Abstract
Numerous G protein-coupled receptors (GPCRs) regulate numerous airway functions and play fundamental roles in normal and aberrant airway and lung physiology. Thus, GPCRs are prime candidates of targeting by disease therapeutics. The intriguing proton-sensing GPCR Ovarian cancer G-protein coupled receptor 1 (OGR1; aka GPR68) has recently been shown capable of regulating airway smooth muscle (ASM) contraction and proliferation. Although the study of OGR1 has been confounded by the fact that the proton is the presumed cognate ligand of OGR1, recent studies have begun to identify novel ligands and modulators capable of regulating the diverse signaling, and functional role, of OGR1. Such studies offer hope for OGR1 targeting drugs as therapeutics for obstructive lung diseases such as asthma. Herein we review the literature to date detailing the receptor biology and pharmacology of OGR1, receptor function in the airway, and describe the potential clinical utility of OGR1-modulating drugs.
Introduction
G protein-coupled receptors (GPCRs) represent the largest protein family in the human genome, are principal regulators of most cellular, tissue, and organ system functions, and are therefore frequent targets of most disease-managing drugs. Multiple GPCRs are expressed in airway cells, including airway smooth muscle (ASM) and the airway epithelium, and serve to regulate airway diameter during both physiological breathing and when excessive airway narrowing/resistance occurs in obstructive lung disease such as asthma and chronic obstructive pulmonary disease (COPD). Two particular GPCRs, the m3 muscarinic acetylcholine receptor (m3AChR) and the beta-2 adrenoceptor (β2AR), have historically dominated the research and drug development efforts focused on control of ASM contraction and thus airway resistance. However, the last 2 decades have revealed dozens of GPCRs that play, if not a physiological role in airway biology, potential pathophysiological and therapeutic roles in obstructive lung diseases.
Bronchodilation/bronchoprotection
Most bronchodilators act through GPCR activation (Gs pathways) or inhibition (Gq pathways) at the receptor locus. We have previously espoused the concept of a dynamic or competitive balance of GPCR signaling in ASM as an important determinant of ASM contractile state, and thus, airway resistance in asthma and COPD [1]. ASM contractile state is largely determined by the competitive activities of pro-contractile and pro-relaxant G protein-coupled receptors (GPCRs) expressed on ASM cells. Under conditions of airway inflammation that occur with asthma, the exaggerated presentation of numerous endogenous GPCR agonists, most pro contractile but some pro-relaxant, tends to shift the competitive balance of GPCR activation toward contractile receptors, resulting in bronchoconstriction.
Although airway resistance is admittedly influenced by additional factors including airway remodeling, airway architecture and tissue mechanics, and other phenomena contributing to impedance, agents that contract and relax airway smooth muscle have a dominant effect on airflow and largely function through ASM GPCRs. As noted above, allergic airway inflammation is typically associated with increased levels of numerous agonists for different pro-contractile ASM GPCRs causing ASM contraction and increased airway resistance. Pro-contractile signaling is typically mediated by Gq-coupled GPCRs (and some Gi-coupled GPCRs) such as histamine H1 receptor (H1R) and muscarinic M3 acetylcholine receptor (m3AChR) that in ASM stimulate intracellular calcium mobilization leading to MLC20 phosphorylation, crossbridge cycling and ASM shortening. One means of preventing or reversing this effect is to deliver to the airway a selective small molecule antagonist of a GPCR causing ASM contraction (for example M3 mAChR or CysLT1R antagonists). However, this strategy can be of limited effect, especially if multiple different receptors are contributing to bronchoconstriction. Conversely, pro-relaxant GPCR signaling is typically mediated by Gs-coupled GPCRs, the β2AR being the most relevant and well-characterized. Activation of Gs-coupled GPCRs leads to production of the second messenger, cyclic AMP (cAMP), which in turn activates protein kinase A (PKA), a critical effector in Gs-coupled receptor mediated inhibition of ASM contraction. PKA can effectively inhibit the contractile machinery by phosphorylating certain Gq-coupled receptors as well as phospholipase C (PLC), and consequently inhibiting Gq-receptor/PLC-mediated generated of phosphoinositide (PI) which is essential for calcium flux. PKA also phosphorylates the inositol 1,4,5-triphosphate (IP3) receptor and reduces its affinity for IP3, thus further limiting mobilization of intracellular calcium. PKA also phosphorylates myosin light chain kinase (MLCK) reducing its affinity to calcium calmodulin, consequently reducing its enzymatic activity and phosphorylation of MLC20. Thus, β-agonists, as well as other PKA-activating agents (e.g., phosphodiesterase inhibitors and agonists of other Gs-coupled receptors discussed below and noted in Fig. 1) have the ability to broncho-relax/protect an airway exposed to numerous and multiple pro-contractile GPCR agonists.
Figure 1. Regulation of contractile (Gq-coupled GPCR) signaling by agonists of Gs-coupled GPCRs.
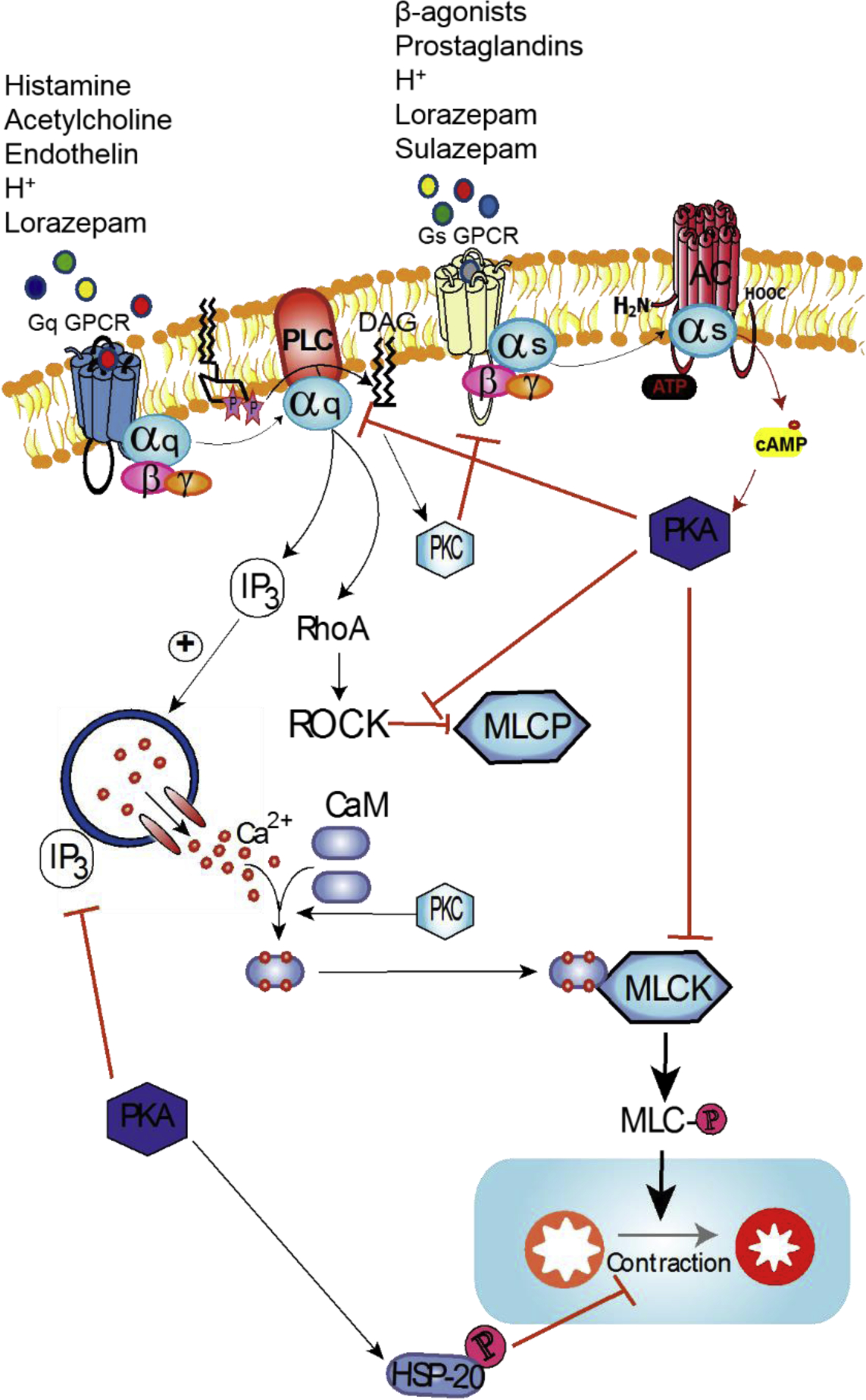
Contractile signaling in ASM is typically initiated through agonist interaction with cognate Gq-coupled GPCR. This signaling progresses through activation of membrane bound phospholipase C beta isoform (PLCβ) which catalyzes conversion of phosphatidylinositol 4,5-bisphosphate (PIP2) into diacyl glycerol (DAG) and inositol triphosphate (IP3). Binding of IP3 to its receptor on the sarcoplasmic reticulum stimulates release of Ca2+ into the cytosol where elevated Ca2+ binds calmodulin, which in turn binds myosin light chain kinase (MLCK) and exposes its catalytic domain, leading to phosphorylation of MLC. Additionally, activation of Rho/Rho kinase (ROCK) supports contractile signaling by inhibition of myosin light chain phosphatase (MLCP) enzyme. Regulation of contractile signaling is primarily achieved by activation of Gs-coupled GPCRs, which activates adenylyl cyclase (AC) which in turn catalyzes cyclization of ATP into cyclic AMP (cAMP), a principal second messenger of Gs-coupled GPCR signaling. cAMP activates PKA, which is a major effector of bronchodilatory outcomes. PKA achieves this by inhibition of contractile machinery at multiple junctions. On the other hand, OGR1 signaling is rather unique in that ligands of OGR1 such as protons (H+) or lorazepam can activate the receptor to Gq-coupled or Gs-coupled signaling, whereas sulazepam induces a Gs-biased signaling downstream of OGR1. Thus, OGR1 is a prime candidate for employing biased ligand pharmacology (i.e., delivery of a Gs-biased OGR1 ligand) in the management of asthma/obstructive lung disease.
The importance of GPCR activation in asthma and COPD is underscored by the fact that most drugs for these diseases target GPCRs either directly (e.g. tiotropium and montelukast inhibiting pro-contractile m3mAChRs and CysLT1Rs, respectively, and beta-agonists activating β2AR) or indirectly (glucocorticoids inhibit the production of pro-contractile GPCR agonists). Many of the GPCRs (and their ligands) that affect airway smooth muscle biology, including the Cysteinyl leukotriene type 1 receptor (CysLTR1), the H1 histamine receptor, E-prostanoid receptors (EP1, EP2, EP3), endothelin type 1 receptor (ET-1), and bradykinin receptors (BK1, BK2) have been the subject of numerous recent reviews [2]. This review will focus on one rather unique GPCR, GPR68 aka the Ovarian cancer G-protein coupled receptor 1 (OGR1 aka GPR68), that belongs to an intriguing subfamily of GPCRs known as proton-sensing GPCRs. Called proton-sensing based on their ability to be activated by low cellular pH with their presumed cognate ligand the proton, OGR1 and its subfamily members (GPR4, GPR65, GPR132) have been extremely difficult to understand and research given the lack of useful pharmacological tools and inherent difficulties in controlling for the promiscuous actions of protons and dynamic changes in pH.
Relevance of pH in the lung and asthma
The recent emergence of an intriguing subfamily of GPCRs that appear capable of activation by low extracellular pH, with protons as their cognate ligand, raises the possibility of a novel, unappreciated pathogenic mechanism in obstructive lung diseases. Under normal physiological conditions, the pH of the airway fluid lining appears to be slightly alkaline [3]. Although considerable debate exists over the appropriateness of various techniques for approximating airway pH, indirect measures using exhaled breath condensate (EBC) [4,5] and tracheobronchial mucous [6] report mean values of ~pH 7.7 and 7.8, respectively. Numerous studies provide evidence that the airway, including the extracellular microenvironment of airway epithelial and ASM cells, is subject to vicissitudes of pH [7–10]. Importantly, several of the events that promote reductions in airway pH are associated with ASM contraction and obstructive lung diseases. “Acid fog” is a prominent environmental factor (the pH in floating fog over Kushiro City, Japan averages pH<5.0) that can cause acute bronchoconstriction and associates with asthma prevalence [11]. There is a long history of research exploring the relation between esophageal reflux disease (and microaspiration of acid reflux) and asthma (reviewed in [3]). Perhaps most importantly, inflammation is known to produce reductions in airway pH, and patients with moderate asthma, COPD, and bronchiectasis have significantly lower exhaled breath condensate (EBC) pH values than those measured in healthy controls [4,12]. Moreover, in these patients EBC pH values significantly correlated with either sputum eosinophilia or neutrophilia, and with oxidative stress [12]. Importantly, resolution of airway inflammation in asthmatics with ICS treatment results in a normalization of airway pH [4].
To date, 2 prominent mechanisms, both neural, have been attributed to bronchoconstriction elicited by acute reductions in airway pH (Figure 2). Termed the “reflex” and “reflux” mechanisms, they involve sensing of low pH by afferent neurons in either the airway or esophagus, which in turn elicit efferent neurons to stimulate ASM with local release of acetylcholine or kinins [13,14]. However, these mechanisms are typically invoked by very low reductions in pH (<pH 6.5). More subtle reductions in airway pH, such as those promoted by airway inflammation, would not be predicted to invoke these mechanisms. Interestingly, the dynamic range of activation of most “proton-sensing” GPCRs has been shown to be ~pH 8.0 -pH 6.8 [15]. Accordingly, proton sensing GPCRs expressed on ASM represent a potential means of controlling ASM contractile state by relevant changes in pH in the ASM microenvironment. Thus, they could either play a role in influencing the obstructive lung diseases such as asthma or be a potential therapeutic target. In addition, as discussed below, the discovered pleiotropic capacity of OGR1 furthers OGR1 as a therapeutic target, irrespective of any pathogenic role OGR1 might play.
Figure 2. Proposed ‘reflex’, ‘reflux’ and ‘direct activation’ arcs contributing to action of proton-sensing receptors in airways.
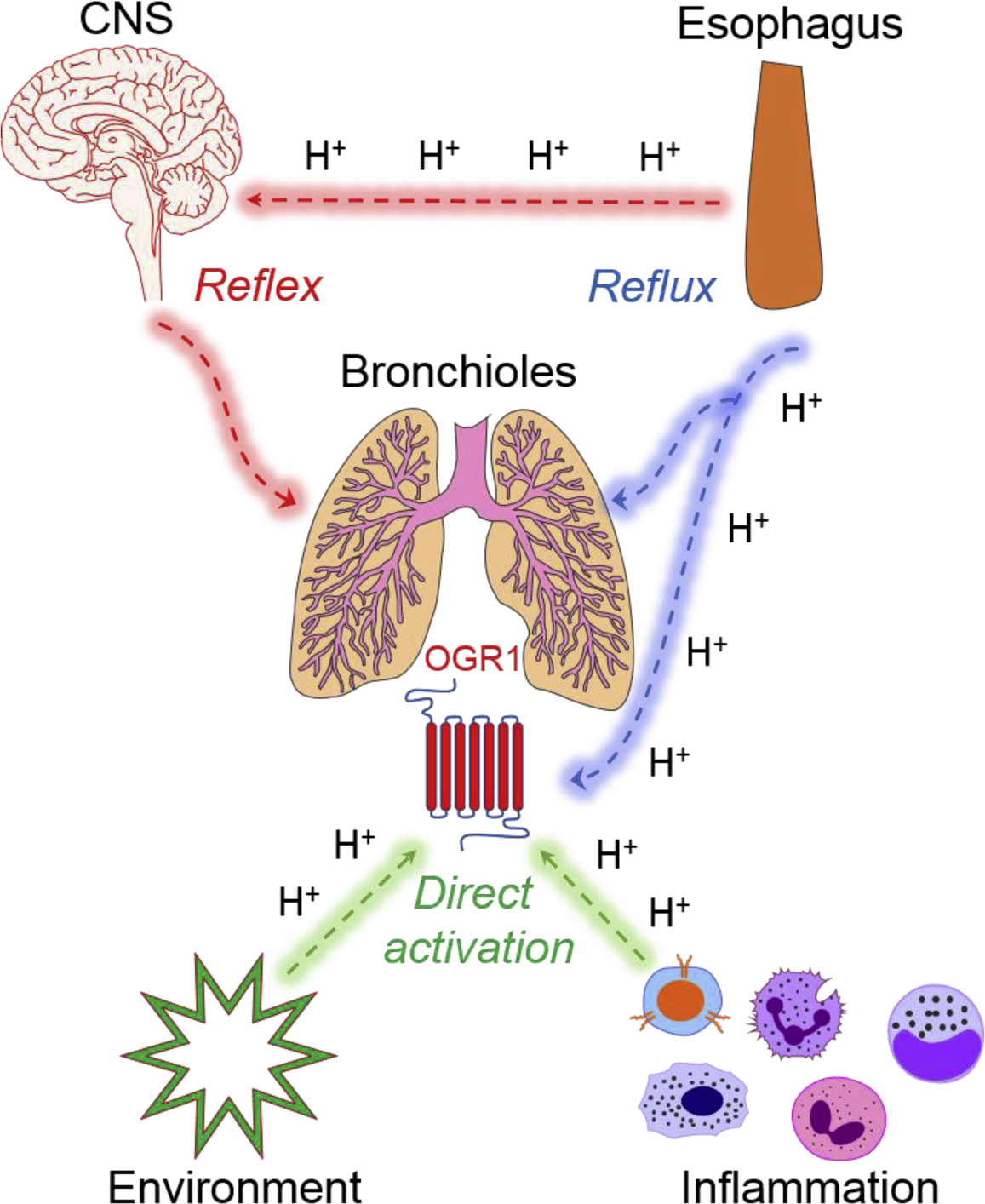
Esophageal acid (protons, H+) is the core component that drives bronchoconstriction [3] in airways through 3 distinct mechanisms. The first mechanism involves activation of the vagal reflex by action of H+ on esophageal afferent sensory neurons that direct a CNS mediated ‘reflex’ response, which culminates in a bronchoconstriction response. The second mechanism involves microaspiration or ‘reflux’ of esophageal acid into the airways which subsequently activates capsaicin-sensitive sensory neurons. Alternatively, aspirated H+ can directly activate OGR1 receptors expressed on the ASM. Finally, bronchoconstriction can also be stimulated through ‘direction activation’ of bronchial OGR1 during airway inflammation or from inhalation of toxic environmental agents (e.g. acid fog).
OGR1 regulates ASM functions critical to the regulation of airway resistance
Not surprisingly, early efforts to establish the functional role of OGR1 (and other proton-sensing GPCRs) focused on putative roles in cancer and bone [16–19]. However, beyond studies suggesting increased OGR1 mRNA expression in certain cancers, evidence for a functional role of OGR1 in either normal or pathophysiology was lacking for years; indeed most studies employing OGR1 knockout mice reported minimal if any positive data [19–21]. However, among the early studies of OGR1 was a report in 2005 [22] of OGR1 expression and signaling in vascular smooth muscle cells, and since this report, studies of OGR1 in smooth muscle cells have arguably provided the most insight into the signaling, function, and regulation of OGR1. Others and we have established OGR1 is expressed in ASM cells and stimulates pro-contractile signaling in response to reductions in extracellular pH [23,24]. Accordingly, these finding place OGR1 within the group of (numerous established and emerging) GPCRs whose therapeutic targeting with agonists, antagonists, or biased ligands (see below) might effectively manage asthma.
Tomura et al. and later Liu et al. demonstrated prostaglandin (PGI2) and cAMP accumulation in response to acidic pH (through OGR1) which appeared to be COX-dependent [22,25]. Saxena et al. [23] showed that modest decreases in extracellular media pH progressively promoted contraction in cultured ASM cells, and effect inhibited by knockdown of OGR1 with siRNA (Figure 3). Subsequent studies have shown that ASM cells secrete IL-6 after being subjected to low extracellular pH [26]. A more recent study demonstrated acid-induced CXCL8 induction in human ASM cells which was PKC-and MEK1/2 dependent, and reversed by dexamethasone pretreatment [27]. The collective signaling analyses across multiple studies [22,23,25,26,28,30] have revealed OGR1 to be somewhat distinct (beyond being a protein-sensing GPCR) in its ability to signal via both the Gq and Gs signaling pathways. Whereas the GPCRs such as BK receptors are also capable in ASM of stimulating Gq-mediated calcium mobilization (which is pro-contractile) and Gs signaling (which is pro-relaxant) simultaneously, they effect Gs signaling by inducing COX-derived prostaglandins including PGE2, which activate Gs via EP2 or EP4 receptors on ASM [31–34]. Although OGR1 can similarly stimulate COX-derived prostaglandins, it also can stimulate cAMP generation in the presence of COX inhibitors (Figure 3) suggesting the ability of OGR1 to couple directly to both Gq and Gs, a fairly rare property also ascribed to the (thromboxane) TP receptor [35]. Importantly, this diversity in signaling raises the possibility of differentially regulating OGR1 by biased ligands.
Figure 3. Acid-mediated effects on signaling and function in primary human ASM cells (and OGR1 requirement).
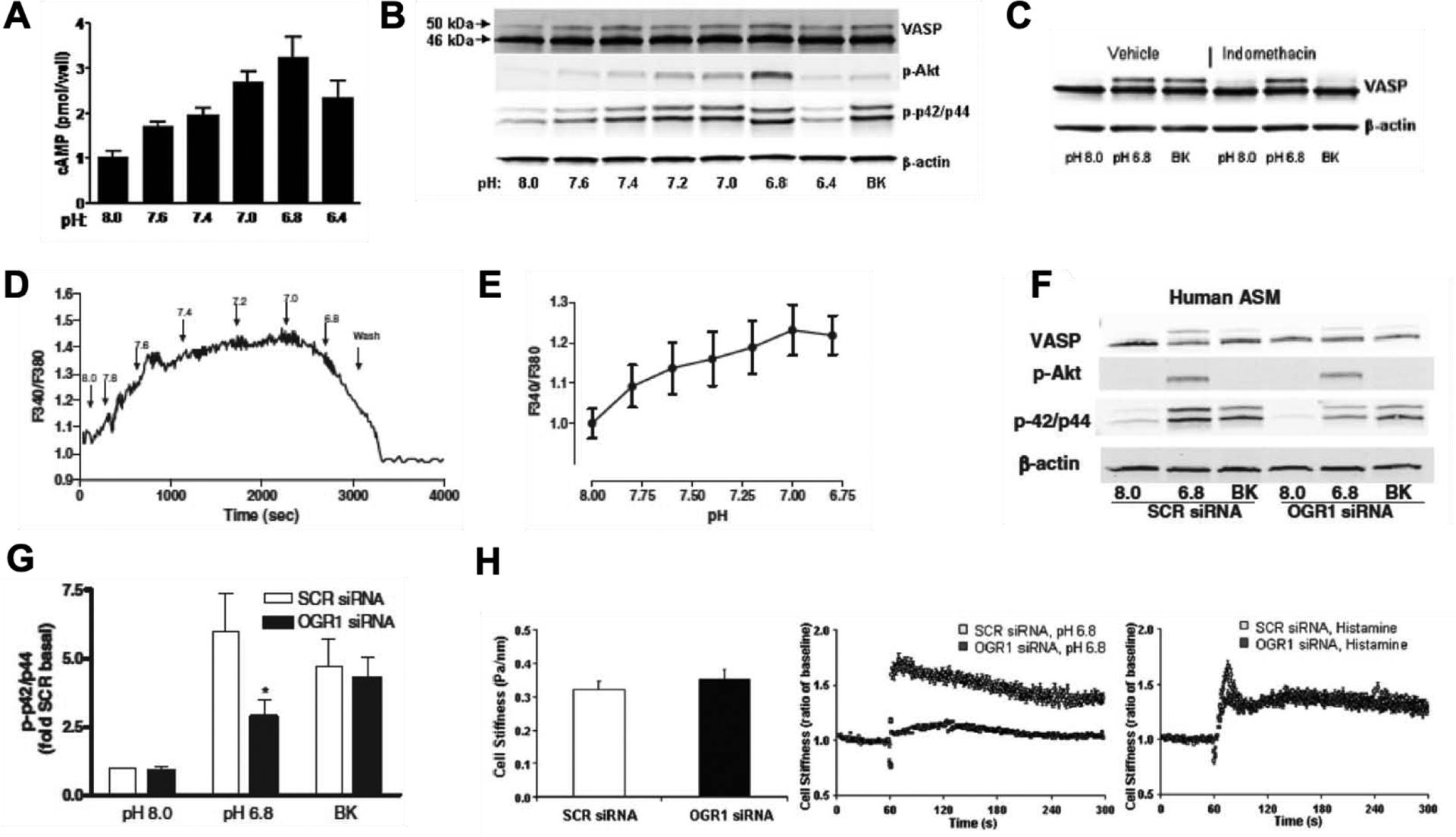
Primary human ASM cells were treated with bradykinin (BK; 100 nM) or HCl to induce ↓pHo conditions as indicated in figure panel. Dose-dependent effects of ↓pHo on A. cellular cAMP levels as determined by radioimmunoassay (RIA) and B. expression of VASP (vasodilator-stimulated phosphoprotein), phospho-p42/44 and phospho-protein kinase B (pAkt), determined by western blot analysis. C. Effect of inhibition of cyclooxygenases (COX) on acid-induced signaling in ASM cells. ASM cells were treated with indomethacin (non-selective COX inhibitor; 10 μM) prior to stimulation with ↓pHo conditions and examined for expression of VASP. D. Single cell mobilization of calcium following stimulation of ASM cells with ↓pHo conditions and E. Mean calcium mobilization ± S.E. (standard error) from 6 different ASM lines. F. ASM cells transfected with OGR1 siRNA on stimulation with ↓pHo conditions demonstrated attenuation of phospho-p42/44 signals and G. Mean phospho-p42/44 ± S.E. from 6 different experiments of OGR1 knockdown. H. OGR1 requirement in acid-mediated effects examined using magnetic twisting cytometry (MTC) approach to study single cell contraction. Baseline cells stiffness in ASM cells transfected with scrambled (SCR) or OGR1 siRNA (left panel), difference in cell stiffness induced by treatment of cells with pH 6.8 conditions (middle panel) and difference in cell stiffness induced by treatment of cells with histamine (right panel). All data sets are reprinted from Saxena et al. 2012 [23].
The challenges in researching OGR1
Beyond the historical lack of research into OGR1 (meaning there is little to build on) and the controversial nature of certain published studies to date (early study has been retracted [36]), the major challenges of studying OGR1 are derived from the assumption that the proton is the cognate ligand of OGR1. Clearly, one of the most ubiquitous and promiscuous things (particle, to be specific) in nature being a GPCR agonist goes against the fundamental principles of GPCR pharmacology and control systems engineering of the human body. Nature typically designs an agonist to enable a significant level of discrimination/selectivity by a given GPCR, enabling a cell, tissue, organ system, or integrated organ system response that meets the homeostasis needs of the organism across a wide range of stimuli. In the study of proton-sensing GPCRs, it serves a practical purpose to start with the assumption that the proton is the cognate receptor for these receptors. However, it is likely that despite its apparent ability to be sufficient to activate proton-sensing GPCRs, the proton is but one of several factors affecting responsiveness of these receptors. Thus, a major challenge of all research into proton-sensing GPCRs is to identity other endogenous (or synthetic) factors that function as orthosteric ligands or allosteric modulators under conditions of varied extracellular pH.
Other challenges, beyond the lack of pharmacological tools, exist in the study of a receptor activated by reduced pH/protons. For example, there exists a need to impose numerous controls to exclude nonspecific actions of ↓pHo or indirect activation of the receptor. The inherent difficulty of working with a low abundance GPCR in a primary cell type has also been challenging. pH changes and protonation may also have effects on plasma membrane and intracellular signaling elements as demonstrated for the Gs protein [37], although this seems unlikely to occur under ↓pHo conditions studied in our recent publication [29]. While stimulation of cells overexpressing OGR1 or endogenous OGR1 results in calcium mobilization, this phenomenon can be influenced by presence of acid-sensing ion channels and transient receptor potential vanilloid receptor (TRPV) and other proton-sensing receptors [38]. Finally, a lack of useful antibodies for OGR1 has limited our ability to study receptor function and regulation in physiological systems or restricted it to analysis of gene expression [23,28,29].
With such challenges stemming for the regulatory role of pH on OGR1 activity, the amount (and reliability) of OGR1 research was limited for years. However, in 2015 using a yeast screening approach of GPCR of GPCR ligand discovery Huang et al. identified a number of benzodiazepines that could activate OGR1 [39]. The discovery of such ligands/allosteric modulators, which could be employed at a set pH, has facilitated the much-needed experimental control in cell-based systems to enable interpretable studies of OGR1 signaling and function. Subsequent studies in our laboratory have revealed the biased nature of GPR1 signaling, with diverse signals linked to qualitatively distinct functional outcomes [28]. Specifically, we demonstrated that while lower pH conditions and lorazepam exhibit no bias (i.e., balanced signaling) for OGR1 coupling to a specific heterotrimeric G protein, sulazepam selectively activates the canonical Gs of the G protein signaling pathway, in heterologous expression systems, as well as in several primary cell types. Finally, although primarily investigated as allosteric modulators of OGR1, lorazepam and sulazepam can signal at high extracellular pH (pH 8.0; OGR1 inactive pH) suggesting that these benzodiazepines can function as orthosteric ligands.
In addition, insight into how OGR1 responsiveness is regulated was enabled by the discovery of lorazepam and sulazepam as OGR1 ligands. Under conditions of sustained agonist exposure, most GPCR undergo the phenomenon of desensitization (reduced responsiveness). However, Russell et al. reported that OGR1 expression increased in response to an acidic environment in a murine myocardial infarction model [40]. Specifically, OGR1 mRNA abundance increased significantly in the border zone, perhaps serving to encapsulate the infarct zone of the myocardium and limit damage from spreading to other parts of the cardiac tissue. The authors postulated that this acidosis-induced sensitization mechanism serves beneficial transcriptional programs that can be targeted by OGR1 activators to manage myocardial infarction.
To explore the robustness of this effect, we studied the regulation of ASM OGR1 in response to acute and chronic activation [29]. Interestingly, we found that chronic treatment of ASM cells with ↓pHo caused desensitization of OGR1 responsiveness, with no upregulation of OGR1 mRNA abundance. Moreover, in contrast to findings presented by Russell et al. [40], we found that myocardial infarction induction in mice resulted in downregulation of OGR1 mRNA in all dissected zones of the infarcted heart tissue. Finally, we also observed that regulation of OGR1 receptor internalization and signaling is agonist dependent. While acute treatment with ↓pHo and lorazepam (unbiased ligands of OGR1) resulted in rapid internalization of the receptor, sulazepam (Gs-biased ligand of OGR1) treatment did not induce receptor internalization.
OGR1 in the integrated airway environment
Most of our current efforts have focused on uncovering nuances of OGR1 signaling and regulation using artificial (HEK cells overexpressing OGR1) and physiologically relevant (endogenous OGR1 in multiple cells types) cell model systems [23,28,29]. However, studies on OGR1 in an integrated airway environment, where distinct cell types interact, are lacking. We have shown that OGR1 and other proton-sensing receptors are expressed in the epithelium [23]. Preliminary findings from our laboratory demonstrate that benzodiazepines can effectively reverse contractile response induced by methacholine (MCh) in human and murine precision cut lung slices [41]. Further, and consistent with the known effects of Gs versus Gq signaling in ASM, sulazepam is more effective than lorazepam in affecting this outcome. Indeed, we previously demonstrated a superior effect of sulazepam in relaxing ASM cells using magnetic twisting cytometry (MTC), suggesting direct activation of OGR1 on ASM dominates the bronchoregulatory effect of OGR1 activation in airway tissue. From a GPCR biology perspective, these observations highlight the power of biased signaling to turn a mixed bag of signaling into a restricted, therapeutically beneficial signal and functional outcome.
The race for additional, and ideally biased, OGR1 ligands
Given the increasing power of screening technologies, along with the increasing capabilities of structural biology, molecular modeling, and bioinformatics approaches to identify existing, and design new ligands and allosteric modulators of GPCRs, the field of GPCR biology is poised to benefit from an exciting new era of drug discovery. Results from recent studies have begun to build the foundation for better understanding OGR1 signaling and function, and the approaches noted above will further advance our armament of OGR1 ligands/allosteric modulators enabling even greater insight into OGR1 function and the therapeutic application of OGR1 drugs. Such promise is exemplified most recently in Foster et al [42], which employed integrated computational approaches to identify peptide activators of multiple GPCRs, including OGR1, which were validated by multiple cell-based models for characterizing receptor activation and signaling.
In summary, OGR1 has emerged as an intriguing regulator of ASM and airway biology relevant to obstructive lung diseases. Further discovery of OGR1 ligands/modulators will enable us to: 1) understand the role of pH and OGR1 in the normal and obstructed airway; 2) be able to link specific cellular signaling events to specific airway cell functions; and 3) ideally, “tune” OGR1 and manipulate ASM signaling such that pathogenic/pro contractile Gq is avoided while pro-relaxant Gs signaling is activated.
Figure 4. Benzodiazepine actions on OGR1 in ASM cells.
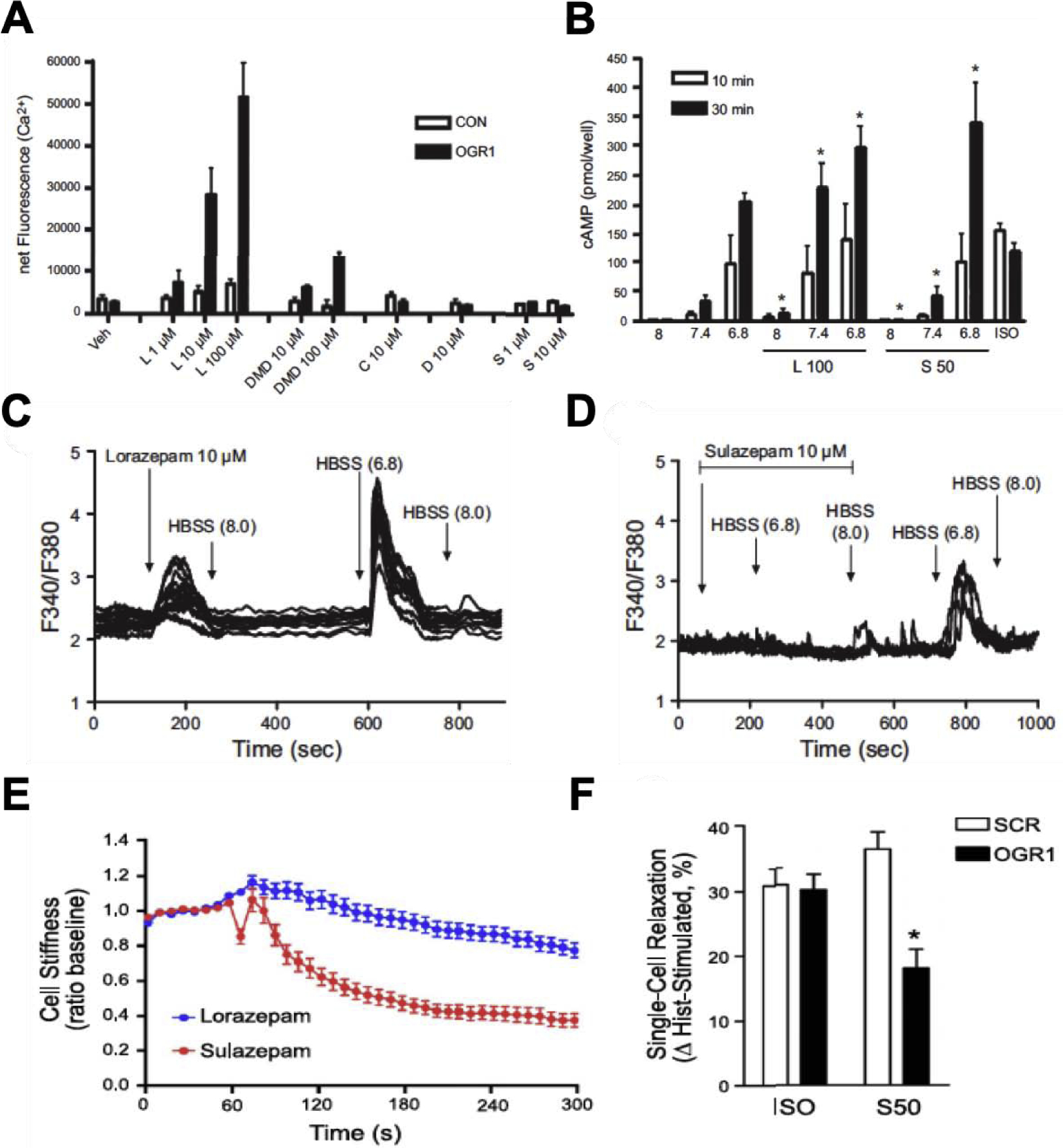
A. Calcium mobilization induced by benzodiazepines. ASM cells were loaded with calcium binding dye (Fluo-4AM) and stimulated with benzodiazepines, lorazepam (L; 1–100 μM), desmethyldiazepam (DMD; 10–100 μM), clobazam (C; 10 μM), diazepam (10 μM) or sulazepam (S; 1–10 μM). Calcium flux was measured using FlexStation. B. cAMP accumulation in ASM cells (measured by ELISA) following stimulation with benzodiazepines. L100: lorazepam 100 μM and S50: sulazepam 50 μM. C. Single cell calcium traces for calcium mobilization in ASM cells stimulated with lorazepam and D. sulazepam. Baseline calcium recordings were measured for cells in HBSS (Hank’s balanced salt solution). Arrows indicate introduction of specific stimulus using the superfusion system. E. Differential regulation of ASM contraction by benzodiazepines examined by single cell contraction assay (MTC). Sulazepam is more effective than lorazepam in inhibition of ASM contraction. F. Requirement of OGR1 for functional regulation of ASM contractile state by sulazepam. Reversal of ASM contraction by sulazepam (S50) is partially mediated by OGR1. SCR: scrambled siRNA and ISO: isoproterenol (1 μM). All data sets are reprinted from Pera et al. 2018 [28].
Acknowledgements
This study was funded by National Institutes of Health Heart, Lung, and Blood Institute Grant P01-HL-114471 (to R. B. P).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimers
Publisher's Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1.Deshpande DA, Penn RB: Targeting G protein-coupled receptor signaling in asthma. Cell Signal 2006, 18:2105–2120. [DOI] [PubMed] [Google Scholar]
- 2.Pera T, Penn RB: Bronchoprotection and bronchorelaxation in asthma: New targets, and new ways to target the old ones. Pharmacol Ther 2016, 164:82–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricciardolo FL, Gaston B, Hunt J: Acid stress in the pathology of asthma. J Allergy Clin Immunol 2004, 113:610–619. [DOI] [PubMed] [Google Scholar]
- 4.Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, Gaston B: Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med 2000, 161:694–699. [DOI] [PubMed] [Google Scholar]
- 5.Vaughan J, Ngamtrakulpanit L, Pajewski TN, Turner R, Nguyen TA, Smith A, Urban P, Hom S, Gaston B, Hunt J: Exhaled breath condensate pH is a robust and reproducible assay of airway acidity. Eur Respir J 2003, 22:889–894. [DOI] [PubMed] [Google Scholar]
- 6.Metheny NA, Stewart BJ, Smith L, Yan H, Diebold M, Clouse RE: pH and concentration of bilirubin in feeding tube aspirates as predictors of tube placement. Nurs Res 1999, 48:189–197. [DOI] [PubMed] [Google Scholar]
- 7.Fischer H, Widdicombe JH: Mechanisms of acid and base secretion by the airway epithelium. J Membr Biol 2006, 211:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hackett AP, Trinick RE, Rose K, Flanagan BF, McNamara PS: Weakly acidic pH reduces inflammatory cytokine expression in airway epithelial cells. Respir Res 2016, 17:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jayaraman S, Song Y, Verkman AS: Airway surface liquid pH in well-differentiated airway epithelial cell cultures and mouse trachea. Am J Physiol Cell Physiol 2001, 281:C1504–1511. [DOI] [PubMed] [Google Scholar]
- 10.Twort CH, Cameron IR: Effects of PCO2, pH and extracellular calcium on contraction of airway smooth muscle from rats. Respir Physiol 1986, 66:259–267. [DOI] [PubMed] [Google Scholar]
- 11.Folinsbee LJ: Human health effects of exposure to airborne acid. Environ Health Perspect 1989, 79:195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostikas K, Papatheodorou G, Ganas K, Psathakis K, Panagou P, Loukides S: pH in expired breath condensate of patients with inflammatory airway diseases. Am J Respir Crit Care Med 2002, 165:1364–1370. [DOI] [PubMed] [Google Scholar]
- 13.Ricciardolo FL, Rado V, Fabbri LM, Sterk PJ, Di Maria GU, Geppetti P: Bronchoconstriction induced by citric acid inhalation in guinea pigs: role of tachykinins, bradykinin, and nitric oxide. Am J Respir Crit Care Med 1999, 159:557–562. [DOI] [PubMed] [Google Scholar]
- 14.Satoh H, Lou YP, Lundberg JM: Inhibitory effects of capsazepine and SR 48968 on citric acid-induced bronchoconstriction in guinea-pigs. Eur J Pharmacol 1993, 236:367–372. [DOI] [PubMed] [Google Scholar]
- 15.Seuwen K, Ludwig MG, Wolf RM: Receptors for protons or lipid messengers or both? J Recept Signal Transduct Res 2006, 26:599–610. [DOI] [PubMed] [Google Scholar]
- 16.Yang M, Mailhot G, Birnbaum MJ, MacKay CA, Mason-Savas A, Odgren PR: Expression of and role for ovarian cancer G-protein-coupled receptor 1 (OGR1) during osteoclastogenesis. J Biol Chem 2006, 281:23598–23605. [DOI] [PubMed] [Google Scholar]
- 17.Pereverzev A, Komarova SV, Korcok J, Armstrong S, Tremblay GB, Dixon SJ, Sims SM: Extracellular acidification enhances osteoclast survival through an NFAT-independent, protein kinase C-dependent pathway. Bone 2008, 42:150–161. [DOI] [PubMed] [Google Scholar]
- 18.Singh LS, Berk M, Oates R, Zhao Z, Tan H, Jiang Y, Zhou A, Kirmani K, Steinmetz R, Lindner D, et al. : Ovarian cancer G protein-coupled receptor 1, a new metastasis suppressor gene in prostate cancer. J Natl Cancer Inst 2007, 99:1313–1327. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Wang D, Singh LS, Berk M, Tan H, Zhao Z, Steinmetz R, Kirmani K, Wei G, Xu Y: Abnormalities in osteoclastogenesis and decreased tumorigenesis in mice deficient for ovarian cancer G protein-coupled receptor 1. PLoS One 2009, 4:e5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan L, Singh LS, Zhang L, Xu Y: Role of OGR1 in myeloid-derived cells in prostate cancer. Oncogene 2014, 33:157–164. [DOI] [PubMed] [Google Scholar]
- 21.D’Souza CA, Zhao FL, Li X, Xu Y, Dunn SE, Zhang L: OGR1/GPR68 Modulates the Severity of Experimental Autoimmune Encephalomyelitis and Regulates Nitric Oxide Production by Macrophages. PLoS One 2016, 11:e0148439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomura H, Wang JQ, Komachi M, Damirin A, Mogi C, Tobo M, Kon J, Misawa N, Sato K, Okajima F: Prostaglandin I(2) production and cAMP accumulation in response to acidic extracellular pH through OGR1 in human aortic smooth muscle cells. J Biol Chem 2005, 280:34458–34464. [DOI] [PubMed] [Google Scholar]
- 23.Saxena H, Deshpande DA, Tiegs BC, Yan H, Battafarano RJ, Burrows WM, Damera G, Panettieri RA, Dubose TD Jr., An SS, et al. : The GPCR OGR1 (GPR68) mediates diverse signalling and contraction of airway smooth muscle in response to small reductions in extracellular pH. Br J Pharmacol 2012, 166:981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Faisy C, Planquette B, Naline E, Risse PA, Frossard N, Fagon JY, Advenier C, Devillier P: Acid-induced modulation of airway basal tone and contractility: role of acid-sensing ion channels (ASICs) and TRPV1 receptor. Life Sci 2007, 81:1094–1102. [DOI] [PubMed] [Google Scholar]
- 25.Liu JP, Komachi M, Tomura H, Mogi C, Damirin A, Tobo M, Takano M, Nochi H, Tamoto K, Sato K, et al. : Ovarian cancer G protein-coupled receptor 1-dependent and -independent vascular actions to acidic pH in human aortic smooth muscle cells. Am J Physiol Heart Circ Physiol 2010, 299:H731–742. [DOI] [PubMed] [Google Scholar]
- 26.Ichimonji I, Tomura H, Mogi C, Sato K, Aoki H, Hisada T, Dobashi K, Ishizuka T, Mori M, Okajima F: Extracellular acidification stimulates IL-6 production and Ca(2+) mobilization through proton-sensing OGR1 receptors in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 2010, 299:L567–577. [DOI] [PubMed] [Google Scholar]
- 27.Kadowaki M, Yamada H, Sato K, Shigemi H, Umeda Y, Morikawa M, Waseda Y, Anzai M, Kamide Y, Aoki-Saito H, et al. : Extracellular acidification-induced CXCL8 production through a proton-sensing receptor OGR1 in human airway smooth muscle cells: a response inhibited by dexamethasone. J Inflamm (Lond) 2019, 16:4. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study further advances the concept of low extracellular pH and OGR1 as mediators of airway smooth muscle synthetic functions that promote airway inflammation. Interestingly, this study reveals the ability of dexamethasone to inhibit acid-induced CXCL8 in cultured ASM, whereas Hunt et al. (ref # 4) previously reported that corticosteroid treatment of asthmatics undergoing an acute asthmatic attack not only treats asthma symptoms but also reverses airway lining fluid acidity. Thus, corticosteroids may affect multiple elements of a complex interplay between ASM and the acid airway microenvironment that modulates airway inflammation.
- 28.Pera T, Deshpande DA, Ippolito M, Wang B, Gavrila A, Michael JV, Nayak AP, Tompkins E, Farrell E, Kroeze WK, et al. : Biased signaling of the proton-sensing receptor OGR1 by benzodiazepines. FASEB J 2018, 32:862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This is the first study detailing biased signaling by OGR1; the benzodiazepine sulazepam was determined to function as a Gs-biased ligand, whereas lorazepam exhibited balanced (Gs, Gq) signaling. The findings highlight the potential power of biased ligand pharmacology for manipulating receptor signaling to preferentially activate therapeutically beneficial pathways.
- 29.Nayak AP, Pera T, Deshpande DA, Michael JV, Liberato JR, Pan S, Tompkins E, Morelli HP, Yi R, Wang N, et al. : Regulation of ovarian cancer G protein-coupled receptor-1 expression and signaling. Am J Physiol Lung Cell Mol Physiol 2019, 316:L894–L902. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This paper details the regulatory (desensitization/sensitization) features of OGR1 using a combination of physiologically relevant cellular and in vivo model systems. OGR1 was shown to desensitize, not sensitize as previously suggested, in an agonist and G protein-dependent manner.
- 30.Tomura H, Wang JQ, Liu JP, Komachi M, Damirin A, Mogi C, Tobo M, Nochi H, Tamoto K, Im DS, et al. : Cyclooxygenase-2 expression and prostaglandin E2 production in response to acidic pH through OGR1 in a human osteoblastic cell line. J Bone Miner Res 2008, 23:1129–1139. [DOI] [PubMed] [Google Scholar]
- 31.Pang L, Knox AJ: PGE2 release by bradykinin in human airway smooth muscle cells: involvement of cyclooxygenase-2 induction. Am J Physiol 1997, 273:L1132–1140. [DOI] [PubMed] [Google Scholar]
- 32.Tilley SL, Hartney JM, Erikson CJ, Jania C, Nguyen M, Stock J, McNeisch J, Valancius C, Panettieri RA Jr., Penn RB, et al. : Receptors and pathways mediating the effects of prostaglandin E2 on airway tone. Am J Physiol Lung Cell Mol Physiol 2003, 284:L599–606. [DOI] [PubMed] [Google Scholar]
- 33.Michael JV, Gavrila A, Nayak AP, Pera T, Liberato JR, Polischak SR, Shah SD, Deshpande DA, Penn RB: Cooperativity of E-prostanoid receptor subtypes in regulating signaling and growth inhibition in human airway smooth muscle. FASEB J 2019, 33:4780–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Misior AM, Deshpande DA, Loza MJ, Pascual RM, Hipp JD, Penn RB: Glucocorticoid- and protein kinase A-dependent transcriptome regulation in airway smooth muscle. Am J Respir Cell Mol Biol 2009, 41:24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Billington CK, Penn RB: Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res 2003, 4:2. [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, Zhu K, Hong G, Wu W, Baudhuin LM, Xiao Y, Damron DS: Sphingosylphosphorylcholine is a ligand for ovarian cancer G-protein-coupled receptor 1. Nat Cell Biol 2000, 2:261–267. [DOI] [PubMed] [Google Scholar]
- 37.Isom DG, Dohlman HG: Buried ionizable networks are an ancient hallmark of G protein-coupled receptor activation. Proc Natl Acad Sci U S A 2015, 112:5702–5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu YL, Mi X, Huang C, Wang HF, Song JR, Shu Q, Ni L, Chen JG, Wang F, Hu ZL: Multiple H(+) sensors mediate the extracellular acidification-induced [Ca(2+)]i elevation in cultured rat ventricular cardiomyocytes. Sci Rep 2017, 7:44951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang XP, Karpiak J, Kroeze WK, Zhu H, Chen X, Moy SS, Saddoris KA, Nikolova VD, Farrell MS, Wang S, et al. : Allosteric ligands for the pharmacologically dark receptors GPR68 and GPR65. Nature 2015, 527:477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russell JL, Goetsch SC, Aguilar HR, Coe H, Luo X, Liu N, van Rooij E, Frantz DE, Schneider JW: Regulated expression of pH sensing G Protein-coupled receptor-68 identified through chemical biology defines a new drug target for ischemic heart disease. ACS Chem Biol 2012, 7:1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nayak AP, Boopathi E, Pan S, Yi R, Javed E, Michael J, Pera T, Tompkins E, Liberato J, Deshpande DA, et al. : Potential role for the peripheral benzodiazepine receptor (PBR) in evoking airway smooth muscle relaxation. In American Thoracic Society: 2018:A1217. [Google Scholar]
- 42.Foster SR, Hauser AS, Vedel L, Strachan RT, Huang XP, Gavin AC, Shah SD, Nayak AP, Haugaard-Kedstrom LM, Penn RB, et al. : Discovery of Human Signaling Systems: Pairing Peptides to G Protein-Coupled Receptors. Cell 2019, 179:895–908 e821. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Using a combination of bioinformatics approach and distinct orthogonal biochemical methods, this study pairs endogenous peptides with OGR1. This is the first report to identify peptides that are involved in physiological and pathophysiological processes as agonists for OGR1, and advances the foundation for future rational drug design of OGR1 ligands.


