Abstract
Objectives:
To determine whether the full spectrum of hypertensive disorders of pregnancy (HDP) -- comprising gestational hypertension; preeclampsia with or without severe features; eclampsia; and Hemolysis, Elevated Liver enzymes, and Low Platelets (HELLP) Syndrome -- is increased at high (≥2500 m, 8250 ft) compared with lower altitudes in Colorado independent of maternal background characteristics, and if so their relationship to neonatal well-being.
Methods:
A retrospective cohort study was conducted using statewide birth-certificate data to compare the frequency of gestational hypertension, preeclampsia (with or without severe features), eclampsia, HELLP Syndrome, or all HDP combined in 617,958 Colorado women who lived at high vs. low altitude (<2500 m) and delivered during the 10-year period, 2007 – 2016. We also compared blood-pressure changes longitudinally during pregnancy and the frequency of HDP in 454 high (>2500 m)- vs. low (<1700 m)-altitude Colorado residents delivering in 2013 and 2014, and matched for maternal risk factors. Data were compared between altitudes using t-tests or chi-square, and by multiple or logistic regression analyses to adjust for risk factors and predict specific hypertensive or neonatal complications.
Results:
Statewide, high-altitude residence increased the frequency of each HDP disorder separately or all combined by 33%. High-altitude women studied longitudinally also had more HDP accompanied by higher blood pressures throughout pregnancy. The frequency of low-birth weight infants (<2500 g), five-minute APGAR scores <7, and NICU admissions were also greater at high than low altitudes statewide, with the latter being accounted for by the increased incidence of HDP.
Conclusions:
Residence at high altitude constitutes a risk factor for HDP and recommends increased clinical surveillance. The increased incidence also makes high altitude a natural laboratory for evaluating the efficacy of predictive biomarkers or new therapies for HDP.
Keywords: Hypoxia, Intrauterine growth restriction, Preeclampsia
Introduction
Residence at high altitude, generally defined as above 2500 m (8250 ft), is associated with an increased incidence of hypertensive disorders of pregnancy (HDP) but unclear is whether only gestational hypertension or the full spectrum of HDP is affected.1,2,3 Over 250,000 Coloradans live at high altitude as do 140 million persons worldwide.4 While residential altitudes are lower in Colorado than in some other regions of the world, Colorado studies have the advantage of complete vital-statistics data and high usage of prenatal care, permitting fuller analysis of the factors contributing to HDP and its outcomes.
Given changes in HDP diagnostic criteria over time and the growing recognition of HDP as a major source of not only perinatal but also life-long morbidity for both mother and child,5–9 we sought to identify the current impact of high-altitude residence on HDP and neonatal well-being. Colorado birth certificates were also revised in 2007 to include more risk factors and the actual altitude of the mother’s residence, thus permitting more accurate assessment of altitude exposure than possible previously. We therefore examined the vital-statistic records for all Colorado residents across a 10-year (2007–2016) period to document the frequency of HDP and their relationships with infant outcomes while controlling for maternal risk factors. Additionally, as a prior smaller study showed that high-altitude residence altered blood-pressure course during pregnancy,10 we accessed longitudinal blood-pressure data from prenatal records for all women seeking prenatal care at a practice serving the highest-altitude regions of Colorado and compared their data to those from matched low- altitude residents to determine if altitude affected blood pressure changes during pregnancy independent of maternal risk factors.
Materials and Methods
Subjects.
This was a retrospective cohort study using birth-certificate data for all Colorado residents delivering during a 10-year (2007 −2016) period, termed “Statewide Cohort”, and data acquired from prenatal records for a subset whose data were examined longitudinally across a 2-year (2013–2014) period, termed “Longitudinal Cohort”. For both cohorts, exclusion criteria were type 1 or type 2 diabetes, chronic hypertension, multiple gestation, or missing demographic study data and, in the case of the Longitudinal Cohort, fewer than four prenatal visits. The longitudinal study protocol was approved by the Colorado Multiple Institutional Review Board and the Catholic Health Initiatives Institute for Research, the relevant IRBs for the Denver and Frisco study sites. The Innovation Institutional Review Board, the ethical oversight group for the Colorado Department of Public Health and Environment, approved the conduct of the statewide studies. Study conduct was in keeping with the STROBE statement.11
Of the 670,017 births statewide from 2007 through 2016, 617,958 included the geocoded altitude of maternal residence and therefore comprised the Statewide Cohort. Altitude was assigned using the latitude and longitude of the mother’s address at the time of delivery12 overlain with the USGS 7.5-minute Digital Elevation Model13, and was accurate to within 15 m.
The Longitudinal Cohort comprised a subset of statewide births whose prenatal records were examined longitudinally under a separate IRB-approved protocol. The high-altitude group was selected by examining the prenatal records of all women seen at a large prenatal practice in Frisco, CO (2766 m) who met our inclusion criteria, lived ≥2500 m (range = 2600 to 3158 m), and delivered in Frisco during 2013–2014. This clinic serves persons residing in multiple towns or regions at Colorado’s highest residential altitudes, and delivers at the highest-altitude labor and delivery unit in the USA. The high-altitude sample was matched for delivery year, maternal age within two years, body mass index (BMI) category, and ethnicity or race (when available) with an equal number of women who obtained prenatal care in metropolitan Denver, delivered there, and lived < 1700 m (range = 1271 to 1682 m). Maternal demographics, prenatal vital signs, HDP diagnoses and infant data were recorded from prenatal and delivery records.
Variables.
For all subjects, data were recorded for maternal altitude of residence (m), age at delivery (year), race (Caucasian or non-Caucasian), ethnicity (Hispanic or non-Hispanic), tobacco use during pregnancy (yes/no), socioeconomic status (qualifying for state Medicaid or federal Women, Infant and Children’s [WIC] benefits, yes/no), parity (primiparous [first delivery] , yes/no), pre-pregnancy body mass index (BMI, kg/m2), weight gain during pregnancy (kg), gestational diabetes (yes/no), and prenatal visits (number). Also, for the Longitudinal Cohort we calculated whether pregnancy weight-gain was appropriate, too little, or too much given her pre-pregnant BMI based on current ACOG guidelines.14 Infant data for both cohorts consisted of birth weight, low birth weight (<2500 g, yes/no), gestational age at delivery (fractional weeks), sex (male or female), whether or not delivery was preterm (<37weeks 0 days gestational age, yes/no), five-minute Apgar scores and, for the Statewide Cohort, whether or not the baby was admitted to a neonatal intensive care unit (NICU, yes/no).
The primary outcome examined was HDP. Statewide birth certificates contained diagnoses for pregnancy-induced hypertension or preeclampsia (with or without severe features), HELLP syndrome (Hemolysis, Elevated Liver enzymes, and Low Platelets), and eclampsia. In the Longitudinal Cohort, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP, DBP + 1/3(SBP-DBP)), and body weight were recorded from each prenatal visit and the maximum values noted. Hypertension during pregnancy was defined as either SBP ≥ 140 mmHg or DBP ≥ 90 mmHg after 20 weeks in a previously normotensive women based on current guidelines5 and the trimester of diagnosis noted. The first trimester was defined as through 14 weeks, second trimester as weeks 15–27, and third trimester as weeks 28 until delivery. Based on these restrictions, HDP refers to gestational hypertension, preeclampsia with or without severe features, eclampsia and HELLP Syndrome in the Statewide Cohort, and to gestational hypertension and preeclampsia with or without severe features in the Longitudinal Cohort as sample sizes were too small to permit analysis of eclampsia or HELLP Syndrome.
Procedure.
Following receipt of the statewide data, the study sample was selected and variables computed. Electronic medical records for the high-altitude Longitudinal Cohort were examined manually and variables of interest entered into the study database. At low altitude, matched participants were identified from patient reports, data abstracted from electronic health records, and entered into the study database.
Statistics.
Following checks for data-entry errors and analysis assumptions, the high- and low-altitude groups from each cohort were compared using student’s t-tests or chi-square analyses as appropriate. Multiple or logistical regression was used to predict specific hypertensive or other complications from altitude of residence while adjusting for risk factors in each cohort. Data are presented as adjusted odds ratios (aOR) with 95% confidence intervals (CI). Babies judged small for gestational age were those weighing less than the 10th percentile for gestational age and sex based on literature values.15 Repeated measures analysis of variance (ANOVA) was used in the Longitudinal Cohort to compare blood-pressure changes during pregnancy at low vs. high altitude in all women, but we were not sufficiently powered to compare altitude groups for those who either remained normotensive or developed HDP. Given that prenatal visits did not occur at the same gestational weeks (w) and days (d) in all women, we averaged MAP values from 8w0d-11w6d, 12w0d-15w6d, 16w0d-19w6d, 20w0d-23w6d, 24w0d-27w6d, 28w0d-31w6d, 32w0d-35w6d, 36w0d-37w6d, and 38w0d-41w6d for examining temporal changes. Results were considered significant when the two-tailed probability associated with the relevant test statistic was < 0.05.
Results
Maternal background characteristics
In the Statewide Cohort, 9,536 (1.5%) lived at high altitude (≥2500 m) and the remainder (608,422) lived at low altitude (<2500 m) whereas, by study design, all 227 women in the high-altitude portion and all 227 in the low-altitude portion of the Longitudinal Cohort lived at high or low altitude, respectively. Compared to the low-altitude women, those living at high altitude in the Statewide cohort were older; had a lower BMI at the start of pregnancy; gained more weight; had fewer prenatal visits; and were more likely to be Caucasian, non-Hispanic, ineligible for WIC or Medicaid, and primiparous (Table 1). Additionally, high-altitude women less often used tobacco or had gestational diabetes. The high- and low-altitude Longitudinal groups were, by definition, of similar age, ethnicity, or BMI but more high-altitude women were Caucasian, qualified for WIC or Medicaid benefits, and had appropriate weight gain (Table 1).
Table 1.
Maternal and infant characteristics by altitude and study cohort.
| STATEWIDE COHORT | LONGITUDINAL COHORT | |||||
|---|---|---|---|---|---|---|
| Low altitude (n=608,422)1 | High altitude (n=9,536)1 | p | Low altitude (n=227) | High altitude (n=227) | p | |
| Maternal background factors | ||||||
| Altitude of residence (m) | 1688±202 | 2752±180 | <.001 | 1651±89 | 2847±126 | <.001 |
| Age (years) | 28.3 ±6.0 | 30.2 ±6.0 | <.001 | 30.7±5.0 | 30.4±5.7 | .452 |
| Race (% Caucasian) | 86.8% | 95.7% | <.001 | 66.8% | 95.3% | <.001 |
| Ethnicity (% Hispanic) | 29.8% | 18.8% | <.001 | 20.3% | 25.3% | .200 |
| WIC and/or Medicaid | 30.5% | 20.8% | <.001 | 19.4% | 44.7% | <.001 |
| Primiparity | 33.1% | 36.7% | <.001 | 40.0% | 41.6% | .731 |
| Pre-preg BMI (kg/m2) | 25.6 ± 5.8 | 24.2 ± 4.8 | <.001 | 25.0±5.8 | 24.4±5.2 | .305 |
| Preg weight gain (kg) | 13.4±6.4 | 13.7±5.8 | <.001 | 12.9±4.8 | 12.7±2.4 | .686 |
| Too much wt gain | - | - | - | 36.1% | 0% | <.001 |
| Prenatal visits (#) | 10.2±3.6 | 9.8±3.6 | <.001 | 11.0±2.3 | 12.6±3.5 | <.001 |
| Preg tobacco use | 7.6% | 6.1% | <.001 | 6.6% | 8.9% | .648 |
| GDM | 3.8% | 3.1% | .001 | 8.4% | 4.6% | .103 |
| Maternal Hypertensive characteristics | ||||||
| Gest HTN/preeclampsia | 3.9% | 4.9% | <.001 | 11.5% | 18.5% | .035 |
| Eclampsia | 0.4% | 0.6% | .006 | - | - | - |
| HELLP | 0.3% | 0.4% | .031 | - | - | - |
| 2nd trimester HTN | - | - | - | 1.8% | 9.3% | <.001 |
| 3rd trimester HTN | - | - | - | 9.3% | 14.5% | .082 |
| Maximum systolic BP | - | - | - | 126±10 | 126±11 | .967 |
| Maximum diastolic BP | - | - | - | 78±8 | 81±9 | .002 |
| Maximum MAP | - | - | - | 92±8 | 94±8 | .007 |
| Infant Outcomes | ||||||
| Birth wt (gm) | 3232±526 | 3115±498 | <.001 | 3264±426 | 3109±406 | <.001 |
| LBW (<2500 gm) | 6.8% | 9.4% | <.001 | 4.0% | 10.4% | .009 |
| GA (wk) | 38.8±1.9 | 38.8±1.9 | .385 | 39.1±2.2 | 39.1±1.2 | .928 |
| Preterm birth (<37 wk) | 7.0% | 7.5% | .058 | 3.5% | 5.4% | .334 |
| 5 min Apgar score <7 | 2.4% | 3.7% | <.001 | 1.8% | 1.0% | .448 |
| NICU admission | 6.8% | 7.4% | .036 | - | - | - |
Data are expressed as means ± standard deviations or percent.
sample sizes (n) are cases with complete data. Hyphens (−) indicate data that were not available. Abbreviations: BMI = body mass index, BP = blood pressure, GA = gestational age, GDM = gestational diabetes, HELLP = hemolysis, elevated liver enzymes & low platelets syndrome, HTN = hypertension, LBW = low birth weight, MAP = mean arterial blood pressure, # = number, NICU = neonatal intensive care unit, Preg = pregnancy, WIC = eligible for the assistance from the supplemental nutrition program for low-income women, wt = weight. Bolded values are statistically significant.
Maternal hypertensive characteristics
Statewide, the incidence of each HDP -- gestational hypertension, preeclampsia, eclampsia, and HELLP syndrome -- was greater in the high- vs. low-altitude women (Table 1). In the Longitudinal Cohort, the women residing at high-compared to low-altitudes more often developed gestational hypertension or preeclampsia, had higher maximum MAP as the result of elevated DBP values, and evidenced 2nd trimester hypertension.
After controlling for the maternal background factors that were significant in Table 1, the Statewide high- vs. low-altitude women had a 33% increased risk of HDP (Table 2). Only gestational diabetes had a greater effect on the incidence of HDP than did altitude. Other factors associated with HDP were greater maternal age, non-Caucasian race, higher pre-pregnancy BMI, greater pregnancy weight gain, and more prenatal visits. Conversely, Hispanic ethnicity, qualifying for WIC or Medicaid, multiparity, and smoking lowered HDP risk. Statewide, high- compared to low-altitude women were 41% more likely to have eclampsia but did not differ with respect to HELLP syndrome after controlling for maternal background characteristics. Other factors that increased the incidence of eclampsia and HELLP Syndrome were greater BMI, more pregnancy weight gain, and gestational diabetes whereas qualifying for WIC or Medicaid and multiparity decreased their incidence.
Table 2.
Logistic and linear regression results predicting other pregnancy hypertensive conditions in the statewide and longitudinal cohorts.
| STATEWIDE COHORT | b | Adj OR | 95% CI | LONGITUDINAL COHORT | B | Adj OR | 95% CI |
|---|---|---|---|---|---|---|---|
| HDP | HDP | ||||||
| Overall model: R2=.057; χ2 =8750.4, p<.001 | Overall model: R2 =.071, χ2=17.38, p=.004 | ||||||
| Maternal age (yr) | .015 | 1.02 | 1.01–1.02 | Non-Caucasian | −.56 | 0.57 | .27–1.47 |
| Non-Caucasian | .084 | 1.09 | 1.04–1.13 | WIC/Medicaid | −.25 | 0.78 | .41–1.47 |
| Hispanic ethnicity | .207 | 0.81 | .79–.84 | Prenatal visits (#) | .11 | 1.11 | 1.02–1.21 |
| WIC/Medicaid | .122 | 0.89 | .86–.92 | Too much wt gain | .78 | 2.17 | .92–5.13 |
| Multiparity | .535 | 0.59 | .57–.60 | High altitude | .66 | 1.93 | .91–4.09 |
| Pre-preg BMI | .081 | 1.08 | 1.08–1.09 | ||||
| Preg wt gain (kg) | .043 | 1.04 | 1.04–1.04 | ||||
| Prenatal visits (#) | .009 | 1.01 | 1.01–1.02 | ||||
| Preg smoking | .063 | 0.94 | .89–.99 | ||||
| GDM | .774 | 2.15 | 2.04–2.26 | ||||
| High altitude | .291 | 1.33 | 1.20–1.48 | ||||
| ECLAMPSIA | HYPERTENSION, 2nd TRIMESTER | ||||||
| Overall model: R2=.026; χ2 = 759.9, p<.001 | Overall model: R2 = χ2= 16.50, p=.006 | ||||||
| Maternal age | .001 | 1.00 | .99–1.01 | Non-Caucasian | −.26 | .77 | .16–3.89 |
| Non-Caucasian | .102 | 1.11 | .98–1.25 | WIC/Medicaid | .74 | 2.09 | .86–5.11 |
| Hispanic ethnicity | .014 | 1.01 | .92–1.12 | Prenatal visits (#) | .09 | 1.10 | .97–1.23 |
| WIC/Medicaid | .138 | .87 | .79–.96 | Too much wt gain | .45 | 1.57 | .21–11.52 |
| Multiparity | −.55 | .58 | .53–.63 | High altitude | 1.48 | 4.38 | .90–21.27 |
| Pre-preg BMI | .066 | 1.07 | 1.06–1.08 | ||||
| Preg wt gain | .031 | 1.03 | 1.03–1.04 | ||||
| Prenatal visits (#) | −.04 | .96 | .95–1.22 | MAXIMUM DBP | |||
| Preg smoking | .048 | 1.05 | .90–1.22 | Overall model: R2=.077; F=6.78, p<.001 | |||
| GDM | .781 | 2.18 | 1.89–2.53 | Non-Caucasian | - | - | .144 |
| 1.66 | |||||||
| High altitude | .340 | 1.41 | 1.04–1.89 | WIC/Medicaid | −.75 | - | .415 |
| Prenatal visits (#) | .54 | - | <.001 | ||||
| Too much wt gain | 1.75 | - | .137 | ||||
| HELLP SYNDROME | High altitude | 1.84 | - | .068 | |||
| Overall model: R2=.036; χ2 = 761.6, p<.001 | |||||||
| Maternal age | .051 | 1.05 | 1.04–1.06 | ||||
| Non-Caucasian | .022 | 1.02 | .88–1.19 | MAXIMUM MAP | |||
| Hispanic ethnicity | −.02 | .98 | .86–1.12 | Overall model: R2=.078, F=6.83, p<.001 | |||
| WIC/Medicaid | .457 | .63 | .55–.73 | Non-Caucasian | - | - | .108 |
| 1.77 | |||||||
| Multiparity | −.94 | .39 | .35–.43 | WIC/Medicaid | .13 | - | .887 |
| Pre-preg BMI | .033 | 1.03 | 1.03–1.04 | Prenatal visits (#) | .52 | - | <.001 |
| Preg wt gain | .012 | 1.01 | 1.01–1.02 | Too much wt gain | 2.18 | - | .057 |
| Prenatal visits (#) | −.11 | .90 | .88–.91 | High altitude | 1.87 | - | .057 |
| Preg smoking | −.23 | .80 | .63–1.00 | ||||
| GDM | .854 | 2.34 | 1.95–2.82 | ||||
| High altitude | .219 | 1.24 | .88–1.76 | ||||
Notes: Shown are unstandardized betas (b) and adjusted odds ratios (OR) with 95% confidence intervals (CI) for all significant variables from Table 1 included as covariates (with the exception of too much weight gain, which was not used due to collinearity with the continuous pregnancy weight gain) and entered in a single step. Abbreviations: DBP = diastolic blood pressure, MAP = mean arterial pressure or see Table 1. Hyphens (−) indicate odds ratios that are not available for linear multiple regression with continuous outcome. Bolded values are statistically significant.
In the Longitudinal Cohort, only the number of prenatal visits was associated with HDP after controlling for maternal background characteristics. MAPs were higher during pregnancy in all women at high vs. low altitude due to the lack of a mid-pregnancy blood pressure fall (Figure 1A). While sample sizes were not sufficient to test for altitudinal differences in the specific subsamples, similar MAP differences between altitudes were evident in the groups who remained either normotensive or became hypertensive when examined separately (Figures 1B, 1C).
Figure 1.
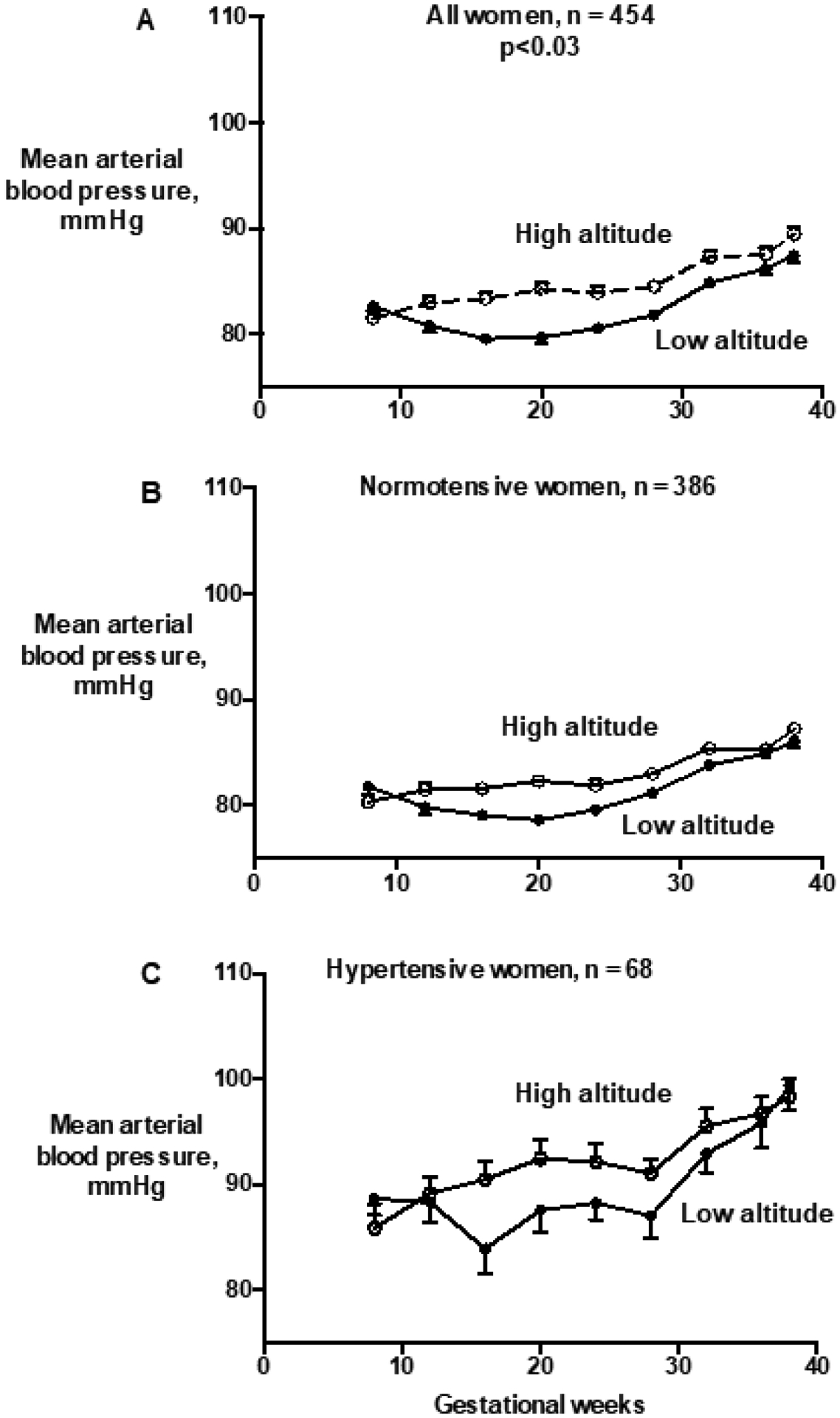
High-(≥2500 m) compared to low-(<1700 m) altitude residents matched for risk factors had higher mean arterial blood pressures (MAPs) throughout pregnancy (repeated measures ANOVA, F for full sample group effect=3.59, p=.03) due, in turn, to the lack of the mid-pregnancy blood-pressure fall seen at low altitude (panel A). Sample sizes (n) are cases with MAP data on at least four occasions. Shown are mean ± standard error of the mean (sem) values for MAP binned by gestational age groups for all longitudinally-studied high- vs. low-altitude residents (panel A), all women whose MAP remained normal (panel B), and the women with HDP (panel C). Sample sizes were not sufficient to compare the subsamples of normotensive or the women with HDP alone between altitudes, but the direction of the altitudinal differences was similar to that seen in all women.
Infant outcomes
Birth weights were lower and LBW more common at high than low altitude in both the statewide and longitudinal cohorts, but there were no significant differences in gestational age or the frequency of preterm births (Table 1). Nearly twice as many high- vs. low-altitude statewide births were small for gestational age (21.0% [20.2, 21.8] vs. 13.6% [13.6, 13.7] respectively, p<0.01). Statewide, infants at high compared to low altitude were 41% more likely to be LBW, 52% more likely to have a five-minute APGAR score <7, and 9% more likely to be admitted to a NICU (Table 3). These increases in the frequencies of LBW or worrisome APGAR scores were little changed whether altitude was considered alone or together with maternal background factors and HDP. However, after the inclusion of HDP in the model, altitude no longer predicted NICU admission which we interpreted as indicating that the link between altitude and NICU admission was driven by HDP. In the Longitudinal Cohort, high altitude increased the risk of having a LBW baby more than two-fold and more than three-fold when the influences of maternal background characteristics and HDP were considered. Sample sizes were insufficient to examine other neonatal outcomes in the Longitudinal Cohort.
Table 3.
Logistic regression results predicting adverse infant outcomes in the statewide and longitudinal cohorts.
| STATEWIDE COHORT | b | Adj OR | 95% CI | LONGITUDINAL COHORT | b | Adj OR | 95% CI |
|---|---|---|---|---|---|---|---|
| LOW BIRTH WEIGHT | LOW BIRTH WEIGHT | ||||||
| Overall model R2= .095, χ2=19694.5, p<.001 | Overall model R2= .062, χ2=10.24, p=.115 | ||||||
| Step 1: Altitude alone | .342 | 1.41 | 1.30–1.52 | Step 1: Altitude alone | 1.02 | 2.78 | 1.20–6.44 |
| Step 2: Plus significant background factors Altitude | .348 | 1.42 | 1.31–1.53 | Step 2: Plus significant background factors Altitude | 1.19 | 3.29 | 1.06–10.21 |
| Step 3: Plus HDP Altitude | .329 | 1.39 | 1.28–1.51 | Step 3: Plus HDP Altitude | 1.18 | 3.25 | 1.04–10.21 |
| 5 MINUTE APGAR SCORE < 7 | |||||||
| Overall model R2= .014, χ2=1422.0, p<.001 | |||||||
| Step 1: Altitude alone | .415 | 1.52 | 1.34–1.72 | ||||
| Step 2: Plus significant background factors Altitude | .420 | 1.52 | 1.34–1.73 | ||||
| Step 3: Plus HDP Altitude | .412 | 1.51 | 1.33–1.71 | ||||
| NICU ADMISSION | |||||||
| Overall model R2= .042, χ2=8722.9, p<.001 | |||||||
| Step 1: Altitude alone | .086 | 1.09 | 1.01–1.19 | ||||
| Step 2: Plus significant background factors Altitude | .075 | 1.08 | 1.01–1.18 | ||||
| Step 3: Plus HDP Altitude | .056 | 1.06 | .97–1.16 | ||||
Note: Shown are unstandardized regression coefficients (b), adjusted odds ratios (OR), 95% confidence intervals (CI) and p values. Step 1 for each regression was entry of altitude of residence alone, step 2 added significant background variables from Table 1 (with the exception of too much weight gain, which was not used in the statewide cohort analysis due to collinearity with pregnancy weight gain), and step 3 added hypertensive disorders of pregnancy (HDP). Bolded values are statistically significant.
Discussion
Colorado residents of high (≥2500 m) compared to low (<2500 m) altitude had a greater incidence of each of the full spectrum of HDP – gestational hypertension, preeclampsia (with or without severe features), eclampsia, and HELLP Syndrome. For all HDP combined, there was a 33% increase at high vs. low altitudes statewide after controlling for differences in maternal background characteristics. Correspondingly, the incidence of gestational hypertension and preeclampsia (with or without severe features) was greater in the subset of high- vs. low-altitude residents matched for known risk factors, and was accompanied by higher MAPs throughout pregnancy due to the lack of a mid-pregnancy blood pressure fall. The lack of mid-pregnancy fall implies that the effect of altitude on increasing the incidence of HDP is not simply due to an effect on women who are otherwise at greater risk of HDP, but rather to alterations in the maternal vascular or other characteristics responsible for the normal pregnancy reductions in blood pressure and systemic vascular resistance. Consistent with the known association between HDP and adverse infant outcomes5, babies born at high vs. low altitudes statewide were more often of LBW, had lower five-minute APGAR scores, and were more frequently admitted to NICUs.
High altitude has long been a natural laboratory for physiological studies of the mechanisms governing oxygen supply. While it is well known that high-altitude residence slows fetal growth and hence lowers birth weight,16 it has been proposed more recently that this was due, in part, to an increased incidence of HDP.2,3,17–19 While prior studies1,2,10 using contemporaneous diagnostic criteria20,21 demonstrated a positive correlation between altitude and gestational hypertension or preeclampsia, this is the first to show that residence at high altitude increased the incidence of all HDP – including eclampsia and HELLP Syndrome – using current HDP-diagnostic criteria5. It is also the first to show that residence at high altitude not only increases the rate of LBW but also NICU admissions, with the latter being due principally to the increased occurrence of HDP.
Our study benefitted from large sample sizes and the availability of statewide birth-certificate data that included the actual altitude of maternal residence and more complete enumeration of risk factors than previously available. While women may have moved to a lower (or higher) altitude during pregnancy, we considered that such moves were unlikely to have affected a large number of persons. Further, more HDP were also observed in the Longitudinal Cohort whose residential altitude was recorded during pregnancy. We recognize that HDP incidence was higher in the Longitudinal than Statewide Cohorts, and propose that this was due to the closer analysis of data permitted by individual chart review.
Our study was limited by the lack of data from sea-level residents, as no point in Colorado is located below 1000 m. We therefore used <2500 m in the Statewide Cohort and <1700 m in the Longitudinal Cohort. We defend the use of different altitude intervals for the Statewide and Longitudinal Cohorts on the basis that most (59%) of the women comprising the low-altitude statewide group lived <1700 m and the medium-altitude regions of Colorado are broadly dispersed and therefore without a single prenatal clinic or delivery site whose records could be accessed. We recognize that different low-altitude cutoffs and years were represented in the two cohorts, and considered reporting their results separately. However, we elected to retain both here since each has unique strengths that permit their combination to convey more information than would be the case if each were published separately. Specifically, the large size and heterogenous nature of the Statewide Cohort allowed us to establish an association between altitude and the relatively rare but life-threatening disorders of eclampsia and HELLP, whereas the unique data on blood pressure course throughout pregnancy in the Longitudinal Cohort permitted us to assess physiological processes likely contributing the adverse effects of altitude. We justify ≥2500 m for defining high altitude based on previous research showing that that is where birth weight and arterial O2 saturation begin to fall.22 We recognize that our statewide analyses were only able to explain ~6% of the variation in HDP, which we interpret as reflecting the many determinants of HDP that are not measured on birth certificates, in medical records, or are as yet unknown. Additionally, we were observing relationships, not causal factors; for example, more prenatal visits were probably associated with HDP because clinicians concerned about HDP increase the number of prenatal visits. Our most serious limitation was the lack of requisite data for distinguishing gestational hypertension from preeclampsia with vs. without severe features (i.e., proteinuria and/or thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema, or cerebral or visual symptoms) since such data are not recorded on birth certificates nor regularly in medical records.
Future studies with better documentation regarding the time of onset and diagnostic signs and symptoms for HDP are needed at high altitude, not only to distinguish preeclampsia from gestational hypertension and preeclampsia with vs. without severe features, but also to assess their relationships to cardiovascular and other diseases later-in-life.23 While the number of high-altitude residents in Colorado is small, some 87 million persons visit Colorado and its mountain locations annually24, and information is currently lacking on the possible effects of short-term altitude exposure on pregnant visitors. Additionally, many individuals live at high altitude in low to middle-income countries (LMIC) worldwide where pregnancy complications are leading causes of high maternal and infant mortality rates.19,25–27 While every pregnancy is at risk for HDP, our data support increased clinical surveillance in high-altitude residents with the overall goal of decreasing morbidity and mortality. Finally and on a different note, while the NIH and other agencies have called for new treatments for HDP, one of the limitations faced by such studies is the large number needed-to-treat when the incidence is low and therefore the substantial reduction in that number at greater baseline-event rates (e.g., 500 [313–1667, 95% CI] needed-to-treat at a 2% vs. 56 [35–185, 95% CI] needed-to-treat at a 18% baseline event rate, respectively28). Based on the current results, high-altitude locales could be attractive study sites for evaluating the efficacy of predictive biomarkers, existing preventive therapies such as low-dose aspirin, or new therapies being identified by our and other’s recent studies29–31.
In conclusion, residence at high altitude constitutes a risk factor for HDP given that it increases the incidence of the full spectrum of HDP, and that this association has persisted over several decades and across multiple diagnostic criteria. The increased HDP incidence impacts maternal health but also adversely affects infant well-being. Thus high-altitude studies provide a natural laboratory for understanding pregnancy complications characterized by intrauterine hypoxia, offer residents important public health information, and could provide valuable study sites for future translational studies.
Acknowledgements
We thank Jared Ahrendsen, Heather Boucher, Yasmine Dakhama, Javier Gutierrez, Pete Julian, Trenton Klimper, Bill Moore, Barbara Neistadt, and Mayi Gnofam for their help with the acquisition of the medical-records data from the longitudinally-studied subjects. We also thank the many physicians and staff members who also helped make the collection of these data possible.
Funding details: This work was supported by the NIH Grants HD088590, HD057022, and the Colorado Center for Women’s Health Research.
Footnotes
Disclosure statement: The authors report no conflicts of interest.
Data availability statement: The data for this study are available from the corresponding author (LGM) upon reasonable request.
Health and Safety: All mandatory laboratory health and safety procedures have been complied with in the course of conducting the studies described in this paper.
References
- 1.Moore LG, Hershey DW, Jahnigen D, Bowes W Jr. The incidence of pregnancy-induced hypertension is increased among Colorado residents at high altitude. American journal of obstetrics and gynecology. 1982;144(4):423–429. [DOI] [PubMed] [Google Scholar]
- 2.Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health. 1997;87(6):1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res. 2003;54(1):20–25. [DOI] [PubMed] [Google Scholar]
- 4.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: regional and life-cycle perspectives. Am J Phys Anthropol. 1998;Suppl 27:25–64. [DOI] [PubMed] [Google Scholar]
- 5.ACOG. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. [DOI] [PubMed] [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. Bmj. 2007;335(7627):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CW, Jaffe IZ, Karumanchi SA. Pre-eclampsia and cardiovascular disease. Cardiovasc Res. 2014;101(4):579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurbasic A, Fraser A, Mogren I, et al. Maternal Hypertensive Disorders of Pregnancy and Offspring Risk of Hypertension: A Population-Based Cohort and Sibling Study. Am J Hypertens. 2019;32(4):331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andraweera PH, Lassi ZS. Cardiovascular Risk Factors in Offspring of Preeclamptic Pregnancies-Systematic Review and Meta-Analysis. The Journal of pediatrics. 2019;208:104–113 e106. [DOI] [PubMed] [Google Scholar]
- 10.Palmer SK, Moore LG, Young D, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. American journal of obstetrics and gynecology. 1999;180(5):1161–1168. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. [DOI] [PubMed] [Google Scholar]
- 12.Desktop PBM. https://www.pitneybowes.com/us/location-intelligence/geographic-information-systems/mapmarker.html. Accessed.
- 13.ESRl. http://www.esri.com. Published Arc GIS software. Accessed. [Google Scholar]
- 14.American College of Obstetricians and Gynecologists CON. Weight gain during pregnancy. Obstet Gynecol 2013;121:210–212. [DOI] [PubMed] [Google Scholar]
- 15.Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstet Gynecol. 2014;124(1):16–22. [DOI] [PubMed] [Google Scholar]
- 16.Lichty JA, Ting RY, Bruns PD, Dyar E. Studies of babies born at high altitudes. I. Relation of altitude to birth weight. American journal of diseases of children. 1957;93(6):666–669. [DOI] [PubMed] [Google Scholar]
- 17.Miller S, Tudor C, Nyima, et al. Maternal and neonatal outcomes of hospital vaginal deliveries in Tibet. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2007;98(3):217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzales GF, Tapia V. Birth weight charts for gestational age in 63,620 healthy infants born in Peruvian public hospitals at low and at high altitude. Acta paediatrica. 2009;98(3):454–458. [DOI] [PubMed] [Google Scholar]
- 19.de la Gálvez Murillo A. Atención obstétrica y complicaciones del embarazo y parto en Bolivia. Cuadernos Hospital de Clínicas. 2009;54(100–107). [Google Scholar]
- 20.Gant NF, Worley RJ. Differential diagnosis of hypertension in pregnancy. Clin Obstet Gynaecol. 1977;4(3):613–633. [PubMed] [Google Scholar]
- 21.ACOG. Hypertension in Pregnancy. ACOG Technical Bulletin No: 219. 1996. [Google Scholar]
- 22.Mortola JP, Frappell PB, Aguero L, Armstrong K. Birth weight and altitude: a study in Peruvian communities. The Journal of pediatrics. 2000;136(3):324–329. [DOI] [PubMed] [Google Scholar]
- 23.Cain MA, Salemi JL, Tanner JP, Kirby RS, Salihu HM, Louis JM. Pregnancy as a window to future health: maternal placental syndromes and short-term cardiovascular outcomes. American journal of obstetrics and gynecology. 2016;215(4):484 e481–484 e414. [DOI] [PubMed] [Google Scholar]
- 24.Wenzel J Colorado’s record tourism growth hits new milestone: 96 million visitors, $1.28 billion in tax revenue. The Denver Post. https://www.denverpost.com/2018/06/28/colorado-tourism-record-2017/. Published 2018. Updated June 29, 2018. Accessed.
- 25.Roost M, Altamirano VC, Liljestrand J, Essen B. Priorities in emergency obstetric care in Bolivia--maternal mortality and near-miss morbidity in metropolitan La Paz. BJOG : an international journal of obstetrics and gynaecology. 2009;116(9):1210–1217. [DOI] [PubMed] [Google Scholar]
- 26.Campero Nava A, Parada Barba C, Mamani Huallpa G, Rios Vacaflor M, Flores Velasco O, Enriquez Nava M. Estudio Nacional de Mortalidad Materna 2011 Bolivia: resumen ejecutivo. In: Md Salud, ed. Vol 44. La Paz, BO: Ministerio de Salud; 2016:1–98. [Google Scholar]
- 27.Toledo-Jaldin L, Bull S, Contag S, et al. Critical barriers for preeclampsia diagnosis and treatment in low-resource settings: An example from Bolivia. Pregnancy Hypertens. 2019;16:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Askie LM, Duley L, Henderson-Smart DJ, Stewart LA, Group PC. Antiplatelet agents for prevention of pre-eclampsia: a meta-analysis of individual patient data. Lancet. 2007;369(9575):1791–1798. [DOI] [PubMed] [Google Scholar]
- 29.Nawathe A, David AL. Prophylaxis and treatment of foetal growth restriction. Best Pract Res Clin Obstet Gynaecol. 2018;49:66–78. [DOI] [PubMed] [Google Scholar]
- 30.Lane SL, Houck JA, Doyle AS, et al. AMP-activated protein kinase activator AICAR attenuates hypoxia-induced fetal growth restriction in mice by improving uterine artery blood flow. The Journal of physiology. 2020:in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lane SL, Dodson RB, Doyle AS, et al. Pharmacological activation of peroxisome proliferator-activated receptor gamma (PPAR-gamma) protects against hypoxia-associated fetal growth restriction. FASEB J. 2019;33(8):8999–9007. [DOI] [PMC free article] [PubMed] [Google Scholar]


