Abstract
Background:
Most endometrial cancer cases are preceded by abnormal uterine bleeding, offering a potential opportunity for early detection and cure of endometrial cancer. While clinical guidelines exist for diagnostic workup of abnormal uterine bleeding, consensus is lacking regarding optimal management for women with abnormal bleeding to diagnose endometrial cancer.
Objective:
We report the baseline data from a prospective clinical cohort study of women referred for endometrial evaluation at the Mayo Clinic, designed to evaluate risk stratification in women at increased risk for endometrial cancer. Here, we introduce a risk-based approach to evaluate diagnostic tests and clinical management algorithms in a population of women with abnormal bleeding undergoing endometrial evaluation at the Mayo Clinic.
Study Design:
A total of 1,163 women aged ≥45 years were enrolled from February 2013-May 2019. We evaluated baseline absolute risks and 95% confidence intervals of endometrial cancer and endometrial intraepithelial neoplasia according to clinical algorithms for diagnostic workup of women with postmenopausal bleeding (assessment of initial vs. recurrent bleeding episode and endometrial thickness measured via transvaginal ultrasound). We also evaluated risks among women with postmenopausal bleeding according to baseline age (<60 vs. 60+ years) as an alternative example. For this approach, biopsy would be conducted for all women aged 60+ years and those aged <60 years with an endometrial thickness of >4mm. We assessed the clinical efficiency of each strategy by estimating the percent of women who would be referred for endometrial biopsy, the percent of cases detected and missed, and the ratio of biopsies per case detected.
Results:
Among the 593 women with postmenopausal bleeding, 18 (3.0%) had endometrial intraepithelial neoplasia and 47 (7.9%) had endometrial cancer and among the 570 premenopausal women with abnormal bleeding, 8 (1.4%) had endometrial intraepithelial neoplasia and 7 (1.2%) had EC. Risks were highest in women aged 60+ years 17.7% (13.0–22.3%), followed by those with recurrent bleeding (14.7%, 11.0–18.3%). Among women with an initial bleeding episode in whom transvaginal ultrasound is recommended, endometrial thickness did not provide meaningful risk stratification: risks of endometrial cancer/endometrial intraepithelial neoplasia were nearly identical in women with an endometrial thickness of >4mm (5.8%; 1.3–10.3%) and ≤4mm (3.6%; 0.9–8.6%). In contrast, among those aged <60 years with an endometrial thickness of >4mm, the risk of endometrial cancer/endometrial intraepithelial neoplasia was 8.4% (4.3–12.5%) and in those with an endometrial thickness of ≤4mm, the risk was 0% (0.0–3.0%; p=0.010). The most efficient strategy was to perform biopsy in all women aged 60+ years and among those aged <60 years with an endometrial thickness of >4mm, with the lowest percentage referred to biopsy while still detecting all cases.
Conclusions:
Current clinical recommendations for endometrial cancer detection in women with abnormal bleeding are not consistent with underlying risk. Endometrial cancer risk factors such as age can provide important risk stratification compared to the assessment of recurrent bleeding. Future research will include a formal assessment of clinical and epidemiologic risk prediction models in our study population as well as validation of our findings in other populations.
Keywords: Endometrial cancer, endometrial intraepithelial neoplasia, abnormal bleeding, postmenopausal bleeding, transvaginal ultrasound, endometrial biopsy, risk assessment
Condensation:
A risk-based approach to evaluating diagnostic testing for endometrial cancer in women with abnormal bleeding suggests that practice recommendations may not reflect underlying risk.
Introduction
Endometrial cancer (EC) incidence and mortality rates have increased over the past decade1, 2, with 61,880 cases and 12,160 deaths occurring in the U.S. in 2019.3 Approximately 80% of ECs present with stage I disease, with high 5-year survival. In contrast, 5-year survival for stage III and stage IV cancers is 69% and 16%, respectively, underscoring the promise of early detection.4–6 Approximately 90% of EC cases are preceded by abnormal uterine bleeding in premenopausal or perimenopausal women (AUB) or postmenopausal bleeding (PMB), offering a potential opportunity to detect and cure ECs that are destined to metastasize with delayed diagnosis..7, 8 However, abnormal bleeding is a common symptom of many benign diseases, and only indicates the presence of EC in approximately 9% of postmenopausal women and 1–2% of premenopausal women7, 9, suggesting that many women at low-risk undergo unnecessary invasive diagnostic procedures to rule out cancer.
In the U.S., methods for endometrial evaluation include transvaginal ultrasound (TVUS) and endometrial sampling, with or without hysteroscopy10–12; however, there is no consensus regarding the optimal sequence or combination of these procedures.8 Absolute risk estimates are important for guiding clinical management and for evaluating new biomarkers and risk prediction tools as they are developed13; however, they have not been systematically evaluated in clinical populations.
We designed a prospective clinical cohort study to evaluate endometrial cancer risk prediction approaches among women with abnormal bleeding undergoing diagnostic evaluation at the Mayo Clinic. We present a baseline analysis evaluating a risk-based approach to clinical management algorithms in this population.
Materials and Methods
Study Population
We conducted a cross-sectional analysis of baseline data from our prospective clinical cohort study of women undergoing endometrial evaluation at the Mayo Clinic.14 Women presenting to the Mayo Clinic’s Department of Obstetrics and Gynecology were prospectively enrolled from February 21, 2013 through May 21, 2019 with passive follow-up via electronic medical record abstraction every 6 to 12 months. The current analysis includes data from the enrollment visit only. Eligible women included those ≥45 years with pre- or perimenopausal AUB or PMB. AUB was defined by symptoms of heavy menstrual bleeding, intermenstrual bleeding, menometrorrhagia, irregular menses, or other AUB among women aged ≥45 years who were not in menopause. Exclusion criteria included prior hysterectomy, prior pelvic radiation, endometrial sampling within the past 3 months, current pregnancy, and inadequate endometrial sampling. This study was approved by the Mayo Clinic and National Cancer Institute Institutional Review Boards; written informed consent was obtained from all participants prior to study enrollment.
Clinical Evaluation
Women underwent clinical evaluation of the endometrium as determined by their care provider. Clinical testing included any combination of the following: TVUS, office hysteroscopy, and office endometrial biopsy. If complete workup in the clinic was not feasible, women underwent assessment under anesthesia via hysteroscopy and dilation and curettage. Hysterectomy was performed if clinically indicated.
Transvaginal Ultrasound.
TVUS was performed as clinically indicated and interpreted by radiologists specialized in pelvic ultrasound. Endometrial stripe thickness (ET) was reported in millimeters (mm).
Hysteroscopy.
Office hysteroscopy using a flexible 3.1 mm diagnostic scope was performed per the clinician’s discretion taking into account patient age, imaging findings, and clinical feasibility.
Endometrial Sampling.
All women enrolled in this study were anticipated to undergo diagnostic evaluation via endometrial sampling. This was performed as a clinic endometrial biopsy with a Pipelle (Pipelle® CooperSurgical, Turnbull, CT) or Endosampler device (MedGyn, Addison, IL) or as a D&C if clinic biopsy was not feasible. Pathology diagnoses were made clinically by gynecologic pathologists.
Outcome Definitions
Among women who did not require a hysterectomy, the final pathology diagnosis of endometrial biopsy or curettage was considered the reference diagnosis. Among women who underwent hysterectomy, the most severe pathology diagnosis on either the endometrial sampling or hysterectomy specimen was considered the final study diagnosis. Women deemed not to require endometrial biopsy were included in the benign group (N=110). Of these, 105 (95.5%) were confirmed to be at very low risk based on being premenopausal (42.7%) or having PMB with an ET of ≤4mm and/or a benign finding on hysteroscopy (52.7%). Removal of the remaining 5 women from risk estimate calculations did not change the results in a sensitivity analysis (not shown).
Final endometrial pathology diagnoses from the baseline visit were classified as: Benign, including women with a normal endometrium, endometrial polyps, disordered proliferative endometrium (DPEM), endometrial hyperplasia without atypia, or other benign histologic findings (e.g., endometritis, fibroids); endometrial intraepithelial neoplasia (EIN), including all diagnoses of hyperplasia with atypia, and EC.
Endometrial Cancer Risk Factors and Clinical Characteristics
Information on clinical risk factors for EC including the indication for endometrial sampling, bleeding type, age, race and ethnicity, body mass index (BMI; categorized as underweight or normal weight (<25 kg/m2), overweight (25 to <30 kg/m2) and obese (≥30 kg/m2))15, hypertension, type II diabetes, and smoking history were abstracted from electronic medical records. Other epidemiologic risk factors including tamoxifen exposure, current hormone use (combined progestin and estrogen formulations), past oral contraception (OC) use, and parity were ascertained via patient interview. Among postmenopausal women, PMB was characterized as either initial episode of bleeding or recurrent bleeding (any occurrence of bleeding after prior evaluation for PMB).
Clinical Algorithms for Evaluating Women with PMB and AUB
We used the current guidelines from the American College of Obstetricians and Gynecologists (ACOG) which recommend either endometrial sampling or TVUS for evaluation of women with an initial episode of PMB. If TVUS is performed and the resulting ET measurement is ≤4mm, endometrial sampling is not required. In women with an ET >4mm, endometrial tissue sampling is recommended. Among women with recurrent PMB, endometrial sampling is recommended regardless of ET.11, 12 For pre- or perimenopausal women with AUB who are ≥45 years, endometrial sampling is recommended as a first-line test.10 In this study, diagnostic evaluations were not performed in the exact sequence as recommended by ACOG, but we simulated the sequential diagnostic process.
Statistical Analysis
We calculated absolute risks of detection at baseline (i.e. prevalent risk) of EC, EIN, and benign outcomes by select baseline characteristics, separately by menopausal status. Absolute risks were compared across baseline characteristics using Chi-square tests. Given that hysterectomy is standard of care for both EIN and EC, we evaluated combined baseline risks (EC/EIN) according to clinical algorithms for diagnostic workup of women with PMB and AUB. For women with PMB, risks were calculated as the proportion of women diagnosed with EC or EIN among all women in sequential strata defined as: bleeding pattern (initial or recurrent) and ET (≤4mm and >4mm). For women with AUB, we calculated the baseline risk of EC or EIN in all women. To illustrate how our analysis can be used to evaluate strategies for risk assessment, we calculated risks among women with PMB dichotomizing baseline age as <60 years vs. ≥60 years (determined a priori based on the average age of women diagnosed with endometrial cancer)16, as an alternative to the assessment of recurrent bleeding. We plotted the risk estimates for each strategy, with marker size weighted by the corresponding number of women in each stratum. For all analyses, we also separately evaluated risks for EC only and among women not currently using hormones. For each analysis, we compared absolute risk estimates using t-tests.
We evaluated the clinical efficiency of each strategy by estimating the percent of women referred for endometrial biopsy, the percent of EC/EIN detected and missed, and the number of biopsies required to detect an EC/EIN. All analyses were performed in Stata 15 and R 3.5.3. Statistical tests were two-sided with p<0.05 considered significant.
Results
In total, 2,782 eligible patients presented for EC evaluation at Mayo Clinic between February 2013 and May 2019. Of 1,460 women enrolled and consented, 1,163 women met inclusion criteria of being 45 years or older (mean 54.6 years) with either PMB or AUB (Figure 1).
Figure 1.
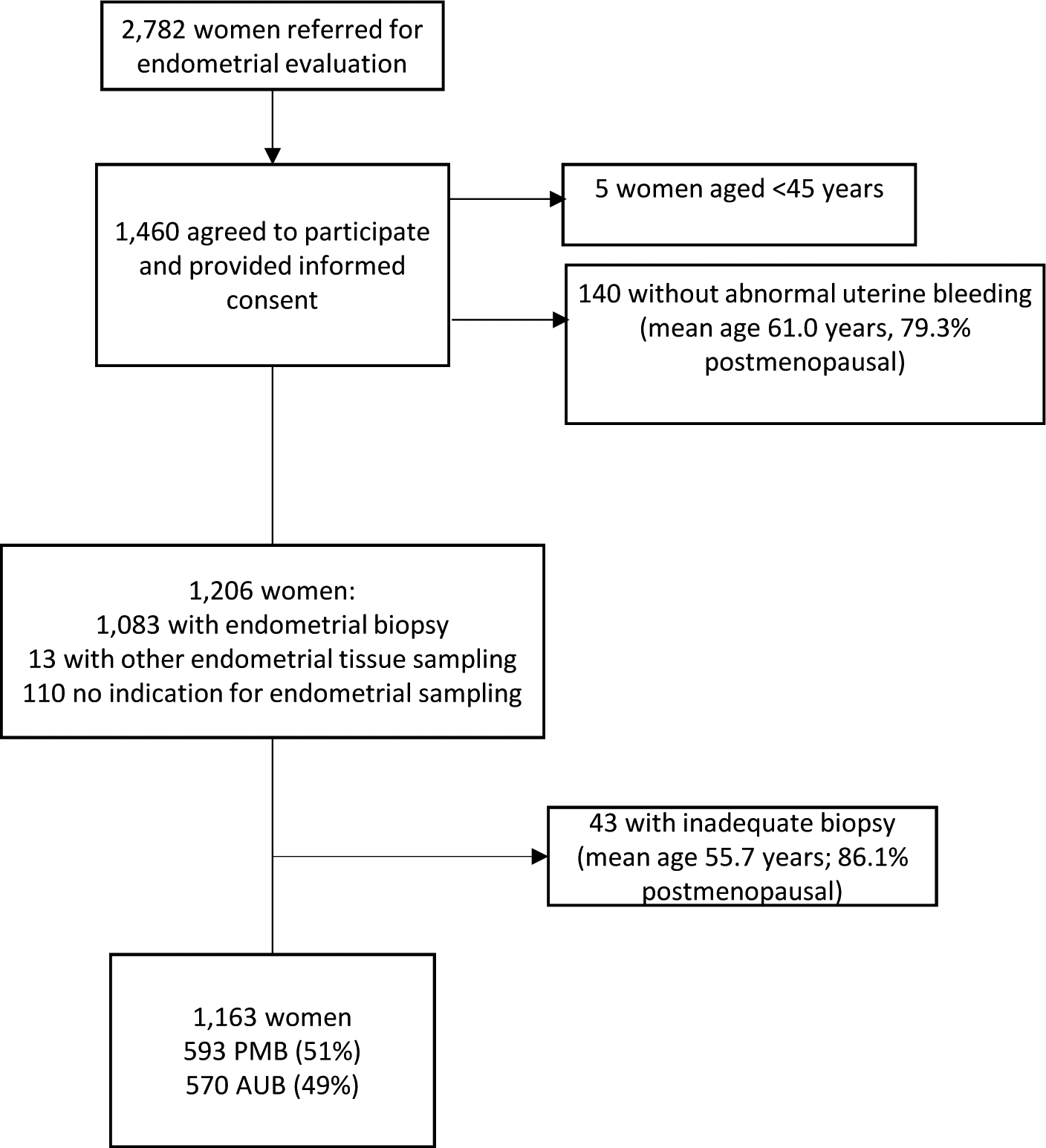
CONSORT Diagram of the study population
Baseline Characteristics and Risk of Worst Outcome Among Women with PMB and AUB
Among 593 women with PMB, 528 (89.0%) had a benign diagnosis, 18 had EIN, and 47 were diagnosed with EC (absolute risks of 3.0% and 7.9%, respectively) (Table 1). Absolute risks of EC were highest in women ≥60 years (14.6%; p-value<0.0001), in those not currently using hormones (10.1%; p-value=0.002), obese women (9.8%; p-value <0.0001), and women with type II diabetes (21.6%; p-value<0.0001). Women with recurrent PMB had higher risks of EIN and EC (4.5% and 10.1%, respectively) compared to those with an initial episode (0.5% and 5.1%, respectively; p-value=0.002). Among women with PMB and available ET measurements (n=430, 72.5%), the risks of EIN and EC were significantly higher in women with an ET of >4mm (4.2% and 10.1%, respectively) compared to those with ≤4mm (0% and 1.6%, respectively; p-value=0.001) (Table 1).
Table 1.
Baseline Characteristics Among Women with Abnormal Uterine Bleeding by Menopausal Status
| Postmenopausal Women (PMB) | Premenopausal Women (AUB) | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Benign | EINEn | EC | Total | Benign | EINEIN | EC | |
| Total | 593 (100.0) | 528 (89.0) | 18 (3.0) | 47 (7.9) | 570 (100.0) | 555 (97.4) | 8 (1.4) | 7 (1.2) |
| Age Group* | ||||||||
| <60 years | 339 (57.2) | 319 (94.1) | 10 (3.0) | 10 (3.0) | ||||
| 60+ years | 254 (42.8) | 209 (82.3) | 8 (3.1) | 37 (14.6) | ||||
| Age Group | ||||||||
| <50 years | 301 | 296 (98.3) | 1 (0.3) | 4 (1.3) | ||||
| 50+ years | 269 | 259 (96.3) | 7 (2.6) | 3 (1.1) | ||||
| Race/Ethnicity | ||||||||
| Non-Hispanic White | 557 (93.9) | 498 (89.4) | 17 (3.1) | 42 (7.5) | 527 (92.5) | 512 (97.2) | 8 (1.5) | 7 (1.3) |
| Non-Hispanic Black | 5 (0.8) | 4 (80.0) | 0 (0.0) | 1 (20.0) | 6 (1.0) | 6 (100.0) | 0 (0.0) | 0 (0.0) |
| Hispanic | 8 (1.4) | 7 (87.5) | 1 (12.5) | 0 (0.0) | 10 (1.8) | 10 (100.0) | 0 (0.0) | 0 (0.0) |
| Other | 23 (3.9) | 19 (82.6) | 0 (0.0) | 4 (17.4) | 27 (4.7) | 27 (100.0) | 0 (0.0) | 0 (0.0) |
| Body Mass Index* | ||||||||
| Normal | 167 (28.4) | 159 (95.2) | 0 (0.0) | 8 (4.8) | 176 | 173 (98.3) | 1 (0.6) | 2 (1.1) |
| Overweight | 165 (28.1) | 151 (91.5) | 1 (0.6) | 13 (7.9) | 167 | 166 (99.4) | 0 (0.0) | 1 (0.6) |
| Obese | 256 (43.5) | 214 (83.6) | 17 (6.6) | 25 (9.8) | 224 | 213 (95.1) | 7 (3.1) | 4 (1.8) |
| Current Hormone Use* | ||||||||
| No | 415 (70.1) | 357 (86.0) | 16 (3.9) | 42 (10.1) | 513 | 499 (97.3) | 7 (1.4) | 7 (1.4) |
| Yes | 177 (29.9) | 170 (96.1) | 2 (1.1) | 5 (2.8) | 55 | 54 (98.2) | 1 (1.8) | 0 (0.0) |
| Type II Diabetes* | ||||||||
| No | 542 (91.4) | 494 (91.1) | 12 (2.2) | 36 (6.6) | 544 | 530 (97.4) | 8 (1.5) | 6 (1.1) |
| Yes | 51 (8.6) | 32 (66.7) | 6 (11.8) | 11 (21.6) | 26 | 25 (96.2) | 0 (0.0) | 1 (3.9) |
| Smoking Status | ||||||||
| Never | 391 (65.9) | 345 (88.2) | 10 (2.6) | 36 (9.2) | 410 | 399 (97.3) | 5 (1.2) | 6 (1.5) |
| Former | 166 (6.1) | 149 (89.8) | 7 (4.2) | 10 (6.0) | 118 | 114 (96.6) | 0 (0.0) | 0 (0.0) |
| Current | 36 (28.0) | 34 (94.4) | 1 (2.8) | 1 (2.8) | 41 | 41 (100.0) | 3 (2.5) | 1 (0.9) |
| Past OC Use | ||||||||
| No | 163 (28.9) | 140 (85.9) | 8 (4.9) | 15 (9.2) | 134 | 129 (96.3) | 2 (1.5) | 3 (2.2) |
| Yes | 402 (71.1) | 366 (91.0) | 9 (2.2) | 27 (6.7) | 427 | 418 (97.9) | 5 (1.2) | 4 (0.9) |
| Parity# | ||||||||
| Nulliparous | 95 (16.0) | 84 (88.4) | 3 (3.2) | 8 (8.4) | 91 | 85 (93.4) | 3 (3.3) | 3 (3.3) |
| Parous | 498 (84.0) | 444 (89.2) | 15 (3.0) | 39 (7.8) | 478 | 469 (98.1) | 1 (1.1) | 4 (0.8) |
| Bleeding Amount (PMB) | ||||||||
| Spotting | 277 (47.6) | 241 (87.0) | 9 (3.3) | 27 (9.8) | ||||
| Light | 184 (31.6) | 169 (91.9) | 5 (2.7) | 10 (5.4) | ||||
| Heavy | 94 (16.2) | 83 (88.3) | 2 (2.1) | 9 (9.6) | ||||
| Other/Not specified | 27 (4.6) | 25 (92.6) | 1 (3.7) | 1 (3.7) | ||||
| Recurrent Bleeding* (PMB) | ||||||||
| No | 216 (37.8) | 204 (94.4) | 1 (0.5) | 11 (5.1) | ||||
| Yes | 355 (62.2) | 303 (85.4) | 16 (4.5) | 36 (10.1) | ||||
| Bleeding Type (AUB) | ||||||||
| Heavy menstrual bleeding | 183 | 180 (98.4) | 3 (1.6) | 0 (0.0) | ||||
| Intermenstrual bleeding | 123 | 121 (98.4) | 0 (0.0) | 2 (1.6) | ||||
| Menometrorrhagia | 216 | 206 (95.4) | 5 (2.3) | 5 (2.3) | ||||
| Irregular menses | 27 | 27 (100.0) | 0 (0.0) | 0 (0.0) | ||||
| Other AUB | 21 | 21 (100.00 | 0 (0.0) | 0 (0.0) | ||||
| Endometrial Thickness >4mm* | ||||||||
| No | 122 (20.6) | 120 (98.4) | 0 (0.0) | 2 (1.6) | 50 | 50 (100.0) | 0 (0.0) | 0 (0.0) |
| Yes | 308 (51.9) | 264 (85.7) | 13 (4.2) | 31 (10.1) | 294 | 283 (96.3) | 6 (2.0) | 5 (1.7) |
| Missing/Not Done | 163 (27.5) | 144 (88.3) | 5 (3.1) | 14 (8.6) | 226 | 222 (98.2) | 2 (0.9) | 2 (0.9) |
Chi2 p-value <0.05 for women with PMB;
Chi-sq p-value for women with AUB
Abbreviations: PMB, postmenopausal bleeding; AUB, abnormal uterine bleeding; EC, endometrial cancer; EIN, Endometrial Intraepithelial Neoplasia; OC, oral contraceptives
Among 570 premenopausal women with AUB, 555 had a benign diagnosis (97.4%), 8 had EIN and 7 had EC, corresponding to risks of 1.4% and 1.2%, respectively. Nulliparity was associated with a statistically significant increased risk of EC (p-value=0.035; Table 1).
Evaluation of Management Algorithms for PMB
We evaluated risks of EC/EIN following a sequential diagnostic process according to ACOG guidelines11. Absolute risk estimates for EC/EIN are shown in Figure 2. Among women with PMB, the risk in those with recurrent bleeding was 14.7% (95% CI=11.0–18.3%) compared to 5.6% (95% CI=2.5–8.6%) in women with an initial bleeding episode (p=0.0008). Among women with an initial bleeding episode, when TVUS is recommended as a first line evaluation, ET did not provide meaningful risk stratification: risks of EC/EIN were not significantly different in women with an ET of >4mm (5.8%; 95% CI=1.3–10.3%) versus those with an ET of ≤4mm (3.6%; 95% CI=0.9–8.6%). In contrast to those with an initial bleeding episode, TVUS provided additional risk stratification among women with recurrent bleeding such that women with an ET >4mm had the highest risk of EC/EIN (19.4%; 95% CI=13.8–25.0%) while in women with an ET of ≤4mm the risk was 0.0% (95% CI=0.0–6.0%; p=0.0001). In women presenting with recurrent bleeding and no ET measurement, the risk was 15.2% (95% CI=8.1–22.2%). We observed similar results for risk of EC alone (Supplemental Figure 1). Risks among women not currently using hormones were generally higher, although risk patterns were similar (Supplemental Figure 2).
Figure 2. Baseline Risk of Endometrial Cancer and Endometrial Intraepithelial Neoplasia according to bleeding type and endometrial thickness.
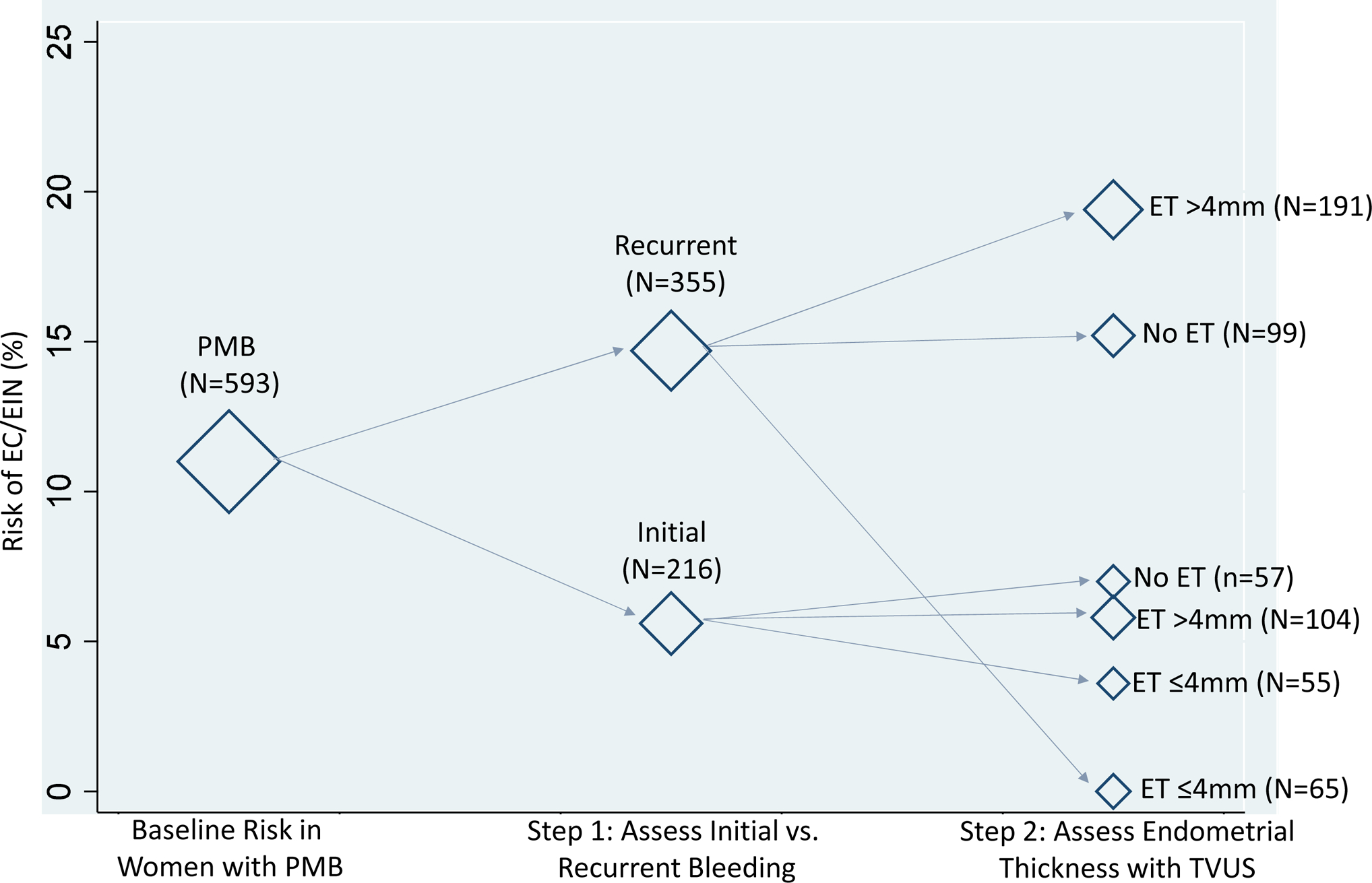
We evaluated the combined baseline risks of endometrial cancer and endometrial intraepithelial neoplasia combined following a sequential diagnostic process according to current guidelines from the American College of Obstetricians and Gynecologists11. Bleeding type was characterized as either initial episode of bleeding or recurrent bleeding (any occurrence of bleeding after prior evaluation for PMB). Endometrial thickness was measured clinically using transvaginal ultrasound. The diamond markers are weighted according to the corresponding number of women within each stratum of diagnostic test results. A total of 22 women are missing bleeding type information; the risk of EC/EIN in these women is 4.6%. Abbreviations: EC, endometrial cancer; EIN, Endometrial Intraepithelial Neoplasia; PMB, postmenopausal bleeding; ET, endometrial thickness; TVUS, transvaginal ultrasound
As an alternative to the current clinical recommendation, we evaluated using age (<60 and ≥60 years) instead of recurrent bleeding as the first risk stratifier (Figure 3). Among women aged ≥60 years, the risk of EC/EIN was 17.7% (95% CI=13.0–22.3%) compared to 5.9% (95% CI=3.4–8.4%) in women aged <60 years (p<0.0001). In those aged <60 years with an ET of >4mm, the risk of EC/EIN was 8.4% (95% CI=4.3–12.5%) and in those with an ET of ≤4mm, the risk was 0% (95% CI=0.0–3.0%; p=0.010). Among women aged ≥60 years with an ET >4mm the risk of EC/ EIN was 22.3% (95% CI=15.2–29.5%), compared to a risk of 4.2% (95% CI=1.2–14.0%) in those with an ET of ≤4mm (p=0.005). We observed similar results for risk of EC alone (Supplemental Figure 3). Risks among women not currently using hormones were generally higher, although risk patterns were similar (Supplemental Figure 4).
Figure 3. Baseline Risk of Endometrial Cancer and Endometrial Intraepithelial Neoplasia according to age and endometrial thickness.
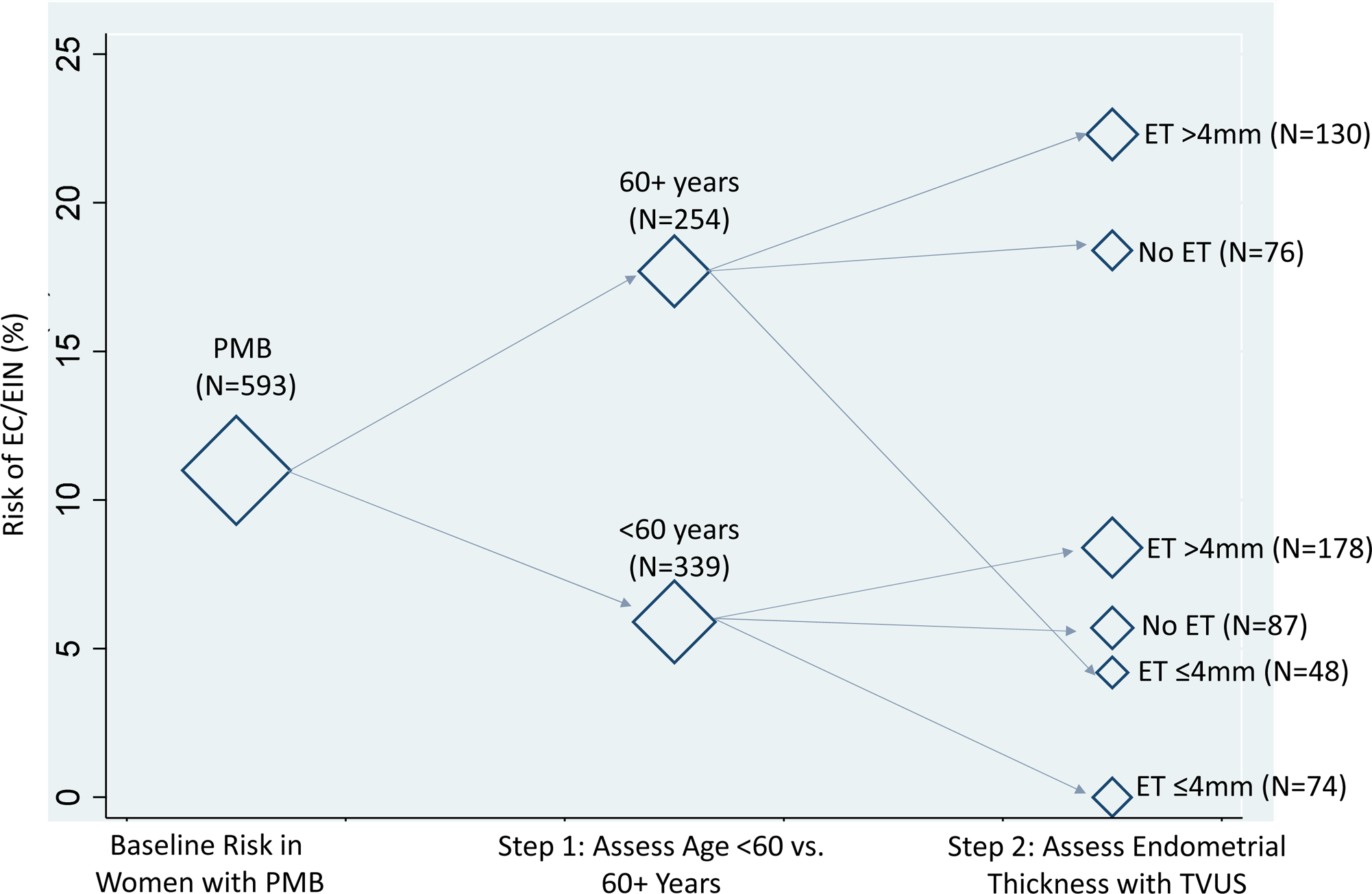
We evaluated the combined baseline risks of endometrial cancer and endometrial intraepithelial neoplasia combined using age at a cutoff of 60 years (<60 and ≥60) as an initial risk stratifier. Endometrial thickness was measured clinically using transvaginal ultrasound. The diamond markers are weighted according to the corresponding number of women within each stratum of diagnostic test results. Abbreviations: EC, endometrial cancer; EIN, Endometrial Intraepithelial Neoplasia; PMB, postmenopausal bleeding; ET, endometrial thickness; TVUS, transvaginal ultrasound
Clinical Efficiency of Algorithms for Diagnostic Evaluation of PMB and AUB
We evaluated implications of different clinical algorithms on detection of EC/EIN and number of biopsies performed among all women. First, we evaluated existing clinical recommendations for women with PMB11: When referring all women with PMB to biopsy, all women would undergo biopsy, with 100% of EC/EIN cases detected at a ratio of 9.7 biopsies per case (Table 2). In the second recommendation of referring all women with recurrent PMB as well as those with initial PMB and ET >4mm to biopsy, 87% of women would receive a biopsy with 97% disease detection at a ratio of 6.7 biopsies per case. In a third scenario that is currently not within ACOG recommendations, when referring all women aged ≥60 years and those aged <60 years with an ET of >4mm to biopsy, referral would be lowest (82%) with 100% disease detection at a ratio of 5.9 biopsies per case. Among premenopausal women aged ≥45 years with AUB, endometrial sampling is the only ACOG recommendation.10 All women would undergo biopsy with 100% of EC/EIN detected at a ratio of 38 biopsies per case.
Table 2.
Simulated Clinical Efficiency of Algorithms for Diagnostic Evaluation Among Women Presenting to Mayo Clinic with PMB and AUB
| Referral Algorithm | Immediate Biopsy | No Biopsy | Women with Biopsy (%) | EC/EIN Detected (%) | EC/EIN Missed (%) | Biopsies per EC/EIN Detected (%) |
|---|---|---|---|---|---|---|
| 1. Biopsy | All with PMB | 100% | 100% | 0 | 9.7 | |
| 2. TVUS in women with initial bleeding | Recurrent bleeding; Initial bleeding with ET >4mm | Initial bleeding, ET≤4mm | 87.3% | 96.7% | 3.3% | 6.7 |
| 3. TVUS in women aged <60 years | Aged 60+ years; Aged <60 years with ET >4mm | Aged <60 years, ET ≤4mm | 82.0% | 100% | 0% | 5.9 |
| 1. Biopsy | All with AUB 45+ years | 100% | 100% | 0 | 38 |
Abbreviations: PMB, postmenopausal bleeding; TVUS, transvaginal ultrasound; ET, endometrial thickness; AUB, abnormal uterine bleeding; EC, endometrial cancer; EIN, Endometrial Intraepithelial Neoplasia
Comment
Principal Findings
We applied a risk-based approach to evaluate diagnostic tests and recommended clinical management algorithms10, 11 to inform decision-making in women with abnormal bleeding. We demonstrate that current diagnostic strategies are not consistent with underlying risk of EC/EIN. For example, TVUS is recommended as a first line test in women with PMB, with biopsy indicated for those with an ET of >4mm.11 However, in our study, women had nearly the same risk of EC/EIN irrespective of ET. In contrast, among women with recurrent bleeding, TVUS provided good risk stratification such that those with a thickness of >4mm had a 1 in 5 risk of EC/EIN while no cases were found in women with a thickness of ≤4mm. To illustrate the principle of risk-based management, we evaluated an alternative approach using age with a cutoff of 60 years followed by TVUS. Among women with PMB aged <60 years, TVUS provided better risk stratification such that women with a thickness of ≤4mm would be safely reassured of 0% EC/EIN risk, while those with a thickness of >4mm had an 8% risk. Women aged ≥60 years with PMB had a EC/EIN risk of 17.7% that was similar to the risk among women with recurrent bleeding and an ET measurement of >4mm (19.4%).
Results in the Context of What is Known
Current guidelines recommend considering clinical risk factors such as age, obesity, and use of unopposed estrogen when evaluating PMB12; however, guidance lacks on how to integrate these factors for clinical-decision making. In our study, using age yielded better risk stratification compared to other clinical risk factors such as BMI; age is a highly reliable risk factor that is easily ascertained. While type II diabetes was associated with a very high absolute risk of EC, the prevalence of type II diabetes was low in our study, and most cancers occurred among women without type II diabetes. Risks among women not currently using hormones were higher compared to hormone users, consistent with the protective effects of combined estrogen/progestin formulations on the endometrium.17 Irregular uterine bleeding is also a common side effect of hormone therapy; therefore, PMB is more frequently secondary to hormone therapy, rather than a symptom of EC/EIN.7 Few clinical risk prediction models have been developed that combine multiple epidemiological and clinical risk factors.18–20 However, these models have not been evaluated in target populations. Currently, there are no established absolute risk thresholds that guide clinical management. Our study shows a path towards developing risk-based management of women with abnormal uterine bleeding.
Clinical Implications
The growing clinical burden of EC, and the possibility to improve survival with early detection, highlight the importance of management guidelines for women with PMB and AUB. Differences in approaches can make it difficult to evaluate the clinical performance of current diagnostic strategies for EC and new tests that are being developed. We assessed the clinical efficiency of these strategies by estimating the percentage of women who would undergo endometrial biopsy, sensitivity of disease detection, and the ratio of biopsies performed to cases detected. In our population, risk assessment including age was most efficient, with the lowest percentage referred to biopsy while still detecting all cases. Results from our analysis emphasize the clinical challenge of managing premenopausal women with AUB, who have very low risks of EC/EIN.9 Current guidelines recommend endometrial biopsy for premenopausal women aged ≥45 years with AUB.10 Only 7 EC and 8 EIN were diagnosed in pre- and perimenopausal women with AUB, corresponding to 2.6% risk of EC/EIN combined, respectively. The efficiency of disease detection was much lower in these women, requiring 38 biopsies per case compared to 5.9–9.7 for strategies in women with PMB. Importantly, we did not observe strong risk stratification for baseline characteristics with risk of EC/EIN in pre- and perimenopausal women with AUB, underscoring the need for biomarkers that can improve risk stratification in this population. While it goes ae scope of this manuscript to evaluate the clinical management of benign causes of abnormal bleeding (e.g., uterine polyps), this will be an important future analysis in our cohort.
Strengths and Limitations
A unique strength of our study is that we enrolled a large clinical population of women with abnormal bleeding undergoing diagnostic evaluation for EC that enables evaluation of risk assessment strategies in this population. Our population is ideally suited to evaluate promising clinical risk models, and ongoing biospecimen collections will enable integration of biomarkers with clinical and epidemiologic risk factors to determine optimal approaches for risk assessment.21 While our study had excellent disease ascertainment, 10% of women did not have endometrial biopsy. We assume that risk among these women is low; however, we cannot rule out the possibility of an underlying EC/EIN diagnosis. It is possible that some patients diagnosed as having a benign endometrium may have a subsequent diagnosis of EC/EIN. Prospective follow-up is a central component of our study and as data become available, they will inform how much reassurance a negative biopsy can provide. Despite a large sample size, the prevalence of EC and EIN in this population were still low, limiting our power to estimate risks with sufficient precision for some scenarios. Further, while our study population reflects real-world clinical practice, results may not be generalizable to other, particularly racially-diverse populations.
Conclusions
In conclusion, we introduce a risk-based approach to guide clinical management and to support evaluation of new biomarkers and risk tools as they are developed.13 We show that using an age cutoff of 60 years improves risk stratification of TVUS in women with PMB. Currently recommended practice does not align with risk of endometrial cancer observed in our study. Risk estimates from our study can serve as a benchmark for future studies of new biomarkers and/or risk prediction tools to assess their clinical significance.
Supplementary Material
AJOG at a Glance:
Why was the study conducted? To assess the clinical utility of strategies for endometrial cancer detection, we applied a risk-based approach to evaluate diagnostic tests and recommended clinical management algorithms to inform clinical decision-making in a population of women with abnormal bleeding at the Mayo Clinic.
What are the key findings? The prevalence of endometrial cancer was 7.9% (47/593) among postmenopausal women and 1.2% (7/570) among pre- or perimenopausal women. Among postmenopausal women with an initial bleeding episode in whom transvaginal ultrasound is recommended, endometrial thickness did not provide meaningful risk stratification. As demonstrated by our example using age with a cutoff of 60 years, clinical variables may improve risk stratification compared to current clinical practice.
What does this study add to what is already known? Current clinical recommendations for endometrial cancer detection may not be consistent with underlying risk.
Acknowledgments:
This research was supported in part by the Mayo Clinic Specialized Program of Research Excellence (SPORE) in Ovarian Cancer, CA136393 from the National Institutes of Health; Mayo Clinic’s NCI Cancer Center Support Grant, P30 CA 15083; and the Intramural Research Program of the National Cancer Institute (Z01CP010124–21). We acknowledge Ann VanOosten for her assistance with study recruitment and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Clarke MA, Devesa SS, Harvey SV, Wentzensen N. Hysterectomy-Corrected Uterine Corpus Cancer Incidence Trends and Differences in Relative Survival Reveal Racial Disparities and Rising Rates of Nonendometrioid Cancers. JCO. 2019;37:1895–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International Patterns and Trends in Endometrial Cancer Incidence, 1978–2013. J Natl Cancer Inst. 2017. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 4.Weiderpass E, Antoine J, Bray FI, Oh JK, Arbyn M. Trends in corpus uteri cancer mortality in member states of the European Union. Eur J Cancer. 2014;50:1675–84. [DOI] [PubMed] [Google Scholar]
- 5.Creasman WT, Odicino F, Maisonneuve P, Quinn MA, Beller U, Benedet JL, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95 Suppl 1:S105–43. [DOI] [PubMed] [Google Scholar]
- 6.UK CR. Uterine cancer statistics: Cancer Research UK; [Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer.
- 7.Clarke MA, Long BJ, Del Mar Morillo A, Arbyn M, Bakkum-Gamez JN, Wentzensen N. Association of Endometrial Cancer Risk With Postmenopausal Bleeding in Women: A Systematic Review and Meta-analysis. JAMA Intern Med. 2018;178:1210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matteson KA, Robison K, Jacoby VL. Opportunities for Early Detection of Endometrial Cancer in Women With Postmenopausal Bleeding. JAMA Intern Med. 2018;178:1222–3. [DOI] [PubMed] [Google Scholar]
- 9.Pennant ME, Mehta R, Moody P, Hackett G, Prentice A, Sharp SJ, et al. Premenopausal abnormal uterine bleeding and risk of endometrial cancer. BJOG : an international journal of obstetrics and gynaecology. 2017;124:404–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ACOG committee opinion no. 557: Management of acute abnormal uterine bleeding in nonpregnant reproductive-aged women. Obstet Gynecol. 2013;121:891–6. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Committee Opinion No. 734: The Role of Transvaginal Ultrasonography in Evaluating the Endometrium of Women With Postmenopausal Bleeding. Obstet Gynecol. 2018;131:e124–e9. [DOI] [PubMed] [Google Scholar]
- 12.Practice Bulletin No. 149: Endometrial cancer. Obstet Gynecol. 2015;125:1006–26. [DOI] [PubMed] [Google Scholar]
- 13.Wentzensen N, Wacholder S. From differences in means between cases and controls to risk stratification: a business plan for biomarker development. Cancer Discov. 2013;3:148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clarke MA, Long BJ, Sherman ME, Lemens MA, Podratz KC, Hopkins MR, et al. A prospective clinical cohort study of women at increased risk for endometrial cancer. Gynecol Oncol. 2020;156:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Organization WH. Obesity: preventing and managing the global epidemic Report of a WHO Consultation.. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 16.Society AC. Key Statistics for Endometrial Cancer 2020. [Available from: https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html.
- 17.Trabert B, Wentzensen N, Yang HP, Sherman ME, Hollenbeck AR, Park Y, et al. Is estrogen plus progestin menopausal hormone therapy safe with respect to endometrial cancer risk? Int J Cancer. 2013;132:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burbos N, Musonda P, Duncan TJ, Crocker SG, Morris EP, Nieto JJ. Estimating the risk of endometrial cancer in symptomatic postmenopausal women: a novel clinical prediction model based on patients’ characteristics. Int J Gynecol Cancer. 2011;21:500–6. [DOI] [PubMed] [Google Scholar]
- 19.Fortner RT, Husing A, Kuhn T, Konar M, Overvad K, Tjonneland A, et al. Endometrial cancer risk prediction including serum-based biomarkers: results from the EPIC cohort. Int J Cancer. 2017;140:1317–23. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer RM, Park Y, Kreimer AR, Lacey JV Jr., Pee D, Greenlee RT, et al. Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: derivation and validation from population-based cohort studies. PLoS Med. 2013;10:e1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarke MA, Long BJ, Sherman ME, Lemens MA, Podratz KC, Hopkins MR, et al. A prospective clinical cohort study of women at increased risk for endometrial cancer. Gynecol Oncol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


