Abstract
Drug addiction is a chronic disease defined by a complex set of characteristics, including loss of control over drug intake and persistent drug craving, which primarily affects a small percentage of people who try drugs. Although many models have been developed to study individual aspects of drug use, there is great translational value in having an animal model that encompasses multiple aspects of the human disease, including the variation in severity observed in humans. Here, we describe an intermittent access model of cocaine self-administration that produces a subset of rats that display many of the core features of addiction, including escalation of drug intake, a binge-like pattern of drug use, robust locomotor sensitization, and high levels of drug-seeking during cue-induced reinstatement. This group is compared to rats that have the same drug history but do not develop this pattern of drug-taking and drug-seeking, as well as rats that undergo a traditional continuous access paradigm. Finally, we observe that high levels of cocaine consumption produce long-term changes in intracellular calcium signaling in the dorsomedial striatum.
1. Introduction
Drug addiction is a chronic, relapsing disease that carries high costs to both individuals and society through its effects on quality of life, productivity, healthcare and crime (Cartwright 2008; Leshner 1997; Robbins and Everitt, 1999). Although drug use is common, only a relatively small number of individuals who try drugs ever develop a substance use disorder (Anthony et al., 1994; Penberthy et al., 2010; Grant and Dawson, 1998). Nonetheless, it is not well-understood why some drug users transition to addiction and others do not. Determining the neural correlates of addiction will help us to address this critical issue; however, it is necessary to first be able to effectively and accurately model in animals the subset of individuals susceptible to drug addiction. This is a challenging task, as addiction is composed of a constellation of symptoms, including compulsive drug-taking and drug-seeking, loss of control over drug intake which leads to binges, and a persistent, sensitized craving for the drug.
Over the years, a range of models have been used to capture key features of addiction. For example, non-contingent models such as locomotor sensitization model the long-lasting effects of drugs associated with addiction, including sensitization of the neural circuits thought to underlie salience attribution (Robinson and Becker, 1986; Paulson et al., 1991; Robinson and Berridge, 1993). In addition, extended access drug self-administration models produce an escalation of drug intake that is translationally appealing, as it is one of the hallmark characteristics that separates recreational users from persons with addiction (Ahmed and Koob, 1998), and prolonged access self-administration paradigms, such as the three-criteria model, are effective at capturing individual differences seen following drug use (Anthony et al., 1994; Penberthy et al., 2010; Grant and Dawson, 1998; Deroche-Gamonet et al., 2004). Of note, self-administration paradigms that report escalation of drug intake have at times failed to induce locomotor sensitization (Ben-Sharar et al., 2004; Knackstedt and Kalivas, 2007; but see Ferrario et al., 2005), suggesting that escalation may reflect a tolerance rather than a sensitization of the neural circuits associated with addiction (Calipari et al., 2014). These self-administration paradigms also have technical drawbacks associated with their long-lasting nature, including catheter patency considerations as well as the time- and resource-intensiveness of the experiments.
Intermittent access (IntA) self-administration paradigms, in which animals go through alternating periods of drug access and no access throughout each session (Zimmer et al., 2012) are intriguing as they seem to mimic the pattern of drug-taking seen in humans (Beveridge et al., 2012). Interestingly, these models have been shown to produce robust incubation of craving, higher motivation to take drug compared to continuous access (ContA) models, and they produce sensitization as opposed to tolerance at the dopamine transporter (Nicolas et al., 2019; Zimmer et al., 2012; Calipari et al., 2013; Calipari et al., 2015). Similar to the three-criteria model, current intermittent access models can last for months (Kawa et al., 2016). However, little has been done to determine if the intermittent access model produces these effects in all animals or if it produces a meaningful variability in addiction susceptibility similar to the three-criteria model. Here, we describe a version of the IntA self-administration model that is shorter in both session length and/or total experiment length than the ~14 week intermittent access model described by Kawa et al. (2016), the extended access model (6h/session), and the three-criteria model (~3 months), but still captures many of the key features of addiction in a subset of individuals including escalation of drug intake and a binge-like pattern of drug use, locomotor sensitization, and high levels of drug-seeking during cue-induced reinstatement.
The dorsomedial striatum (DMS) is an important brain region for the transition to addiction from initial to compulsive use (Robbins & Everitt, 2002; Caprioli et al., 2017). Within the dorsal striatum, cocaine exposure produces a number of changes to intracellular signaling cascades, in part by modulating regulation of calcium (Yang & Choe, 2014; Kim et al., 2015; Kim et al., 2009; Schierberl et al., 2012; Shen et al., 2018). In addition, intracellular calcium signaling in the striatum modulates cocaine reward and sensitization (Barr et al., 2015; Mizuno et al., 2013; Yasui and Su, 2016). Sustained administration of cocaine alters intracellular calcium signaling producing an endoplasmic reticulum (ER) stress response in the dorsal striatum, which may play a role in the neurotoxic effects of psychostimulants (Choe et al., 2011). It is unclear how different models of cocaine self-administration may differentially affect calcium signaling in the dorsal striatum. Here, we provide evidence through 2-photon calcium imaging following self-administration that high levels of cocaine consumption during continuous access administration are capable of producing significant and long-lasting alterations in intracellular calcium signaling in DMS.
2. Materials and Methods
2.1. Animals
All experiments were approved by the Seattle Children’s Research Institute Institutional Animal Use and Care Committee and adhered to NIH guidelines. Male Sprague-Dawley rats (n = 89, Envigo) weighing 250–274g upon arrival were single-housed in a temperature- and humidity-controlled vivarium on a 12 h light/dark cycle. Food was available ad libitum except during fixed ratio (FR) training when animals were mildly food restricted and given 20g chow per day. Water was available ad libitum. All procedures and experiments took place during the light cycle.
2.2. Drugs
Cocaine hydrochloride was obtained from the National Institute of Drug Abuse and dissolved in 0.9% sterile saline.
2.3. Viral Vectors
A GCaMP6m viral vector driven by the human synapsin promoter (AAV5-hSyn-GCaMP6m-WPRE-SV40) and packaged in adenoassociated virus serotype 5 (AAV5) was obtained from the University of Pennsylvania viral vector core with a titer of ~1×109 viral genomes/μL.
2.4. Surgical Techniques
Rats were anesthetized with isoflurane (2–4%, inhalation, Patterson Veterinary) for all surgical procedures. Rats received an injection of meloxicam (0.2 mg/kg sc, Patterson Veterinary) prior to surgery for analgesia. Rats underwent post-operative monitoring for at least 3 days following each surgical procedure.
Rats were implanted with chronic indwelling catheters placed into the right jugular vein and attached to a back-mounted port as previously described (Crombag et al., 2000). Catheters were flushed daily with 0.2 mL of gentamicin (5 mg/mL, Patterson Veterinary) dissolved in sterile saline to prevent occlusions and infections. Rats received injections of 0.2 mL sodium brevital dissolved in sterile saline (10 mg/mL iv, Patterson Veterinary) to determine catheter patency prior to the first session, and after the last session, of self-administration. Catheters were considered patent if rats became ataxic within 5 seconds of the infusion.
For calcium imaging experiments, one week prior to catheter surgery, animals received viral infusions of hSyn-GCaMP6m obtained from the University of Pennsylvania viral vector core using standard stereotaxic procedures. Briefly, 27-gauge stainless steel needles attached to gas-tight syringes (Hamilton Company) were used to infuse hSyn-GCaMP6m (1.0 μL) into the dorsomedial striatum (DMS; coordinates relative to bregma and from skull surface, A/P: + 0.2 mm, M/L: ± 2.0 mm, D/V: − 4.1 mm) at a rate of 0.4 μL/min. Needles were left in place for an additional 5 min infusion to allow for diffusion away from the infusion site.
2.5. Cocaine Self-Administration
2.5.1. Self-Administration Chambers
Self-administration occurred in standard operant chambers (Med Associates) equipped with two retractable levers, two white stimulus lights (one located above each lever), a white house light located on the back of the chamber, a house fan, and metal grid floor. A syringe pump located outside of the box delivered cocaine via tubing attached to a suspended swivel and the catheter backport, allowing for free movement of the rats.
2.5.2. Cocaine Self-Administration Procedure
At least 5 days after surgery, rats were trained to lever press for cocaine on an FR1 schedule for 5 days. Each training session began with the insertion of the two retractable levers. A lever press on the active lever resulted in a cocaine infusion (0.4 mg/kg/inf in 50 μL over 2.8s) and illumination of the white stimulus light above the active lever (4s). Additional presses on the active lever during the light cue presentation were recorded, but did not result in additional infusions. Pressing on the inactive lever had no programmed consequences (i.e., no cocaine and no light). The location of the active lever was counterbalanced across animals. The session ended after 3h or after the rat had received 10 infusions, whichever came first. Following completion of 5 days of FR1 training, rats were given 14 sessions (one session per day for 14 days) of either intermittent (n = 53) or continuous (n = 28) access to cocaine on a FR1 schedule of reinforcement. A single IntA session consisted of 5 min access to cocaine followed by a 25 min time-out period where the levers were retracted, and cocaine was not available. This cycle repeated for a total of 155 minutes resulting in 6 drug-available periods separated by 5 drug-unavailable periods. For ContA, rats had access to the levers and cocaine for the entire 155 minutes of each session.
2.6. Extinction
A subset of intermittent and ContA rats (n = 24) underwent extinction training at the conclusion of the self-administration sessions. Rats were put into the operant chambers for 60 minutes per session, and responses on the active and inactive levers had no programmed consequences (i.e., no drug infusion or illumination of cue light). Rats underwent 10 sessions of extinction training. At the end of extinction training all rats had made fewer than 30% of the active lever responses that they had made on their first day of extinction.
2.7. Cue-induced Reinstatement
After rats had achieved the extinction criteria, they underwent a 60-minute cue-induced reinstatement test of cocaine-seeking. Rats were put into the operant chamber, and the session began with the extension of the levers and the illumination of the cue light above the active lever for 4 sec. Each active lever press resulted in a 4 sec presentation of the cue-light, but no drug infusion.
2.8. Locomotor Sensitization
A subset of intermittent and ContA rats (n = 38) underwent testing to assess for the development of locomotor sensitization. Prior to the start of self-administration, rats underwent an initial locomotor test where they were placed into locomotor activity boxes (San Diego Instruments) and allowed to habituate for 30 minutes. The rats then received an injection of saline (0.9%, ip), followed 30 minutes later by an injection of cocaine (10 mg/kg, ip) and behavior was recorded for an additional 60 minutes. Twenty-four hours later rats began FR1 training followed by either 14 sessions of intermittent (n = 27) or continuous (n = 11) access self-administration. Two weeks after the last self-administration session, rats underwent a second locomotor test in order to assess locomotor sensitization.
2.9. Calcium Imaging
2.9.1. Slice Preparation
Naive animals (n = 8) or animals that had undergone IntA or ContA self-administration (n = 18) were anesthetized and perfused with an ice cold, high-sucrose artificial cerebrospinal fluid (aCSF) solution (in mM: 210 sucrose, 5 KCl, 1.25NaH2PO4·H2O, 3.5MgSO4·7H2O, 0.5CaCl2·2H2O, 26 NaHCO3 and 10 D-glucose, osmolarity ~300 mOsm) 7–10 days after the end of self-administration. Rats were decapitated and their brains quickly removed and transferred to a chamber of cold, high-sucrose aCSF. Three hundred micron thick coronal slices were taken with a vibrating microtome, while the brains were submerged in cold, high-sucrose aCSF. Slices were then transferred to a chamber filled with N-methyl-D-glucamine protective recovery aCSF (in mM: 93 N-methyl-D-glucamine, 93 HCl, 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 HEPES, 25 glucose, 5 sodium ascorbate, 2 thiourea, 3 sodium pyruvate, 10MgSO4·7H2O and 0.5CaCl2·2H2O, osmolarity ~300 mOsm) for less than 15 minutes. Finally, slices were transferred to a chamber filled with room temperature aCSF (in mM: 119 NaCl, 5 KCl, 1.3MgSO4·7H2O, 2.5CaCl2·2H2O, 1NaH2PO4·H2O, 16.2 NaHCO3, 11 D-glucose and 10 HEPES, osmolarity ~300 mOsm) and allowed to rest for ~1 hour. All solutions were bubbled with a 95 percent O2–5 percent CO2 mixture.
2.9.2. Image Acquisition and Processing
Slices were submerged in oxygenated aCSF (32°C), which was superfused throughout the experiments. Fluorescence images were captured at 5 Hz with an Olympus FV1000 upright microscope equipped with 25× 1.05 NA Ultra-objectives driven by a femtosecond pulsed MaiTai DeepSee laser (Spectra Physics) tuned to 890-nm excitation. After acquisition, images were processed using FIJI and FluoroSNNAP (Patel et al., 2015). ROIs were identified from image stacks, which were motion corrected. The ΔF/F record was calculated by subtracting raw fluorescence values with the mean of the lower 50 percent of previous 10-second values and dividing by the mean of the lower 50 percent of previous 10-second values (Patel et al., 2015). Calcium events were detected using a deconvolution method with a detection threshold of 3 standard deviations above the baseline.
2.10. Statistical Analysis
Brain levels of cocaine were modeled based on a two-compartment model as previously described (Zimmer et al., 2011). The estimation was based on the equation:
which estimates the brain cocaine concentration (c) by accounting for the dose of cocaine (d), the transport of cocaine between the blood and brain(k = 0.233 min−1), the brain volume (v = 0.151 kg−1), and the removal of cocaine from circulation via redistribution (α = 0.642min−1) and elimination (β = 0.097min−1).
GraphPad Prism 8 was used for all statistical analyses. Unpaired t-tests were used to analyze differences in total drug intake. Instead of providing an experimenter-assigned cutoff to demarcate escalation, linear regressions were used to analyze active presses across days and separate animals into escalators and non-escalators. The linear regression was run on each individual animal to determine if the presses by the animal across days had a significantly positive slope (escalation). During the acquisition phase of self-administration, cocaine injections were capped at 10 maximum infusions. This was done to ensure that all animals acquired self-administration without significant differences in total consumption prior to beginning the intermittent or continuous paradigms. The first day of the intermittent or continuous paradigm is the first day that the animals can take as much cocaine as they choose without a maximum infusion cap. This typically results in very high intake on day 1 as they binge in absence of the infusion cap. This is followed by a strong reduction in intake after they experience their binge. In order to prevent the escalation determination from being confounded due to this instability produced by the transition from the acquisition phase to the intermittent or continuous phase, Days 1–3 were excluded from the escalation analysis. Repeated measures two-way ANOVAs were used to analyze group differences in extinction and reinstatement. Pearson correlations were used to analyze the relatedness of extinction responding (on Day 1), reinstatement responding, and drug intake at the end of self-administration (average infusions over last 3 days). Repeated measures two-way ANOVAs were used to analyze group differences in infusions during ContA following IntA, and locomotor responding during habituation, following saline injection, and following cocaine injection. One-way ANOVAs were used to analyze group differences in calcium event rates, calcium event rise times, and calcium event fall times. For all comparisons, α ≤ 0.05. Hedge’s g was used for effect size analysis. Mauchly’s test was used to validate the assumption of sphericity where relevant and a Greenhouse-Geisser correction was applied when the assumption was violated. Tukey or Sidak post-hoc tests were performed where appropriate. Data is graphed as mean ±SEM.
3. Results
3.1. Escalation in IntA self-administration as a marker of addiction severity
After an initial 5-day training period, rats underwent 14 days of self-administration under a ContA or IntA schedule (Fig. 1a). All rats acquired cocaine self-administration and learned to discriminate between the active and inactive levers (Fig. 1b–d). Linear regressions were performed on active lever presses over sessions for each animal (data not shown), and analysis revealed that a subset of the IntA rats significantly increased active lever presses across sessions, indicating an escalation of drug intake. Accordingly, IntA animals were then placed into two groups: IntA escalators (n = 21) and IntA non-escalators (n = 32). As expected from the linear regressions, analysis of group data found that both the ContA group and the IntA non-escalator group maintained stable levels of cocaine self-administration, as active lever presses did not change over time (one-way RM ANOVAs, ContA: F(4.149, 112.0) = 1.18, p = 0.32, Mauchly’s test p < 0.0001, eps = 0.41, Fig. 1b; IntA non-escalators: F(6.28, 194.5) = 1.37, p = 0.23, Mauchly’s test p < 0.0001, eps = 0.63, Fig. 1c). In contrast, the IntA escalator group significantly increased their active presses across self-administration sessions (one-way RM ANOVA, F(4.08, 81.52) = 23.71, p < 0.0001, Mauchly’s test p < 0.0001, eps = 0.41, Fig. 1d). A significant difference in the escalation behavior of the three groups was confirmed upon direct comparison (two-way RM ANOVA, main effect of time F(10,780) = 8.807, p < 0.0001; main effect of group F(2,78) = 58.29, p < 0.0001; effect of group × time interaction F(20,780) = 2.99, p < 0.0001). Due to the strong association between escalation of intake and addiction, we hypothesized that escalation behavior would reliably predict performance of other addiction-related behaviors and sought to validate this distinction by comparing the behavioral responses of the IntA escalators to the non-escalators and ContA groups during drug-taking, drug-seeking, and locomotor sensitization.
Figure 1. ContA and IntA paradigms produce different patterns of cocaine intake.
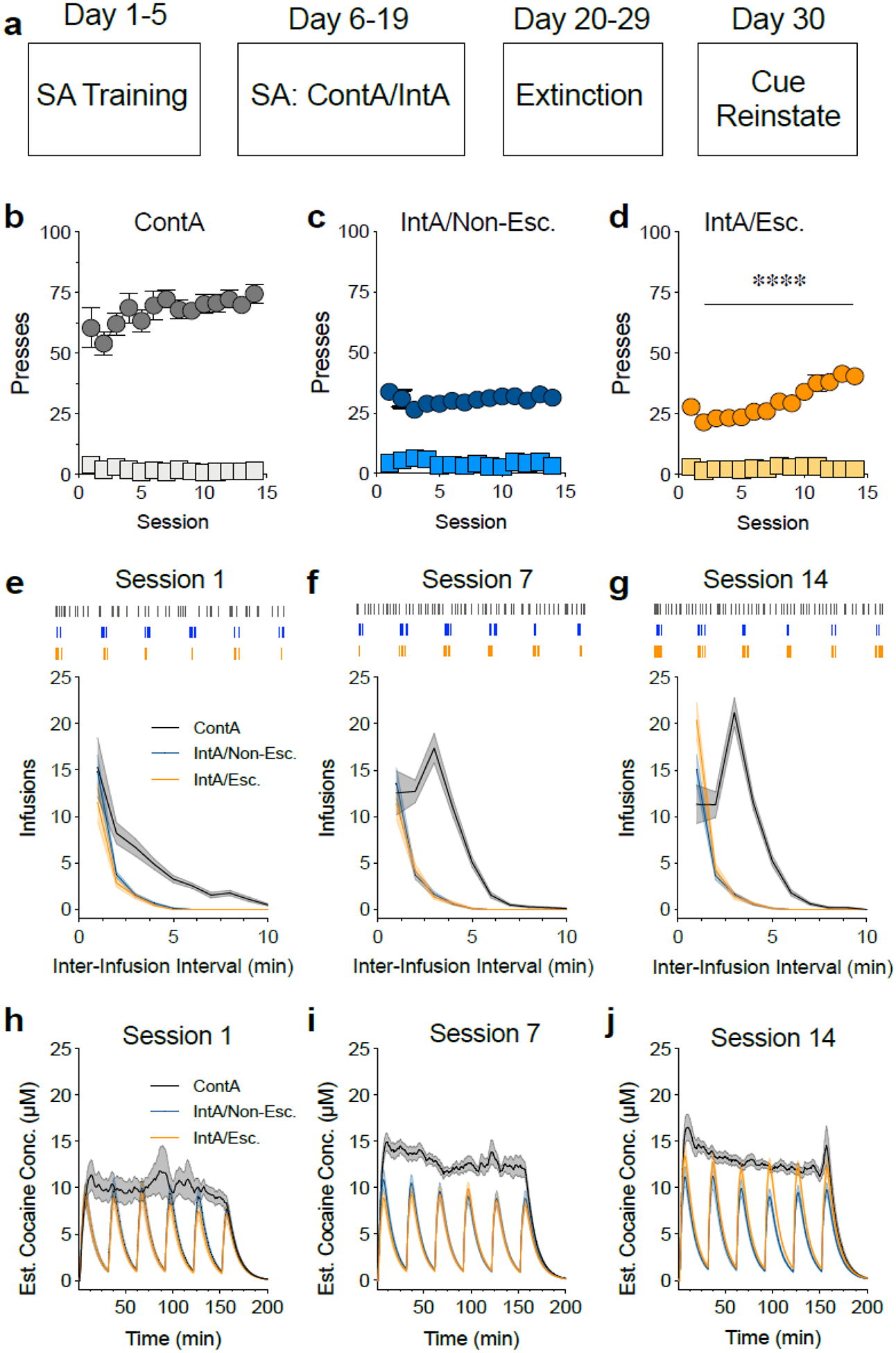
(a) Illustration of the self-administration paradigm. (b-d) Total active (circles) and inactive (squares) lever presses during self-administration training for the ContA group (b), the IntA non-escalator group (c) and the IntA escalator group (d). Although the ContA group had the greatest number of active presses, only the IntA escalator group significantly increased their drug intake across sessions (****p < 0.0001). (e-g) The total infusions earned at different inter-infusion intervals on the first (e), seventh (f) and fourteenth (g) session of self-administration. The IntA escalator group took significantly more infusions with short inter-infusion intervals compared to the IntA non-escalator group (p < 0.0001). Shaded portions represent SEM. Hash marks above the graphs indicate lever presses of representative animals. Gray symbols/lines represent the ContA group, blue symbols/lines represent the IntA non-escalator group and orange symbols/lines represent the IntA escalator group. (h-j) Estimated cocaine concentrations on the first (h), seventh (i) and fourteenth (j) session of self-administration. Cocaine concentrations were relatively stable in the ContA group following the loading phase whereas the IntA groups showed spikes in cocaine concentrations coinciding with the drug-available periods. Shaded portions represent SEM. N=21–32/group.
3.2. Escalation in IntA rats produces an altered pattern of drug consumption
A core premise of the intermittent access model is that the pattern and kinetics of drug consumption may play a meaningful role in long-term behavioral adaptations leading to addiction. We therefore hypothesized that if the IntA escalators represented a subset of animals more prone to addiction, then they may also engage in an altered pattern of consumption compared to non-escalators. Additionally, it has previously been shown that a fast pattern of self-administration can predict vulnerability to addiction (Belin et al., 2009). In order to characterize the pattern of drug consumption across groups, we examined the inter-infusion interval patterns during self-administration. The number of infusions at each inter-infusion interval for the IntA escalator and non-escalator groups were not different during session 1 (two-way RM ANOVA, no main effect of group F(1,51) = 2.39, p = 0.13; main effect of time F(19,969) = 85.57, p < 0.0001; no effect of group × time interaction F(19,969) = 1.36, p = 0.14, Fig. 1e) or session 7 (two-way RM ANOVA, no main effect of group F(1,51) = 0.48, p = 0.49; main effect of time F(19,969) = 93.88, p < 0.0001; no effect of group × time interaction F(19,969) = 0.75, p = 0.76, Fig. 1f). However, the inter-infusion intervals were significantly different on session 14, with the escalator group showing a greater number of infusions at 0–1 min inter-infusions intervals (i.e., a “burst” pattern of lever pressing) (two-way RM ANOVA, main effect of group F(1,51) = 4.26, p = 0.04; main effect of time F(19,969) = 175.4, p < 0.0001; effect of group × time interaction F(19,969) = 3.90, p < 0.0001, Fig. 1g).
The ContA group also showed a change in drug consumption pattern over time, as their inter-infusion interval was significantly different on session 14 compared to session 1 (two-way RM ANOVA, main effect of time F(19,513) = 54.68, p < 0.0001; main effect of session F(1,27) = 18.8, p = 0.0002; effect of session × time interaction F(19,513) = 19.24, p < 0.0001, Fig. 1e,g). This was due to a drug intake pattern marked by a high level of presses with short intervals between them (reflecting a loading phase) as well as a high level of presses with an intermediate interval between them (reflecting a maintenance phase). Finally, this group took significantly more drug over the course of self-administration compared to the IntA groups (escalators and non-escalators), likely due to the increased time of access (one-way ANOVA, main effect F(2,103) = 20.96, p < 0.0001, post-hoc ContA vs IntA escalators p < 0.0001, post-hoc ContA vs IntA non-escalators p < 0.0001 Fig. 4c). However, post-hoc analysis revealed no differences in drug intake between the IntA escalator and non-escalator groups (p = 0.9986). The differences in intake patterns between the ContA and IntA groups are also illustrated by examining estimated brain cocaine concentrations, with both IntA groups showing multiple peaks in cocaine concentrations compared to a steady-state level that is achieved over the course of each session with ContA (Fig. 1h–j).
Figure 4. Calcium signaling in dorsomedial striatum is altered following ContA self-administration.
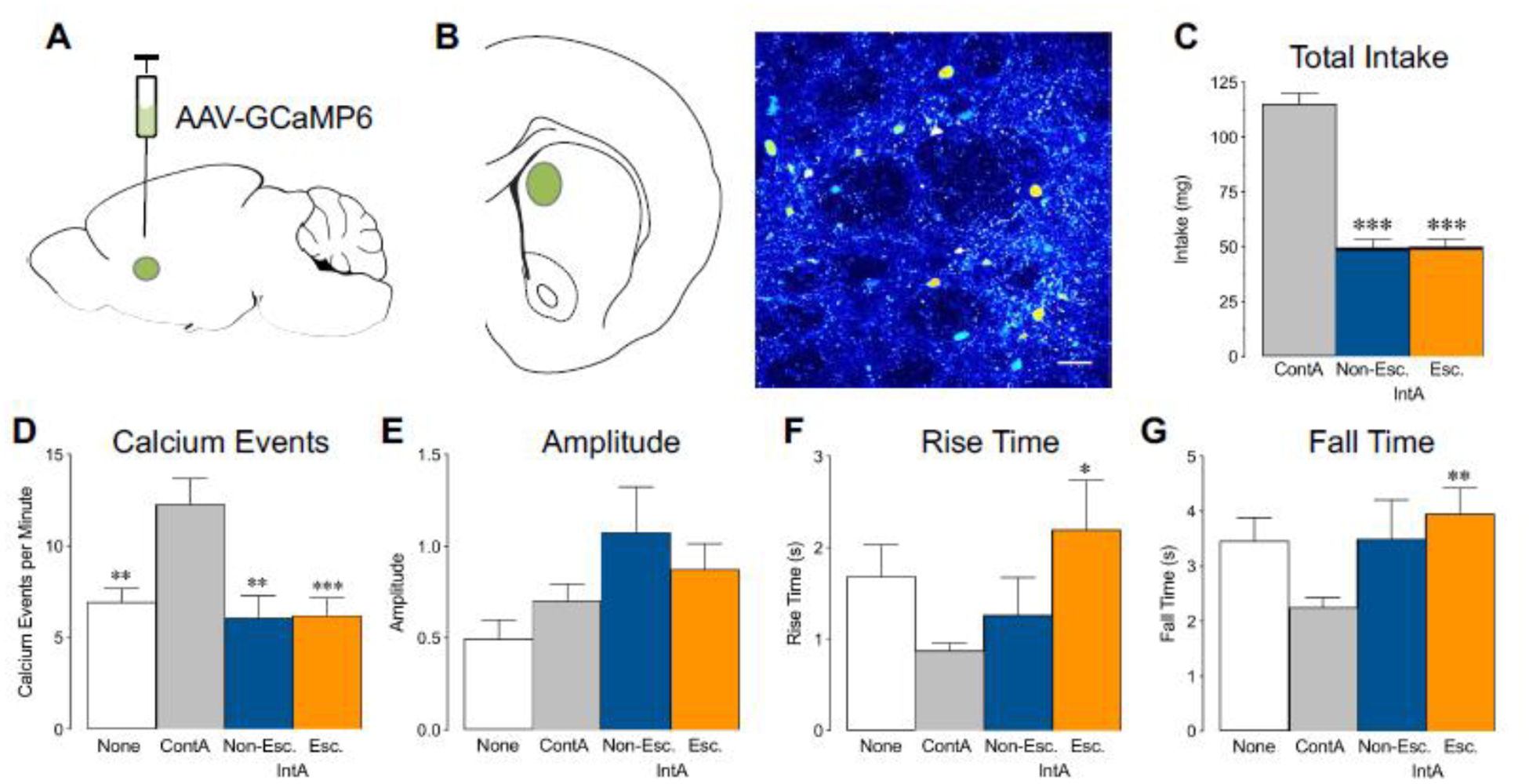
(a) Illustration of the viral vector approach. An AAV vector expressing GCaMP6m was injected into the dorsomedial striatum. (b) Representative section of GCaMP6m fluorescent expression from the area of dorsomedial striatum depicted in green. Scale bar, 20 μm. (c) Total cocaine intake during self-administration was significantly higher in the ContA group, but there were no differences in intake between the IntA groups (***p < 0.0001). (d) There were significantly more calcium events in the ContA group compared to the other groups (**p<0.01; ***p<0.001). (e-g) Although there were no group differences in amplitude (e), the ContA group had significantly shorter rise times (f) and fall times (g) of calcium events compared to the IntA escalator group (*p<0.05; **p<0.01). White bars represent the drug naïve group, gray bars represent the ContA group, blue bars represent the IntA non-escalator group and orange bars represent the IntA escalator group. N=14–34 cells/group.
3.3. IntA escalator group show higher cue-induced reinstatement of drug-seeking
Next, we predicted that a group more susceptible to addiction (IntA escalators) would be especially vulnerable to relapse following presentation of a drug-associated cue. We tested the effect of self-administration pattern (IntA vs ContA) on relapse susceptibility by examining cue-induced reinstatement of drug-seeking following extinction. During extinction training, all groups decreased responding on the active lever over sessions, and there were no differences between groups (two-way RM ANOVA, no main effect of group F(2,22) = 0.38, p = 0.69; main effect of time F(2.537,55.82) = 51.7, p < 0.0001, Mauchly’s p < 0.0001, eps = 0.28; no effect of group × time interaction F(18,198) = 0.81, p = 0.69; Fig. 2a). Although all groups increased their number of active lever presses during cue-induced reinstatement compared to extinction (two-way RM ANOVA, main effect of phase F(1,21) = 129.9, p < 0.0001, eps = 1.0; main effect of group F(2,21) = 3.75, p = 0.04; no effect of group × time interaction F(2,21) = 3.00, p = 0.07, Fig. 2b), post-hoc tests revealed that the IntA access escalator group showed significantly higher levels of reinstatement compared to both the ContA group (p = 0.04) and the IntA non-escalator group (p = 0.002). In addition, the IntA escalator group showed an increased pattern of “burst” pressing, with a significantly higher number of presses at 0–1 min inter-press intervals compared to the other groups (two-way RM ANOVA, no main effect of group F(2,21) = 3.34, p = 0.06; main effect of time F(1.037,21.78) = 122.2, p < 0.0001, Mauchly’s Test p < 0.0001, eps = 0.115; effect of group × time interaction F(18,189) = 3.32, p < 0.0001, Fig. 2c).
Figure 2. Cue-induced reinstatement is greatest in the IntA escalator group.
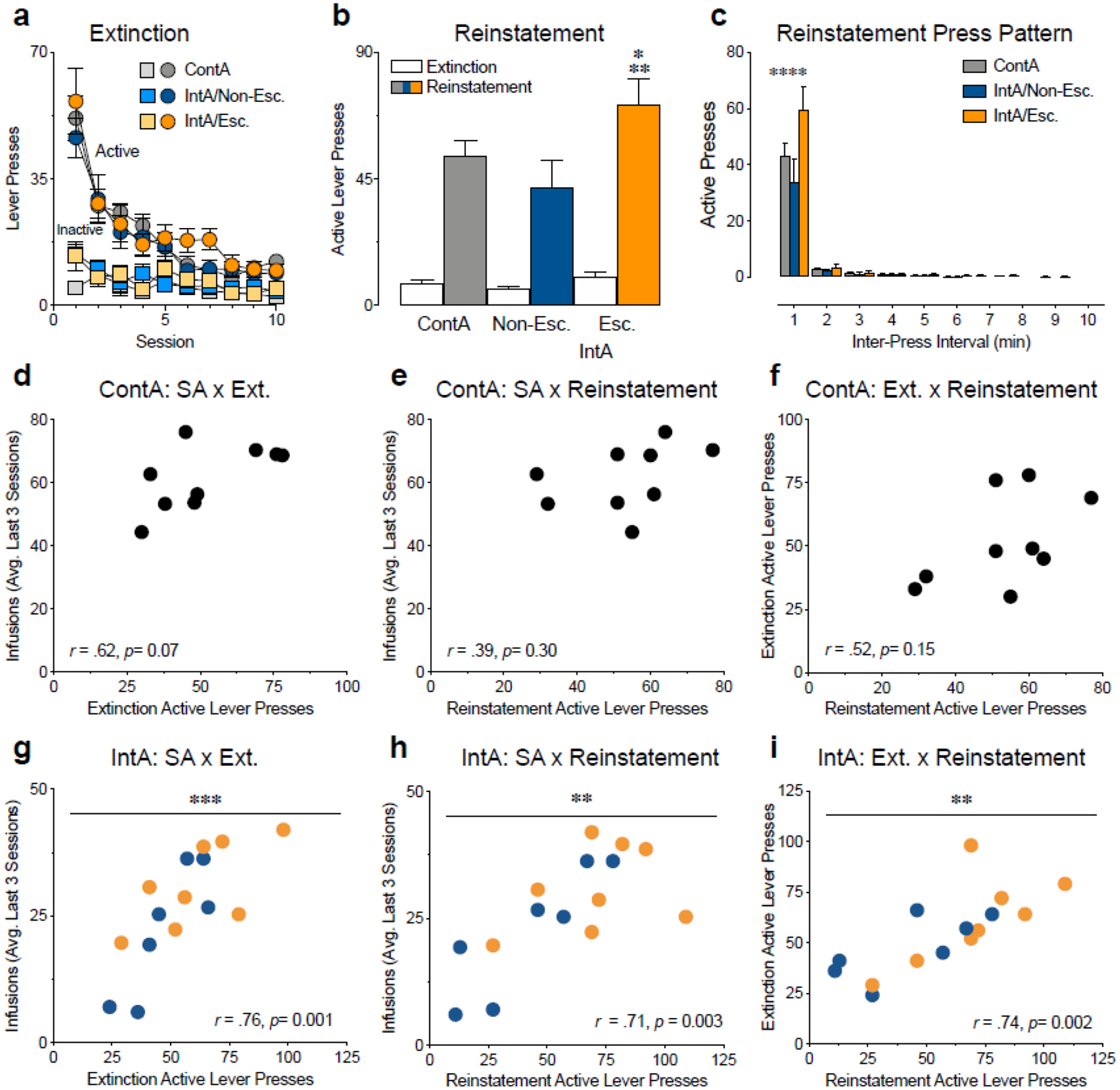
(a) Total active (circles) and inactive (squares) lever presses during extinction. All groups extinguished responding over sessions. (b) All groups showed a significant increase in the number of active lever presses made during cue-induced reinstatement compared to extinction; however, the IntA escalator group showed a greater level of cue-induced reinstatement compared to the ContA group (p = 0.04) and the IntA non-escalator group (**p = 0.002). (c) The total number of active lever presses made at different inter-press intervals during cue-induced reinstatement. The IntA escalator group made significantly more lever presses at short inter-press intervals compared to the other groups (****p < 0.0001). (d-f) There were no correlations in the ContA group between drug infusions at the end of self-administration and active lever presses on the first day of extinction (d) or active lever presses during cue-induced reinstatement (e), or between active lever presses on the first day of extinction and during reinstatement (f). (e-g) In the IntA groups, drug infusions at the end of self-administration were positively correlated to active lever presses on the first day of extinction (g, ***p = 0.001) and active lever presses during cue-induced reinstatement (h, **p = 0.01), and active lever presses on the first day of extinction were positively correlated to active lever presses during cue-induced reinstatement test (i, **p = 0.002). Gray symbols represent the ContA group, blue symbols represent the IntA non-escalator group and orange symbols represent the IntA escalator group. N=7–9/group.
3.4. Variability in behaviors related to addiction severity
When analyzed as a whole, ContA produces groups of animals with high levels of drug-taking and seeking; however, it is not clear if the behavior of the individual animals varies in a meaningful way, such that those with the highest consumption are also the most vulnerable to drug-seeking during extinction and reinstatement as might be expected of an individual with addiction. We tested whether the two self-administration paradigms were producing coherent, consistent models of addiction by examining the relatedness of these three factors. There was no correlation in rats that underwent ContA self-administration between the amount of drug taken during the last 3 days of self-administration and the number of active presses on the first day of extinction (Pearson correlation, r = 0.62, p = 0.07, Fig. 2d), or the number of active presses on the cue-induced reinstatement test (Pearson correlation, r = 0.39, p = 0.30, Fig. 2e). There was also no correlation between the number of active presses on the first day of extinction and the number of active presses on the cue-induced reinstatement test (Pearson correlation, r = 0.52, p = 0.15, Fig. 2f) in this group. In contrast, there were significant correlations between the amount of drug taken during the last 3 days of self-administration and the number of active presses on the first day of extinction (Pearson correlation, r = 0.73, p = 0.001, Fig. 2g), as well as the number of active presses on the cue-induced reinstatement test (Pearson correlation, r = 0.63, p = 0.01, Fig. 2h) in rats that underwent IntA self-administration. The number of active presses on the first day of extinction and the number of active presses on the cue-induced reinstatement test were also significantly correlated (Pearson correlation, r = 0.74, p = 0.002, Fig. 2i) in this group. These correlations provide evidence that while ContA models individual features of addiction well at a population level, the IntA paradigm more successfully produces a spread in behavior consistent with individual differences in addiction severity.
3.5. IntA facilitates the development of locomotor sensitization
Having shown that IntA self-administration produces meaningful individual variability in several aspects of addiction, including escalation of intake, consumption pattern, and drug-seeking, we next sought to determine if it would also produce a long-lasting sensitization of striatal function. We examined locomotor sensitization in groups that underwent either ContA or IntA cocaine self-administration by administering cocaine 24h prior to and two weeks following self-administration. There were no differences across groups in the number of ambulations made during habituation (two-way RM ANOVA, no main effect of group F(2,35) = 0.26, p = 0.77; main effect of test F(1,35) = 5.74, p = 0.02, eps = 1.0; no group × test interaction F(2,35) = 1.91, p = 0.16, Fig. 3b) or following an injection of saline (two-way RM ANOVA, no main effect of group F(2,35) = 0.86, p = 0.43; main effect of test F(1,35) = 19.67, p < 0.0001, eps = 1.0; no group × test interaction F(2,35) = 0.76, p = 0.48, Fig. 3c). However, there were significant differences in the development of locomotor sensitization as a function of group (two-way RM ANOVA, no main effect of group F(2,35) = 0.01, p = 0.99; main effect of test F(1,35) = 26.02, p < 0.0001, eps = 1.0; effect of group × test interaction F(2,35) = 4.06, p = 0.03, Fig. 3d). Post-hoc tests revealed that there were no differences in ambulations between Test 1 and Test 2 in the ContA group (p = 0.61), whereas there was a small but significant difference in the IntA non-escalator group (p = 0.03) and a robust difference in the IntA escalator group (p = 0.0002). Although it may appear that the IntA escalator group has an attenuated initial locomotor response to cocaine, which could support the notion of pre-existing differences between individuals, post-hoc analyses failed to detect any differences between IntA escalators and the other groups on Test 1 (IntA escalator vs non-escalator, p = 0.44, IntA escalator vs continuous, p = 0.29). These results indicate that sensitization did not develop following ContA self-administration but did develop following IntA self-administration with an especially strong response in the IntA escalator group (effect size analysis; Hedge’s g = 4.03). In addition, these findings further support the idea that escalation of intake during an IntA paradigm acts as a valid and useful marker for addiction severity.
Figure 3. Locomotor sensitization to cocaine is most robust in the IntA escalator group.
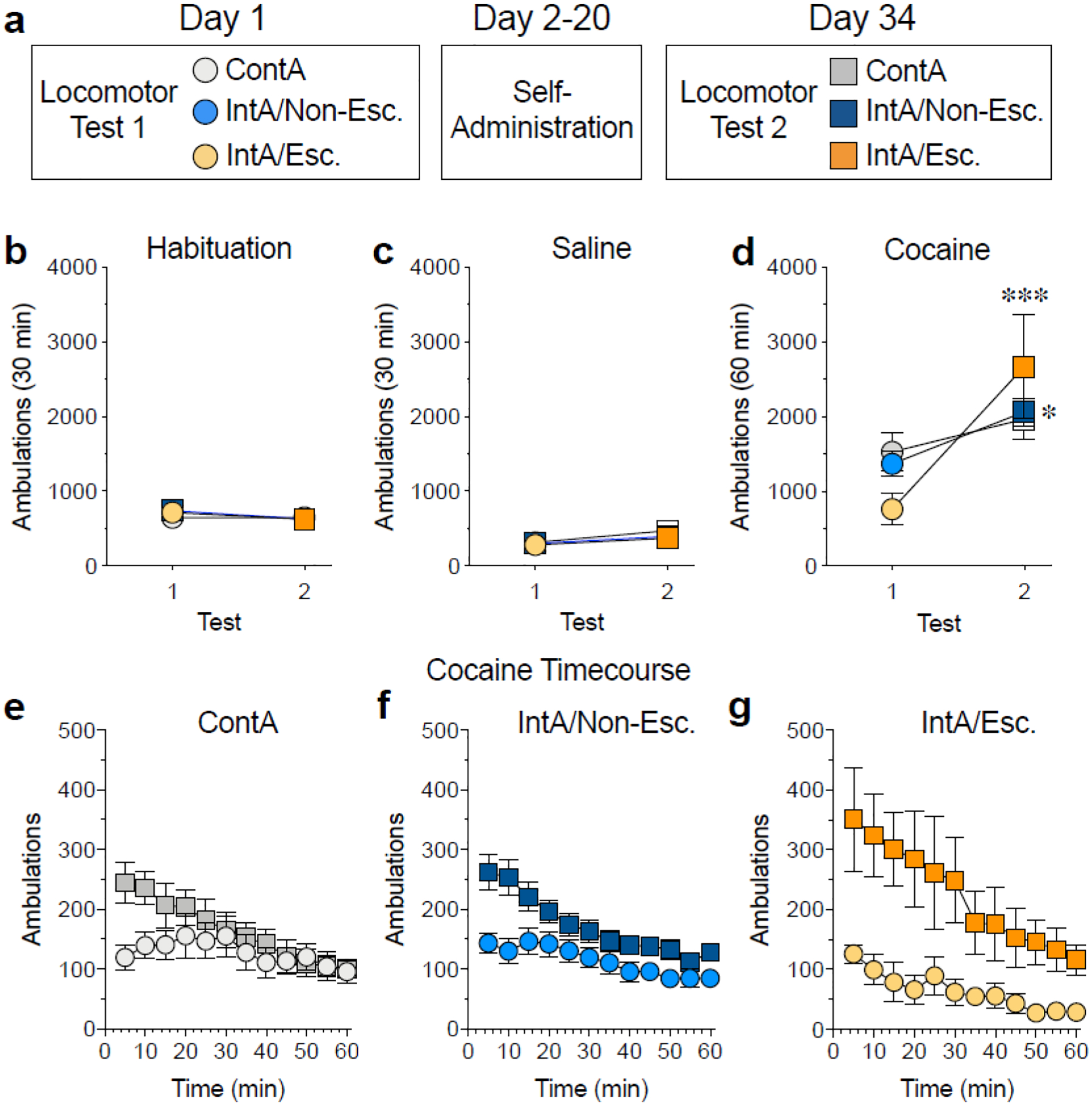
(a) Illustration of the locomotor sensitization paradigm. Rats underwent two cocaine locomotor tests, one 24h prior to self-administration and the second one 14 days after the end of self-administration. (b,c) There were no group differences in ambulations during either habituation (a) or following an injection of saline (c). (d-g) Locomotor sensitization, as measured by an increase in ambulations over the two tests, did not occur following ContA self-administration but was seen following IntA self-administration. Locomotor sensitization was most robust in the IntA escalator group (*p<0.05, ***p<0.001 vs. Test 1). Total ambulations are shown in d and the timecourse of ambulations is shown for each group in e-f. Gray symbols represent the ContA group, blue symbols represent the IntA non-escalator group and yellow/orange symbols represent the IntA escalator group. Circles represent Test 1 and squares represent Test 2. N=7–20/group.
3.6. Calcium events in dorsomedial striatum after self-administration
The DMS has been shown to play a role in several of the addiction measures studied here, including locomotor sensitization, self-administration, and craving (Kim et al., 2013; Murray et al., 2012; Caprioli et al., 2017). Cocaine self-administration also leads to a host of neural alterations in the striatum, including changes in intracellular signaling (Graham et al., 2007; Ghasemzadeh et al., 2009; Fasano & Brambilla, 2002; Hoffmann et al., 2017). We hypothesized that the IntA and ContA paradigms would produce not only behavioral differences, but also long-term basal differences in DMS signaling. Thus, we performed 2-photon calcium imaging in dorsomedial striatal slices from rats 10–14 days after the end of cocaine self-administration to determine how self-administration pattern affects baseline calcium signaling. We found that the ContA self-administration group had significantly more calcium events in the dorsomedial striatum compared to drug-naïve controls and both IntA groups (one-way ANOVA, F(3,104) = 7.33, p = 0.0002, Fig. 4b). In addition, although there were no group differences in the amplitude of calcium events (one-way ANOVA, F(3,87) = 2.51, p = 0.06, Fig. 4e), calcium events in the ContA group had significantly shorter rise times (one-way ANOVA, F(3,87) = 3.16, p = 0.03, IntA escalator vs continuous, p = 0.03, Fig. 4f) and fall times (one-way ANOVA, F(3,87) = 4.43, p = 0.006, IntA escalator vs continuous, p = 0.002, Fig. 4g) compared to the IntA escalator group. In contrast, there were no differences in calcium events, including rise and fall times, between drug-naïve controls and the IntA groups (Fig. 4d–g).
4. Discussion
Investigating individual differences in susceptibility to addiction is a major issue facing the addiction field. Here, we examined the ability of two 14-day self-administration paradigms (a ContA schedule and an IntA schedule) to elicit addiction-like patterns of behavior. We found that the IntA paradigm produced an escalation of intake across sessions that was not seen with the ContA model. Interestingly, however, only ~40% of rats that were in the IntA group showed escalation of drug intake, while the remaining ~60% maintained stable intake levels across sessions. Given that escalation of intake is a hallmark of addiction (Ahmed and Koob, 1998), we sought to assess the value of escalation as a marker for an addiction-like phenotype by its ability to predict behavioral outcomes in other critical components of addiction, including patterns of drug-seeking and consumption, susceptibility to cue-induced relapse, and locomotor sensitization.
Indeed, we found that the IntA escalator group had a more binge-like pattern of drug consumption during self-administration and a greater degree of locomotor sensitization compared to the other groups. In addition, the escalator group showed the highest levels of cue-induced reinstatement, with the most pressing at short inter-press intervals, suggesting a unique, rapid pattern of drug-seeking, as previously described (Allain & Samaha, 2019; Algallal et al., 2018). The enhanced susceptibility to cue-induced reinstatement present in the IntA escalator group indicates that these animals may have alterations in glutamatergic regions with strong projections to striatum, such as prefrontal cortex, amygdala, and ventral hippocampus, which all play a role in cue-induced reinstatement (Kalivas & McFarland, 2003; Stefanik et al., 2013; Stefanik & Kalivas, 2013; Rogers & See, 2007). Of note, total cocaine intake over the 14-day period did not differ between the IntA escalator and non-escalator groups; therefore, these behavioral differences are not simply the result of increased cocaine use. Finally, we found that drug intake at the end of self-administration, drug-seeking during the first extinction session, and cue-induced drug-seeking were all highly correlated in the IntA paradigm, with no correlation in the ContA paradigm. These data are consistent with previous work that found that IntA access self-administration leads to higher levels of motivation on a progressive ratio test compared to ContA (Zimmer et al., 2012). Our findings do not negate the value of the ContA model of self-administration in the study of individual components of drug addiction; however, they do suggest that the IntA model is more effective at producing meaningful behavioral variability allowing for the identification of a subset of animals that display a coherent, comprehensive addiction symptomology.
The escalation behavior that was seen in the IntA paradigm was characterized by enhanced drug intake during the second half of the self-administration session. This is a departure from the escalation pattern that typically occurs with extended access models, which produce escalation at the beginning of the session (Ahmed and Koob, 1998). However, the pattern of escalation seen with the IntA group may be better reflective of people with a substance use disorder, as they typically increase their drug consumption as a drug-taking session progresses (Gawin & Kleber, 1986). It is thought that this pattern is likely an attempt to counteract the rapid decrease in the physical and psychological effects that occur initially, reflective of acute tolerance (Trinkoff et al., 1990; Brower et al., 1986). However, it is also possible that the increased drug intake is due to sensitization of drug-craving systems, or a combination of both processes.
The circuits that regulate locomotor activity heavily overlap with those that mediate drug craving; accordingly, locomotor sensitization has been used as a behavioral read-out of the neural adaptations thought to underlie the heightened incentive salience and craving produced by repeated drug use in susceptible individuals (Robinson and Berridge, 1993). In the present work, we found that locomotor sensitization occurred in both IntA groups, but was more robust in the escalator group, and was not present following ContA self-administration. This data builds on recent work that reported that rats under an IntA schedule show sensitization within the self-administration sessions (Allain and Samaha, 2019; Algallal et al., 2018) by demonstrating that the sensitization that develops can persist for weeks after the end of self-administration.
The dorsomedial striatum is an important region for goal-directed actions, drug-seeking and craving, and the transition to drug addiction (Robbins & Everitt, 2002; Yin et al., 2005; Nonomura et al., 2018; Ito et al., 2002; Caprioli et al., 2017). Alterations in striatal intracellular calcium signaling have also been implicated in both drug reward and sensitization (Barr et al., 2015; Mizuno et al., 2013; Yasui and Su, 2016). Sustained, but not acute, cocaine administration leads to activation of the c-Jun N-terminal kinase (JNK) pathway in the dorsal striatum via altered calcium signaling, which may partially underlie the neurotoxic effects of psychostimulant use (Choe et al., 2011). Thus, we hypothesized that the behavioral differences in addiction severity produced by the IntA paradigm would be reflected by long-lasting basal adaptations in the dorsomedial striatum. To address this, we examined alterations in intracellular calcium signaling. Unexpectedly, we did not observe any differences in baseline calcium signaling events between the escalator and non-escalator IntA groups, nor between these groups and naïve controls. However, there were many differences in calcium signaling at baseline in the ContA group. In particular, we found that the calcium events in this group were more frequent, but had an altered waveform characterized by abbreviated rise and fall times compared to the other groups. These data indicate that the DMS is susceptible to long-term cocaine-induced changes in baseline calcium signaling; however, it requires either very high levels of drug consumption or more consistent cocaine levels, as achieved during ContA, to produce these changes. These data imply that such changes are not responsible for the behavioral differences observed between the IntA escalators and non-escalators. However, it may be that they are relevant to other behaviors that we did not compare IntA escalators and non-escalators on, but are well-modeled by the ContA paradigm. It is also possible that the increased frequency of calcium signaling in the DMS of continuous access animals produced important changes that are not as readily apparent in behavioral readouts, such as an increased susceptibility to the neurotoxic effects of psychostimulant use.
5. Conclusions
Of the many people who try drugs in their life, only a small proportion ever transition from recreational use to addiction (Anthony et al., 1994; Penberthy et al., 2010; Grant and Dawson, 1998). It seems likely that these individuals have a pre-disposed susceptibility to addiction, and it is critical that we find methods to identify and research this vulnerable population. The results from these experiments indicate that a relatively-short (i.e., 14 day) IntA model of cocaine self-administration is sufficient to produce a meaningful variance in behavioral data that can be used to probe individual differences in addiction severity. Specifically, this paradigm produces a subset of animals that escalate their drug intake across sessions, show binge-like patterns of drug-taking, and have high levels of cue-induced drug-seeking and locomotor sensitization. Importantly, these animals can be compared to a group that undergoes the equivalent drug history but does not show the same behavioral phenotype. Thus, this paradigm is likely to have significant translational and practical utility in future addiction research.
Funding Disclosure
This work was supported by grants from the National Institute on Drug Abuse (T32DA007278 to AFG and R01DA036582 to SMF). The authors declare no competing financial interests.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Ahmed SH, & Koob GF (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science, 282(5387), 298–300. [DOI] [PubMed] [Google Scholar]
- Algallal H, Allain F, Ndiaye NA, & Samaha AN (2018). Sex differences in cocaine self-administration behaviour under long access versus intermittent access conditions. Addiction biology, e12809. [DOI] [PubMed] [Google Scholar]
- Allain F, & Samaha AN (2019). Revisiting long-access versus short-access cocaine self-administration in rats: intermittent intake promotes addiction symptoms independent of session length. Addiction Biology. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner LA, & Kessler RC (1994). Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Experimental and clinical psychopharmacology, 2(3), 244. [Google Scholar]
- Barr JL, Deliu E, Brailoiu GC, Zhao P, Yan G, Abood ME, … & Brailoiu E (2015). Mechanisms of activation of nucleus accumbens neurons by cocaine via sigma-1 receptor–inositol 1, 4, 5-trisphosphate–transient receptor potential canonical channel pathways. Cell Calcium, 58(2), 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, & Deroche-Gamonet V (2009). Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biological psychiatry, 65(10), 863–868. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, & Ettenberg A (2004). The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain research, 995(1), 46–54. [DOI] [PubMed] [Google Scholar]
- Beveridge TJR, Wray P, Brewer A, Shapiro B, Mahoney JJ, & Newton TF (2012). Analyzing human cocaine use patterns to inform animal addiction model development. College on Problems of Drug Dependence, Palm Springs, CA. [Google Scholar]
- Brower KJ, Hierholzer R, & Maddahian E (1986). Recent trends in cocaine abuse in a VA psychiatric population. Psychiatric Services, 37(12), 1229–1234. [DOI] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, & Jones SR (2014). Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor stimulating effects of cocaine. Journal of neurochemistry, 128(2), 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, & Jones SR (2013). Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology, 38(12), 2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Siciliano CA, Zimmer BA, & Jones SR (2015). Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking. Neuropsychopharmacology, 40(3), 728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli D, Venniro M, Zhang M, Bossert JM, Warren BL, Hope BT, & Shaham Y (2017). Role of dorsomedial striatum neuronal ensembles in incubation of methamphetamine craving after voluntary abstinence. Journal of Neuroscience, 37(4), 1014–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright WS (2008). Economic costs of drug abuse: Financial, cost of illness, and services. Journal of Substance Abuse Treatment, 34(2), 224–233. [DOI] [PubMed] [Google Scholar]
- Choe ES, Ahn SM, Yang JH, Go BS, & Wang JQ (2011). Linking cocaine to endoplasmic reticulum in striatal neurons: role of glutamate receptors. Basal Ganglia, 1(2), 59–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE (2000) The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behavioural Brain Research 116:1–22. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Belin D, & Piazza PV (2004). Evidence for addiction-like behavior in the rat. Science, 305(5686), 1014–1017. [DOI] [PubMed] [Google Scholar]
- Fasano S, & Brambilla R (2002). Cellular mechanisms of striatum-dependent behavioral plasticity and drug addiction. Current molecular medicine, 2(7), 649 665. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, & Robinson TE (2005). Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biological psychiatry, 58(9), 751–759. [DOI] [PubMed] [Google Scholar]
- Gawin FH, & Kleber HD (1986). Abstinence symptomatology and psychiatric diagnosis in cocaine abusers: clinical observations. Archives of general psychiatry, 43(2), 107–113. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, & Mantsch JR (2009). Neuroadaptations in the cellular and postsynaptic group 1 metabotropic glutamate receptor mGluR5 and Homer proteins following extinction of cocaine self-administration. Neuroscience letters, 452(2), 167–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, & Self DW (2007). Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nature neuroscience, 10(8), 1029. [DOI] [PubMed] [Google Scholar]
- Grant BF, & Dawson DA (1998). Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. Journal of substance abuse, 10(2), 163–173. [DOI] [PubMed] [Google Scholar]
- Hoffmann HM, Crouzin N, Moreno E, Raivio N, Fuentes S, McCormick PJ, … and Vignes M (2016). Long-lasting impairment of mGluR5-activated intracellular pathways in the striatum after withdrawal of cocaine self- administration. International Journal of Neuropsychopharmacology, 20(1), 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Dalley JW, Robbins TW, & Everitt BJ (2002). Dopamine release in the dorsal striatum during cocaine-seeking behavior under the control of a drug-associated cue. Journal of Neuroscience, 22(14), 6247–6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, & McFarland K (2003). Brain circuitry and the reinstatement of cocaine seeking behavior. Psychopharmacology, 168(1–2), 44–56. [DOI] [PubMed] [Google Scholar]
- Kawa AB, Bentzley BS, & Robinson TE (2016). Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology, 233(19–20), 3587–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, & Kalivas PW (2007). Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. Journal of Pharmacology and Experimental Therapeutics, 322(3), 1103–1109. [DOI] [PubMed] [Google Scholar]
- Kim SM, Ahn SM, Go BS, Wang JQ, & Choe ES (2009). Alterations in AMPA receptor phosphorylation in the rat striatum following acute and repeated cocaine administration. Neuroscience, 163(2), 618–626. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim W, Baik JH, & Yoon BJ (2013). Different locomotor sensitization responses to repeated cocaine injections are associated with differential phosphorylation of GluA1 in the dorsomedial striatum of adult rats. Behavioural brain research, 257, 71–76. [DOI] [PubMed] [Google Scholar]
- Kim J, Ryu IS, Seo SY, & Choe ES (2015). Activation of Protein Kinases and Phosphatases Coupled to Glutamate Receptors Regulates the Phosphorylation State of DARPP32 at Threonine 75 After Repeated Exposure to Cocaine in the Rat Dorsal Striatum in a Ca2+-Dependent Manner. International Journal of Neuropsychopharmacology, 18(12), pyv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI (1997). Addiction is a brain disease, and it matters. Science, 278(5335), 45–47. [DOI] [PubMed] [Google Scholar]
- Mizuno K, Kurokawa K, & Ohkuma S (2013). Regulatory mechanisms and pathophysiological significance of IP3 receptors and ryanodine receptors in drug dependence. Journal of pharmacological sciences, 123(4), 306–311. [DOI] [PubMed] [Google Scholar]
- Murray JE, Belin D, & Everitt BJ (2012). Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology, 37(11), 2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas C, Russell TI, Pierce AF, Maldera S, Holley A, You ZB, … & Ikemoto S (2019). Incubation of cocaine craving after intermittent-access self administration: sex differences and estrous cycle. Biological psychiatry, 85(11), 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonomura S, Nishizawa K, Sakai Y, Kawaguchi Y, Kato S, Uchigashima M, … & Sano H (2018). Monitoring and updating of action selection for goal-directed behavior through the striatal direct and indirect pathways. Neuron, 99(6), 1302–1314. [DOI] [PubMed] [Google Scholar]
- Patel TP, Man K, Firestein BL, Meaney DF (2015). Automated quantification of neuronal networks and single-cell calcium dynamics using calcium imaging. Journal Neuroscience Methods 243:26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, & Robinson TE (1991). Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology, 103(4), 480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penberthy JK, Ait-Daoud N, Vaughan M, & Fanning T (2010). Review of treatment for cocaine dependence. Current drug abuse reviews, 3(1), 49–62. [DOI] [PubMed] [Google Scholar]
- Robbins TW, & Everitt BJ (1999). Drug addiction: bad habits add up. Nature, 398(6728), 567. [DOI] [PubMed] [Google Scholar]
- Robbins TW, & Everitt BJ (2002). Limbic-striatal memory systems and drug addiction. Neurobiology of learning and memory, 78(3), 625–636. [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Becker JB (1986). Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain research reviews, 11(2), 157–198. [DOI] [PubMed] [Google Scholar]
- Robinson TE, & Berridge KC (1993). The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain research reviews, 18(3), 247–291. [DOI] [PubMed] [Google Scholar]
- Rogers JL, & See RE (2007). Selective inactivation of the ventral hippocampus attenuates cue-induced and cocaine-primed reinstatement of drug-seeking in rats. Neurobiology of learning and memory, 87(4), 688–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierberl K, Giordano T, Satpute S, Hao J, Kaur G, Hofmann F, … & Rajadhyaksha A (2012). Cav1. 3 L-type Ca2+ channels mediate long-term adaptation in dopamine D2L-mediated GluA1 trafficking in the dorsal striatum following cocaine exposure. Channels, 6(1), 11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Jin S, Duan Y, Liang J, Zhang M, Jiang F, & Sui N (2018). Distinctive Changes of L-Type Calcium Channels and Dopamine Receptors in the Dorsomedial and Dorsolateral Striatum after the Expression of Habitual Cocaine Seeking Behavior in Rats. Neuroscience, 370, 139–147. [DOI] [PubMed] [Google Scholar]
- Stefanik MT, & Kalivas PW (2013). Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine seeking. Frontiers in behavioral neuroscience, 7, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Moussawi K, Kupchik YM, Smith KC, Miller RL, Huff ML, … & LaLumiere RT (2013). Optogenetic inhibition of cocaine seeking in rats. Addiction biology, 18(1), 50–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkoff AM, Ritter C, & Anthony JC (1990). The prevalence and self-reported consequences of cocaine use: an exploratory and descriptive analysis. Drug and alcohol dependence, 26(3), 217–225. [DOI] [PubMed] [Google Scholar]
- Yang JH, & Choe ES (2014). Protein kinase G regulates β-synuclein in response to repeated exposure to cocaine in the rat dorsal striatum in a Ca2+-dependent manner. Neuroscience letters, 582, 6–11. [DOI] [PubMed] [Google Scholar]
- Yasui Y, & Su TP 2016. Potential molecular mechanisms on the role of the sigma-1 receptor in the action of cocaine and methamphetamine. Journal of drug and alcohol research, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, & Balleine BW (2005). The role of the dorsomedial striatum in instrumental conditioning. European Journal of Neuroscience, 22(2), 513–523. [DOI] [PubMed] [Google Scholar]
- Zimmer BA, Dobrin CV, & Roberts DC (2011). Brain-cocaine concentrations determine the dose self-administered by rats on a novel behaviorally dependent dosing schedule. Neuropsychopharmacology 36, 2741–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer BA, Oleson EB, & Roberts DC (2012). The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology, 37(8), 1901. [DOI] [PMC free article] [PubMed] [Google Scholar]


