Abstract
Background
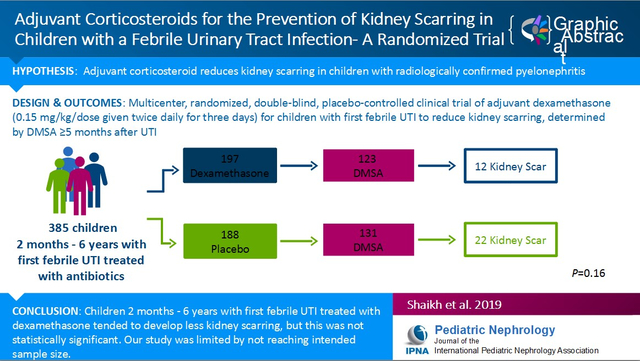
To evaluate the efficacy of adjuvant systemic corticosteroids in reducing kidney scarring. A previous study suggested that use of adjuvant systemic corticosteroids reduces kidney scarring in children radiologically confirmed to have extensive pyelonephritis. Efficacy of corticosteroids for children with febrile urinary tract infection (UTI) has not been studied.
Methods
Children aged 2 months to 6 years with their first febrile UTI were randomized to corticosteroids or placebo for 3 days (both arms received antimicrobial therapy); kidney scarring was assessed using 99mTc-dimercaptosuccinic acid kidney scan 5–24 months after the initial UTI.
Results
We randomized 546 children of which 385 had a UTI and 254 had outcome kidney scans (instead of the 320 planned). Rates of kidney scarring were 9.8% (12/123) and 16.8% (22/131) in the corticosteroid and placebo groups, respectively (p=0.16), corresponding to an absolute risk reduction of 5.9% (95% confidence interval: −2.2, 14.1).
Conclusion
While children randomized to adjuvant corticosteroids tended to develop fewer kidney scars than children who were randomized to receive placebo, a statistically significant difference was not achieved. However, the study was limited by not reaching its intended sample size.
Clinical Trial Registration
Clinicaltrials.gov, NCT01391793, Registered 7/12/2011
Keywords: Children, pyelonephritis, UTI, dexamethasone, Clinical Trials, DMSA, kidneys
Graphical Abstract
Introduction
Acquired kidney scarring from childhood UTI, albeit rarely, is associated with hypertension, preeclampsia, and kidney failure later in life [1–4]. Antibiotic treatment alone is insufficient to eliminate kidney scarring [5–14]. Animal studies suggest that the inflammatory response, rather than direct damage from bacterial infection, is the most important factor in the formation of kidney scars [15–17]. This hypothesis has led to studies evaluating the use of systemic corticosteroids in combination with antimicrobial therapy (hereafter referred to as adjuvant corticosteroids) to reduce kidney scarring. This strategy has shown potential benefit in animal studies [6, 11] and in one small study in children with pyelonephritis confirmed by performing a 99mTc-dimercaptosuccinic acid (DMSA) kidney scan at the time of UTI diagnosis [18]. However, in many settings it is costly and impractical to obtain DMSA scans on children at the time of diagnosis of UTI. Therefore, in the STARRS study (Steroids to Actively Reduce Renal Scarring), we sought to evaluate the effectiveness of adjuvant corticosteroids in preventing kidney scarring in all young febrile children with a first UTI.
Methods
Eligibility and enrollment
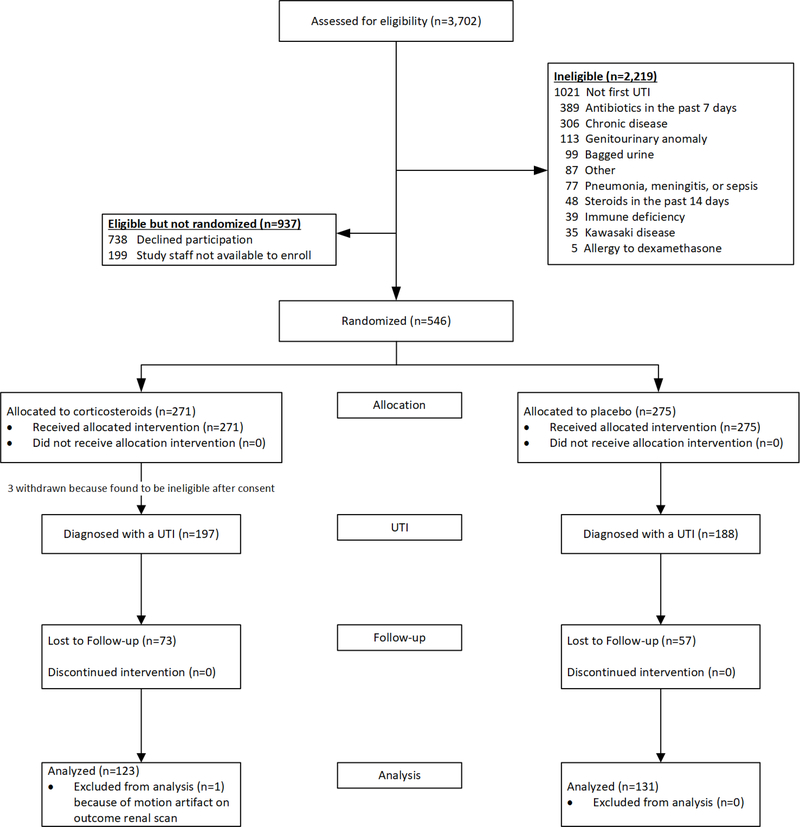
Between October 2011 and August 2017, we conducted a double-blind, placebo-controlled randomized trial at the Children’s Hospital of Pittsburgh, PA; Nationwide Children’s Hospital, in Columbus, OH; American Family Children’s Hospital in Madison, WI; Children’s National Health System, in Washington, DC.; Hasbro Children’s Hospital in Providence, RI; and Primary Children’s Hospital in Salt Lake City, UT. The study was approved by the Institutional Review Boards of the respective institutions and registered at ClinicalTrials.gov (NCT01391793) prior to patient enrollment; written informed consent was obtained from a parent of each enrolled child. A detailed study protocol can be found in the supplement. Eligible children were aged 2 months to 6 years with a documented temperature of ≥38.3°C who were treated for their first presumed UTI based on a urinalysis exhibiting pyuria (≥10 WBC/mm3 in an uncentrifuged specimen, ≥5 WBC/hpf in a centrifuged specimen, or ≥1+ leukocyte esterase on dipstick). Exclusion criteria are listed in Figure 1.
Figure 1.
Enrollment, randomization, and follow-up of children in the study
Most children were enrolled at the time of diagnosis of UTI. In some cases, parents were approached a day or two (up to 48 hours) after starting antimicrobial therapy. This enrollment strategy was supported by an earlier study [18] in which adjuvant corticosteroids appeared to be effective despite a delay in administration of approximately 48 hours in the majority of patients.
Randomization
We stratified children according to duration of fever prior to diagnosis (<48 hours vs. ≥48 hours) and site of enrollment. At each study site, within each stratum, we randomly assigned children in blocks of four in a 1:1 ratio to receive a 3-day course of either oral corticosteroid (dexamethasone 0.15 mg per kg per dose), or placebo, each twice daily. Dexamethasone and placebo suspensions were prepared by the Investigational Drug Service at the University of Pittsburgh Research Pharmacy and were similar in appearance and taste; containers were sequentially numbered and opened sequentially. Assignment to treatment groups remained undisclosed to parents, research personnel, and health care providers throughout the study. Parents were advised to administer acetaminophen as needed for symptomatic relief and advised against the use of ibuprofen due to its anti-inflammatory effects. All children received 10 days of antibiotics (chosen by the treating physician) for UTI.
Data collection and follow-up
We collected data on the following potential predictors and confounders of kidney scarring at the baseline evaluation: demographics (sex, race, ethnicity, age), symptoms (type and duration), past medical history (including result of prenatal ultrasound), medications (including non-steroidal anti-inflammatory drug use), and family history (vesicoureteral reflux (VUR), UTI, scarring, kidney disease). We performed an age-appropriate evaluation for bladder or bowel dysfunction [19], and constipation [20]. Urine was processed per standard of care at the local clinical microbiology laboratory. To monitor the child’s wellbeing and to ensure compliance (defined as taking at least 80% of scheduled doses) with the study product, we contacted parents by telephone (up to two attempts daily) for 3 days after enrollment or until fever resolved. Children with a positive culture (defined as a catheterized specimen with growth of one or more uropathogens at ≥50,000 CFU/mL or clean catch urine specimen with growth of one or more uropathogens at ≥100,000 CFU/mL) remained in the study. Children with a negative urine culture were withdrawn from the study; parents were instructed to stop the study product and no outcome assessments were performed. As per standard of care, voiding cystourethrograms were not performed routinely.
All outcome DMSA scans were obtained 5 to 24 months after enrollment. In children with febrile reinfections, the scan was obtained at least 4 months after the most recent febrile recurrence of UTI. DMSA scans during acute infection were obtained only in a subset of children who were enrolled in a concurrent observational study. Ninety minutes to 3 hours after intravenous administration of 50–75 μCi/kg of 99mTc-DMSA (maximum dose 3 miCi) high-resolution magnified posterior and posterior-oblique images of the kidneys were obtained using a high-resolution pinhole collimator. A single posterior planar image acquired with low-energy collimator image was used to calculate differential kidney function. We did not perform a DMSA scan during the acute phase to ascertain the presence of pre-existing scarring because we expected such scars to be very rare and to be equally distributed in the two treatment groups (see discussion section for more detail).
Throughout the study, parents were called monthly and instructed to contact study personnel or their primary care provider for any fever or urinary symptoms. Children with recurrent febrile UTIs were retreated with antibiotics and, in a blinded fashion, the same study product (corticosteroid or placebo) we provided at the time of their first UTI.
Outcomes
Our two primary outcome measures, which we specified a priori, were kidney scarring and severe kidney scarring. Kidney scarring was determined by the majority of DMSA diagnoses of three reference radiologists/nuclear medicine physicians who were blinded to treatment assignment. Scarring was defined as photopenic cortical defect(s) with or without loss of contour or volume because all outcome DMSA scans were obtained at least 4 months after febrile UTI, thereby eliminating acute inflammation as the cause of the defect. To assess the severity of scarring each renal cortex was divided into 12 equal segments; severe kidney scarring was defined as having greater than 4 affected renal segments or global atrophy (i.e. diffuse scarring or shrunken kidney)[21]. To test for differences in the proportion of children with kidney scarring in the two treatment groups, we used logistic regression. Kidneys judged as being atrophied were considered to have all 12 segments affected. To test for differences in the proportion of children with severe kidney scarring, a relatively rare outcome, we assumed the number of cases in each group would follow a Poisson distribution and conditioned on the total number of events in both groups combined. In addition to the above prespecified analyses, we also examined whether the proportion of scarred kidneys differed in the two treatment groups (i.e. using kidneys, not children, as the unit of analysis) using a logistic regression model appropriate for clustered data.
We also investigated whether selected variables collected at baseline (1) were associated with kidney scarring, (2) were confounders, or (3) had an interaction with treatment. Children remained in the study for approximately 4 to 24 months and all adverse events occurring during this time were recorded. We examined adverse events occurring within 2 weeks of initiation of study product.
Our planned sample size of 160 evaluable children (i.e., children with a completed late DMSA kidney scan) in each treatment group assumed an α=0.05 and afforded the ability to detect a 10% absolute reduction (from 15% [22] to 5%) in the proportion of children with kidney scarring with a statistical power of 80%. We aimed to detect a difference of 10% because this would translate to a number needed to treat of 10; we felt that detecting smaller differences was not a priority in this initial study. All analyses were based on the intention-to-treat principle and used two-sided tests. One interim analysis was completed when 33% of patients had been accrued and at that time the Data Safety Monitoring Board approved continuation of the study.
Results
Study population
Of 3,702 children screened, 1,483 were eligible; of these, we enrolled and randomized 546 (Figure 1). There were no statistically significant differences in gender (p=0.30) or race (p=0.71) between enrolled children and children whose parents declined enrollment (data not shown); however, children enrolled tended to be younger (1.58 vs. 1.90 years, p<0.001) and less likely to be Hispanic (p<0.001) than children not enrolled. After withdrawal of children with negative urine cultures, 197 children randomized to adjuvant corticosteroid and 188 children randomized to placebo were followed prospectively (Figure 1). The large majority of randomized children were enrolled in Pittsburgh (360 of 385). Selected demographic and clinical characteristics of randomized children diagnosed with a UTI are shown in Table 1; no significant differences between treatment groups were apparent except that children in the corticosteroid group were significantly younger (see footnote to Table 1). Accordingly, the analysis of the primary outcome for kidney scarring was adjusted for age in addition to the study stratification variable (duration of fever prior to diagnosis). The confidence interval for the difference in proportion with kidney scarring in the two treatment groups adjusted for those covariates and was constructed using the Delta method [23]. Of the children with data on time of study product administration, 82% (310/377) received study product within 4 hours of urine collection. The highest recorded mean (SD) temperature (°C) was similar in the two treatment groups [39.8 (0.7) vs. 39.8 (0.6) in the corticosteroid and placebo groups, respectively]. Of the children with data on compliance, 93.4% (171/183) of children randomized to corticosteroid and 95.1% (176/185) of children randomized to placebo reportedly received at least 80% of scheduled study product doses (p=0.63). The proportion of evaluable children (i.e. children who returned for a DMSA kidney scan) in each group were 62.9% (124/197) and 69.7% (131/188) for the corticosteroid and placebo groups, respectively (p=0.20, Figure 1). There were no significant differences between children who returned for a DMSA kidney scan and those who did not, with regards to age, sex, race, maternal education, health insurance, and duration of fever; however, a significantly higher percentage of Hispanic children did not return for a DMSA kidney scan (58.6% vs 31.7%, p=0.006). The median (inter-quartile range) time from the initial UTI to the DMSA kidney scan was 6.1 (5.1–6.3) months. The proportion of children with one or more recurrences of febrile UTI during the follow-up period were similar in the corticosteroid and placebo groups (8.8% and 9.3%, respectively; p=0.89); 86.7% and 85.7% of febrile reinfections were re-treated with study product in the corticosteroid and placebo groups, respectively. One child’s DMSA scan was uninterpretable due to motion artifact (Figure 1). Among the 254 evaluable children, 240 (94%) were treated with cefdinir.
Table 1.
Selected Demographic and Clinical Characteristics of Randomized Children with a Urinary Tract Infection According to Treatment Groupa
| Characteristic | Corticosteroid Group (N=197) | Placebo Group (N=188) |
|---|---|---|
| No. of children (percent) | ||
| Age at entry, months | ||
| 2–23 | 145 (73.6) | 132 (70.2) |
| 24–71 | 52 (26.4) | 56 (29.8) |
| Sex | ||
| Maleb | 14 (7.1) | 17 (9.0) |
| Female | 183 (92.9) | 171 (91.0) |
| Race | ||
| Caucasian | 128 (65.0) | 126 (67.0) |
| African American | 41 (20.8) | 28 (14.9) |
| Other/multiracial | 28 (14.2) | 34 (18.1) |
| Health insurance status | ||
| Private | 81 (41.1) | 97 (51.6) |
| Medicaid | 108 (54.8) | 86 (45.7) |
| None | 6 (3.0) | 3 (1.6) |
| Unknown | 2 (1.0) | 2 (1.1) |
| Fever duration before diagnosis | ||
| <48 hours | 110 (55.8) | 96 (51.1) |
| ≥48 hours | 87 (44.2) | 92 (48.9) |
No significant differences noted between the two treatment groups except for mean age (17.2 months vs. 20.8 months in the corticosteroid and placebo groups, respectively, p=0.046).
13 of 31 (42.0%) of included males were circumcised
Kidney scarring
Evidence of kidney scarring in children according to treatment group is shown in Table 2. Overall, 34 children (39 kidneys) exhibited evidence of scarring; in all cases, volume loss/contour change was present (i.e. no child had photopenia without contour change). Kidney scarring was present in 16.8% (22/131) of children randomized to placebo and in 9.8% (12/123) of children randomized to adjuvant corticosteroids (p=0.16 adjusting for duration of fever and age; absolute risk reduction of 5.9% [95% CI: −2.2, 14.1]). When the analysis was repeated using kidney as the experimental unit, the percentage of scarred kidneys in the placebo and adjuvant corticosteroids groups was 9.9% and 5.3%, respectively (p=0.11). Three children, all in the placebo group, had severe kidney scarring (p=0.25; absolute risk reduction of 2.3% [95% CI, −0.3, 4.9]). Of these, none had infection with an organism that was resistant to the antibiotic initially prescribed and two had a single recurrent infection prior to the outcome DMSA. Of all children in the study, only one had generalized kidney atrophy; all other children had well localized scars. Agreement between radiologists on the presence of scarring on the outcome DMSA kidney scan was moderate (Fleiss Kappa=0.52) [24]. In kidneys determined to have kidney scarring, scarring affected an average of 1.9 segments and 2.4 segments in the corticosteroid and placebo groups, respectively (p=0.22).
Table 2.
Renal Scarring According to Treatment Group
| Renal scarring | Corticosteroid Treatment Group | Placebo Treatment Group | Absolute Difference in Risk in % (95% CI) | P value |
|---|---|---|---|---|
| No. of children with renal scarring/total (%) | Percentage points | |||
| Overall | 12/123(9.8%) | 22/131 (16.8%) | 5.9% (−2.2, 14.1) | 0.16a |
| Severe | 0/123 (0%) | 3/131 (2.3%) | 2.3% (−0.3, 4.9) | 0.25 |
Adjusted for duration of fever before presentation (our stratification variable) and age. Unadjusted p-value was 0.10
The only predictor of kidney scarring was age (Table 3); older children were more likely to exhibit kidney scarring (the odds [CI] of kidney scarring was 2.8 [1.3 – 5.8] fold higher in children ≥24 months compared to younger children).
Table 3.
Predictors of Renal Scarring in Children with an Outcome Renal Scan
| Characteristic at Entry* | Corticosteroid (N=123) | Placebo (N=131) | Odds ratio (95%CI) | P valuea |
|---|---|---|---|---|
| No. of children with renal scarring/total (%) | ||||
| Age | .008 | |||
| < 24 months | 8/95 (8.4) | 10/91 (11.0) | Reference | |
| ≥24 months | 4/28 (14.3) | 12/40 (30.0) | 2.75 (1.30–5.80) | |
| Gender | .39 | |||
| Female | 10/114 (8.8) | 20/120 (16.7) | Reference | |
| Male | 2/9 (22.2) | 2/11 (18.2) | 1.67 (0.52–5.38) | |
| Race | .48 | |||
| White | 6/74 (8.1) | 18/89 (20.2) | 1.33 (0.60–2.95) | |
| Other race | 6/49 (12.2) | 4/42 (9.5) | Reference | |
| Fever duration before presentation | .48 | |||
| <48 hours | 6/68 (8.8) | 10/67 (14.9) | Reference | |
| ≥48 hours | 6/55 (10.9) | 12/64 (18.8) | 1.30 (0.63–2.68) | |
| Fever duration before study product dispensed | .70 | |||
| <48 hours | 6/63 (9.5) | 9/59 (15.3) | Reference | |
| ≥48 hours | 6/60 (10.0) | 13/72 (18.1) | 1.16 (0.56–2.41) | |
| Ibuprofen use within 24 hours of enrollment | .56 | |||
| Yes | 4/66 (6.1) | 14/78 (17.9) | Reference | |
| No | 8/57 (14.0) | 8/53 (15.1) | 1.24 (0.60–2.57) | |
| Ibuprofen use during treatment | .70 | |||
| Yes | 2/31 (6.5) | 5/29 (17.2) | Reference | |
| No | 10/92 (10.9) | 17/102 (16.7) | 1.20 (0.49–2.92) | |
| Leukocyte esterase | .21 | |||
| 1+ or less | 3/18 (16.7) | 3/16 (18.8) | Reference | |
| 2+ | 2/15 (13.3) | 7/28 (25.0) | 1.12 (0.35–3.57) | |
| 3+ | 7/90 (7.8) | 12/87 (13.8) | 0.55 (0.20–1.51) | |
| Infecting organism | .20 | |||
| E. coli | 11/117 (9.4) | 19/121 (15.7) | Reference | |
| Other organism | 1/6 (16.7) | 3/10 (30.0) | 2.18 (0.65–7.27) | |
P-values listed correspond to prediction of renal scarring; for this, treatment groups were combined and adjusted for. P values for interaction (not listed) were all non-significant for the above variables.
Complications and adverse events
Details concerning selected adverse events in children who were dispensed the allocated study product are summarized in Table 4. Children randomized to adjuvant corticosteroids were more likely to exhibit fussiness (p=0.004). No child developed a kidney abscess. Rates of unplanned hospitalizations (from any cause) in both arms were comparable.
Table 4.
Distribution of Selected Adverse Events Within 2 Weeks of Taking Study Product in Randomized Children who Received Allocated Intervention
| Adverse Event | Treatment Group | P value | |
|---|---|---|---|
| Corticosteroid (n=271) | Placebo (n=275) | ||
| Number (percent) of children | |||
| Non-serious adverse events | |||
| Diarrhea | 19 (7.0) | 16 (5.8) | 0.69 |
| Fussiness | 25 (9.2) | 8 (2.9) | 0.004 |
| Vomiting | 2 (0.7) | 7 (2.5) | 0.18 |
| Sleepier than usual | 4 (1.5) | 1 (0.4) | 0.21 |
| Apparent abdominal pain | 1 (0.4) | 0 | 0.50 |
| Serious adverse events | |||
| Hospitalization (any cause)a | 6 (2.2) | 7 (2.5) | >0.99 |
| Bacteremia | 1 (0.4) | 1 (0.4) | >0.99 |
| Renal abscess | 0 | 0 | N/A |
In children initially managed as outpatients
Discussion
Previous animal studies [6, 11] and one Taiwanese study that included hospitalized children with severe pyelonephritis provide preliminary support for the efficacy of corticosteroids in preventing kidney scarring in children with proven pyelonephritis [18]. In contrast with the Taiwanese study in which pyelonephritis was confirmed in all children using DMSA kidney scans at the time of diagnosis of UTI, we included all febrile children with UTI. We found that children 2 months to 6 years of age with a first febrile UTI who were randomized to receive adjuvant corticosteroids tended to develop fewer kidney scars than children who were randomized to receive placebo; however, a statistically significant difference was not achieved. Although our data alone do not support the routine use of adjuvant corticosteroids in all children with a febrile UTI at this time, our study was limited by not reaching its intended sample size. Thus, given the findings of the other studies to date, further study examining this question may be warranted.
Our study was underpowered; we reached 79% of the target sample size; funding and time to complete the study ran out before we could reach our targets. Accordingly, our findings should not be taken as definitive evidence that adjuvant corticosteroids are ineffective at reducing scarring in children with a febrile UTI.
Regarding predictors of kidney scarring (Table 3), we found that children 2 years of age and older have significantly greater odds of kidney scarring, which is consistent with previous studies [25–27]. Ibuprofen use before or after diagnosis of UTI was not associated with increased risk of kidney scarring.
By design, we did not perform a DMSA scan during the acute phase because, in a previous study [28], we found that the proportion of children with pre-existing lesions that could be confused with kidney scarring in children with a documented first febrile UTI (i.e. examination of all the child’s medical records since birth revealed no previous UTI) was likely to be extremely low (0 of 50 such children in our previous study had pre-existing lesions on early DMSA). Furthermore, even if a few children happened to have pre-existing lesions, likely equal numbers would have been randomly assigned to the two treatment arms. Although differentiation of acquired kidney scarring and congenital dysplasia can be difficult, the latter is more often present in males and often is characterized by generalized kidney atrophy. Only one child (a 24-month-old female) in this study had generalized atrophy; this child had 2 febrile infections during the study period (both treated with placebo). Thus, in this study, the vast majority of lesions detected at the time of the late DMSA scan likely represent acquired kidney scarring secondary to UTIs.
Our study had several limitations. Although this is the largest study to date, the sample size was inadequate. One reason for this was the high rate of false positive urinalyses (i.e. 158 febrile children with presumed UTI were enrolled but then withdrawn because of a negative urine culture, see Figure 1), which speaks to the inadequacies of the available screening tests for UTI. Attrition rate was also relatively high; it was difficult to keep some parents engaged in the study and willing to follow through with the DMSA scan, which usually occurred at least 6 months after their child’s UTI; other parents were concerned about the time required to travel to the tertiary regional hospital, the need for intravenous access, and exposure to radiation. However, attrition seemed to have largely been based on parent availability and interest, and therefore unlikely to have biased the results. The small proportion of male children is another limitation. Strengths of our study include avoidance of specimens collected using a perineal bag and use of three independent radiologists/nuclear medicine physicians to interpret the DMSA kidney scans.
Because this study was limited by not reaching its intended sample size, future randomized trials, especially those focusing on children at high risk of pyelonephritis, identified using noninvasive biomarkers when such markers become available, may be warranted.
Supplementary Material
What does it add to existing literature
A previous study suggested that use of adjuvant systemic corticosteroids reduces kidney scarring in children radiologically confirmed to have extensive pyelonephritis. Efficacy of corticosteroids for children with febrile urinary tract infection (UTI) has not been studied.
What is the impact
Rates of kidney scarring were 9.8% (12/123) and 16.8% (22/131) in the corticosteroid and placebo groups, respectively (p=0.16). While corticosteroid administration did not significantly reduce kidney scarring, the study was limited by not reaching its intended sample size.
Key message
Corticosteroid administration did not significantly reduce kidney scarring; however, the study was limited by not reaching its intended sample size.
Acknowledgements
We would like to acknowledge the efforts of Marcia Pope, Jennifer Nagg, Mary Ann Haralam, Megan Skae, Linette Milkovich, and Rose Azrak in recruiting and enrolling patients for the study. Additionally, we would like to acknowledge the efforts of Christi McElheny for sample processing and Drs. Uri Alon, Adam Hersh, and Eugene Shapiro for serving on the Data Safety Monitoring Board for the study.
Funding Support: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R01DK087870)
Footnotes
Conflict of Interest: The authors have no conflicts of interest to disclose
Declarations
Availability of Data: Data is available at clinicaltrials.gov. No datasets will be provided
Financial Disclosures: The authors have no financial relationships relevant to this article to disclose
IRB Approval: The study was approved by the Institutional Review Boards of the respective institutions
Category of Study: Clinical
Patient Consent: All patients and parents were consented prior to enrollment
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Wennerstrom M, Hansson S, Jodal U, Sixt R, Stokland E (2000) Renal function 16 to 26 years after the first urinary tract infection in childhood. Arch Pediatr Adolesc Med 154:339–345 [DOI] [PubMed] [Google Scholar]
- 2.Wennerstrom M, Hansson S, Hedner T, Himmelmann A, Jodal U (2000) Ambulatory blood pressure 16–26 years after the first urinary tract infection in childhood. J Hypertens 18:485–491 [DOI] [PubMed] [Google Scholar]
- 3.Martinell J, Lidin-Janson G, Jagenburg R, Sivertsson R, Claesson I, Jodal U (1996) Girls prone to urinary infections followed into adulthood. Indices of renal disease. Pediatr Nephrol 10:139–142 [DOI] [PubMed] [Google Scholar]
- 4.Jacobson SH, Eklof O, Eriksson CG, Lins LE, Tidgren B, Winberg J (1989) Development of hypertension and uraemia after pyelonephritis in childhood: 27 year follow up. BMJ 299:703–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haraoka M, Matsumoto T, Mizunoe Y, Ogata N, Takahashi K, Kubo S, Tanaka M, Kumazawa J (1993) Effect of prednisolone on renal scarring in rats following infection with Serratia marcescens. Ren Fail 15:567–571 [DOI] [PubMed] [Google Scholar]
- 6.Haraoka M, Matsumoto T, Takahashi K, Kubo S, Tanaka M, Kumazawa J (1994) Suppression of renal scarring by prednisolone combined with ciprofloxacin in ascending pyelonephritis in rats. J Urol 151:1078–1080 [DOI] [PubMed] [Google Scholar]
- 7.Hoberman A, Wald ER, Hickey RW, Baskin M, Charron M, Majd M, Kearney DH, Reynolds EA, Ruley J, Janosky JE (1999) Oral versus initial intravenous therapy for urinary tract infections in young febrile children. Pediatrics 104:79–86 [DOI] [PubMed] [Google Scholar]
- 8.Huang A, Palmer LS, Hom D, Anderson AE, Kushner L, Franco I (1999) Ibuprofen combined with antibiotics suppresses renal scarring due to ascending pyelonephritis in rats. J Urol 162:1396–1398 [PubMed] [Google Scholar]
- 9.Meylan PR, Markert M, Bille J, Glauser MP (1989) Relationship between neutrophil-mediated oxidative injury during acute experimental pyelonephritis and chronic renal scarring. Infect Immun 57:2196–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Omrod D, Cawley S, Miller T (1986) Neutrophil-mediated tissue destruction in experimental pyelonephritis. Univ of Chicago, Chicago, Illinois. [Google Scholar]
- 11.Pohl HG, Rushton HG, Park JS, Chandra R, Majd M (1999) Adjunctive oral corticosteroids reduce renal scarring: the piglet model of reflux and acute experimental pyelonephritis. J Urol 162:815–820 [DOI] [PubMed] [Google Scholar]
- 12.Roberts JA, Roth JK Jr., Domingue G, Lewis RW, Kaack B, Baskin G (1983) Immunology of pyelonephritis in the primate model. VI. Effect of complement depletion. J Urol 129:193–196 [DOI] [PubMed] [Google Scholar]
- 13.Shimamura T (1981) Mechanisms of renal tissue destruction in an experimental acute pyelonephritis. Exp Mol Pathol 34:34–42 [DOI] [PubMed] [Google Scholar]
- 14.Calderon-Margalit R, Golan E, Twig G, Leiba A, Tzur D, Afek A, Skorecki K, Vivante A (2018) History of Childhood Kidney Disease and Risk of Adult End-Stage Renal Disease. N Engl J Med 378:428–438 [DOI] [PubMed] [Google Scholar]
- 15.Bille J, Glauser MP (1982) Protection against chronic pyelonephritis in rats by suppression of acute suppuration: effect of colchicine and neutropenia. J Infect Dis 146:220–226 [DOI] [PubMed] [Google Scholar]
- 16.Roberts JA, Roth JK Jr., Domingue G, Lewis RW, Kaack B, Baskin G (1982) Immunology of pyelonephritis in the primate model. V. Effect of superoxide dismutase. J Urol 128:1394–1400 [DOI] [PubMed] [Google Scholar]
- 17.Glauser MP, Lyons JM, Braude AI (1978) Prevention of chronic experimental pyelonephritis by suppression of acute suppuration. J Clin Invest 61:403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang YY, Chen MJ, Chiu NT, Chou HH, Lin KY, Chiou YY (2011) Adjunctive oral methylprednisolone in pediatric acute pyelonephritis alleviates renal scarring. Pediatrics 128:e496–504 [DOI] [PubMed] [Google Scholar]
- 19.Farhat W, Bagli DJ, Capolicchio G, O’Reilly S, Merguerian PA, Khoury A, McLorie GA (2000) The dysfunctional voiding scoring system: quantitative standardization of dysfunctional voiding symptoms in children. J Urol 164:1011–1015 [DOI] [PubMed] [Google Scholar]
- 20.Benninga M, Candy DC, Catto-Smith AG, Clayden G, Loening-Baucke V, Di Lorenzo C, Nurko S, Staiano A (2005) The Paris Consensus on Childhood Constipation Terminology (PACCT) Group. J Pediatr Gastroenterol Nutr 40:273–275 [DOI] [PubMed] [Google Scholar]
- 21.Keren R, Carpenter MA, Hoberman A, Shaikh N, Matoo TK, Chesney RW, Matthews R, Gerson AC, Greenfield SP, Fivush B, McLurie GA, Rushton HG, Canning D, Nelson CP, Greenbaum L, Bukowski T, Primack W, Sutherland R, Hosking J, Stewart D, Elder J, Moxey-Mims M, Nyberg L (2008) Rationale and design issues of the Randomized Intervention for Children With Vesicoureteral Reflux (RIVUR) study. Pediatrics 122 Suppl 5:S240–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaikh N, Ewing AL, Bhatnagar S, Hoberman A (2010) Risk of renal scarring in children with a first urinary tract infection: a systematic review. Pediatrics 126:1084–1091 [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Liu F (2019) Baseline-Covariate Adjusted Confidence Interval for Proportional Difference Between Two Treatment Groups in Clinical Trials. Stat Biopharm Res 11:292–300 [Google Scholar]
- 24.Fleiss J (1971) Measuring nominal scale agreement among many raters. Psychol Bull 76:378–382 [Google Scholar]
- 25.Hum SW, Shaikh N (2019) Risk Factors for Delayed Antimicrobial Treatment in Febrile Children with Urinary Tract Infections. J Pediatr 205:126–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaikh N, Mattoo TK, Keren R, Ivanova A, Cui G, Moxey-Mims M, Majd M, Ziessman HA, Hoberman A (2016) Early Antibiotic Treatment for Pediatric Febrile Urinary Tract Infection and Renal Scarring. JAMA Pediatr 170:848–854 [DOI] [PubMed] [Google Scholar]
- 27.Shaikh N, Craig JC, Rovers MM, Da Dalt L, Gardikis S, Hoberman A, Montini G, Rodrigo C, Taskinen S, Tuerlinckx D, Shope T (2014) Identification of children and adolescents at risk for renal scarring after a first urinary tract infection: a meta-analysis with individual patient data. JAMA Pediatr 168:893–900 [DOI] [PubMed] [Google Scholar]
- 28.Shaikh N, Haralam MA, Kurs-Lasky M, Hoberman A (2019) Association of Renal Scarring With Number of Febrile Urinary Tract Infections in Children. JAMA Pediatr. DOI: 10.1001/jamapediatrics.2019.2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.