Abstract
Epidemiological evidence suggests that females have an advantage over males in cases of melanoma incidence, progression, and survival. However, the biological mechanisms underlying these sex differences remain unclear. With the knowledge that females generally have a more robust immune system than males, we investigated sex differences in melanoma progression in a B16-F10/BL6 syngeneic mouse model. We observed significantly less tumor volume and growth rate over 14 days in female mice compared to male mice. Furthermore, higher populations of CD4+ and CD8+ T cells, which indicate adaptive immune responses, were found in the circulating blood and tumors of females and corresponded with less tumor growth, and vice versa in males. Our results highlight a mouse model that represents melanoma progression in the human population and displays a higher immune response to melanoma in females compared to males. These findings suggest that the immune system may be one of the mechanisms responsible for sex differences in melanoma.
Electronic supplementary material
The online version of this article (10.1007/s00262-020-02643-3) contains supplementary material, which is available to authorized users.
Keywords: Melanoma, Sex differences, Male, Female, Immunity
Introduction
Skin cancer is the most common form of cancer in the United States, with melanoma being the deadliest [1]. Exposure to environmental ultraviolet radiation has been associated with about 86% of melanoma cases [2]. Current estimates show that melanoma incidence is increasing approximately 3% every year, with males projected to account for 60% of melanoma incidences and 67% of melanoma-related deaths in 2020 [1]. This biological sex difference has been reported in epidemiological studies and cancer registries over the years, with females showing a clear advantage over males [1]. This is despite the prevalence of tanning and sun-seeking activities in females relative to males [3, 4]. Despite these lifestyle differences which should disfavor females, it is remarkable that females have the advantage over males in melanoma incidence, disease progression, and survival [5, 6]. Nevertheless, the mechanism(s) responsible for these sex differences are not fully understood.
The progression of melanoma from initiation to metastasis is driven mainly by proliferation events resulting from genetic mutations [7]. The genetic alterations in melanomas create antigenic epitopes such as TYRP-2 and MART-1 that are recognized by the host's adaptive immune CD4+ (helper) and CD8+ (cytotoxic) T cells [8]. In this study, we focused on the CD4 and CD8 markers, because the adaptive immune response is one of the major defense mechanisms against melanoma. Interestingly, females are been shown to have higher CD4+ and CD8+ T cell populations across different species compared to males [9]. Hence, we investigated whether sex differences in the adaptive immune response would explain the disparity in melanoma progression between males and females. We used a syngeneic B16-F10/BL6 mouse model, whose tumors are similar to humans in aggressiveness and metastatic potential, to demonstrate our concept and observe immune response to the progression (growth) of melanoma.
Materials and methods
Animal experiments
All animal procedures were in accordance with the National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee of Washington State University. Male and female C57BL/6 wild-type mice (8–10 weeks) were obtained from the Jackson Laboratories. The mice were maintained under 12 h/12 h light/dark cycle and given ad libitum food and water in the animal facility at Washington State University, Spokane. For subcutaneous injection, B16-F10 melanoma cells were purchased from ATCC and cultured in RPMI-1640 + 10% FBS. For each mouse, 200,000 cells in 50% serum-free RPMI-1640 and 50% matrigel (Corning, Cat # 354248) were injected into the lower right flank region. By the visible appearance of tumors in all mice, tumor volumes were measured using a digital caliper and calculated using the formula: V = (W2 × L)/2 [10]. All mice were sacrificed when the largest tumor reached a designated endpoint of 1000 mm3. Concurrently, control mice (non-tumor bearing) were sacrificed. Blood and tumors were harvested and immediately processed for immunophenotyping.
Lymphocyte isolation
Lymphocytes were isolated from peripheral blood using Lympholyte-Mammal (Cedarlane Laboratories) as previously described by us [11]. Briefly, whole blood was centrifuged for 3 min at 5000 rpm to separate plasma. All centrifugation steps were carried out at room temperature. The remaining blood fraction was resuspended in 2 mL of PBS prewarmed at 37 °C. Density separation of lymphocytes was achieved by adding 4 mL of Lympholyte-Mammal (CedarLane labs, Cat # CL5115) to the diluted blood and centrifuging for 20 min at 2000 rpm. The lymphocytes at the interface between the suspension medium and Lympholyte were collected and any contaminating red blood cells were lysed using 1× RBC lysis buffer (0.15 M NH4Cl, 0.01 M NaHCO3, 1 mM EDTA) for 5 min. Afterwards, 10 mL of cold PBS was added to stop the lysis reaction. The cells were then counted and resuspended in appropriate amount of PBS for flow cytometry.
Tumor-infiltrating lymphocyte isolation
Tumor-infiltrating lymphocytes were isolated from melanoma as previously described by us [10]. Briefly, tumors were isolated and washed with ice-cold PBS + 0.1% BSA, pushed through a wire mesh strainer, resuspended in ice-cold PBS + 0.1% BSA, and centrifuged at 4 °C for 1 min at 480 rpm. The supernatant was collected and centrifuged at 4 °C for 10 min at 1200 rpm. The pellet was resuspended in 37.5% Percoll (GE Healthcare, Cat # 17089101), then centrifuged at 2,000 rpm for 30 min. The lymphocytes were collected, treated with 1X RBC lysis buffer, counted, and resuspended in an appropriate amount of PBS + 0.1% BSA for flow cytometry.
Flow cytometry and analysis
The general flow cytometry method was used as previously described [11]. Isolated cells were incubated with anti-CD4 and anti-CD8 conjugated with PE-Cy7 and e610 fluorophores, respectively (eBioscience, clone #s GK1.5 and 53-6.7, respectively) and flow cytometry was performed using a Beckman Coulter Gallios model A94291. Data was analyzed using Kaluza Analysis Software v1.5. Double-stained populations were excluded in these analyses.
Statistical analysis
Tumor volumes were compared by repeated measures Two-way ANOVA and corrected for multiple comparisons using Sidak’s test. Tumor growth rate was determined by linear regression and one-way ANOVA comparison of the slopes from day 8 to 14. Immune differences were analyzed using Students’ t test and also expressed in terms of CD4+ : CD8+ ratio. Outlying data points were determined using the upper and lower bound limits of data range in Excel (Microsoft) and subsequently removed from analyses. With the exception of the outlier test, all analyses were performed using Prism version 6.01 (GraphPad software).
Results
Sex differences in the growth of melanoma
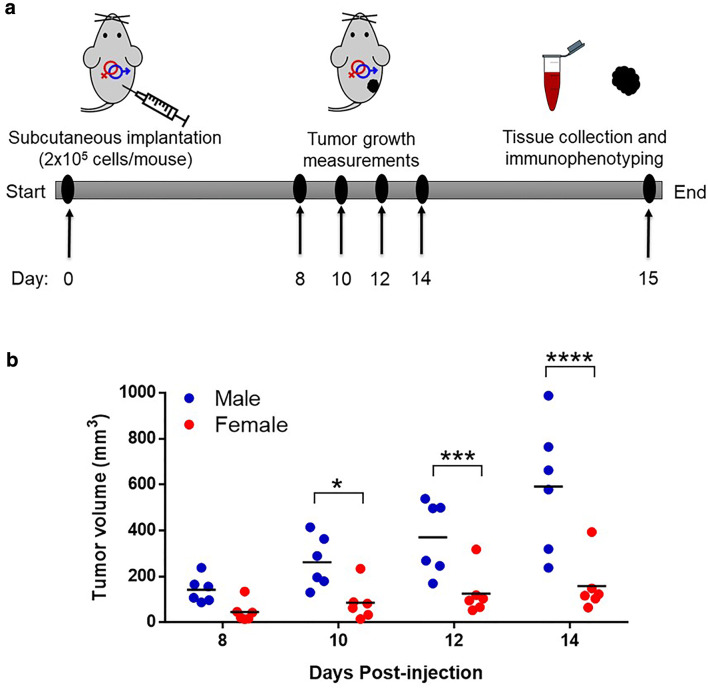
We carried out our studies with wild-type male and female mice according to the experimental timeline in Fig. 1a. B16-F10 melanoma cells were subcutaneously injected to the lower right flank region, and on day 8 all mice had visible measurable tumors. These mice were monitored daily and measurements were taken every 2 days. There were no signs of toxicity or discomfort observed throughout the experiment. By measurement of tumor volumes, we observed an interesting phenotype showing clear distinction in the growth of tumors. Tumors had significantly less volumes, by up to threefold, in the female mice compared to the male mice from day 10 through 14 (Fig. 1b). Furthermore, tumor growth rate calculated by linear regression revealed a significantly lower tumor growth rate in females (p < 0.0029) compared to males (Fig. S1). This phenotype corroborates human data which showed that females had less rapid tumor growth and were at lower risk of progression compared to males [6, 12]. In summary, our melanoma tumor model showed less melanoma growth in female compared to male mice, which represents melanoma progression events in the human population.
Fig. 1.
Sex differences in melanoma growth. a Description of timeline. Male and female wild-type C57BL/6 mice were injected (subcutaneously) with 200,000 B16-F10 melanoma cells. Tumor growth was measured from day 8 through 14. When any tumor sizes approached a maximum of 1000 mm3, all animals were sacrificed, and tissue samples were collected for further processing. b Tumor volumes were measured on day 8 through 14 for male and female mice. Statistical comparison was performed using repeated measures Two-way ANOVA with Sidak’s correction. n = 6 mice for each group. *p < 0.05, ***p < 0.001, ****p < 0.0001
Sex differences in adaptive immune response to melanoma
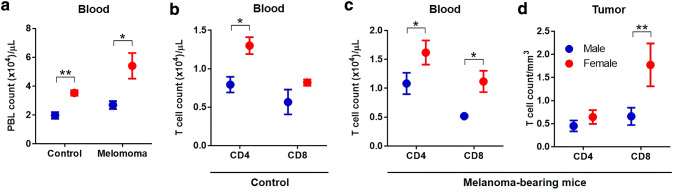
After observing that female mice had less tumor growth compared to male mice, we wanted to see if the response of the adaptive immune system could explain any of this phenotypic difference. Our B16-F10 melanoma tumor cells express melanoma-associated antigens, like Tyrp-2 and mutated p53, that are recognized by the host’s CD4+ (helper and recruitment) and CD8+ (cytotoxic) T cells [8, 13, 14]. In addition, we had a non-tumor (control) group. All animals were sacrificed 15 days after tumor implantation (for the tumor-bearing group). Therefore, we used immunophenotyping by flow cytometry to investigate circulating CD4+ and CD8+ T cells in the blood and infiltrating lymphocytes in the tumors. In the control mice, the number of peripheral blood lymphocytes (PBL) per µL of blood in female mice was higher compared to male mice (p = 0.0061) (Fig. 2a). In analyzing subsets of lymphocytes, we observed significantly higher CD4+ T cells in females (p = 0.028) compared to males, whereas the CD8+ T cell population showed no statistical difference (Fig. 2b). In melanoma tumor-bearing mice, the number of circulating PBLs per µL of blood was increased by approximately 1.5 fold, indicating a response to the tumors compared to control mice. Again, the number of PBLs in female mice was significantly higher than male mice (p = 0.016) (Fig. 2a). When comparing sex differences of circulating T cells in tumor-bearing mice, we observed significantly higher CD4+ and CD8+ T cell populations in female mice compared to male mice (p = 0.035 and 0.020, respectively) (Fig. 2c). Furthermore, we measured tumor-filtrating lymphocytes from harvested tumors and did not see a difference with CD4+ T cells but saw a significant increase in CD8+ T cells per mm3 of tumor in female mice compared to male mice (p = 0.0081) (Fig. 2d). In addition, we calculated lower CD4/CD8 T cell ratios in the blood and tumors of tumor-bearing female mice compared to male mice (Table 1). A lower ratio is often associated with favorable prognosis of tumor progression and survival. Collectively, we see that the response of adaptive T cells to melanoma is increased in female mice compared to male mice, corresponding to sex differences in tumor growth.
Fig. 2.
Sex differences in immune response to melanoma. Melanoma tumor-bearing mice were sacrificed on day 15 after tumor implantation, alongside control mice with no tumors. Lymphocytes were isolated from blood (a–c) and tumors (d), counted and immunophenotyped for CD4+ and CD8+ T cell markers and analyzed by flow cytometry. Lymphocytes from blood were represented per µL of blood and from tumors were represented per mm3 of tumor volume. Statistical analysis was done using student’s t test between sex. n = 5–6 for tumor bearing mice and n = 3 for control mice. *p < 0.05, **p < 0.01. Error bars = S.E.M
Table 1.
CD4/CD8 T cell ratios in tumor-bearing mice
| Tissue/sex | Male | Female |
|---|---|---|
| Blood | 2.09 | 1.45 |
| Tumor | 0.69 | 0.36 |
Discussion
In this study, we report an interesting finding on sex differences in immune response to melanoma tumor growth in a B16-F10/BL6 mouse model. Until now, there have been no reports into the mechanistic links between sex and the disparity in melanoma incidences, growth, and survival. One assumption used to explain these differences is that behaviors that inform decisions to detect melanomas and visit to health care providers are more associated with females than males [15]. However, this hypothesis does not account for progression after disease onset which shows less aggressive metastasis in females [6]. Our mouse model omits variability caused by diagnosis-seeking behavior and still displayed a striking phenotype of less tumor growth in females (Fig. 1b and S1). Although differences in immune function have been broadly proposed as a mechanism to explain the sex disparity in melanoma, it has not been fully investigated [16].
T cells are important players in melanoma response, and helper T cells specifically are important for protection against malignancy by activating and recruiting antigen-specific effector T cells [17]. This T cell-mediated response requires CD4+ in the priming stage and CD8+ in the effector stage [8]. These T cells release functional cytokines such as interferon gamma (IFNγ) and interleukin-2 (IL-2) which are important for anti-tumor immunity [8]. Our study utilized a melanoma model that elicits responses by both CD4+ and CD8+ T cells via Tyrp-2 recognition on B16-F10 cells [8]. Our findings suggest that the sexual dimorphism in the immune system of females and males, as seen in the healthy population, could be relevant in the context of melanoma progression and outcomes [9, 16]. In a healthy population, the adaptive immune system is generally stronger in females compared to males [9], a phenomenon we were able to confirm in our control mice (Fig. 2a). This difference in immune system strength was similar between the sexes in the context of melanoma (Fig. 2b, c). Previous studies using mouse models have further shown that helper T cells produce IFNγ at higher levels in female mice compared to male mice in response to parasitic infections [18]. Even in humans, females produced higher IFNγ upon stimulation of naïve CD4+ T cells compared to males [19]. In melanoma patients, a clinical study identified an increased number of antigen-specific helper T cells in females compared to males [20]. Taken together, the body of evidence suggests the possibility of greater anti-tumor immune activity in females compared to males. However, further work is still needed to characterize circulating and tumor-infiltrating T cells by subsets and functionality, memory versus effector T cells and cytokine activity, in our model. Nevertheless, our findings provide a framework for future mechanistic studies for understanding sex differences in immune responses against melanoma growth rate.
A possible confounder in this study is the endocrine system, and more specifically sex steroids, which can influence immune responses by binding to specific-hormone receptors on immune cells [21]. In particular, estrogen is widely hypothesized to be a key player in the sex disparity in melanoma [22]. The influence of estrogen could be direct, as estrogen receptor alpha (ERα) is differentially expressed by T cells, allowing for direct classical regulation by estrogen [23]; or indirect through non-classical signaling that can occur via protein–protein interactions between estrogen receptors and estrogen receptor element-independent transcription factors like NFκB [24]. Estrogen has been shown to enhance IFNγ secretion, by increasing Ifng transcription or upregulating the Th-1 transcription factor, T-bet, which promotes T cell responses and cell-mediated immunity [25, 26]. Furthermore, exogenous estrogen has been demonstrated to enhance the expansion of regulatory T cell populations in vivo, introducing additional environmental confounders [27]. These links suggest a secondary regulation of the immune system, and potentially the immune response, through sex steroids like estrogen. It is important to uncouple the mechanisms involved with sex differences in immune response against melanoma, but it will require further investigation as to whether these differences are independent of hormonal signaling.
Finally, the sex differences in immune response hold implications for immunotherapy, which is widely used in melanoma treatment. A common therapeutic strategy is using anti-PD-1/PD-L1 antibodies as checkpoint inhibitors to bolster the host’s immune response [28]. Interestingly, there were sex differences observed in the use of PD-L1 blockade in B16 melanoma tumor-bearing mice, where the females responded better to treatment by showing greater decrease in regulatory T cells which increased cytotoxic T cells. Consequently, this resulted in reduced tumor growth in females compared to males [29]. Despite similar expression levels, the difference in PD-L1 signaling between males and females remains unclear. The identification of sex-dependent differences in immune regulation and response will be important in identifying novel treatment strategies against melanoma.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1—Determination of B16-F10 tumor growth rates. A linear regression model was fit to the tumor growth data for each sex from Fig. 1 and generated the equation y = mx + b, where m = slope. The difference in the slopes was calculated using one-way ANOVA to test the null hypothesis that the slopes are identical. Supplementary material 1 (JPEG 40 kb)
Acknowledgements
This work was supported by grants from the Congressionally Directed Medical Research Program Award CA171123 (S.G.), in part by the National Institutes of Health R01ES030113, R21CA227381 (S.G.) and Melanoma Research Alliance Team Science Award (S.G.).
Author contributions
Concept and supervision by SG. Animal studies, assays, and analysis performed by PD, KP, AL, and SG. Immunology expertise, training, and antibodies from AL and HZ. Writing of the original draft by PD. Reviewing and editing by all authors.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.American Cancer Society Cancer facts & figures 2020. The Society, Atlanta, GA [DOI] [PMC free article] [PubMed]
- 2.Parkin DM, Mesher D, Sasieni P. 13. Cancers attributable to solar (ultraviolet) radiation exposure in the UK in 2010. Br J Cancer. 2011;105(Suppl 2):S66–S69. doi: 10.1038/bjc.2011.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koh HK, Bak SM, Geller AC, Mangione TW, Hingson RW, Levenson SM, Miller DR, Lew RA, Howland J. Sunbathing habits and sunscreen use among white adults: results of a national survey. Am J Public Health. 1997;87(7):1214–1217. doi: 10.2105/ajph.87.7.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi K, Lazovich D, Southwell B, Forster J, Rolnick SJ, Jackson J. Prevalence and characteristics of indoor tanning use among men and women in the United States. Arch Dermatol. 2010;146(12):1356–1361. doi: 10.1001/archdermatol.2010.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu-Smith F, Farhat AM, Arce A, Ziogas A, Taylor T, Wang Z, Yourk V, Liu J, Wu J, McEligot AJ, Anton-Culver H, Meyskens FL. Sex differences in the association of cutaneous melanoma incidence rates and geographic ultraviolet light exposure. J Am Acad Dermatol. 2017;76(3):499–505. doi: 10.1016/j.jaad.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joosse A, de Vries E, Eckel R, Nijsten T, Eggermont AM, Holzel D, Coebergh JW, Engel J, Munich Melanoma G. Gender differences in melanoma survival: female patients have a decreased risk of metastasis. J Invest Dermatol. 2011;131(3):719–726. doi: 10.1038/jid.2010.354. [DOI] [PubMed] [Google Scholar]
- 7.Miller AJ, Mihm MC., Jr Melanoma. N Engl J Med. 2006;355(1):51–65. doi: 10.1056/NEJMra052166. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez-Montagut T, Turk MJ, Wolchok JD, Guevara-Patino JA, Houghton AN. Immunity to melanoma: unraveling the relation of tumor immunity and autoimmunity. Oncogene. 2003;22(20):3180–3187. doi: 10.1038/sj.onc.1206462. [DOI] [PubMed] [Google Scholar]
- 9.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 10.Dakup PP, Porter KI, Little AA, Gajula RP, Zhang H, Skornyakov E, Kemp MG, Van Dongen HPA, Gaddameedhi S. The circadian clock regulates cisplatin-induced toxicity and tumor regression in melanoma mouse and human models. Oncotarget. 2018;9(18):14524–14538. doi: 10.18632/oncotarget.24539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Meadows GG. Chronic alcohol consumption in mice increases the proportion of peripheral memory T cells by homeostatic proliferation. J Leukoc Biol. 2005;78(5):1070–1080. doi: 10.1189/jlb.0605317. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Dowling JP, Murray WK, McArthur GA, Thompson JF, Wolfe R, Kelly JW. Rate of growth in melanomas: characteristics and associations of rapidly growing melanomas. Arch Dermatol. 2006;142(12):1551–1558. doi: 10.1001/archderm.142.12.1551. [DOI] [PubMed] [Google Scholar]
- 13.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mansour M, Pohajdak B, Kast WM, Fuentes-Ortega A, Korets-Smith E, Weir GM, Brown RG, Daftarian P. Therapy of established B16-F10 melanoma tumors by a single vaccination of CTL/T helper peptides in VacciMax. J Transl Med. 2007;5:20. doi: 10.1186/1479-5876-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Courtenay WH. Constructions of masculinity and their influence on men’s well-being: a theory of gender and health. Soc Sci Med. 2000;50(10):1385–1401. doi: 10.1016/s0277-9536(99)00390-1. [DOI] [PubMed] [Google Scholar]
- 16.Dronca RS, Dong H. A gender factor in shaping T-cell immunity to melanoma. Front Oncol. 2015;5:8. doi: 10.3389/fonc.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother. 2005;54(8):721–728. doi: 10.1007/s00262-004-0653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts CW, Walker W, Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev. 2001;14(3):476–488. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, Akkermann R, Stanczyk FZ, Prat A, Steinman L, Dunn SE. Peroxisome proliferator-activated receptor (PPAR)alpha and -gamma regulate IFNgamma and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci USA. 2012;109(24):9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wesa AK, Mandic M, Taylor JL, Moschos S, Kirkwood JM, Kwok WW, Finke JH, Storkus WJ. Circulating type-1 anti-tumor CD4(+) T cells are preferentially pro-apoptotic in cancer patients. Front Oncol. 2014;4:266. doi: 10.3389/fonc.2014.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gubbels Bupp MR, Potluri T, Fink AL, Klein SL. The confluence of sex hormones and aging on immunity. Front Immunol. 2018;9:1269. doi: 10.3389/fimmu.2018.01269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caruntu C, Mirica A, Rosca AE, Mirica R, Caruntu A, Tampa M, Matei C, Constantin C, Neagu M, Badarau AI, Badiu C, Moraru L. The role of estrogens and estrogen receptors in melanoma development and progression. Acta Endocrinol (Buchar) 2016;12(2):234–241. doi: 10.4183/aeb.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phiel KL, Henderson RA, Adelman SJ, Elloso MM. Differential estrogen receptor gene expression in human peripheral blood mononuclear cell populations. Immunol Lett. 2005;97(1):107–113. doi: 10.1016/j.imlet.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Kovats S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell Immunol. 2015;294(2):63–69. doi: 10.1016/j.cellimm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fox HS, Bond BL, Parslow TG. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991;146(12):4362–4367. [PubMed] [Google Scholar]
- 26.Karpuzoglu E, Phillips RA, Gogal RM, Jr, Ansar Ahmed S. IFN-gamma-inducing transcription factor, T-bet is upregulated by estrogen in murine splenocytes: role of IL-27 but not IL-12. Mol Immunol. 2007;44(7):1808–1814. doi: 10.1016/j.molimm.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H. Cutting edge: estrogen drives expansion of the CD4 + CD25 + regulatory T cell compartment. J Immunol. 2004;173(4):2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 28.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin PY, Sun L, Thibodeaux SR, Ludwig SM, Vadlamudi RK, Hurez VJ, Bahar R, Kious MJ, Livi CB, Wall SR, Chen L, Zhang B, Shin T, Curiel TJ. B7-H1-dependent sex-related differences in tumor immunity and immunotherapy responses. J Immunol. 2010;185(5):2747–2753. doi: 10.4049/jimmunol.1000496. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1—Determination of B16-F10 tumor growth rates. A linear regression model was fit to the tumor growth data for each sex from Fig. 1 and generated the equation y = mx + b, where m = slope. The difference in the slopes was calculated using one-way ANOVA to test the null hypothesis that the slopes are identical. Supplementary material 1 (JPEG 40 kb)