Abstract
The Netrin-1/DCC guidance cue pathway plays a critical role in guiding growing axons towards the prefrontal cortex (PFC) during adolescence and in the maturational organization and adult plasticity of PFC connectivity. In this review we put forward the idea that alterations in PFC architecture and function, which are intrinsically linked to the development of major depressive disorder (MDD), originate in part from the dysregulation of the Netrin-1/DCC pathway by a mechanism that involves the microRNA, miR-218. We discuss evidence derived from mouse models of stress and human postmortem brain and genome-wide association studies (GWAS), indicating an association between the Netrin-1/DCC pathway and MDD. We propose a potential role of circulating miR-218 as a biomarker of stress vulnerability and MDD.
Keywords: Prefrontal cortex, microRNA, Neurodevelopment, Resilience, Adolescence, Biomarkers
I. INTRODUCTION
Major depressive disorder (MDD) is a debilitating and potentially fatal psychiatric condition affecting ~5% of the world population and contributing significantly to the global burden of disease and disability(1). The treatments for MDD remain inadequately effective for roughly half of patients due to our limited understanding of the molecular mechanisms underlying this disorder and the heterogeneity of its symptomalogy(2). which can be experienced as early as in periadolescence and remains present across the lifespan(3–7). Postmortem and neuroimaging studies have consistently demonstrated that increased vulnerability to depression is associated with altered organization of the prefrontal cortex (PFC) circuitry, including its reciprocal connections with the ventral tegmental area (VTA), nucleus accumbens (NAcc), hippocampus (HPC), and amygdala, among other regions (8–12). These structural alterations comprise impaired signaling of proteins that are essential for neuronal morphology and synaptic plasticity, resulting in cortical dysfunction (13,15,17).
The fine organization of neuronal circuits is achieved during embryonic and postnatal life through the action of guidance cues, families of proteins that direct growing neurites (e.g., axons or dendrites) toward their intended targets(16,18–20). Guidance cues, including the netrin, ephrin, slit, and semaphorin families, bind to specific receptors located at the tip of the growth cone (the enlarged protrusion of the growing neurite) to promote neurite extension (attraction) or collapse (repulsion) by a mechanism involving dynamic remodeling of the actin cytoskeleton(21).
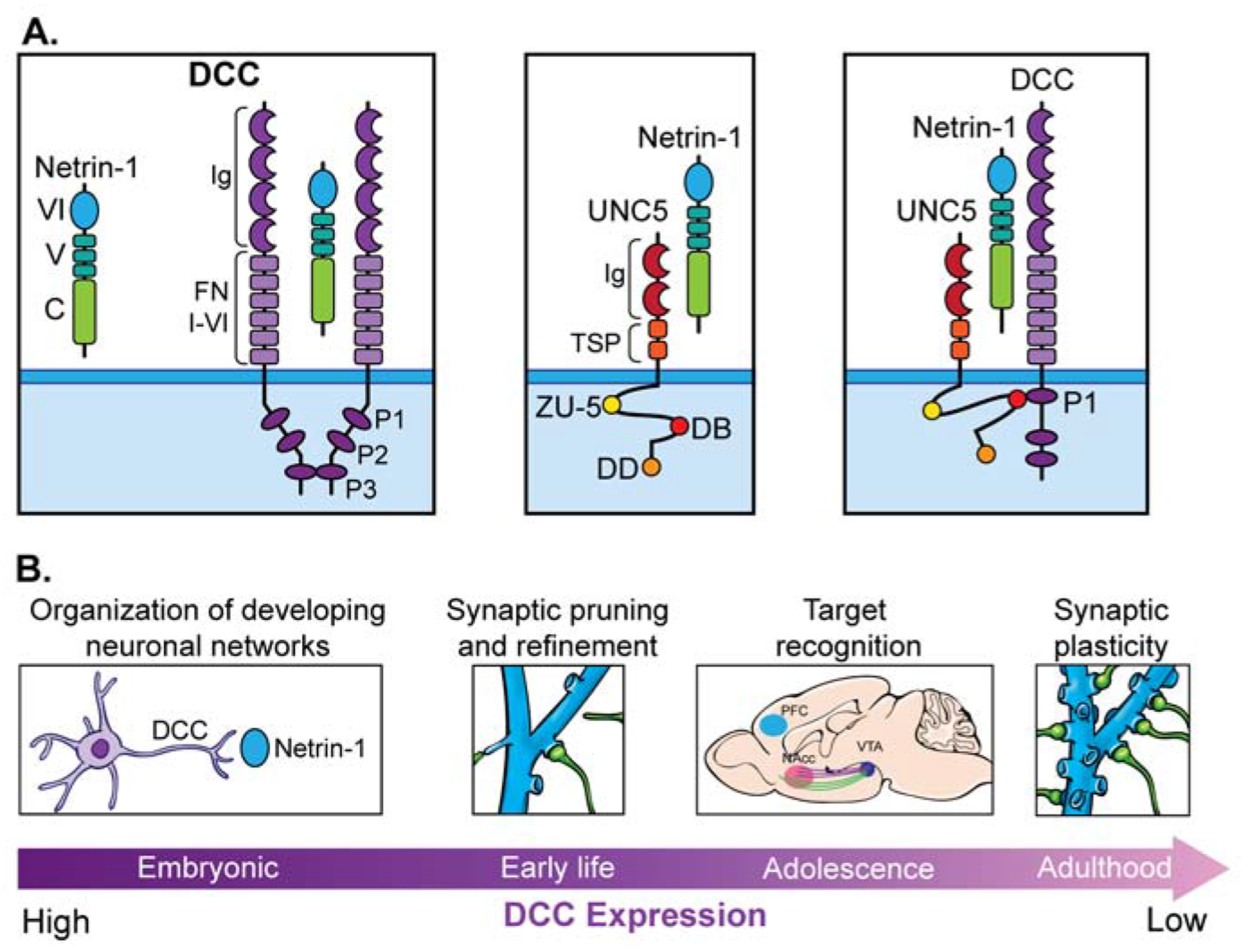
Netrin-1, the most characterized member of the netrin family and a member of the laminin superfamily, organizes neural network connectivity bifunctionally—promoting attraction or repulsion, depending on the activation of different receptors or receptor complexes(19,22,23). There are two main families of Netrin-1 receptors, DCC (deleted in colorectal cancer) and UNC5 (uncoordinated-5) (Figure 1A). DCC receptors account for attraction, whereas UNC5 receptors alone or as DCC/UNC5 complexes mediate repulsion (Figure 1A)(24–26). Netrin-1 influences axonal navigation by signaling whether, when, and where to grow (27–29) and also plays a critical role in post-axonal pathfinding wiring events, including axon arborization, dendritic growth, and synapse formation(30–36). These functions appear to be specific to particular developmental periods and maturational states (Figure 1B) (37,38) and maybe be sensitive to positive and negative environmental events(38–43).
Figure 1. The structure and function of Netrin-1 and its receptor DCC.
(A) Netrin-1 is a member of the laminin superfamily and is a secreted protein associated with the extracellular matrix. Its terminal laminin VI domain is globular and is followed by domain V, which is composed of three epidermal growth factor (EGF) repeats. The carboxy-terminal, C-domain (called NTR), is enriched in basic amino acids and binds heparin in the extracellular matrix through interactions with heparin sulfate proteoglycans. The VI and V domains are responsible for the binding of receptors. The DCC receptor is a single pass transmembrane protein, with an extracellular fragment composed of four immunoglobulin-like (Ig-like) domains folding into a horseshoe conformation, and six fibronectin type III (FN) domains where Netrin-1 binds to FN4 and FN5 domains. The cytosolic portion of DCC contains three domains, P1, P2 and P3, responsible for signaling. Netrin-1 has 3 binding sites for DCC, two of these sites can be replaced by other Netrin-1 receptors, including UNC5. Upon Netrin-1 binding, DCC receptor molecules form a signaling complex by interacting through their cytosolic P3 domains. This initiates the recruitment of intracellular components, including members of the NCK family of adaptor proteins and focal adhesion kinases, which in turn activate Src family kinases, Rho GTPases, the release of Ca2+, protein translation and reorganization of the actin cytoskeleton. The UNC5 receptor is a transmembrane protein with two Ig-like domains (most likely responsible for Netrin-1 binding), two thrombospondin domains (Tsp), and a large cytoplasmic tail composed of three domains, a ZO-1/Unc5 (ZU-5) domain, a DCC binding motif (DB), and a death domain (DD). When binding to Netrin-1, UNC5 and DCC proteins form receptor complexes by interacting through DCC’s cytosolic P1 domain and UNC5’s ZU5 and DB domains. (B) DCC-mediated Netrin-1 signaling influences the growth, targeting, and arborization of axons, dendritic growth, and synapse formation. These functions are restricted or predominant to specific periods of development and neuronal circuits. Netrin-1 and DCC continue to be expressed in the adult brain and are involved in refining neuronal structural organization and synaptic plasticity. Netrin-1 and DCC expression decrease from early life to adulthood. This switch in expression levels presumably coincides with the transition of their role as organizers of the connectivity of large neuronal networks to the refinement of established local circuits. DCC-mediated Netrin-1 signaling is thought to mediate predominantly neuron-neuron connectivity, although these proteins are expressed in other cell types, including glia, depending on the CNS region.
Variations in Netrin-1 or DCC/UNC5 receptors during critical periods for the establishment of motivation- and reward-relevant circuitries are likely to contribute to individual differences in susceptibility to develop psychiatric disorders(44). In this review, we highlight the role of the Netrin-1/DCC pathway in the development and adult plasticity of the PFC as a key mediator of depression in humans and stress vulnerability in mice. We hypothesize that alterations in the Netrin-1/DCC pathway prime the central nervous system to disruption by environmental factors such as stress. This renders individuals more susceptible to develop behavioral abnormalities.
II. THE NETRIN-1/DCC SIGNALING PATHWAY
Netrin-1 is a secreted protein that binds to cell surfaces and to the extracellular matrix to direct growth of DCC-expressing axons along an adhesive surface (haptotaxis)(27,28,45). It was described as a guidance cue attracting growing axons towards intended targets, where they can establish synaptic contacts(46), but subsequently was also identified as a repellent cue(47,48). The DCC:UNC5 ratio at the cell surface determines Netrin-1 signaling, leading to the recruitment of downstream proteins that induce cytoskeletal reorganization(49–51). Netrin-1, DCC, and UNC5 continue to be expressed in the adult brain and are involved in the reorganization of neuronal structure and synaptic plasticity within local circuits (Figure 1B)(32,34,52) In this review we focus on the Netrin-1/DCC pathway.
A. DCC receptors
The DCC gene is on chromosome 18q and encodes a ~185 kDa protein (Figure 1A)(46). DCC receptors are expressed by selective neuronal populations, including cingulate 1, prelimbic and infralimbic sub-regions of the PFC and dopamine (DA) neurons in the VTA and substantia nigra pars compacta, across the lifespan and across species, including humans(53–56). Their levels decrease drastically from embryonic life and early adolescence to adulthood (Figure 1B)(32,34,52) and are regulated epigenetically via DNA methylation(57,58) and microRNAs (miRNAs)(42,59–62). For example, hypermethylation at the promoter site of the DCC gene leads to reduced DCC protein expression(57,58), whereas hypomethylation results in increased protein(64). In addition, two miRNAs, miR-218 and miR-9, have been shown to bind directly to the three prime untranslated region (3’UTR) of the DCC mRNA and decrease its expression in vitro and in vivo(42,59,61). DCC protein and miR-218 selectively colocalize in pyramidal neurons of human and mouse PFC and in VTA DA neurons, with their expression levels correlating negatively across postnatal development(42,59).
B. DCC signaling and maturation of the PFC
In humans, magnetic resonance studies have found that gray matter thickness in the PFC decreases across adolescence before stabilizing in adulthood, while white matter volume increases(14,63). This results from modifications occurring at the cellular level (e.g., neuronal architecture, synapse density, and neurotransmitter concentration)(65,66), and coincides with the acquisition of complex behavioral and cognitive abilities(67–69). In rodents, PFC maturation involves synaptic pruning, structural modifications of pyramidal neuron structure, DA axon ingrowth, and changes in expression/sensitivity of DA receptors(41,67,69–73) DCC receptors are highly expressed in cell bodies and dendrites of PFC pyramidal neurons and, to a lesser extent, GABAergic interneurons(56). Netrin-1 and DCC receptors facilitate cortical synaptogenesis by increasing the structural complexity of pyramidal neurons and by promoting axon–dendritic adhesive contacts(33). Both proteins are enriched at synapses where they regulate synaptic plasticity and trigger the potentiation of excitatory synaptic transmission via the insertion of GluA1 AMPARs into the postsynaptic membrane of pyramidal neurons in the adult forebrain(32,34). Whether and how altered levels of Netrin-1/DCC signaling in PFC pyramidal neurons within the fully differentiated adult brain lead to changes in their structure and functioning remains to be determined. Initial evidence suggests that such alterations might induce changes in dendritic spine morphology (74).
DCC receptors are also expressed by VTA DA neurons that innervate non-cortical targets, including the NAcc. Our work in mice shows that, in adolescence, the interaction between DCC receptors in mesolimbic DA axons, and Netrin-1 expressed by dendrites of NAcc neurons and located in the surrounding extracellular matrix, induces DA axons to recognize this region as their final target. Mesocortical DA axons lack or have scant levels of DCC receptors, and instead of recognizing the NAcc as their final target, they continue to grow towards the PFC, including its orbital portion, across adolescence(41,71). By preventing mesolimbic DA axons from continuing to grow ectopically to the PFC, DCC receptors in DA neurons determine the structural and functional maturation of adult PFC pyramidal neurons(41,71). Amphetamine exposure during adolescence dysregulates DCC receptor expression in mesolimbic DA axons as well as Netrin-1 in the NAcc, profoundly disrupting PFC development and cognitive processing in rodents(40,43,75,76). These effects are restricted to recreational, but not therapeutic-like, doses of amphetamine, and are initiated by dysregulation of the Dcc repressor, miR-218(42,43). Whether stress in adolescence induces enduring alterations in PFC structure and function by targeting DCC receptors in DA neurons remains to be established.
III. RODENT MODELS OF STRESS SUSCEPTIBILITY
Depression is a heterogeneous disorder accompanied by a variety of symptoms, including recurrent episodes of sadness, hopelessness, lack of interest or pleasure (anhedonia), social avoidance, and suicidal ideation, among many others(2). Risk for MDD is mediated by the complex interaction between genetic and environmental factors, with the subjective nature of the depressive symptomatology representing a significant challenge for the understanding of its etiology, diagnosis, and treatment(77,78). Stressful life events, such as physical abuse, personal loss, or financial problems, are the main environmental risk factor for MDD in vulnerable individuals(79,80). This epidemiological observation has fostered the development of rodent stress models to induce behavioral alterations that resemble some of the core symptoms of human depression, including learned helplessness, anhedonia, reduced grooming, and changes in appetite, sleep, circadian rhythms, and social behavior(81–83). These models also recapitulate the molecular, cellular, and circuit signatures observed in MDD(2,83,84).
Rodent models of chronic stress involve the sustained or repeated activation of stress systems, including the hypothalamic-pituitary-adrenal (HPA) axis and many others, in which animals are subjected to a specific paradigm, including chronic administration of pharmacological activators of the stress response (e.g., corticosterone), chronic physical restraint, or chronic exposure to a range of physical stressors (e.g., footshock), over a period that ranges from 1 to 12 weeks. Chronic variable stress entails the application of several of these stressors to avoid the animals’ habituation to a single type of stress. At the completion of the paradigms, animals are assessed in a battery of behavioral tests that measure phenotypic traits such as reward sensitivity, social interaction, sucrose preference, and time spent grooming (for an extensive review, see(81,82,85)). Stress-induced alterations in many of these behaviors can be reversed by chronic treatment with monoamine-based antidepressants which are typically used to treat depression in humans(81).
The chronic social defeat stress (CSDS) paradigm has more recently emerged as a validated mouse model that provides the advantage of segregating socially-defeated mice into susceptible and resilient populations based on a social interaction test (SIT) (86–88). The CSDS protocol involves an experimental mouse, usually from the C57BL/6J strain, being exposed to repeated bouts of social subordination (defeat) by an aggressive CD-1 mouse during several consecutive days(87). At the completion of the CSDS procedure, mice are evaluated in the SIT to assess CSDS-induced social avoidance towards a social target (e.g., a novel CD-1 mouse)(87). Mice showing high social avoidance are classified as susceptible and show a range of other behavioral deficits, including reduced sucrose preference. Resilient mice do not exhibit most of these impairments, but instead display a series of behavioral and molecular adaptations that protect them from developing stress susceptibility. Although the CSDS protocol was originally standardized for male mice, novel protocols have been adapted for use in female mice, allowing the use of CSDS for studies on sex differences and vulnerability to stress(89–93).
IV. DCC RECEPTOR EXPRESSION IN THE PFC IS DYSREGULATED IN MDD AND DETERMINES SUSCEPTIBILITY TO CSDS
To understand the role of Netrin-1/DCC signaling pathway in depression, we have taken a translational approach using postmortem human brain tissue of adult subjects who were previously diagnosed with MDD and the CSDS mouse model. We measured DCC mRNA expression in the PFC of two separate and independent cohorts of depressed adult subjects who died by suicide and were antidepressant-free for at least three months before death. Depressed subjects exhibit significantly higher DCC mRNA levels (~50%) in comparison to psychiatrically healthy controls(39,59), which significantly and negatively correlated with a reduction in miR-218 expression (~50%)(59), suggesting miRNA mechanisms linking alterations in the Netrin-1/DCC pathway and MDD. Using the CSDS model, we found that adult mice that exhibit susceptibility to CSDS also display upregulation of Dcc mRNA and DCC protein in the PFC in comparison to control and resilient mice(59). Furthermore, susceptibility to CSDS is prevented by viral-mediated downregulation of Dcc in PFC pyramidal neurons, a manipulation that does not alter anxiety-like behaviors, learning, or locomotor activity(59). Adult exposure to CSDS does not lead to changes in Dcc mRNA levels in the VTA(59), a brain region highly associated with vulnerability to stress (86,88,94,96).
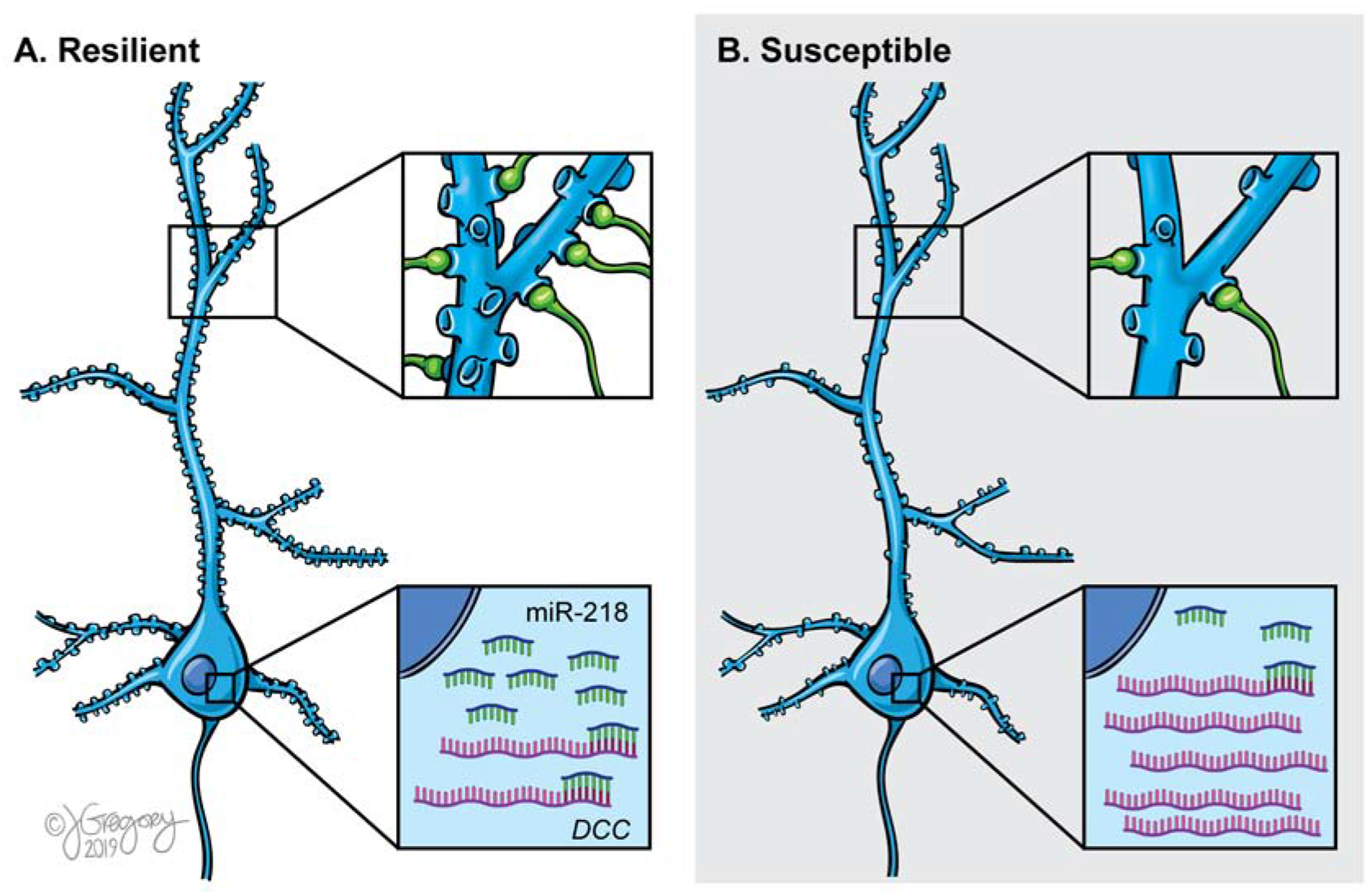
Our rodent studies further suggest that miR-218 in the PFC may act as a molecular switch of stress vulnerability because bidirectionally manipulating its expression leads to opposite CSDS-induced behavioral outcomes. Dampening miR-218 expression in the adult PFC using an antagomiR (antimiR-218) promotes susceptibility to a single exposure to social defeat(75). Furthermore, intranasal infusion of antimiR-218 turns resilient mice into susceptible, when assessed in a second SIT(75). In contrast, overexpression of miR-218 selectively in PFC pyramidal neurons protects against CSDS-induced social avoidance(75). These effects seem to involve DCC-dependent remodeling of dendritic structure because miR-218 overexpression in PFC pyramidal neurons of socially-defeated mice increases their dendritic spine density and prevents stress-induced reductions in thin spine density, most likely by promoting the formation of immature or nascent spines (Figure 2)(75). This finding is consistent with the role of DCC receptors in experience-dependent reorganization of synaptic connectivity in the adult brain(32,34,52).
Figure 2. miR-218 levels in the PFC determine susceptibility versus resilience to chronic stress in adulthood.

(A) Experimentally-induced increased miR-218 expression in adult PFC pyramidal neurons prevents stress-induced DCC upregulation, maintaining dendritic structure and optimal synaptic connectivity. (B) Stress-induced miR-218 downregulation in adult PFC pyramidal neurons results in DCC upregulation, causing dendritic structure modifications and aberrant synaptic connectivity.
Exposure to CSDS in adulthood may alter DCC expression in the forebrain regions. For example, a wholegenome bisulfite sequencing study of DNA methylation in the NAcc revealed greater hypomethylated sites in non-CpG sequences of the Dcc gene body of susceptible mice, suggesting enhanced expression(95). An analysis of the two paralogs of miR-218(97), miR-218–1 and miR-218–2, showed no differential methylation within 20kb of miR-218–1, but identified two hypermethylated CpGs sites approximately 2kb downstream of miR-218–2, suggesting methylation mechanisms controlling both miR-218–2 and Dcc expression in this region.
V. GENETIC VARIANTS IN NETRIN-1 AND DCC ARE ASSOCIATED WITH MDD
Twin studies and genome-wide association studies (GWAS) have estimated the heritability of MDD to range between ~30 to 40%(77,78). This heritability is thought to be highly complex with potentially many hundreds of genetic variations being involved, each having miniscule effects. Only a few genetic variants have been identified and consistently replicated in large human cohorts(78). Despite the relatively low heritability of MDD compared to other psychiatric disorders(78,98), increasing numbers of GWAS find that specific single-nucleotide polymorphisms (SNPs) within the DCC gene are linked to MDD or to traits associated with depression (Table 1).
Table 1.
Summary of published GWAS linking single nucleotide polymorphisms within the NETRIN1/DCC signaling pathway with MDD, Depressive Symptoms or Depression-associated traits. Human genes are designated in italicized uppercase letters.
| Author | Year | Total N | Cases | Controls | Replication Sample Cases | Replication Sample Control | Meta-analysis sample | Ancestry | MDD DEFINITION | GWAS hits | Putative Genes DCC or Netrin | SNP - location | Functional consequence | Notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Okbay et al.(105) | 2016 | 75306 | 16471 | 58835 | 105739 | E | Frequency, in the past two-weeks, in which the respondent experienced feelings of unenthusiasm or disinterest and feelings of depression or hopelessness. | 2 | DCC | rs62100776 | intron variant | |||
| Smith et al.(112) | 2016 | 91370 (47196 ♀ /44174 ♂) | 15346 | E | Eysenck Personality Questionnaire-Revised (EPQ R-S) Short Form’s Neuroticism scale | 9 | Netrin-1 SNP, rs8081460, was associated with neuroticism in the UK Biobank sample. This SNP effect did not replicate in independent samples | |||||||
| Zeng et al.(102) | 2017 | 6455 | 1123 | 5332 | 9240 | 9519 | E | Structured Clinical Interview | 1 | Netrin 1 | Polygenic risk scores (PRSs) calculated for netrin-1 pathway more accurately predicted MDD in one of the cohorts compared with PRS calculated for the whole genome | |||
| Dunn et al.(99) | 2016 | 0 | 7179 AA, 3138 Hs | AA, Hs | Center for Epidemiological Studies of Depression Scale (CES-D) | 0 | The top signals in AA were rs73531535 (located 20kb from GPR139, p=5.75×10–8) and rs75407252 (intronic to CACNA2D3, p=6.99×10–7). In Hs, the top signals were rs2532087 (located 27kb from CD38, p=2.44×10–7) and rs4542757 (intronic to DCC, p=7.31×10–7). | |||||||
| Ward et al.(108) | 2017 | 113968 | 53525 | 60443 | Mood instability measured by a single question: ‘Does your mood often goes up and down? | 4 | DCC | rs8084280 | intron variant | |||||
| Wray et al.(107) | 2018 | 480359 | 135458 | 344901 | E | Diagnostic Interview. Cases were required to meet international consensus criteria (DSM-IV, ICD-9, or ICD-10 | 44 | DCC | rs11663393 | intron variant | ||||
| Amare et al. (140) | 2019 | 336753 | 90150 | 246603 | NR | NR | E Ch ♀ | Self-reported MDD (srMDD), recurrent MDD(rMDD), | 1 | rMDD significant SNP was not replicated in the replication sample | ||||
| Howard et al. (141) | 2019 | 807553 | 246363 | 561190 | 414055 | E | Clinical interview and broader criteria | 102 | DCC | rs7227069 | intron variant | |||
| Arnau-Soler et al.(109) | 2019 | 4919 (2990 ♀ /1929 ♂) | 99057 | E/Sc | Questionnaire and psychological assessment | 0 | Post-GWAS gene-based test identified six genes assocaited with the Patient Health Questionnaire validated to screen mental illness: DCC, ACSS3, DRD2, STAG1, FOXP2 and KYNU | |||||||
| Barbu et al.(103) | 2019 | 6420 | E | 0 | PRS from the Netrin-1 signaling pathway is significantly and specifically associated with white matter integrity in thalamic radiations, namely lower fractional anisotropy, and higher mean diffusivity | |||||||||
| Roberson-Nay et al.(114) | 2018 | 150 pairs of MZ twins | E | DSM-V criteria for MD | 0 | 0 | 0 | Identified altered methylation in Netrin-1. Gene enrichment analyses implicated genes related to neuron structures and neurodevelopmental processes including cell-cell adhesion genes (e.g., CDHs, PCDHAs, PCDHA1C/2C). Genes previously implicated in mood and psychiatric disorders as well as chronic stress (e.g., HDAC4, NRG1) | ||||||
| Strawbridge et al.(110) | 2019 | 122822 | 39265 | 83557 | 15735 & 23923 | 84499 & 84167 | E | Questionnaire to assess suicidality phenotype | 3 | The gene-based analysis was used to identify genes containing potential composite association signals that were not identified by the individual SNP analysis, but which might nevertheless contribute to biological mechanisms underlying suicidality. The gene-based analysis highlighted CNTN5, ADCK3/COQ8A, CEP57, and FAM76B and DCC for suicidality. EIF4A1 and SENP3 and DCC for deliberate self-harm | ||||
| Ward et al.(106) | 2019 | 375275 | Anhedonia | 11 | DCC | rs72923287 | intron variant | PRS for anhedonia was associated with poorer brain white matter integrity, smaller total grey matter volume, and smaller volumes of brain regions linked to reward and pleasure processing, including nucleus accumbens, caudate, and medial frontal cortex. A locus in the DCC gene was the most significant hit associated with anhedonia | ||||||
| Lee et al.(115) | 2019 | 727126 | 232964 | 494162 | E | Cross psychiatric disorders | 23 associated with at least four of the disorders | DCC | rs8084351 | intron variant | The region surrounding SNP rs8084351 at the gene DCC featured the most pleiotropic association. This region showed association with all eight psychiatric dissordes studied (MD, SCZ, BIP, ADHD, ASD, TS, ANO, OCD) |
E=european
Sc=Scottish
rMDD=recurrent major depression
ASD=Autism spectrum disorder
AA= African American
BIP=Bipolar Disorder
MD= Major Depression
TS=tourette syndrome
Hs: Hispanic
TS= two samples
SCZ= Schizophrenia
ANO=anorexia nervosa
Ch= Chinese
PRS=Poygenetic Risk Score
ADHD= Attention deficit hyperactivity disorder
OCD=Obsessive–compulsive disorder
One pioneer GWAS examined the interaction between genetic variants and environmental factors, such as social support and stressful life events, in a small sample of African-American and Hispanic/Latino women. There were no SNPs that achieved genome-wide significance, but an intron variant within the DCC gene, rs4542757, had the strongest association signal with MDD(99). Subsequently, a GWAS comprising non–hypothesis-driven pathway analysis and regional heritability analyses was performed to identify gene networks that were previously linked to MDD in two independent and large human cohorts(100–102) The polygenic risk score (PRS) found the Netrin-1 signaling pathway as the pathway most consistently associated with MDD(102). Closer analysis revealed that variants within the DCC gene region encoding Netrin-1 binding sites had the greatest heritability (Figure 1A)(102).
A follow-up study(103) assessed the white matter integrity of individuals with high PRS for MDD derived from the Netrin-1 signaling pathway identified previously(102). PRSs for the Netrin-1 pathway were associated with alterations in the superior longitudinal fasciculus, a fiber tract that connects the frontal, temporal, and parietal lobes(103). Another study that combined genetic analysis and functional brain imaging reported a total of 11 novel loci highly correlated with anhedonia and MDD, and, to a lesser degree, with schizophrenia and bipolar disorder. The most significant hit was at the DCC locus on chromosome 18. Anhedonia was associated with smaller total grey matter volume, and reduced white matter integrity in the NAcc, caudate, and PFC(106). These GWAS are consistent with our findings showing that humans who develop with DCC haploinsufficiency exhibit decreased striatal volume and decreased mesocorticolimbic anatomical connectivity(104).
Polymorphisms within the DCC and NETRIN-1 genes have also been linked to psychological traits related to MDD, including neuroticism, subjective well-being, or anhedonia (Table 1). For example, the intronic variants of the DCC gene, rs62100776(105) and rs11663393(107) are associated with depressive symptoms, in large GWAS meta-analyses, whereas the DCC SNPs, rs8084280 and rs62099230, localized within the region encoding the receptor binding sites, are linked to mood instability and suicidality(108,110). In addition, the NETRIN-1 SNP, rs8081460, is associated with neuroticism; however, this SNP effect has not been replicated in two independent smaller samples(112). Finally, a study conducting gene-based tests identified DCC among six genes associated with depressive symptoms in UK Biobank participants(109).
Consistent with our postmortem results, a blood microarray analysis of two independent case-control studies identified overexpression of the DCC mRNA, among 165 differentially expressed genes in MDD(111) and a methylome-wide CpG-SNP analysis found reduced methylation within the DCC gene in blood samples of MDD patients(113). Altered methylation of the Netrin-1 gene has also been associated with depression in a study with monozygotic twins with and without a lifetime history of early-onset depression(114), suggesting that variations in the methylation levels of these genes may add to what can be explained by the sequence variation.
The convergence between human postmortem studies, rodent studies, and GWAS suggests that the relevance of the Netrin1/DCC pathway in the etiology of MDD results from its developmental role in the spatiotemporal organization of circuitries involved in cognition and emotion. Perhaps it is not surprising that the largest genome-wide meta-analysis of psychiatric disorders conducted to date, with more than 725,000 cases-controls, across eight disorders, found the intronic DCC SNP, rs8084351, to have the most significant and pleiotropic effect(115). Overall, the identified SNPs are present within regions important for the function of the DCC protein or within intronic regions, likely leading to altered DCC gene transcription, possibilities that now warrant direct investigation. The link between alterations in this pathway and MDD may occur in the absence of stress but may be exacerbated by adverse experiences, including those occurring in adulthood, as our studies in rodents indicate(59,75). DCC protein expression decreases from early life to adulthood(33,42,54); stress-induced alterations in the Netrin1/DCC pathway seem to have different outcomes depending on the developmental stage when this is experienced (Figure 2 and Figure 3).
Figure 3. Stress in adolescence may alter ongoing PFC development by disrupting DCC expression in dopamine axons.
(A) Sagittal representation of the adult mesocorticolimbic system where DA axons expressing DCC receptors (in purple) innervate the NAcc, whereas DA axons that lack DCC receptors (in green) innervate the PFC. (B) Stress in adolescence may alter the topographic organization of mesolimbic and mesocortical DA axons in adulthood. For example, stress may reduce DCC expression in DA axons that normally recognize the NAcc as their final target, inducing their ectopic growth to the PFC.
VI. BIOMARKERS OF VULNERABILITY TO MDD: POTENTIAL ROLE OF MIR-218
Biomarkers can provide information about traits that could predict the onset and course of a disease, confirm the clinical status of an individual, and be used to track the effectiveness of a treatment regimen. Biomarkers range from the blood pressure to complex biochemical analysis of blood and other peripheral tissues(116,117). Genetic, behavioral, and neuroimaging indices have also been introduced as potential biomarkers of neuropsychiatric disorders(118–120), but to date there is no bona fide biomarker for any psychiatric syndrome that is used clinically to help guide diagnosis, treatment, or prognosis.
The use of miRNAs as non-invasive biomarkers of depression and potential mediators of pharmacological and behavioral interventions is gaining interest(121,123). miRNAs can be accessed via bodily fluids, are highly stable due to their biological packaging, and may represent a signature of alterations occurring in the brain(123,125). Interestingly, given the diverse etiology of MDD, microarrays or small RNA sequencing studies have identified a few miRNAs as potential biomarkers of MDD, including miR-1202, miR-135, miR-146a/b-5, miR-425–3p, miR-24–3p, miR-941, and miR-589 (for reviews see: (117,121,123), which have also been found to be altered in postmortem brain tissue and plasma or blood-derived cells of MDD subjects(122,124,126–129)). These correlational studies, however, do not provide direct evidence that brain miRNA alterations can be detected in blood and that miRNAs are functionally implicated in MDD vulnerability.
To begin filling this gap, we showed that circulating miR-218 levels may serve as a biomarker of stress vulnerability through a mechanism that involves the PFC. Adult susceptible mice to CSDS exhibit reduced blood levels of miR-218, and these levels correlate with stress-induced social avoidance(75). These findings are consistent with a recent small RNA sequencing study reporting miR-218 as one of the miRNAs differentially downregulated in blood obtained from MDD patients(130) and with our report that miR-218 is decreased in the PFC of MDD individuals and of CSDS-induced susceptible mice(75). We further showed that experimentally-induced miR-218 downregulation in the PFC associates with a parallel reduction in circulating levels of miR-218 in mice. Conversely, viral-mediated upregulation of miR-218 selectively in PFC pyramidal neurons causes increased miR-218 levels in blood(75).
Circulating miRNAs exist in high-density lipoproteins and exosomes that prevent them from degradation before they reach target cells(131,132). Recent evidence demonstrates that motor neurons can release miR-218 to the extracellular space to be taken up by astrocytes(133). Our findings(75) raise the possibility that PFC pyramidal neurons may release exosomes containing miR-218, reaching the peripheral circulation. It is necessary to determine whether viral-mediated manipulations of miR-218 in brain regions other than the PFC, including the HPC, would also result in measurable changes of this miRNA in blood. Future experiments are required to definitively establish this novel mechanism as well as the potential of miR-218, and presumably other miRNAs, to serve as validated biomarkers for aspects of MDD.
VII. CONCLUSIONS AND FUTURE DIRECTIONS
A clear understanding of the pathophysiology of MDD becomes urgently necessary for developing novel preventive strategies and generating evidence-based and rapid-acting therapeutic interventions. This review creates a translational bridge between human and rodent research that points towards Netrin-1 and its DCC receptor as important contributors to the pathophysiology of MDD and opens a new venue for future studies. From the preclinical side, we report studies showing that alterations in DCC protein expression induced by adult stress confer vulnerability to depression-like behaviors. From the human side, we highlight evidence from GWAS showing that DCC polymorphisms not only are associated with depressive symptoms but also with alterations in brain circuitry. We propose a role for miR-218, readily measured in blood, as a potential biomarker of stress vulnerability. The consistency between studies is striking, considering the heterogeneous methodologies and samples in humans and mice.
Adolescent and adult women are twice as likely as men to develop MDD and other stress-related disorders. They begin to manifest depressive symptoms early in adolescence and continue to be at heightened risk throughout life. Yet, the great majority of preclinical studies have been conducted primarily on males, neglecting important aspects of sex differences, such as sex hormones, chromosomal differences, behavioral coping strategies, and stress reactivity. There is a need to study the role of Dcc and miR-218 in female mice exposed to chronic stress during adolescence and adulthood following protocols that are now widely validated. GWAS should also examine the specific interaction between DCC polymorphisms and sex in large and independent cohorts of men and women suffering from MDD(99).
Biomarkers can be observed at baseline conditions to predict the outcome of an illness or response to a treatment (predictors), or can change over the course of an illness or as the result of a specific treatment (mediators)(134). Our results show that mice that become susceptible to CSDS display low levels of circulating miR-218(59). Whether circulating miR-218 can predict stress vulnerability or the outcome of antidepressant treatment remains to be answered. Our preliminary data suggest that circulating miR-218 during adolescence predicts susceptibility to adult exposure to CSDS (135). miR-218 levels in blood exhibit a very interesting developmental pattern that mirrors PFC miR-218 expression, and our data demonstrate that primary changes in miR-218 expression in PFC neurons per se can cause parallel changes in circulating levels of this miRNA(75). Future studies will provide an understanding of the potential role of circulating miR-218 as a developmental predictor and mediator of stress vulnerability. Targeting miR-218 or directly the Netrin-1/DCC pathway in early life (e.g., during adolescence) may be a promising venue for the development of prevention and intervention strategies.
Perinatal stress, including prenatal and early stress, increases the risk for depression and other stress-related disorders later in life by two- to four-fold(136). This effect may be mediated by changes in the Netrin/DCC signaling pathway. For example, Dcc and Unc5c mRNA were among 916 transcripts differentially expressed in the HPC of adult rats exposed to prenatal stress(137). We propose that stress across the lifespan dysregulate the Netrin-1 system in selective PFC-limbic circuits, via epigenetic mechanisms. In this context, the alterations in DCC and miR218 expression observed in the adult MDD postmortem brain studies may already be present earlier in development and/or result from early life adversity. Longitudinal studies collecting peripheral tissue samples may be able to address this important issue(111).
Our work on the adolescent development of the mesocorticolimbic system shows that DCC signaling within DA neurons of the VTA determines the extent of their innervation to the PFC and the organization and function of the PFC local circuits in adulthood(71). This effect is mediated by miR-218 regulation of Dcc in the VTA(42) and can be modified by exposure to drugs of abuse during adolescence(40,71,76,138,139). Whether chronic stress in adolescence regulates DCC receptor and miR-218 expression in the VTA, disrupting the development of the DA innervation to the PFC, and whether this effect is sex-specific, needs to be determined. We hypothesize that stress in adolescence alters DCC expression in mesolimbic DA axons, triggering their mistargeting in the NAcc and their ectopic growth to the PFC, remodeling adult PFC synaptic circuitry (Figure 3).
In conclusion, the work summarized here demonstrates the importance of the Netrin-1/DCC signaling pathway in controlling susceptibility to chronic stress and the development of MDD, with converging evidence obtained from humans and mouse models. The work underscores the importance of unbiased approaches with both species in identifying novel pathogenic mechanisms of MDD.
ACKNOWLEDGMENTS AND DISCLOSURES
C.F. is supported by the National Institute on Drug Abuse (R01DA037911), the Canadian Institute for Health Research (MOP-74709; MOP-119543), the Natural Science and Engineering Research Council of Canada (2982226). E.J.N. is supported by the National Institute of Mental Health (P50MH096890; R01MH051399) and the Hope for Depression Research Foundation (HDRF). C.F. is a research scholar of the Fonds de Recherche du Québec - Santé. A.T.B. received the Integrated Program in Neuroscience fellowship. We thank Jill Gregory for assistance with figure preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
VIII. References
- 1.Malhi GS, Mann JJ (2018): Depression. Lancet (London, England) 392: 2299–2312. [DOI] [PubMed] [Google Scholar]
- 2.Akil H, Gordon J, Hen R, Javitch J, Mayberg H, McEwen B, et al. (2018): Treatment resistant depression: A multi-scale, systems biology approach. Neurosci Biobehav Rev 84: 272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62: 593–602. [DOI] [PubMed] [Google Scholar]
- 4.Rice F, Riglin L, Thapar AK, Heron J, Anney R, O’Donovan MC, Thapar A (2019): Characterizing Developmental Trajectories and the Role of Neuropsychiatric Genetic Risk Variants in Early-Onset Depression. JAMA psychiatry 76: 306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paus T, Keshavan M, Giedd JN (2008): Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9: 947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustün TB (2007): Age of onset of mental disorders: a review of recent literature. Curr Opin Psychiatry 20: 359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee FS, Heimer H, Giedd JN, Lein ES, Šestan N, Weinberger DR, Casey BJ (2014): Mental health. Adolescent mental health--opportunity and obligation. Science 346: 547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tottenham N, Galván A (2016): Stress and the adolescent brain: Amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci Biobehav Rev 70: 217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, Cui R (2017): The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast 2017: 6871089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Zhao Y, Chen Z, Long J, Dai J, Huang X, et al. (2019): Meta-analysis of cortical thickness abnormalities in medication-free patients with major depressive disorder. Neuropsychopharmacology. 10.1038/s41386-019-0563-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD (2002): Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biol Psychiatry 51: 342–344. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS, Morrison JH (2013): The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 79: 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duman RS, Aghajanian GK, Sanacora G, Krystal JH (2016): Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat Med 22: 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A 101: 8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, et al. (2012): Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18: 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stoeckli ET (2018): Understanding axon guidance: are we nearly there yet? Development 145 10.1242/dev.151415 [DOI] [PubMed] [Google Scholar]
- 17.Duman RS, Aghajanian GK (2012): Synaptic dysfunction in depression: Potential therapeutic targets. Science (80-) 338: 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beamish IV, Hinck L, Kennedy TE (2018): Making Connections: Guidance Cues and Receptors at Nonneural Cell-Cell Junctions. Cold Spring Harb Perspect Biol 10 10.1101/cshperspect.a029165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanoue V, Cooper HM (2019): Branching mechanisms shaping dendrite architecture. Dev Biol 451: 16–24. [DOI] [PubMed] [Google Scholar]
- 20.Van Battum EY, Brignani S, Pasterkamp RJ (2015): Axon guidance proteins in neurological disorders. Lancet Neurol 14: 532–46. [DOI] [PubMed] [Google Scholar]
- 21.Dent EW, Gupton SL, Gertler FB (2011): The growth cone cytoskeleton in axon outgrowth and guidance. Cold Spring Harb Perspect Biol 3 10.1101/cshperspect.a001800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai Wing Sun K, Correia JP, Kennedy TE (2011): Netrins: versatile extracellular cues with diverse functions. Development 138: 2153–69. [DOI] [PubMed] [Google Scholar]
- 23.Boyer NP, Gupton SL (2018): Revisiting Netrin-1: One Who Guides (Axons). Front Cell Neurosci 12: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finci L, Zhang Y, Meijers R, Wang J-H (2015): Signaling mechanism of the netrin-1 receptor DCC in axon guidance. Prog Biophys Mol Biol 118: 153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meijers R, Smock RG, Zhang Y, Wang J-H (2019): Netrin Synergizes Signaling and Adhesion through DCC. Trends Biochem Sci. 10.1016/j.tibs.2019.10.005 [DOI] [PubMed] [Google Scholar]
- 26.Finci LI, Krüger N, Sun X, Zhang J, Chegkazi M, Wu Y, et al. (2014): The crystal structure of netrin-1 in complex with DCC reveals the bifunctionality of netrin-1 as a guidance cue. Neuron 83: 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dominici C, Moreno-Bravo JA, Puiggros SR, Rappeneau Q, Rama N, Vieugue P, et al. (2017): Floor-plate-derived netrin-1 is dispensable for commissural axon guidance. Nature 545: 350–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varadarajan SG, Kong JH, Phan KD, Kao T-J, Panaitof SC, Cardin J, et al. (2017): Netrin1 Produced by Neural Progenitors, Not Floor Plate Cells, Is Required for Axon Guidance in the Spinal Cord. Neuron 94: 790–799.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore SW, Biais N, Sheetz MP (2009): Traction on immobilized netrin-1 is sufficient to reorient axons. Science 325: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen K, Cowan CW (2010): Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol 2: a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manitt C, Nikolakopoulou AM, Almario DR, Nguyen SA, Cohen-Cory S (2009): Netrin participates in the development of retinotectal synaptic connectivity by modulating axon arborization and synapse formation in the developing brain. J Neurosci 29: 11065–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horn KE, Glasgow SD, Gobert D, Bull S-J, Luk T, Girgis J, et al. (2013): DCC expression by neurons regulates synaptic plasticity in the adult brain. Cell Rep 3: 173–85. [DOI] [PubMed] [Google Scholar]
- 33.Goldman JS, Ashour MA, Magdesian MH, Tritsch NX, Harris SN, Christofi N, et al. (2013): Netrin-1 promotes excitatory synaptogenesis between cortical neurons by initiating synapse assembly. J Neurosci 33: 17278–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Glasgow SD, Labrecque S, Beamish IV., Aufmkolk S, Gibon J, Han D, et al. (2018): Activity-Dependent Netrin-1 Secretion Drives Synaptic Insertion of GluA1-Containing AMPA Receptors in the Hippocampus. Cell Rep 25: 168–182.e6. [DOI] [PubMed] [Google Scholar]
- 35.Colón-Ramos DA, Margeta MA, Shen K (2007): Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science 318: 103–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poon VY, Klassen MP, Shen K (2008): UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature 455: 669–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flores C (2011): Role of netrin-1 in the organization and function of the mesocorticolimbic dopamine system. J Psychiatry Neurosci 36: 296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoops D, Flores C (2017): Making Dopamine Connections in Adolescence. Trends Neurosci 40: 709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manitt C, Eng C, Pokinko M, Ryan RT, Torres-Berrío A, Lopez JP, et al. (2013): dcc orchestrates the development of the prefrontal cortex during adolescence and is altered in psychiatric patients. Transl Psychiatry 3: e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds LM, Yetnikoff L, Pokinko M, Wodzinski M, Epelbaum JG, Lambert LC, et al. (2018): Early Adolescence is a Critical Period for the Maturation of Inhibitory Behavior. Cereb Cortex 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoops D, Reynolds LM, Restrepo-Lozano J-M, Flores C (2018): Dopamine Development in the Mouse Orbital Prefrontal Cortex Is Protracted and Sensitive to Amphetamine in Adolescence. eNeuro 5: ENEURO.0372–17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuesta S, Restrepo-Lozano JM, Silvestrin S, Nouel D, Torres-Berrío A, Reynolds LM, et al. (2018): Non-Contingent Exposure to Amphetamine in Adolescence Recruits miR-218 to Regulate Dcc Expression in the VTA. Neuropsychopharmacology 43: 900–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuesta S, Restrepo-Lozano JM, Popescu C, He S, Reynolds LM, Israel S, et al. (2019): DCC-related developmental effects of abused-versus therapeutic-like amphetamine doses in adolescence. Addict Biol e12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vosberg DE, Leyton M, Flores C (2019): The Netrin-1/DCC guidance system: dopamine pathway maturation and psychiatric disorders emerging in adolescence. Mol Psychiatry. 10.1038/s41380-019-0561-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Z, Makihara S, Yam PT, Teo S, Renier N, Balekoglu N, et al. (2019): Long-Range Guidance of Spinal Commissural Axons by Netrin1 and Sonic Hedgehog from Midline Floor Plate Cells. Neuron 101: 635–647.e4. [DOI] [PubMed] [Google Scholar]
- 46.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M (1996): Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87: 175–85. [DOI] [PubMed] [Google Scholar]
- 47.Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M (1997): Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature 386: 833–8. [DOI] [PubMed] [Google Scholar]
- 48.Manitt C, Kennedy TE (2002): Where the rubber meets the road: netrin expression and function in developing and adult nervous systems. Prog Brain Res 137: 425–42. [DOI] [PubMed] [Google Scholar]
- 49.Bartoe JL, McKenna WL, Quan TK, Stafford BK, Moore JA, Xia J, et al. (2006): Protein interacting with C-kinase 1/protein kinase Calpha-mediated endocytosis converts netrin-1-mediated repulsion to attraction. J Neurosci 26: 3192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Muramatsu R, Nakahara S, Ichikawa J, Watanabe K, Matsuki N, Koyama R (2010): The ratio of “deleted in colorectal cancer” to “uncoordinated-5A” netrin-1 receptors on the growth cone regulates mossy fibre directionality. Brain 133: 60–75. [DOI] [PubMed] [Google Scholar]
- 51.Bouchard J-F, Moore SW, Tritsch NX, Roux PP, Shekarabi M, Barker PA, Kennedy TE (2004): Protein kinase A activation promotes plasma membrane insertion of DCC from an intracellular pool: A novel mechanism regulating commissural axon extension. J Neurosci 24: 3040–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yetnikoff L, Eng C, Benning S, Flores C (2010): Netrin-1 receptor in the ventral tegmental area is required for sensitization to amphetamine. Eur J Neurosci 31: 1292–302. [DOI] [PubMed] [Google Scholar]
- 53.Osborne PB, Halliday GM, Cooper HM, Keast JR (2005): Localization of immunoreactivity for deleted in colorectal cancer (DCC), the receptor for the guidance factor netrin-1, in ventral tier dopamine projection pathways in adult rodents. Neuroscience 131: 671–81. [DOI] [PubMed] [Google Scholar]
- 54.Manitt C, Labelle-Dumais C, Eng C, Grant A, Mimee A, Stroh T, Flores C (2010): Peri-pubertal emergence of UNC-5 homologue expression by dopamine neurons in rodents. PLoS One 5: e11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reyes S, Fu Y, Double KL, Cottam V, Thompson LH, Kirik D, et al. (2013): Trophic factors differentiate dopamine neurons vulnerable to Parkinson’s disease. Neurobiol Aging 34: 873–86. [DOI] [PubMed] [Google Scholar]
- 56.Manitt C, Mimee A, Eng C, Pokinko M, Stroh T, Cooper HM, et al. (2011): The netrin receptor DCC is required in the pubertal organization of mesocortical dopamine circuitry. J Neurosci 31: 8381–8394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park HL, Kim MS, Yamashita K, Westra W, Carvalho AL, Lee J, et al. (2008): DCC promoter hypermethylation in esophageal squamous cell carcinoma. Int J cancer 122: 2498–502. [DOI] [PubMed] [Google Scholar]
- 58.Derks S, Bosch LJW, Niessen HEC, Moerkerk PTM, van den Bosch SM, Carvalho B, et al. (2009): Promoter CpG island hypermethylation- and H3K9me3 and H3K27me3-mediated epigenetic silencing targets the deleted in colon cancer (DCC) gene in colorectal carcinogenesis without affecting neighboring genes on chromosomal region 18q21. Carcinogenesis 30: 1041–8. [DOI] [PubMed] [Google Scholar]
- 59.Torres-Berrío A, Lopez JP, Bagot RC, Nouel D, Dal Bo G, Cuesta S, et al. (2017): DCC Confers Susceptibility to Depression-like Behaviors in Humans and Mice and Is Regulated by miR-218. Biol Psychiatry 81: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore LD, Le T, Fan G (2013): DNA methylation and its basic function. Neuropsychopharmacology 38: 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang X, Chen Q, Yi S, Liu Q, Zhang R, Wang P, et al. (2019): The microRNAs let-7 and miR-9 down-regulate the axon-guidance genes Ntn1 and Dcc during peripheral nerve regeneration. J Biol Chem 294: 3489–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jang HS, Shin WJ, Lee JE, Do JT (2017): CpG and Non-CpG Methylation in Epigenetic Gene Regulation and Brain Function. Genes (Basel) 8 10.3390/genes8060148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW (2003): Mapping cortical change across the human life span. Nat Neurosci 6: 309–15. [DOI] [PubMed] [Google Scholar]
- 64.Zhou C, Ye M, Ni S, Li Q, Ye D, Li J, et al. (2018): DNA methylation biomarkers for head and neck squamous cell carcinoma. Epigenetics 13: 398–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petanjek Z, Judaš M, Šimic G, Rasin MR, Uylings HBM, Rakic P, Kostovic I (2011): Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci U S A 108: 13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS (2010): Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry 167: 1479–1488. [DOI] [PubMed] [Google Scholar]
- 67.Caballero A, Granberg R, Tseng KY (2016): Mechanisms contributing to prefrontal cortex maturation during adolescence. Neurosci Biobehav Rev 70: 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larsen B, Luna B (2018): Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci Biobehav Rev 94: 179–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Naneix F, Marchand AR, Di Scala G, Pape J-R, Coutureau E (2012): Parallel maturation of goal- directed behavior and dopaminergic systems during adolescence. J Neurosci 32: 16223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tarazi FI, Baldessarini RJ (2000): Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci 18: 29–37. [DOI] [PubMed] [Google Scholar]
- 71.Reynolds LM, Pokinko M, Torres-Berrío A, Cuesta S, Lambert LC, Del Cid Pellitero E, et al. (2018): DCC Receptors Drive Prefrontal Cortex Maturation by Determining Dopamine Axon Targeting in Adolescence. Biol Psychiatry 83: 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shaw GA, Dupree JL, Neigh GN (2020): Adolescent maturation of the prefrontal cortex: Role of stress and sex in shaping adult risk for compromise. Genes Brain Behav 19: e12626. [DOI] [PubMed] [Google Scholar]
- 73.Delevich K, Okada NJ, Rahane A, Zhang Z, Hall CD, Wilbrecht L (2020): Sex and Pubertal Status Influence Dendritic Spine Density on Frontal Corticostriatal Projection Neurons in Mice. Cereb Cortex. 10.1093/cercor/bhz325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Reynolds LM, Makowski CS, Yogendran SV, Kiessling S, Cermakian N, Flores C (2015): Amphetamine in Adolescence Disrupts the Development of Medial Prefrontal Cortex Dopamine Connectivity in a dcc-Dependent Manner. Neuropsychopharmacology 40: 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Torres-Berrío A, Nouel D, Cuesta S, Parise EM, Restrepo-Lozano JM, Larochelle P, et al. (2019): MiR-218: a molecular switch and potential biomarker of susceptibility to stress. Mol Psychiatry. 10.1038/s41380-019-0421-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reynolds LM, Flores C (2019): Guidance cues: linking drug use in adolescence with psychiatric disorders. Neuropsychopharmacology 44: 225–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Levinson DF (2006): The genetics of depression: a review. Biol Psychiatry 60: 84–92. [DOI] [PubMed] [Google Scholar]
- 78.Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium, Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, et al. (2013): A mega-analysis of genome-wide association studies for major depressive disorder. Mol Psychiatry 18: 497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kendler KS, Karkowski LM, Prescott CA (1999): Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry 156: 837–41. [DOI] [PubMed] [Google Scholar]
- 80.Liu RT, Alloy LB (2010): Stress generation in depression: A systematic review of the empirical literature and recommendations for future study. Clin Psychol Rev 30: 582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nestler EJ, Hyman SE (2010): Animal models of neuropsychiatric disorders. Nat Neurosci 13: 1161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Czéh B, Fuchs E, Wiborg O, Simon M (2016): Animal models of major depression and their clinical implications. Prog Neuropsychopharmacol Biol Psychiatry 64: 293–310. [DOI] [PubMed] [Google Scholar]
- 83.Bale TL, Abel T, Akil H, Carlezon WA, Moghaddam B, Nestler EJ, et al. (2019): The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology 44: 1349–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. (2017): Sex-specific transcriptional signatures in human depression. Nat Med 23: 1102–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Willner P (2017): The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiol Stress 6: 78–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. (2006): Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311: 864–8. [DOI] [PubMed] [Google Scholar]
- 87.Golden SA, Covington HE, Berton O, Russo SJ (2011): A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6: 1183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krishnan V, Han M-H, Graham DL, Berton O, Renthal W, Russo SJ, et al. (2007): Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131: 391–404. [DOI] [PubMed] [Google Scholar]
- 89.Harris AZ, Atsak P, Bretton ZH, Holt ES, Alam R, Morton MP, et al. (2018): A Novel Method for Chronic Social Defeat Stress in Female Mice. Neuropsychopharmacology 43: 1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Newman EL, Covington HE, Suh J, Bicakci MB, Ressler KJ, DeBold JF, Miczek KA (2019): Fighting Females: Neural and Behavioral Consequences of Social Defeat Stress in Female Mice. Biol Psychiatry 86: 657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takahashi A, Chung J-R, Zhang S, Zhang H, Grossman Y, Aleyasin H, et al. (2017): Establishment of a repeated social defeat stress model in female mice. Sci Rep 7: 12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yohn CN, Ashamalla SA, Bokka L, Gergues MM, Garino A, Samuels BA (2019): Social instability is an effective chronic stress paradigm for both male and female mice. Neuropharmacology 160: 107780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Iñiguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, et al. (2018): Vicarious Social Defeat Stress Induces Depression-Related Outcomes in Female Mice. Biol Psychiatry 83: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koo JW, Chaudhury D, Han M-H, Nestler EJ (2019): Role of Mesolimbic Brain-Derived Neurotrophic Factor in Depression. Biol Psychiatry 86: 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.O’Toole N, Zhang T-Y, Wen X, Diorio J, Silveira PP, Labonté B, et al. (2019): Epigenetic signatures of chronic social stress in stress-susceptible animals. bioRxiv 690826. [Google Scholar]
- 96.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. (2013): Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 493: 532–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Small EM, Sutherland LB, Rajagopalan KN, Wang S, Olson EN (2010): MicroRNA-218 regulates vascular patterning by modulation of Slit-Robo signaling. Circ Res 107: 1336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wray NR, Pergadia ML, Blackwood DHR, Penninx BWJH, Gordon SD, Nyholt DR, et al. (2012): Genome-wide association study of major depressive disorder: new results, meta-analysis, and lessons learned. Mol Psychiatry 17: 36–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dunn EC, Wiste A, Radmanesh F, Almli LM, Gogarten SM, Sofer T, et al. (2016): Genome-wide association study (gwas) and genome-wide by environment interaction study (gweis) of depressive symptoms in african american and hispanic/latina women. Depress Anxiety 33: 265–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium (2015): Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci 18: 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Smith BH, Campbell H, Blackwood D, Connell J, Connor M, Deary IJ, et al. (2006): Generation Scotland: the Scottish Family Health Study; a new resource for researching genes and heritability. BMC Med Genet 7: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng Y, Navarro P, Fernandez-Pujals AM, Hall LS, Clarke TK, Thomson PA, et al. (2017): A Combined Pathway and Regional Heritability Analysis Indicates NETRIN1 Pathway Is Associated With Major Depressive Disorder. Biol Psychiatry 81: 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Barbu MC, Zeng Y, Shen X, Cox SR, Clarke T-K, Gibson J, et al. (2019): Association of Whole-Genome and NETRIN1 Signaling Pathway-Derived Polygenic Risk Scores for Major Depressive Disorder and White Matter Microstructure in the UK Biobank. Biol psychiatry Cogn Neurosci neuroimaging 4: 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vosberg DE, Zhang Y, Menegaux A, Chalupa A, Manitt C, Zehntner S, et al. (2018): Mesocorticolimbic Connectivity and Volumetric Alterations in DCC Mutation Carriers. J Neurosci 38: 4655–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Okbay A, Baselmans BML, De Neve J-E, Turley P, Nivard MG, Fontana MA, et al. (2016): Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet 48: 624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ward J, Lyall LM, Bethlehem RAI, Ferguson A, Strawbridge RJ, Lyall DM, et al. (2019): Novel genome-wide associations for anhedonia, genetic correlation with psychiatric disorders, and polygenic association with brain structure. bioRxiv 656298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. (2018): Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 50: 668–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ward J, Strawbridge RJ, Bailey MES, Graham N, Ferguson A, Lyall DM, et al. (2017): Genome-wide analysis in UK Biobank identifies four loci associated with mood instability and genetic correlation with major depressive disorder, anxiety disorder and schizophrenia. Transl Psychiatry 7: 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arnau-Soler A, Macdonald-Dunlop E, Adams MJ, Clarke T-K, MacIntyre DJ, Milburn K, et al. (2019): Genome-wide by environment interaction studies of depressive symptoms and psychosocial stress in UK Biobank and Generation Scotland. Transl Psychiatry 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Strawbridge RJ, Ward J, Ferguson A, Graham N, Shaw RJ, Cullen B, et al. (2019): Identification of novel genome-wide associations for suicidality in UK Biobank, genetic correlation with psychiatric disorders and polygenic association with completed suicide. EBioMedicine 41: 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Leday GGR, Vértes PE, Richardson S, Greene JR, Regan T, Khan S, et al. (2018): Replicable and Coupled Changes in Innate and Adaptive Immune Gene Expression in Two Case-Control Studies of Blood Microarrays in Major Depressive Disorder. Biol Psychiatry 83: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Smith DJ, Escott-Price V, Davies G, Bailey MES, Colodro-Conde L, Ward J, et al. (2016): Genome-wide analysis of over 106 000 individuals identifies 9 neuroticism-associated loci. Mol Psychiatry 21: 749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Aberg KA, Shabalin AA, Chan RF, Zhao M, Kumar G, van Grootheest G, et al. (2018): Convergence of evidence from a methylome-wide CpG-SNP association study and GWAS of major depressive disorder. Transl Psychiatry 8: 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Roberson-Nay R, Wolen AR, Lapato DM, Lancaster EE, Webb BT, Verhulst B, et al. (2018): Twin Study of Early-Onset Major Depression Finds DNA Methylation Enrichment for Neurodevelopmental Genes. bioRxiv 422345. [Google Scholar]
- 115.Lee PH, Anttila V, Won H, Feng Y-CA, Rosenthal J, Zhu Z, et al. (2019): Genomic Relationships, Novel Loci, and Pleiotropic Mechanisms across Eight Psychiatric Disorders. Cell 179: 1469–1482.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yuan H, Mischoulon D, Fava M, Otto MW (2018): Circulating microRNAs as biomarkers for depression: Many candidates, few finalists. J Affect Disord 233: 68–78. [DOI] [PubMed] [Google Scholar]
- 117.Gururajan A, Clarke G, Dinan TG, Cryan JF (2016): Molecular biomarkers of depression. Neurosci Biobehav Rev 64: 101–33. [DOI] [PubMed] [Google Scholar]
- 118.Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, et al. (2017): Erratum: Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23: 264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ruan Q, D’Onofrio G, Sancarlo D, Bao Z, Greco A, Yu Z (2016): Potential neuroimaging biomarkers of pathologic brain changes in Mild Cognitive Impairment and Alzheimer’s disease: a systematic review. BMC Geriatr 16: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Strawbridge R, Young AH, Cleare AJ (2017): Biomarkers for depression: recent insights, current challenges and future prospects. Neuropsychiatr Dis Treat 13: 1245–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Issler O, Chen A (2015): Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci 16: 201. [DOI] [PubMed] [Google Scholar]
- 122.Gheysarzadeh A, Sadeghifard N, Afraidooni L, Pooyan F, Mofid MR, Valadbeigi H, et al. (2018): Serum-based microRNA biomarkers for major depression: MiR-16, miR-135a, and miR-1202. J Res Med Sci 23: 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tavakolizadeh J, Roshanaei K, Salmaninejad A, Yari R, Nahand JS, Sarkarizi HK, et al. (2018): MicroRNAs and exosomes in depression: Potential diagnostic biomarkers. J Cell Biochem 119: 3783–3797. [DOI] [PubMed] [Google Scholar]
- 124.Fiori LM, Lopez JP, Richard-Devantoy S, Berlim M, Chachamovich E, Jollant F, et al. (2017): Investigation of miR-1202, miR-135a, and miR-16 in Major Depressive Disorder and Antidepressant Response. Int J Neuropsychopharmacol 20: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, et al. (2008): Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18: 997–1006. [DOI] [PubMed] [Google Scholar]
- 126.Lopez JP, Lim R, Cruceanu C, Crapper L, Fasano C, Labonte B, et al. (2014): MiR-1202 is a primate-specific and brain-enriched microRNA involved in major depression and antidepressant treatment. Nat Med. 10.1038/nm.3582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Issler O, Haramati S, Paul ED, Maeno H, Navon I, Zwang R, et al. (2014): MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron 83: 344–360. [DOI] [PubMed] [Google Scholar]
- 128.Belzeaux R, Bergon A, Jeanjean V, Loriod B, Formisano-Tréziny C, Verrier L, et al. (2012): Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl Psychiatry 2: e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lopez JP, Fiori LM, Cruceanu C, Lin R, Labonte B, Cates HM, et al. (2017): MicroRNAs 146a/b-5 and 425–3p and 24–3p are markers of antidepressant response and regulate MAPK/Wnt-system genes. Nat Commun 8: 15497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mendes-Silva AP, Diniz BS, Tolentino Araújo GT, de Souza Nicolau E, Pereira KS, Silva Ferreira CM, Barroso LS (2017): Mirnas and their role in the correlation between major depressive disorder, mild cognitive impairment and alzheimer’s disease. Alzheimer’s Dement 13: P1017–P1018. [Google Scholar]
- 131.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT (2011): MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13: 423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu G, Drescher KM, Chen XM (2012): Exosomal miRNAs: Biological properties and therapeutic potential. Front Genet 3: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hoye ML, Regan MR, Jensen LA, Lake AM, Reddy LV, Vidensky S, et al. (2018): Motor neuron-derived microRNAs cause astrocyte dysfunction in amyotrophic lateral sclerosis. Brain 141: 2561–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Belzeaux R, Lin R, Turecki G (2017): Potential Use of MicroRNA for Monitoring Therapeutic Response to Antidepressants. CNS Drugs 31: 253–262. [DOI] [PubMed] [Google Scholar]
- 135.Torres-Berrío A, Morgunova A, Flores C (2018): Adolescent levels of circulating miR-218 predict susceptibility to chronic social defeat stress in adult mice. Society for Neuroscience. Retrieved from https://www.abstractsonline.com/pp8/#!/4649/presentation/33240 [Google Scholar]
- 136.Scott KM, McLaughlin KA, Smith DAR, Ellis PM (2012): Childhood maltreatment and DSM-IV adult mental disorders: comparison of prospective and retrospective findings. Br J Psychiatry 200: 469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cattaneo A, Cattane N, Malpighi C, Czamara D, Suarez A, Mariani N, et al. (2018): FoxO1, A2M, and TGF-β1: three novel genes predicting depression in gene X environment interactions are identified using cross-species and cross-tissues transcriptomic and miRNomic analyses. Mol Psychiatry 23: 2192–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yetnikoff L, Pokinko M, Arvanitogiannis A, Flores C (2014): Adolescence: a time of transition for the phenotype of dcc heterozygous mice. Psychopharmacology (Berl) 231: 1705–14. [DOI] [PubMed] [Google Scholar]
- 139.Yetnikoff L, Almey A, Arvanitogiannis A, Flores C (2011): Abolition of the behavioral phenotype of adult netrin-1 receptor deficient mice by exposure to amphetamine during the juvenile period. Psychopharmacology (Berl) 217: 505–14. [DOI] [PubMed] [Google Scholar]
- 140.Amare AT, Vaez A, Hsu Y-H, Direk N, Kamali Z, Howard DM, et al. (2019): Bivariate genome-wide association analyses of the broad depression phenotype combined with major depressive disorder, bipolar disorder or schizophrenia reveal eight novel genetic loci for depression. Mol Psychiatry. 10.1038/s41380-018-0336-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Howard DM, Adams MJ, Clarke T-K, Hafferty JD, Gibson J, Shirali M, et al. (2019): Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 22: 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]


