Abstract
Purpose
To evaluate changes in intraocular pressure (IOP) following cataract surgery among patients with or without glaucoma, using automated extraction of data from electronic health records (EHR).
Design
Retrospective cohort study.
Participants
Adults who underwent cataract surgery without another concurrent eye surgery at a single academic center from 2009–2018.
Methods
Patient information was identified from procedure and billing codes, demographic tables, medication orders, clinical notes, and eye examination fields captured in the EHR. A previously validated natural language processing pipeline was utilized to identify laterality of cataract surgery from operative notes and laterality of eye medications from medication orders. Cox proportional hazards modeling evaluated factors associated with sustained postoperative IOP reduction through 14 months or last visit while taking the same or fewer glaucoma medications.
Main Outcome Measures
Sustained post-cataract surgery IOP reduction, measured at 14 months or last follow-up while using equal or fewer glaucoma medications compared to baseline, and without additional glaucoma laser or surgery on the operative eye.
Results
7574 eyes of 4883 patients underwent cataract surgery with median follow-up of 244 days. The mean preoperative baseline IOP for all patients was 15.2 mmHg (SD 3.4), which decreased to 14.20mmHg (SD 3.0) at 12 months postoperatively. Patients with IOP ≥21.0 mmHg had mean postoperative IOP reduction ranging from −6.2 to −6.9 mmHg. Cataract surgery was more likely to yield sustained IOP reduction for patients with primary open angle glaucoma (HR 1.19, 95%CI 1.05–1.36) or narrow angles/angle closure (HR 1.21, 95%CI 1.08–1.34) compared to patients without glaucoma. Those with a higher baseline IOP were more likely to achieve sustained postoperative IOP reduction (HR 1.06 per one mmHg increase in baseline IOP, 95%CI 1.05–1.07).
Conclusions
Our results suggest that patients with primary open angle glaucoma or narrow angles/chronic angle closure were more likely to achieve sustained IOP reduction after cataract surgery. Patients with higher baseline IOP had increasingly higher odds of achieving reduction in IOP. This evidence demonstrates the potential utility of a pipeline for automated extraction of ophthalmic surgical outcomes from EHR to answer key clinical questions on a large scale.
Introduction
Cataract extraction has been shown to decrease intraocular pressure (IOP) in patients with narrow angles, as well as in patients with open angles and ocular hypertension.1 Among patients with narrow angles, the IOP-lowering effect of cataract surgery is at least in part thought to be related to widening of the angle due to removal of the crystalline lens.2,3 In patients with open angles, changes in anterior segment anatomy due to cataract surgery may also contribute to lower IOP.2–4 Some have also suggested that the IOP-lowering effect may be related to phacoemulsification energy5 and related effects on the trabecular meshwork.6
Large studies of IOP-lowering in patients with diverse preoperative diagnoses are generally limited to data collected from registries, such as the Swedish National Cataract Register, which has reported on follow-up data collected from a single postoperative visit,7 or an electronic health records registry in the UK, which was also limited to data from a single postoperative visit and did not distinguish between different glaucoma subtypes or include information on the use of glaucoma medications.8 Such granular ata for multiple follow-up visits over long periods of time are relatively time-consuming to collect, making it difficult to perform large prospective or even retrospective chart review studies of IOP changes after surgery. Furthermore, administrative databases such as insurance billing claims are limited due to lack of surgical laterality and clinical information they include. Randomized controlled trials have also provided evidence for IOP-lowering in patients with open angle glaucoma,9–11 or ocular hypertension,12 but these studies by design do not have patients with diverse preoperative diagnoses and are subject to strict trial protocols, medication washout regimes, and inclusion/exclusion criteria, which make it difficult to infer the effects of cataract surgery on IOP in the real-world setting. To better understand the effect of cataract surgery on IOP across a broad range of patients, more granular data are needed.
We created a text-processing pipeline to automatically extract surgical laterality using free-text cataract surgery operative notes in electronic health records (EHR), thus enabling accurate evaluation of postoperative clinical measures in the operative eye.13 In this investigation, we used our developed system for automated EHR data extraction to evaluate change in intraocular pressure (IOP) after cataract surgery in a large cohort of patients, comparing IOP outcomes among those with versus without baseline glaucoma.
Methods
Data Source and Population
We have developed a relational database that captures data from a hospital EHR system, an EPIC system installed in 2008 (Epic Systems, Verona, WI). The database contains structured data, including patient demographics, diagnosis and procedure codes, and medication orders (mapped to RxNorm14); eye examination findings (semi-structured data); and clinical narrative text (unstructured data).15
We identified cataract surgeries which occurred at Stanford University Medical Center between 8/25/2009 and 1/04/2018 using Current Procedural Terminology (CPT16) codes 66982 or 66984. Cataract surgeries performed with additional concurrent eye surgeries were identified by existence of CPT codes for other ophthalmic surgeries on the same day; these surgeries were excluded. Laterality of surgery was determined from the free-text operative notes as previously described by a validated text-processing algorithm13,17; if laterality was indeterminate or the operative note was missing (N=379), then those surgeries were excluded. Surgeries without at least one preoperative visit with identifiable intraocular pressure of the surgical eye were excluded (N=94).
The Stanford Institutional Review Board approved the study.
Measures
Available measures included demographics such as patient age, sex, and race/ethnicity. Patients’ glaucoma-related diagnoses were determined by ICD-9 and ICD-10 (International Classification of Diseases, 9th and 10th editions billing codes18 associated with the surgical encounter or clinical encounters on or before surgery. If there was more than one glaucoma-related diagnosis, they were prioritized in the following order: narrow or closed angles (365.2-, 365.02, 365.06, H402-, H400.3, H400.6), secondary glaucomas (365.03, 365.3–365.6-, 365.81–3, 365.13–365.14, H400.4, H404-H406-, H408.1-H408.3-, H401.3, H401.4, Q150), primary open angle glaucoma (365.10–365.12, 365.15, 365.89, 365.9, H401.0-H401.2-, H401.5-), open angle suspect / ocular hypertension (365.00, 365.01, 365.04–365.05, H400.0-H400.2-, H400.5-).
Main Outcome
The main outcome was reduction in IOP after cataract surgery, sustained through 14 months or last follow-up while using the same or reduced glaucoma medications compared to baseline, and without further glaucoma laser or surgery on the operative eye. Baseline and postoperative IOP were automatically extracted from semi-structured fields within the EHR. In the case of multiple IOP measurements in a given day, the first applanation measurement was included; where no applanation was documented, the first non-applanation measurement was included. IOPs were excluded if measured after any further glaucoma procedures on the operative eye (which occurred in N=61 eyes), as identified by CPT codes (0191T,0192T,0253T, 0376T, 0449T, 0474T, 65820, 65850, 65855, 66160,66170, 66172,66174, 66175,66179, 66180, 66183, 66710, 66711). Glaucoma medications and their laterality were determined from medication orders and automated text-processing of associated medication signatures available within the EHR, as previously described.13 This text-processing algorithm recognizes right, left, and bilateral laterality from eye medication signatures (e.g. “Take 1 drop in the right eye twice daily”) and had 100% agreement with manual validation on a random sample of medication signatures.13 The number of glaucoma medications was totaled at baseline and for each postoperative visit date. Combination medications (e.g., dorzolamide-timolol, brimonidine-timolol) were considered as two medications. IOPs were excluded if measured after patients received additional glaucoma medications compared to baseline (N=704 eyes).
Manual Validation of Glaucoma Diagnoses and Medications
Validation of glaucoma diagnosis categories derived from billing codes was performed by a manual review of a random sample of charts from 1000 cataract surgeries, including 500 with no glaucoma-related billing code and 500 with a glaucoma-related billing code. The glaucoma diagnosis category for the preoperative eye was determined and accuracy compared to the diagnosis category determined from billing codes. Validation of medication information was undertaken by manual review of the same random sample. Baseline number of medications was determined by review of preoperative free-text notes by two independent reviewers. The outcome of whether the patient was on the same or fewer glaucoma medications compared to baseline in the operative eye postoperatively was determined by review of postoperative free-text notes by two independent reviewers. Interrater agreement was calculated using the kappa statistic.
Statistical Analyses
Descriptive statistics were performed using means and standard deviations for continuous measures and counts and percentages for categorical measures. Mean IOP was evaluated at baseline and at postoperative months 1 (day 28, range 21–56 days), 3 (day 84, range 70–112 days), 6 (day 180, range 150–210 days), and 12 (day 365, range 300–420 days). As the timing of visits could vary in individual follow-up, a range of postoperative days were considered for each of the prespecified postoperative visits with the IOP at the visit closest to that time point being chosen. The proportion of patients with lower IOP was also assessed for each postoperative time point.
Cox proportional hazards models were constructed to identify possible predictors associated with the main outcome, reduction in IOP after cataract surgery sustained through 14 months or last follow-up while using the same or fewer glaucoma medications, and without further glaucoma procedural intervention. Covariates included in the Cox models included age, sex, race/ethnicity, glaucoma diagnosis type, and baseline IOP. Patients who had a transient decrease in IOP which was not sustained through last follow-up or through 14 months (if follow-up was longer) were considered not to have achieved the outcome. Patients whose IOP increased after cataract surgery were considered not to have achieved the outcome. Patients who underwent additional glaucoma procedural interventions were censored at that time point. Model standard errors accounted for within-patient clustering when patients contributed to the population multiple times due to having undergone cataract surgery in both eyes. Analyses were performed with Stata version 12 and p-value less than 0.05 was considered statistically significant.
Results
Population Characteristics
A total of 4883 unique patients undergoing 7574 eligible cataract surgeries were included. The mean age at first surgery was 70.2 (SD 11.7) years. A total of 21.3% (N=1042) of patients had a glaucoma-related diagnosis at baseline, including 5.1% (N=259) with POAG and 5.3% (N=259) with narrow or closed angle (Table 1). The mean baseline preoperative intraocular pressure was 15.2 mmHg (SD 3.4) and 4.8% (N=361) were taking one glaucoma medication at baseline, while 4.6% (N=350) were taking 2 or more glaucoma medications at baseline. Median follow-up after cataract surgery was 244 days, with an interquartile range (IQR) of 38–871 days, 182 days (IQR 34–782) for eyes with glaucoma-related diagnosis, and 494 days (IQR 110–1162) for eyes without a glaucoma-related diagnosis.
Table 1.
Population characteristics of patients who underwent cataract surgery
| Total population N=4883 | |||
|---|---|---|---|
| Mean | SD | ||
| Age at first surgery (years) | 70.2 | 11.7 | |
| N | % | ||
| Female | 2734 | 56.0% | |
| Glaucoma Diagnosis | Total | 1042 | 21.3% |
| Narrow or Closed Angle | 259 | 5.3% | |
| Primary Open Angle Glaucoma | 248 | 5.1% | |
| Secondary Glaucomas | 123 | 2.5% | |
| Open Angle Glaucoma Suspect / Ocular Hypertension | 412 | 8.4% | |
| Race/Ethnicity | |||
| White | 2246 | 46.0% | |
| Black | 139 | 2.8% | |
| Asian or Pacific Islander | 1223 | 25.0% | |
| Hispanic/Latino | 646 | 13.2% | |
| Other/Unknown | 629 | 12.9% | |
SD = Standard Deviation.
Manual Validation of Glaucoma Diagnoses and Medications
Among those presumed to have no glaucoma based on billing codes, 2.8% (N=14) had a documented glaucoma-related diagnosis, of which half (N=7) were a glaucoma suspect or ocular hypertension diagnosis. Among those with a glaucoma-related billing code, 7.4% (N=37) had a discrepancy between billing code and free-text note documentation, including 2.6% (N=13) with no evident glaucoma-related diagnosis in the notes and 4.8% (N=24) with a different glaucoma-related diagnosis category documented in the notes. At baseline, prior to surgery, only 15 cases (1.5%) had a different number of glaucoma medications in the eye that underwent surgery by manual chart review, and only 4 cases (0.4%) were using more glaucoma medications than at baseline when previously recorded to be the same or fewer number of glaucoma medications than baseline, or vice versa. Kappa for interrater agreement was 0.9552 for diagnoses, 0.9112 for number of medications.
IOP lowering after cataract surgery, by glaucoma diagnosis
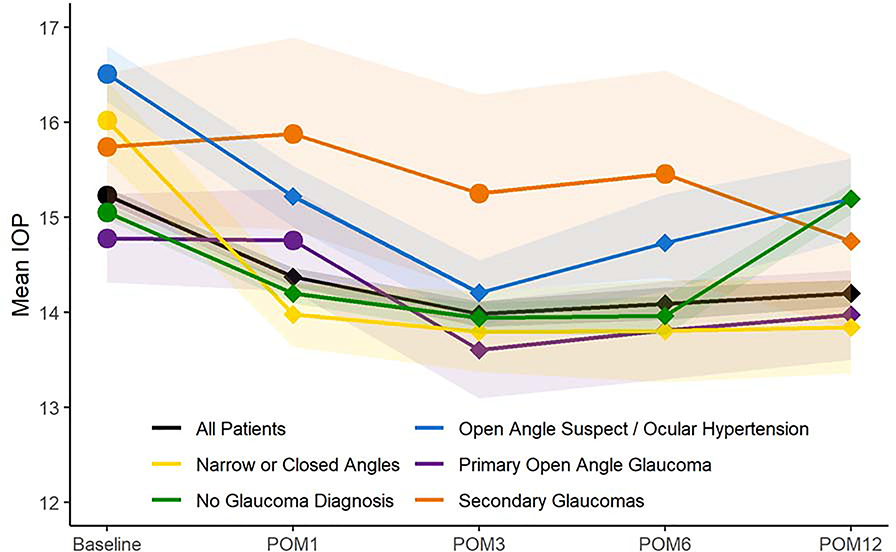
The mean IOP at baseline for all patients was 15.2 mmHg (SD 3.4) which decreased to 14.4 mmHg (SD 3.3) at one month, 14.0 mmHg (SD 3.2) at 3 months, 14.1 mmHg (SD 3.4) at 6 months, and 14.2 mmHg (SD 3.0) at 12 months (P<0.0001 for all postoperative time points compared to baseline, paired t-tests). Figure 1 depicts the mean intraocular pressure at baseline and postoperative months 1, 3, 6, and 12 for patients with different subtypes of glaucoma-related diagnoses, or no glaucoma-related diagnosis. Across all glaucoma patients, there was a mean decline in IOP from baseline which was statistically significant at all time points (P<0.05, paired t-tests), except for among patients with secondary glaucomas (only statistically significant at 12 months postoperatively) and among patients with POAG (statistically significant at 3, 6, and 12 months but not at 1 month postoperatively).
Figure 1. Mean Intraocular Pressure Before and After Cataract Surgery by Glaucoma-Related Diagnosis.
Legend: The figure depicts the mean intraocular pressure at baseline before cataract surgery, and at postoperative months 1, 3, 6, and 12 for all patients and stratified by glaucoma-related diagnosis. Diamond-shaped markers at postoperative time points indicate a statistically significant change in mean IOP compared to baseline for that group (P<0.05, paired t-tests). Bands indicate 95% confidence intervals.
POM = postoperative month. IOP = intraocular pressure.
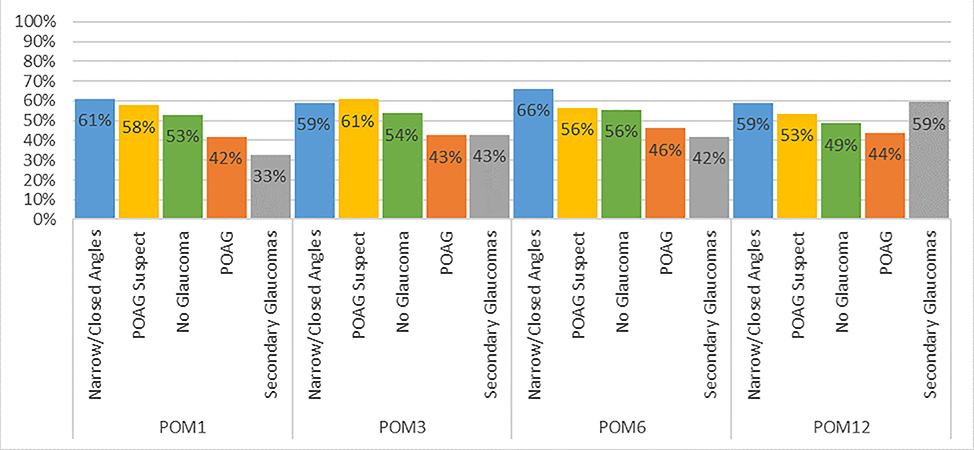
Figure 2 depicts the percentage of patients with each glaucoma subtype achieving lower postoperative IOP using the same or fewer glaucoma medications at postoperative months 1, 3, 6, and 12. Across all time points, 33–66% of patients achieved this outcome, with a generally higher percentage (59–66%) of patients with narrow or closed angles achieving this outcome (P<0.05, chi-squared tests for all time points).
Figure 2. Percent of Patients with Lower Intraocular Pressure on the Same or Fewer Glaucoma Medications After Cataract Surgery.
Legend: The figure depicts percentage of patients with each glaucoma-related diagnosis who had lower intraocular pressure after cataract surgery at postoperative months 1, 4, 6, and 12, while using the same or fewer glaucoma medications.
POM = postoperative month.
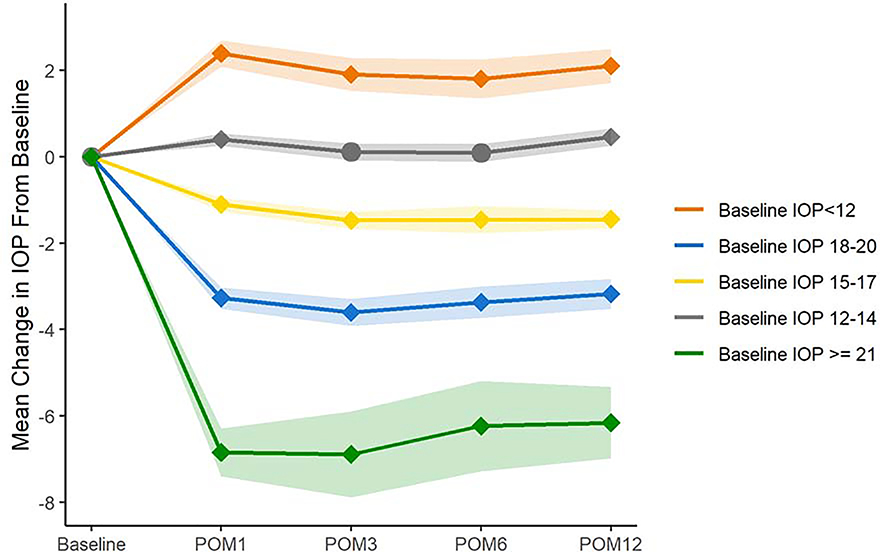
IOP lowering after cataract surgery, by baseline IOP
We also investigated the changes in IOP after cataract surgery, stratified by baseline preoperative intraocular pressure, divided into 5 groups: baseline IOP <12, 12–14, 15–17, 18–20, ≥21mmHg. Figure 3 depicts the mean change in IOP from baseline for patients in each of these groups and shows that those with higher baseline IOP had greater mean reduction in IOP after cataract surgery. Notably, those with baseline IOP ≥21 mmHg had mean postoperative IOP reduction ranging from −6.2 to −6.9 mmHg. For all groups except for those with baseline IOP 12–14 mmHg, the change in IOP from baseline was statistically significant at all time points (P<0.0001, paired t-test). For patients with baseline IOP 12–14 mmHg, IOPs at postoperative months 3 and 6 were not statistically significantly different from baseline.
Figure 3. Mean Change in Intraocular Pressure After Cataract Surgery Stratified by Baseline Intraocular Pressure.
Legend: The figure depicts the mean change in intraocular pressure after cataract surgery at postoperative months 1, 3, 6, and 12, Patients were stratified by baseline IOP. Diamond-shaped markers indicate a statistically significant difference in mean IOP compared to baseline for that group (P<0.0001, paired t-tests). Bands indicate 95% confidence intervals.
POM = postoperative month. IOP = intraocular pressure.
Cox Proportional Hazards Modeling
Multivariable Cox proportional hazards modeling investigated factors associated with sustained reduction of IOP through 14 months or last follow-up after cataract surgery, while using the same or fewer glaucoma medications and without further glaucoma procedural intervention(s). Compared to patients with no glaucoma diagnosis, increased hazard ratios for achieving reduction of IOP after cataract surgery were seen for those with POAG (HR 1.19, 95% CI 1.05–1.36) and for those with narrow or closed angles (HR 1.20, 95% CI 1.08–1.34). Baseline IOP was a strong predictor of postoperative IOP reduction; for every 1 mmHg increase in the baseline IOP, there was a 6% increase in the hazard for achieving reduction in IOP (HR 1.06, 95% CI 1.05–1.07).
Discussion
In this population-based study using automated extraction of clinical data from electronic health records, we identified over 7500 cataract surgeries in a single academic center between 2009–2018. We found that patients with primary open angle glaucoma or narrow/closed angles were more likely to achieve sustained IOP reduction after cataract surgery while using the same or fewer glaucoma medications and without further glaucoma laser or surgery, compared to those without a glaucoma-related diagnosis. Furthermore, we found that IOP reduction after cataract surgery varied based on preoperative IOP; patients with higher baseline IOP measurements had greater postoperative reduction in IOP. This study fills an important gap in the literature, by examining large-scale real-world evidence of IOP reduction after cataract surgery in a variety of glaucoma subtypes.
IOP lowering after cataract surgery in patients with narrow angles is well-documented and is thought to be related to widening of the angle after removal of the lens.1 Reassuringly, our analysis based on automated extraction of thousands of IOP measurements from the electronic health records also found that those with narrow angles averaged significantly lower IOP after cataract surgery, with approximately 60% of patients achieving lower IOPs through the first year postoperatively, with an overall mean decrease of 2.05–2.23 mmHg postoperatively. Longitudinal regression analyses also confirmed that those with narrow angles had higher odds of a sustained decrease in IOP after cataract surgery than those without documented glaucoma diagnosis or narrow angles.
IOP lowering after cataract surgery in patients with open-angle glaucoma has been documented,7,19–24 but the extent of IOP lowering has been variable and sometimes considered insignificant. The mechanism for IOP reduction in open angle glaucoma is not fully elucidated, but it is hypothesized that cataract surgery may lower the IOP by affecting the trabecular meshwork, ciliary body, or anterior segment anatomy.1 We found that those with POAG in our study also had significant IOP reduction after cataract surgery, though smaller magnitude than patients with narrow/closed angles. This is similar to IOP decreases in open angle glaucoma patients reported in previous studies, on the order of 1–2 mmHg.7,19–24 However, these previous studies also suggested that less than half of open angle glaucoma patients achieved lower postoperative IOP after cataract surgery. Nevertheless, in our multivariable analysis, those with POAG were significantly more likely to achieve sustained lowering of IOP after cataract surgery without additional medications. More recent studies of IOP lowering after cataract surgery in patients with open angle glaucoma have been in the context of randomized controlled trials comparing minimally invasive glaucoma surgeries against cataract surgery alone, such as trials for iStent,10 Cypass,11 or Hydrus.9 In these cases the magnitude of the IOP decrease is typically greater, up to around 5–6 mmHg, but are measured with medication washout, so that the average baseline IOP of 24–25 mmHg is much higher than in our study (14.8 mmHg among POAG patients, medicated per usual care).
We found that baseline IOP strongly affected magnitude of IOP reduction after cataract surgery. For those patients in our study with the highest level of preoperative IOPs (≥21 mmHg), we also found that postoperative IOP fell on average by over 6 mmHg, which is similar in magnitude to the minimally invasive glaucoma surgery trials. In contrast, patients with baseline IOPs 12–14 mmHg saw very little if any clinically significant IOP lowering on average. While regression to the mean may contribute to these findings, this dependence on baseline IOP has also been reported in the literature7, including OHTS12, which had a rigorous study design and protocol designed to minimize the effects of regression to the mean.25 Another study which measured the IOP of fellow nonoperative eyes found no difference in their IOPs after cataract surgery, suggesting that the IOP-lowering effect of surgery on the operative eyes was not simply due to an unusual outlying measurement at baseline.26
The overall significance and strengths of our study include the unique usage of a validated automated text-processing pipeline for electronic health records to determine laterality of surgery and associated clinical findings to answer key clinical questions. Working with a large sample size drawn directly from electronic health records allowed us to work directly with clinical information (such as IOP, surgical laterality) which would be unavailable in other large datasets such as insurance claims. Furthermore, extracting a large volume of clinical measurements directly from the electronic health records minimizes the time-consuming chart review of multiple patient visits for thousands of patients, reducing reviewer transcription error and increasing the feasibility of recording more accurate results, as all patient visits may be included rather than only a selection.
This work has important limitations that need to be considered when interpreting the results. Reflective of the local patient population, the study population had a high proportion of Asian patients and patients with narrow or closed angles, which may not reflect a more general US population. Our study was performed in the setting of an academic tertiary care center with many glaucoma providers, resulting in a high proportion of cataract patients with glaucoma-related diagnoses, which may not be as reflective of the general population of patients undergoing cataract surgery. In addition, another limitation is that some patients could have had glaucoma laser or incisional surgery done by an outside provider while continuing to have follow-up with their cataract surgeon. However, we might expect this to mitigate the differences reported in IOP between those with and without glaucoma rather than accentuate them. Due to sparsity in individual billing codes, we grouped billing codes together in groups of related diagnoses, including glaucoma suspects and ocular hypertensives, despite potential differences between these populations. However, our model does include baseline IOP and can be expected to control for elevated baseline IOP. EHRs themselves can have data-entry errors, such as from outdated medication lists, previous information carried forward, miscoding, and so on.27 However, key ophthalmic examination findings (such as IOP) would be expected to be updated, verified, and to have higher accuracy at each visit since they are an important element of clinical decision making, especially for patients with glaucoma. Limitations such as miscoding also exist in other types of large datasets such as insurance claims, but the advantage of EHR data is that the full medical record is available for reference, which we have utilized for validation. Future work can target the understanding of unstructured data in free-text clinical notes to corroborate structured data fields within the EHR and to identify additional information about patients not available in the structured billing codes, such as prior surgical history. In addition, our automated clinical information extraction pipeline was developed based on cases from a single academic center. Future work will also focus on expanding and validating these tools for wider use.
In conclusion, in this large observational study of over 7500 cataract surgeries, those with POAG or narrow or closed angles were more likely to achieve sustained IOP reduction after cataract surgery than patients without a glaucoma-related diagnosis. The effect of cataract surgery on IOP was significantly impacted by baseline IOP. These results demonstrate the potential utility of a pipeline for automated extraction of ophthalmic clinical information to investigate key clinical questions on a large scale. Further research is needed to elucidate the mechanism by which cataract surgery achieves IOP-lowering in patients with open angle glaucoma.
Table 2.
Clinical characteristics associated with cataract surgeries
| Total surgeries N=7574 | |||
|---|---|---|---|
| Median | IQR | ||
| Follow-up (days) | 244 | 38–871 | |
| Mean | SD | ||
| Baseline IOP | 15.2 | 3.4 | |
| N | % | ||
| Eye Laterality | Right | 3813 | 50.3% |
| Left | 3761 | 49.7% | |
| Baseline number of ocular antihypertensive medications | 0 | 6863 | 90.6% |
| 1 | 361 | 4.8% | |
| ≥2 | 350 | 4.6% | |
IQR = interquartile range. SD = standard deviation.
Table 3.
Cox proportional hazards model for sustained reduction of intraocular pressure after cataract surgery while using the same or fewer ocular antihypertensive medications
| Hazard Ratio | 95% CI | P-value | |
|---|---|---|---|
| Age (per year increase) | 1.00 | (0.99–1.00) | 0.310 |
| Female (Reference=Male) | 1.00 | (0.95–1.06) | 0.857 |
| Glaucoma diagnosis (Reference=no glaucoma) | |||
| Primary Open Angle Glaucoma | 1.19 | (1.05–1.36) | 0.008 |
| Narrow or Closed Angle | 1.20 | (1.08–1.34) | 0.001 |
| Secondary Glaucomas | 1.15 | (0.94–1.41) | 0.176 |
| Open Angle Glaucoma Suspect or Ocular Hypertension | 1.04 | (0.95–1.13) | 0.434 |
| Race/Ethnicity (Reference=White) | |||
| Black | 1.17 | (1.02–1.34) | 0.023 |
| Asian or Pacific Islander | 1.05 | (0.98–1.12) | 0.112 |
| Hispanic/Latino | 1.08 | (1.00–1.17) | 0.046 |
| Other/Unknown | 0.98 | (0.90–1.06) | 0.568 |
| Baseline IOP (per 1-point increase) | 1.06 | (1.05–1.07) | <0.001 |
CI = Confidence Interval. IOP = Intraocular Pressure.
Acknowledgments
Financial support: National Institute on Aging R03-AG056453 (SP); departmental support from Research to Prevent Blindness and National Eye Institute P30–026877 (SP and SYW); National Library of Medicine T15 LM 007033 (SYW); Stanford Medical Scholars Research Program (ADA)
The sponsor or funding organization had no role in the design or conduct of this research.
Footnotes
Conflict of Interest: No conflicting relationship exists for any author.
Meeting Presentations: American Academy of Ophthalmology Annual Meeting, 2018, Chicago; American Glaucoma Society Annul Meeting, 2019, San Francisco
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Masis Solano M, Lin SC. Cataract, phacoemulsification and intraocular pressure: Is the anterior segment anatomy the missing piece of the puzzle? Progress in retinal and eye research. 2018. [DOI] [PubMed] [Google Scholar]
- 2.Perez CI, Chansangpetch S, Nguyen A, et al. How to Predict Intraocular Pressure Reduction after Cataract Surgery? A Prospective Study. Current eye research. 2019;44(6):623–631. [DOI] [PubMed] [Google Scholar]
- 3.Huang G, Gonzalez E, Peng P-H, et al. Anterior chamber depth, iridocorneal angle width, and intraocular pressure changes after phacoemulsification: narrow vs open iridocorneal angles. Archives of ophthalmology (Chicago, Ill. : 1960). 2011;129(10):1283–1290. [DOI] [PubMed] [Google Scholar]
- 4.Hsia YC, Moghimi S, Coh P, Chen R, Masis M, Lin SC. Anterior segment parameters as predictors of intraocular pressure reduction after phacoemulsification in eyes with open-angle glaucoma. Journal of cataract and refractive surgery. 2017;43(7):879–885. [DOI] [PubMed] [Google Scholar]
- 5.DeVience E, Chaudhry S, Saeedi OJ. Effect of intraoperative factors on IOP reduction after phacoemulsification. International ophthalmology. 2017;37(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang N, Chintala SK, Fini ME, Schuman JS. Ultrasound Activates the TM ELAM-1/IL-1/NF-κB Response: A Potential Mechanism for Intraocular Pressure Reduction after Phacoemulsification. Investigative ophthalmology & visual science. 2003;44(5):1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zetterström C, Behndig A, Kugelberg M, Montan P, Lundström M. Changes in intraocular pressure after cataract surgery: analysis of the Swedish National Cataract Register Data. Journal of cataract and refractive surgery. 2015;41(8):1725–1729. [DOI] [PubMed] [Google Scholar]
- 8.Leal I, Chu CJ, Yang YY, Manasses DM, Sebastian RT, Sparrow JM. Intraocular Pressure Reduction After Real-world Cataract Surgery. Journal of glaucoma. 2020. [DOI] [PubMed] [Google Scholar]
- 9.Jones J, Koch DD, Vold S, et al. Results from the United States cohort of the HORIZON trial of a Schlemm canal microstent to reduce intraocular pressure in primary open-angle glaucoma. Journal of cataract and refractive surgery. 2019;45(9):1305–1315. [DOI] [PubMed] [Google Scholar]
- 10.Samuelson TW, Sarkisian SR, Lubeck DM, et al. Prospective, Randomized, Controlled Pivotal Trial of an Ab Interno Implanted Trabecular Micro-Bypass in Primary Open-Angle Glaucoma and Cataract: Two-Year Results. Ophthalmology. 2019;126(6):811–821. [DOI] [PubMed] [Google Scholar]
- 11.Vold S, Ahmed IIK, Craven ER, et al. Two-Year COMPASS Trial Results: Supraciliary Microstenting with Phacoemulsification in Patients with Open-Angle Glaucoma and Cataracts. Ophthalmology. 2016;123(10):2103–2112. [DOI] [PubMed] [Google Scholar]
- 12.Mansberger SL, Gordon MO, Jampel H, et al. Reduction in intraocular pressure after cataract extraction: the Ocular Hypertension Treatment Study. Ophthalmology. 2012;119(9):1826–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang SY, Pershing S, Hernandez-Boussard T. Automated Extraction of Ophthalmic Surgery Outcomes from the Electronic Health Record. International journal of medical informatics. 2019. [DOI] [PubMed] [Google Scholar]
- 14.RxNorm: META2017AB Full Update 2018_03_05. Bethesda, MD: National Library of Medicine. [Google Scholar]
- 15.Seneviratne MG, Seto T, Blayney DW, Brooks JD, Hernandez-Boussard T. Architecture and Implementation of a Clinical Research Data Warehouse for Prostate Cancer. EGEMS (Washington, DC). 2018;6(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Medical Association. CPT® (Current Procedural Terminology). 2017. Available at: https://www.ama-assn.org/practice-management/cpt-current-procedural-terminology. Accessed December 14, 2017.
- 17.eyelovedata/opnotefunctions: v1.0.1: Zenodo; 2019.
- 18.World Health Organization. International Classification of Diseases. 2016. Available at: http://www.who.int/classifications/icd/en/. Accessed December 14, 2017.
- 19.Kim WJ, Kim JM, Kim KN, Kim CS. Effect of Preoperative Factor on Intraocular Pressure after Phacoemulsification in Primary Open-angle Glaucoma and Primary Angle-closure Glaucoma. Korean journal of ophthalmology : KJO. 2019;33(4):303–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pohjalainen T, Vesti E, Uusitalo RJ, Laatikainen L. Phacoemulsification and intraocular lens implantation in eyes with open-angle glaucoma. Acta ophthalmologica Scandinavica. 2001;79(3):313–316. [DOI] [PubMed] [Google Scholar]
- 21.Shah AA, Ling J, Nathan NR, et al. Long-term intraocular pressure changes after femtosecond laser-assisted cataract surgery in healthy eyes and glaucomatous eyes. Journal of cataract and refractive surgery. 2019;45(2):181–187. [DOI] [PubMed] [Google Scholar]
- 22.Yoo C, Amoozgar B, Yang K-S, Park J-H, Lin SC. Glaucoma severity and intraocular pressure reduction after cataract surgery in eyes with medically controlled glaucoma. Medicine. 2018;97(42):e12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Majstruk L, Leray B, Bouillot A, et al. Long term effect of phacoemulsification on intraocular pressure in patients with medically controlled primary open-angle glaucoma. BMC ophthalmology. 2019;19(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arthur SN, Cantor LB, WuDunn D, et al. Efficacy, safety, and survival rates of IOP-lowering effect of phacoemulsification alone or combined with canaloplasty in glaucoma patients. Journal of glaucoma. 2014;23(5):316–320. [DOI] [PubMed] [Google Scholar]
- 25.Gordon MO, Kass MA. The Ocular Hypertension Treatment Study: design and baseline description o fthe participants. Archives of ophthalmology (Chicago, Ill. : 1960). 1999;117(5):573–583. [DOI] [PubMed] [Google Scholar]
- 26.Yang HS, Lee J, Choi S. Ocular biometric parameters associated with intraocular pressure reduction after cataract surgery in normal eyes. American journal of ophthalmology. 2013;156(1):89–94.e1. [DOI] [PubMed] [Google Scholar]
- 27.Hersh WR, Weiner MG, Embi PJ, et al. Caveats for the use of operational electronic health record data in comparative effectiveness research. Medical care. 2013;51(8 Suppl 3):S30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]