Abstract
Introduction
Thyroid-associated ophthalmopathy (TAO) is a disfiguring, potentially blinding, and sub-optimally managed autoimmune condition. Current therapy of active TAO consists most frequently of glucocorticoid steroids, orbital radiation, or B-cell depletion; all of which are associated with substantial side effects. Teprotumumab (Tepezza) is a human monoclonal antibody against the insulin-like growth factor type I receptor (IGF-IR), recently evaluated in 2 clinical trials for active moderate-to-severe TAO that was recently approved by the United States Food and Drug Administration (FDA) for use in TAO.
Areas Covered
This article reviews phase II and III placebo controlled, double masked, prospective, multicenter studies assessing the efficacy and safety of teprotumumab for the treatment of active, moderate-to-severe TAO.
Expert Opinion
Teprotumumab has demonstrated substantial and rapid improvement in Clinical Activity Score and proptosis reduction in TAO compared to placebo. Subjective diplopia and quality of life were also improved in both clinical trials. Teprotumumab exhibited a favorable safety profile, with transient hyperglycemia, muscle cramps, and auditory side effects being associated with the drug; these were usually transient. The trial findings indicate that teprotumumab is a promising, potential first line therapy for treating active TAO.
Keywords: IGF-IR, Graves’ disease, thyroid associated ophthalmopathy, thyroid eye disease, R1507, RV001, teprotumumab, Tepezza
1. Introduction
Thyroid-associated ophthalmopathy (TAO) is a progressive autoimmune disease that leads to the enlargement of orbital fat and extraocular muscles, potentially disrupting orbital architecture and causing vision loss. TAO is the most common, and most serious, extra-thyroidal manifestation of Grave’s Disease (GD). Clinically apparent TAO is observed in approximately one-half of patients with GD [1]. Of those, the majority exhibits only mild disease. In the United States, the annual incidence of TAO is 16/100,000 in females and 2.9/100,000 in men [2].
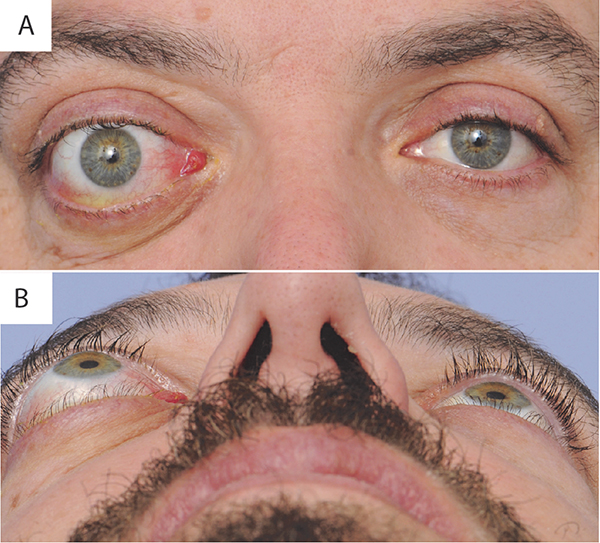
The clinical course of TAO was first described by Francis Rundle in 1945 [3]. The initial, active phase of TAO is characterized by inflammation and expansion of the periorbital and orbital tissues. Clinically, this manifests as ocular protrusion (proptosis), orbital pain, eyelid swelling, conjunctival injection and edema (chemosis), increased scleral show (eyelid retraction), ocular surface dryness, restricted eye movements, double vision (diplopia), and rarely, compressive optic neuropathy (Figure 1). The active phase most frequently lasts between 1 and 3 years before the inflammation plateaus and eventually leads to the stable phase, which may be dominated by the consequences of fibrosis and an absence of clinical change [3,4]. An ophthalmic examination is performed to quantify the severity of clinical signs (mild, moderate-to-severe, or sight-threatening) and to determine the activity of TAO. The latter is frequently reported using scoring systems such as the Clinical Activity Score (CAS) [5]. 5–10% of all cases of TAO are characterized as being moderate-to-severe [6]. Once the inactive phase is reached, clinical signs rarely remit, leaving patients with lasting functional and cosmetic deficits that may require orbital, extraocular muscle, and/or eyelid surgery.
Figure 1:
Thyroid associated ophthalmopathy. A) Portrait view demonstrates axial anterior displacement of the right globe (proptosis), conjunctival injection most prominent along the rectus muscle insertions, lower eyelid retraction, upper eyelid temporal flare, and caruncular edema. B) Worm’s eye view demonstrating the degree of right proptosis relative to the left eye. Note: patient permission was obtained.
Orbital fibroblasts play an essential role in the pathogenesis of TAO. These cells demonstrate the potential to differentiate into adipocytes and myofibroblasts and to produce hyaluronic acid [7–10]. The increased volume of fat and muscle, and elevated water content in the orbit leads to many of the clinical manifestations of TAO. Additionally, orbital fibroblasts can interact with immune cells and propagate an inflammatory response, largely as a consequence of a fibroblast subset comprising CD34+ fibrocytes [11–13].
Orbital fibroblasts express the thyroid-stimulating hormone receptor (TSHR), the immune tolerance of which when lost results in the production of activating autoantibodies against the receptor. Thyroid-stimulating immunoglobulins (TSIs) are a subset of antibodies against the TSHR. Although the relationship is not completely understood at this time, the activation of TSHR displayed on orbital fibroblasts by TSIs may be involved in the pathogenesis of TAO [11].
Orbital fibroblasts also express the insulin-like growth factor receptor type 1 (IGF-1R), which forms a physical and functional complex with TSHR [14]. Inhibiting IGF-1R attenuates the downstream effects of IGF-I- and TSH-dependent signaling in orbital fibroblasts [14, 15]. Therefore, IGF-1R is a potentially useful therapeutic target for the management of TAO. This review summarizes clinical data supporting the use of teprotumumab (also known as RV001, R1507), an inhibitory human monoclonal antibody with absolute specificity for IGF-IR, for treating active TAO.
2. Overview of the Market
Glucocorticoid steroids are the most commonly used drugs for treatment of active TAO [16]. The side effects of systemic steroids are substantial, including impaired glucose tolerance, hypertension, iatrogenic Cushing’s syndrome, osteoporosis, psychiatric maladies, impaired renal function, gastrointestinal side effects, glaucoma, cataract formation, and infection [16]. High-dosage pulse intravenous steroids are more effective than oral steroids and are better tolerated with fewer side effects [16]. These high-dosage steroids are associated with severe, occasionally fatal liver toxicity [16–18].
Use of external beam radiotherapy is advocated by some, both as standalone treatment and in combination with glucocorticoids [19]. Radiotherapy appears to be equivalent to that of glucocorticoids [20], with the combination of treatments potentially being more effective than either modality alone [21]. Orbital radiation has been shown to be rather safe overall, but can cause significant ocular side effects including cataracts, retinopathy, and a theoretical potential for radiation-induced malignancy continues to be concerning [22, 23].
Biological medications have been repurposed for use in active TAO. Effectiveness of rituximab (anti-CD20) remains uncertain, with one recent prospective double masked study demonstrating CAS improvement relative to glucocorticoids [24], while another finding no difference versus placebo [25]. Tocilizumab (anti-IL6 receptor monoclonal antibody inhibitor) demonstrated improvement in CAS in moderate-to-severe active TAO but failed to reduce proptosis [26]. Mycophenolate mofetil (inosine monophosphate dehydrogenase inhibitor) as monotherapy or in combination with glucocorticoids may prove superior to glucocorticoid monotherapy [27].
3. Teprotumumab Overview
Teprotumumab is a 150 kDa fully human monoclonal IgG1 antibody with absolute specificity against IGF-1R [4]. It binds to the extracellular region of the IGF-1R in a cysteine-rich domain with high affinity, blocking IGF-I and IGF-II from binding to the receptor [4]. After binding, the IGF-1R-teprotumumab complex is internalized and degraded [4].
Teprotumumab administered at a dosage of 9mg/kg has a half-life of 4–8 days [28]. The clearance of teprotumumab follows a dual elimination model that is non-linear (saturable) at low concentrations and linear (non-saturable) kinetics at higher concentrations [28].
4. Clinical Efficacy
A multicenter, double-masked, placebo-controlled phase II clinical trial (NCT01868997) studying the use of teprotumumab in active, moderate-to-severe TAO demonstrated clinically meaningful responses [29]. The primary outcome was the rate of clinical response, defined as a reduction in CAS by ≥ 2 points and a reduction in proptosis by ≥ 2 mm in the more severely affected eye, without worsening in the contralateral eye at week 24. During the study period, infusions of placebo or teprotumumab (initial 10mg/kg dose followed by 20mg/kg if tolerated) were given every three weeks. In the intention-to-treat cohort, 69% (29/42 patients) receiving teprotumumab vs. 20% (9/45 patients) receiving placebo had a clinical response at 24 weeks (p < 0.001) [29]. Treatment onset was rapid with 43% of patients receiving teprotumumab vs. 4% of patients receiving placebo reaching the primary outcome at 6 weeks [29].
A phase III confirmatory trial (NCT03298867; “OPTIC”) was recently completed in the spring of 2019 [30]. Inclusion criteria were identical to the phase II study. The primary outcome was reduction of proptosis (≥2mm) at week 24. In this study, 83% receiving teprotumumab vs. 10% receiving placebo achieved the primary outcome at 24 weeks (p < 0.001) [30]. Proptosis non-responders in the OPTIC study were allowed to enter an open label extension study (NCT03461211; “OPTIC-X”) to receive eight additional teprotumumab infusions over 24 weeks. Completion of the OPTIC-X study is anticipated in 2022.
5. Safety and Tolerability
Teprotumumab was developed for treatment of solid malignancies. The safety, pharmacodynamics and tolerability of teprotumumab were therefore initially studied in the cancer space. In a phase 1 clinical trial, thirty-seven patients with advanced solid tumors not amenable to standard therapy received up to 9mg/kg of weekly teprotumumab without evidence of dose-limiting toxicity [28], although two patients developed serious adverse events (hyperbilirubinemia and cerebral ischemia). A larger phase 1b trial involving teprotumumab in combination with other chemotherapeutics also demonstrated that the medication was generally well tolerated [31]. The most frequent severe adverse effects were fatigue, pancytopenia, and gastrointestinal distress. On the basis of several trials, teprotumumab was judged to be well tolerated but without sufficient efficacy to continue its developmental program for advanced solid tumors [32]. Despite the apparent tolerability of the drug, virtually all developmental programs for cancer therapeutics have been discontinued.
Both clinical trials of TAO also demonstrated the safety of teprotumumab. Hyperglycemia, muscle cramps and hearing abnormalities were identified as treatment-related adverse events [29,30]. Elevated blood glucose was reversible and usually managed easily with blood glucose monitoring and medication adjustment [29,30]. Hyperglycemia is commonly experienced with IGF-1R inhibition [29,30,33]. Importantly, these adverse events were most frequently transient.
6. Regulatory Affairs
No medications have been approved for management of this condition in the past. Prior to approval, the FDA designated teprotumumab breakthrough, orphan drug, and fast track status for use in TAO. A Biologics License Application was submitted to the FDA on July 10, 2019. On January 21st, 2020 the FDA approved teprotumumab for the treatment of active TAO.
7. Conclusions
Teprotumumab is a therapeutic human monoclonal antibody inhibitor of IGF-1R that has been evaluated in two recently completed clinical trials in active, moderate-to-severe TAO. Teprotumumab is currently the only FDA approved medication for use in TAO. The drug reduced both the activity and severity of TAO compared to placebo when administered every three weeks over a 24-week treatment period in phase II and phase III clinical trials. A crossover phase III study is currently underway to demonstrate drug effectiveness in non-responders and those who relapsed after the 24-week therapeutic phase. Teprotumumab exhibits a favorable safety profile. Follow-up assessment of patients is currently underway to determine the durability of the benefits of teprotumumab.
8. Expert Opinion
The low annual incidence, heterogeneity of presentation, and the historic inability of preclinical animal models to replicate human disease with high fidelity have resulted in incomplete understanding of and development of effective treatment. Consequently, inflammation associated with TAO is treated with high-dose glucocorticoids, external beam radiotherapy, or biological agents repurposed from other diseases. These therapies can improve symptoms is some patients but are unreliable and fail to alter the disease course or need for surgical intervention. Further, all carry substantial side effects. Therefore, an unmet need has existed for better treatment of TAO.
The discovery of a physical and functional relationship between IGF-IR and TSHR and the dependence of TSHR signaling on the activity of IGF-IR represents a pivotal development in our understanding of the pathogenesis of TAO [14]. It identified IGF-IR as a putative therapeutic target, not only for TAO but potentially for other autoimmune diseases as well [35]. Therefore, teprotumumab, a monoclonal antibody inhibitor of IGF-IR repurposed from the cancer space became, a plausible therapeutic agent for TAO.
In phase II and phase III clinical trials, teprotumumab demonstrated a rapid reduction of both proptosis and clinical activity score compared to placebo for active, moderate-to-severe TAO. The medication was well tolerated. Although some experts in the field advocate that a randomized trial comparing teprotumumab to intravenous steroids is needed [36], the aggregate findings concerning teprotumumab prompt the authors to conclude that teprotumumab represents a potential first line and paradigm changing medical treatment for active TAO. With our current knowledge, the drug may be best suited for active disease where steroids are being considered, especially those at a high dosage. The efficacy of teprotumumab in inactive disease is undetermined. The drug offers an advantage over the currently available pharmacological and surgical options by its restoration of function and appearance in many cases. Although data from the phase III extension study and longer-term assessments are pending, teprotumumab appears to alter the natural progression of disease over the duration studied in the clinical studies already published [29, 30]. The side effect profile of teprotumumab appears to be considerably better than that of steroids, radiotherapy, and rituximab. Given its recent approval by the FDA, we proffer that the drug will gain wide acceptance in medical and patient communities.
9. Drug Summary
-
9.1
Drug Name (generic): Teprotumumab (RV001, R1507)
-
9.2
Clinical Phase: FDA Approved
-
9.3
Indication: Active thyroid associated ophthalmopathy (Janupaiary 21st, 2020)
-
9.4
Pharmacology description / mechanism of action: human monoclonal inhibitory antibody against the insulin-like growth factor I receptor (IGF-1R).
-
9.5
Route of administration: intravenous infusion
-
9.6
Chemical structure: Proprietary
-
9.7
Pivotal trials:
-
9.7.1
NCT01868997 (Phase 2) [29]
-
9.7.2
NCT03298867 (Phase 3) – OPTIC [30]
-
9.7.3
NCT03461211 (Phase 3) – OPTIC-X
-
9.7.1
Article Highlights.
Teprotumumab is a therapeutic monoclonal antibody that inhibits the insulin-like growth factor type I receptor (IGF-IR).
It was studied in phase II and phase III trials for moderate-to-severe active thyroid-associated ophthalmopathy (TAO).
Teprotumumab demonstrated substantial and rapid improvement in clinical activity, diplopia and proptosis reduction.
Teprotumumab is the only FDA approved therapy for TAO.
Teprotumumab exhibited an overall favorable safety profile.
Its long-term durability is currently being evaluated.
Funding
T Smith has received funding for his work from the following: NIH grant EY008976, NIH grant UM1 Al 110498 05, NEI Core grant EY007003
Footnotes
Declaration of interest
T Smith was issued U.S. patents for the use of inhibitors of IGF-IR in Graves’ disease, TAO and other autoimmune diseases. US Patents covered therapeutic targeting of IGF-I receptor)10/140003, 13710635.8, 03/00187, 10/046,651, 10/038509. These are held by the Los Angeles Biomedical Foundation and UCLA School of Medicine. He serves as a paid consultant for Horizon Therapeutics and Immunovant. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
One reviewer has participated in the trials referred to in this paper. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1).Smith TJ, Hegedus L. Graves’ disease. New Engl J Med 2016;375:1552–1565. [DOI] [PubMed] [Google Scholar]
- 2).Bartley GB. The epidemiologic characteristics and clinical course of ophthalmopathy associated with autoimmune thyroid disease in Olmsted County, Minnesota. Transactions of the American Ophthalmological Society 1994;92:477–588. [PMC free article] [PubMed] [Google Scholar]
- 3).Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clin Sci 1945;5(3–4):177–194. [PubMed] [Google Scholar]
- 4).Smith TJ. Challenges in orphan drug development: identification of effective therapy for thyroid-associated ophthalmopathy. Annu Rev Pharmacol Toxicol 2019;59:129–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Stan MN, Garrity JA, Bahn RS. The evaluation and treatment of Graves’ ophthalmopathy. Med Clin North Am 2012;96(2):311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Bartalena L, Fatourechi V. Extrathyroidal manifestations of Graves’ disease: a 2014 update. J Endocrinol Invest. 2014;37(8):691–700. [DOI] [PubMed] [Google Scholar]
- 7).Sorisky A, Pardasani D, Gagnon A, et al. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab 1996;81(9):3428–3431. [DOI] [PubMed] [Google Scholar]
- 8).Smith TJ. Potential roles of CD34+ fibrocytes masquerading as orbital fibroblasts in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2019;104:581–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Smith TJ, Koumas L, Gagnon A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2002;87(1):385–392. [DOI] [PubMed] [Google Scholar]
- 10).Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-1 receptor. J Clin Endocrinol Metab 2004;89(10):5076–5080. [DOI] [PubMed] [Google Scholar]
- 11).Mohyi M, Smith TJ. IGF1 receptor and thyroid-associated ophthalmopathy. J Mol Endocrinol 2018;61(1):T29–T43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Douglas RS, Afifivan NF, Hwang CJ, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab 2010;95(1):430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Smith TJ. TSH-receptor-expressing fibrocytes and thyroid-associated ophthalmopathy. Nat Rev Endocrinol 2015;11(3):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves’ disease. J Immunol 2008;181:4397–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Smith TJ, Janssen JAMJL. Insulin-like growth factor-1 receptor and thyroid-associated ophthalmopathy. Endocrine Reviews 2019;40:236–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Bartalena L, Krassas GE, Wiersinga W, et al. Efficacy and safety of three different cumulative doses of intravenous methylprednisolone for moderate to severe and active Graves’ orbitopathy. J Clin Endocrinol Metab 2012;97(12):4454–4463. [DOI] [PubMed] [Google Scholar]
- 17).Sisti E, Coco B, Menconi F, et al. Intravenous glucocorticoid therapy for Graves’ ophthalmopathy and acute liver damage: an epidemiological study. Eur J Endorcrinol 2015;172:269–276. [DOI] [PubMed] [Google Scholar]
- 18).Walasik-Szemplinska D, Kaminski G, Sudol-Szopinska L. Life-threatening complications of high doses of intravenous methylprednisolone for treatment of Graves’ orbitopathy. Thyroid Res 2019:23:12–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Nicosia L, Reverberi C, Agolli L, et al. Orbital radiotherapy plus concomitant steroids in moderate-to-severe Graves’ Ophthalmopathy: good results after long-term follow-up. Int J Endocrinol Metab 2019;17(1):e84427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Prummel MF, Mourits MP, Blank L, et al. Randomized double-blind trial of prednisone versus radiotherapy in Graves’ ophthalmopathy. Lancet 1993;342(8877):949–954. [DOI] [PubMed] [Google Scholar]
- 21).Kim JW, Han SH, Son BJ, et al. Efficacy of combined orbital radiation and systemic steroids in the management of Graves’ orbitopathy. Grafes Arch Clin Exp Ophthalmol 2016;254(5):991–998. [DOI] [PubMed] [Google Scholar]
- 22).Wakelkamp IMMJ, Tan H, Saeed P, et al. Orbital irradiation for Graves’ ophthalmopathy: is it safe? A long-term follow-up study. Ophthalmology 2004;111:1557–1562. [DOI] [PubMed] [Google Scholar]
- 23).Snijders-Keilholz A, De Keizer RJ, Goslings BM, et al. Probable risk of tumour induction after retro-orbital irradiation for Graves’ ophthalmopathy. Radiother Oncol 1996;38(1):69–71. [DOI] [PubMed] [Google Scholar]
- 24).Salvi M, Vannuchi G, Curro N, et al. Efficacy of B-cell targeted therapy in rituximab in patients with active moderate to severe Graves’ orbitopathy: a randomized controlled study. J Clin Endocrinol Metab 2015;100:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25).Stan MN, Garrity JA, Carranza Leon BG, et al. Randomized controlled trial of rituximab in patients with Graves’ orbitopathy. J Clin Endocrinol Metab 2015;100:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Perez-Moreiras JV, Gomez-Reino JJ, Maneiro JR, et al. Efficacy of tocilizumab in patients with moderate-to-severe corticosteroid-resistant graves’ orbitopathy: a randomized clinical trial. Am J Ophthalmol 2018;195:181–190. [DOI] [PubMed] [Google Scholar]
- 27).Kahaly GJ, Riedl M, Konig J, et al. Mycophenolate plus methylprednisolone versus methylprednisolone alone in active, moderate-to-severe Graves’ orbitopathy (MINGO): a randomised, observer-masked, multicentre trial. Lancet Diabetes Endocrinol 2018;6(4):287–298. [DOI] [PubMed] [Google Scholar]
- 28).Kurzrock R, Patnaik A, Aisner J, et al. A phase 1 study of weekly R1507, a human monoclonal antibody insulin-like growth factor-1 receptor antagonist, in patients with advanced solid tumors. Clin Cancer Res;16(8):2458–2465. [DOI] [PubMed] [Google Scholar]
- 29).Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med 2017;376(18):1748–1761.** a. Annotated Bibliography: Phase II multicenter, double masked, randomized, placebo-controlled clinical trial demonstrating the efficacy and safety profile of teprotumumab for use in TAO.
- 30).Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. New Engl J Med 2020;382:341–352.** a. Annotated Bibliography: Phase III confirmatory clinical trial confirming the efficacy and safety profile of teprotumumab for use in TAO.
- 31).Mahadevan D, Sutton GR, Arteta-Bulos R, et al. Phase 1b study of safety, tolerability and efficacy of R1507, a monoclonal antibody to IGF-1R in combination with multiple standard oncology regimens in patients with advanced solid malignancies. Cancer Chemother Pharmacol 2014;73:467–473. [DOI] [PubMed] [Google Scholar]
- 32).Pappo AS, Vassal G, Crowley JJ, et al. A phase 2 trial of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF-1R), in patients with recurrent of refractory rhabomyosarcoma, osteosarcoma, synovial sarcoma, and other soft tissue sarcomas: results of a sarcoma alliance for research through collaboration study. Cancer 2014;120(16):2448–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Goldman JW, Mendenhall MA, Rettinger SR. Hyperglycemia associated with targeted oncologic treatment: mechanisms and management. Oncologist 2016;21(11):1326–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Smith TJ, Huetwell FGL, Hegedus L, et al. Role of IGF-1 pathway in the pathogenesis of Graves’ orbitopathy. Best Pract Res Clin Endocrinol Metab 2012;26(3):291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Smith TJ. Insulin-like growth factor-1 regulation of immune function: a potential therapeutic target in autoimmune disease? Pharmacol Rev 2010;62(2):199–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36).Smith TJ, Bartalena L. Will biological agents supplant systemic glucocorticoids as the first-line treatment for thyroid-associated ophthalmopathy? Eur J Endocrinol 2019;181(5):D27–D43. [DOI] [PMC free article] [PubMed] [Google Scholar]