Abstract
Sleep disturbance and decreased daytime activity are well-described among people with chronic heart failure (HF) who suffer from disabling daytime symptoms and poor function. Alterations in the circadian rhythmicity of rest-activity may also be associated with these outcomes. However, little is known about the associations between rest-activity rhythms (RARS), symptoms and functional performance or the extent to which they are explained by sleep characteristics among people with HF. The purpose of this study is to evaluate parametric and non-parametric circadian characteristics of RARs and the associations between these variables, daytime symptoms, and functional performance among patients with stable heart failure (HF). We recruited adults with stable HF from HF disease management programs. Participants wore wrist actigraphs for 3 d, completed one night of unattended polysomnography and the Six Minute Walk Test, and reported daytime symptoms and physical function. We performed cosinor, non-parametric, and spectral analyses to evaluate the rest-activity rhythms and computed bivariate correlations between the rest-activity rhythm, demographics, daytime symptoms, and functional performance. We conducted multiple regression analysis to examine how RARs contribute to daytime symptoms and functional performance after controlling for insomnia and covariates. The sample included 135 participants [Mean age = 60.6 (16.1) y, n = 88 (65.2%) male]. Older age, greater comorbidity, and poorer New York Heart Association (NYHA) Class, and more EEG arousals were associated with greater intra-daily variability of the RAR. More robust rhythmicity represented by the circadian quotient was associated with better NYHA class and less sleep fragmentation. A higher circadian quotient was significantly associated with lower fatigue, depression, and sleepiness and better functional performance after controlling for insomnia and clinical and demographic characteristics. Circadian parameters of rest-activity are associated with symptoms and functional performance among people with HF independent of insomnia or sleep disordered breathing. Interventions targeted at improving the stability and strength of rest-activity rhythms may improve symptom and functional outcomes for these patients.
Keywords: circadian rest-activity rhythm, sleep, heart failure, cosinor analysis, fatigue, insomnia
Introduction
People with chronic heart failure (HF), a group of over 26 million people worldwide (Savarese and Lund 2017), experience high symptom burden, functional disability, poor quality of life, morbidity and mortality, and excessive health costs. Despite advances in drug and device therapy, people with HF continue to experience symptoms, low levels of daytime activity, and poor functional performance, and there is a critical need to understand factors contributing to these outcomes, ways to monitor them, and interventions to improve them. Chronic sleep disturbance, insomnia, and sleep-disordered breathing are common among HF patients (Redeker and Stein 2006; Redeker et al. 2010a), and contribute to poor quality of life, functional performance, and daytime symptoms (Redeker et al. 2010b; Jeon and Redeker 2016; Brostrom et al. 2004). Although sleep characteristics are closely linked with both circadian rhythmicity and homeostatic processes, little is known about daily RARs or their associations with sleep, symptoms, or daytime function among HF patients.
RARs reflect both endogenous circadian rhythms (e.g., melatonin rhythm), and exogenous circadian entrainment (e.g., 24 h light-darkness cycle). Desynchronized RARs, as demonstrated by the MESOR (24 h times series mean) and amplitude (magnitude of 24 h variation explained by rhythm), are associated with fatigue, depressive symptom, frailty, and impairment in physical and cognitive performance (Berger et al. 1999, 2012; Liu et al. 2013; Costanzo et al. 2016; Manousakis et al. 2018; Carvalho-Bos et al. 2007; Maekawa & Kume 2019), while robust RARs are associated with better health outcomes and longer survival time in cancer patients (Mormont et al. 2000, 2002a, 2002b; Innominato et al. 2009; Levi F et al. 2014; Tranah et al. 2011; Sultan et al. 2017; Cash et al. 2018).
Non-parametric measures of RARs add valuable insight beyond the contributions of circadian rhythmicity because they do not make assumptions about the robustness of the rhythms, which may be less pronounced in older adults. Decrements in nonparametric characteristics of RARs (i.e., increased intra-daily variability and decreased inter-daily stability) measured by wrist actigraph recordings, but not sleep measures, predicted death among older adults in the Rotterdam study (Zuurbier et al. 2015). More regular RARs, with higher inter-daily stability and lower intra-daily variability, were observed in older individuals and females (Mitchell et al. 2017).
Although numerous studies have addressed the contributions of RARs in a variety of populations, as noted above, little is known about RARs among people with HF, a group at high risk for poor sleep, fragmented and low levels of activity, high levels of symptom burden, and poor functional performance. Notably, a group of 20 HF patients had significantly less robust RARs manifested by lower MESOR and amplitude than those of a healthy control group (Liebzeit et al. 2017). However, the associations between these characteristics and symptoms and functional performance were not evaluated. The purposes of this study, a secondary analysis of data from our larger study (Redeker et al. 2010a, 2010b), were to evaluate parametric and non-parametric circadian characteristics of RARs and the associations between these variables and clinical and demographic patient characteristics, sleep characteristics, daytime symptoms, and functional performance among patients with stable HF.
Methods
Design.
The study employed a cross-sectional design. We obtained human subjects’ approval, and all participants provided written informed consent. We previously reported details regarding the setting, participant recruitment, variables, and measures (Redeker et al. 2010a, 2010b, 2012). Details are summarized below as pertinent to this report.
Participants.
We recruited 173 stable HF patients from HF disease management programs in the Northeastern United States between 2003 and 2007. Included participants were ≥18 ys of age and had stable chronic New York Heart Association Functional Classification (NYHA) I - IV HF with either reduced or preserved ejection fraction, based on echocardiographic measurements of left ventricular ejection fraction (LVEF) (Redeker et al. 2010a, 2010b). The current report includes 135 participants who had 3 d of actigraph data, polysomnography (PSG), and symptom, and functional performance measures available for analysis.
Procedures.
Participants completed sleep and symptom questionnaires and wrist actigraphy. They completed one night of unattended polysomnography (PSG) and a Six Minute Walk Test (6MWT) in the clinical setting.
We evaluated insomnia (difficulty initiating or maintaining sleep, or waking too early in the morning, DIMS) with 3 items from the Sleep Habits Questionnaire developed for the Sleep Heart Health Study (Baldwin et al. 2001). The three items were rated on a 5-point Likert scale which represents as “never” to “almost always.” Based on the previous study (Baldwin et al. 2001), the presence of insomnia symptoms was identified when patients responded often or almost always on one or more of the three items. We dichotomized for individuals who rated with a score of ≥3 on at least one item as experiencing insomnia.
We collected 4 d of wrist actigraphy on each participant with the Actiwatch-64 actigraph (Minimitter Inc., Bend OR) in one-minute epochs. Participants were instructed to wear the actigraph continuously for 4 d with 1 night overlapping with the night of polysomnographic recording, depress the event marker at “lights out” and “lights on” times, and remove it only for bathing. Only the 3 nights that did not occur on the night of the PSG recording were used for the current study. Full details of the actigraph sleep scoring were previously reported (Jeon et al. 2019). Data was collected in 1 min epochs, and we computed total sleep time (TST), sleep efficiency (SE), sleep-onset latency (SOL), and the fragmentation index with the Actiware Sleep V5 program (Respironics Mini Mitter, Inc., Bend, OR) with the medium sensitivity setting. The sleep parameters for each night were averaged for the analysis. A review by the American Academy of Sleep Medicine (AASM) Task Force concluded that actigraph recordings are valid for identifying circadian disorders, sleep disturbance, and predicting melatonin and body temperature rhythms (Sack et al. 2007), and circadian and other temporal rhythms are discernable in the time series data available through actigraphy.
We obtained one night of unattended PSG in participants’ homes. A sleep technologist visited each patient in his/her home, applied the sensors and equipment, and returned in the morning to retrieve it. Standard methods were used to score the sleep data, including score and rescore reliability. Variables used in this study include TST, SOL, percent of rapid eyeball movement (REM) sleep over TST, electroencephalogram (EEG) arousals, and sleep-disordered breathing (respiratory disturbance index – RDI, oxygen desaturation). Full details of the PSG analyses have been reported (Redeker et al. 2010a).
Daytime symptoms.
Fatigue, depressive symptoms, and sleepiness were evaluated with the Multi-Dimensional Assessment of Fatigue Scale (MAF) (Belza 1990), the Center for Epidemiology Studies Depression (CES-D) (Radloff 1977, 1986, 1991), and the Epworth Sleepiness Scale (ESS) respectively (Johns 1991, 1992). We calculated “sensory fatigue” by averaging responses on items for degree, severity, and distress in the MAF (Belza 1990). The psychometric properties of these scales in our study were previously reported (Redeker et al. 2010b).
Functional performance was evaluated with an objective (Six Minute Walk Test) (Lipkin et al. 1986) and a self-report measure (physical function scale of the Medical Outcomes Study Short Form-36) (Ware 1992, 1993, 1994). Physical functioning showed excellent validity to measure physical health with a correlation of 0.85 and a reliability with Cronbach’s alpha >0.80 (Ware 1994).
We used the Charlson Comorbidity Index (Charlson et al. 1987, 1994) to measure comorbidity and the NYHA Classification (New York Heart Association 1964) as a measure of the severity of HF. Data for these indices were obtained from medical records.
Rest-activity rhythms.
We exported the time series activity data from the wrist actigraphy. To maintain a consistent start time for evaluation of rhythms, activity data before midnight of the initial date were truncated. The RAR for each participant was estimated using the regression model for a single-component cosinor model as:
Where Mi, Ai, and φi are the MESOR (Midline Estimating Statistic of the Rhythm: rhythm-adjusted average activity count), the amplitude (a measure of the half range of the predicted variation of a cycle), and the acrophase (time of the high peak of the cycle) for participant i respectively. For the 24 h cycle of the rhythm, based on the average activity counts per 10 min, the period (τ) is 144 (6/h × 24 h) hours. Those parameters were estimated with a least squares approach (Cornelissen 2014).
We calculated the timing of the peak activity (Ø) with the equation, . Although the strength of circadian rhythms has been measured in several ways, including the autocorrelation of activity counts around 24 h, the ratio of nighttime activity to daytime activity, the circadian quotient, and the rhythm quotient (Ancoli-Israel et al. 1997a, 1997b; Levin et al. 2005; Grutsch et al. 2011), we used the circadian quotient, the ratio of the amplitude to the MESOR. The circadian quotient provides a normalized amplitude compared to the individual average activity level (i.e. MESOR) that accounts for daytime as well as nighttime activity: thus, it allows comparison of the strength of circadian rhythm between patients with different activity levels.
Inter-daily stability (IS) and intra-daily variability (IV).
We calculated the inter-daily stability and intra-daily variability with nonparametric methods (Witting et al. 1990) using hourly total activity counts (24 data points per day). The IS ranges from 0 to 1, and is closer to 1 for stronger adherence to a circadian rhythm over consecutive days with consistent daily rest-activity rhythms. The IV quantifies fragmentation of the circadian rhythm within a 24 h cycle and transitions between rest and activity (Zuurbier et al. 2015). Higher IV represents a weaker rest-activity rhythm and is close to two for Gaussian noise. On the other hand, the IV would be close to zero for a strong rest-activity rhythm.
Statistical Analysis.
The periodicity of the RAR was assessed with individual periodograms obtained by PROC SPECTRA in SAS version 9.4. The periodogram, which represents spectral energy, is the maximum at the suggested period of the cycle for the RAR. We assessed the distribution of periods with a maximum periodogram (i.e., maximum spectral energy) and tested whether the mean period equaled 24 h. We developed a SAS macro to estimate the parameters of the cosinor model and the nonparametric metrics of the RARS for each individual using the general linear model (GLM) in SAS as the statistical procedure. We computed bivariate correlations between the circadian parameters (i.e., MESOR, amplitude, acrophase, circadian quotient, IS, and IV) and the demographic and clinical characteristics (i.e., age, Charlson Comorbid Index, NYHA Classification, and left ventricular ejection fraction[LVEF]), daytime symptoms, and functional performance. The additional contributions of the circadian parameters (i.e., circadian quotient, inter-daily stability, intra-daily variability) to the variance in daytime symptoms (sensory fatigue, sleepiness, depression) and functional performance (6MWT distance and physical function) were examined. To handle type I error inflation due to multiple tests with the correlations, we calculated the false discovery rate (FDR) using PROC MULTTEST, SAS version 9.3 (Glickman et al. 2014).
We controlled for insomnia symptoms and clinical and demographic covariates in the GLM to examine the unique contributions of the RAR based on our previous finding that insomnia was independently associated with daytime symptoms and functional performance in this sample (Redeker et al. 2010b). We created a parsimonious model using the previously controlled covariates (Redeker et al. 2010b), including age, gender, BMI, Charlson comorbidity index, RDI, and the percent of time at oxygen saturation < 90% as the base-model for each of the daytime symptoms and functional performance and selected the models with a conventional stepwise approach. The log-transformed circadian quotient and the presence of insomnia symptom (Yes vs. No) were added to each of the selected base-models. To compare to the predictable potential of the circadian quotient, the same GLM approach was repeated for the IS, IV, and PSG sleep efficiency replacing the circadian quotient.
Results
Table 1 shows the descriptive statistics for the demographic and clinical characteristics of the sample and sleep characteristics, symptoms, and functional performance. The sample included 135 participants [(Mean age = 60.6 SD = 16.1 ys; N = 88 (65.2%) male]. The majority were in class II or III of the New York Heart Association Functional Classification (87%) and had LVEF <45 (78%). The average comorbidity index was 2.4 (SD = 1.5), and half of the participants had insomnia. More than half of the participants had hypertension (57%), and some anti-hypertensive medications, including ACE Inhibitors (54.5%), angiotension receptor blockers (31.8%), and beta alpha blockers (59.1%), were used for hypertension or heart failure.
Table 1.
Demographic and Clinical Characteristics of the Sample (N=135)
| Mean (SD) / N (%) | |
|---|---|
| Demographic Variables | |
| Age (years) | |
| Gender | 60.6 (16.1) |
| Male | 88 (65.2%) |
| Female | 47 (34.8%) |
| Race | |
| White | 87 (64.4%) |
| Minority | 48 (35.6%) |
| Clinical Variables | |
| LVEF (%) | 33.2 (15.0) |
| LVEF<45 | 100 (78.1%) |
| NYHA classification | |
| I | 5 (3.7) |
| II | 78 (57.8%) |
| III | 41 (30.4%) |
| IV | 11 (8.1%) |
| Body Mass Index | 30.6 (8.4) |
| Charlson Comorbidity Index | 2.4 (1.5) |
| Hypertension | 77 (57.0%) |
| Anti-hypertensives | |
| ACE Inhibitors | 72 (54.5%) |
| Angiotension Receptor Blockers | 42 (31.8%) |
| Beta Alpha Blockers | 78 (59.1%) |
| Sleep Habits Questionnaire | |
| Insomnia (DIMS) | |
| Yes | 67 (49.6%) |
| Actigraphic Sleep Characteristics* | |
| Total Sleep Time (min) | 378.9 (87.5) |
| Sleep Onset Latency (min) | 29.3 (38.4) |
| Sleep Efficiency (%) | 76.9 (12.8) |
| Sleep Fragmentation index | 41.6 (21.3) |
| PSG Sleep Characteristics | |
| Total Sleep time (min) | 319.6 (94.2) |
| Sleep Latency (min) | 29.1 (32.0) |
| Sleep Efficiency (%) | 71.1 (15.3) |
| % Sleep Stage 1 over TST | 28.3 (13.4) |
| % Sleep Stage 2 over TST | 51.2 (11.1) |
| % Sleep Stage 3&4 over TST | 6.6 (7.4) |
| % REM Sleep over TST | 13.9 (6.8) |
| % Time at O2 saturation <90% | 12.2 (19.0) |
| Respiratory Disturbance Index (RDI) | 23.8 (17.9) |
| EEG Arousal index | 22.1 (9.9) |
| Daytime Symptoms | |
| Sensory Fatigue (MAF) | 5.0 (2.5) |
| Sleepiness (Epworth Scale) | 7.9 (4.4) |
| Depression (CES-D) | 16.2 (10.5) |
| Functional Performance | |
| Six-Minute Walk | 1007 (436) |
| Physical Function (SF-36) | 22.7 (2.2) |
Note.
Actigraph sleep characteristics are averaged scores of observed variables during 3 nights; PSG = Polysomnography (single night) ; LVEF = left ventricular ejection fraction: NYHA = New York Heart Association Functional Classification; TST = total sleep time; MAF = Multi-dimensional assessment of fatigue scale; CESD = Centers for the Epidemiological Study of Depression Scale
Sleep duration, measured with actigraphy and polysomnography, was shorter than the recommended sleep duration of 7–8 h for adults (Hirshkowitz et al. 2015; Watson et al. 2015), and ranged from 319 to 379 min. The average sleep efficiency was poor, being 76.9% and 71.1% by for actigraph and PSG assessments, respectively. The average RDI was 23.8 (SD =17.9). Approximately half of the participants had insomnia, as in our larger sample (Redeker et al. 2010b).
The means of suggested periods with a maximum spectral energy are 24.7 h (95% CI=22.6 – 26.8) and 27.3 h (95% CI=24.3 – 30.2) in the participants without and with insomnia, respectively, but they are not statistically different (p=.15) Figure 1 displays the distributions of periods with a maximum spectral energy by insomnia status and shows most RARs follow the periodicity of 24 h.
Figure 1.
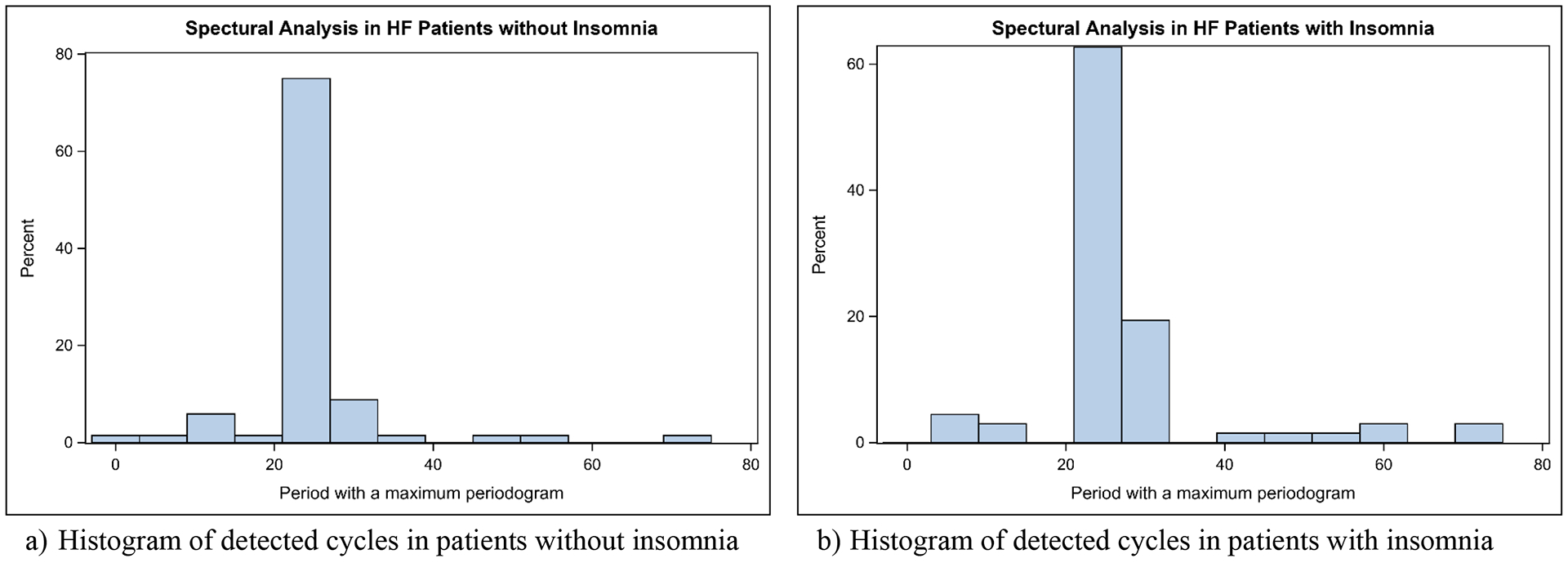
Distributions of Periods with a Maximum Periodogram by Insomnia Status
The MESOR was 141.6 (SD = 81.2), and the acrophase was approximately 15:00h. We calculated the average amplitude (M = 110.8; SD = 78.5) and the circadian quotient (0.75: SD = 0.21) (Table 2). The means of the inter-daily stability (IS) and intra-daily variability (IV) were 0.63 (SD = 0.14) and 0.93 (SD = 0.35), respectively. Participants with insomnia symptoms had statistically lower IS compared to those without insomnia (p=.0344), but there were no differences in the IV between people with and without insomnia. All of the other measures were not statistically different between participants with and without insomnia
Table 2.
Descriptive Statistics for the Circadian Rhythm Variables Computed from Wrist Actigraphy
| Variables | All subjects (N=135) | Insomnia (DIMS) | |
|---|---|---|---|
| Yes (N=67) | No (N=68) | ||
| Mean (SD), [1st quartile – 3rd quartile] | |||
| Cosinor Model | |||
| MESOR | 141.6 (81.2), [83.7, 182.1] | 150.2 (78.8), [91.8, 182.7] | 133.0 (83.1), [70.7, 180.1] |
| Amplitude | 110.8 (78.5), [54.0, 153.0] | 113.9 (74.0), [62.0, 154.0] | 107.7 (83.1), [50.5, 144.0] |
| Acrophase | 14.8 (2.0), [13.6, 15.8] | 15.0 (1.6), [13.5, 16.1] | 14.7 (2.2), [13.7, 15.7] |
| Circadian Quotient | 0.75 (0.21), [0.61, 0.92] | 0.73 (0.20), [0.60, 0.90] | 0.65 (0.14), [0.56, 0.76] |
| Nonparametric measures of variability | |||
| Interdaily Stability (IS)* | 0.63 (0.14), [0.54, 0.74] | 0.60 (0.14), [0.51, 0.69] | 0.65 (0.14), [0.56, 0.76] |
| Intradaily Variability (IV) | 0.93 (0.35), [0.67, 1.14] | 0.93 (0.33), [0.67, 1.14] | 0.94 (0.37), [0.67, 1.12] |
Note.
indicates significantly difference between insomnia and no insomnia at 5% significance level.
Figure 2 provides examples of estimates of the parametric (cosinor analysis) and nonparametric (IS & IV) RAR measures selected to illustrate the variability in the RARs. Case I had frequent activity over the day and nighttime, which yielded a weak circadian quotient of 0.26, low IS (0.34) and high IV (1.47). Case II had a high level of activity during the nighttime that produced a low circadian quotient of 0.47, with better IS (0.61) and IV (0.71). Cases I and II had poor actigraph-recorded sleep efficiency of 67.1% and 74.8%, respectively. Cases III and IV demonstrated robust circadian rhythms with circadian quotients of 0.87 and 0.97, but Case IV had a lower IV (0.41). The acrophases for Cases II and IV were ~13:00h (earlier than the overall sample mean), and they had sleep efficiency of 80.7% and 87.4%, respectively. Only Case III had insomnia symptoms.
Figure 2.
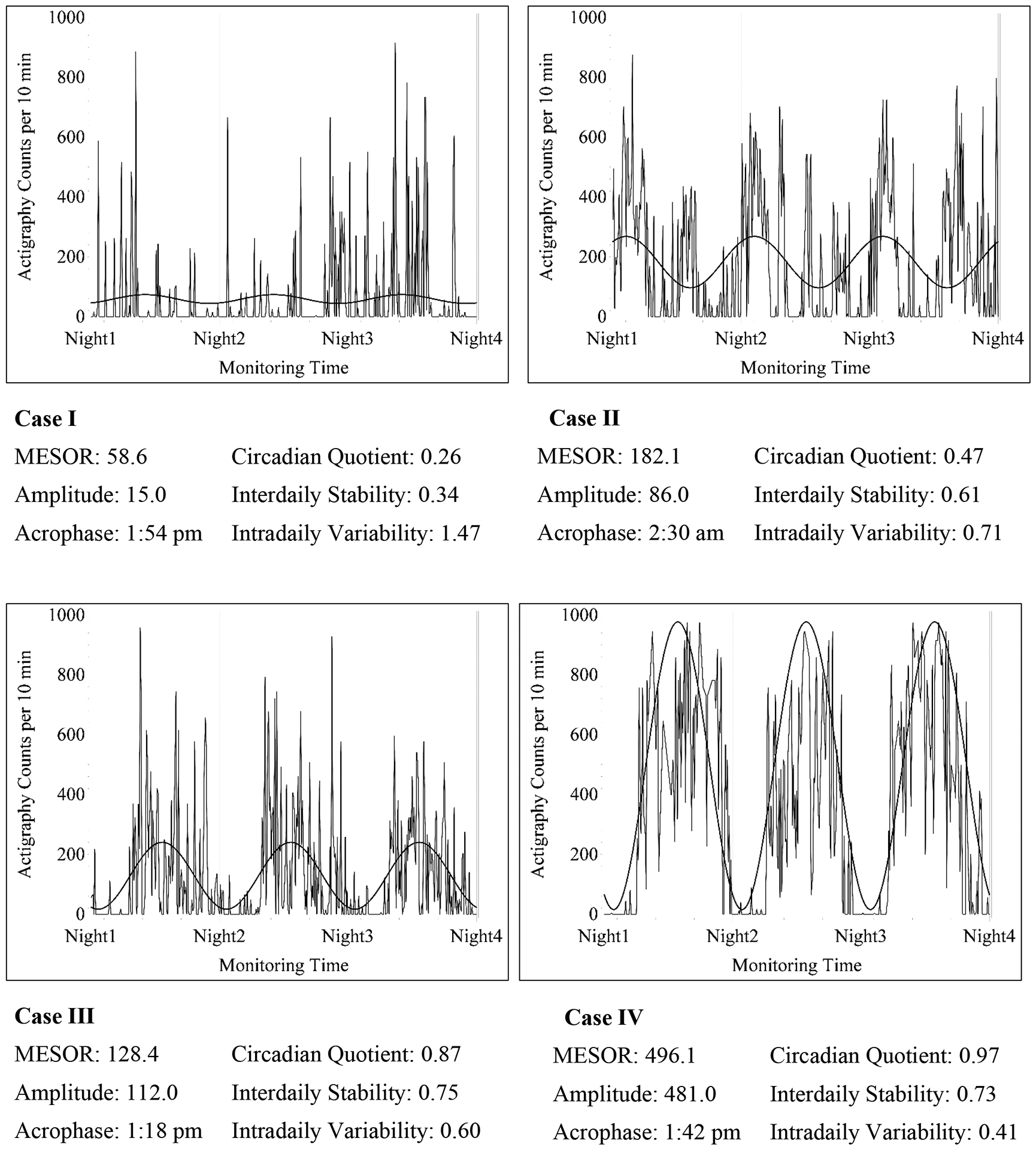
Examples of Rest-activity Patterns with Circadian Rhythm and Sleep Outcomes
The circadian quotient was positively associated with IS (r = 0.51) and negatively associated with IV (r = −0.62). The correlations between the demographic and clinical characteristics of the sample and the circadian parameters are shown in Table 3. Older age was associated with lower MESOR and amplitude, earlier acrophase, and greater IV, but not circadian quotient. A higher comorbidity index and higher NYHA Class, indicating more symptomatic HF, were associated with lower MESOR and amplitude, circadian quotient, and greater IV. Higher LVEF, indicative of better cardiac function (LVEF>45%), was not associated with the circadian quotient, IS, or IV.
Table 3.
Correlations Between the Circadian Rhythm Variables and Demographic Characteristics (Pearson correlations)
| Circadian quotienta) r | Interdaily Stability r | Intradaily Variability r | |
|---|---|---|---|
| Age | −0.01 | 0.03 | *0.25 |
| Race (White vs. Minority)b) | 0.14 | 0.09 | 0.09 |
| Charlson Comorbid Index | −0.16 | −0.14 | *0.24 |
| NYHA Classification | **−0.26 | −0.13 | **0.28 |
| LVEF < 45%b) | −0.12 | −0.02 | 0.03 |
| Sleep Fragmentation (actigraph) | **−0.31 | −0.15 | 0.18 |
| Respiratory Disturbance Index | −0.07 | −0.05 | 0.10 |
| % Time at O2 saturation <90% | −0.07 | −0.08 | 0.00 |
| EEG Arousal Index | −0.17 | −0.06 | *0.24 |
| % REM sleep over TST | 0.17 | 0.02 | −0.18 |
Note. *, **, *** indicate False Discovery Rate <.05, .01, and .001. NYHA: New York Heart Association Functional Classification.
Circadian quotient was log-transformed.
Point-Biserial Correlation Coefficient was used for Race (Binary) and the positive coefficients represents higher scores in white than minority.
A higher sleep fragmentation index, measured with wrist actigraphy, was associated with lower circadian quotient (r = −0.31) and higher IV (r = 0.18), while the respiratory disturbance index and % time at oxygen saturation < 90% were not associated the circadian parameters. Sleep fragmentation had a strong negative correlation with the circadian quotient (r=−0.44), IS (r=−0.29), and IV (r=0.29) only in participants aged 60 and older, while these correlations were −0.13 to 0.10 in younger participants. A higher EEG arousal index (r = 0.24) and lower percentage of REM sleep (r = −0.18) were associated with greater IV.
Bivariate correlations between the circadian parameters, daytime symptoms, and functional performance are presented in Table 4. The MESOR and amplitude were positively associated with greater functional performance but were not associated with daytime symptoms. A more robust circadian quotient was significantly positively associated with lower levels of daytime symptoms (r = −0.21, −0.21, and −0.30 for sensory fatigue, sleepiness, and depression, respectively) and positively associated with greater functional performance (r = 0.26 and 0.23 for 6 min walk and physical function, respectively). Greater IS (r = 0.29) and lower IV (r = −0.37) were significantly associated with longer distance in 6MWT. Greater IS was associated with less depression (r = −0.23). We also examined the correlations between the daytime symptoms and functional performance and the PSG sleep characteristics. Only higher PSG-recorded sleep efficiency was significantly associated with greater functional performance (r = 0.21), but no other PSG sleep characteristics were associated with daytime symptoms or functional performance.
Table 4.
Correlations Between the Circadian Rhythm Variables, Daytime Symptoms, and Functional Performance (Pearson correlation)
| Variables | Circadian quotienta) r | Interdaily Stability r | Intradaily Variability r |
|---|---|---|---|
| Daytime Symptoms | |||
| Sensory Fatigue | *−0.21 | −0.10 | 0.10 |
| Sleepiness | **−0.21 | −0.08 | 0.17 |
| Depression | **−0.30 | *−0.23 | 0.14 |
| Functional Performance | |||
| Six-Minute Walk | **0.26 | **0.29 | ***−0.37 |
| Physical Function | **0.23 | 0.16 | *−0.22 |
Note. *, **, *** indicate False Discovery Rate <.05, .01, and .001. Objective sleep variables were obtained from actigraph data of 3 consecutive days except % REM sleep which was obtained from PSG data obtained on one night.
Circadian quotient was log-transformed.
Table 5 shows the estimated coefficients and standard errors of the contributions of the log-transformed circadian quotient and insomnia symptoms to daytime symptoms and functional performance after controlling for covariates (age, gender, NYHA, BMI, comorbidity, RDI, and percentage of time at oxygen saturation < 90%). As in our previous report of the larger sample for this study (Redeker et al. 2010b), insomnia symptoms were significantly associated with greater daytime symptoms and lower physical functioning. The log-transformed circadian quotient was negatively associated with fatigue (p =.03), daytime sleepiness (p =.06), and depression (p <.01), after controlling for insomnia and the covariates. Given the lack of an association between the rest-activity parameters and insomnia severity, these associations were independent of the contribution of insomnia. The circadian quotient was positively associated with Six-Minute Walk distance (p < .01) and not significantly associated with self-reported physical function (p = .15), after controlling for the covariates.
Table 5.
Contributions of the Circadian Quotient to Daytime Symptoms and Functional Performance With Covariates
| Predictors | Outcome Variables | ||||
|---|---|---|---|---|---|
| Daytime Symptoms | Functional Performance | ||||
| Sensory Fatigue | Sleepiness | Depression | Six-Minute Walk | Physical Function | |
| Coeff±StdErr (p-value) | Coeff±StdErr (p-value) | Coeff±StdErr (p-value) | Coeff±StdErr (p-value) | Coeff±StdErr (p-value) | |
| Circadian Quotienta) | −1.324±0.601 (.0296) | −2.061±1.075 (.0575) | −7.120±2.293 (.0024) | 291.5±95.8 (.0029) | 0.693±0.483 (.1539) |
| Insomnia (DIMS)b) | 0.947±0.404 (.0208) | 1.310±0.735 (.0774) | 5.936±1.549 (.0002) | −34.7±65.8 (.5987) | −0.552±0.328 (.0951) |
| Covariates | |||||
| Age | −0.029±0.014 (.0481) | - | −0.189±0.056 (.0009) | −7.6±2.1 (.0005) | - |
| Gender (Male) | −1.384±0.428 (.0016) | - | −4.315±1.633 (.0093) | 353.7±67.5 (<.0001) | 1.530±0.343 (<.0001) |
| NYHA Classification | 0.374±0.306 (.2244) | - | 1.453±1.243 (.2448) | −141.0±52.2 (.0081) | −0.960±0.259 (.0003) |
| Body Mass Index | −0.048±0.027 (.0826) | - | −0.158±0.103 (.1280) | - | - |
| Charlson Comorbid Index | - | 0.359±0.256 (.1631) | 1.368±0.582 (.0202) | −42.6±26.1 (.1057) | −0.332±0.123 (.0080) |
| Respiratory Disturbance Index (RDI) | - | 0.050±0.021 (.0172) | - | - | −0.016±0.009 (.0907) |
| LVEF (%) | - | - | - | −3.9±2.3 (.0975) | - |
Note.
Circadian quotient was log-transformed.
Insomnia symptom was dichotomized based on DIMS. With replacement of circadian quotient, lower IS and higher IV were associated with less depression (p=.0494 & .0643) and greater six-minute walk (p=.0112 & .0042) in the adjusted models.
Inclusion of the IS and IV as independent variables in the model, rather than the circadian quotient, revealed that IS had a small association with depressive symptoms (p =.05), and there was a small and not statistically significant association between IV and depressive symptoms (p =.06) and longer Six-Minute Walk distance (p =.01 for IS and p<.01 for IV) after controlling for the contributions of insomnia and the covariates. However, neither the IS nor the IV added significantly to the variance in the symptom or functional performance measures beyond that of the circadian quotient. While there were significant bivariate correlations between Six-Minute Walk Test distance and physical function and PSG sleep efficiency, these relationships were not statistically significant after controlling for the same covariates.
Discussion
To our knowledge, we are the first to characterize the associations between rhythmic characteristics, circadian quotient, intra-daily variability, and inter-daily stability, of the rest-activity rhythm among patients with stable HF and their HF symptoms and functional performance. Our findings demonstrated small, but quite consistent associations, between the circadian quotient, a measure of the strength of the circadian rhythm, daytime symptoms, and both objective and self-report measures of functional performance. Notably, the associations between the circadian quotient, daytime symptoms, and 6MWT distance persisted after statistically controlling for important clinical, demographic, and sleep variables, including indicators of insomnia and sleep-disordered breathing that are common in this population. These findings suggest that the associations between rest-activity and symptoms and function are independent from those of sleep.
Our findings extend past studies in other populations. For example, depressive symptoms were associated with more fragmented and less robust circadian rhythms in the general population (Luik et al. 2013; Smagula et al. 2015), and the strength of the RAR was associated with fatigue (Berger et al. 2012) and self-reported physical function among cancer patients (Innominato et al. 2018; Mornont et al. 2002; Roscoe et al. 2002; Ancoli-Israel et al. 2006). Although the MESOR and amplitude in our study were similar to those of healthy adults (Brown et al. 1990), a previous study showed that RARs were more robust in healthy adults than HF patients (Liebzeit et al. 2017), and all of the circadian attributes demonstrated a high degree of variability in our sample. The inter-daily stability and the intra-daily variability were lower and higher, respectively, than found for the cohort of the Rotterdam study, a study of older adults, that revealed that these parameters predicted death (Zuurbier et al. 2015). Taken together with these levels, the associations between intra-daily variability and 6MWT, NYHA classification, comorbidity, physical function, and depression found in our study suggest the possibility that these metrics also have negative prognostic value in HF patients, although further longitudinal study is needed to extend these cross-sectional findings.
The lower amplitude, MESOR, and IV in older age and higher NYHA may reflect fragmentation of the rest-activity rhythm due to age-related declines and illness (Zuurbier et al. 2015) and decreased daily activity levels that are common in HF patients. These findings are consistent with past studies that found decreased amplitude and earlier acrophase, and increased IV and IS, among older adults (Robillard et al. 2014). The lack of an association between age and the circadian quotient may reflect the fact that older adults have low average daytime activity, which is reflected in the low MESOR, accompanied by an amplitude that may be lower due to reduced sleep fragmentation detected in the analyses conducted separately in older and younger adults. Among the younger adults, the circadian quotient was primarily influenced by peak daytime activity counts rather than sleep fragmentation. These findings, obtained as secondary analyses, should be replicated in a larger study prospectively designed to evaluate age-related differences.
We previously reported that insomnia, but not sleep-disordered breathing, was associated with daytime symptoms and functional performance in the HF patients of this study, and we are conducting a randomized controlled trial of the effects of cognitive behavioral therapy for insomnia (CBT-I) in this population (Redeker et al. 2010a, 2010b, 2017). However, our cross-sectional findings suggest that circadian rhythms of rest-activity are independently associated with symptoms and functional performance, and together these findings underscore the potential importance of both sleep and the 24 h activity rest-activity rhythm to these outcomes.
Future longitudinal study is needed to determine the extent to which rest-activity rhythms predict important HF outcomes. The significance of this work is underscored by recent evidence of the importance circadian rhythmicity in this population. For example, recent evidence suggests that melatonin, a hormone that reflects circadian rhythmicity, is important to HF and fatigue (Melamud et al. 2012; Kwon et al. 2015). Lower melatonin levels were associated with poorer function among HF patients (Dzida et al. 2013), poor survival in rats (Simko et al. 2014), and lack of reversed left ventricular remodelling in response to cardiac resynchronization therapy (Dominguez-Rodriguez et al. 2016). Given emerging understanding of the contributions of low melatonin levels to cardiac function (Simo et al. 2014; Dominguez-Rodriguez et al. 2014; Sehirli et al. 2013), and associations of less robust circadian rhythms with higher blood pressure and inflammatory biomarkers (Morris et al. 2012, 2016, 2017), future studies should evaluate the role of variation in the melatonin rhythm and exogenous lighting and other zeitgebers, as well as rest-activity in these patients. Interventions focused on manipulating rest-activity (e.g., timed bright light, increased social interactions and physical activity, regularizing bedtimes, and/or use of melatonin) may also improve symptom outcomes. The possible benefits of intervention are underscored by the findings that improvements in the rest-activity rhythm among cancer patients corresponded to improvements in symptoms (Roscoe et al. 2002), but this has not been examined among HF patients.
Strengths of this study included the well characterized sample and the availability of actigraphic, polysomnographic, symptom, and functional performance measures for the analyses. However, the availability of only 3 d of actigraphy may have limited our ability to fully assess inter-daily stability over a longer period of time, and this may have influenced the small correlations noted between comorbidity and other variables. Our study was also limited by its cross-sectional design and the absence of information on light and melatonin levels, as well as specific types of exercise or physical activity that may influence activity counts. The participants in this study were community-residing adults, and we did not control for important exogenous influences that may have contributed to rhythmicity. Although the RAR reflects endogenous rhythmic as well as exogenous influences, and thus is not a pure circadian measure, there is considerable evidence that the rest-activity rhythm has prognostic potential (Tranah et al. 2011; Levi F et al. 2014; Zuurbier et al. 2015; Cash et al. 2018). By integrating biological measures, such as dim light melatonin or OMIC indicators, we would estimate more accurate functions of circadian rhythm on daytime symptoms and functioning in future research with a large sample size.
Our study suggests associations between indices of the rest-activity rhythm and important symptom and functional outcomes among patients with stable HF. Future longitudinal and experimental studies are needed to evaluate the causal relationships among rest-activity rhythm and HF outcomes and the potential role of intervention focused on the rest-activity rhythm that may improve these outcomes.
Acknowledgments
Financial Supports: R01NR016191 (Redeker, PI); P20NR014126 (Redeker, PI); R01NR008022 (Redeker, PI)
Footnotes
All listed authors have seen and approved the manuscript
Disclosure of interest: Nancy Redeker (presence of financial support and absence of conflict of interest), Sangchoon Jeon (presence of financial support and absence of conflict of interest), Samantha Conley (presence of financial support and absence of conflict of interest)
References
- Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason W. 1997b. Use of wrist activity for monitoring sleep/wake in demented nursing-home patients. Sleep. 20(1):24–27. doi: 10.1093/sleep/20.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ancoli-Israel S, Klauber MR, Jones DW, Kripke DF, Martin J, Mason W, Pat-Horenczyk R, Fell R. 1997a. Variations in circadian rhythms of activity, sleep, and light exposure related to dementia in nursing-home patients. Sleep. 20(1):18–23. [PubMed] [Google Scholar]
- Ancoli-Israel S, Liu L, Marler MR, Parker BA, Jones V, Sadler GR, Dimsdale J, Cohen-Zion M, Fiorentino L. 2006. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 14(3):201–209. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin CM, Griffith KA, Nieto FJ, O’Connor GT, Walsleben JA, Redline S. 2001. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 24(1):96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- Belza BL. 1990. Multidimensional Assessment of Fatigue (MAF) Scale Users Guide. Seattle: University of Washington. [Google Scholar]
- Berger AM, Farr L. 1999. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncol Nurs Forum. 26(10):1663–1671. [PubMed] [Google Scholar]
- Berger AM, Hertzog M, Geary CR, Fischer P, Farr L. 2012. Circadian rhythms, symptoms, physical functioning, and body mass index in breast cancer survivors. J Cancer Surviv. 6(3):305–314. doi: 10.1007/s11764-012-0218-x. [DOI] [PubMed] [Google Scholar]
- Broström A, Stromberg A, Dalstrom U, Fridlund B. 2004. Sleep difficulties, daytime sleepiness, and health-related quality of life in patients with chronic heart failure. J Cardiovasc Nurs. 19(4):234–242. doi: 10.1097/00005082-200407000-00003. [DOI] [PubMed] [Google Scholar]
- Brown AC, Smolensky MH, D’Alonzo GE, Redman DP. 1990. Actigraphy: a means of assessing circadian patterns in human activity. Chronobiol Int. 7(2):125–133. [DOI] [PubMed] [Google Scholar]
- Cash E, Duck CR, Brinkman C, et al. Depressive symptoms and actigraphy-measured circadian disruption predict head and neck cancer survival. Psychooncology. 2018;27(10):2500–2507. doi: 10.1002/pon.4862. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, McKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- Charlson ME, Szatrowski TP, Peterson J, Gold J. 1994. Validation of a combined comorbidity index. J Clin Epidemiol. 47(11):1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- Cornelissen G 2014. Cosinor-based rhythmometry. Theor Biol Med Model. 11:16. doi: 10.1186/1742-4682-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo ES, Juckett MB, Coe CL, et al. Inflammation, circadian rest-activity rhythms, and behavioral sequelae of chronic graft-versus-host disease. Brain, Behavior, and Immunity. 2017;66:e1. [Google Scholar]
- Dominguez-Rodriguez A, Abreu-Gonzalez P, Piccolo R, Galasso G, Reiter RJ. 2016. Melatonin is associated with reverse remodeling after cardiac resynchronization therapy in patients with heart failure and ventricular dyssynchrony. Int J Cardiol. 221:359–363. doi: 10.1016/j.ijcard.2016.07.056. [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez A, Abreu-Gonzalez P, Reiter RJ. 2014. The potential usefulness of serum melatonin level to predict heart failure in patients with hypertensive cardiomyopathy. Int J Cardiol. 174(2):415–417. doi: 10.1016/j.ijcard.2014.04.044. [DOI] [PubMed] [Google Scholar]
- Dzida G, Prystupa A, Lachowska-Kotowska P, Kadas T, Kamieński P, Kimak E, Hałabiś M, Kiciński P. 2013. Alteration in diurnal and nocturnal melatonin serum level in patients with chronic heart failure. Ann Agr Envi Med. 20(4):745–748. [PubMed] [Google Scholar]
- Glickman ME, Rao SR, Schultz MR. 2014. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 67(8):850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Grutsch JF, Wood PA, Du-Quiton J, Reynolds JL, Lis CG, Levin RD, Ann Daehler M, Gupta D, Quiton DF, Hrushesky WJ. 2011. Validation of actigraphy to assess circadian organization and sleep quality in patients with advanced lung cancer. J Circ Rhythms. 9:4. doi: 10.1186/1740-3391-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghayegh S, Khoshnevis S, Smolensky MH, Diller KR, Castriotta RJ 2019. Performance comparison of different interpretative algorithms utilized to derive sleep parameters from wrist actigraphy data. Chronobiol Int. 36(12): 1752–1760. doi: 10.1080/07420528.2019.1679826. [DOI] [PubMed] [Google Scholar]
- Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, Hazen N, Herman J, Adams Hillard PJ, Katz ES, et al. 2015. National Sleep Foundation’s updated sleep duration recommendations: final report. Sleep Health. 1(4):233–243. doi: 10.1016/j.sleh.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Hori H, Koga N, Hidese S, et al. 24-h activity rhythm and sleep in depressed outpatients. J Psychiatr Res. 2016;77:27–34. doi: 10.1016/j.jpsychires.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Innominato PF, Komarzynski S, Palesh OG, Dallmann R, Bjarnason GA, Giacchetti S, Ulusakarya A, Bouchahda M, Haydar M, Ballesta A, et al. 2018. Circadian rest-activity rhythm as an objective biomarker of patient-reported outcomes in patients with advanced cancer. Cancer Med. 7(9):4396–4405. doi: 10.1002/cam4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innominato PF, Mormont MC, Rich TA, Waterhouse J, Levi FA, Bjarnason GA. 2009. Circadian disruption, fatigue, and anorexia clustering in advanced cancer patients: implications for innovative therapeutic approaches. Integr Cancer Ther. 8(4):361–370. doi: 10.1177/1534735409355293. [DOI] [PubMed] [Google Scholar]
- Jeon S, Conley S, Redeker NS. 2019. Discrepancy between wrist-actigraph and polysomnographic measures of sleep in patients with stable heart failure and a novel approach to evaluating discrepancy. J Sleep Res. 28(2):e12717. doi: 10.1111/jsr.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S, Redeker NS. 2016. Sleep Disturbance, Daytime Symptoms, and Functional Performance in Patients With Stable Heart Failure: A Mediation Analysis. Nurs Res. 65(4):259–267. doi: 10.1097/NNR.0000000000000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns MW. 1991. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- Johns MW. 1992. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 15(4):376–381. doi: 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- Kwon KJ, Lee EJ, Kim MK, Jeon SJ, Choi YY, Shin CY, Han SH. 2015. The potential role of melatonin on sleep deprivation-induced cognitive impairments: implication of FMRP on cognitive function. Neuroscience. 301:403–414. doi: 10.1016/j.neuroscience.2015.05.079. [DOI] [PubMed] [Google Scholar]
- Levi F, Dugue PA, Innominato P, et al. Wrist actimetry circadian rhythm as a robust predictor of colorectal cancer patients survival. Chronobiol Int. 2014;31(8):891–900. doi: 10.3109/07420528.2014.924523. [DOI] [PubMed] [Google Scholar]
- Levin RD, Daehler MA, Grutsch JF, et al. Circadian function in patients with advanced non-small-cell lung cancer. Brit J Cancer. 2005;93(11):1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebzeit D, Phelan C, Moon C, Brown R, Bratzke L. 2017. Rest-Activity Patterns in Older Adults with Heart Failure and Healthy Older Adults. J Aging Phys Act. 25(1):116–122. doi: 10.1123/japa.2016-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkin DP, Scriven AJ, Crake T, Poole-Wilson PA. 1986. Six minute walking test for assessing exercise capacity in chronic heart failure. Br Med J (Clin Res Ed). 292(6521): 653–655. doi: 10.1136/bmj.292.6521.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Rissling M, Neikrug A, Fiorentino L, Natarajan L, Faierman M, Sadler GR, Dimsdale JE, Mills PJ, Parker BA, et al. 2013. Fatigue and Circadian Activity Rhythms in Breast Cancer Patients Before and After Chemotherapy: A Controlled Study. Fatigue. 1(1–2):12–26. doi: 10.1080/21641846.2012.741782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Tiemeier H. 2013. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int. 30(10):1223–1230. doi: 10.3109/07420528.2013.813528. [DOI] [PubMed] [Google Scholar]
- Maekawa H, Kume Y. Imbalance of nonparametric rest-activity rhythm and the evening-type of chronotype according to frailty indicators in elderly community dwellers. Chronobiol Int. 2019;36(9):1208–1216. doi: 10.1080/07420528.2019.1626416. [DOI] [PubMed] [Google Scholar]
- Manousakis JE, Scovelle AJ, Rajaratnam SMW, Naismith SL, Anderson C. Advanced Circadian Timing and Sleep Fragmentation Differentially Impact on Memory Complaint Subtype in Subjective Cognitive Decline. Journal of Alzheimer’s disease : JAD. 2018;66(2):565–577. doi: 10.3233/JAD-180612. [DOI] [PubMed] [Google Scholar]
- Melamud L, Golan D, Luboshitzky R, Lavi I, Miller A. 2012. Melatonin dysregulation, sleep disturbances and fatigue in multiple sclerosis. J Neurol Sci. 314(1–2):37–40. doi: 10.1016/j.jns.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Mitchell JA, Quante M, Godbole S, James P, Hipp JA, Marinac CR, Mariani S, Cespedes Feliciano EM, Glanz K, Laden F, et al. 2017. Variation in actigraphy-estimated rest-activity patterns by demographic factors. Chronobiol Int. 34(8):1042–1056. doi: 10.1080/07420528.2017.1337032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mormont MC, Langouet AM, Claustrat B, Bogdan A, Marion S, Waterhouse J, Touitou Y, Lévi F. 2002a. Marker rhythms of circadian system function: a study of patients with metastatic colorectal cancer and good performance status. Chronobiol Int. 19(1):141–155. doi: 10.1081/cbi-120002593. [DOI] [PubMed] [Google Scholar]
- Mormont MC, Waterhouse J. Contribution of the rest-activity circadian rhythm to quality of life in cancer patients. Chronobiol Int. 2002b; 19(1): 313–323. doi: 10.1081/cbi-120002606. [DOI] [PubMed] [Google Scholar]
- Mormont MC, Waterhouse J, Bleuzen P, Giacchetti S, Jami A, Bogdan A, Lellouch J, Misset JL, Touitou Y, Lévi F. 2000. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 6(8):3038–3045. [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Hu K, Scheer FA. 2016. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Natl Acad Sci U S A. 113(10):E1402–1411. doi: 10.1073/pnas.1516953113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Mistretta J, Hu K, Scheer F. 2017. Circadian Misalignment Increases C-Reactive Protein and Blood Pressure in Chronic Shift Workers. J Biol Rhythms. 32(2):154–164. doi: 10.1177/0748730417697537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Yang JN, Scheer FA. 2012. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 199:337–358. doi: 10.1016/B978-0-444-59427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New York Heart Association. 1964. Diseases of the heart and blood vessels: Nomenclature and criteria for diagnosis (6th ed). Boston: Little-Brown. [Google Scholar]
- Radloff LS. 1977. The CES-D Scale: A self report depression scale for research in a general population. Appl Psychol Meas. 1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Radloff LS. 1991. The use of the Center for Epidemiological Studies Depression Scale in adolescents and young adults. J Youth Adol. 20(2):149–166. doi: 10.1007/BF01537606. [DOI] [PubMed] [Google Scholar]
- Radloff LS, Teri L. 1986. Use of the Center for Epidemiological Studies-Depression Scale with older adults. Clin Gerontol. 5(1–2):119–136. doi: 10.1300/J018v05n01_06. [DOI] [Google Scholar]
- Redeker NS, Adams L, Berkowitz R, Blank L, Freudenberger R, Gilbert M, Walsleben J, Zucker MJ, Rapoport D. 2012. Nocturia, sleep and daytime function in stable heart failure. J Card Fail. 18(7):569–575. doi: 10.1016/j.cardfail.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeker NS, Jeon S, Muench U, Campbell D, Walsleben J, Rapoport DM. 2010b. Insomnia symptoms and daytime function in stable heart failure. Sleep. 33(9):1210–1216. doi: 10.1093/sleep/33.9.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeker NS, Knies AK, Hollenbeak C, Klar Yaggi H, Cline J, Andrews L, Jacoby D, Sullivan A, O’Connell M, Iennaco J, et al. 2017. Cognitive behavioral therapy for insomnia in stable heart failure: Protocol for a randomized controlled trial. Contemp Clin Trials. 55:16–23. doi: 10.1016/j.cct.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeker NS, Muench U, Zucker MJ, Walsleben J, Gilbert M, Freudenberger R, Chen M, Campbell D, Blank L, Berkowitz R et al. 2010a. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep. 33(4):551–560. doi: 10.1093/sleep/33.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeker NS, Stein S. 2006. Characteristics of sleep in patients with stable heart failure versus a comparison group. Heart Lung. 35(4):252–261. doi: 10.1016/j.hrtlng.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Robillard R, Naismith SL, Smith KL, Rogers NL, White D, Terpening Z, Ip TK, Hermens DF, Whitwell B, Scott EM, et al. 2014. Sleep-wake cycle in young and older persons with a lifetime history of mood disorders. PLoS One. 9(2):e87763. doi: 10.1371/journal.pone.0087763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, Andrews PL. 2002. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 10(4):329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP Jr, Vitiello MV, Zhdanova IV; American Academy of Sleep Medicine. 2007. Circadian rhythm sleep disorders: part I, basic principles, shift work and jet lag disorders. An American Academy of Sleep Medicine review. Sleep. 30(11):1460–1483. doi: 10.1093/sleep/30.11.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese G, Lund LH. 2017. Global Public Health Burden of Heart Failure. Card Fail Rev. 3(1):7–11. doi: 10.15420/cfr.2016:25:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehirli AO, Koyun D, Tetik S, Özsavcı D, Yiğiner Ö, Çetinel Ş, Tok OE, Kaya Z, Akkiprik M, Kılıç E, et al. 2013. Melatonin protects against ischemic heart failure in rats. J Pin Res. 55(2):138–148. doi: 10.1111/jpi.12054. [DOI] [PubMed] [Google Scholar]
- Simko F, Bednarova KR, Krajcirovicova K, Hrenak J, Celec P, Kamodyova N, Gajdosechova L, Zorad S, Adamcova M. 2014. Melatonin reduces cardiac remodeling and improves survival in rats with isoproterenol-induced heart failure. J Pin Res. 57(2): 177–184. doi: 10.1111/jpi.12154. [DOI] [PubMed] [Google Scholar]
- Smagula SF, Ancoli-Israel S, Blackwell T, Boudreau R, Stefanick ML, Paudel ML, Stone KL, Cauley JA; Osteoporotic Fractures in Men (MrOS) Research Group. 2015. Circadian rest-activity rhythms predict future increases in depressive symptoms among community-dwelling older men. Am J Geriatr Psychiatry. 23(5):495–505. doi: 10.1016/jagp..2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan A, Choudhary V, Parganiha A. Worsening of rest-activity circadian rhythm and quality of life in female breast cancer patients along progression of chemotherapy cycles. Chronobiol Int. 2017;34(5):609–623. doi: 10.1080/07420528.2017.1286501. [DOI] [PubMed] [Google Scholar]
- Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol. 2011;70(5):722–732. doi: 10.1002/ana.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE. 1993. Health Survey Manual and Interpretation Guide. Boston: Health Institute, New England Medical Center. [Google Scholar]
- Ware JE. 1994. SF-36 Physical and Mental Health Summary Scales: A User’s Manual. Boston: The Health Institute, New England Medical Center. [Google Scholar]
- Ware JE Jr., Sherbourne CD 1992. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 30(6):473–483. [PubMed] [Google Scholar]
- Watson NF, Badr MS, Belenky G, Bliwise DL, Buxton OM, Buysse D, Dinges DF, Gangwisch J, Grandner MA, Kushida C, et al. 2015. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 38(6):843–844. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. 1990. Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biol Psychiatry. 27(6):563–572. doi: 10.1016/0006-3223(90)90523-5. [DOI] [PubMed] [Google Scholar]
- Zuurbier LA, Luik AI, Hofman A, Franco OH, Van Someren EJ, Tiemeier H. 2015. Fragmentation and stability of circadian activity rhythms predict mortality: the Rotterdam study. Am J Epidemiol. 181(1):54–63. doi: 10.1093/aje/kwu245. [DOI] [PubMed] [Google Scholar]


