Abstract
The second messenger molecule 3’5’-cyclic adenosine monophosphate (cAMP) imparts several beneficial effects in lung diseases such as asthma, chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF). While cAMP is bronchodilatory in asthma and COPD, it also displays anti-fibrotic properties that limit fibrosis. Phosphodiesterases (PDEs) metabolize cAMP and thus regulate cAMP signaling. While some existing therapies inhibit PDEs, there are only broad family specific inhibitors. The understanding of cAMP signaling compartments, some centered around lipid rafts/caveolae, has led to interest in defining how specific PDE isoforms maintain these signaling microdomains. The possible altered expression of PDEs, and thus abnormal cAMP signaling, in obstructive lung diseases has been poorly explored. We propose that inhibition of specific PDE isoforms can improve therapy of obstructive lung diseases by amplifying specific cAMP signals in discreet microdomains.
Introduction
Chronic obstructive pulmonary disease (COPD), asthma and idiopathic pulmonary fibrosis (IPF) represent leading causes of morbidity and mortality [1–3]. These diseases are characterized by airway obstruction, chronic inflammation and airway remodeling, though the extent of each component varies by disease. Airway obstruction leading to a shortness of breath is a common feature of COPD, asthma and IPF. In asthma, such obstruction is associated with airway hyperresponsiveness but in all three diseases bronchodilators are used to open the airways [4]. Inflammation in COPD is characterized by increased numbers of CD8+ T-lymphocytes, macrophages and neutrophils, while in asthma inflammation is characterized by eosinophils, CD4+ T-lymphocytes and mast cells [4]. The asthma-COPD disease overlap syndrome (ACOS) adds another level of complexity representing disease phenotypes. For example, severe asthmatics can display increased numbers of neutrophils and COPD patients suffering from exacerbations typically have increased numbers of eosinophils. These circumstances make it a major challenge to manage the therapy of patients with ACOS [5•].
In the etiology of IPF, the role of inflammation is disputed and largely considered a by-product of fibrosis [3,6,7]. Since anti-inflammatory treatments show minimal therapeutic benefit in patients with IPF, research has shifted focus to viewing the diesease as a defect in the healing and repair process with concomitant inflammation [6]. Upon alveolar epithelial injury, transforming growth factor (TGF)-β induces fibroblast cells to transition into a-smooth muscle actin expressing myofibroblasts, which produce excess collagen and extracellular matrix proteins [8]. Due to continuous alveolar epithelial microinjury, a dysregulated response occurs where myofibroblasts persist and remain active for an indefinite period of time and lose the ability to undergo apoptosis [9,10]. Normally after alveolar epithelial type I cells are damaged, alveolar epithelial type II cells regenerate the damage [11]. Abnormal healing responses causes an inability of alveolar epithelial type II cells to regenerate epithelial cells leading to permanent loss of functional lung tissue [12].
All these obstructive lung diseases are caused by a diverse subset of genetic and environmental factors including allergens, cigarette smoke and air pollution [13•]. Air pollution emanating from primarily diesel automotive engines is linked to metabolic dysregulation, metabolic reprogramming, and mitochondrial dysfunction in each disorder [14•, 15,16]. Taken together, these three represent serious pulmonary diseases that possess both common and unique characteristics. The heterogenous nature of these obstructive lung disorders hinders an effective treatment of all patient groups. New therapies tailored to the heterogeneity of the patient cohorts need to be developed based on novel insights that integrate underlying molecular mechanisms into translational pharmacological research. 3’5’-cyclic adenosine monophosphate (cAMP) acts as a key regulator of all cells in the body. In the lung, cAMP regulates airway smooth muscle tone, cell proliferation, differentiation, apoptosis, inflammatory mediator synthesis and release, deposition of extracellular matrix, and the maintenance of barrier function in both endothelial and epithelial cells [17,18]. In this review, we will discuss multi-component therapies based on the concept of compartmentalization of cellular cAMP signaling pathways with a focus on strategies that target phosphodiesterases (PDEs).
Limitations of current treatment guidelines
The extent of airway obstruction, chronic inflammation and airway remodeling varies in COPD, asthma and IPF [1–3], resulting in disease specific treatment guidelines. In the treatment and management of COPD, approved therapies include the usage of bronchodilators (β2-adrenoceptor (β2AR) agonists, anticholinergics and broad PDE inhibitors), and a combination therapies of inhaled corticosteroid plus long-acting β2-AR agonists or anticholinergics and long-acting β2-AR agonists. The broad PDE4 inhibitor, roflumilast, has been approved as add-on treatment to these therapies in patients with severe COPD associated with bronchitis and a history of frequent exacerbations [1,19]. In the treatment and management of asthma, combined therapies of (short-acting or long-acting) β2AR agonists and inhaled corticosteroid are primarily used. Orally administered roflumilast has been proposed as a beneficial add-on therapy for use in patients with moderate-to-severe asthma but is not currently approved for this use [2,4,19]. Theophyl-line, a broad-spectrum PDE inhibitor and adenosine receptor antagonist, has fallen out of favor for asthma maintenance due to a wide array of off-target effects.
The treatment of IPF primarily relies on current antifibrotic therapeutics such as pirfenidone and nintedanib, both considered to have anti-inflammatory and anti-fibrotic action [3,6,7]. Importantly, it has been reported recently that Gαs-coupled receptors such as the β2-AR and receptors for prostaglandin E2 (PGE2) are repressed in IPF lungs [20–22]. Therapies targeting receptors coupled to cAMP in lung fibroblasts have some degree of specificity based on more limited expression of receptors across lung cell types. However, no therapeutic strategy currently exploits the antifibrotic effects of cAMP.
It has long been recognized that β2AR agonists, and the receptors themselves, contribute to asthma mortality [23,24]. These findings have led many to discount the therapeutic potential of cAMP signaling due to its association with β2AR as their canonical second messenger. However, some of these adverse effects of β2AR can be attributed to cAMP independent β-arrestin signaling by these receptors [25]. Inhibition of PDE, particularly in combination with β2AR agonists, is a logical means to increase cAMP-induced bronchodilation without enhancing maladaptive β-arrestin signaling.
A current limitation is existing PDE inhibitors lack specificity due to the broad expression patterns of PDE isoforms (Table 1 and Figure 1). Expression and function of phosphodiesterases (primarily PDE4 and PDE3) are altered in lung pathologies, particularly upon cigarette smoke exposure [26,27•]. As it is now well established that increasing cAMP can improve lung dysfunction [4,17–19], constraining the endogenous enzymes that degrade cAMP could have profound impact on the treatment of obstructive lung diseases. Current cyclic nucleotide-based therapies induce broad effects through their action on many cell types, including airway smooth muscle, (myofibroblasts, bronchial epithelium and endothelium, and via inhibition of broad classes of PDE. Systemic or inhaled administration targets widely expressed proteins to induce therapeutic effects and unwanted side effects (Figure 1). These cyclic nucleotide-based therapies neglect the impact of spatio-temporal dynamics of cAMP signaling and lead to global (many cells and tissues) and bulk (many subcellular domains) elevations of cAMP. However, research over the past 15 years pointed to the existence of cAMP-sensing multiprotein complexes composed of a distinct subset of cAMP-producing receptors and PDEs, leading to the accepted concept of compartmentalized cyclic nucleotide signalling [4,17–19]. Intriguingly, recent studies in human fibroblasts from IPF patients illustrate the importance of monitoring the kinetics and localization of intracellular cAMP signals [28,29]. We will highlight some key findings of how the understanding of cAMP signaling compartments have evolved substantially, emphasizing the fact that no therapies yet exist to leverage localized cAMP signaling.
Table 1.
Mammalian phosphodiesterase isoforms, their cellular compartments and inhibitors
| PDE family | Isoforms | Substrate | Cellular compartment | Receptors | Inhibitor/drugs | References |
|---|---|---|---|---|---|---|
| PDE1 | PDE1A | cAMP/cGMP | Cytosolic | β2-AR | 8-methoxymethyl-IBMX; vinpocetine; nimodipine; IC86340; IC295; | [65–67] |
| PDE1B | ||||||
| PDE1C | ||||||
| PDE2 | PDE2A | cAMP/cGMP | Membrane or cytosolic, mitochondria | EP2 β2-AR | BAY 60-7550; PDP; EHNA; IC933; oxindole; ND-7001; | [55,68] |
| PDE3 | PDE3A | cAMP/cGMP | Membrane or cytosolic | β2-AR | Olprinone; cilostamide; milronone; cilostazol; milrinone; siguazodan; enoximone; motapizone; SK&F94120; org9935; | [26,69] |
| PDE3B | ||||||
| PDE4 | PDE4A | cAMP | Membrane or cytosolic | EP2 | Rolipram; roflumilast; cilomast; RP73401; Ro20–1724; CHF6001; GPD-1116; ASP3258; YM976; | [70–72] |
| PDE4B | ||||||
| PDE4C | ||||||
| PDE4D | ||||||
| PDE5 | PDE5A | cGMP | Cytosolic | β2-AR | Zaprinast; DMPPO; sildenafil; tadalafil; vardenafil; dipyridamole; E4021; avanafil; | [73] |
| PDE6 | PDE6A | cGMP | Cytosolic | β2-AR | Zaprinast; DMPPO; sildenafil; vardenafil; | [74] |
| PDE6B | ||||||
| PDE6C | ||||||
| PDE6D | ||||||
| PDE7 | PDE7A | cAMP | Cytosolic | β2-AR | BRL 50481; IC242; T-2585; compound 21a; | [75] |
| PDE7B | ||||||
| PDE8 | PDE8A | cAMP | Membrane or cytosolic, mitochondria | β2-AR | PF-4957325; dipyridamole; | [62•] |
| PDE8B | ||||||
| PDE9 | PDE9A | cGMP | Cytosolic or nuclear | β2-AR | BAY-73–6691; PF-04447943; | [76] |
| PDE10 | PDE10A | cAMP/cGMP | Cytosolic | β2-AR | Papaverine; TP-10; MP-10 (PF-2545920); | [77] |
| PDE11 | PDE11A | cAMP/cGMP | Cytosolic | β2-AR | BC11-38; | [78] |
Figure 1.
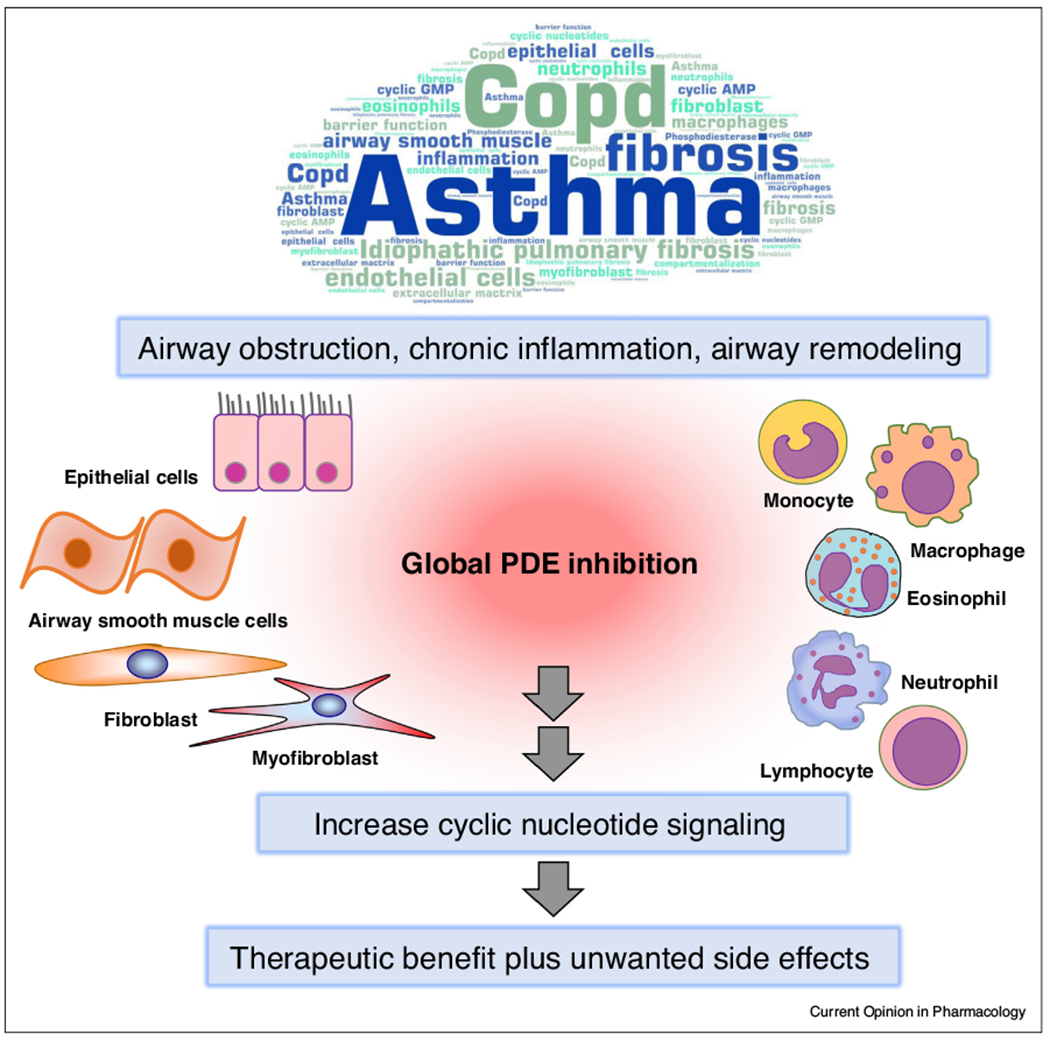
PDE inhibition in obstructive lung diseases.
Pulmonary diseases such as COPD, asthma and IPF are characterized a certain degree of airway obstruction, chronic inflammation and airway remodeling. Global PDE inhibition increases cyclic nucleotide signaling and therefore provides therapeutic benefit. However, the widespread expression of PDEs in all types of lung cells causes broad inhibition of a family of isoforms and eventually also leads to unwanted side effects. Specific inhibitors of single PDE isoforms, particularly those acting specifically in particular subcellular signaling microdomains, have the potential to be more effective and specific. See text for further detail.
The role of PDEs in compartmentized cAMP signaling
cAMP signaling compartments were first proposed by Buxton and Brunton based on early observations of disparate effects of cAMP signals in cells [30]. G protein-coupled receptors (GPCRs) initiate the signaling cascade leading to second messenger generation and their localization in plasma membrane microdomains are critical elements in the subsequent localization of a cAMP signal [31]. The G protein, Gαs, appears to be expressed uniformly across membrane microdomains and is in stoichiometric excess of both GPCRs and adenylylcyclases (AC) [32]. AC isoforms reside in caveolar/lipid raft microdomains or in non-raft plasma membrane domains [31]. However, the generation of an intracellular signal in a microdomain losses its spatial specificity if the signal freely diffuses through the cell. The fact that cAMP signals can remain membrane-delimited was first reported by Rich et al. [33]. FRET-based cAMP biosensors have provided direct evidence that cAMP is compartmentized [34•,35•]. Ample evidence has emerged since these reports to support the concept that cAMP does not freely diffuse inside cells [36]. Hydrolysis of cAMP by PDEs, in some cases existing in multiprotein complexes including receptors and A-kinase anchoring proteins (AKAPs) [4,17], physical barriers to diffusion by intracellular structures [37] and the buffering effects of PKA [38] all play roles in limiting the mobility of cAMP (Figure 2).
Figure 2.
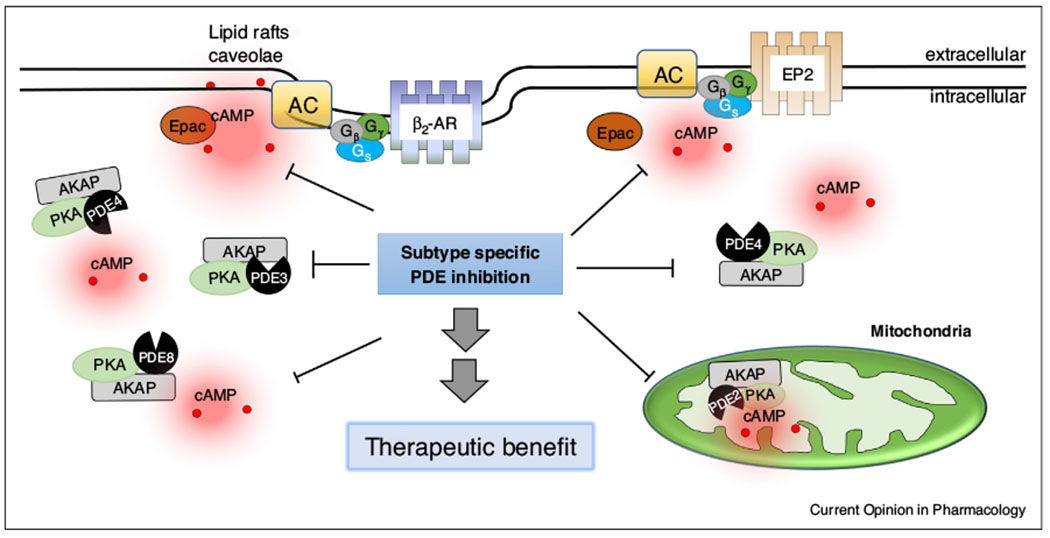
PDEs facilitate formation and maintenance of cAMP signaling compartments.
Individual PDE isoforms are expressed in discrete subcellular locations where they regulate cyclic nucleotide signaling in specific signaling compartments. Some of these specific findings are illustrated here. Understanding the microdomains where these PDEs are expressed and the cellular functions they regulate can lead to more specific therapeutic approaches. See text for further detail.
PDEs, by virtue of their ability to metabolize cAMP, play critical roles the establishment and maintenance of compartmentized cAMP signaling domains. Recent reviews discuss these effects of PDEs in more detail [39,40•]. Very few reports have examined the distinct localization of specific PDE isoforms in the subcellular microdomains where these cAMP pools operate, and these investigations have focused primarily on PDE4 [41]. PDE4D is of particular interest because it binds to β-arrestin, which brings it into close association with a recently activated GPCR [42]. PDEs also bind to certain AKAP isoforms to target their enzymatic activity to specific signaling complexes. These types of interactions expand the potential roles for AKAPs in the formation of macromolecular signal transduction complexes that create cAMP compartments (Figure 2). Recent work by the group of Baillie and others using cell-permeable peptide disrupters provided novel insights into the concept of compartmentized cAMP signaling, particularly focusing on different PDE4 isoforms. For example, direct interaction between PDE4A5 and the p75 neurotrophin receptor was implicated in extracellular matrix remodeling and tissue repair, processes potentially important to target fibrinolysis in COPD [43]. Direct interaction between PDE4D5 and integrin α5 was implicated in endothelial proinflammatory functions, processes known to impact obstructive lung diseases [44]. Disruptor peptides have been of tremendous value to provide proof-of-concept, although their development into drug-like tools still in its infancy.
Dynamic alterations of PDE expression in lung diseases: focus on PDE4
The PDE superfamily consists of 11 gene families with more than 21 individual genes, each with a distinct profile of substrate specificities, molecular structures and subcellular localizations (Table 1). PDE4, PDE7 and PDE8 are cAMP-specific; PDE5, PDE6 and PDE9 are cGMP-specific; PDE1, PDE2, PDE3, PDE10 and PDE11 are dual-specific [4,45]. Intriguingly, we reported recently that PDE family member mRNA levels exhibit a differential sensitivity to cigarette smoke in nasal epithelium, bronchial epithelium and lung tissue from patient cohorts [27•]. The gene expression of both PDE1A and PDE11A were decreased in bronchial epithelium and lung tissue. PDE7A was decreased in bronchial epithelium and nasal epithelium whereas PDE6A was increased in bronchial epithelium and lung tissue. These data suggest that these PDE isoforms, largely neglected in current lung research, are of central importance in pathophysiological processes induced by cigarette smoke, the latter primarily linked to COPD and IPF pathologies [13•].
Of note, PDE4D had a contrasting pattern of change (decreased in bronchial epithelium and increased in lung tissue), implying that this PDE isoform may be subject to alternative regulation in the different elements of the respiratory tract. Alternatively, cell type specific expression of PDE4D and alterations in these cell types may cause the shift in expression levels during smoke exposure. We also reported that changes in PDE3A and PDE4D protein expression were restricted to distinct lung compartments (bronchial epithelium versus total lung homogenates) [27•]. Our findings correlate with the existence of a unique amino-terminus of the PDE4 family shown to contribute to the generation of distinct signalling complexes that seem to maintain local cAMP gradients [19,45]. Indeed, in primary human airway smooth muscle cells PDE4D, to be more precise PDE4D5, is subject to cAMP-induced upregulation at the level of gene expression, protein expression and activity [46], implicating this isoform as an important cAMP negative feedback node. In human airway smooth muscle PGE2 upregulates PDE4D5 expression and leads to pro-asthmatic changes, including β2AR desensitization, reduced cellular cAMP and increased airway smooth muscle responsiveness to acetylcholine [47]. Moreover, human airway smooth muscle from asthmatic patients exhibited an increased PDE4D expression and a subsequent reduction of cAMP production by β2AR agonists [48]. Together these studies indicate that alterations in spatio-temporal dynamics of cAMP may contribute to key features of obstructive lung diseases and further points to the necessity to accelerate research to successfully target PDE4 family subtypes, such as PDE4D, to overcome limitations in current therapies. Recent studies also demonstrate that a subset of PDE4s, such as PDE4A-D, are specifically linked to Gαs-coupled receptors and cAMP production (Table 1). Targeting PDE4D might increase the therapeutic benefit for patients suffering from cigarette smoke-related obstructive lung diseases such as COPD and IPF [13•].
Focus on PDE2 and PDE3 as novel players in lung disease
There are several dual-specific PDE isoforms that are important for maintaining the crosstalk between the cyclic nucleotides cAMP and cGMP. Of particular interest is PDE3, also known as cGMP-inhibited cAMP-specific PDE. PDE3 exhibits a higher affinity and lower catalytic hydrolysis rate for cGMP compared to cAMP, thus cGMP acts as a competitive inhibitor and subsequently leads to inhibition of cAMP hydrolysis by PDE3 [49,50]. On the other hand, binding of cGMP to the amino-terminus of the allosteric regulatory site of PDE2 increases the hydrolysis rate of cAMP by 10-fold, leading to a profound drop in cellular cAMP [51,52•]. Recent research identified PDE3A as a novel anti-inflammatory target in allergic airway inflammation [53•], and as shown for PDE4s, a subset of PDE3s are specifically linked to Gαs-coupled receptors and thus cAMP (Table 1). In addition to these interesting features, PDE2 is known to localize in mitochondria and linked to cardiac and lung injury models [54,55] (Figure 2). Lung mitochondrial dysfunction, linked to metabolic dysregulation and metabolic reprogramming [56,57•], is caused by air pollution primarily originating from diesel burning automotive engines and it is envisioned as a primary cause for lung dysfunction [58–60]. Distinct groups of people are more susceptible to the health effects of air pollution. Of particular concern are elderly, children and people with pre-existing obstructive lung diseases, specifically the groups suffering from exacerbations [14•, 15,16]. Several types of pollutants are released during diesel fuel combustion including but not limited to particulate matter, metals and polycyclic aromatic hydrocarbons [58–60]. In primary murine tracheal epithelial and airway smooth muscle cells polycyclic aromatic hydrocarbons reduce cellular cAMP production by β2AR agonists [61•]. Such studies correlate air pollution-induced mitochondrial dysfunction with symptoms of asthma, COPD and IPF. Future studies should focus on targeting and stabilizing subcellular microdomain-specific PDEs to overcome the current limitations in PDE-based therapies of these obstructive lung diseases.
Focus on PDE8 as novel player in lung disease
While the cAMP signaling community has largely focused on PDE3 and PDE4 isoforms, other PDEs capable of regulating cAMP levels are encoded in the human genome. PDE8A, known as an IBMX-insensitive PDE, is expressed in human airway smooth muscle cells [62•]. This PDE isoform localizes in lipid rafts and specifically regulates cAMP signaling stimulated by β2AR with no effect on cAMP signaling stimulated by PGE2 ([62•] and Figure 2). Inhibition of PDE8 may make an advantaged therapeutic approach since this isoform is not widely expressed in the body and it does not regulate global cAMP signaling [63]. Specific inhibitors of PDE8, particularly those with proven pharmacokinetic or safety profiles in animal studies and/or clinical trials, do not exist. More work is needed to determine if PDE8 inhibition would provide a therapeutic benefit, but it is tempting to speculate that it will be particularly effective in combination with β2AR agonists.
Conclusions and future perspectives
Other than the few reports outlined above, the localization of PDE isoforms in specific cAMP compartments is unknown. We and others have postulated that some PDEs act as global regulators of cAMP signaling while other PDEs are specifically localized (Figure 2). It is further likely that some PDE isoforms regulate a specific signaling complex in a contextuall manner by moving to a location based on particular stimuli [64]. Lung fibroblasts provide evidence for this type of regulation. PGE2, an important endogenous regulator of lung fibroblasts, displays diminished cAMP responses following chronic exposure to PGE2. Our recent data show that PGE2 pretreatment induces expression of PDE3A, 4B and 4D but that similar treatment with β2AR agonist only induced expression of PDE4D [64]. This indicates that EP2 receptors induce qualitatively different signals than β2AR despite both coupling to increases in cAMP. Moreover, the induction of PDE expression by PGE2 pretreatment reduced cAMP signaling by EP2 receptors but had no effect on cAMP responses stimulated by β2AR. The logical conclusion from these results is that the induced PDE isoform (likely PDE4B) selectively participates in EP2R signaling complexes and is excluded from the compartment where β2AR act. This highly specific regulation by one PDE isoform provides an opportunity for new therapeutics that are more effective with fewer adverse effects. The fact that broad PDE inhibitors have enjoyed some clinical success, such as the PDE3 family inhibitor cilostazol and the PDE4 family inhibitor roflumilast, should provide motivation to develop small molecules that are more selective. Other approaches for disrupting the complexes where PDE isoforms act are also possible, as recently reviewed by Blair and Baillie [40•] and outlined in detail above.
Obstructive pulmonary diseases continue to represent leading causes of morbidity and mortality. Current bronchodilatory treatments for asthma and COPD leverage cAMP signaling. The fact that roflumilast, a PDE4 inhibitor, is an effective add-on therapy for COPD and asthma shows the importance of the cAMP pathway and the utility of enhancing these signals via inhibition of PDE activity. However, broad inhibition of an entire PDE family, such as PDE3 or PDE4, will have numerous effects based on the widespread expression of these isoforms in many cells and tissues. Therapeutics that specifically inhibit limited PDE isoforms, such as PDE3A or PDE4D, will likely enjoy greater clinical benefits with fewer adverse effects. Furthermore, certain lung pathologies may alter PDE expression or localization, causing degradation of cAMP signals to be distorted. As discussed above, many lung pathologies show altered PDE expression upon exposure to cigarette smoke. Such changes would mean the current therapies can’t reach full therapeutic potential in some patients, but they may also provide therapeutic opportunities if highly specific inhibitors can be developed to target upregulated PDE isoforms in the critical cell type and avoid inhibition of other isoforms.
In addition to improving specificity of PDE inhibitors, more research into how specific PDE isoforms play key roles in distinct subcellular compartments is needed. As discussed above, PDE isoforms PDE3A, PDE4D and PDE8A are differentially expressed in distinct lung compartments. It is likely that other isoforms also have specific localization. It is our thesis that inhibiting a specific PDE isoform that resides in a distinct locale would increase intracellular cAMP signaling that couples to a subset of all possible effects of cyclic nucleotides. This targeted action would have more specific therapeutic effects but would also boost the effectiveness of existing therapies. To reach these goals we need to fully define the molecules in each cAMP signaling microdomain (including GPCR, AC isoforms, AKAPs and PDEs), characterize the cellular functions that each microdomain regulate, and understand the role each one plays in lung pathophysiology. Targeting of subcellular cAMP domains using a variety of cAMP sensors has been achieved [35•] and the use of peptide disruptors has provided the proof-of-concept of therapeutic approaches that focus on subtype specific targeting of PDEs [40•]. Completing our understanding of cAMP signaling microdomains and the PDE isoforms therein should then provide impetus to develop new drugs with PDE isoform selectivity.
Acknowledgements
This work was supported by grants from the Deutsche Forschungsgemeinschaft (M.S.), the Brazilian Federal Agency for Support and Evaluation of Graduate Education – CAPES (055/14) (I.C.), the N.I.H. National Heart, Lung and Blood Institute grant HL058506 and the National Institute of General Medical Sciences grant GM107094 (R.O.).
Footnotes
Conflicts of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
- 1.Mirza S, Clay RD, Koslow MA, Scanlon PD: COPD guidelines: a review of the 2018 GOLD report. Mayo Clin Proc 2018, 93:1488–1502. [DOI] [PubMed] [Google Scholar]
- 2.Boulet LP, Reddel HK, Bateman E, Pedersen S, FitzGerald JM, O’Byrne PM: The global initiative for asthma (GINA): 25 years later. Eur Respir J 2019, 54. [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer Dj, Behr J, Cottin V, Danoff Sk, Morell F et al. : Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ ALAT clinical practice guideline. Am J Respir Crit Care Med 2018, 198:e44–e68. [DOI] [PubMed] [Google Scholar]
- 4.Zuo H, Cattani-Cavalieri I, Musheshe N, Nikolaev VO, Schmidt M: Phosphodiesterases as therapeutic targets for respiratory diseases. Pharmacol Ther 2019, 197:225–242. [DOI] [PubMed] [Google Scholar]
- 5.Albertson TE, Chenoweth JA, Pearson SJ, Murin S: The pharmacological management of asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS). Expert Opin Pharmacother 2020, 21:213–231. [DOI] [PubMed] [Google Scholar]; •This paper redefines the current insights into the treatment of ACOS.
- 6.Heukels P, Moor CC, von derThusen JH, Wijsenbeek MS, Kool M:Inflammation and immunity in IPF pathogenesis and treatment. Respir Med 2019, 147:79–91. [DOI] [PubMed] [Google Scholar]
- 7.Saito S, Alkhatib A, Kolls JK, Kondoh Y, Lasky JA: Pharmacotherapy and adjunctive treatment for idiopathic pulmonary fibrosis (IPF). J Thorac Dis 2019, 11:S1740–S1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phan SH: The myofibroblast in pulmonary fibrosis. Chest 2002, 122:286S–289S. [DOI] [PubMed] [Google Scholar]
- 9.Kropski JA, Blackwell TS: Progress in understanding and treating idiopathic pulmonary fibrosis. Annu Rev Med 2019, 70:211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fastres A, Felice F, Roels E, Moermans C, Corhay JL, Bureau F, Louis R, Clercx C, Guiot J: The lung microbiome in idiopathic pulmonary fibrosis: a promising approach for targeted therapies. Int J Mol Sci 2017, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasper M, Barth K: Potential contribution of alveolar epithelial type I cells to pulmonary fibrosis. Biosci Rep 2017, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, Randell SH, Noble PW, Hogan BL: Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 2013, 123:3025–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maghsoudloo M, Azimzadeh Jamalkandi S, Najafi A, Masoudi-Nejad A: Identification of biomarkers in common chronic lung diseases by co-expression networks and drug-target interactions analysis. Mol Med 2020, 26:9. [DOI] [PMC free article] [PubMed] [Google Scholar]; •This important paper is the first to identify biomarkers common in asthma, COPD and IPF.
- 14.Annesi-Maesano I: Air pollution and chronic obstructive pulmonary disease exacerbations: when prevention becomes feasible. Am J Respir Crit Care Med 2019, 199:547–548. [DOI] [PubMed] [Google Scholar]; •This paper gives important insights into exacerbations in COPD and their link to air pollution.
- 15.Bontinck A, Maes T, Joos G: Asthma and air pollution: recent insights in pathogenesis and clinical implications. Curr Opin Pulm Med 2020, 26:10–19. [DOI] [PubMed] [Google Scholar]
- 16.Sese L, Annesi-Maesano I, Nunes H: Impact of particulate matter on the natural history of IPF: a matter of concentrations? Chest 2018, 154:726–727. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt M, Dekker FJ, Maarsingh H: Exchange protein directly activated by cAMP (epac): a multidomain cAMP mediator in the regulation of diverse biological functions. Pharmacol Rev 2013, 65:670–709. [DOI] [PubMed] [Google Scholar]
- 18.Sayner SL: Emerging themes of cAMP regulation of the pulmonary endothelial barrier. Am J Physiol Lung Cell Mol Physiol 2011, 300:L667–L678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC: Advances in targeting cyclic nucleotide phosphodiesterases. Nat Rev Drug Discov 2014, 13:290–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Insel PA, Sriram K, Gorr MW, Wiley SZ, Michkov A, Salmeron C, Chinn AM: GPCRomics: an approach to discover GPCR drug targets. Trends Pharmacol Sci 2019, 40:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang SK, Fisher AS, Scruggs AM, White ES, Hogaboam CM, Richardson BC, Peters-Golden M: Hypermethylation of PTGER2 confers prostaglandin E2 resistance in fibrotic fibroblasts from humans and mice. Am J Pathol 2010, 177:2245–2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haak AJ, Ducharme MT, Diaz Espinosa AM, Tschumperlin DJ: Targeting GPCR signaling for idiopathic pulmonary fibrosis therapies. Trends Pharmacol Sci 2020, 41:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen LP, Lin R, Parra S, Omoluabi O, Hanania NA, Tuvim MJ, Knoll BJ, Dickey BF, Bond RA: Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci USA 2009, 106:2435–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suissa S, Ernst P, Boivin JF, Horwitz RI, Habbick B, Cockroft D, Blais L, McNutt M, Buist AS, Spitzer WO: A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care Med 1994, 149:604–610. [DOI] [PubMed] [Google Scholar]
- 25.Deshpande DA, Theriot BS, Penn RB, Walker JK: Beta-arrestins specifically constrain beta2-adrenergic receptor signaling and function in airway smooth muscle. FASEBJ 2008, 22:2134–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuo H, Han B, Poppinga WJ, Ringnalda L, Kistemaker LEM, Halayko AJ, Gosens R, Nikolaev vO, Schmidt M: Cigarette smoke up-regulates PDE3 and PDE4 to decrease cAMP in airway cells. BrJ Pharmacol 2018, 175:2988–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zuo H, Faiz A, van den Berge M, Mudiyanselage S, Borghuis T, Timens W, Nikolaev VO, Burgess JK, Schmidt M: Cigarette smoke exposure alters phosphodiesterases in human structural lung cells. Am J Physiol Lung Cell Mol Physiol 2020, 318:L59–L64. [DOI] [PubMed] [Google Scholar]; •This paper describes the impact of environmental particles on the expression profile of PDEs.
- 28.Rosethorne EM, Charlton SJ: Airway remodeling disease: primary human structural cells and phenotypic and pathway assays to identify targets with potential to prevent or reverse remodeling. J Exp Pharmacol 2018, 10:75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts MJ, Broome RE, Kent TC, Charlton SJ, Rosethorne EM: The inhibition of human lung fibroblast proliferation and differentiation by Gs-coupled receptors is not predicted by the magnitude of cAMP response. Respir Res 2018, 19:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buxton IL, Brunton LL: Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem 1983, 258:10233–10239. [PubMed] [Google Scholar]
- 31.Johnstone TB, Agarwal SR, Harvey RD, Ostrom RS: cAMP signaling compartmentation: adenylyl cyclases as anchors of dynamic SIGNALING complexes. Mol Pharmacol 2018, 93:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Post SR, Hilal-Dandan R, Urasawa K, Brunton LL, Insel PA:Quantification of signalling components and amplification in the beta-adrenergic-receptor-adenylate cyclase pathway in isolated adult rat ventricular myocytes. Biochem J 1995, 311:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW:Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion [see comments]. J Gen Physiol 2000, 116:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musheshe N, Schmidt M, Zaccolo M: cAMP: from long-range second messenger to nanodomain signalling. Trends Pharmacol Sci 2018, 39:209–222. [DOI] [PubMed] [Google Scholar]; •This review shapes novel insight into the localized cAMP signaling in time and space.
- 35.Schleicher K, Zaccolo M: Defining a cellular map of cAMP nanodomains. Mol Pharmacol 2020. [Google Scholar]; •This review discusses integrated approaches for defining cAMP signaling domains.
- 36.Agarwal SR, Clancy CE, Harvey RD: Mechanisms restricting diffusion of intracellular cAMP. Sci Rep 2016, 6:19577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saucerman JJ, Greenwald EC, Polanowska-Grabowska R: Mechanisms of cyclic AMP compartmentation revealed by computational models. J Gen Physiol 2014, 143:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang PC, Boras BW, Jeng MT, Docken SS, Lewis TJ, McCulloch AD, Harvey RD, Clancy CE: A computational modeling and simulation approach to investigate mechanisms of subcellular cAMP compartmentation. PLoS Comput Biol 2016, 12:e1005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kokkonen K, Kass DA: Nanodomain regulation of cardiac cyclic nucleotide signaling by phosphodiesterases. Annu Rev Pharmacol Toxicol 2017, 57:455–479. [DOI] [PubMed] [Google Scholar]
- 40.Blair CM, Baillie GS: Reshaping cAMP nanodomains through targeted disruption of compartmentalised phosphodiesterase signalosomes. Biochem Soc Trans 2019, 47:1405–1414. [DOI] [PubMed] [Google Scholar]; •This paper highlights novel molecular mechanisms for targeting subcellular cyclic nucleotide domains.
- 41.Houslay MD, Adams DR: PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem J 2003, 370:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baillie GS, Sood A, McPhee I, Gall I, Perry SJ, Lefkowitz RJ, Houslay MD: Beta-arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs toGi. Proc Natl Acad Sci U S A 2003,100:940–945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Houslay KF, Fertig BA, Christian F, Tibbo AJ, Ling J, Findlay JE, Houslay MD, Baillie GS: Phosphorylation of PDE4A5 by MAPKAPK2 attenuates fibrin degradation via p75 signalling. J Biochem 2019, 166:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Yun S, Budatha M, Dahlman JE, Coon BG, Cameron RT, Langer R, Anderson DG, Baillie G, Schwartz MA: Interaction between integrin alpha5 and PDE4D regulates endothelial inflammatory signalling. Nat Cell Biol 2016, 18:1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houslay MD: Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci 2010, 35:91–100. [DOI] [PubMed] [Google Scholar]
- 46.Le Jeune IR, Shepherd M, Van Heeke G, Houslay MD, Hall IP: Cyclic AMP-dependent transcriptional up-regulation of phosphodiesterase 4D5in human airway smooth muscle cells. Identification and characterization of a novel PDE4D5 promoter. J Biol Chem 2002, 277:35980–35989. [DOI] [PubMed] [Google Scholar]
- 47.Hu A, Diener BL, Josephson MB, Grunstein MM: Constitutively active signaling by the G protein betagamma-subunit mediates intrinsically increased phosphodiesterase-4 activity in human asthmatic airway smooth muscle cells. PLoS One 2015, 10:e0118712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trian T, Burgess JK, Niimi K, Moir LM, Ge Q, Berger P, Liggett SB, Black JL, Oliver BG: Beta2-agonist induced cAMP is decreased in asthmatic airway smooth muscle due to increased PDE4D. PloS One 2011. 6:e20000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Degerman E, Belfrage P, Manganiello VC: Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3). J Biol Chem 1997, 272:6823–6826. [DOI] [PubMed] [Google Scholar]
- 50.Shakur Y, Holst LS, Landstrom TR, Movsesian M, Degerman E, Manganiello V: Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog Nucleic Acid Res Mol Biol 2001, 66:241–277. [DOI] [PubMed] [Google Scholar]
- 51.Martinez SE, Wu AY, Glavas NA, Tang XB, Turley S, Hol WG, Beavo JA: The two GAF domains in phosphodiesterase 2A have distinct roles in dimerization and in cGMP binding. Proc Natl Acad Sci USA 2002, 99:13260–13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pavlaki N, Nikolaev VO: Imaging of PDE2- and PDE3-mediated cGMP-to-cAMP cross-talk in cardiomyocytes. J Cardiovasc Dev Dis 2018, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]; •This paper highlights the crosstalk between cAMP and cGMP in diseas settings.
- 53.Beute J, Lukkes M, Koekoek EP, Nastiti H, Ganesh K, de Bruijn MJ, Hockman S, van Nimwegen M, Braunstahl GJ, Boon L et al. : A pathophysiological role of PDE3 in allergic airway inflammation. JCI Insight 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]; •This paper redefines the cellular function of PDE3.
- 54.Liu D, Wang Z, Nicolas V, Lindner M, Mika D, Vandecasteele G, Fischmeister R, Brenner C: PDE2 regulates membrane potential, respiration and permeabilitytransition of rodent subsarcolemmal cardiac mitochondria. Mitochondrion 2019, 47:64–75. [DOI] [PubMed] [Google Scholar]
- 55.Witzenrath M, Gutbier B, Schmeck B, Tenor H, Seybold J, Kuelzer R, Grentzmann G, Hatzelmann A, van Laak V, Tschernig T et al. : Phosphodiesterase 2 inhibition diminished acute lung injury in murine pneumococcal pneumonia. Crit Care Med 2009, 37:584–590. [DOI] [PubMed] [Google Scholar]
- 56.Prakash YS, Pabelick CM, Sieck GC: Mitochondrial dysfunction in airway disease. Chest 2017, 152:618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cloonan SM, Choi AM: Mitochondria in lung disease. J Clin Invest 2016, 126:809–820. [DOI] [PMC free article] [PubMed] [Google Scholar]; •This paper recaptures the importance of mitochondria function in lun disorders.
- 58.Lelieveld J, Evans JS, Fnais M, Giannadaki D, Pozzer A: The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525:367–371. [DOI] [PubMed] [Google Scholar]
- 59.Annesi-Maesano I: The air of Europe: where are we going? Eur Respir Rev 2017, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu W, Jin Y, Carlsten C: Inflammatory health effects of indoor and outdoor particulate matter. J Allergy Clin Immunol 2018, 141:833–844. [DOI] [PubMed] [Google Scholar]
- 61.Factor P, Akhmedov AT, McDonald JD, Qu A, Wu J, Jiang H, Dasgupta T, Panettieri RA Jr, Perera F, Miller RL: Polycyclic aromatic hydrocarbons impair function of beta2-adrenergic receptors in airway epithelial and smooth muscle cells. Am J Respir Cell Mol Biol 2011, 45:1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]; •This paper provides first molecular insights into the dysfunction of th beta2AR under air pollution exposure.
- 62.Johnstone TB, Smith KH, Koziol-White CJ, Li F, Kazarian AG, Corpuz ML, Shumyatcher M, Ehlert FJ, Himes BE, Panettieri RA Jr et al. : PDE8 is expressed in human airway smooth muscle and selectively regulates cAMP signaling by beta2-adrenergic receptors and adenylyl cyclase 6. Am J Respir Cell Mol Biol 2018, 58:530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]; •This paper is the first to report PDE8A expression and function in the lunc and shows this isoform is specifically localized in lipid raft microdomains
- 63.Zuo H, Schmidt M, Gosens R: PDE8: a novel target in airway smooth muscle. Am J Respir Cell Mol Biol 2018, 58:426–427. [DOI] [PubMed] [Google Scholar]
- 64.Nunez FJ, Schulte NA, Fogel DM, Michalski J, Rennard SI, Penn RB, Toews ML, Ostrom RS: Agonist-specific desensitization of PGE2-stimulated cAMP signaling due to upregulated phosphodiesterase expression in human lung fibroblasts. Naunyn Schmiedebergs Arch Pharmacol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brown DM, Hutchison L, Donaldson K, MacKenzie SJ, Dick CA, Stone V: The effect of oxidative stress on macrophages and lung epithelial cells: the role of phosphodiesterases 1 and 4. Toxicol Lett 2007. 168:1–6. [DOI] [PubMed] [Google Scholar]
- 66.Kogiso H, Hosogi S, Ikeuchi Y, Tanaka S, Shimamoto C, Matsumura H, Nakano T, Sano KI, Inui T, Marunaka Y et al. : A low [Ca(2+)]i-induced enhancement of cAMP-activated ciliary beating by PDE1A inhibition in mouse airway cilia. Pflugers Arch 2017, 469:1215–1227. [DOI] [PubMed] [Google Scholar]
- 67.Murray F, Patel HH, Suda RY, Zhang S, Thistlethwaite PA, Yuan JX, Insel PA: Expression and activity of cAMP phosphodiesterase isoforms in pulmonary artery smooth muscle cells from patients with pulmonary hypertension: role for PDE1. Am J Physiol Lung Cell Mol Physiol 2007, 292:L294–303. [DOI] [PubMed] [Google Scholar]
- 68.Bubb KJ, Trinder SL, Baliga RS, Patel J, Clapp LH, MacAllister RJ, Hobbs AJ: Inhibition of phosphodiesterase 2 augments cGMP and cAMP signaling to ameliorate pulmonary hypertension. Circulation 2014, 130:496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mokra D, Drgova A, Pullmann R Sr, Calkovska A: Selective phosphodiesterase 3 inhibitor olprinone attenuates meconium-induced oxidative lung injury. Pulm Pharmacol Ther 2012, 25:216–222. [DOI] [PubMed] [Google Scholar]
- 70.Armani E, Amari G, Rizzi A, De Fanti R, Ghidini E, Capaldi C, Carzaniga L, Caruso P, Guala M, Peretto I et al. : Novel class of benzoic acid ester derivatives as potent PDE4 inhibitors for inhaled administration in the treatment of respiratory diseases. J Med Chem 2014, 57:793–816. [DOI] [PubMed] [Google Scholar]
- 71.Kubo S, Kobayashi M, Iwata M, Takahashi K, Miyata K, Shimizu Y:Disease-modifying effect of ASP3258, a novel phosphodiesterase type 4 inhibitor, on subchronic cigarette smoke exposure-induced lung injury in guinea pigs. Eur J Pharmacol 2011, 659:79–84. [DOI] [PubMed] [Google Scholar]
- 72.Mori H, Nose T, Ishitani K, Kasagi S, Souma S, Akiyoshi T, Kodama Y, Mori T, Kondo M, Sasaki S et al. : Phosphodiesterase 4 inhibitor GPD-1116 markedly attenuates the development of cigarette smoke-induced emphysema in senescence-accelerated mice P1 strain. Am J Physiol Lung Cell Mol Physiol 2008, 294:L196–204. [DOI] [PubMed] [Google Scholar]
- 73.Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR:Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation 2003, 107:3230–3235. [DOI] [PubMed] [Google Scholar]
- 74.Nikolova S, Guenther A, Savai R, Weissmann N, Ghofrani HA, Konigshoff M, Eickelberg O, Klepetko W, Voswinckel R, Seeger W et al. : Phosphodiesterase 6 subunits are expressed and altered in idiopathic pulmonary fibrosis. Respir Res 2010, 11:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith SJ, Brookes-Fazakerley S, Donnelly LE, Barnes PJ, Barnette MS, Giembycz MA: Ubiquitous expression of phosphodiesterase 7A in human proinflammatory and immune cells. Am J Physiol Lung Cell Mol Physiol 2003, 284: L279–289. [DOI] [PubMed] [Google Scholar]
- 76.Tajima T, Shinoda T, Urakawa N, Shimizu K, Kaneda T: Phosphodiesterase 9 (PDE9) regulates bovine tracheal smooth muscle relaxation. J Vet Med Sci 2018, 80:499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu B, Lindsey A, Li N, Lee K, Ramirez-Alcantara V, Canzoneri JC, Fajardo A, Madeira da Silva L, Thomas M, Piazza JT et al. :Phosphodiesterase 10A is overexpressed in lung tumor cells and inhibitors selectively suppress growth by blocking beta-catenin and MAPK signaling. Oncotarget 2017, 8:69264–69280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Makhlouf A, Kshirsagar A, Niederberger C: Phosphodiesterase 11: a brief review of structure, expression and function. Int J Impot Res 2006, 18:501–509. [DOI] [PubMed] [Google Scholar]


