281. [DOI] [PubMed] [Google Scholar]
- 15.Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JRC, Deckersbach T, Trivedi MH (2015): Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am J Psychiatry 172: 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tozzi L, Goldstein-Piekarski AN, Korgaonkar MS, Williams LM (2020): Connectivity of the Cognitive Control Network During Response Inhibition as a Predictive and Response Biomarker in Major Depression: Evidence From a Randomized Clinical Trial. Biol Psychiatry 87: 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizzagalli DA (2011): Frontocingulate Dysfunction in Depression: Toward Biomarkers of Treatment Response. Neuropsychopharmacology 36: 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pizzagalli DA, Webb CA, Dillon DG, Tenke CE, Kayser J, Goer F, et al. (2018): Pretreatment Rostral Anterior Cingulate Cortex Theta Activity in Relation to Symptom Improvement in Depression: A Randomized Clinical Trial. JAMA Psychiatry 75: 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dunkin JJ, Leuchter AF, Cook IA, Kasl-Godley JE, Abrams M, Rosenberg-Thompson S (2000): Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord 60: 13–23. [DOI] [PubMed] [Google Scholar]
- 20.Taylor BP, Bruder GE, Stewart JW, McGrath PJ, Halperin J, Ehrlichman H, Quitkin FM (2006): Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed outpatients. Am J Psychiatry 163: 73–78. [DOI] [PubMed] [Google Scholar]
- 21.Gudayol-Ferré E, Herrera-Guzmán I, Camarena B, Cortés-Penagos C, Herrera-Abarca JE, Martínez-Medina P, et al. (2010): The role of clinical variables, neuropsychological performance and SLC6A4 and COMT gene polymorphisms on the prediction of early response to fluoxetine in major depressive disorder. J Affect Disord 127: 343–351. [DOI] [PubMed] [Google Scholar]
- 22.Etkin A, Patenaude B, Song YJC, Usherwood T, Rekshan W, Schatzberg AF, et al. (2015): A cognitive-emotional biomarker for predicting remission with antidepressant medications: a report from the iSPOT-D trial. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 40: 1332–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexopoulos GS, Manning K, Kanellopoulos D, McGovern A, Seirup JK, Banerjee S, Gunning F (2015): Cognitive control, reward-related decision making and outcomes of late-life depression treated with an antidepressant. Psychol Med 45: 3111–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sneed JR, Roose SP, Keilp JG, Krishnan KRR, Alexopoulos GS, Sackeim HA (2007): Response Inhibition Predicts Poor Antidepressant Treatment Response in Very Old Depressed Patients. Am J Geriatr Psychiatry 15: 553–563. [DOI] [PubMed] [Google Scholar]
- 25.Kalayam B, Alexopoulos GS (2003): A Preliminary Study of Left Frontal Region Error Negativity and Symptom Improvement in Geriatric Depression. Am J Psychiatry 160: 2054–2056. [DOI] [PubMed] [Google Scholar]
- 26.Herrera-Guzmán I, Gudayol-Ferré E, Lira-Mandujano J, Herrera-Abarca J, Herrera-Guzmán D, Montoya-Pérez K, Guardia-Olmos J (2008): Cognitive predictors of treatment response to bupropion and cognitive effects of bupropion in patients with major depressive disorder. Psychiatry Res 160: 72–82. [DOI] [PubMed] [Google Scholar]
- 27.Bruder GE, Alvarenga JE, Alschuler D, Abraham K, Keilp JG, Hellerstein DJ, et al. (2014): Neurocognitive predictors of antidepressant clinical response. J Affect Disord 166: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mikoteit T, Hemmeter U, Eckert A, Brand S, Bischof R, Delini-Stula A, et al. (2015): Improved Alertness Is Associated with Early Increase in Serum Brain-Derived Neurotrophic Factor and Antidepressant Treatment Outcome in Major Depression. Neuropsychobiology 72: 16–28. [DOI] [PubMed] [Google Scholar]
- 29.Cléry-Melin M-L, Gorwood P (2017): A simple attention test in the acute phase of a major depressive episode is predictive of later functional remission: C léry -M elin et al. Depress Anxiety 34: 159–170. [DOI] [PubMed] [Google Scholar]
- 30.Murrough JW, Wan L-B, lacoviello B, Collins KA, Solon C, Glicksberg B, et al. (2013): Neurocognitive effects of ketamine in treatment-resistant major depression: association with antidepressant response. Psychopharmacology (Berl). 10.1007/s00213-013-3255-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shiroma PR, Albott CS, Johns B, Thuras P, Wels J, Lim KO (2014): Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 17: 1805–1813. [DOI] [PubMed] [Google Scholar]
- 32.Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Kalayam B, Katz R, Kanellopoulos D, et al. (2007): Event-related potentials in an emotional go/no-go task and remission of geriatric depression. Neuroreport 18: 217–221. [DOI] [PubMed] [Google Scholar]
- 33.Gudayol-Ferré E, Herrera-Guzmán I, Camarena B, Cortés-Penagos C, Herrera-Abarca JE, Martínez-Medina P, et al. (2012): Prediction of remission of depression with clinical variables, neuropsychological performance, and serotonergic/dopaminergic gene polymorphisms: PREDICTION OF REMISSION OF DEPRESSION. Hum Psychopharmacol Clin Exp 27: 577–586. [DOI] [PubMed] [Google Scholar]
- 34.Murrough JW, Burdick KE, Levitch CF, Perez AM, Brallier JW, Chang LC, et al. (2015): Neurocognitive Effects of Ketamine and Association with Antidepressant Response in Individuals with Treatment-Resistant Depression: A Randomized Controlled Trial. Neuropsychopharmacology 40: 1084–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jha MK, Minhajuddin A, Gadad BS, Greer T, Grannemann B, Soyombo A, et al. (2017): Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 78: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jha MK, Minhajuddin A, Gadad BS, Greer TL, Mayes TL, Trivedi MH (2017): Interleukin 17 selectively predicts better outcomes with bupropion-SSRI combination: Novel T cell biomarker for antidepressant medication selection. Brain Behav Immun 66: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jha MK, Minhajuddin A, Gadad BS, Trivedi MH (2017): Platelet-Derived Growth Factor as an Antidepressant Treatment Selection Biomarker: Higher Levels Selectively Predict Better Outcomes with Bupropion-SSRI Combination. Int J Neuropsychopharmacol 20: 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Collaborating Centre for Mental Health (UK) (2010): Depression: The Treatment and Management of Depression in Adults (Updated Edition). Leicester (UK): British Psychological Society; Retrieved August 8, 2019, from http://www.ncbi.nlm.nih.gov/books/NBK63748/ [PubMed] [Google Scholar]
- 39.Fredman SJ, Fava M, Kienke AS, White CN, Nierenberg AA, Rosenbaum JF (2000): Partial response, nonresponse, and relapse with selective serotonin reuptake inhibitors in major depression: a survey of current “next-step” practices. J Clin Psychiatry 61: 403–408. [DOI] [PubMed] [Google Scholar]
- 40.Ruhé HG, Huyser J, Swinkels JA, Schene AH (2006): Switching antidepressants after a first selective serotonin reuptake inhibitor in major depressive disorder: a systematic review. J Clin Psychiatry 67: 1836–1855. [DOI] [PubMed] [Google Scholar]
- 41.Papakostas GI, Fava M, Thase ME (2008): Treatment of SSRI-resistant depression: a meta-analysis comparing within- versus across-class switches. Biol Psychiatry 63: 699–704. [DOI] [PubMed] [Google Scholar]
- 42.Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML (2005): The Neural Correlates of Anhedonia in Major Depressive Disorder. Biol Psychiatry 58: 843–853. [DOI] [PubMed] [Google Scholar]
- 43.Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, et al. (2006): Reward-related decision-making in pediatric major depressive disorder: an fMRI study. J Child Psychol Psychiatry 47: 1031–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH (2008): Neural responses tomonetary incentives in major depression. Biol Psychiatry 63: 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pizzagalli DA, losifescu D, Hallett LA, Ratner KG, Fava M (2008): Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res 43: 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smoski MJ, Felder J, Bizzell J, Green SR, Ernst M, Lynch TR, Dichter GS (2009): fMRI of alterations in reward selection, anticipation, and feedback in major depressive disorder. J Affect Disord 118: 69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD (2008): Abnormal temporal difference reward-learning signals in major depression. Brain 131: 2084–2093. [DOI] [PubMed] [Google Scholar]
- 48.Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, et al. (2011): Expected value and prediction error abnormalities in depression and schizophrenia. Brain 134: 1751–1764. [DOI] [PubMed] [Google Scholar]
- 49.Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC (2012): Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am J Psychiatry 169: 152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pizzagalli DA, Evins AE, Schetter EC, Frank MJ, Pajtas PE, Santesso DL, Culhane M (2008): Single dose of a dopamine agonist impairs reinforcement learning in humans: behavioral evidence from a laboratory-based measure of reward responsiveness. Psychopharmacology (Berl) 196: 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pergadia ML, Der-Avakian A, D’Souza MS, Madden PAF, Heath AC, Shiffman S, et al. (2014): Association between nicotine withdrawal and reward responsiveness in humans and rats. JAMA Psychiatry 71: 1238–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Der-Avakian A, D’Souza MS, Pizzagalli DA, Markou A (2013): Assessment of reward responsiveness in the response bias probabilistic reward task in rats: implications for cross-species translational research. Transl Psychiatry 3: e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser RH, Treadway MT, Wooten DW, Kumar P, Goer F, Murray L, et al. (2018): Frontostriatal and Dopamine Markers of Individual Differences in Reinforcement Learning: A Multi-modal Investigation. Cereb Cortex N Y N 1991 28: 4281–4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fletcher K, Parker G, Paterson A, Fava M, losifescu D, Pizzagalli DA (2015): Anhedonia in melancholic and non-melancholic depressive disorders. J Affect Disord 184: 81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pizzagalli DA, Jahn AL, O’Shea JP (2005): Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry 57: 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, Dahl RE (2010): Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Neurosci 10: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Webb CA, Trivedi MH, Cohen ZD, Dillon DG, Fournier JC, Goer F, et al. (2019): Personalized prediction of antidepressant v. placebo response: evidence from the EMBARC study. Psychol Med 49: 1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenberg T, Fournier JC, Stiffler R, Chase HW, Almeida JR, Aslam H, et al. (2019): Reward related ventral striatal activity and differential response to sertraline versus placebo in depressed individuals. Mol Psychiatry. 10.1038/s41380-019-0490-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Everitt BJ, Robbins TW (2005): Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8: 1481–1489. [DOI] [PubMed] [Google Scholar]
- 60.Haber SN, Knutson B (2010): The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 35: 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, et al. (2016): Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): Rationale and design. J Psychiatr Res 78: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nestler EJ, Carlezon WA (2006): The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59: 1151–1159. [DOI] [PubMed] [Google Scholar]
- 63.Dunlop BW, Nemeroff CB (2007): The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64: 327–337. [DOI] [PubMed] [Google Scholar]
- 64.Huys QJ, Pizzagalli DA, Bogdan R, Dayan P (2013): Mapping anhedonia onto reinforcement learning: a behavioural meta-analysis. Biol Mood Anxiety Disord 3: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. (2002): Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage 15: 273–289. [DOI] [PubMed] [Google Scholar]
- 66.Knutson B, Adams CM, Fong GW, Hommer D (2001): Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci Off J Soc Neurosci 21: RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW (2004): Incentive-elicited brain activation in adolescents: similarities and differences from young adults. J Neurosci Off J Soc Neurosci 24: 1793–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schreiter S, Spengler S, Willert A, Mohnke S, Herold D, Erk S, et al. (2016): Neural alterations of fronto-striatal circuitry during reward anticipation in euthymic bipolar disorder. Psychol Med 46: 3187–3198. [DOI] [PubMed] [Google Scholar]
- 69.Weiland BJ, Welsh RC, Yau W-YW, Zucker RA, Zubieta J-K, Heitzeg MM (2013): Accumbens functional connectivity during reward mediates sensation-seeking and alcohol use in high-risk youth. Drug Alcohol Depend 128: 130–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Knutson B, Cooper JC (2005): Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol 18: 411–417. [DOI] [PubMed] [Google Scholar]
- 71.Garrison J, Erdeniz B, Done J (2013): Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci Biobehav Rev 37: 1297–1310. [DOI] [PubMed] [Google Scholar]
- 72.Luijten M, Schellekens AF, Kühn S, Machielse MWJ, Sescousse G (2017): Disruption of Reward Processing in Addiction: An Image-Based Meta-analysis of Functional Magnetic Resonance Imaging Studies. JAMA Psychiatry 74: 387–398. [DOI] [PubMed] [Google Scholar]
- 73.Berridge KC (2012): From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci 35: 1124–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robinson TE, Berridge KC (2008): Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci 363: 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salgado S, Kaplitt MG (2015): The Nucleus Accumbens: A Comprehensive Review. Stereotact Funct Neurosurg 93: 75–93. [DOI] [PubMed] [Google Scholar]
- 76.Duff EP, Cunnington R, Egan GF (2007): REX: response exploration for neuroimaging datasets. Neuroinformatics 5: 223–234. [DOI] [PubMed] [Google Scholar]
- 77.Hamilton M (1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.JASP Team (2018): JASP, version 0.11.1. Retrieved from https://jasp-stats.org/
- 79.Jeffreys H (1998): Theory of Probability, 3rd ed. Oxford [Oxfordshire] : New York: Clarendon Press; Oxford University Press. [Google Scholar]
- 80.Stahl SM, Pradko JF, Haight BR, Modell JG, Rockett CB, Learned-Coughlin S (2004): A Review of the Neuropharmacology of Bupropion, a Dual Norepinephrine and Dopamine Reuptake Inhibitor. Prim Care Companion J Clin Psychiatry 6: 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McMakin DL, Olino TM, Porta G, Dietz LJ, Emslie G, Clarke G, et al. (2012): Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry 51: 404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, et al. (2013): Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry 73: 639–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stewart JW, Thase ME (2007): Treating DSM-IV depression with atypical features. J Clin Psychiatry 68: e10. [DOI] [PubMed] [Google Scholar]
- 84.Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, et al. (2016): Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. J Affect Disord 203: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walsh E, Carl H, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T, et al. (2017): Attenuation of Frontostriatal Connectivity During Reward Processing Predicts Response to Psychotherapy in Major Depressive Disorder. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol 42: 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whitton A, Reinen J, Slifstein M, McGrath P, losifescu D, Abi-Dargham A, et al. (2018): 253. Utilizing a Behavioral Assay of Reward Learning to Predict Clinical Response to a Dopamine Agonist in Individuals With Depression. Biol Psychiatry 83: S102. [Google Scholar]
- 87.Whitton A, Reinen J, Slifstein M, Ang Y-S, McGrath J, losifescu D, et al. (in press): Baseline reward processing and ventrostriatal dopamine function is associated with pramiprexole response in depression. Brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Medicine Use and Spending in the U.S. - A Review of 2017 and Outlook to 2022 (n.d.): Retrieved August 20, 2019, from https://www.iqvia.com/institute/reports/medicine-use-and-spending-in-the-us-review-of-2017-outlook-to-2022
- 89.Furukawa TA, Onishi Y, Hinotsu S, Tajika A, Takeshima N, Shinohara K, et al. (2013): Prescription patterns following first-line new generation antidepressants for depression in Japan: A naturalistic cohort study based on a large claims database. J Affect Disord 150: 916–922. [DOI] [PubMed] [Google Scholar]
- 90.Corruble E, Guelfi JD (2000): Does increasing dose improve efficacy in patients with poor antidepressant response: a review. Acta Psychiatr Scand 101: 343–348. [DOI] [PubMed] [Google Scholar]
- 91.Adli M, Baethge C, Heinz A, Langlitz N, Bauer M (2005): Is dose escalation of antidepressants a rational strategy after a medium-dose treatment has failed? A systematic review. Eur Arch Psychiatry Clin Neurosci 255: 387–400. [DOI] [PubMed] [Google Scholar]
- 92.Ruhé HG, Huyser J, Swinkels JA, Schene AH (2006): Dose escalation for insufficient response to standard-dose selective serotonin reuptake inhibitors in major depressive disorder: systematic review. Br J Psychiatry J Ment Sci 189: 309–316. [DOI] [PubMed] [Google Scholar]
- 93.Dold M, Bartova L, Rupprecht R, Kasper S (2017): Dose Escalation of Antidepressants in Unipolar Depression: A Meta-Analysis of Double-Blind, Randomized Controlled Trials. Psychother Psychosom 86: 283–291. [DOI] [PubMed] [Google Scholar]
- 94.Bschor T, Kern H, Henssler J, Baethge C (2018): Switching the Antidepressant After Nonresponse in Adults With Major Depression: A Systematic Literature Search and Meta-Analysis. J Clin Psychiatry 79 10.4088/JCP.16r10749 [DOI] [PubMed] [Google Scholar]
- 95.Zhou X, Ravindran AV, Qin B, Del Giovane C, Li Q, Bauer M, et al. (2015): Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J Clin Psychiatry 76: e487–498. [DOI] [PubMed] [Google Scholar]
- 96.Henssler J, Bschor T, Baethge C (2016): Combining Antidepressants in Acute Treatment of Depression: A Meta-Analysis of 38 Studies Including 4511 Patients. Can J Psychiatry Rev Can Psychiatr 61: 29–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ferreri M, Lavergne F, Berlin I, Payan C, Puech AJ (2001): Benefits from mianserin augmentation of fluoxetine in patients with major depression non-responders to fluoxetine alone. Acta Psychiatr Scand 103: 66–72. [DOI] [PubMed] [Google Scholar]
- 98.Licht RW, Qvitzau S (2002): Treatment strategies in patients with major depression not responding to first-line sertraline treatment. A randomised study of extended duration of treatment, dose increase or mianserin augmentation. Psychopharmacology (Berl) 161: 143–151. [DOI] [PubMed] [Google Scholar]
- 99.Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, et al. (2006): Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med 354: 1231–1242. [DOI] [PubMed] [Google Scholar]
- 100.Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin F, Warden D, et al. (2006): Medication augmentation after the failure of SSRIs for depression. N Engl J Med 354: 1243–1252. [DOI] [PubMed] [Google Scholar]
- 101.Mohamed S, Johnson GR, Chen P, Hicks PB, Davis LL, Yoon J, et al. (2017): Effect of Antidepressant Switching vs Augmentation on Remission Among Patients With Major Depressive Disorder Unresponsive to Antidepressant Treatment: The VAST-D Randomized Clinical Trial. JAMA 318: 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kato T, Furukawa TA, Mantani A, Kurata K, Kubouchi H, Hirota S, et al. (2018): Optimising first- and second-line treatment strategies for untreated major depressive disorder - the SUN D study: a pragmatic, multi-centre, assessor-blinded randomised controlled trial. BMC Med 16: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williams LM, Rush AJ, Koslow SH, Wisniewski SR, Cooper NJ, Nemeroff CB, et al. (2011): International Study to Predict Optimized Treatment for Depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.