Abstract
Rationale.
One hallmark of tumor-derived exosomes (TEX) is the promotion of cancer progression by stimulating angiogenesis. This study was performed to evaluate the role of adenosine receptors in TEX-induced angiogenesis.
Methods.
TEX produced by UMSCC47 head and neck cancer cell line were isolated by mini size exclusion chromatography (mini-SEC). Enzymatic activity of ectonucleotidases CD39/CD73 carried by TEX was measured by HPLC. Adenosine content of TEX was measured by UPLC-MS/MS. Primary human macrophages were co-incubated with TEX or exosomes derived from the plasma of head and neck cancer patients and their marker expression profile was analysed by flow cytometry. The macrophage secretome was analysed by angiogenesis arrays. The in vitro angiogenic potential of TEX was evaluated in endothelial growth studies. Results were validated in vivo using basement membrane extract plug assays in A1R−/−, A2AR−/− and A2BR−/− rats. Vascularization was analysed by hemoglobin quantification and immunohistology with vessel and macrophage markers.
Results.
TEX carried enzymatically-active CD39/CD73 and adenosine. TEX promoted A2BR-mediated polarization of macrophages towards an M2-like phenotype (p < 0.05) and enhanced their secretion of angiogenic factors. Growth of endothelial cells was stimulated directly by TEX and indirectly via macrophage-reprogramming dependent on A2BR signalling (p < 0.01). In vivo, TEX stimulated the formation of defined vascular structures and macrophage infiltration. This response was absent in A2BR−/− rats (p < 0.05).
Conclusion.
This report provides the first evidence for adenosine production by TEX in order to promote angiogenesis via A2BR. A2BR antagonism emerges as a potential strategy to block TEX-induced angiogenesis.
Keywords: exosomes, TEX, angiogenesis, adenosine, A2BR
Introduction
The role of tumor-derived exosomes (TEX) in disease progression is of great current interest. Exosomes are a subset of small extracellular vesicles (EVs) sized at 30–150 nm that are found in body fluids of all individuals. Cancer patients’ body fluids are enriched in TEX, which play a key role in mediating intercellular communication [1]. One hallmark of TEX is their pro-angiogenic properties and the ability to stimulate blood vessel formation in the tumor microenvironment (TME) [2, 3]. The cargo of TEX is enriched in pro-angiogenic factors, such as angiogenic proteins, mRNAs and miRNAs, which functionally reprogram endothelial cells (ECs) [4]. It has been recognized that TEX cargo mimics the content of the parent tumor cells [5]. Also, tumor cells grown under hypoxic conditions release TEX with pro-angiogenic functions [6]. Interestingly, TEX interact avidly with ECs, either by surface/ligand interactions or by endocytosis-mediated internalization, ultimately leading to the recipient cell reprogramming and expression of the angiogenic phenotype [7].
It was previously shown that exosomes isolated from plasma of normal donors (NDs) or cancer patients carry enzymatically-active CD39 and CD73, two ectonucleotidases, which are important components of the adenosine (ADO) pathway. Exosomes are able to hydrolyze extracellular ATP (eATP) to ADO, which makes them an important source of ADO in the TME [8, 9]. It was also shown, that exosomes suppress T cell functions through ADO production in the TME [9]. However, besides its immunosuppressive effects, ADO has been recognized as a potent stimulator of angiogenesis [10]. ADO plays a critical role in promoting EC proliferation and migration by increasing levels of VEGF and other angiogenic growth factors [11]. Studies of the ADO receptor subtypes 1, 2A and 2B (A1R, A2AR and A2BR, respectively) revealed that the pro-angiogenic effects of ADO on ECs are mainly mediated by A2BR [12]. However, adenosine receptors (ADORs) can also act in a functionally cooperative fashion to promote angiogenesis by a paracrine mechanism involving the differential expression and secretion of angiogenic factors from other cell types [13, 14]. Treatment of macrophages and mast cells with ADO upregulated the production of VEGF, IL-8, angiopoetin-1 and thrombospondin-1, resulting in stimulation of angiogenesis [14, 15]. The purpose of this study was to establish whether TEX induce ADOR signalling which results in promotion of angiogenesis.
Materials and Methods
Adenosine receptor antagonists
The following ADOR antagonists were used: PSB-36 (A1R, 1μM, Tocris), ZM 241385 (A2AR, 1μM, Sigma) and MRS 1754 (A2BR, 0.3μM, Tocris).
Cell line
The HPV(+) head and neck squamous cell carcinoma (HNSCC) cell line UMSCC47 was established by Dr. Thomas Carey (University of Michigan, Ann Arbor, MI) and obtained from Robert L. Ferris (UPMC Hillman Cancer Center, Pittsburgh, PA). Cells were authenticated prior to their use and were grown in DMEM (Lonza Inc.) supplemented with 1% (v/v) penicillin/streptomycin and 10% (v/v) FBS (Gibco, Thermo Fisher Scientific) at 37°C and in the atmosphere of 5% CO2 in air. FBS was depleted of exosomes by ultracentrifugation at 100,000xg for 3h. For exosome isolation, 2.5 x 106 UMSCC47 cells were seeded with 25mL media in 150 cm2 cell culture flasks as previously described [16]. Supernatants were collected after 72 hours.
TEX isolation by mini-SEC
Processing of supernatants and exosome isolation by mini-SEC was performed as previously described [17]. Briefly, cell culture supernatants were centrifuged at room temperature (RT) for 10 min at 2000×g, transferred to new tubes for centrifugation at 10,000×g at 4 °C for 30 min and filtrated using a 0.22 μm bacterial filter. Afterwards, aliquots of supernatants were concentrated by using Vivacell 100 concentrators at 2000×g. 1 mL of concentrated supernatant was loaded on a 10 cm-long Sepharose 2-B column and individual 1 mL fractions were collected. Fraction #4 containing non-aggregated TEX was used in subsequent assays.
TEX characterization
Determination of protein concentrations, transmission electron microscopy (TEM), tunable resistive pulse sensing (TRPS) and western blotting was performed as previously described in detail [17].
Bead-assisted flow cytometry
The presence of CD39 and CD73 was confirmed by bead-assisted flow cytometry as previously described [18]. Briefly, 10 μg of TEX in 100 μl PBS were captured with biotin-labeled anti-CD63 mAb (1:50, Biolegend, San Diego, CA) for 2 h at RT. TEX were then incubated with 10 μL of ExoCap™ Streptavidin magnetic beads (MBL International, Woburn, MA) for 2 h at RT. The beads/anti-CD63Ab/TEX complexes were washed using a magnet and re-suspended in 200 μl of PBS. 100 μl of the sample was stained with an anti-CD39-FITC (1:25, 328206, Biolegend), anti-CD73-FITC (1:25, 344016, Biolegend) or with the appropriate isotype control for 1 h at RT. Analysis was performed with the Gallios flow cytometer and the Kaluza 1.0 software (Beckman Coulter, Krefeld, Germany). 10.000 events were acquired by gating on the bead fraction in the forward/sideward light scatter.
Dot blot analysis
Dot blot analysis of TEX was performed as previously described [19]. Briefly, various concentrations of isolated TEX were absorbed onto nitrocellulose membranes at room temperature for 30 min. The membranes were blocked with 5% non-fat milk in PBS in the absence or presence of 0.1% (v/v) Tween-20 (PBST) at RT for 1 h. Anti-CD39 (1:1000, sc-33558, Santa Cruz Biotechnology) and anti-CD73 (1:1000, sc-25603, Santa Cruz Biotechnology) antibodies were incubated on the membranes in PBS or PBST blocking buffer at 4°C overnight followed by HRP-conjugated secondary antibody incubation at RT for 1 h. Blots were developed with ECL detection reagents (GE Healthcare Biosciences).
Analysis of enzymatic activity of exosomal CD39/CD73
Ten μg of exosomes in 100 μl of PBS were incubated at 37°C for 60 min with either 20 μM of N6-etheno-ATP (substrate for CD39) or 20 μM of N6-etheno-AMP (substrate for CD73 activity). N6-etheno-ATP, N6-etheno-ADP, N6-etheno-AMP, N6-etheno-adenosine, and N6-etheno-adenine were measured by high performance liquid chromatography (HPLC) using an Agilent (Santa Clara, CA) HPLC system (HP Agilent Technologies 1100 series HPLC chromatograph equipped with an Agilent 1260 Infinity fluorescence detector). Aliquots of samples were injected onto a C-18 reverse phase column (Agilent Eclipse Plus C18, 5 μm, 4.6 x 250 mm), and N6-etheno-purines were separated by HPLC in gradient mode [buffer A: 0.2 M KH2PO4 in water; buffer B: 0.2 M KH2PO4 in 15% acetonitrile; linear gradient (% B): at 0 min 5.0%; from 0 to 5 min, 5.0%; from 5 to 30 min to 100.0%; from 30 to 40 min, 100.0%; from 40 to 41 min to 5.0%; from 41 to 50 min, 5.0%]. The flow rate was 1.0 ml/min. Fluorescence of adenine nucleotides in the eluate was monitored at an emission of 420 nm, after excitation at 280 nm. Chromatograms were processed and stored in digital form with Agilent OpenLAB CDS software. Standard curves were generated from authentic N6-etheno-ATP, N6-etheno-ADP, N6-etheno-AMP, N6-etheno-adenosine, and N6-etheno-adenine obtained from the BioLog Life Science Institute (Hayward, CA; catalog numbers E 004, E 007, E 005, E 011 E 012, respectively).
UPLC-MS/MS for adenosine and inosine
Hundred μg of exosomes in PBS were mixed with internal standards (13C10-adenosine and 15N4-inosine) and injected onto a Waters (Milford, MA) Acquity ultraperformance liquid chromatograph (UPLC) connected to a Waters UPLC BEH C18 column (1.7 μm beads; 2.1 × 150 mm). Adenosine and inosine were quantified by selected reaction monitoring using a triple quadrupole mass spectrometer (TSQ Quantum-Ultra; ThermoFisher Scientific, San Jose, CA) with a heated electrospray ionization source. The mobile phase was a linear gradient flow rate (300 uL/min) of 1% acetic acid in water (pH, 3; mobile phase A) and 100% methanol (mobile phase B). The gradient (A/B) settings were: from 0 to 2 minutes, 99.6%/0.4%; from 2 to 3 minutes, to 98.0%/2.0%; from 3 to 4 minutes, to 85.0%/15.0%; from 4 to 6.5 minutes, to 99.6%/0.4%. The instrument parameters were: sample tray temperature, 10°C; column temperature, 50°C; ion spray voltage, 4.0 kilovolts; ion transfer tube temperature, 350°C; source vaporization temperature, 320°C; Q2 CID gas, argon at 1.5 mTorr; sheath gas, nitrogen at 60 psi; auxiliary gas, nitrogen at 35 psi; Q1/Q3 width, 0.7/0.7 units full-width half-maximum; scan width, 0.6 units; scan time, 0.01 seconds. The following mass transitions (selected reaction monitoring) were obtained: adenosine (368 → 136 m/z, retention time (RT) = 3.29 min); 13C10-adenosine (376 → 141 m/z, RT = 3.29); inosine (269 → 137 m/z, RT = 3.10 min); and 15N4-inosine (273 → 141 m/z, RT = 3.10 min).
Patients
Peripheral venous blood specimens were collected from 12 patients with HNSCC with active disease (AD) seen at the UPMC Otolaryngology Clinic in 2018 or 2019. Blood specimen from 5 normal donors (ND) served as control. Informed consent from all individuals was obtained and the study was approved by the institutional review board of the University of Pittsburgh (UPCI 09-069/IRB991206). The blood samples were delivered to the laboratory and were centrifuged at 1,000xg for 10 minutes to separate the plasma from blood components. Exosomes were isolated by mini-SEC as described above. The characterization of exosomes derived from plasma of patients with HNSCC was previously described by us in detail [17, 20]. The presence of ectonucleotidases CD39 and CD73 and their enzymatic activity were also previously documented by us [8].
Primary macrophage culture
Blood samples were obtained from healthy donors. Informed consent from all individuals was obtained and the study was approved by the institutional review board of the University of Pittsburgh (UPCI 09-069/IRB991206). Peripheral blood mononuclear cells (PBMCs) were isolated as previously described in detail [17], 7.5×106PBMCs (10% monocytes) were seeded in 6-well plates. After 3 h of incubation, cells were washed gently 5 times with PBS (pH 7.4) for removal of non-adherent cells, and adherent cells were considered monocytes. For the differentiation into macrophages, 2 mL/well of the growth medium RPMI1640 supplemented with 10% (v/v) heat-inactivated FBS and 50 ng/mL GM-CSF (300-3, Peprotech) was used. Medium was renewed after 4 days and differentiation was completed after 7 days.
After differentiation, macrophages were maintained in RPMI1640 supplemented with 10% (v/v) heat-inactivated FBS for 72 h. Cells were treated either with PBS (Mϕ Naïve) or TEX (25 μg/mL), or were pre-treated for 30 min with ADOR antagonists before treatment with TEX.
After 72 h incubation, the phenotype was assessed by flow cytometry after surface or intracellular staining with anti-CD80-PE (1:3, 557227, BD Bioscience), anti-CD86-PE (1:3, PNIM2729U, IO Test), anti-HLA-DR-FITC (1:3, PNIMO436U, IO Test), anti-EGFR-APC (1:10, 352906, Biolegend), anti-CD206-FITC (1:3, 551135, BD Bioscience), anti-IL-10-PE (1:3, 559330, BD Bioscience), anti-LAP-PE (1:3, 349604, Biolegend), anti-Arginase-1-FITC (1:5, IC5868F, R&D Systems), anti-CD39-FITC (1:25, 328206, Biolegend), anti-CD73-FITC (1:25, 344016, Biolegend) and with appropriate isotype controls in staining buffer (PBS + 3 % BSA) for 30 min at RT with a minimum of two washes with PBS after each incubation. Cell fluorescence was measured with an Accuri flow cytometer according to the manufacturer’s instructions (BD Biosciences, San Jose, CA, USA).
Collection of conditioned medium
Macrophages were generated and treated as described above. Conditioned medium (CM) was collected after 72 h of incubation and centrifuged at 2,000xg for 10 min. CM was either used fresh or stored at −80°C until use.
Angiogenesis antibody arrays
The relative levels of human angiogenesis–related proteins in CM was measured using a Human Angiogenesis Array Kit (R&D Systems Inc.). Aliquots of CM (500 μL) were added to the array, and the results were analyzed with the ImageJ software (http://rsbweb.nih.gov/ij/).
Raw-Blue™ NF-κB Reporter Assay
NF-κB expression assay was performed as previously described [21]. Briefly, RAW-Blue™ cells (Invivogen, San Diego, CA) were grown and maintained in high-glucose DMEM supplemented with 10% (v/v) heat-inactivated FBS, 1% (v/v) penicillin/streptomycin and 100 μg/mL Normicin™ (Invivogen, San Diego, CA). RAW-Blue™ cells (20,000 per well) were seeded in a 96-well plate and treated with indicated antagonists for 30 min at 37°C. Afterwards, 20 μg/mL TEX and as positive control 100 ng/mL lipopolysaccharide (LPS; Sigma-Aldrich, St. Louis, MO) were added to the cells in triplicate and incubated for 24 h under culture conditions. Post incubation, 20 μL of conditioned media was collected, incubated with 200 μL QUANTI-blue™ reagent (Invivogen, San Diego, CA) and optical density at 655 nm was measured using TECAN spectrophotometer (TECAN, Mannedorf, Switzerland).
Endothelial cell growth studies
HUVECs obtained from Cell Systems were cultured in EGM™ Endothelial Cell Growth BulletKit™ (Lonza) and used at 4–6 passage. HUVECs were plated at the density of 5×103 cells/well in 96-well tissue culture dishes and allowed to attach overnight. Cells were then growth arrested by feeding EBM™ containing 0.5% albumin for 48 h. Afterwards, medium was changed to EBM™ containing platelet-derived growth factor–BB (PDGF-BB) (25 ng/mL) and the treatment was added. Cells were either treated with 2-chloroadenosine (CADO, 1 μM, Tocris) every 24 h, 10 μg of TEX or 100 μL of CM from macrophage cultures. In some experiments, cells were pre-incubated with an A2BR antagonist for 30 min before actual treatment was added. After 48 h of incubation, the MTS cell proliferation assay was performed according to the manufacturer’s instructions (Abeam). Absorbance was measured at 490 nm.
Animal study
Protocols for animal experiments were approved by the Institutional Animal Care and Use Committee under the reference number 18114203. Male or female SS/JrHsdMcwi rats with the following genetic backgrounds were used: A1R−/− (n = 4), A2AR−/− (n = 4), A2BR−/− (n = 6). Rats were generated by the MCW Gene Editing Rat Resource Program (Dr. Aron M. Geurts, Department of Physiology and Human Molecular Genetics Center, Medical College of Wisconsin, Milwaukee, WI) and extensively characterized by Jackson and colleagues [22].
Basement Membrane Extract Plug Assay
Growth-factor depleted Cultrex® (500 μL; Trevigen, MD, USA) was mixed with 100 μL PBS (Neg. CTRL), 100 μg total exosomal protein in a volume of 100 μL PBS (Exosomes) or 1 μg of EGF in 100 μL of PBS (Pos. CTRL) and incubated for 24 h on ice. Rats were anesthetized and the skin overlying the area of injection was gently shaved. Each rat received 3 subcutaneous injections (Neg. CTRL, Exosomes, Pos. CTRL) in the midventral abdominal region, which were permitted to solidify. Plugs were harvested after 7 d and photographed. Each plug was cut into two halves; one half was used for hemoglobin quantification and the other half was used for tissue histology.
Hemoglobin quantification
Implants were harvested, minced and digested using the human tumor digestion kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. As an indirect measure of vascularization, hemoglobin content in the supernatants from the digested plugs was quantified using Drabkin’s reagent (Sigma Aldrich, Waltham, MA) as previously described [23, 24].
Tissue histology
Excised implants were placed in 4% paraformaldehyde for 24 h, and subsequently in 30% sucrose (Sigma-Aldrich) for 24 h. Samples were embedded in OCT compound (Thermo Fisher Scientific) and stored at −80°C for subsequent sectioning. Cryostat sections (6 μm) were cut and immunofluorescence staining was performed by incubating sections with a rabbit anti-rat CD31 mAb (1:100, ab222783, abcam), a rabbit anti-rat Arginase-1 mAb (1:50, 93668, Cell Signaling) or a goat anti-rat CD68 Ab (1:100, sc-7084, Santa Cruz) overnight at 4°C. After washing, tissue sections were incubated with Cy™3-conjugated AffiniPure F(ab’)2 Fragment Donkey Anti-Rabbit IgG (1:400, 711-166-152, Jackson Immuno Research) or DyLight™649-conjugated AffiniPure F(ab’)2 Fragment Donkey Anti-Goat IgG (1:400, 705-496-147, Jackson Immuno Research) for 1 h at RT. Negative controls were stained in parallel with the secondary antibodies alone. Sections were counterstained and mounted with DAPI Fluoromount-G® (SouthernBiotech) and imaged using an Olympus BX51 microscope.
Statistical analysis
All data were analysed using the GraphPad Prism software (v7.0). Values are expressed as mean ± SEM. Differences between groups were assessed by Student t test and differences were considered significant at p< 0.05.
Results
Characteristics of TEX released by UMSCC47 cells
The exosome #4 fractions isolated from supernatants of the UMSCC47 cell line by mini-SEC showed the typical size and vesicular morphology which is associated with exosomes (Fig. 1A). The qNano analysis of TEX revealed a size distribution ranging from 55 to 150nm (Fig. 1B). TEX carried the exosomal markers TSG101 and CD9 whereas they did not carry Grp94, a negative marker for exosomes (Fig. 1C). The exosome characteristics were defined according with the criteria specified by MISEV(2018) for EV studies [25]. The analysis with UPLC-MS/MS revealed that UMSCC47-derived exosomes carry ADO as well as the ADO metabolite inosine (Fig. 1D).
Figure 1.
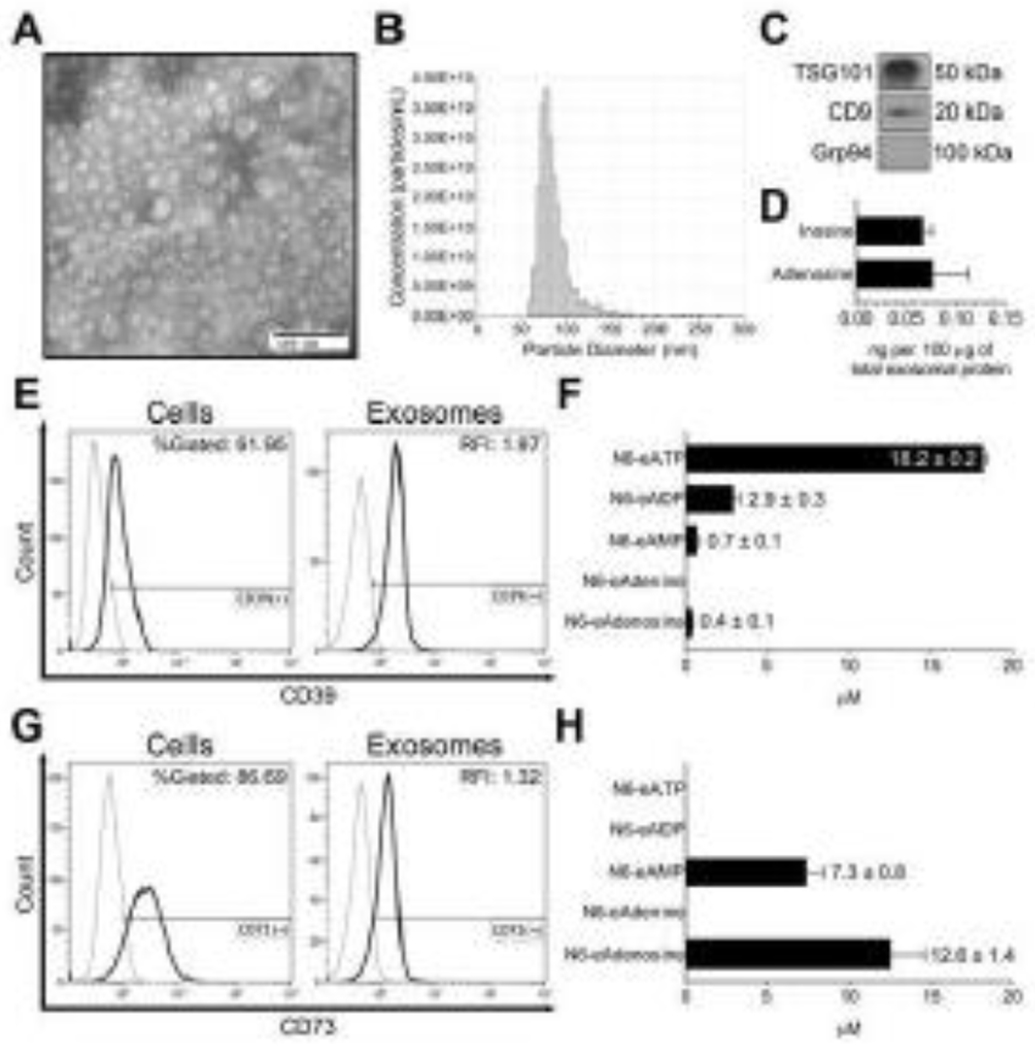
Characterization of TEX. UMSCC47 HPV(+) head and neck cancer cells were cultured and used for TEX generation. (A) Representative TEM image of UMSCC47-derived exosomes. (B) Representative TRPS (qNano) size and concentration distribution plot of UMSCC47-derived exosomes. (C) Western blots of UMSCC47-derived exosomes for exosome markers TSG101 and CD9 as well as negative marker Grp94 carried by UMSCC47-derived exosomes. Each lane was loaded with 5 μg protein of exosome lysate. (D) Quantification of adenosine and inosine in UMSCC47-derived exosomes by UPLC-MS/MS. (E) Representative histograms for CD39 expression of UMSCC47 cells (left panel) and TEX deriving from UMSCC47 cells (right panel) analyzed by flow cytometry. (F) CD39 enzymatic activity of UMSCC47-derived exosomes. (G) Representative histograms for CD73 expression of UMSCC47 cells (left panel) and TEX deriving from UMSCC47 cells (right panel). (H) CD73 enzymatic activity of UMSCC47-derived exosomes. Values represent means ± SEM.
UMSCC47 cells were chosen for this study because they express ectonucleotidases CD39 and CD73. 62% and 87% of the cells expressed these ectonucleotidases, respectively (Fig. 1E and G). Bead-assisted flow cytometry revealed that TEX carried CD39 and CD73 on their surface, reflecting the enzymatic content of UMSCC47 cells (Fig. 1E and G). However, besides its presence on the surface, CD39 was also present inside the vesicles. CD73 was found to be mostly surface bound (Fig. S1). Additionally, these enzymes were shown to be functionally active, as illustrated in Fig. 1F and H. Especially CD73 showed a high activity and converted approximately 65% of the added N6-etheno-AMP to N6-etheno-ADO (Fig. 1H).
Our data consistently show that TEX produced by UMSCC47 cells and isolated by SEC from UMSCC47 supernatants have the typical characteristics of exosomes and are potent producers of ADO by carrying functionally-active ectonucleotidases and ADO as well as ADO metabolites in their lumen.
TEX reprogram macrophages to an angiogenic phenotype via A2BR signalling
To investigate indirect effects of TEX which promote angiogenesis in recipient cells, TEX were co-incubated with macrophages. Representative phase-contrast images of macrophages are presented in Fig. 2B. A panel of established M1 and M2 markers, as well as the functional markers CD39 and CD73, were used to evaluate changes of macrophages in response to co-incubation with TEX. Overall, treatment with TEX resulted in a mixed phenotype, since the M1 markers CD80, CD86 and EGFR were significantly upregulated (p < 0.05; Fig. 2A) as were the M2 markers, CD206, LAP and Arginase-1 (p < 0.05; Fig. 2A). The functional marker CD73 was enhanced after TEX treatment (p < 0.05), indicating enhanced potential of macrophages for producing ADO (Fig. 2A). No changes were observed for the CD39 expression (Fig. 2A).
Figure 2.
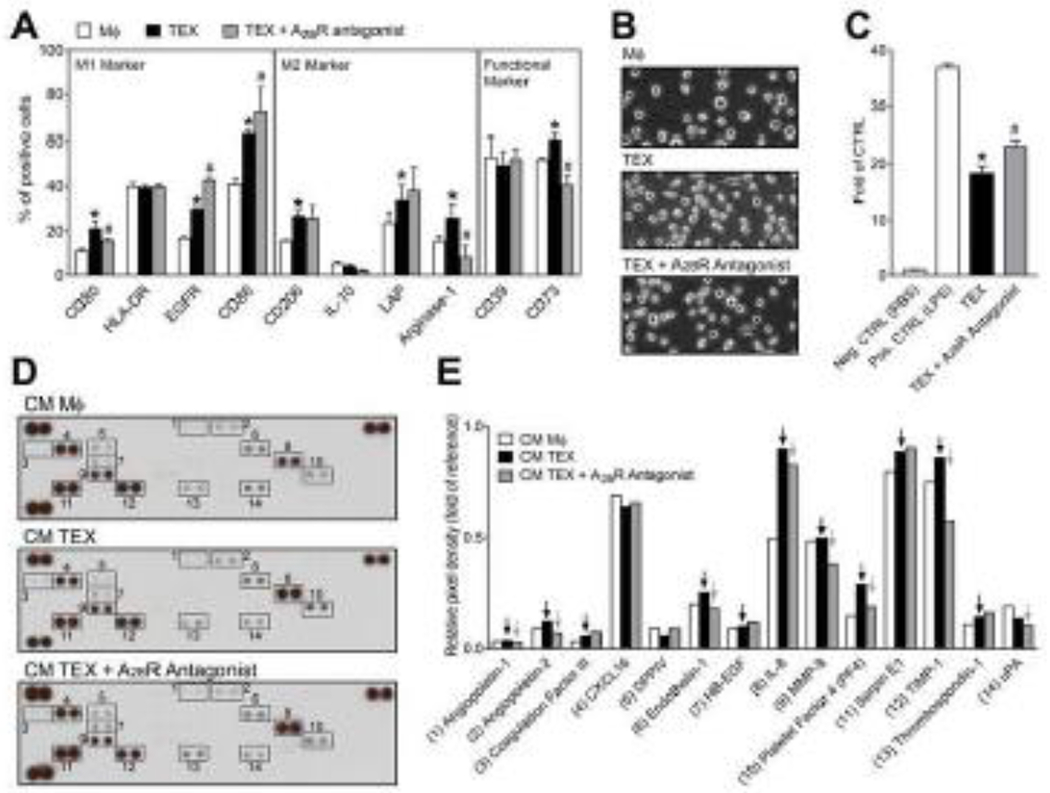
TEX interact with macrophages via A2BR and promote their secretion of pro-angiogenic factors. (A) PBMCs were isolated from healthy donors and monocytes were cultured for 7 days in the presence of GM-CSF to differentiate them into macrophages. After differentiation, macrophages were treated with TEX in the presence or absence of an antagonist for A2BR. Marker expression of a panel of established M1- and M2-markers and functional markers was analyzed by flow cytometry. (B) Representative phase contrast images of macrophages. (C) Raw-Blue™ NF-κB reporter cells were treated PBS (Neg. CTRL), LPS (100 ng/mL, Pos. CTRL), TEX (20 μg/mL exosomal protein) and TEX in the presence of an A2BR antagonist. (D) Conditioned medium of macrophages was collected and analyzed by human angiogenesis arrays. (E) The arrays were quantified using ImageJ. Values are normalized to reference spots on the membranes (top left, top right, and bottom left). Black arrows indicate increase compared to control. Grey arrows indicate factors which secretion was blocked by the A2BR antagonist. All values in this figure represent means ± SEM. *p < 0.05 vs. CTRL; #p < 0.05 vs. TEX.
Macrophages were also treated with TEX in combination with pharmacological antagonists for A1R, A2AR and A2BR to evaluate the influence of ADOR signalling on polarization and activation of macrophages. Overall, the treatment with the A2BR antagonist resulted mainly in polarization towards M1 macrophages and less so towards M2, as evidenced by a significant upregulation of the M1 markers, CD86 and EGFR, and a significant downregulation of the M2 markers, IL-10 and Arginase-1 (p < 0.05; Fig. 2A). Antagonists for A1R and A2AR had only minor effects on macrophage polarization induced by TEX (Fig. S2).
Additionally, an NF-κB-SEAP reporter cell line was used to assess NF-κB activation after TEX treatments. TEX stimulated NF-κB activation (p < 0.05), and activity was significantly enhanced by pre-treatment with an A2BR antagonist (p < 0.05). Pre-treatment with A1R or A2AR antagonists reduced activation of the NF-κB pathway (Fig. S2K and S2L).
The heatmap which is presented in Fig. S2L summarizes the phenotypical changes of macrophages in response to TEX treatment. The TEX treatment alone resulted in a mixed phenotype, whereas the combined treatment of TEX and an A2BR antagonist resulted in more M1-like macrophages, indicating that TEX induce A2BR signalling, which results in the polarization towards pro-angiogenic M2-like macrophages.
To validate these results, we investigated pro-angiogenic factors in the macrophage secretome by using human angiogenesis arrays. Naïve macrophages secreted several angiogenic factors, which were detected after 72 h of culture (Fig. 2D). Treatment with TEX resulted in the elevated secretion of several pro-angiogenic factors compared to naïve cells, such as Angiopoietin-1, Endothelin-1, Platelet Factor 4 and Serpin E1 (Fig. 2E). Interestingly, the secretion of IL-8 was highly stimulated by TEX. Pre-treatment with an A2BR antagonist in combination with TEX partially blocked the upregulation of these factors. Specifically, the upregulation of Angiopoietin-1, Endothelin-1, IL-8 and Platelet Factor 4 was dependent on A2BR signalling (Fig. 2E).
Our group previously showed, that exosomes isolated from the plasma of HNSCC patients carry enzymatically-active CD39 and CD73 [8]. Therefore, we co-incubated plasma-derived exosomes from cancer patients or normal donors with naive macrophages and analysed the marker expression profile to validate our cell line-based findings. The expression of M1 markers CD86 and EGFR showed no differences compared to naïve macrophages after treatment with cancer patient- or normal donor-derived exosomes (Fig. 3A and B). The M2 markers CD206 and Arginase-1 were significantly upregulated after co-incubation with plasma-derived exosomes from HNSCC patients (p < 0.001 and p < 0.01, respectively). No significant changes were observed for normal donor-derived exosomes (Fig. 3C and D). This indicates, that circulating exosomes in the plasma of HNSCC patients with active disease reprogram macrophages to a pro-angiogenic M2-like phenotype, similar to pure TEX from a HNSCC cell line.
Figure 3.
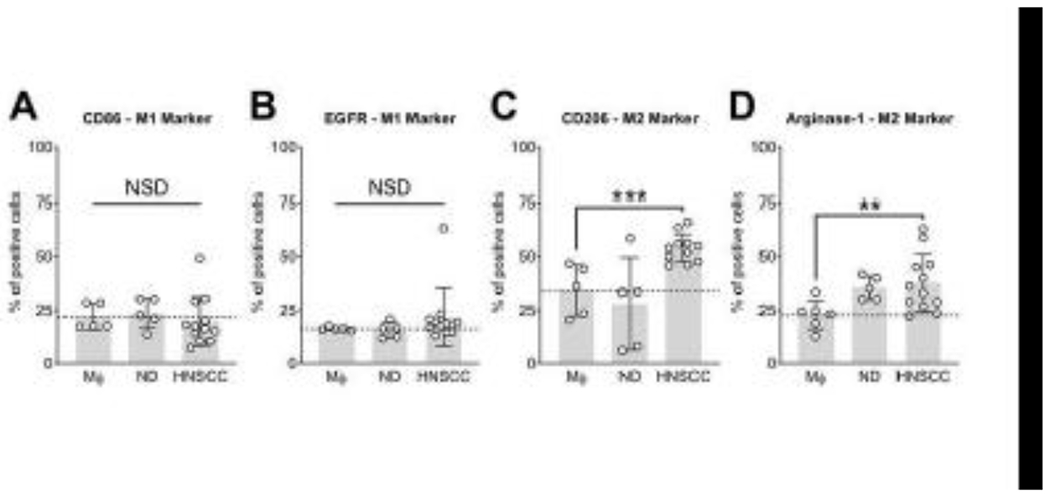
Exosomes from the plasma of HNSCC patients promote a M2-like phenotype in primary macrophages. Macrophages were treated with exosomes from the plasma of normal donors (ND), patients with active disease (HNSCC) or with the equivalent volume of PBS (Mϕ). After 72 h of incubation the marker expression profile of M1 markers CD86 (A) and EGFR (B) and M2 markers CD206 (C) and Arginase-1 (D) was analyzed by flow cytometry. Values represent means ± SD. **p < 0.01 vs. Mϕ; **p < 0.001 vs. Mϕ.
TEX directly and indirectly stimulate endothelial cell (EC) growth
To assess direct and indirect reprogramming of ECs by TEX, growth studies were performed. First, ECs were treated with CADO, a stable analogue of ADO, which resulted in a statistically significant promotion of EC proliferation (p < 0.01; Fig. 4A). This stimulation was blocked by using an A2BR antagonist as demonstrated in Fig. 4A (p < 0. 05) indicating that the stimulation of EC growth is A2BR-mediated. Next, we treated ECs with 10 μg of TEX which caused a significant increase in EC proliferation (p < 0.05; Fig. 4B). The A2BR antagonist blocked this effect similarly to the previous experiment with CADO (p < 0.05; Fig. 4B). ECs were also co-incubated with CM derived from naïve macrophages and macrophages cultured with TEX in the presence or absence of the A2BR antagonist. As described above, macrophages co-incubated with TEX released higher levels of pro-angiogenic factors. Here, this was validated functionally in the growth study, since the CM TEX group showed the biggest stimulation of EC proliferation (p < 0.05; Fig. 4C). The group with the A2BR antagonist showed a lower response compared to TEX only (Fig. 4C). These results demonstrate, that ADO stimulates EC growth via A2BR signalling. Adenosine-rich TEX cause a similar A2BR-mediated effect on ECs. Also, macrophages are reprogrammed via A2BR signalling and induce EC growth by secreting pro-angiogenic factors. This emphasizes that TEX are capable to directly and indirectly promote angiogenesis in the TME via A2BR-mediated signalling.
Figure 4.
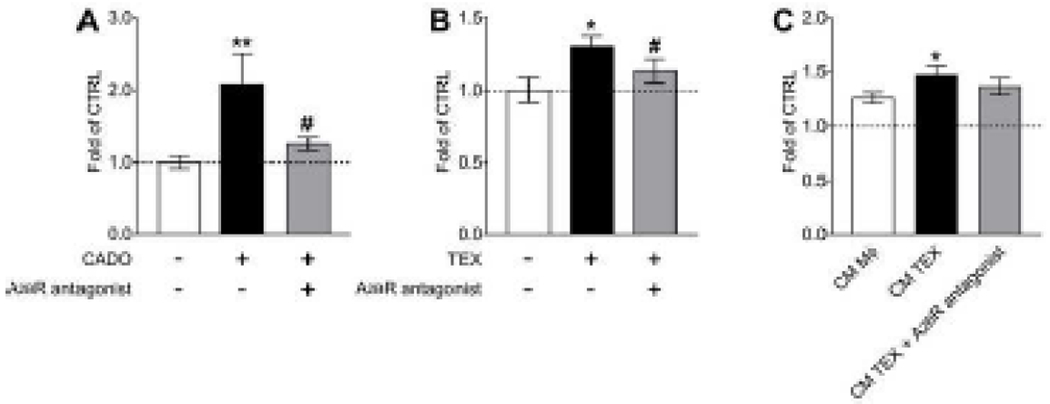
TEX directly and indirectly stimulate A2BR-dependent endothelial cell growth. (A) Proliferation of HUVECs in response to the treatment with CADO (1 μM) in the presence or absence of an A2BR antagonist (0.3 μM). (B) Proliferation of HUVECs in response to the treatment with TEX (10μg exosomal protein) in the presence or absence of an A2BR antagonist (0.3 μM). (C) Proliferation of HUVECs in response to the treatment with conditioned medium from Mϕ macrophages, macrophages treated with TEX and macrophages treated with TEX in the presence of an A2BR antagonist. Values are presented as fold of CTRL. All values in this figure represent means ± SEM. *p < 0.05 vs. CTRL; **p < 0.01 vs. CTRL; #p < 0.05 vs. CADO or TEX.
TEX promote A2BR-dependent vascularization in vivo
To assess the pro-angiogenic potential of TEX in vivo and the influence of ADOR on exosome-induced angiogenesis, TEX were mixed with growth-factor depleted Cultrex and were injected subcutaneously into A1R−/−, A2AR−/− and A2BR−/− rats. The implanted control plugs did not show any signs of vascularization, whereas the plugs with TEX induced a grossly obvious, enhanced vascularization in the A1R−/−, A2AR−/−, but not in the A2BR−/− rats (Fig. 5A). The positive CTRL which was a mixture of Cultrex and EGF induced a similar effect compared to TEX (Fig. 5A). Results were validated by immunostainings for the endothelial marker CD31 (Fig. 5C) and the functional vascular content was measured by quantifying the amount of hemoglobin inside of the plugs (Fig. 5B). Hemoglobin content of TEX and Pos. CTRL plugs was significantly higher compared to Neg. CTRL (p < 0.05) in A1R−/−, A2AR−/− rats. No significant differences were observed in the A2BR−/− rats (Fig. 5B). Quantification of immunostaining for CD31-positive signals gave similar results (Fig. 5D). Additionally, CD68/Arginase-1 immunostaining was performed in order to evaluate the infiltration of M2 macrophages. Vascularization of the plugs was associated with enhanced macrophage infiltration. The negative CTRL plugs did not contain double-positive CD68/Arginase-1 cells, whereas a remarkable and statistically-significant infiltration of cells with this phenotype was observed in the exosome and positive CTRL groups of A1R−/−, A2AR−/− rats (p < 0.05; Fig. 5E and F). In contrast, A2BR−/− rats only showed minimal macrophage infiltration.
Figure 5.
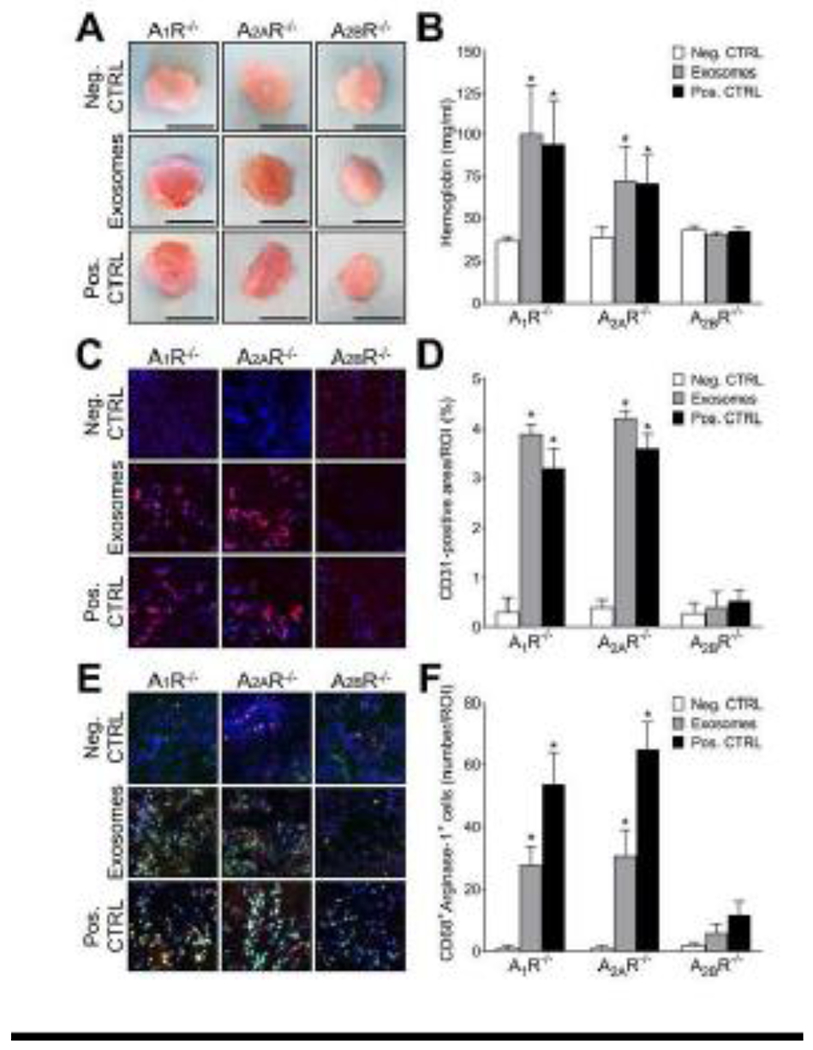
TEX promote A2BR-dependent angiogenesis in vivo. A1R−/−, A2AR−/− and A2BR−/− rats received subcutaneous injections of growth-factor depleted Cultrex®. Each animal received 3 plugs (Negative CTRL: 500 μl Cultrex® + 100 μl PBS; Exosomes: 500 μl Cultrex® +100 μg exosomal protein in 100 μl PBS; Positive CTRL: 500 μl Cultrex® + 1 μg of EGF in 100 μl PBS). Plugs were harvested after 7 days. (A) Representative photographs of harvested plugs. (B) Hemoglobin content in plugs. (C) Representative images of immunofluorescence staining for CD31 (red) and DAPI (blue). (D) Quantitative analysis of CD31+ signals by immunofluorescent staining. Data are expressed as the percentage of the area that was positively stained from the region of interest (% ROI). (E) Representative images of immunofluorescence staining for CD68 (green), Arginase-1 (red) and DAPI (blue). (F) Quantitative analysis of CD68+Arginase-1+ cells by immunofluorescent staining. Data are expressed as number of double positive cells per region of interest (% ROI). Data is presented as means ± SEM. Asterisks indicate a significant difference (p < 0.05) compared to Neg. CTRL.
Discussion
This communication emphasizes the important role of TEX as carriers and producers of ADO as well as the importance of TEX-associated ADO to promote angiogenesis via stimulation of the A2BR as visualized in Fig. 6. Our studies show that not only do TEX carry CD39/CD73 to autonomously produce ADO, but also directly deliver ADO and inosine as part of their cargo. This exosomal storehouse of adenosine and inosine represents a heretofore unknown mechanism for distal delivery of these purines; by protecting them from rapid uptake and metabolism, exosomes can shuttle adenosine and inosine across cells, tissues and organ systems. This is a novel and important finding.
Figure 6.
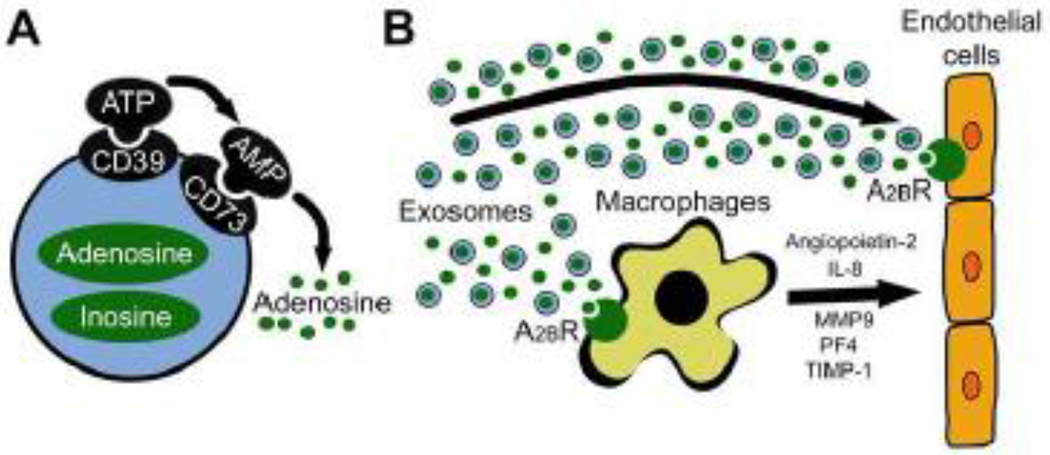
A schema of endothelial cells reprogramming by tumor-derived exosomes (TEX). (A) TEX carry ectonucleotidases CD39 and CD73 on their surface, which enzymatically produce adenosine. In addition to these surface-bound molecules, exosomes encapsulate adenosine and inosine. (B) TEX interact directly with endothelial cells by delivering/producing adenosine and inducing an A2BR-mediated stimulation of endothelial cell growth. Also, TEX reprogram other cells in the tumor microenvironment to release pro-angiogenic factors. Specifically, TEX-associated adenosine binds to A2BR on macrophages and stimulates the secretion of angiopoietin-2, interleukin 8 (IL-8), matrix metallopeptidase 9 (MMP9), platelet factor 4 (PF4) and tissue inhibitor of matrix metalloproteinases 1 (TIMP-1) which ultimately stimulate angiogenesis.
The promotion of angiogenesis by TEX is of great current interest. After Skog et al. reported that TEX contain angiogenic proteins, mRNAs and miRNAs and that they deliver messages to ECs [2], an increasing number of publications were dedicated to the pro-angiogenic functions of TEX. Since then, a variety of pathways have been uncovered, including surface/ligand interactions between TEX and ECs, such as ephrin receptor signalling or VEGF receptor signalling pathways [3, 26]. Moreover, genetic reprogramming of ECs is discussed in the literature, especially translation of miRNAs delivered by TEX resulting in the promotion of blood vessel formation [27]. Compared to these well-described pathways, the ADO pathway has not been studied in the context of TEX-induced angiogenesis so far. The importance of this pathway is supported by the almost ubiquitous expression of ADORs in healthy and neoplastic tissue and by currently on-going clinical trials in patients with cancer which specifically target components of the ADO pathway [28].
Most recent studies of pro-angiogenic TEX functions have mainly addressed direct interactions between TEX and ECs. However, other reports have shown that TEX function by paracrine and distant signalling to cells in the TME or at distant sites. Further, numerous publications describe TEX-induced stimulation of cancer-associated fibroblasts, suppression of various immune cell subsets and the TEX-driven formation of a pre-metastatic niche [9, 29, 30]. It is likely that “TEX-reprogrammed” cells in the TME act in a functionally cooperative fashion to promote angiogenesis by paracrine mechanisms, involving the secretion of angiogenic factors or the expression of specific ligands. In this study, macrophages were chosen to show these indirect pro-angiogenic functions of TEX which involve cellular reprogramming.
Our data indicate that the angiogenic effects of TEX are mainly mediated by A2BR signalling, which is in agreement with several other publications. Dubey et al. demonstrated that A2BR stimulate growth of ECs [12], and Du et al. reported similar results, showing that A2BR-driven angiogenesis leads to VEGF production and upregulation of eNOS in ECs [31]. There is also evidence, that ADO stimulates macrophages to release pro-angiogenic factors via A2BR, confirming the results of our study [15]. In aggregate, these findings indicate that A2BR plays a critical role in regulating the vascular remodelling associated with EC proliferation in angiogenesis, collateral vessel development, and recovery after vascular injury [12]. However, the other ADORs have also been reported to modulate angiogenesis, although evidence for direct stimulation of ECs is lacking. It has been shown that agonists of all ADOR types in tumor-bearing mice enhance the infiltration of macrophages into tumors and stimulate vascularization and production of pro-angiogenic factors in the TME [32]. Although the relative importance of each receptor subtype remains unclear, it appears that all ADOR subtypes participate in mediating the angiogenic actions of ADO, working in a coordinated fashion involving indirect and direct actions on ECs, smooth muscle cells, fibroblasts, and resident immune cells including monocytes, macrophages, and mast cells [13]. Our study shows that TEX promote angiogenesis by a direct interaction with ECs and also indirectly via macrophages. Interestingly, the same A2BR-mediated pathway promoted angiogenesis involving both cell types, emphasizing the important role of A2BR signalling in TEX-mediated angiogenesis.
Future studies will be necessary to fully evaluate the effects of TEX on A2BR-mediated angiogenesis in the TME. The experiments with macrophages and plasma-derived exosomes were performed with a small cohort of patients, and a larger patient cohort needs to be investigated in future studies. Additionally, the complex mixture of TEX and non-TEX (exosomes with origins different from tumor cells) in the plasma of cancer patients requires further analysis. The separation of TEX and non-TEX by immune capture, as illustrated in our reports [33] would be an elegant approach to enhance the understanding of macrophage-mediated reprogramming locally in the TME and at distant sites. We recently showed, that HNSCC-derived exosomes can be captured by using the marker CD44v3 [34]. Co-incubation of macrophages and endothelial cells with CD44v3+ and CD44v3− plasma-derived exosomes would further demonstrate the physiological relevance of TEX in this pathway.
Our in vivo study shows the angiogenic potential of TEX under physiologic conditions in immunocompetent rats. However, the altered immune landscape, cytokine levels and other tumor-associated factors, which are observed in the TME and at distant sites, are also likely to have an impact on the promotion of angiogenesis by TEX. Cekic et al. antagonized A2BR in an immunocompetent xenograft model and observed a decrease in tumor growth and in angiogenesis [35]. Thus, the blockade of A2BR may be an effective strategy to silence pro-angiogenic effects of TEX and generally decrease the neovascularization inside tumors.
In summary, TEX appear to play an important role in angiogenesis and thus may contribute to tumor growth and metastasis. Our data suggest that the complex cargo of TEX, which includes ADO and ADO metabolites, as well as enzymatically-active ectonucleotidases are mainly responsible for the stimulation of blood vessel growth. The A2BR-mediated signalling is a newly discovered pathway for exosome-induced angiogenesis, which results in reprogramming of ECs to an angiogenic phenotype by direct interaction, but also by reprogramming other cell types present in the TME, such as macrophages (Fig. 6). Blockade of A2BR may result in effective inhibition of exosome-driven angiogenesis and may further improve existing therapeutic modalities and long-term therapeutic results for patients with HNSCC or other types of cancer.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health grants HL109002, DK091190, HL069846, DK068575, and DK079307 to EKJ and the NIH grant R01-CA 1686288 to TLW. NL was supported by the Leopoldina Fellowships LPDS 2017-12 and LPDR 2019-02 from German National Academy of Sciences Leopoldina. JHA was supported by the Programa de Doutorado Sanduíche no Exterior (PDSE) 88881.188926/2018-01 from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Declaration of interest
No potential conflicts of interest were disclosed.
References
- 1.Whiteside TL (2016) Tumor-Derived Exosomes and Their Role in Cancer Progression. Adv Clin Chem 74:103–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skog J, Wurdinger T, Rijn S Van, et al. (2012) Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato S, Vasaikar S, Eskaros A, et al. (2019) EPHB2 carried on small extracellular vesicles induces tumor angiogenesis via activation of ephrin reverse signaling. JCI insight 4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludwig N, Whiteside TL (2018) Potential Roles of Tumor-derived Exosomes in Angiogenesis. Expert Opin Ther Targets 22:409–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kucharzewska P, Christianson HC, Welch JE, et al. (2013) Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci U S A 110:7312–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umezu T, Tadokoro H, Azuma K, et al. (2014) Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood 124:3748–3757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludwig N, Yemeni SS, Razzo BM, Whiteside TL (2018) Exosomes from HNSCC Promote Angiogenesis through Reprogramming of Endothelial Cells. Mol Cancer Res 16:1798–1808 [DOI] [PubMed] [Google Scholar]
- 8.Schuler PJ, Saze Z, Hong C-S, et al. (2014) Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin Exp Immunol 177:531–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clayton A, Al-Taei S, Webber J, et al. (2011) Cancer Exosomes Express CD39 and CD73, Which Suppress T Cells through Adenosine Production. J Immunol 187:676–683 [DOI] [PubMed] [Google Scholar]
- 10.Ethier MF, Chander V, Dobson JG Jr. (1993) Adenosine stimulates proliferation of human endothelial cells in culture. AmJ Physiol 265:H131–H138 [DOI] [PubMed] [Google Scholar]
- 11.Adair TH (2005) Growth regulation of the vascular system: an emerging role for adenosine. AJP Regul Integr Comp Physiol 289:R283–R296 [DOI] [PubMed] [Google Scholar]
- 12.Dubey RK, Gillespie DG, Jackson EK (2002) A(2B) Adenosine Receptors Stimulate Growth of Porcine and Rat Arterial Endothelial Cells. Hypertension 39:530–536 [DOI] [PubMed] [Google Scholar]
- 13.Auchampach J (2007) Adenosine Receptors and Angiogenesis. Circ Res 101:1057–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I (2003) Mast cell-mediated stimulation of angiogenesis: Cooperative interaction between A2B and A3 adenosine receptors. Circ Res 92:485–492 [DOI] [PubMed] [Google Scholar]
- 15.Emens I, Bousquenaud M, Lenoir B, et al. (2015) Adenosine stimulates angiogenesis by up-regulating production of thrombospondin-1 by macrophages. J Leukoc Biol 97:9–18 [DOI] [PubMed] [Google Scholar]
- 16.Ludwig N, Razzo BM, Yemeni SS, Whiteside TL (2019) Optimization of cell culture conditions for exosome isolation using mini-size exclusion chromatography (mini-SEC). Exp Cell Res 378:149–157 [DOI] [PubMed] [Google Scholar]
- 17.Ludwig N, Hong C-S, Ludwig S, et al. (2019) Isolation and Analysis of Tumor-Derived Exosomes. Curr Protoc Immunol 127:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma P, Ludwig S, Muller L, et al. (2018) Immunoaffinity-based isolation of melanoma cell-derived exosomes from plasma of patients with melanoma. J Extracell Vesicles 7:1435138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sung BH, Weaver AM (2017) Exosome secretion promotes chemotaxis of cancer cells. Cell Adhes Migr 11:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludwig S, Floros T, Theodoraki M-N, et al. (2017) Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin Cancer Res 23:4843–4854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yemeni SS, Whiteside TL, Weiss LE, Campbell PG (2019) Bioprinting exosome-like extracellular vesicle microenvironments. Bioprinting 13:1–12 [Google Scholar]
- 22.Jackson EK, Gillespie DG, Mi Z, Cheng D (2018) Adenosine receptors influence hypertension in dahl salt-sensitive rats: Dependence on receptor subtype, salt diet, and sex. Hypertension 72:511–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson NE, Discafani CM, Downs EC, et al. (1991) A Quantitative in Vivo Mouse Model Used to Assay Inhibitors of Tumor-induced Angiogenesis. Cancer Res 51:1339–1344 [PubMed] [Google Scholar]
- 24.Ekambaram P, Lee JL, Hubei NE, et al. (2018) The CARMA3-Bell0-MALT1 Signalosome Drives NF-κB Activation and Promotes Aggressiveness in Angiotensin II Receptor-positive Breast Cancer. Cancer Res 78:1225–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thery C, Witwer KW, Aikawa E, et al. (2019) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 8:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ekstrom EJ, Bergenfelz C, von Biilow V, et al. (2014) WNT5A induces release of exosomes containing pro-angiogenic and immunosuppressive factors from malignant melanoma cells. Mol Cancer 13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eimezu T, Ohyashiki K, Kuroda M, Ohyashiki JH (2013) Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene 32:2747–2755 [DOI] [PubMed] [Google Scholar]
- 28.Azambuja JH, Ludwig N, Braganhol E, Whiteside TL (2019) Inhibition of the adenosinergic pathway in cancer rejuvenates innate and adaptive immunity. Int J Mol Sci 20: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webber J, Steadman R, Mason MD, et al. (2010) Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res 70:9621–9630 [DOI] [PubMed] [Google Scholar]
- 30.Keklikoglou I, Cianciaruso C, Gii9 E, et al. (2019) Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat Cell Biol 21:190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du X, Ou X, Song T, et al. (2015) Adenosine A2B receptor stimulates angiogenesis by inducing VEGF and eNOS in human microvascular endothelial cells. Exp Biol Med 240:1472–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koszalka P, Golubska M, Urban A, et al. (2016) Specific activation of A3, A2a and Al adenosine receptors in CD73-knockout mice affects B16F10 melanoma growth, neovascularization, angiogenesis and macrophage infiltration. PLoS One 11:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma P, Diergaarde B, Ferrone S, et al. (2020) Melanoma cell-derived exosomes in plasma of melanoma patients suppress functions of immune effector cells. Sci Rep 10:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Theodoraki M, Matsumoto A, Beccard I, et al. (2020) CD44v3 protein-carrying tumor-derived exosomes in HNSCC patients’ plasma as potential noninvasive biomarkers of disease activity. Oncoimmunology 9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cekic C, Sag D, Li Y, et al. (2012) Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J Immunol 188: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


