Abstract
Introduction:
Slit2 is an extracellular matrix protein that regulates migration of developing axons during central nervous system (CNS) development. Roundabout (Robo) receptors expressed by various cell types in the CNS, mediate intracellular signal transduction pathways for Slit2. Recent studies indicate that Slit2 plays important protective roles in a myriad of processes such as cell migration, immune response, vascular permeability and angiogenesis in CNS pathologies.
Areas Covered:
This review provides an overview of the diverse functions of Slit2 in CNS disorders and discusses the potential of Slit2 as a therapeutic target. We reviewed preclinical studies reporting the role of Slit2 in various CNS disease models, transgenic animal research and rodent models that utilized Slit2 as a therapy.
Expert Opinion:
Slit2 exerts a wide array of beneficial effects ranging from anti-migration, blood brain barrier (BBB) protection, inhibition of peripheral immune cell infiltration and anti-apoptosis in various disease models. However, a dual role of Slit2 in endothelial permeability has been observed in transgenic animals. Further research on Slit2 will be crucial including key issues such as effects of transgenic overexpression versus exogenous Slit2, function of Slit2 dependent on cellular expression of Robo receptors and the underlying pathology for potential clinical translation.
Keywords: Anti-migration, Apoptosis, Blood brain barrier, Glioma, Ischemic stroke, Neuroinflammation, Roundabout, Slit2, Surgical brain injury, Traumatic brain injury
1. INTRODUCTION TO SLITS AND ROBO
Slit proteins are key regulators in guiding the migration of axons and neuronal precursors by preventing abnormal midline crossings during the development of embryonic nervous system [1–3]. Slits are evolutionarily conserved extracellular matrix proteins which were discovered to be synthesized by glial cells in the central nervous system (CNS) of Drosophila [3, 4]. There are three known isoforms of Slit proteins in the vertebrates which includes Slit1, Slit2 and Slit3 [5–7]. Among the Slit proteins, Slit1 is predominantly expressed in the developing nervous system whereas Slit2 and Slit3 are also expressed outside the nervous system including kidney, lungs, heart and immune cells [7–9]. Slit2 was originally identified in Drosophila melanogaster as a chemorepellent preventing inappropriate midline crossing by the developing commissural neurons, which played an important role in guiding axonal path of the developing embryonic nervous system [2, 3, 10, 11]. Slit2 exerts its function by binding to the Roundabout (Robo) receptors [6, 12]. Slit-Robo signaling mediates various biological processes ranging from axonal guidance and CNS development to organogenesis, angiogenesis, vascular development, endothelial cell migration, leukocyte chemotaxis, tumor metastasis and CNS pathologies. See figure 1.
Figure 1.
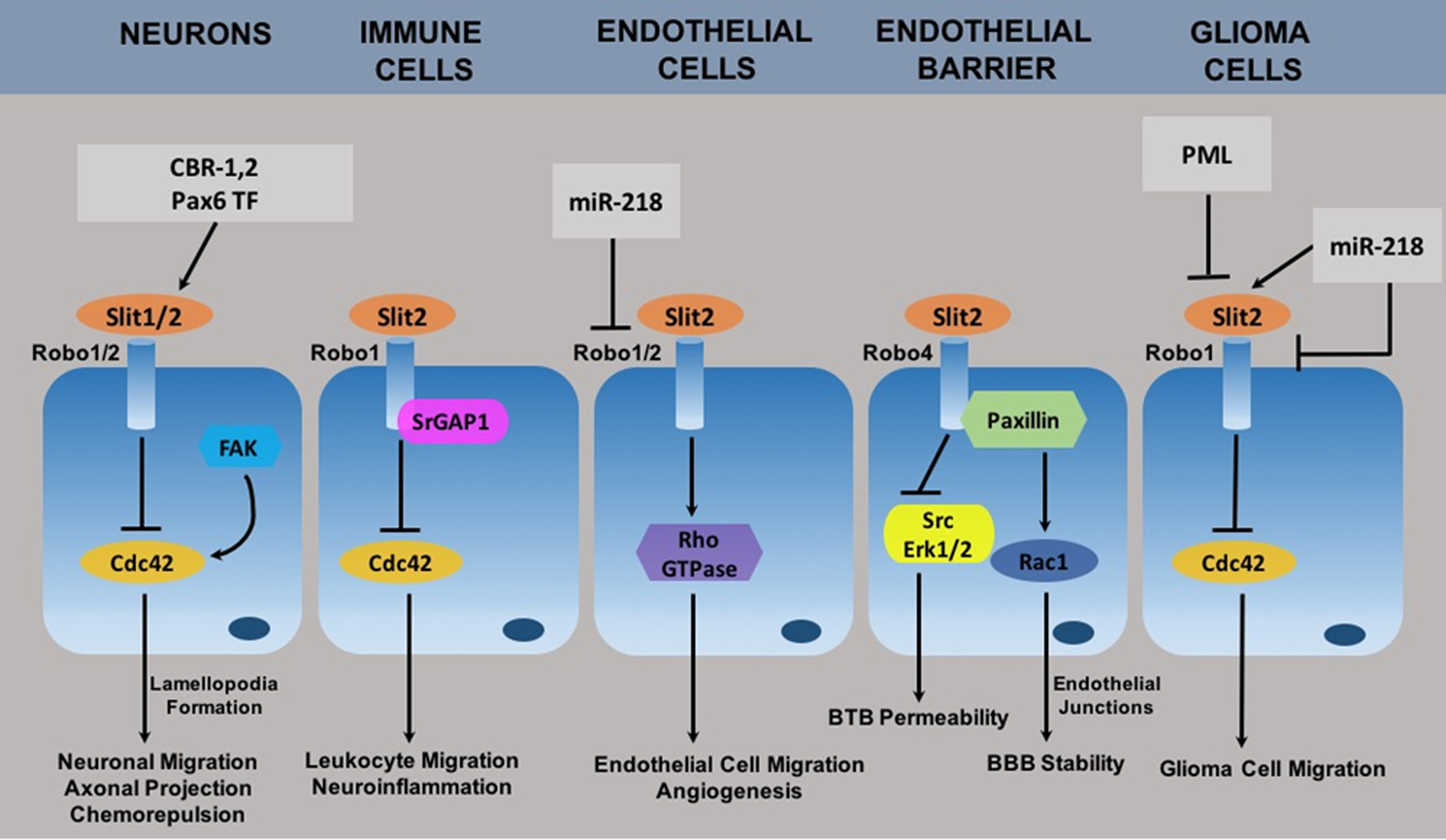
Schematic representation of Slit-Robo signaling mechanisms in CNS disorder. Abbreviations: CBR-1,2, cannabinoid receptor 1 and 2; Pax6 TF, Pax6 transcription factor; FAK, focal adhesion kinase; SrGAP1, Slit Robo GTPase activating protein 1; miR-218, micro RNA 218; Erk1/2, extracellular signal regulated kinase 1/2; PML, protein promyelocytic leukemia.
Although Slit2 was originally identified as an axonal repellent, recent publications show that the function of Slit2-Robo extends beyond physiological regulation of the developing CNS to regulate disease processes. Emerging evidence shows that among the members of Slit family of proteins, Slit2 has important roles in CNS disorders too but there have been no review articles focused on the role of Slit2 specifically in CNS disorders. Previously published reviews on Slit-Robo signaling have focused on CNS development [6, 13], axonal guidance and neuronal migration [14], renal disorders [15], cancers [16, 17], and signaling crosstalk [18]. This review is focused on the diverse functions of Slit2-Robo signaling in CNS pathologies.
2. STRUCTURE OF SLITS AND ROBO
The mammalian Slit protein contains an N-terminal signal peptide, four leucine-rich repeat (LRR) domains, nine epidermal growth factor (EGF)-like repeats, a laminin G-like domain, and a cysteine rich C terminus [5, 11].. The Robo family of transmembrane receptors consists of four members Robo1, Robo2, Robo3/Rig-1 and Robo4/magic Roundabout that mediate intracellular signal transduction pathways for Slit ligands. The structure and function of Robo1–3 are similar, and these are single-pass transmembrane proteins which belong to the immunoglobulin superfamily [12, 19]. The extracellular region of Robo receptors contain five immunoglobulin (Ig) domains and three fibronectin (FN) type III repeats, a transmembrane domain and four conserved proline-rich cytoplasmic motifs (CC0-CC3) [6, 12, 19]. Among Robo receptors, the structure of Robo4 is unique compared to other members of the Robo family. Robo4 contains two extracellular Ig and FN3 domains and only two intracellular domains CC0 and CC2 [12, 20].
Among the Slit proteins, Slit2 has been most widely studied during pathological states in the CNS. Slit2 is expressed by neurons in the normal adult brain in rats and human brain samples [1, 6]. However, in response to an injury Slit2 is expressed by glial cells in addition to neurons [21–23], which implicates distinct functions of Slit2 in the adult brain following an injury. Among the Robo family, Robo1 receptor was mainly expressed by neurons in the normal cerebral cortex while glial cells were either negative or faintly positive for Robo1 expression [24]. In addition, Robo1 is also highly expressed by non-neuronal cells such as the peripheral immune cells [8, 9]. Robo4 is an endothelial-specific transmembrane receptor which has vascular stabilization and anti-angiogenic functions [25–28]. The Robo4 receptor was found to be expressed by brain microvascular endothelial cells [29].
3. SLIT BINDING CHARACTERISTICS TO ROBO AND OTHER RECEPTORS
Robo receptors are known to mediate the critical repulsive guidance cues upon binding of Slits. However, the molecular interaction between Slit and Robo is not completely understood and how this interaction leads to downstream signal transduction has not been fully elucidated. Published studies provide some evidence to the manner in which Slit binds to and activates Robo receptors. In addition to the effects of Slit mediated via binding to different Robo receptors, other receptors have also been shown to play a role in Slit functions.
Slit proteins are cleaved within the fifth EGF region which releases the ~110–140 kDa N-terminal fragment (Slit2-N) that binds to Robo receptors and the ~55 kDa C-terminal fragment (Slit2-C) [12, 13]. The second LRR domain of Slit2 directly binds with Ig domain of Robo to transduce downstream signaling pathways [12]. Biochemical and structural studies revealed that the Ig1 domain of Robo1 primarily interacts with Slit2 while the Ig2 domain could be a secondary binding site for Slit2 [30]. Additionally, the domain 4 (D4) Ig4 region of Robo-mediated dimerization was identified to be key for Robo activation, and auto-inhibition of this region prevented dimerization and premature activation of Robo receptors [31, 32]. Slit releases the auto-inhibition of Robo and thereby mediates the activation of Robo receptors [31]. Also, Slit2-N binding can induce conformation change in the inactive Robo1 dimers which is required for receptor activation [33].
In addition, heparan sulfate/heparin has been shown to play an integral role in Slit-Robo signaling [34]. The second LRR domain of Slit was shown to have a binding site for heparin/heparan sulfate at the C-terminal cap region [34]. Biochemical and functional studies revealed that heparin, the highly sulfated variant of heparan sulfate enhanced the affinity of Slit-Robo interaction [34]. Furthermore, Robo structure-based mutagenesis studies using Robo mutants identified heparan sulfate/heparin binding sites in Robo Ig domains which contributed to Slit-Robo signaling [35].
Existing literature supports that Slit2 can elicit its functions by signaling via Robo1 and Robo2 receptors, but there is controversy whether it can bind Robo4. Furthermore, Robo3 does not bind to Slit ligands with high affinity [36] and there is conflicting evidence regarding whether Slit2 can bind to Robo4 [28, 37–40]. Furthermore, Robo4 can also bind to another endothelial-specific receptor Unc5B to mediate its vascular effects [27].
Although the Slit2-N fragment has been implicated to be the active fragment that contributes to Slit functions, recent study showed that the C-terminal fragment of Slit2 also participates in its biological functions indicating that the effects of Slit2 can be independent of the Robo receptor-binding N-terminal domain [41]. Additionally, a recent study identified that Slit C-terminal fragment specifically can bind to the sempahorin-receptor PlexinA1 to elicit repulsive guidance cues for the commissural axons, and the interaction between the two did not require Robo receptor [42]. Thus, these findings indicate that not only full-length and N-terminal region of Slits but the C-terminal fragment can mediate functions of Slits via interacting with diverse receptors.
4. DOWNSTREAM TARGETS OF SLIT2-ROBO SIGNALING IN THE CNS
4.1. Small Rho-GTPase Cdc42
Various axon guidance cues regulate actin polymerization at the leading edge of growth cones during cell motility or axon pathfinding by modulating the activity of cytoskeletal regulatory proteins. Cdc42 is a member of the small Rho-GTPases that regulates actin cytoskeletal polymerization during cellular motility. Modulation of Cdc42 activity by various guidance cue molecules has been implicated in directing cell migration [25]. Yinn et al. showed that Slit2 regulated Cdc42 activity in glioma cells dependent on Robo1 receptor in vitro [43]. The expression of Slit2 in glioma cells attenuated Cdc42 activity, and this effect was reversed with immunodepletion of Slit2. Likewise, immunodepletion of Slit2 also reversed the effects of Slit2 to inhibit glioma cell migration [43]. Additionally when glioma cells were transfected with Robo1 small interfering RNA (siRNA), the effects of recombinant Slit2 to inhibit cell migration and reduce Cdc42 activity were reversed suggesting that Slit2 inhibited glioma cell migration by attenuating Cdc42 activity dependent on Robo1 receptor [43]. Additionally, inhibition of Cdc42 activity promoted the anti-migratory effects of Slit2 thereby preventing peripheral immune cells from reaching the injured perisurgical brain tissue surrounding the surgical resection site in a rat model of surgical brain injury (SBI) [44]. However, the activation of Cdc2 and PAK was downstream of Nck1 and 2 in response to Slit2-Robo1 activation which mediated sprouting angiogenesis in the postanatal retina and during embryonic development [45].
4.2. Focal Adhesion Kinase (FAK)
Previous study showed that Slit2 regulated neuronal motility in a focal adhesion kinase (FAK) and Cdc42-dependent manner in vitro [25]. Positive guidance cues such as brain derived neurotrophic factor (BDNF) and laminin increased Cdc42 activity and enhanced cellular protrusion, whereas Slit2 decreased Cdc42 activity and lamellipodia formation in the growth cones thereby reducing neuronal migration, which was disrupted with the expression of constitutively active FAK [25]. These findings suggested that Slit2 regulated neuronal migration by modulating Cdc42 activity in a FAK-dependent manner. The downstream effectors of Cdc42 such as p21-activated kinase (PAK) and N Wiskott Aldrich Syndrome Protein (N-WASP) modulate actin polymerization and therefore may affect cell migration [25].
4.3. Src and Extracellular Signal-regulated Kinase (Erk) 1/2
Previous study using a glioma blood-tumor barrier (BTB) model in vitro showed that Slit2 had anti-permeability effect on endothelial cells derived from the BTB. Robo4 knockdown increased BTB endothelial permeability by disrupting tight junction proteins which was mediated via the activation of Src-Erk1/2 and matrix metalloproteinase 9 (MMP-9) signaling pathway [29]. Inhibitors of Src and Erk partially reversed the increase in BTB permeability and MMP-9 activity induced by Robo4 knockdown, which showed that Src and Erk1/2 are downstream mediators involved in endothelial Robo4 activation.
5. REGULATION OF SLIT2-ROBO IN THE CNS
5.1. Transcription Factors Pax6
Various regulatory genes control the expression of axonal guidance molecules and thereby regulate the path that axonal fibers travel. Among few studies that have explored the role of regulatory genes and transcription factors that control expression of Slit and its receptor Robo, the transcription factor Pax6 seems to play a role in axonal pathfinding during brain development by regulating Slit proteins [46]. The expressions of Slit1 and Slit2 were downregulated whereas netrin-1 was upregulated in the dorsal thalamus of Pax6 deficient mice [46]. This was associated with aberrant pattern of axonal projections from mammillary bodies to thalamus and midbrain in Pax6 mutant mice [46]. The expression of Slit3 did not change, which was low in wild type and mutant mice. However, expression patterns of the receptor for netrin-1, deleted in colorectal cancer (DCC), and Robo1–3 was similar between Pax mutant and wild type embryonic mice [46]. Mutations in genes that encode Pax6 may lead to reduced Slit1 and Slit2 expression resulting in abnormal axonal pathfinding.
5.2. MicroRNA 218
MicroRNAs (miRNA) are short noncoding single-stranded RNAs, ~22 nucleotides in length, that inhibit the expression of target mRNAs. The intron of the Slit2 and Slit3 genes encode microRNA 218 (miR-218) [47, 48]. The expression of miR-218 was found to be at high levels in the adult mice brain as well as in vascular organs such as the heart, lungs and eyes and showed similar expression patterns to the Slits. Furthermore, miR-218 repressed the expressions of Robo1 and Robo2, and negatively influenced endothelial cell migration which implicated critical roles of miR-218 and Slit-Robo balance for maintaining vascular pattern [47, 48].
MicroRNAs have been shown to function as oncogenes or tumor suppressors in various types of cancers. MicroRNA 218 in particular was downregulated in glioma cell lines and in human glioma tissues [26]. Transfection of glioma cells with miR-218 inhibited the migration and invasion of glioma cells through modulation of Slit2-Robo1 pathway [26], and overexpression of miR-218 inhibited tumorigenesis and proliferation of glioma cells [49]. MicroRNA 218 transfection was associated with increased expression of Slit2 but downregulated the expression of Robo1 in glioma cells by targeting the 3′-untranslated region of Robo1 [26]. These findings are similar to previous studies in which miR-218 was shown to have a tumor suppressive function by inhibiting invasion and progression of gastric and nasopharyngeal cancer cells by downregulating Slit2-Robo1 pathway [50, 51]. MicroRNAs may therefore be important therapeutic targets to reduce the invasiveness of glioma cells by regulating the expression of Slit2 and its receptor Robo.
5.3. Nuclear Scaffold Protein Promyelocytic Leukemia (PML)
The Slit proteins have been implicated to play a role the migration of neural cells as well neoplastic cells in the CNS [52, 53]. Cell migration is an important process both during neurogenesis and glioblastoma multiforme (GBM) aggressiveness. Previous study identified the nuclear scaffold protein promyelocytic leukemia (PML) upstream of Slits in regulating the migration of neural precursor cells (NPCs) and GBM cells. The NPCs derived from PML−/− mice displayed increased proliferation and impaired migration in vitro [52]. Among the various genes involved in regulation of cell migration that were analyzed, Slit2 gene was found to be upregulated in PML−/− cells. Furthermore, the study suggested that the pro-migratory role of PML was mediated via Polycomb repressive complex 2 (PRC2)-mediated repression of Slit genes [52]. The PRC2 induces histone modification and DNA methylation of tumor suppressor genes [53]. This study provides insights into possible upstream regulator of Slit2 that can be targeted in malignant tumors.
5.4. Endocannabinoids and Cannabinoid Receptors
The upstream factors that regulate Slit-Robo signaling, expression and function remains poorly explored. In the developing brain, Slit2 regulates the direction of migrating axons specifically by preventing midline crossing and maintaining the dorsoventral positioning of axons. Endocannabinoids and cannabinoid receptors 1 and 2 have been implicated as upstream regulators of Slit2-Robo1interaction during development of the forebrain axonal tracts [54]. Cannabinoid receptor activation increased the accumulation of Slit2 in the oligodendrocyte end feet and Robo1 at the axonal growth cones, thereby promoting Slit2-Robo interactions during axonal tract formation. These findings have important implications in utero exposure to cannabis as well as in stroke and cerebrovascular diseases in which cannabinoid receptors have been implicated to play important roles.
6. ROLES OF SLIT2 DEPENDENT ON CELL TYPES IN THE CNS
6.1. Slit2 in Endothelial Cells
Previous studies that have examined the role of Slit2 on endothelial cells show that Slit2 provides endothelial protective function in the CNS. Robo4 receptor is the endothelial specific receptor for Slit2 that is expressed in various organs and in human brain microvascular endothelial cells. The vascular protective function of Slit2 has been explored in various models, and Slit2 was found to reduce vascular hyperpermeability which depended on Robo4 receptor in mouse models of pulmonary inflammation and retinopathy [37, 55]. Robo4 null mice had increased severity of lung injury than Robo4+/+ mice when subjected to lung inflammation [55], which proposed a protective function for Slit2-Robo4. An in vitro model of BTB model was utilized to explore the role of Slit2-Robo4 in maintaining BTB stability [29]. The expression of Robo4 was upregulated in human glioma tissues compared to normal brain tissues as well as in glioma cocultured endothelial cells derived from the BTB model [29]. Slit2 pretreatment reduced BTB endothelial permeability but the protective effect of Slit2 was not seen in the presence of Robo4 knockdown. Additionally, Robo4 overexpression reduced BTB permeability which was associated with reduced MMP-9 activity and a corresponding increase in endothelial tight junction proteins [29]. Whereas, Robo4 knockdown increased endothelial BTB permeability 1.41 times higher compared to the control group and elicited the opposite effect on MMP-9 activity and tight junction protein expression. Furthermore, Src and Erk1/2 were downstream mediators involved in regulation of MMP-9 activity by Robo4 [29]. Likewise, we explored the potential BBB protective function of Slit2 following surgical brain injury (SBI), and we observed that Slit2 protected BBB stability in SBI rat model which was dependent on Robo4 receptor signaling pathway [44]. Recombinant Slit2 administration prior to inducing neurosurgical injury reduced post-operative brain edema, BBB permeability and endothelial tight junction protein disruption following SBI compared to control group [44].
However, a dual role of Slit2 has been implicated in the vascular endothelial cells outside of CNS. The endothelial protective function of Slit2-Robo4 was observed following intraocular injections of Slit2 in a retinal vascular hyperpermeability model in mice [37]. Slit2 reduced vascular hyperpermeability in rodent model of lung inflammation [55] and inhibited Andes virus induced disruption of endothelial adherens junction proteins in human pulmonary microvascular endothelial cells (PMECs) [56]. However, vascular permeability was increased in Slit2 transgenic mice compared to wild type mice subjected to laser-induced choroidal neovascularization along with upregulation of Slit2, Robo1, vascular endothelial growth factor receptor 2 (VEGFR2), and phosphorylated Erk 1/2 [57].
Furthermore, Slit-Robo has been implicated in regulation of endothelial cell migration and angiogenesis. Migration and biochemical analysis suggest that Slit2 binds endothelial cells expressing Robo1 and Robo4 and inhibits endothelial migration in vitro [39]. Endothelial cell knockout of Robo4 abrogated this effect leading to a pro-migratory response. However, in vivo studies indicate that Slit mediates angiogenic signals via Robo1 and Robo2, while there is no indication that binding of Slit-Robo4 regulates angiogenesis. Studies outside the CNS indicate that Slit proteins can modulate angiogenesis with Slit2 and Slit3 being implicated to have pro-angiogenic effects [58, 59]. The study by Rama et al. used Slit2 conditional knockout mice which showed that Slit2 was required for retinal angiogenesis. Furthermore, using mice with genetic loss of function of the Robo receptors, the study showed that Slit2 potently promoted retinal angiogenesis via Robo1 and Robo2 receptors in the postnatal mouse retina and in mouse model of oxygen-induced retinopathy [58]. This study indicated that blockade of Slit binding to Robo1 and Robo2 receptors may inhibit pathological angiogenesis in ocular neovascular diseases. In primary osteoblasts, Slit3 was identified as an angiogenic factor negatively regulated by the adaptor protein Schnurri3 (SHN3), a suppressor of osteoblast activity. The study found that Shn3−/− osteoblasts had higher levels of Slit3, and conditioned media derived from Shn3−/− osteoblasts induced endothelial migration and capillary tube formation, which was abrogated with Slit3 blocking antibody. Furthermore, Robo1 receptor was found to mediate the endothelial response to Slit3 in promoting osteogenic response [59].
Various molecular mechanisms involved in Slit-Robo signaling in endothelial cells migration and angiogenesis have been reported. The adaptor proteins Nck1 and 2 binding to Robo1 was required for endothelial cell polarity and migration which contributed to Slit2 mediated sprouting angiogenesis in the postnatal retina and embryonic development [45]. Endocytosis and subsequent receptor signaling has emerged as a mechanism of VEGF signaling during angiogenesis. Slit2 was shown to be involved in VEGFR2 endocytosis, which provides insights into how it may regulate angiogenesis [60]. Slit2-Robo1/2 promoted EndophilinA2-mediated endocytosis of VEGFR2 and downstream PAK activation which promoted endothelial cells migration during angiogenesis [60]. However, in the absence of VEGF the internalized VEGFR was inactive. This may partly explain why Slit2 may have a dual role during angiogenesis, wherein Slit2 mediated internalization of VEGFR reduces receptor availability for signaling and on the other hand, in the presence of VEGF with Slit2 there is enhanced VEGFR2 internalization and downstream activation [60].
Moreover, the endothelial response to Slit2 seems to be dependent on tissue specific expression of the type of Robo receptors. Studies from systemic pathologies indicate that the expression and function of endothelial Robo4 may be context dependent and can vary depending on the pathology. For instance, Robo4 was upregulated in endothelial cells in various neoplasms [29]. The expression of Robo4 was downregulated in virus-infected human PMECs, and the PMECs predominantly expressed Robo4 but not Robo1 [56]. Slit2 inhibited viral-induced permeability in PMECs by interactions with Robo4. However, the anti-permeability effect of Slit2 was not observed in human umbilical vein endothelial cells (HUVECs) which express similar levels of Robo4 and Robo1 receptors [56]. These findings suggest that the endothelial response to Slit2 is dependent on tissue specific endothelial cell expression of Robo receptors.
6.2. Slit2 in Pericytes
Pericytes are special cell types that provide structural support to capillaries and interact with endothelial cells by making direct cell-cell contacts and through paracrine signals. Pericytes play a critical role during physiological and pathological angiogenesis [61]. During initial stage of angiogenesis, pericytes detach from the endothelial cells which enables endothelial cells to migrate and proliferate into surrounding extracellular matrix giving rise to sprouting new vessels which are stabilized by the newly recruited pericytes [62]. The surface of pericytes exhibited higher levels of Robo1 and low levels of Robo4 receptors. Slit2 inhibited platelet derived growth factor (PDGF) induced human brain vascular pericyte motility in an in vitro wound healing assay [63]. Slit2 also inhibited PDGF-induced lamellipodia formation in the pericytes, features suggestive of a pro-migratory phenotype [63]. The pericyte chemorepellent effect of Slit2 was partially decreased but not abolished by antibodies blocking Robo1 and Robo4 receptors [63]. Additionally, pericytes were found to secrete high levels of Slit2 while negligible amounts of Slit1 and Slit3 suggesting that Slit2 secreted by pericytes may act on Robo receptors in an autocrine manner to regulate pericyte function during angiogenesis. These findings provide insights into angiogenic processes that depend not only on endothelial cells but also on pericyte migration. The effect of Slit2 on pericyte function also seems to determine angiogenic regulation by Slit2 which cannot be overlooked.
6.3. Slit2 Inhibition of Leukocyte Migration
The repulsion effect of Slit on migration appears to be conserved across varying cell types. Slit2 has been known for its role in repelling the migration of axons and neurons during CNS development. Additionally, the inhibitory effect of Slit2 on leukocyte migration was demonstrated in vitro studies [9] as well as in systemic diseases such as lung inflammation and glomerulonephritis in rodent models, see table 1 [15, 55]. In the CNS, a few studies have shown that Slit2 modulates cerebrovascular inflammatory response following injury. Altay et al. reported that systemically administered exogenous human Slit protein attenuated cerebral microvessel leukocyte-endothelial adherence following TNFα inflammatory response and after global cerebral ischemia in mice [64]. In the SBI rodent model, we observed that exogenous recombinant Slit2 reduced peripheral leukocyte infiltration to the perisurgical brain tissue, and this anti-migratory effect was mediated via Robo1 and srGAP1 dependent mechanisms [65]. These findings suggest that Slit2 may be a potential anti-inflammatory target in CNS pathologies. Interestingly, a recent study discovered that different isoforms of Slit can have differential effects on neutrophil chemotaxis. The study showed that ~140-kDa N-terminal Slit2 fragment (Slit2-N) had a chemoattractant effect whereas the ~110-kDa N-terminal Slit2 fragment (Slit2-S) was chemorepellent for human neutrophils [66]. Both isoforms mediated their effects via Robo1 receptor activation though downstream signaling pathway was different. This finding has important implications when designing therapeutics given that different immune cells may express Robo receptors differently, and the chemotactic response may therefore depend on the immune cell type and the type of Slit2 fragment.
Table 1.
Summary of studies related to Slit2 in rodent models of CNS disorders.
| Experimental Models | Parameters Evaluated and Treatment | Observations and Results | References |
|---|---|---|---|
| Glioma mouse model (intracranial xenograft) | Slit2, Robo1 expression RT-PCR Intracranial implantation of glioma cells expressing Slit2 gene; histology at 7, 14, 20 days post-implantation Glioma cells transfected with Slit2; in vitro assay |
Slit2 inhibited glioma cell migration and invasion in vitro and in vivo Slit2 downregulated Cdc42 activity through Robo1 receptor |
Yiin et al., Neuro-oncology, 2009 |
| Global cerebral ischemia mouse model (bilateral common carotid artery occlusion) TNFα-induced neuroinflammation mouse model |
Slit subcutaneous delivery osmotic minipump (0.5 μg/kg/h) immediately after BCCAO Human Slit protein (3.0 μg/kg) concomitant administration with TNFα (3.0 μg/kg) intraperitonal Intravital microscopy for leukocyte brain microvessel adherence |
Cerebral microvessel leukocyte adherence 79% ↓ at 24 hours after global cerebral ischemia Leukocyte adherence 98% ↓ at 4 hours after TNFα exposure |
Altay et al., Experimental Neurology, 2007 |
| Transient forebrain ischemia rat model (4 vessel occlusion) | Sit2, Robo1, Robo4 expressions Immunohistochemistry and western blots at days 7, 14, 21, 28 |
Slit2 ↑, Robo1 ↑, Robo4 ↑ Slit2, Robo1 and Robo4 expression increased progressively at days 7–28 in reactive astrocytes of the ischemic hippocampus but not in neurons or microglia |
Park et al., Brain Research, 2016 |
| Transient focal cerebral ischemia rat model (middle cerebral artery occlusion-60 min) | Slit2, Robo1, Robo2, Robo4 expressions Immunofluorescence staining at 3 days, 1, 2, 3, 4 and 10 weeks after ischemia |
Slit2 ↑, Robo (1, 2, 4) ↑ Expression was induced days 7–14 after reperfusion and progressively increased up to 10 weeks after ischemia Astroglial induction of Slit2, Robo occurred in peri-infarct area |
Jin et al., Neurochemical Research, 2016 |
| Neonatal hypoxic ischemic encephalopathy (HIE) rat pup model | Slit2, Robo1 expressions and cellular localization Western blot at 6, 12, 24, 72 hours post-HIE Recombinant Slit2 (3 μg/kg, 10 μg/kg, 30 μg/kg) intranasal treatment 1 hour post-HIE |
Slit2 ↑, Robo1 ↑ (peak at 72 hours), co-localized with neurons Recombinant Slit2 (10 μg/kg) reduced infarct area, neuron apoptosis and improved neurofunction up to 4 weeks Recombinant Slit2 downregulated RhoA, Bax and CC3, which was reversed with recombinant Robo1 or sRGAP1 siRNA |
Kaur et al., Neuropharmacology, 2019 |
| Traumatic brain injury (TBI) mouse model (cryo injury) | Slit1-Slit3 expression Immunohistochemistry and Combined double fluorescence in situ hybridization at days 2, 7, 14 post-injury |
Slit2 predominantly expressed surrounding necrotic tissue, Slit1 and Slit3 much weaker expression Slit2 mRNA ↑ by day 2, peaked at day 7 followed by decline at day 14 post-injury Slit2 was expressed by proximal reactive astrocytes surrounding the lesion but not distal astrocytes, microglia or oligodendrocytes |
Hagino et al, Glia, 2003 |
| Surgical brain injury (SBI) rat model (partial frontal lobe resection) | Slit2, Robo1 expressions and cellular localization ELISA, Western blot at 24, 72 hours and day 7 post-SBI Recombinant Slit2 (1 μg/kg, 3 μg/kg, 10 μg/kg) intraperitoneal pretreatment 1 hour before SBI |
Slit2 ↑, Robo1 ↑ in perisurgical brain tissue at 24, 72 hours and day 7 post-SBI Slit2 expressed by neurons, astrocytes, and Robo1 expressed by neutrophils, leukocytes Recombinant Slit2 (10 μg/kg) reduced brain edema, neuroinflammation and improved neurobehavior after SBI Recombinant Slit2 reduced CD45, MPO and Cdc42 activity, which was reversed with recombinant Robo1 or srGAP1 siRNA |
Sherchan et al., Neurobiology of Disease, 2016 |
| Surgical brain injury (SBI) rat model (partial frontal lobe resection) | Slit2, Robo4 expressions and cellular localization Western blot 6, 12, 24, 72 hours post-SBI Recombinant Slit2 (10 μg/kg) intraperitoneal pretreatment 1 hour before SBI |
Slit2 ↑ in perisurgical brain tissue at 6 to 72 hours after SBI and Robo4 ↔ Robo4 expressed by endothelial cells, astrocytes and neurons Recombinant Slit2 reduced BBB permeability and restored tight junction proteins after SBI Slit2 increased Rac1 activity, which was reversed by Robo4 and Paxillin siRNA |
Sherchan et al., Scientific Reports, 2017 |
| Epilepsy rat model (lithium-pilocarpine induced) Intractable temporal lobe epilepsy (TLE) patients |
Slit2 expression in rat brain samples at 1, 7, 14, 30 and 60 days post-seizure Slit2 expression in temporal neocortex samples from TLE patients Immunohistochemistry and Western blots |
Slit2 ↓ days 1–14 after seizure followed by Slit2 ↑ with peak at days 30 and 60 after seizures Slit2 was expressed by neurons in acute phase after epilepsy and expressed mainly by astrocytes in chronic phase Slit2 expressed in neurons in controls and Slit2 ↑ in TLE patients, expressed by neurons and astrocytes |
Fang et al., Brain Research, 2010 |
| Spinal cord injury rat model Spinal cord injury female rat model Spinal cord injury mouse model |
Slit2, Robo1 mRNA and protein expression Slit2-N treatment Inhibition of Robo1 or RhoA Slit1–3 and Robo1–3 expressions |
Slit mRNA and protein ↑ at day 7, peaked day 14, normal levels by day 21 Robo1 ↔ Slit2 ↓, Robo1 and RhoA ↑ Slit2-N treatment and inhibition of Robo1 or RhoA enhanced synapse formation at 6 weeks following injury and promoted functional recovery over 8-week period after the injury Slit1 and Slit3 mRNAs were detected at the center of lesion 8 days after injury but not Slit2 |
Liu et al., Acta Histochemica, 2011 Li et al., Neuroreport, 2017 Wehrle et al., European Journal of Neuroscience, 2005 |
| Intracerebral hemorrhage (ICH) mouse model (collagenase induced) Slit2 overexpressing transgenic (Slit2-Tg) mice |
BBB permeability in vivo Miles assay and amyloid-β permeability Choroid plexus structure and electron microscopy Lateral ventricle area and pressure |
Increased brain microvessel density and permeability in Slit2-Tg mice compared to wild type mice Lateral ventricle area and pressure increased in Slit2-Tg mice Slit2-Tg mice had larger ICH volume compared to control mice |
Han and Geng., Acta Pharmacologica Sinica, 2011 |
| Slit2 overexpressing transgenic mice | BBB permeability Neuronal apoptosis Amyloid-β deposition |
BBB permeability, hippocampal neuronal apoptosis, Aβ deposition, acetylcholinesterase expression increased in Slit2 transgenic mice | Li et al., Journal of Alzheimers Disease, 2015 |
| Mild traumatic brain injury (TBI) mouse model (closed-skull compression) Slit2 overexpressing transgenic (Slit2-Tg) mice |
In vivo two-photon imaging BBB leakage of Rhodamine B Cell death, propidium iodide (PI) staining 6 and 24 hours after TBI |
Increased BBB leakage and cell death occurred after TBI in Slit2-Tg mice compared to C57 control mice | Li et al., Neuroscience Letters, 2016 |
| Slit2 overexpressing transgenic (Slit2-Tg) aged mice (15-month old) | In vivo two-photon fluorescence imaging Immunofluorescence staining Morris water maze test |
Paravascular pathway clearance improved by inhibition of astrocyte activation and maintenance of AQP4 polarity in Slit2-Tg aging mice BBB disruption and Aβ accumulation reduced, spatial memory improved in Slit2-Tg aging mice |
Li et al., International Journal of Molecular Medicine, 2018 |
Table 1.Summary of studies examining role of Slit2 in preclinical models of CNS pathology. Abbreviations: Robo, roundabout; RT-PCR, reverse transcriptase polymerase chain reaction; BCCAO, bilateral common carotid artery occlusion; HIE, hypoxic-ischemic encephalopathy; CC3, cleaved caspase 3; srGPA1, Slit-Robo GTPase activating protein 1; siRNA, small interfering ribonucleic acid; TBI, traumatic brain injury; SBI, surgical brain injury; ELISA, enzyme linked immunosorbent assay; MPO, myeloperoxidase; TLE, temporal lobe epilepsy; ICH, intracerebral hemorrhage; Slit2-Tg, Slit2 transgenic; BBB, blood brain barrier; AQP4, aquaporin 4. The following are descriptions of symbols used in the table: increased expression ↑, decreased expression ↓, no significant change in expression ↔
7. PRECLINICAL STUDIES OF SLIT2 AND ROBO IN THE CNS
7.1. Role of Slit2 During CNS Development
7.1.1. Slit2 in Guidance of Axonal Migration and Neurogenesis
Multiple factors are involved in guiding the axons projecting from diverse neuronal populations in the brain towards appropriate direction during CNS development [67]. Among such factors, Slit2-Robo signaling has emerged as a conserved system that guides path of axons in the developing nervous system across species. Slit proteins in the brain are crucial during development of forebrain axons since it maintains the proper position, extension and crossing of axons and axonal tracts in the forebrain [13]. During development the forebrain exhibits complementary pattern in the expression of Slit1 and Slit2 proteins with the expressions of Robo1 and Robo2. The projecting axons avoid regions expressing high levels of Slit2 mRNA during development of the axonal tract projections in embryonic mice [13]. Slit2 therefore acts as an axonal repellant thereby, guiding migration of axons in the proper direction. Additionally, Slit mutant mice exhibited abnormal patterns and positioning of axonal tract projections during development [13]. Studies using Slit or Robo knockout mice showed that axons abnormally approached and crossed the midline during development in different regions of the brain [68–70]. Additionally, Robo2 knockdown led to severe loss of forebrain descending tract fibers. There is some evidence that in addition to Slit2, other Slits also aid in guiding the developing axons. For instance, Slit2 antisense morpholino oligonucleotides that targeted translation of Slit2 mRNA reduced the formation of axonal projections, and simultaneous inhibition of both Slit2 and Slit3 function further reduced the projecting axons [67].
Recent paper showed that Slit-Robo signaling can also modulate cortical neurogenesis. The study discovered that inhibition of Robo signaling drives indirect neurogenesis, a process which involves the production of intermediate progenitor cells (IPCs) that ultimately divide to produce neurons. This study suggested that attenuation of Robo receptor signaling as the mechanism underlying the evolutionary expansion of complex mammalian cerebral cortex [71].
7.2. Role of Slit2 in CNS Disorders
7.2.1. Slit2 in Glioma
Given the repellant function of Slit2, its role in the migration and invasiveness of glioma cells have been explored in rodents. Malignant glioma is the most common primary brain tumor in adults and is associated with high mortality and poor prognosis [26]. The invasive nature of glioma increases the risk of its recurrence despite surgical removal, and invasiveness is the most common indicator for poor prognosis in glioma patients. Dysregulation of cell motility has been postulated to be involved in glioma cell infiltration thereby promoting invasive nature of the tumor [26, 43]. While the role of Slit-Robo system in axon and neuronal migration is well established, fewer studies have examined the role of Slit2 in CNS tumors. Slit2 is normally expressed by neurons and astrocytes in the normal brain and evidence from published studies indicate that Slit2 has tumor suppressive function [24, 72]. In human glioma cells, Slit2 was expressed at very low levels and the expression was lowered further more so with malignant progression [24, 43, 72]. However, Robo1 protein and mRNA was highly expressed in different grades of glioma and the percent of tumors that expressed Robo1 increased as tumor invasiveness progressed [24, 72]. Mertsch et al. suggested that hypermethylation of SLIT2 promoter associated with increasing grade of glioma may be responsible for the lower expression of Slit2 in high grades tumors similar to what has been reported in peripheral tumors outside the CNS including colorectal, cervical and renal cancers [73–75].
There is some supporting evidence that methylation of SLIT2 gene regulates its expression in glioma cells. While normal brain samples lacked any methylation at the SLIT2 promoter region, the CpG island on SLIT2 promoter was frequently hypermethylated in 59% (37/63) of glioma tumors analyzed and in 71% (5/7) glioma cell lines with a corresponding loss in Slit2 expression, which was restored after treatment with demethylating agent 5-aza-2’-deoxycytidine in glioma cell lines [76]. This was associated with reduced expression of Slit2 depending on the degree of methylation of the promoter. Furthermore, treatment with demethylating agent restored SLIT2 gene expression in the methylated glioma cell lines but did not change the expression of SLIT2 in unmethylated glioma cell lines indicating that hypermethylation of SLIT2 promoter reduced the expression of Slit2 in the tumors [76].
The Slit-Robo system has been shown to be important regulators in guiding motility of various cell types; few studies examined the involvement of Slit2 and Robo in tumor cell migration in the CNS. Yinn et al. observed that glioma cells treated with recombinant Slit2 or stably transfected with Slit2 gene exhibited reduced capacity to migrate and invade when compared to controls in vitro [43]. In addition, the capacity of tumor cells to infiltrate and invade into brain parenchyma was impaired when glioma cells transfected with Slit2 gene were implanted intracranially in the mouse brain [43]. When glioma cells were transfected with Robo siRNA, the anti-migratory effects of Slit2 was reversed suggesting that Slit2 inhibited glioma cell migration in a Robo1 dependent manner [43]. Similar findings were observed by Mertsch et al. in which Slit2 reduced migration of glioma cell lines which was neutralized by knockdown of Robo1 in vitro [24]. These observations suggest that Slit2-Robo1 has a critical role in glioma cell migration which provides important knowledge in understanding the invasive nature of gliomas. Since glioma cell invasion into brain parenchyma is a major contributor to tumor recurrence, the tumor suppressive role of Slit2 may provide novel treatment strategies against malignant gliomas.
7.2.2. Slit2 in Cerebral Ischemia
The expression of Slit2 and Robo receptors has been shown to be altered after cerebral ischemia in rodent models. In a study by Park et al., the temporal changes and cellular expression of Slit2, Robo1 and Robo4 was characterized for up to 28 days after transient forebrain ischemia in a rat model [22]. Slit2 was constitutively expressed by neurons in control animals with negligible expression in glial cells. Following ischemic injury, Slit2 immunoreactivity was increased in the ischemic hippocampus which was evident at day 3 after injury with progressive increases seen at days 7, 14 and 28 after injury. Slit2 expression was selectively induced in reactive astrocytes with negligible expression in microglia in the ischemic hippocampus [22]. The spatiotemporal distribution of Robo was similar to that of Slit2. In sham animals, Robo1 and Robo4 was localized to the neurons but were absent in astrocytes or microglia. However, in the ischemic hippocampus, the expression of Robo1 and Robo4 was induced in reactive astrocytes in a similar pattern as Slit2 which was noted at day 3 and further increased at days 7, 14 and 28 after reperfusion [22]. The induction of Slit2 and Robo expression coincided with reactive astrocytosis after transient ischemia, and the study suggested that Slit2-Robo potentially modulate glial response during the recovery phase. Additionally, these findings suggest a novel role for Robo4 in reactive astrogliosis and long-term recovery after ischemic injury in addition to its conventional role in modulating the vascular system.
In a study by Jin et al., the spatiotemporal distribution pattern of Slit2 and Robo receptors Robo1, Robo2 and Robo4 were evaluated for up to 10 weeks following transient cerebral ischemia in a middle cerebral artery occlusion (MCAO) rat model [23]. Slit2 and Robo receptors shared an overlapping expression patterns in the ischemic striatum. Slit2 and Robo were expressed by neurons in sham rats, and following transient cerebral ischemia the expression of Slit2 and Robo receptors was observed predominantly in reactive astrocytes in the peri-infarct area which was evident by day 3 after reperfusion and progressively increased during 10 week observation period [23]. The long-lasting induction of Slit2 and Robo predominantly occurred in reactive astrocytes surrounding the infarct area, suggesting that Slit2 and Robo are related to reactive astrocytes after ischemic stroke. Reactive gliosis can provide a protective barrier and limit the progression of injury but on the other hand it may hinder axonal regeneration which may be detrimental. Additionally, the outcome of astroglial response may also vary depending on the time point when Slit2-Robo induction and reactive gliosis occurs following an injury, which is essential to be considered when designing therapeutics.
Slit2 also modulated leukocyte migration to the brain following cerebral ischemia. Slit2 can inhibit the migration of various cell types including neurons, pericytes, endothelial cells, and peripheral leukocytes [5, 77]. The inhibitory effect of Slit2 on peripheral leukocyte migration to the brain has been a recent addition to the spectrum of anti-migratory effects of Slit2 on various cell types. Altay et al. explored the role of Slit to reduce cerebral leukocyte migration in rodent models [64]. Exogenous Slit administration decreased leukocyte adherence by 98% in cerebral microvessels 4 hours following TNFα exposure [64]. Similar effects were observed after experimental global cerebral ischemia in which 79% reduction in leukocyte adherence was observed 24 hours following bilateral common carotid artery occlusion induced global cerebral ischemia in mouse model [64]. Administration of RoboN, the soluble receptor for Slit reversed the inhibitory effect of Slit on leukocyte migration to cerebral microvessels after global ischemia. Additionally, neutralizing endogenous Slit exacerbated TNFα induced cerebral microvessel leukocyte recruitment suggesting an anti-migratory role of both endogenous and exogenous Slit following cerebrovascular inflammation [64]. A recently published study from our lab explored the role of Slit2 in hypoxic-ischemic encephalopathy (HIE) injury in a neonatal rat model [78]. Deprivation of oxygen supply to the neonatal brain due to asphyxia during perinatal period causes brain injury which can lead to long term neurological disabilities including mental retardation, cerebral palsy, seizures and even mortality in some cases [79]. Neuronal apoptosis is a major cause of neuronal cell death following hypoxic-ischemic injury to the brain [78, 80]. Administration of recombinant Slit2 reduced neuronal apoptosis and improved long-term neurological deficits up to 4 weeks after neonatal HIE in rat pups. There was reduction in brain infarct volume, cell death and improved neurological function in HIE rats that were treated with recombinant Slit2. Furthermore, the study utilized recombinant Robo1 as a decoy receptor and knocked down Slit-Robo GTPase activating protein 1 (srGAP1) which reversed the protective effects of Slit2. This study elicited that the neuroprotective role of Slit2 was potentially mediated through activation of Slit2-Robo1 and srGAP1 pathway [78]. These studies provide evidence of the diverse functions of Slit2 which can target critical pathologies commonly encountered after ischemic brain injuries.
7.2.3. Slit2 in Traumatic and Surgical Brain Injury
The role of Slit2 after brain injury has been examined in a few studies. In a cryo-injured traumatic brain injury (TBI) mouse model Slit2 was found to be highly expressed by neurons and astrocytes surrounding the lesion [21]. Slit2 was the predominant member of Slit family proteins expressed in the surrounding necrotic tissue following cryo-injury, while Slit1 and Slit3 showed much weaker expression [21]. Slit2 mRNA was elevated during the early phase of injury by day 2 and reached a peak at day 7 followed by a decline in expression on day 14 post-injury. The proximal reactive astrocytes surrounding the lesion expressed Slit2 but not the distal astrocytes, microglia or oligodendrocytes [21]. Furthermore, Slit2 co-localized with the heparan sulfate proteoglycan, glypican-1, indicating a possible involvement of Slit2 and glypican-1 interaction during recovery after TBI. The heparin sulfate chain of glypican-1 binds with high affinity to Slit2 and the authors postulated that such a binding may increase the interaction of Slit2 with Robo during recovery after injury.
Likewise, we observed that Slit2 increased in the surrounding perisurgical brain tissue following SBI in a rat model [65]. The SBI rat model represents a clinical scenario of injury to the brain that occurs due to inadvertent injury that can be inflicted to the perisurgical brain tissue during neurosurgical procedures in the brain [81–83]. We observed that Slit2 played a role against neuroinflammation and endothelial protection in SBI rodent model. Slit2 was expressed by both neurons and astrocytes in the perisurgical brain tissue surrounding the neurosurgical injury [65]. Slit2 was increased as an endogenous protective response to neurosurgical injury, since knockdown of Slit2 worsened outcomes in SBI rat model. Additionally, recombinant Slit2 administration improved outcomes after surgical brain injury by reducing brain edema and neuroinflammation in the perisurgical brain tissue [65]. Slit2 reduced the infiltration of peripheral immune cells as indicated by reduced leukocytes and neutrophils infiltration at the injury site [65]. The protective effects of Slit2 against immune cell infiltration were mediated via Robo1-srGAP1 mediated inhibition of Cdc42 activity [65]. Recombinant Slit2 also preserved BBB tight junction proteins following SBI [44]. The protective mechanism of Slit2 against BBB disruption was mediated via Robo4 receptor and Paxillin dependent activation of Rac1 signaling pathway, thereby preserving the expression of endothelial junction proteins [44]. These findings demonstrate pleiotropic effects of Slit2 which makes it a potential therapeutic target for CNS disorders given that Slits and Robo are expressed by all cell types in the neurovascular unit. These studies indicate that Slit2 is increased in response to injury possibly as the body’s protective response to aid with the recovery. These findings have direct clinical implications since neurosurgical injury is often overlooked and therapeutic targets will provide opportunities for treatments to reduce potential post-surgical complications.
7.2.4. Slit2 in Epilepsy
The expression and spatiotemporal change of Slit2 in relation to synaptic plasticity after temporal lobe epilepsy was characterized by Fang et al. The study evaluated the expression of Slit2 after temporal lobe epilepsy using temporal neocortex samples from patients with intractable temporal lobe epilepsy and in experimental animals [84]. While Slit2 was mainly expressed by neurons in normal human controls, samples taken from patients with intractable temporal lobe epilepsy showed that Slit2 was increased and expressed by both neurons and astrocytes [84]. Similar findings were observed in the experimental rat model with lithium-pilocarpine administration induced seizures [84]. Slit2 gradually decreased in the acute phase from days 1 to 14 post-seizure compared to controls and then increased to reach peak levels at 30 and 60 days [84]. Slit2 was expressed by neurons in control rats and during the acute and latent phase of epilepsy in the rat model. However, during the chronic phase at 30 and 60 days, Slit2 was mainly expressed by astrocytes [84]. This study suggests that Slit2 could be an important guidance molecule to direct axonal sprouting towards the correct target during remodeling phase after epilepsy [84]. Changes in Slit2 expression during the course of epilepsy may affect the formation of neural circuits and mossy fiber projections, and this can have important implications in the plasticity and recovery after epilepsy. The authors suggest that downregulation of Slit2 during the acute phase after epilepsy may facilitate remodeling that promotes seizures, whereas upregulation of Slit2 during the chronic phase may prevent abnormal sprouting. Further investigation into the role of Slit2 after epilepsy is necessary to delineate its function after epilepsy.
7.2.5. Slit2 in Spinal Cord Injury
The role of Slits in the spinal cord injury remains less studied compared to brain injuries. During recovery after spinal cord injury the environmental repellent and attractant cues have been implicated to play important roles to guide the axonal growth cones. Few studies have examined the role of Slit and Robo in axonal pathfinding and synapse formation during spinal cord injury. The expression of a number of guidance and synaptogenic molecules including Slits were found to be present in the spinal cord in healthy adult mice and following injury [85]. In a rat model of traumatic spinal cord injury, the expression profiles of Slit2 and Robo1 was evaluated which showed that both the mRNA and protein levels of Slit2 was upregulated while Robo1 remained unchanged following injury [86]. Slit2 expression was increased at day 7 and peaked at day 14 after spinal cord injury and returned to normal levels 21 days after injury in the rat model [86]. Slit2 was detected in the neuronal plasma membrane but did not co-localize with glial fibrillary acidic protein (GFAP)-positive astrocytes [86]. Furthermore, Slit2 was identified to promote synaptogenesis during recovery after spinal cord injury in a female rat model. Slit2 was decreased but Robo1 and RhoA increased after injury, possibly delaying recovery after the injury. The study demonstrated that treatment with Slit2-N or inhibition of Robo1 or RhoA enhanced synapse formation at 6 weeks following injury and promoted functional recovery over 8-week period after the injury [87].
In the mouse spinal cord injury model, Slit1 and Slit3 mRNAs were detected at the center of the lesion 8 days after injury but not Slit2 [88]. Furthermore, the Slits and Robo1–3 were expressed by macrophagic or fibroblastic cells. The study suggested that Slits and other chemotactic factors possibly control the migration of macrophages, angiogenesis and lesion scarring, which may inhibit axonal outgrowth following injury.
Likewise, in a mouse model of sciatic nerve transection injury the changes in Slit1–3 and Robo1–3 expressions in the spinal cord, dorsal root ganglia (DRG) and sciatic nerve was evaluated during 4 to 14 days after injury [89]. Although there were no significant changes in Slit2 and Robos in the spinal cord, a dynamic change in their expressions was observed in the peripheral nervous system following injury. Slit2 was downregulated in the distal nerve stump after the injury and gradually upregulated in the proximal stump upon axonal regeneration. Slit3 was the major ligand expressed in the nerve bridge and distal sciatic nerve stump, and Robo1 and Robo2 changes were observed after injury [89]. The study indicated that Slit3-Robo signaling played important role in peripheral nerve regeneration and Slit2 maintains homeostasis of peripheral nerves but may not play important roles in the early stage of peripheral nerve regeneration [89].
7.3. Transgenic Overexpression of Slit2 in CNS Pathology
Few studies have explored CNS pathology using Slit2 transgenic animals. Transgenic mice overexpressing Slit2 had increased brain microvessel density compared to wild type mice, and transgenic mice were more susceptible to collagenase induced intracerebral hemorrhage (ICH) [90]. Transgenic mice exhibited larger hemorrhagic areas and increased hemorrhage volume compared to control mice indicating that the vessels in Slit2 transgenic mice were fragile and susceptible to greater injury given the same degree of insult [90]. There was increased brain microvessel permeability in Slit2 transgenic mice demonstrated by Evans blue dye extravasation and Amyloid-β 40 peptides leakage from serum into the brains of Slit2 transgenic mice but not in the wild type mice [90]. Likewise, transgenic mice overexpressing human Slit2 exhibited increased BBB permeability and Alzheimer’s disease-like alterations characterized by behavioral changes, hippocampal neuron apoptosis, and amyloid-β protein deposition [91]. Similarly, transgenic overexpression of Slit2 in mice increased BBB permeability and cell death compared to control mice following mild traumatic brain injury in a closed-skull TBI model [92]. On the other hand, transgenic overexpression of Slit2 in aged mice showed that Slit2 overexpression improved the function of paravascular pathway and BBB integrity by inhibiting astrocyte activation and maintaining aquaporin 4 (AQP4) polarity in 15-month old Slit2 transgenic mice [93]. This study has important clinical implications for aging individuals and neurodegenerative disorders in which defects in paravascular pathways have been associated with increased amyloid-β deposition [94]. Further understanding of the role of Slit2 in aging will provide potential novel strategies against neurodegenerative disorders. These studies highlight an important point that the duration and extent of Slit2 expression should be taken into consideration when designing Slit2 based therapeutics. Additionally, the expression and function of Slit2 may vary dependent on age.
8. CONCLUSIONS
The role of Slit2 in CNS development has been well established; however, its regulation in CNS diseases is a new field that has been explored in the recent decade with preclinical studies in various models such as ischemic stroke, glioma, neurosurgical brain injury and traumatic brain injury. These studies indicate that Slit2 has a wide range of biological effects including inhibition of peripheral immune cell migration, BBB protection, anti-apoptotic function and anti-migratory effects in CNS pathology. As regards the endothelial function of Slit2, findings from transgenic mice studies and exogenous Slit2 administration should be taken into consideration when determining the endothelial effects of Slit2. Various disease models showed endothelial protective function of exogenous Slit2 administration including in the brain. However, transgenic mice studies indicate that Slit2 overexpression increased brain microvessel density and promoted BBB permeability [90–92]. The variability in Robo receptor types expressed by different tissues and the underlying pathology may determine endothelial effects of Slit2, with predominantly Robo4 expression indicative of protective endothelial function. Additionally, existing research also suggests that endogenous Slit2 overexpression and exogenous Slit2 possibly have diverse functions and mechanisms on the endothelial cells. In Slit2 transgenic mice, blood vessels were more susceptible to collagenase induced ICH and had larger hemorrhagic volumes compared to control mice [90]. The findings in this study conflict with the protective effects of Slit2 observed in other animal models of CNS disorder [24, 64, 65, 78]. These findings could be attributed to differences in the animal models used, differential mechanism of endogenous versus exogenous Slit2, and differences in the magnitude of exposure to Slit2 in transgenic animals as opposed to exogenous recombinant Slit2 administration. Although the temporal pattern and duration of Slit2 expression in transgenic animals subjected to injury and the mechanism of injury was not explored, these findings necessitate further exploration of the function of Slit2 in CNS disorders.
9. EXPERT OPINION
9.1. Roles of Other Members of Slit Proteins
Most of the studies in CNS disorders have focused on the roles of Slit2 even though there are three known isoforms of Slit proteins (Slit1-Slit3). This review focused Slit2 studies but other Slit members regulate various processes which may be relevant to CNS disorders too. For instance, Slit1 is predominantly expressed in the developing CNS and Slit3 has been reported to have anti-permeability effects following VEGF induced retinal hyperpermeability [1, 37]. It is likely that Slit1 and Slit3 may be involved during recovery after CNS injury. A detailed examination of all members of Slit family proteins is warranted.
9.2. Exploration of Robo Receptor Subtypes in CNS Pathology
The effects of Slit2 may depend on the type of receptors predominantly expressed following an injury. The expression and function of Robo receptors may be tissue specific and context dependent. For instance, Slit2 reduced permeability in human PMECs that predominantly express Robo4, but the anti-permeability effect of Slit2 was not observed in HUVECs which express similar levels of Robo4 and Robo1 receptors [56]. Therefore, an in depth understanding of Robo receptors and changes that may occur in CNS pathology requires further exploration
9.3. Role of Alternate Signaling Pathways in Slit2 Mediated Protection
Few downstream targets of Slit2-Robo signaling pathways have been identified in the CNS which provides a glimpse into intracellular mechanisms by how Slit2 exerts its effects. Downstream effectors such as Abelson kinase (Abl) and Enabled (Ena) can interact with the intracellular motif of Robo and regulate cell migration. Abl antagonizes the repulsive effects of Robo whereas, Ena enhances Robo mediated repulsive signaling pathway [12]. Other small GTPases including RhoA and Rac1 regulate cellular actin organization and motility, which can impact cell motility and endothelial function of Slit2 [95, 96]. Slit2 can also inhibit chemokine induced Src kinase activity and Lck kinase activity which have been reported to regulate cell migration [8]. Slit2 can therefore possibly activate various intracellular signaling pathways, which may play a role in the diverse functions of Slit2 in CNS pathologies.
9.4. Potential Upstream Regulators of Slit2
Preclinical studies in CNS animal models showed that endogenous Slit2 levels increased after SBI and neonatal hypoxic ischemic encephalopathy as a protective response to counteract the consequences injury. However, much remains unknown about how the expression of Slit2 is regulated following CNS injury. Some upstream regulators have been identified that modulate the expression of Slit-Robo in the CNS. The transcription factor such as Pax6 has been shown to regulate the expression of Slit2 [46]. Additionally, changes in SLIT2 gene, for instance hypermethylation of the gene in systemic tumors and glioma have been observed [73, 76]. The upstream regulators of Slit2 could be important targets for potential therapeutic manipulation. Further exploration into the endogenous regulation of Slit2 is essential to understand its role and response in CNS pathology.
Current research suggests that Slit2 can target a wide array of pathophysiological consequences, which extends beyond anti-migratory function, in various preclinical models of CNS disorders. Few areas remain relatively unexplored such as the potential role of other members of Slit proteins and how they compare to Slit2, detailed exploration of the function of Robo receptor types, potential mechanisms and regulators of Slit2. Further research is crucial to understand key issues including transgenic overexpression versus exogenous Slit2 effects, cell dependent functions of Slit2 and the effects of Slit2 dependent on the underlying pathology for potential translation as a therapeutic target in CNS pathologies.
ARTICLE HIGHLIGHTS.
Upregulation of Slit2 expression and prominent astroglial induction of Slit2 has been observed in various CNS pathologies.
Slit2 affects a wide range of biological processes following injury including anti-inflammatory, anti-migratory, anti-apoptotic and BBB protection, which makes it a potential therapeutic target.
Exogenous Slit2 administration has been utilized as a therapeutic strategy in various preclinical models.
The effects of Slit2 are induced via Robo receptors-mediated activation of intracellular signaling pathways and dependent on the type of Robo receptors expressed by the cells.
Further studies are required to fully elucidate the complex roles of Slits and Robo in CNS disorders for potential therapeutic application.
Funding
The work of the author was partially supported by NIH grant NS082184 to JHZ.
LIST OF ABBREVIATIONS
- CNS
Central nervous system
- Robo
Roundabout
- BBB
Blood brain barrier
- LRR
Leucine-rich repeats
- EGF
Epidermal growth factor
- Ig
Immunoglobulin
- FN
Fibronectin
- siRNA
Small interfering ribonucleic acid
- FAK
Focal adhesion kinase
- BDNF
Brain derived neurotrophic factor
- PAK
p21-activated kinase
- N-WASP
N Wiskott Aldrich syndrome protein
- Erk1/2
Extracellular signal-regulated kinase
- BTB
Blood tumor barrier
- MMP-9
Matrix metalloproteinase 9
- miRNA
MicroRNA
- miR-218
MicroRNA 218
- PML
Promyelocytic leukemia
- GBM
Gliobalstoma multiforme
- NPC
Neural precursor cell
- PRC2
Polycomb repressive complex 2
- SBI
Surgical brain injury
- PMECs
Pulmonary microvascular endothelial cells
- VEGFR2
Vascular endothelial growth factor receptor 2
- SHN3
Schnurrin 3
- VEGF
Vascular endothelial growth factor
- HUVECs
Human umbilical vein endothelial cells
- PDGF
Platelet derived growth factor
- IPCs
Intermediate progenitor cells
- MCAO
Middle cerebral artery occlusion
- HIE
Hypoxic-ischemic encephalopathy
- srGAP1
Slit-Robo GTPase activating protein 1
- TBI
Traumatic brain injury
- GFAP
Glial fibrillary acidic protein
- DRG
Dorsal root ganglia
- AQP4
Aquaporin 4
- DCC
Deleted in colorectal cancer
- ICH
Intracerebral hemorrhage
- Abl
Abelson kinase
- Ena
Enabled
Footnotes
Declarations of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Marillat V, Cases O, Nguyen-Ba-Charvet KT, Tessier-Lavigne M, Sotelo C, Chedotal A: Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol 2002, 442(2):130–155. [DOI] [PubMed] [Google Scholar]
- 2.Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S: slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev 1990, 4(12A):2169–2187. [DOI] [PubMed] [Google Scholar]; **This article describes the extracellular matrix protein Slit and its role in axonal guidance.
- 3.Rothberg JM, Hartley DA, Walther Z, Artavanis-Tsakonas S: slit: an EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system. Cell 1988, 55(6):1047–1059. [DOI] [PubMed] [Google Scholar]
- 4.Yuen DA, Robinson LA: Slit2-Robo signaling: a novel regulator of vascular injury. Curr Opin Nephrol Hypertens 2013, 22(4):445–451. [DOI] [PubMed] [Google Scholar]
- 5.Wong K, Park HT, Wu JY, Rao Y: Slit proteins: molecular guidance cues for cells ranging from neurons to leukocytes. Curr Opin Genet Dev 2002, 12(5):583–591. [DOI] [PubMed] [Google Scholar]
- 6.Guan KL, Rao Y: Signalling mechanisms mediating neuronal responses to guidance cues. Nat Rev Neurosci 2003, 4(12):941–956. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi S, Robinson LA: Slit2-Robo signaling in inflammation and kidney injury. Pediatr Nephrol 2015, 30(4):561–566. [DOI] [PubMed] [Google Scholar]
- 8.Prasad A, Qamri Z, Wu J, Ganju RK: Slit-2/Robo-1 modulates the CXCL12/CXCR4-induced chemotaxis of T cells. J Leukoc Biol 2007, 82(3):465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu JY, Feng L, Park HT, Havlioglu N, Wen L, Tang H, Bacon KB, Jiang Z, Zhang X, Rao Y: The neuronal repellent Slit inhibits leukocyte chemotaxis induced by chemotactic factors. Nature 2001, 410(6831):948–952. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This article identified the leukocyte chemotaxis inhibitory role of Slit.
- 10.Rothberg JM, Artavanis-Tsakonas S: Modularity of the slit protein. Characterization of a conserved carboxy-terminal sequence in secreted proteins and a motif implicated in extracellular protein interactions. J Mol Biol 1992, 227(2):367–370. [DOI] [PubMed] [Google Scholar]
- 11.Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T: Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 1999, 96(6):795–806. [DOI] [PubMed] [Google Scholar]
- 12.Ballard MS, Hinck L: A roundabout way to cancer. Adv Cancer Res 2012, 114:187–235. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This article is a comprehensive review of Robo receptors.
- 13.Nguyen-Ba-Charvet KT, Chedotal A: Role of Slit proteins in the vertebrate brain. J Physiol Paris 2002, 96(1–2):91–98. [DOI] [PubMed] [Google Scholar]
- 14.Blockus H, Chedotal A: The multifaceted roles of Slits and Robos in cortical circuits: from proliferation to axon guidance and neurological diseases. Current opinion in neurobiology 2014, 27:82–88. [DOI] [PubMed] [Google Scholar]
- 15.Kanellis J, Garcia GE, Li P, Parra G, Wilson CB, Rao Y, Han S, Smith CW, Johnson RJ, Wu JY et al. : Modulation of inflammation by slit protein in vivo in experimental crescentic glomerulonephritis. The American journal of pathology 2004, 165(1):341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong M, Jun T, Nie Y, Hao J, Fan D: The Role of the Slit/Robo Signaling Pathway. Journal of Cancer 2019, 10(12):2694–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koohini Z, Teimourian S: Slit/Robo Signaling Pathway in Cancer; a New Stand Point for Cancer Treatment. Pathology oncology research : POR 2019, 25(4):1285–1293. [DOI] [PubMed] [Google Scholar]
- 18.Blockus H, Chedotal A: Slit-Robo signaling. Development 2016, 143(17):3037–3044. [DOI] [PubMed] [Google Scholar]; **This article is a detailed review highlighting the recent advances in the roles of Slit-Robo.
- 19.Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G: Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell 1998, 92(2):205–215. [DOI] [PubMed] [Google Scholar]
- 20.Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R: Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics 2002, 79(4):547–552. [DOI] [PubMed] [Google Scholar]; ** This study identified Robo4 receptor/Magic roundabout as endothelial specific receptor.
- 21.Hagino S, Iseki K, Mori T, Zhang Y, Hikake T, Yokoya S, Takeuchi M, Hasimoto H, Kikuchi S, Wanaka A: Slit and glypican-1 mRNAs are coexpressed in the reactive astrocytes of the injured adult brain. Glia 2003, 42(2):130–138. [DOI] [PubMed] [Google Scholar]
- 22.Park JH, Pak HJ, Riew TR, Shin YJ, Lee MY: Increased expression of Slit2 and its receptors Robo1 and Robo4 in reactive astrocytes of the rat hippocampus after transient forebrain ischemia. Brain research 2016, 1634:45–56. [DOI] [PubMed] [Google Scholar]
- 23.Jin X, Shin YJ, Riew TR, Choi JH, Lee MY: Increased Expression of Slit2 and its Robo Receptors During Astroglial Scar Formation After Transient Focal Cerebral Ischemia in Rats. Neurochemical research 2016, 41(12):3373–3385. [DOI] [PubMed] [Google Scholar]
- 24.Mertsch S, Schmitz N, Jeibmann A, Geng JG, Paulus W, Senner V: Slit2 involvement in glioma cell migration is mediated by Robo1 receptor. J Neurooncol 2008, 87(1):1–7. [DOI] [PubMed] [Google Scholar]
- 25.Myers JP, Robles E, Ducharme-Smith A, Gomez TM: Focal adhesion kinase modulates Cdc42 activity downstream of positive and negative axon guidance cues. Journal of cell science 2012, 125(Pt 12):2918–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu JJ, Gao GZ, Zhang SM: miR-218 inhibits the migration and invasion of glioma U87 cells through the Slit2-Robo1 pathway. Oncol Lett 2015, 9(4):1561–1566. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 27.Zhang F, Prahst C, Mathivet T, Pibouin-Fragner L, Zhang J, Genet G, Tong R, Dubrac A, Eichmann A: The Robo4 cytoplasmic domain is dispensable for vascular permeability and neovascularization. Nature communications 2016, 7:13517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suchting S, Heal P, Tahtis K, Stewart LM, Bicknell R: Soluble Robo4 receptor inhibits in vivo angiogenesis and endothelial cell migration. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2005, 19(1):121–123. [DOI] [PubMed] [Google Scholar]
- 29.Cai H, Liu W, Xue Y, Shang X, Liu J, Li Z, Wang P, Liu L, Hu Y, Liu Y: Roundabout 4 regulates blood-tumor barrier permeability through the modulation of ZO-1, Occludin, and Claudin-5 expression. J Neuropathol Exp Neurol 2015, 74(1):25–37. [DOI] [PubMed] [Google Scholar]
- 30.Morlot C, Thielens NM, Ravelli RB, Hemrika W, Romijn RA, Gros P, Cusack S, McCarthy AA: Structural insights into the Slit-Robo complex. Proceedings of the National Academy of Sciences of the United States of America 2007, 104(38):14923–14928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barak R, Yom-Tov G, Guez-Haddad J, Gasri-Plotnitsky L, Maimon R, Cohen-Berkman M, McCarthy AA, Perlson E, Henis-Korenblit S, Isupov MN et al. : Structural Principles in Robo Activation and Auto-inhibition. Cell 2019, 177(2):272–285 e216. [DOI] [PubMed] [Google Scholar]
- 32.Yom-Tov G, Barak R, Matalon O, Barda-Saad M, Guez-Haddad J, Opatowsky Y: Robo Ig4 Is a Dimerization Domain. J Mol Biol 2017, 429(23):3606–3616. [DOI] [PubMed] [Google Scholar]
- 33.Aleksandrova N, Gutsche I, Kandiah E, Avilov SV, Petoukhov MV, Seiradake E, McCarthy AA: Robo1 Forms a Compact Dimer-of-Dimers Assembly. Structure 2018, 26(2):320–328 e324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain SA, Piper M, Fukuhara N, Strochlic L, Cho G, Howitt JA, Ahmed Y, Powell AK, Turnbull JE, Holt CE et al. : A molecular mechanism for the heparan sulfate dependence of slit-robo signaling. The Journal of biological chemistry 2006, 281(51):39693–39698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuhara N, Howitt JA, Hussain SA, Hohenester E: Structural and functional analysis of slit and heparin binding to immunoglobulin-like domains 1 and 2 of Drosophila Robo. The Journal of biological chemistry 2008, 283(23):16226–16234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zelina P, Blockus H, Zagar Y, Peres A, Friocourt F, Wu Z, Rama N, Fouquet C, Hohenester E, Tessier-Lavigne M et al. : Signaling switch of the axon guidance receptor Robo3 during vertebrate evolution. Neuron 2014, 84(6):1258–1272. [DOI] [PubMed] [Google Scholar]
- 37.Jones CA, London NR, Chen H, Park KW, Sauvaget D, Stockton RA, Wythe JD, Suh W, Larrieu-Lahargue F, Mukouyama YS et al. : Robo4 stabilizes the vascular network by inhibiting pathologic angiogenesis and endothelial hyperpermeability. Nature medicine 2008, 14(4):448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This article elicited the role of Robo4 in vascular endothelial stability. Slit2-Robo4 stabilized endotheiial vascular permeability in retinopathy, and Robo4 knockout mice had imparierd vascular integrity and increased vascular leakage.
- 38.Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY: Robo4 is a vascular-specific receptor that inhibits endothelial migration. Developmental biology 2003, 261(1):251–267. [DOI] [PubMed] [Google Scholar]
- 39.Kaur S, Samant GV, Pramanik K, Loscombe PW, Pendrak ML, Roberts DD, Ramchandran R: Silencing of directional migration in roundabout4 knockdown endothelial cells. BMC cell biology 2008, 9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bedell VM, Yeo SY, Park KW, Chung J, Seth P, Shivalingappa V, Zhao J, Obara T, Sukhatme VP, Drummond IA et al. : roundabout4 is essential for angiogenesis in vivo. Proceedings of the National Academy of Sciences of the United States of America 2005, 102(18):6373–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Svensson KJ, Long JZ, Jedrychowski MP, Cohen P, Lo JC, Serag S, Kir S, Shinoda K, Tartaglia JA, Rao RR et al. : A Secreted Slit2 Fragment Regulates Adipose Tissue Thermogenesis and Metabolic Function. Cell metabolism 2016, 23(3):454–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Delloye-Bourgeois C, Jacquier A, Charoy C, Reynaud F, Nawabi H, Thoinet K, Kindbeiter K, Yoshida Y, Zagar Y, Kong Y et al. : PlexinA1 is a new Slit receptor and mediates axon guidance function of Slit C-terminal fragments. Nature neuroscience 2015, 18(1):36–45. [DOI] [PubMed] [Google Scholar]
- 43.Yiin JJ, Hu B, Jarzynka MJ, Feng H, Liu KW, Wu JY, Ma HI, Cheng SY: Slit2 inhibits glioma cell invasion in the brain by suppression of Cdc42 activity. Neuro Oncol 2009, 11(6):779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherchan P, Huang L, Akyol O, Reis C, Tang J, Zhang JH: Recombinant Slit2 Reduces Surgical Brain Injury Induced Blood Brain Barrier Disruption via Robo4 Dependent Rac1 Activation in a Rodent Model. Scientific reports 2017, 7(1):746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubrac A, Genet G, Ola R, Zhang F, Pibouin-Fragner L, Han J, Zhang J, Thomas JL, Chedotal A, Schwartz MA et al. : Targeting NCK-Mediated Endothelial Cell Front-Rear Polarity Inhibits Neovascularization. Circulation 2016, 133(4):409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsuchiya R, Takahashi K, Liu FC, Takahashi H: Aberrant axonal projections from mammillary bodies in Pax6 mutant mice: possible roles of Netrin-1 and Slit 2 in mammillary projections. Journal of neuroscience research 2009, 87(7):1620–1633. [DOI] [PubMed] [Google Scholar]
- 47.Small EM, Sutherland LB, Rajagopalan KN, Wang S, Olson EN: MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circulation research 2010, 107(11):1336–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fish JE, Wythe JD, Xiao T, Bruneau BG, Stainier DY, Srivastava D, Woo S: A Slit/miR-218/Robo regulatory loop is required during heart tube formation in zebrafish. Development 2011, 138(7):1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu JJ, Gao GZ, Zhang SM: MiR-218 inhibits the tumorgenesis and proliferation of glioma cells by targeting Robo1. Cancer Biomark 2016, 16(3):309–317. [DOI] [PubMed] [Google Scholar]
- 50.Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S, Guo X, Wang B, Gang Y, Zhang Y et al. : MiR-218 inhibits invasion and metastasis of gastric cancer by targeting the Robo1 receptor. PLoS genetics 2010, 6(3):e1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alajez NM, Lenarduzzi M, Ito E, Hui AB, Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J et al. : MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res 2011, 71(6):2381–2391. [DOI] [PubMed] [Google Scholar]
- 52.Amodeo V,AD, Betts J, Bartesaghi S, Zhang Y, Richard-Londt A, Ellis M, Roshani R, Vouri M, Galavotti S et al. : A PML/Slit Axis Controls Physiological Cell Migration and Cancer Invasion in the CNS. Cell reports 2017, 20(2):411–426. [DOI] [PubMed] [Google Scholar]
- 53.Villa R, Pasini D, Gutierrez A, Morey L, Occhionorelli M, Vire E, Nomdedeu JF, Jenuwein T, Pelicci PG, Minucci S et al. : Role of the polycomb repressive complex 2 in acute promyelocytic leukemia. Cancer cell 2007, 11(6):513–525. [DOI] [PubMed] [Google Scholar]
- 54.Alpar A, Tortoriello G, Calvigioni D, Niphakis MJ, Milenkovic I, Bakker J, Cameron GA, Hanics J, Morris CV, Fuzik J et al. : Endocannabinoids modulate cortical development by configuring Slit2/Robo1 signalling. Nature communications 2014, 5:4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, Chen L, Kaminoh Y, Chan AC, Passi SF et al. : Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Science translational medicine 2010, 2(23):23ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorbunova EE, Gavrilovskaya IN, Mackow ER: Slit2-Robo4 receptor responses inhibit ANDV directed permeability of human lung microvascular endothelial cells. Antiviral research 2013, 99(2):108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li S, Huang L, Sun Y, Bai Y, Yang F, Yu W, Li F, Zhang Q, Wang B, Geng JG et al. : Slit2 Promotes Angiogenic Activity Via the Robo1-VEGFR2-ERK1/2 Pathway in Both In Vivo and In Vitro Studies. Investigative ophthalmology & visual science 2015, 56(9):5210–5217. [DOI] [PubMed] [Google Scholar]
- 58.Rama N, Dubrac A, Mathivet T, Ni Charthaigh RA, Genet G, Cristofaro B, Pibouin-Fragner L, Ma L, Eichmann A, Chedotal A: Slit2 signaling through Robo1 and Robo2 is required for retinal neovascularization. Nature medicine 2015, 21(5):483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu R, Yallowitz A, Qin A, Wu Z, Shin DY, Kim JM, Debnath S, Ji G, Bostrom MP, Yang X et al. : Targeting skeletal endothelium to ameliorate bone loss. Nature medicine 2018, 24(6):823–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Genet G, Boye K, Mathivet T, Ola R, Zhang F, Dubrac A, Li J, Genet N, Henrique Geraldo L, Benedetti L et al. : Endophilin-A2 dependent VEGFR2 endocytosis promotes sprouting angiogenesis. Nature communications 2019, 10(1):2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bergers G, Song S: The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol 2005, 7(4):452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raza A, Franklin MJ, Dudek AZ: Pericytes and vessel maturation during tumor angiogenesis and metastasis. American journal of hematology 2010, 85(8):593–598. [DOI] [PubMed] [Google Scholar]
- 63.Guijarro-Munoz I, Cuesta AM, Alvarez-Cienfuegos A, Geng JG, Alvarez-Vallina L, Sanz L: The axonal repellent Slit2 inhibits pericyte migration: potential implications in angiogenesis. Experimental cell research 2012, 318(4):371–378. [DOI] [PubMed] [Google Scholar]
- 64.Altay T, McLaughlin B, Wu JY, Park TS, Gidday JM: Slit modulates cerebrovascular inflammation and mediates neuroprotection against global cerebral ischemia. Experimental neurology 2007, 207(2):186–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sherchan P, Huang L, Wang Y, Akyol O, Tang J, Zhang JH: Recombinant Slit2 attenuates neuroinflammation after surgical brain injury by inhibiting peripheral immune cell infiltration via Robo1-srGAP1 pathway in a rat model. Neurobiology of disease 2016, 85:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pilling D, Chinea LE, Consalvo KM, Gomer RH: Different Isoforms of the Neuronal Guidance Molecule Slit2 Directly Cause Chemoattraction or Chemorepulsion of Human Neutrophils. J Immunol 2019, 202(1):239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang C, Gao J, Zhang H, Sun L, Peng G: Robo2--slit and Dcc--netrin1 coordinate neuron axonal pathfinding within the embryonic axon tracts. J Neurosci 2012, 32(36):12589–12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagri A, Marin O, Plump AS, Mak J, Pleasure SJ, Rubenstein JL, Tessier-Lavigne M: Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 2002, 33(2):233–248. [DOI] [PubMed] [Google Scholar]
- 69.Andrews W, Liapi A, Plachez C, Camurri L, Zhang J, Mori S, Murakami F, Parnavelas JG, Sundaresan V, Richards LJ: Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development 2006, 133(11):2243–2252. [DOI] [PubMed] [Google Scholar]
- 70.Lopez-Bendito G, Flames N, Ma L, Fouquet C, Di Meglio T, Chedotal A, Tessier-Lavigne M, Marin O: Robo1 and Robo2 cooperate to control the guidance of major axonal tracts in the mammalian forebrain. J Neurosci 2007, 27(13):3395–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cardenas A, Villalba A, de Juan Romero C, Pico E, Kyrousi C, Tzika AC, Tessier-Lavigne M, Ma L, Drukker M, Cappello S et al. : Evolution of Cortical Neurogenesis in Amniotes Controlled by Robo Signaling Levels. Cell 2018, 174(3):590–606 e521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu Y, Li WL, Fu L, Gu F, Ma YJ: Slit2/Robo1 signaling in glioma migration and invasion. Neurosci Bull 2010, 26(6):474–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dallol A, Morton D, Maher ER, Latif F: SLIT2 axon guidance molecule is frequently inactivated in colorectal cancer and suppresses growth of colorectal carcinoma cells. Cancer Res 2003, 63(5):1054–1058. [PubMed] [Google Scholar]
- 74.Narayan G, Goparaju C, Arias-Pulido H, Kaufmann AM, Schneider A, Durst M, Mansukhani M, Pothuri B, Murty VV: Promoter hypermethylation-mediated inactivation of multiple Slit-Robo pathway genes in cervical cancer progression. Mol Cancer 2006, 5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Astuti D, Da Silva NF, Dallol A, Gentle D, Martinsson T, Kogner P, Grundy R, Kishida T, Yao M, Latif F et al. : SLIT2 promoter methylation analysis in neuroblastoma, Wilms’ tumour and renal cell carcinoma. Br J Cancer 2004, 90(2):515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dallol A, Krex D, Hesson L, Eng C, Maher ER, Latif F: Frequent epigenetic inactivation of the SLIT2 gene in gliomas. Oncogene 2003, 22(29):4611–4616. [DOI] [PubMed] [Google Scholar]
- 77.Wong K, Ren XR, Huang YZ, Xie Y, Liu G, Saito H, Tang H, Wen L, Brady-Kalnay SM, Mei L et al. : Signal transduction in neuronal migration: roles of GTPase activating proteins and the small GTPase Cdc42 in the Slit-Robo pathway. Cell 2001, 107(2):209–221. [DOI] [PubMed] [Google Scholar]
- 78.Kaur H, Xu N, Doycheva DM, Malaguit J, Tang J, Zhang JH: Recombinant Slit2 attenuates neuronal apoptosis via the Robo1-srGAP1 pathway in a rat model of neonatal HIE. Neuropharmacology 2019, 158:107727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arteni NS, Salgueiro J, Torres I, Achaval M, Netto CA: Neonatal cerebral hypoxia-ischemia causes lateralized memory impairments in the adult rat. Brain research 2003, 973(2):171–178. [DOI] [PubMed] [Google Scholar]
- 80.Nakajima W, Ishida A, Lange MS, Gabrielson KL, Wilson MA, Martin LJ, Blue ME, Johnston MV: Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci 2000, 20(21):7994–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frontczak-Baniewicz M, Walski M, Madejska G, Sulejczak D: MMP2 and MMP9 in immature endothelial cells following surgical injury of rat cerebral cortex--a preliminary study. Folia neuropathologica 2009, 47(4):338–346. [PubMed] [Google Scholar]
- 82.Jadhav V, Matchett G, Hsu FP, Zhang JH: Inhibition of Src tyrosine kinase and effect on outcomes in a new in vivo model of surgically induced brain injury. Journal of neurosurgery 2007, 106(4):680–686. [DOI] [PubMed] [Google Scholar]
- 83.Jadhav V, Solaroglu I, Obenaus A, Zhang JH: Neuroprotection against surgically induced brain injury. Surgical neurology 2007, 67(1):15–20; discussion 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fang M, Liu GW, Pan YM, Shen L, Li CS, Xi ZQ, Xiao F, Wang L, Chen D, Wang XF: Abnormal expression and spatiotemporal change of Slit2 in neurons and astrocytes in temporal lobe epileptic foci: A study of epileptic patients and experimental animals. Brain research 2010, 1324:14–23. [DOI] [PubMed] [Google Scholar]
- 85.Jacobi A, Schmalz A, Bareyre FM: Abundant expression of guidance and synaptogenic molecules in the injured spinal cord. PloS one 2014, 9(2):e88449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu JB, Jiang YQ, Gong AH, Zhang ZJ, Jiang Q, Chu XP: Expression of Slit2 and Robo1 after traumatic lesions of the rat spinal cord. Acta histochemica 2011, 113(1):43–48. [DOI] [PubMed] [Google Scholar]
- 87.Li Y, Gao Y, Xu X, Shi R, Liu J, Yao W, Ke C: Slit2/Robo1 promotes synaptogenesis and functional recovery of spinal cord injury. Neuroreport 2017, 28(2):75–81. [DOI] [PubMed] [Google Scholar]
- 88.Wehrle R, Camand E, Chedotal A, Sotelo C, Dusart I: Expression of netrin-1, slit-1 and slit-3 but not of slit-2 after cerebellar and spinal cord lesions. The European journal of neuroscience 2005, 22(9):2134–2144. [DOI] [PubMed] [Google Scholar]
- 89.Chen B, Carr L, Dun XP: Dynamic expression of Slit1–3 and Robo1–2 in the mouse peripheral nervous system after injury. Neural regeneration research 2020, 15(5):948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Han HX, Geng JG: Over-expression of Slit2 induces vessel formation and changes blood vessel permeability in mouse brain. Acta pharmacologica Sinica 2011, 32(11):1327–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li JC, Han L, Wen YX, Yang YX, Li S, Li XS, Zhao CJ, Wang TY, Chen H, Liu Y et al. : Increased permeability of the blood-brain barrier and Alzheimer’s disease-like alterations in slit-2 transgenic mice. Journal of Alzheimer’s disease : JAD 2015, 43(2):535–548. [DOI] [PubMed] [Google Scholar]
- 92.Li S, Li H, He XF, Li G, Zhang Q, Liang FY, Jia HH, Li JC, Huang R, Pei Z et al. : Transgenic over-expression of slit2 enhances disruption of blood-brain barrier and increases cell death after traumatic brain injury in mice. Neuroscience letters 2016, 631:85–90. [DOI] [PubMed] [Google Scholar]
- 93.Li G, He X, Li H, Wu Y, Guan Y, Liu S, Jia H, Li Y, Wang L, Huang R et al. : Overexpression of Slit2 improves function of the paravascular pathway in the aging mouse brain. International journal of molecular medicine 2018, 42(4):1935–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bakker EN, Bacskai BJ, Arbel-Ornath M, Aldea R, Bedussi B, Morris AW, Weller RO, Carare RO: Lymphatic Clearance of the Brain: Perivascular, Paravascular and Significance for Neurodegenerative Diseases. Cellular and molecular neurobiology 2016, 36(2):181–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wojciak-Stothard B, Ridley AJ: Rho GTPases and the regulation of endothelial permeability. Vascular pharmacology 2002, 39(4–5):187–199. [DOI] [PubMed] [Google Scholar]
- 96.Waschke J, Baumgartner W, Adamson RH, Zeng M, Aktories K, Barth H, Wilde C, Curry FE, Drenckhahn D: Requirement of Rac activity for maintenance of capillary endothelial barrier properties. American journal of physiology Heart and circulatory physiology 2004, 286(1):H394–401. [DOI] [PubMed] [Google Scholar]


