Abstract
An extra X chromosome occurs in ~1 in 1,000 females, resulting in a karyotype 47,XXX also known as trisomy X syndrome (TXS). Women with TXS appear to be at increased risk for premature ovarian insufficiency, however very little research on this relationship has been conducted. The objective of this case-control study is to compare ovarian function, as measured by anti-mullerian hormone (AMH) levels, between girls with TXS and controls. Serum AMH concentrations were compared between 15 females with TXS (median age 13.4 years) and 26 controls (median age 15.1 years). Females with TXS had significantly lower serum AMH compared to controls (0.7 ng/mL (IQR 0.2–1.7) vs 2.7 (IQR 1.3–4.8), p<0.001). Additionally, girls with TXS were much more likely to have an AMH below the 2.5th percentile for age with 67% of them meeting this criteria (OR 11, 95% CI 2.3–42). Lower AMH concentrations in females with TXS may represent an increased risk for primary ovarian insufficiency in these patients and potentially a narrow window of opportunity to pursue fertility preservation options. Additional research is needed to understand the natural history of low AMH concentrations and future risk of premature ovarian insufficiency in girls with TXS.
Keywords: trisomy X syndrome (TXS), primary ovarian insufficiency (POI), anti-mullerian hormone (AMH), sex chromosome aneuploidy, fertility
INTRODUCTION
Trisomy X syndrome (TXS) is a sex chromosome aneuploidy caused by the presence of an extra X chromosome, yielding a karyotype of 47,XXX in affected females [1]. This chromosomal aneuploidy occurs in approximately 1 in 1,000 live female births [2]. Despite the high prevalence of this condition, it is suspected that only about 10% of females with TXS are actually diagnosed [3, 4]. A wide range of medical and psychological conditions are found in association with TXS, however some girls and women may be minimally affected. The most common clinical features of TXS include tall stature, hypotonia in infancy, clinodactyly, epicanthal folds, and constipation [5]. Less frequent, but still present in up to 1220%, include seizure disorders, genitourinary malformations, intention tremor, and congenital hip dysplasia [6]. There is also an increased risk of neuropsychological difficulties compared to unaffected females, including developmental delays, particularly speech and language deficits, learning disabilities, attention deficit hyperactivity disorder, anxiety, depression, adjustment disorders, autism spectrum disorder, and social immaturity [7–9].
Diminished ovarian reserve and an acceleration of the loss of ovarian function have long been described in women with TXS. The initial case report of TXS by Jacobs et al in 1959 described a woman who presented at age 19 with secondary amenorrhea [1]. Since then, a dozen case reports have described premature ovarian insufficiency (POI) in TXS [10–20], however there is a paucity of data on its prevalence in women with TXS. The clinical definition of POI requires four months of amenorrhea along with elevated Follicle Stimulating Hormone (FSH) prior to the age of 40, and is associated with infertility, osteoporosis, and psychological distress [21, 22]. Studies have found that up to 3% of women with POI have a 47,XXX karyotype [17], however the prevalence of ovarian dysfunction among individuals with TXS is unknown. Birth cohorts from the 1960–70s report the majority of girls with TXS experience normal pubertal onset, menses, and fertility; however there were also reports of both early and delayed puberty as well as secondary amenorrhea [23]. A cross-sectional study of 15 peripubertal girls with TXS reported abnormal hypothalamic-pituitary-gonadal function with higher basal and stimulated gonadotropins as well as decreased ovarian volumes compared to controls [24]. Overall, the literature points to a relationship between TXS and ovarian dysfunction, however the data are limited.
Anti-mullerian hormone (AMH) is produced by ovarian follicles from childhood through the adult years, with a decline to undetectable levels at menopause. AMH is a widely used biomarker of ovarian reserve as it indirectly reflects the primordial follicle pool and subsequently the remaining length of a woman’s reproductive lifespan [25]. AMH is produced by granulosa cells of growing ovarian follicles beginning at the secondary stage and the serum concentrations are largely independent of gonadotropins or the menstrual cycle, making it more reliable and consistent relative to other biomarkers of ovarian reserve. AMH is currently being evaluated for its efficacy in estimating ovarian reserve and predicting POI in other at-risk pediatric populations, most notably Turner syndrome [26–28]. To our knowledge, no studies have evaluated concentrations of AMH in females with TXS, and thus the objective of this study was to assess AMH as a biomarker of ovarian function in girls with this condition.
METHODS
Study design and sample population
This is a case-control study comparing participants with TXS to female controls without TXS. Cases (N=15) included females with TXS who were seen in the eXtraordinarY Kids Clinic at Children’s Hospital Colorado [29] between December 2015 and August 2019 who had AMH measured as part of a comprehensive clinical assessment. Controls (N=26) included females who were enrolled in a study to assess cardiovascular health and all had regular menses. Females taking oral contraception, had a history of pregnancy, or a diagnosis of polycystic ovarian syndrome were excluded from this study for both cases and controls [30]. In addition, females with a known monosomy X cell line (ie Turner syndrome) were excluded as this is well-known to affect ovarian function. The studies were approved by the local institutional review board (COMIRB #08–0512 and #10–1288) and all parents provided written informed consent and girls provided assent as age appropriate.
Study procedures
The diagnosis of TXS syndrome in cases was confirmed by review of the patient’s karyotype and/or chromosomal microarray. Hormone concentrations were measured from standard venipuncture serum samples. When possible, labs for post-menarchal females were obtained on the third day of the menstrual cycle. Additional data collection from review of medical records included age at menarche, menstrual pattern, and medical and neurodevelopmental/psychological comorbidities.
All AMH and estradiol (E2) concentrations for both cases and controls were measured at Esoterix Laboratory Services, Calabasas Hills, CA between May 2009 and May 2019. Prior to June 2014, AMH was measured via a commercially available Beckman Coulter Gen II AMH ELISA assay; the lower limit of detection for this assay was 0.13 ng/mL, intra- and inter-assay CV were <12% and <18% respectively. In July 2014, Esoterix switched to an in-house electrochemiluminescent assay with lower limit of quantification of 0.015ng/mL and intra- and inter-assay CV <3% and <13% respectively. Comparison of the two assays demonstrated good correlation (Deming regression y = 0.94x − 0.0498, R=0.97). AMH values obtained prior to July 2014 were adjusted based on the equation derived by the laboratory to allow comparison. E2 concentrations, when obtained, were measured via high-powered liquid chromatography tandem mass spectroscopy. Lower limit of quantification for this assay is 1.0 pg/mL, intra- and inter-assay CV are <9% and <12% respectively. FSH and LH levels, when obtained, were measured in-house via Siemens Immulite 2000XPI assays at the Children’s Hospital Colorado chemistry laboratory in Aurora, CO, with lower limits of quantification of 0.10 and 0.10, and with intra- and inter-assay CV of 4.52% /2.89% and 4.91%/4.80% respectively.
Statistical Analysis
Data were examined for normality by plotting and Shapiro-Wilk test; outliers were not excluded given the small sample size. Median and interquartile ranges (IQR) were used to summarize data. A two-sided Wilcoxon Signed Rank test was used to compare the AMH and estradiol concentrations between groups. The proportion of patients with an AMH below the laboratory provided normal range for females 7–25 years (<1.05ng/ml) was calculated and compared between groups using Fisher’s exact test. If more than one AMH was obtained for a single participant, only the first one was used in these analyses. Finally, a receiver operating characteristic (ROC) curve was created to identify the sensitivity and specificity of AMH for predicting TXS. Analyses were conducted using GraphPad Prism version 8.1.1 for MacOS, GraphPad Software, San Diego, California USA, www.graphpad.com.
RESULTS
Descriptive characteristics for the cases (n=15) and controls (n=26) are reported in Table 1. Nine of the 15 girls with TXS (60%) had a prenatal TXS diagnosis. The median age of TXS diagnosis in those diagnosed after birth was 8.5 years; one patient was diagnosed at 12 months of age, five patients were diagnosed between 8 and 12 years of age for reasons that included concern for developmental delays and/or dysmorphic features. None of the patients were diagnosed for pubertal or reproductive concerns. Body mass index (BMI) was lower in TXS participants and the prevalence neuropsychological conditions were higher. Co-existing medical conditions were present in 60% of the TXS group and included atopic conditions, eosinophilic esophagitis, hypothyroidism, constipation, type 2 diabetes, epilepsy, hypertension, pulmonary embolism, stroke, ventriculoseptal defect, dysplastic kidney, and postural orthostatic tachycardia syndrome. Medical conditions affected 46% of the control group and included atopic conditions, hypothyroidism, migraines, gastroesophageal reflux disease, obstructive sleep apnea, and non-alcoholic steatohepatitis. Every patient with TXS had one or more neuropsychological diagnosis including attention deficit hyperactivity disorder, anxiety, autism spectrum disorder, intellectual disability, depression, learning disability, trichotillomania, obsessive compulsive disorder, and bipolar disorder. In comparison, only 23% of controls had documented neuropsychological conditions including anxiety, depression, and learning disabilities.
Table 1.
Participant characteristics
| TXS N=15 | Control N=26 | P value | |
|---|---|---|---|
| Age in years, median (range) | 13.4 (5.4–24.2) | 15.0 (13.2–17.0) | 0.500 |
| Height SDS, mean (SD) | 1.05 (0.9) | 0.01 (1.2) | 0.004 |
| BMI SDS, mean (SD) | −0.42 (1.5) | 1.6 (0.78) | <0.001 |
| Chronic medical conditions, N (%) | 9 (60) | 12 (46) | 0.400 |
| Chronic ND/psych conditions, N (%) | 15 (100) | 6 (23) | <0.001 |
TXS= Trisomy X syndrome; AMH= Anti-mullerian hormone; ND= neurodevelopmental; SDS=standard deviation score
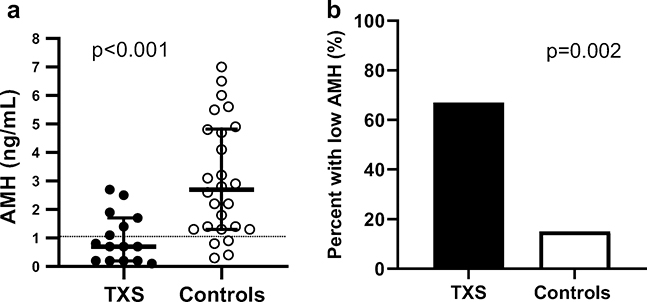
The median AMH for TXS participants was significantly lower than that of controls (0.7 ng/mL (IQR 0.2–1.7) vs 2.7 (IQR 1.3–4.9), p<0.001, Figure 1). All 15 of the participants with TXS had serum AMH concentrations well below the median for the assay and this did not correlate with age (Figure 2a). In addition, girls with TXS had 11 times greater odds of having a low AMH (< 2.5th percentile for the assay) compared to controls (95% CI 2.3–42, p=0.001, Figure 2b). Using the standard laboratory cutoff for low AMH (<1.05 ng/dl) had a 60% sensitivity and 85% specificity for TXS. The AMH cutoff value that gave the highest sensitivity and specificity for TXS was an AMH <1.75 ng/dl (sensitivity 80% with 95%CI 55–93% and specificity 65% with 95%CI 46–81%). The overall ROC model was significant with R=0.83, p<0.0004. Four girls with TXS had repeat AMH measured. Repeat AMH remained in the normal range for the one patient who had an initial AMH in the normal range and remained low for the other three patients who had a low initial AMH. Gonadotropin concentrations (LH and FSH) were within normal ranges for the nine TXS participants who had them measured, despite their low AMH. Controls did not have gonadotropins measured so this could not be compared. Estradiol concentrations were similar between cases and controls (median 42 (IQR 27–66) vs 46 (IQR 29–74, p>0.1).
Figure 1.
AMH concentrations in adolescents with trisomy X syndrome (black circles) and controls (open circles) was not related to age. The light gray shaded area between dashed lines represents normal range of AMH for the assay. Cases < 11 years (n=2) and >19 years (n=1) were excluded from this figure but AMH concentrations for these individuals can be found in Table 2. AMH= anti-mullerian hormone; TXS= trisomy X syndrome.
Figure 2.
(A) Median Anti-Mullerian Hormone (AMH) concentrations in girls with trisomy X syndrome (TXS; black circles) are significantly lower than controls (open circles). Bars represent the median and IQR. (B) Girls with TXS have an 11 times greater odds of having an AMH below 1.05 ng/ml (<2.5th percentile) compared to controls.
Twelve of the 15 TXS participants had spontaneous menarche between 11.5 and 14 years of age (median 12.5 IQR 11.5, 12.8). The three girls who were premenarchal were 5.4 years, 9.7 years, and 13.4 years old. The 13.4 year old had not yet had thelarche, consistent with a diagnosis of delayed puberty. Pubertal onset and tempo was otherwise within the normal range. Of the 12 girls who were menstruating, eight reported having regular menstrual cycles while four reported irregular menstrual cycles, although two of the four were within two years of menarche therefore irregular cycles may be normal. Participant-level details for menarche, menstrual pattern, and hormone concentrations for girls with TXS are reported in Table 2. By inclusion criteria, all girls in the control group had menarche within a normal age range and were having regular menses at the time of assessment.
Table 2.
AMH, gonadotropin, and estradiol levels in patients with TXS
| Patient | Age at AMH (y) | AMH (ng/mL) | FSH (mIU/mL) | LH (mIU/mL) | E2 (pg/mL) | Menarche (y) | Menstrual pattern |
|---|---|---|---|---|---|---|---|
| 1 | 5.4 | 0.7 | - | - | - | N/A | N/A |
| 2 | 9.7 | 1.4 | 4.3 | 0.32 | 8.8 | N/A | N/A |
| 3 | 11.4 | 0.8 | 6.1 | 3.1 | 37 | 13.3 | Regular |
| 4 | 12.4 | 2.7 | 7.4 | 4.77 | 52 | 12 | Regular |
| 13.5 | 3.2 | 3.3 | 1.4 | 20 | - | ||
| 15.0 | 3.2 | - | - | - | - | ||
| 5 | 12.9 | 0.2 | 7.4 | 3.4 | - | 11.5 | Irregular |
| 13.0 | 0.5 | 6.2 | 4.8 | 49 | - | ||
| 6 | 13.1 | 0.7 | - | - | - | 11.5 | Regular |
| 7 | 13.3 | 0.2 | 12.4 | 8.28 | 42 | 12.8 | Regular |
| 14.5 | 0.2 | 12.2 | 7.0 | 11 | - | ||
| 8 | 13.4 | 0.2 | 7.8 | 2.61 | - | N/A | N/A |
| 9 | 13.9 | 0.1 | - | - | - | 12.5 | Irregular |
| 10 | 14.6 | 1.1 | - | 3.24 | 27 | 13 | Regular |
| 11 | 16.7 | 1.9 | 2.8 | 3.6 | - | 12.5 | Irregular |
| 12 | 16.9 | 1.7 | - | - | - | 14 | Regular |
| 13 | 17.9 | 0.2 | - | - | - | 12.5 | Regular |
| 14 | 18.5 | 0.7 | 5.5 | 4.44 | 85 | 11.5 | Irregular |
| 18.7 | 0.3 | 6.0 | 2.0 | 59 | - | ||
| 15 | 19.7 | 2.5 | 5.5 | 6.57 | 66 | 11 | Regular |
| Median | 13.4 | 0.7 | 6.1 | 3.5 | 42.0 | 12.5 | |
| IQR | 12.4–16.9 | 0.2–1.7 | 4.9–7.6 | 3.0–5.2 | 27–66 | 11.5–12.8 |
AMH= anti-mullerian hormone; FSH= follicle stimulating hormone; LH= luteinizing hormone; E2= estradiol; TXS= trisomy X syndrome. Dashes in FSH, LH, and E2 columns indicate that these labs were not drawn for respective participants. Dashes in menarche column indicate date of menarche not recorded in medical charts of respective participants.
DISCUSSION
This case-control study is the first report of AMH concentrations in girls with TXS, concluding AMH is lower in this cohort than in female adolescent controls. As AMH is a biomarker of ovarian follicular reserve and women with TXS are known to be an at-risk population, it is likely these lower AMH concentrations represent diminished ovarian reserve and an increased risk for future POI [31, 32]. Notably, two thirds of girls with TXS in this cohort had AMH levels below the range of normal for age. Therefore, these data strongly support that diminished ovarian reserve is common in youth with TXS, and that the inclusion of AMH measurement in this population may be highly informative for reproductive counseling.
There has been minimal prior investigation into ovarian function in youth with TXS. In 2016, Stagi et. al published a cross-sectional study evaluating the hypothalamic-pituitary-gonadal axis in 15 girls age 7–12 years with TXS compared to age- and pubertal-stage matched females without TXS undergoing evaluation for possible early puberty [24]. They found that girls with TXS had higher basal and stimulated LH and FSH concentrations and lower estradiol and inhibin B. Girls with TXS also had significantly smaller ovarian volumes than controls. Although they did not measure AMH, these data are congruent with our study suggesting frequent ovarian dysfunction in girls with TXS. That study also found earlier pubertal onset, defined as development of pubertal changes before 9 years of age, in the girls with TXS, which has also been reported in longitudinal birth cohorts. Although one girl in our study had premature thelarche, normal or even delayed pubertal onset was observed in the others. Prior to the study by Stagi et al and our current data, most observational studies have reported normal puberty and fertility in girls with TXS, including many pregnancies and live births [33]. However these studies were small and assessments may not have been sensitive enough to detect more subtle differences. Additional research is needed to understand the natural history of ovarian function in longitudinal cohorts of girls with TXS throughout the lifespan.
Potential etiologies of POI in TXS have been proposed, including congenital malformations resulting in abnormal ovarian development or localized ovarian autoimmunity [16, 17, 15, 24, 34], however structural defects and autoimmune conditions affect a minority of females with TXS. Additional possible explanations for POI in this population include disrupted regulation or mutations, duplications, or deletions in POI candidate genes on the X chromosome, such as POF1B, STS, XNPEP2, FMR-1 and USP9X to name a few [35–37]. Overexpression of pro-apoptotic genes on the X chromosome, such as in AIFM1, or under-expression of anti-apoptotic genes, such as in BCORL1 may also contribute to POI in sex chromosome abnormalities [37–39]. Finally, tissue-specific mosaicism for a monosomy X cell line is difficult to rule out. It is clear that further investigation is needed to understand the ovarian pathophysiology occurring in females with TXS.
Fertility preservation options, including oocyte cryopreservation, are clinically available for post-menarchal females at high risk of POI, and experimental options such as ovarian cryopreservation are available in some centers [40]. Recommendations for fertility counseling and preservation exist for patients at high risk for infertility due to iatrogenic etiologies such as radiation, chemotherapy, or other causes [41]. Currently, there is far less information available to assist providers in facilitating long-term discussions surrounding future fertility for patients with genetic conditions known to be associated with higher rates of infertility at earlier ages [42]. Previous studies exploring attitudes and healthcare experiences in patients at risk for infertility have shown that patients and their parents are often confused or uncertain about fertility questions. Many report feeling uncomfortable broaching these discussions and thus rely on the pediatric provider to initiate the conversation [42, 43]. Furthermore, absence of disclosure of or discussion about fertility topics by pediatric providers can lead to serious distress later in life for the affected adolescent/adult [42]. It is important that pediatric providers be able to identify pediatric populations at-risk for future infertility and to discuss what is known in terms of their specific risk with affected families. This needs to be done in coordination with specialists who can discuss fertility preservation options before the window of opportunity to pursue these options has run out. Further, consideration of the neurodevelopmental and psychological comorbidities in TXS in the approach to counseling and potential medical procedures associated with fertility preservation is important and may require adaptations and collaborations across different disciplines to support individuals with TXS and their families in decision-making. This study and available literature suggest that TXS is a condition in need of additional research on patient-centered outcomes for fertility counseling and preservation.
Strengths of this study include the relatively large cohort of young females with TXS and measurement of AMH at the same commercial laboratory. The most significant limitation of this study is the lack of longitudinal clinical outcomes, such as amenorrhea, consecutive FSH levels, antral follicle count, or infertility, that would be needed to confirm future POI in the girls with low AMH. Therefore, we can only infer that low AMH in TXS has the same implications as for typical females. Another limitation is that our cohort of girls with TXS had lower BMI and a greater number of comorbidities than our controls, which is not unexpected for this population. However, BMI alone without a concurrent diagnosis of PCOS does not appear to be associated with AMH concentrations [44, 45]. Psychological stress may be associated with lower AMH concentrations according to a recent study, which may have implications in our cohort given the high rates of psychological diagnoses [46]. Likewise, females with chronic kidney disease as well as chronic rheumatological conditions have been reported to have lower AMH concentrations, and the impact of other chronic illnesses on AMH is largely unknown [47, 48]. Whether medical or psychological comorbidities contribute to differences in serum AMH or future ovarian function in TXS is unknown and more work is needed to understand these relationships. Despite these limitations, AMH is widely accepted as a reliable marker of ovarian follicular reserve and our data are an important addition to the literature.
CONCLUSION
In conclusion, children and adolescents with TXS have lower than normal serum AMH concentrations despite normal menarche and menstrual patterns. Low AMH, as a biomarker of ovarian reserve, may indicate an increased risk for eventual POI in these patients and potentially a narrow window of opportunity to pursue fertility preservation options. While further investigation is needed to determine significance of this finding and its role in clinical practice, clinical evaluation of AMH could be considered in post-menarchal females with TXS, especially if they experience irregular menses or secondary amenorrhea, with referral to a gynecologist or reproductive endocrinologist for further counseling. Additional research is needed to understand the natural history of low AMH concentrations in girls with TXS, the mechanisms and pathophysiology of diminished ovarian reserve, and correlation with clinical outcomes such as fertility, age of menopause, and potentially additional impacts on psychologic, cardiometabolic, and bone health.
ACKNOWLEDGEMENTS
The authors wish to thank the study participants in the eXtraordinarY Kids Clinic and their families. We also acknowledge Esoterix Laboratories, in particular Walt Chandler, PhD, Kelly Chun, PhD, and Steve Phagoo, PhD. Additionally, we acknowledge funding from the National Center for Advancing Translational Sciences (NIH/NCATS) Colorado CTSA (UL1TR002535), the National Institute of Child Health and Human Development (NIH/NICHD) (K23HD092588), the American Heart Association (13CRP14120015), the National Institute of Digestion, Diabetes, and Kidney Disease (NIH/NIDDK) (K23DK107871), the University of Colorado Departments of Obstetrics and Gynecology and Pediatrics. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views.
Funding: This work was financially supported by the following: National Institute of Child Health and Human Development (NIH/NICHD) (K23HD092588), National Institute of Digestion, Diabetes, and Kidney Disease (NIH/NIDDK) (K23DK107871), National Center for Advancing Translational Sciences (NIH/NCATS) Colorado CTSA (UL1TR002535), American Heart Association (13CRP14120015), University of Colorado Department of Obstetrics and Gynecology Research Funds, and University of Colorado Department of Pediatrics. Contents are the authors’ sole responsibility and do not necessarily represent official NIH or AHA views.
Footnotes
DECLARATIONS
Conflicts of interest/Competing interests: NT and SD serve as medical advisors and SH on the board of directors for the advocacy group AXYS (Association for X&Y syndromes).
Consent to participate: The local institutional review board (COMIRB #08–0512 and #10–1288) approved these protocols and all parents provided written informed consent and girls provided assent as age appropriate. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent for publication: N/A
Availability of data and material: All data generated or analyzed during this study are included in this published article.
Code availability: N/A
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Jacobs P, Baikie A, Brown W, Macgregor T, Maclean N, Harnden D. Evidence for the existence of the human “superfemale”. Lancet. 1959:423–5. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs P The incidence and etiology of sex chromosome abnormalities in man. Birth defects original article series. 1979;15:3–14. [PubMed] [Google Scholar]
- 3.Nielsen J, Wohlert M. Sex chromosome abnormalities found among 34,910 newborn children: results from a 13-year incidence study in Arhus, Denmark. Birth defects original article series. 1990;26(4):209–23. [PubMed] [Google Scholar]
- 4.Berglund A, Viuff MH, Skakkebaek A, Chang S, Stochholm K, Gravholt CH. Changes in the cohort composition of turner syndrome and severe non-diagnosis of Klinefelter, 47,XXX and 47,XYY syndrome: a nationwide cohort study. Orphanet journal of rare diseases. 2019;14(1):16. doi: 10.1186/s13023-0180976-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tartaglia NR, Howell S, Sutherland A, Wilson R, Wilson L. A review of trisomy X (47,XXX). Orphanet journal of rare diseases. 2010;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tartaglia N Medical Problems and Follow-up in Trisomy X Syndrome. KS&A Scientific Advisory Committee Conference; Los Angeles2009. [Google Scholar]
- 7.Tartaglia NR, Ayari N, Hutaff-Lee C, Boada R. Attention-deficit hyperactivity disorder symptoms in children and adolescents with sex chromosome aneuploidy: XXY, XXX, XYY, and XXYY. Journal of developmental and behavioral pediatrics : JDBP. 2012;33(4):309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linden MG, Bender BG. Fifty-one prenatally diagnosed children and adolescents with sex chromosome abnormalities. American journal of medical genetics. 2002;110(1):11–8. doi: 10.1002/ajmg.10394. [DOI] [PubMed] [Google Scholar]
- 9.Otter M, Schrander-Stumpel CT, Didden R, Curfs LM. The psychiatric phenotype in triple X syndrome: new hypotheses illustrated in two cases. Dev Neurorehabil. 2012;15(3):233–8. doi: 10.3109/17518423.2012.655799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugawara N, Maeda M, Manome T, Nagai R, Araki Y. Patients with 47, XXX karyotype who experienced premature ovarian failure (POF): two case reports. Reprod Med Biol. 2013;12(4):193–5. doi: 10.1007/s12522-013-0158-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skalba P, Cygal A, Gierzynska Z. A case of premature ovarian failure (POF) in a 31-year-old woman with a 47,XXX karyotype. Endokrynol Pol. 2010;61(2):217–9. [PubMed] [Google Scholar]
- 12.Itu M, Neelam T, Ammini AC, Kucheria K. Primary amenorrhoea in a triple X female. Aust N Z J Obstet Gynaecol. 1990;30(4):386–8. doi: 10.1111/j.1479-828x.1990.tb02039.x. [DOI] [PubMed] [Google Scholar]
- 13.Menon V, Edwards RL, Butt WR, Bluck M, Lynch SS. Review of 59 patients with hypergonadotrophic amenorrhoea. Br J Obstet Gynaecol. 1984;91(1):63–6. doi: 10.1111/j.1471-0528.1984.tb05280.x. [DOI] [PubMed] [Google Scholar]
- 14.Smith HC, Seale JP, Posen S. Premature ovarian failure in a triple X female. J Obstet Gynaecol Br Commonw. 1974;81(5):405–9. doi: 10.1111/j.1471-0528.1974.tb00488.x. [DOI] [PubMed] [Google Scholar]
- 15.Holland CM. 47,XXX in an adolescent with premature ovarian failure and autoimmune disease. Journal of pediatric and adolescent gynecology. 2001;14(2):77–80. doi: 10.1016/s1083-3188(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 16.Michalak DP, Zacur HA, Rock JA, Woodruff JD. Autoimmunity in a patient with 47,XXX karyotype. Obstet Gynecol. 1983;62(5):667–9. [PubMed] [Google Scholar]
- 17.Goswami R, Goswami D, Kabra M, Gupta N, Dubey S, Dadhwal V. Prevalence of the triple X syndrome in phenotypically normal women with premature ovarian failure and its association with autoimmune thyroid disorders. Fertility and sterility. 2003;80(4):1052–4. doi: 10.1016/s0015-0282(03)01121-x. [DOI] [PubMed] [Google Scholar]
- 18.Tungphaisal S, Jinorose U. True 47,XXX in a patient with premature ovarian failure: the first reported case in Thailand. J Med Assoc Thai. 1992;75(11):661–5. [PubMed] [Google Scholar]
- 19.Villanueva AL, Rebar RW. Triple-X syndrome and premature ovarian failure. Obstet Gynecol. 1983;62(3 Suppl):70s–3s. [PubMed] [Google Scholar]
- 20.Kodandapani S, Pai MV, Nambiar J, Moka R. Premature ovarian aging in primary infertility: Triple X syndrome. J Hum Reprod Sci. 2011;4(3):153–4. doi: 10.4103/0974-1208.92292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Visser JA, Schipper I, Laven JS, Themmen AP. Anti-Mullerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nature reviews Endocrinology. 2012;8(6):331–41. doi: 10.1038/nrendo.2011.224. [DOI] [PubMed] [Google Scholar]
- 22.Nelson LM. Clinical practice. Primary ovarian insufficiency. The New England journal of medicine. 2009;360(6):606–14. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ratcliffe S, Butler G, Jones M. Edinburgh study of growth and development of children with sex chromosome abnormalities. Birth defects original article series. 1990;26(4):1–44. [PubMed] [Google Scholar]
- 24.Stagi S, di Tommaso M, Scalini P, Lapi E, Losi S, Bencini E et al. Triple X syndrome and puberty: focus on the hypothalamus-hypophysis-gonad axis. Fertility and sterility. 2016;105(6):1547–53. doi: 10.1016/j.fertnstert.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 25.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertility and sterility. 2011;95(1):170–5. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Hagen CP, Aksglaede L, Sorensen K, Main KM, Boas M, Cleemann L et al. Serum levels of anti-Mullerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. The Journal of clinical endocrinology and metabolism. 2010;95(11):5003–10. doi: 10.1210/jc.2010-0930. [DOI] [PubMed] [Google Scholar]
- 27.Hamza RT, Mira MF, Hamed AI, Ezzat T, Sallam MT. Anti-Mullerian hormone levels in patients with turner syndrome: Relation to karyotype, spontaneous puberty, and replacement therapy. American journal of medical genetics Part A. 2018;176(9):1929–34. doi: 10.1002/ajmg.a.40473. [DOI] [PubMed] [Google Scholar]
- 28.Lunding SA, Aksglaede L, Anderson RA, Main KM, Juul A, Hagen CP et al. AMH as Predictor of Premature Ovarian Insufficiency: A Longitudinal Study of 120 Turner Syndrome Patients. The Journal of clinical endocrinology and metabolism. 2015;100(7):E1030–8. doi: 10.1210/jc.2015-1621. [DOI] [PubMed] [Google Scholar]
- 29.Tartaglia N, Howell S, Wilson R, Janusz J, Boada R, Martin S et al. The eXtraordinarY Kids Clinic: an interdisciplinary model of care for children and adolescents with sex chromosome aneuploidy. J Multidiscip Healthc. 2015;8:323–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pigny P, Merlen E, Robert Y, Cortet-Rudelli C, Decanter C, Jonard S et al. Elevated serum level of anti-mullerian hormone in patients with polycystic ovary syndrome: relationship to the ovarian follicle excess and to the follicular arrest. The Journal of clinical endocrinology and metabolism. 2003;88(12):5957–62. doi: 10.1210/jc.2003-030727. [DOI] [PubMed] [Google Scholar]
- 31.Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. The Journal of clinical endocrinology and metabolism. 2011;96(8):2532–9. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 32.Castillo S, Lopez F, Tobella L, Salazar S, Daher V. [The cytogenetics of premature ovarian failure]. Rev Chil Obstet Ginecol. 1992;57(5):341–5. [PubMed] [Google Scholar]
- 33.Otter M, Schrander-Stumpel CT, Curfs LM. Triple X syndrome: a review of the literature. Eur J Hum Genet. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collen RJ, Falk RE, Lippe BM, Kaplan SA. A 48,XXXX female with absence of ovaries. American journal of medical genetics. 1980;6(4):275–8. doi: 10.1002/ajmg.1320060404. [DOI] [PubMed] [Google Scholar]
- 35.Prueitt RL, Ross JL, Zinn AR. Physical mapping of nine Xq translocation breakpoints and identification of XPNPEP2 as a premature ovarian failure candidate gene. Cytogenet Cell Genet. 2000;89(1–2):44–50. doi: 10.1159/000015560. [DOI] [PubMed] [Google Scholar]
- 36.Bione S, Rizzolio F, Sala C, Ricotti R, Goegan M, Manzini MC et al. Mutation analysis of two candidate genes for premature ovarian failure, DACH2 and POF1B. Human reproduction (Oxford, England) 2004;19(12):2759–66. doi: 10.1093/humrep/deh502. [DOI] [PubMed] [Google Scholar]
- 37.Quilter CR, Karcanias AC, Bagga MR, Duncan S, Murray A, Conway GS et al. Analysis of X chromosome genomic DNA sequence copy number variation associated with premature ovarian failure (POF). Human reproduction (Oxford, England). 2010;25(8):2139–50. doi: 10.1093/humrep/deq158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saito M, Novak U, Piovan E, Basso K, Sumazin P, Schneider C et al. BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2009;106(27):11294–9. doi: 10.1073/pnas.0903854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erhart LM, Lankat-Buttgereit B, Schmidt H, Wenzel U, Daniel H, Goke R. Flavone initiates a hierarchical activation of the caspase-cascade in colon cancer cells. Apoptosis. 2005;10(3):611–7. doi: 10.1007/s10495-005-1895-y. [DOI] [PubMed] [Google Scholar]
- 40.Johnson EK, Finlayson C, Rowell EE, Gosiengfiao Y, Pavone ME, Lockart B et al. Fertility Preservation for Pediatric Patients: Current State and Future Possibilities. The Journal of urology. 2017;198(1):186–94. doi: 10.1016/j.juro.2016.09.159. [DOI] [PubMed] [Google Scholar]
- 41.Committee opinion no. 605: primary ovarian insufficiency in adolescents and young women. Obstet Gynecol. 2014;124(1):193–7. doi: 10.1097/01.AOG.0000451757.51964.98. [DOI] [PubMed] [Google Scholar]
- 42.Nahata L, Quinn GP, Tishelman AC, Section On E. Counseling in Pediatric Populations at Risk for Infertility and/or Sexual Function Concerns. Pediatrics. 2018;142(2). doi: 10.1542/peds.2018-1435. [DOI] [PubMed] [Google Scholar]
- 43.Dennis A, Howell S, Cordeiro L, Tartaglia N. “How should I tell my child?” Disclosing the diagnosis of sex chromosome aneuploidies. J Genet Couns. 2015;24(1):88–103. doi: 10.1007/s10897-014-9741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lefebvre T, Dumont A, Pigny P, Dewailly D. Effect of obesity and its related metabolic factors on serum anti-Mullerian hormone concentrations in women with and without polycystic ovaries. Reprod Biomed Online. 2017;35(3):325–30. doi: 10.1016/j.rbmo.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Simoes-Pereira J, Nunes J, Aguiar A, Sousa S, Rodrigues C, Sampaio Matias J et al. Influence of body mass index in anti-Mullerian hormone levels in 951 non-polycystic ovarian syndrome women followed at a reproductive medicine unit. Endocrine. 2018;61(1):144–8. doi: 10.1007/s12020-018-1555-y. [DOI] [PubMed] [Google Scholar]
- 46.Dong YZ, Zhou FJ, Sun YP. Psychological stress is related to a decrease of serum anti-mullerian hormone level in infertile women. Reprod Biol Endocrinol. 2017;15(1):51. doi: 10.1186/s12958-0170271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henes M, Froeschlin J, Taran FA, Brucker S, Rall KK, Xenitidis T et al. Ovarian reserve alterations in premenopausal women with chronic inflammatory rheumatic diseases: impact of rheumatoid arthritis, Behcet’s disease and spondyloarthritis on anti-Mullerian hormone levels. Rheumatology (Oxford) 2015;54(9):1709–12. doi: 10.1093/rheumatology/kev124. [DOI] [PubMed] [Google Scholar]
- 48.Szydlowska I, Marciniak A, Brodowska A, Lisak M, Przysiecka S, Rozanski J. Assessment of ovarian reserve as an indicator of fertility and health consequences in patients with chronic kidney disease stages 3–4. Gynecol Endocrinol. 2018;34(11):944–8. doi: 10.1080/09513590.2018.1473364. [DOI] [PubMed] [Google Scholar]