Abstract
The purpose of this study was to investigate the differences in CT characteristics and disease spread patterns between ROS1-rearranged adenocarcinomas and epidermal growth factor receptor (EGFR)-mutant or anaplastic lymphoma kinase (ALK)-rearranged adenocarcinomas. Patients with stage IIIb/IV adenocarcinoma with ROS1 rearrangement, EGFR mutations, or ALK rearrangement were retrospectively identified. Two radiologists evaluated CT features and disease spread patterns. A multivariable logistic regression model was applied to determine the clinical and CT characteristics that can discriminate between ROS1-rearranged and EGFR-mutant or ALK-rearranged adenocarcinomas. A cohort of 169 patients was identified (ROS1 = 23, EGFR = 120, and ALK = 26). Compared to EGFR-mutant adenocarcinomas, ROS1-rearranged adenocarcinomas were less likely to have air-bronchogram (p = 0.011) and pleural retraction (p = 0.048) and more likely to have pleural effusion (p = 0.025), pericardial metastases (p < 0.001), intrathoracic and extrathoracic nodal metastases (p = 0.047 and 0.023, respectively), and brain metastases (p = 0.017). Following multivariable analysis, age (OR = 1.06; 95% CI: 1.01, 1.12; p = 0.024), pericardial metastases (OR = 10.50; 95% CI: 2.10, 52.60; p = 0.005), and nodal metastases (OR = 8.55; 95% CI: 1.14, 62.52; p = 0.037) were found to be more common in ROS1-rearranged tumors than in non-ROS1-rearranged tumors. ROS1-rearranged adenocarcinomas appeared as solid tumors and were associated with young age, pericardial metastases and advanced nodal metastases relative to tumors with EGFR mutations or ALK rearrangement.
Subject terms: Cancer, Diagnostic markers
Introduction
Non-small cell lung cancer (NSCLC) is a disease of ambiguity regarding its molecular heterogeneity and variable histologic subtypes1,2. Owing to recent advances in the field of genetic analysis, lung adenocarcinomas have been characterized into clinically significant molecular subsets3–5. Epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) gene rearrangements are currently the most well-known actionable mutations. Target agents, such as EGFR tyrosine kinase inhibitors and ALK inhibitors, have revolutionized treatment for NSCLC harboring these driver mutations.
ROS1 gene rearrangements are another actionable driver mutation identified in 1–2% of patients with advanced stage NSCLC. Patients with ROS1-rearranged lung cancer show similar characteristics to those with ALK rearrangement, such as predilections for younger age, female gender, non-smoker status, and lung adenocarcinoma histology6. In addition, crizotinib, the first generation inhibitor for ALK-rearranged NSCLC, demonstrated an overall response rate of 72% as well as a median progression-free survival of 19.2 months in patients with ROS1-rearranged lung cancer7 and was approved as front-line therapy for ROS1-rearranged NSCLC in 2016.
The most recent National Comprehensive Cancer Network (NCCN) guidelines recommend genetic testing in all patients with advanced NSCLC before initial treatment8. However, molecular testing may not be feasible because of insufficient tissue samples from small biopsies or be inaccurate owing to intra- and intertumoral heterogeneity9,10. In addition, rebiopsy for genomic evaluation during treatment may not be feasible in some patients with advanced disease. Recent studies have shown that imaging features suggest certain molecular alterations in NSCLC, such as EGFR mutations and ALK rearrangement11–13. However, to date, limited studies have evaluated the imaging features of ROS1-rearranged lung cancer14,15. Therefore, the purpose of our study was to investigate the differences in CT characteristics and disease spread patterns between patients with lung adenocarcinoma who have ROS1 rearrangement and those with EGFR mutations or ALK rearrangement.
Results
Patient characteristics
Twenty-three patients who had lung adenocarcinoma with ROS1 rearrangement [5 men and 18 women; mean age of 56 years (range of 31–76 years)] were identified. For the control groups, 120 patients with EGFR-mutant lung adenocarcinoma [40 men and 80 women; mean age of 62 years (range of 28–83 years)] were randomly chosen based on the prevalence of genetic mutations in the lung cancer population study16. 26 patients with ALK-rearranged adenocarcinoma [9 men and 17 women; mean age of 56 years (range of 30–83 years)] were also included in this study. The mean age of the 169 patients was 59.4 years (range of 28–83 years). Clinicopathologic characteristics of these patients are summarized in Table 1. Patients with ROS1 rearrangement were younger [mean age of 56 years (range of 31–76 years)] than those with EGFR mutations [mean age of 62 years (range of 28–83 years); p = 0.006]. No significant difference was observed in gender or smoking status between patients with ROS1 rearrangement and those with EGFR mutations or ALK rearrangement (Table 1).
Table 1.
Demographic findings and Tumor, Node, Metastasis (TNM) staging according to genetic mutation type.
| ROS1 | EGFR | ALK | P-value | ||
|---|---|---|---|---|---|
| ROS1 vs EGFR | ROS1 vs ALK | ||||
| No. of patients | 23 | 120 | 26 | ||
| Age, years* | 56 (31–76) | 62 (28–83) | 56 (30–83) | 0.006 | 0.882 |
| Sex | 0.273 | 0.319 | |||
| M | 5 (22) | 40 (33) | 9 (35) | ||
| F | 18 (78) | 80 (66) | 17 (65) | ||
| Smoking | 0.076 | 0.885 | |||
| Never | 17 (74) | 79 (66) | 20 (77) | ||
| Ex-smoker | 4 (17) | 39 (33) | 5 (19) | ||
| Current | 2 (9) | 2 (2) | 1 (4) | ||
| Pack years* | 4 (0–25) | 6 (0–40) | 2 (0–30) | ||
| T stage | 0.380 | 0.060 | |||
| T1 | 8 (35) | 25 (21) | 6 (23) | ||
| T2 | 9 (39) | 56 (47) | 6 (23) | ||
| T3 | 1 (4) | 16 (13) | 9 (35) | ||
| T4 | 5 (22) | 23 (19) | 5 (19) | ||
| N stage | 0.047 | 0.803 | |||
| N0 | 2 (9) | 39 (33) | 5 (19) | ||
| N1 | 1 (4) | 8 (7) | 1 (4) | ||
| N2 | 4 (17) | 24 (20) | 4 (15) | ||
| N3 | 16 (70) | 49 (41) | 16 (62) | ||
| M stage | 0.042 | 0.159 | |||
| 0 | 2 (9) | 2 (2) | 0 | ||
| 1a | 8 (35) | 25 (21) | 6 (23) | ||
| 1b | 13 (57) | 93 (78) | 20 (77) | ||
Unless otherwise indicated, data are presented as number of patients with the percentage in parentheses.
*Data are presented as the median with the range in parentheses.
CT evaluation
CT features of the primary tumor and disease spread patterns according to the three genotypes are summarized in Table 2. Lung adenocarcinomas with ROS1 rearrangement were mainly solid in density (19 of 23, 83%) (Figs. 1, 2 and 3), similar to EGFR-mutant (73%) or ALK-rearranged (88%) tumors, and tended to have a lobulated border (15 of 23, 65%). Compared with EGFR-mutant tumors, ROS1-rearranged tumors were less likely to have air-bronchogram (p = 0.011) and pleural retraction (p = 0.048) but more likely to have pleural effusion (p = 0.025), pericardial metastases (p < 0.001) (Fig. 3B), intrathoracic and extrathoracic lymph node metastases (p = 0.047 and 0.023, respectively) (Figs. 1, 2, 3), and brain metastases (p = 0.017). ROS1- and ALK-rearranged tumors showed similar CT features and no significant differences except for pericardial metastasis, which was more frequent in ROS1-rearranged tumors but statistically insignificant (p = 0.060).
Table 2.
CT features and disease spread patterns according to genetic mutation type.
| Features | ROS1 | EGFR | ALK | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ROS1 vs EGFR | ROS1 vs ALK | |||||||||
| Primary tumor | ||||||||||
| Size (mm)* | 32 (14–100) | 35 (1–100) | 40 (15–100) | 0.196 | 0.370 | |||||
| Density | Solid | 20 (87) | 92 (77) | 24 (92) | 0.408 | 0.655 | ||||
| Subsolid | 3 (13) | 28 (23) | 2 (8) | |||||||
| Location | Central | 14 (61) | 75 (63) | 20 (77) | 0.883 | 0.224 | ||||
| Peripheral | 9 (39) | 45 (37) | 6 (23) | |||||||
| Border | Smooth | 4 (17) | 14 (12) | 2 (8) | ||||||
| Lobulated | 15 (65) | 56 (47) | 15 (58) | 0.111 | 0.347 | |||||
| Spiculated | 4 (17) | 50 (42) | 9 (34) | |||||||
| Air-bronchogram | 3 (13) | 49 (41) | 6 (23) | 0.011 | 0.472 | |||||
| Pleural retraction | 11 (48) | 83 (69) | 13 (50) | 0.048 | 0.879 | |||||
| Central low-attenuation | 7 (30) | 38 (32) | 10 (38) | 0.907 | 0.556 | |||||
| Calcification | 1 (4) | 20 (17) | 5 (19) | 0.198 | 0.194 | |||||
| Lymph node metastases | 21 (91) | 96 (80) | 21 (81) | |||||||
| N0 | 2 (9) | 24 (20) | 5 (19) | |||||||
| N1 | 1 (4) | 5 (4) | 1 (4) | 0.047 | 0.803 | |||||
| N2 | 4 (17) | 22 (18) | 3 (12) | |||||||
| N3 | 16 (70) | 69 (58) | 17 (65) | |||||||
| Distant metastases | ||||||||||
| Lung metastasis | Miliary | 0 | 10 (8) | 0 | ||||||
| Scattered | 5 (22) | 36 (30) | 9 (35) | 0.145 | 0.895 | |||||
| Lymphangitic | 5 (22) | 14 (12) | 4 (15) | |||||||
| Aerogeneous | 1 (4) | 1 (1) | 1 (4) | |||||||
| Pleural | 14 (61) | 55 (46) | 16 (62) | 0.186 | 0.962 | |||||
| Pericardial | 7 (30) | 2 (2) | 2 (8) | < 0.001 | 0.060 | |||||
| Pleural effusion | 12 (52) | 34 (28) | 10 (29) | 0.025 | 0.336 | |||||
| Extrathoracic | Liver | 4 (17) | 18 (15) | 6 (23) | 0.756 | 0.730 | ||||
| Adrenal | 3 (13) | 15 (13) | 5 (19) | 1.000 | 0.707 | |||||
| Brain | 3 (13) | 47 (39) | 7 (27) | 0.017 | 0.299 | |||||
| Lymph nodes | 4 (17) | 4 (3) | 5 (19) | 0.023 | 1.000 | |||||
| Bone | 7 (30) | 50 (42) | 13 (50) | 0.360 | 0.245 | |||||
Unless otherwise indicated, data are presented as number of patients with the percentage in parentheses.
*Data are presented as the median with the range in parentheses.
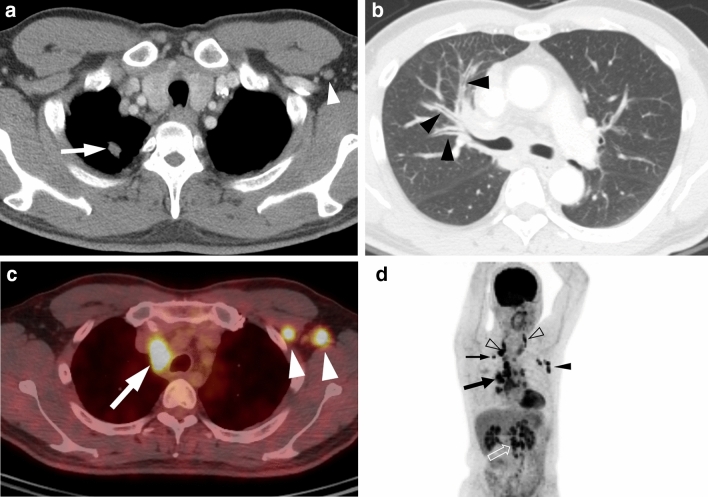
Figure 1.
A 44-year-old man with ROS1-rearranged lung adenocarcinoma with extensive lymph node metastases. (a) Transverse mediastinal CT image demonstrates a small solid nodule (arrow) in the right upper lobe, which is presumed to be a primary tumor. Left axillary lymph node enlargement (arrowhead) is also noted. (b) Transverse lung window CT image shows diffuse bronchial wall thickening (arrowheads), which represents lymphangitic carcinomatosis. (c) Fused PET/CT image demonstrates fluorodeoxyglucose (FDG)-avid right paratracheal (arrow) and left axillary (arrowheads) lymph nodes. (d) Maximum intensity projection image of PET shows intense FDG uptake in the primary tumor (thin arrow), cervical (open arrowheads), mediastinal (thick arrow), left axillary (arrowhead), and intraabdominal (open arrow) lymph node metastases.
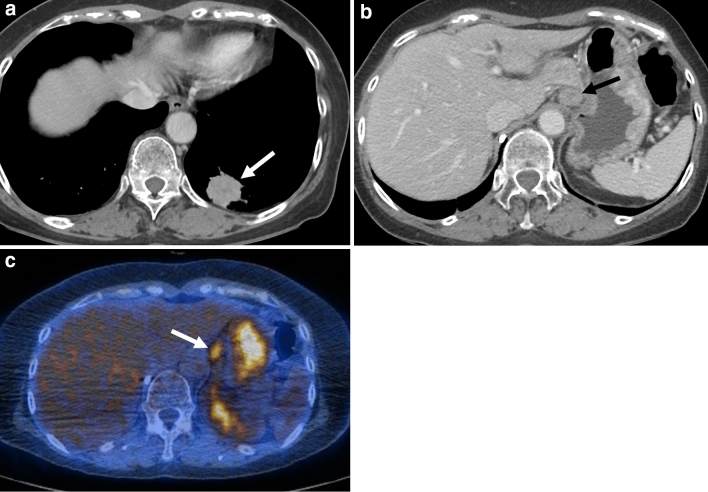
Figure 2.
A 55-year-old woman with ROS1-rearranged lung adenocarcinoma with distant (abdominal) lymph node metastases. (a, b) Chest CT images demonstrate a peripheral solid mass (arrow) in the left lower lobe, which was shown to be adenocarcinoma from the percutaneous core needle biopsy. Note the enlarged left gastric lymph node (arrow in b). (c) Fused PET/CT image demonstrates FDG-avid left gastric lymph node (arrow), which was revealed to be metastasis from the endobronchial ultrasound-guided needle biopsy.
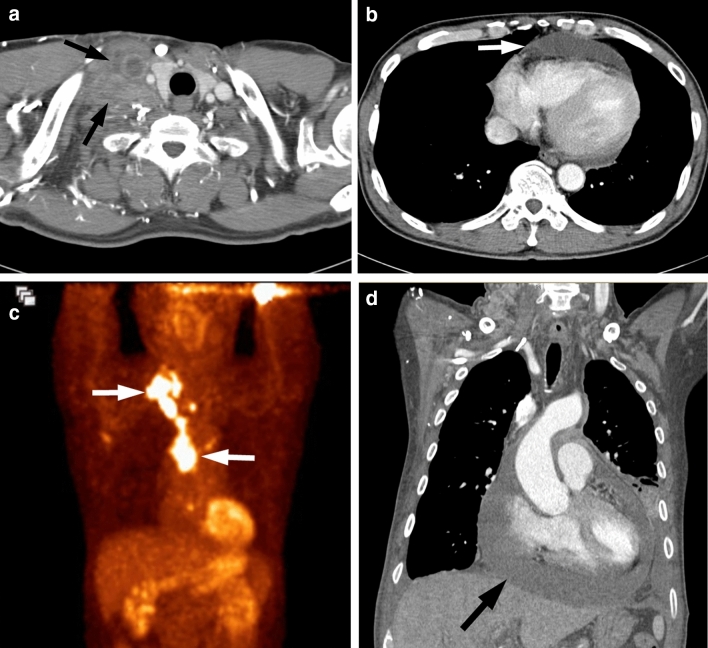
Figure 3.
A 44-year-old man with ROS1-rearranged lung adenocarcinoma with pericardial and lymph node metastases. (a, b) Chest CT images demonstrate conglomerated metastatic lymph nodes in the right supraclavicular region (white arrows), which were shown to be metastatic adenocarcinoma from the lymph node core biopsy. Note the moderate amount of pericardial effusion (white arrow), which represents pericardial metastases. (c) Maximum intensity projection image of PET displays intense FDG uptake in the right supraclavicular and mediastinal lymph metastases (arrows). (d) Follow-up CT performed for marked hypotension illustrates an increased amount of pericardial effusion (arrow), which is consistent with impending cardiac tamponade.
Multivariable logistic regression model for ROS1 versus non-ROS1 tumors
In the univariable analysis, age (p = 0.026), pericardial metastasis (p < 0.001), air-bronchogram (p = 0.030), presence of nodal metastases (p = 0.025), and pleural effusion (p = 0.041) were statistically significant. In the multivariable analysis, age [odds ratio (OR) = 1.06; 95% confidence interval (CI): 1.01, 1.12; p = 0.024], pericardial metastases (OR = 10.50; 95% CI: 2.10, 52.60; p = 0.005), and nodal metastases (OR = 8.55; 95% CI: 1.14, 62.52; p = 0.037) were more common in patients with ROS1 rearrangement than in those with non-ROS1 rearrangement (EGFR mutations and ALK rearrangement) (Table 3).
Table 3.
Univariate and multivariate analyses for significant predictors of ROS1-rearranged adenocarcinomas.
| Variables | Univariable analysis | Multivariable analysis* | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |||
| Size | 0.99 (0.97, 1.01) | 0.244 | ||||
| Age | 1.04 (1.01, 1.08) | 0.026 | 1.06 (1.01, 1.12) | 0.024 | ||
| Location | 0.84 (0.34, 2.06) | 0.191 | ||||
| Air-bronchogram | 0.25 (0.07, 0.87) | 0.030 | 0.34 (0.01, 1.43) | 0.142 | ||
| Calcification | 4.55 (0.59, 35.32) | 0.148 | ||||
| Pleural retraction | 0.48 (0.20, 1.16) | 0.102 | ||||
| Central low-attenuation | 0.89 (0.34, 2.32) | 0.820 | ||||
| Pleural metastasis | 0.61 (0.25, 1.49) | 0.278 | ||||
| Pericardial metastasis | 15.60 (4.10, 58.81) | < 0.001 | 10.50 (2.10, 52.60) | 0.005 | ||
| Bone metastasis | 0.53 (0.19, 1.52) | 0.234 | ||||
| Nodal metastasis | 5.46 (1.23, 24.52) | 0.025 | 8.55 (1.14, 62.52) | 0.037 | ||
| Pleural effusion | 2.53 (1.04, 6.17) | 0.041 | 2.63 (0.81, 8.55) | 0.107 | ||
HR hazard ratio, CI confidence interval.
*P-values < 0.1 in the univariate analysis were involved in the multivariate analysis.
Correlation between the predictors of ROS1-rearranged tumors and response to crizotinib
Among 23 patients with ROS1-rearranged tumor, 20 patients who received at least one dose of crizotinib were included in the analyses of overall response. The overall responses included 4 complete response (CR) (20%), 8 partial response (PR) (40%), 6 stable disease (30%), and 2 progressive disease (10%). The overall response rate was 60% (12 of 20). The area under the curve (AUC) of the model was 0.725 (95% CI: 0.66, 0.78), indicating moderate predictive performance (Fig. 4)17.
Figure 4.
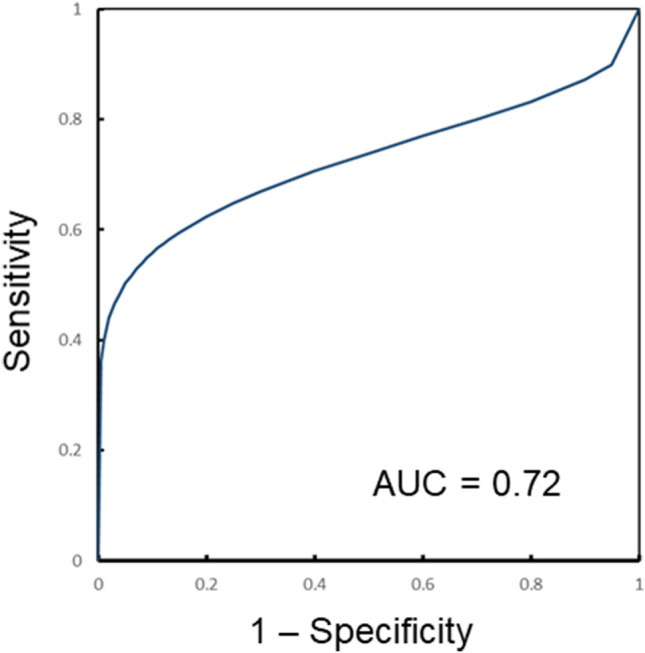
Receiver operating characteristic (ROC) curve for the prediction model of a best overall response of complete response or partial response to crizotinib. Area under the ROC curve was 0.72 (95% confidence interval: 0.66, 0.78), indicating moderate predictive performance.
Discussion
Our study showed that in a cohort of patients with advanced adenocarcinomas, patients with ROS1-rearranged tumors exhibit characteristic clinical and radiologic features compared to those with EGFR mutations or ALK rearrangement. As per our findings, it is proposed that young age and disease spread patterns, including pericardial metastasis and nodal metastasis, are important predictors of ROS1-rearranged tumors.
Current guidelines recommend that all patients with adenocarcinomas be tested for routine biomarkers, including EGFR mutations, ALK rearrangement, and ROS1 rearrangement, because FDA-approved agents for lung cancer are available for these biomarkers8,18. However, in clinical practice, molecular testing in patients with advanced lung cancer may not always be feasible for several reasons, such as nondiagnostic or inconclusive results from small biopsy specimens, inconsistency among the various molecular tests, or intra- and intertumoral heterogeneity of genetic mutations19–21. Recent studies have demonstrated that imaging features may suggest certain molecular alterations in NSCLC, such as EGFR mutations and ALK rearrangement11–13. Therefore, these specific imaging features combined with clinical features may help identify patients who could benefit from expedited testing for genetic mutations or rebiopsy after nondiagnostic results.
In our study, CT features of the primary tumor among the three genotypes showed substantial overlap. In terms of lesion density, ROS1-rearranged tumors were mainly solid, similar to EGFR-mutant or ALK-rearranged tumors. Previous studies have reported that ALK-rearranged tumors typically appear as a solid lesion with a lobulated contour and hypoattenuation on a contrast-enhanced CT representing histologic features, such as abundant intra- or extracellular mucin and a solid signet-ring cell pattern22,23. Notably, the majority of EGFR-mutant tumors in our cohort were also solid in density, although several studies suggested a close association between EGFR-mutant tumors and the presence of ground-glass opacity components11,12. These findings suggest that the density of the tumor may not be unique across the genetic mutations, especially in advanced adenocarcinomas. ROS1-rearranged tumors were less likely to have air-bronchogram and pleural retraction, which are well-recognized imaging features favoring EGFR-mutant adenocarcinomas12,24. It is also noteworthy that no significant difference was observed in imaging features of the primary tumor between ROS1- and ALK-rearranged tumors, which may be attributed to the fact that these tumors have substantial similarities in both clinical attributes and response to crizotinib therapy.
With regard to imaging features other than the primary tumor, ROS1-rearranged tumors more frequently showed advanced intra- and extrathoracic lymph node metastases, pleural effusion, and pericardial metastases compared to EGFR-mutant tumors, although these differences were not observed between ROS1- and ALK-rearranged tumors. This tendency toward lymphangitic spread of ROS1-rearranged tumors, such as advanced lymphadenopathy and pericardial metastases, is also similar to that of ALK-rearranged tumors, which has been reported in a previous study comparing ALK-rearranged and EGFR-mutant advanced adenocarcinomas11. In addition, ROS1-rearranged tumors were less likely to be associated with brain metastases compared with other mutation groups, which is corroborated by previous studies25,26. The mechanism of lower incidence of brain metastasis in ROS1-rearranged tumors compared with other mutations groups is not yet fully understood but might be partly explained by the propensity for lymphangitic tumor spread rather than hematogenous spread.
Given the results of our study, clinical and imaging features suggest the possibility of ROS1 rearrangement and prioritize appropriate genetic testing in advanced lung cancer. This has substantial clinical implications because the prevalence of ROS1 rearrangement (1–2%) is much lower compared to that of EGFR mutations, which is known to be 20–30% in Western countries and 50–65% in East Asian countries27.
Our study has several limitations. First, the number of patients with ROS1-rearranged tumors was small mainly due to the overall rarity of this mutation in lung cancer. Second, our study is a retrospective study based on a single large tertiary referral center, and the findings of our study may not be generalizable. In addition, there may have been a bias in the selection of patients for our study. Additional prospective studies with a large number of patients are needed for further validation of the current results. Third, it is difficult to distinguish ROS1- from ALK-rearranged tumors with clinical and imaging features alone as these tumors have considerable overlaps in clinicoradiologic features as well as treatment regimen. Therefore, appropriate genetic testing should be guaranteed at initial diagnosis for effective personalized treatment. Finally, although our study suggested that imaging features might be helpful in distinguishing ROS1 rearrangement from other mutations, the mechanism underlying the differences still remains to be elucidated.
In summary, despite shared clinical and imaging features, ROS1-, ALK-, and EGFR-positive advanced adenocarcinomas differ in certain imaging features of the primary tumor and disease spread patterns. ROS1-rearranged adenocarcinomas are more likely to be associated with younger age and distribution of metastatic disease, including pericardial and nodal metastases.
Methods
This retrospective study was approved by the Institutional Review Board and the Ethics Committee of Samsung Medical Center. Informed consent was waived from the patient and all methods in the study were performed in accordance with the relevant guidelines and regulations.
Patients and data selection
From July 2009 to June 2015, a total of 7033 patients with NSCLC underwent genetic mutation studies at our institution. We selected all patients who had advanced adenocarcinoma (stage IIIb/IV) with ROS1 rearrangement to participate in this study. For comparison with patients with ROS1 rearrangement, we also identified patients who had advanced adenocarcinoma with EGFR mutations and ALK rearrangement during the study time frame. Only the patients who satisfied the following criteria were included in this study: (1) aged 18 years or older; (2) histologically proven adenocarcinoma at clinical or pathological stage IIIb/IV; (3) positive for ROS1 rearrangement, ALK rearrangement, or EGFR mutations; (4) no history of previous treatment; and (5) available for a pretreatment chest CT study. In all patients, the histologic diagnoses were made by a pathologist (with 23 years of experience in thoracic pathology) by means of a percutaneous core needle and/or bronchoscopic biopsy. Chest CT studies were performed within one month prior to lung biopsy. Clinical and pathologic data were obtained from electronic medical records, including age at diagnosis; gender; smoking status (never smoker, ex-smoker, and current smoker); and Tumor, Node, Metastasis (TNM) staging based on the 8th edition of the TNM Classification of Malignant Tumors28.
Image acquisition and analysis
Chest CT studies were performed using various helical CT scanners (Light Speed VCT and Discovery CT750 HD, GE Healthcare, WI, USA; Somatom Definition Flash, Siemen Medical System, Erlangen, Germany). CT images were obtained from the lung apices to the middle portion of both kidneys. Reconstructed images were interfaced directly to a picture archiving and communication system (PACS) (Centricity 4.0; GE Healthcare, Mt. Prospect, IL, USA). Two radiologists (with 27 and 17 years of experience in thoracic imaging interpretation, respectively) who were blinded to the clinical and pathologic data as well as mutation statuses reviewed the CT images independently, and the final conclusion was reached in consensus.
Tumor characteristics were evaluated by the two radiologists on the basis of a review of transverse images, including tumor size (maximum axial diameter); density (solid or subsolid); location; border (smooth, lobulated, or spiculated); and the presence or absence of calcification, air-bronchogram, and pleural retraction.
Metastatic lymphadenopathy was confirmed histologically (endobronchial ultrasound-guided lymph node aspiration biopsy) or determined by imaging studies. Lymph nodes that measured more than 10 mm in short axis diameter and/or displayed increased glucose uptake [higher than that of the surrounding tissue and with a maximum standardized uptake value (SUV) of more than 3.5 as determined by quantitative analysis] on PET/CT scans were considered malignant29.
Intrathoracic metastases were recorded as follows: intrapulmonary, pleural, pericardial, or bone. Intrapulmonary metastases were classified as miliary (< 5 mm), nodular scattered (> 5 mm), or lymphangitic carcinomatosis. Intrathoracic bone metastases were determined by a decrease in tumor size after chemotherapy or target therapy on follow-up imaging studies (5). Extrathoracic metastases were evaluated by CT of the abdomen and/or pelvis as well as a brain MRI for each patient. PET/CT scans were also reviewed for the presence of distant metastases if available.
Statistical analysis
All data were recorded as means ± standard deviations for continuous variables or frequencies and as percentages for categorical variables. To explore discriminative imaging features between the mutation groups, we used the two sample t-test, Fisher's exact test, the Wilcoxon rank sum test, and the chi-square test for univariate analysis. A multivariable logistic regression model was created with the factors that demonstrated a p-value < 0.1 in the univariate analysis. To evaluate the correlation between the predictors of ROS1-rearranged tumors and antitumor activity of crizotinib, the performance of the model in predicting a best overall response of CR or PR to crizotinib was assessed by calculating the area under the receiver operating characteristic (ROC) curve. Best overall response was derived from investigator assessment using RECIST v1.1 criteria. Statistical analyses were performed with SPSS software (version 26.0, SPSS, Chicago, IL, USA). A p-value < 0.05 was considered to indicate a significant difference.
Acknowledgements
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare (1520230), Republic of Korea.
Author contributions
T.J.K. conceived of the original idea for the study. J.H.W. and T.J.K. participated in the study design. J.H.W., T.J.K., T.S.K., and J.H. performed the data acquisition. J.H.W. and T.J.K. participated in the statistical analyses. All authors participated in the data interpretation. J.H.W. drafted the first version of the report. All authors revised and approved the final draft of the report.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kerr KM. Pulmonary adenocarcinomas: Classification and reporting. Histopathology. 2009;54:12–27. doi: 10.1111/j.1365-2559.2008.03176.x. [DOI] [PubMed] [Google Scholar]
- 2.Travis, W. D. Reporting lung cancer pathology specimens. Impact of the anticipated 7th Edition TNM classification based on recommendations of the IASLC Staging Committee. Histopathology54, 3–11, http://doi.org/10.1111/j.1365-2559.2008.03179.x (2009). [DOI] [PubMed]
- 3.Horn L, Pao W. EML4-ALK: Honing in on a new target in non-small-cell lung cancer. J. Clin. Oncol. 2009;27:4232–4235. doi: 10.1200/jco.2009.23.6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 5.Paez JG, et al. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 6.Bergethon K, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J. Clin. Oncol. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaw AT, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N. Engl. J. Med. 2014;371:1963–1971. doi: 10.1056/NEJMoa1406766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network. NCCN practice guidelines in oncology: Non-small cell lung cancer. National Comprehensive Cancer Network website. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf. Accessed 23 March 2020.
- 9.Nam BD, et al. Transthoracic rebiopsy for mutation analysis in lung adenocarcinoma: Outcomes and risk factors for the acquisition of nondiagnostic specimens in 199 patients. Clin. Lung Cancer. 2019;20:e309–e316. doi: 10.1016/j.cllc.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 10.Jekunen AP. Role of rebiopsy in relapsed non-small cell lung cancer for directing oncology treatments. J. Oncol. 2015;2015:809835. doi: 10.1155/2015/809835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi CM, Kim MY, Hwang HJ, Lee JB, Kim WS. Advanced adenocarcinoma of the lung: Comparison of CT characteristics of patients with anaplastic lymphoma kinase gene rearrangement and those with epidermal growth factor receptor mutation. Radiology. 2015;275:272–279. doi: 10.1148/radiol.14140848. [DOI] [PubMed] [Google Scholar]
- 12.Hong SJ, et al. Radiogenomic correlation in lung adenocarcinoma with epidermal growth factor receptor mutations: Imaging features and histological subtypes. Eur. Radiol. 2016;26:3660–3668. doi: 10.1007/s00330-015-4196-z. [DOI] [PubMed] [Google Scholar]
- 13.Kim, T. J., Lee, C.-T., Jheon, S. H., Park, J.-S. & Chung, J.-H. Radiologic characteristics of surgically resected non-small cell lung cancer with ALK rearrangement or EGFR mutations. Ann. Thorac. Surg.101, 473–480, http://doi.org/10.1016/j.athoracsur.2015.07.062 (2016). [DOI] [PubMed]
- 14.Yoon HJ, et al. Decoding tumor phenotypes for ALK, ROS1, and RET fusions in lung adenocarcinoma using a radiomics approach. Medicine. 2015;94:e1753. doi: 10.1097/md.0000000000001753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plodkowski AJ, et al. From genotype to phenotype: Are there imaging characteristics associated with lung adenocarcinomas harboring RET and ROS1 rearrangements? Lung Cancer. 2015;90:321–325. doi: 10.1016/j.lungcan.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JY, Jang SH. Epidemiology of lung cancer in Korea: Recent trends. Tuberculosis Respir. Dis. 2016;79:58–69. doi: 10.4046/trd.2016.79.2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greiner M, Pfeiffer D, Smith RD. Principles and practical application of the receiver-operating characteristic analysis for diagnostic tests. Prevent. Vet. Med. 2000;45:23–41. doi: 10.1016/s0167-5877(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 18.Planchard, D. et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol29, iv192–iv237, http://doi.org/10.1093/annonc/mdy275 (2018). [DOI] [PubMed]
- 19.Ren S, et al. Atypical negative ALK break-apart FISH harboring a crizotinib-responsive ALK rearrangement in non-small-cell lung cancer. J. Thorac. Oncol. 2014;9:e21–e23. doi: 10.1097/JTO.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun, J.-M. et al. A dramatic response to crizotinib in a non–small-cell lung cancer patient with IHC-positive and FISH-negative ALK. J. Thorac. Oncol.7, e36–e38, 10.1097/JTO.0b013e318274694e (2012). [DOI] [PubMed]
- 21.Li, W. et al. Combinational analysis of FISH and immunohistochemistry reveals rare genomic events in ALK fusion patterns in NSCLC that responds to Crizotinib treatment. J. Thorac. Oncol.12, 94–101, 10.1016/j.jtho.2016.08.145 (2017). [DOI] [PubMed]
- 22.Yoshida A, et al. Comprehensive histologic analysis of ALK-rearranged lung carcinomas. Am. J. Surg. Pathol. 2011;35:1226–1234. doi: 10.1097/pas.0b013e3182233e06. [DOI] [PubMed] [Google Scholar]
- 23.Jokoji R, et al. Combination of morphological feature analysis and immunohistochemistry is useful for screening of EML4-ALK-positive lung adenocarcinoma. J. Clin. Pathol. 2010;63:1066–1070. doi: 10.1136/jcp.2010.081166. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, et al. CT features associated with epidermal growth factor receptor mutation status in patients with lung adenocarcinoma. Radiology. 2016;280:271–280. doi: 10.1148/radiol.2016151455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gainor, J. F. et al. Patterns of metastatic spread and mechanisms of resistance to Crizotinib in ROS1-positive non-small-cell lung cancer. JCO Precis. Oncol, 1–13, http://doi.org/10.1200/po.17.00063 (2017). [DOI] [PMC free article] [PubMed]
- 26.Wu Y-L, et al. Phase II study of Crizotinib in East Asian patients with ROS1-positive advanced non-small-cell lung cancer. J. Clin. Oncol. 2018;36:1405–1411. doi: 10.1200/jco.2017.75.5587. [DOI] [PubMed] [Google Scholar]
- 27.Kwak EL, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldstraw, P. et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J. Thorac. Oncol.2, 706–714, http://doi.org/10.1097/JTO.0b013e31812f3c1a (2007). [DOI] [PubMed]
- 29.Shim SS, et al. Non-small cell lung cancer: Prospective comparison of integrated FDG PET/CT and CT alone for preoperative staging. Radiology. 2005;236:1011–1019. doi: 10.1148/radiol.2363041310. [DOI] [PubMed] [Google Scholar]