Abstract
Aims
To evaluate the associations between retinal blood flow (RBF) and optical coherence tomography (OCT) structural measurements in normal-tension glaucoma (NTG) eyes with single-hemifield visual field (VF) damage by the Doppler OCT.
Methods
The Doppler OCT was used to measure temporal artery (TA) RBF and temporal vein (TV) RBF. Retinal nerve fibre layer thickness (RNFLT) was measured by spectral-domain OCT.
Results
Forty-three consecutive eyes of 43 patients with NTG with VF defect confined to a single hemifield and 24 eyes of 24 age-matched healthy subjects were studied. TA and TV RBF and RNFLT were reduced in the damaged hemisphere compared with the normal hemisphere (mean (SD), 3.61 (1.68) vs 5.86 (2.59) μL/min, p<0.001; 5.61 (2.51) vs 6.94 (2.83) μL/min, p=0.010; 69.0 (19.7) vs 99.7 (22.8) μm, p<0.001). Those values in the normal hemisphere of NTG eyes also decreased compared with the healthy hemisphere of the healthy eyes (8.40 (3.36) μL/min, p<0.001; 9.28 (4.47) μL/min, p<0.002; 122.8 (20.2) μm, p<0.001). Multivariate model showed that normal and damaged hemispheres and RNFLT were associated with RBF reduction. In addition, the RBF in the normal hemisphere was lower than that in the healthy hemisphere even after adjusting for RNFLT.
Conclusion
In NTG eyes with single-hemifield damage, the RBF was significantly reduced in the damaged hemisphere compared with the normal one. The RBF decreased in the normal and damaged hemispheres of NTG eyes compared with the healthy hemisphere independent from RNFLT.
INTRODUCTION
Glaucoma is commonly defined as an optic neuropathy characterised by progressive loss of retinal ganglion cells and also associated with structural damage to the optic nerve and visual field (VF) loss.1 In primary open-angle glaucoma (POAG), increased intraocular pressure (IOP) is considered the most important risk factor. However, in normal-tension glaucoma (NTG), the deterioration of the optic nerve fibres occurs under statistically normal IOP.2 Although NTG is considered as a form of POAG and IOP is still widely recognised as a major risk factor for progression,3 whether NTG and high-tension glaucoma differ in their pathogenesis remains controversial. Therefore, vascular factors (eg, vasospasms, small vessel disease or autoregulatory dysfunction) have been suggested to significantly contribute to the development and progression of glaucomatous optic neuropathy and VF loss.4,5
Blood flow can be regulated in response to changing metabolic demands and local vascular resistance.4 When the demand for blood flow in the local tissue decreases, the blood flow is reduced through autoregulatory mechanisms. Previous studies have examined the relationship between the retinal vascular diameter and retinal nerve fibre layer (RNFL) thickness in patients with POAG. Jonas et al6,7 reported that parapapillary retinal vessel diameters may reflect the demand of vascular supply in the corresponding superficial retinal area, and it decreases with advanced glaucomatous optic nerve damage. Similarly, Kim et al8 demonstrated statistically significant associations between central retinal vessel diameters and RNFL defects in the quadrant in patients with NTG and that the vessel narrowing is due to the decreased demand for blood flow in the areas of the retina damaged by glaucoma. But Hwang et al9 reported that there was no correlation or paradoxical correlation between blood flow and structure using the Doppler optical coherence tomography (DOCT) by the double circular scan method. Whether there is a correlation between blood flow and structure is still controversial.
Recently, a novel velocimetry technique using optical coherence tomography (OCT) technology, namely DOCT, has been developed.10–12 OCT can detect morphological images and a Doppler shift of reflected light, which provides information regarding flow and movement.13,14 We previously developed a DOCT instrument with novel software, referred to as a segmental scan method,15–17 which enables continuous measurement of the retinal blood flow (RBF) in the retinal arterioles venules during one cardiac cycle.
In this study, we investigated the associations between RBF and structural measurements in normal and damaged hemispheres of NTG eyes with single-hemifield VF defects.
METHODS
Study population
A total of 43 Japanese patients with NTG with single-hemifield VF defect and 24 age-matched healthy subjects without retinal diseases participated in this study (1 April 2017 to 31 January 2020). Written informed consent was obtained from all subjects using the consent forms.
All volunteers underwent a comprehensive ophthalmological examination, including a review of the medical history, best corrected visual acuity, slit-lamp biomicroscopy, IOP measurement through Goldman applanation tonometry, gonioscopy, ultrasound pachymetry, funduscopic examination using a 90-D lens, funds and optic disk photography. The axial length of each eye was measured using an A-mode ultrasound system (IOL Master 500; Carl Zeiss Meditec, Jena, Germany). Mean arterial blood pressure (MABP) and heart rate (HR) were measured using an electronic sphygmomanometer (EP-88 Si; Colin, Tokyo, Japan). MABP was calculated as 1/3 systolic blood pressure (SBP) +2/3 diastolic blood pressure (DBP). Mean ocular perfusion pressure (OPP) was calculated as 2/3 MABP − IOP.18 Standard automated perimetry (SAP) was performed using the Swedish Interactive Threshold Algorithm standard 30–2 threshold test (Humphrey Field Analyzer 750 II-I; Carl Zeiss Meditec, Tokyo, Japan). Measurements of circumpapillary RNFL thickness (RNFLT) and macular ganglion cell complex thickness (GCCT) were performed using spectral-domain OCT (3D OCT-1 Maestro; Topcon Corp., Tokyo, Japan). Only good quality circumpapillary RNFL and macular ganglion cell complex images, defined by scans with a manufacture signal index ≥45, without segmentation failure or motion artefacts (ie, missing or blank areas), were included for the analysis.19
Inclusion criteria, common to both the healthy and NTG groups, consisted of reliable SAP defined as <20% fixation losses and <33% false-positive and false-negative errors, spherical equivalent refractive error between −6.00 and +3.00 D sphere, best corrected visual acuity of 20/40 or better, age ≥40 and≤80 years, and no prior history of intraocular surgery.
Inclusion criteria for the healthy group were IOP≤21 mm Hg, normal appearance of the optic nerve head and RNFL, normal SAP defined as a glaucomatous hemifield test within normal limits, and pattern SD (PSD) within 95% CI limits.
Inclusion criteria for the NTG group consisted of glaucomatous optic neuropathy and corresponding abnormal VF defined as abnormal glaucomatous hemifield test and PSD outside 95% normal limits. The patients required one normal visual hemifield, and the glaucomatous VF damage had to be confined to a single hemifield. All patients were familiar with SAP testing from earlier exposure to at least two VF examinations. The glaucomatous hemifield required the presence of a cluster of at least three contiguous test locations at p<0.05 on the pattern deviation plot, with at least one test location at p<0.01, except for the test locations in the most peripheral part. The sensitivity of each test location was converted to a linear scale of 1/Lambert (1/Lambert = 100.1 × dB value).20 The retinal sensitivity (RS) values in 1/Lambert were calculated in each hemifield using the average of 38/76 VF locations. Finally, the average of the 1/Lambert scale was converted to the dB scale.
Exclusion criteria for both the groups were history of intraocular/laser surgery, existing retinal pathologies, non-glaucomatous optic neuropathy, uveitis, ocular trauma or diabetes. Participants with systemic hypertension were included unless they were diagnosed with hypertensive retinopathy. Participants with unreliable VF results and poor-quality spectral-domain OCT scans were excluded from this study.
RBF measurements of DOCT
As we previously described, the DOCT flowmeter is based on a commercially available spectral-domain OCT system (3D OCT-1 Maestro; Topcon Corp.) operated at an 800 nm wavelength range.
In DOCT, the flow velocity, v(z), can be derived from the Doppler shift incurred by the moving blood:
where z is the depth location, is the phase difference at the same depth location between adjacent profiles after Fourier transform, λ0 is the centre wavelength, n is the refractive index of blood, τ is the time interval between the adjacent profiles and θ is the Doppler angle between the flow vector and the incident probe beam.
A detailed description of blood flow measurement has been previously published.15 Besides the measurement of the phase difference, the Doppler angle θ must be known to calculate the velocity. In the retina, most blood vessels run nearly parallel to the retinal surface, except at the area around the optic nerve head. As θ approaches 90°, the measured velocity becomes very sensitive to the accuracy of θ. To minimise this potential velocity measurement error, our Doppler blood flow measurements were performed where the Doppler angle θ is considerably <90°, at approximately 80°. Automated alignment software was integrated to seek the proper Doppler angle. Such autoalignment software traces the boundary of the internal limiting membrane in real time along the blood vessel to determine the Doppler angle. If the Doppler angle was out of range, the OCT beam was shifted farther away from the pupillary centre. This alignment was repeated until the estimated Doppler angle was calculated. The Doppler angle estimation routine was performed in locations where a pair of B-scan images was captured 100 μm apart across the blood vessels. This measurement was repeated eight times, and the median value was used to define the Doppler angle. Since the time interval for capturing a pair of B-scan images is only 10 ms, usually there are no eye motion artefacts. Obtaining the median value of eight repeated imaging measurements ensured more reliable and accurate results. DOCT imaging was performed for 2 s, followed by colour fundus imaging. The blood vessels were automatically detected and identified from the OCT structured and phase images. The vessel diameter was also measured using OCT phase images. The Image J software was used to describe the blood flow profile.
DOCT image acquisition and processing
The pupil was dilated with one drop of 0.5% tropicamide and 0.5% phenylephrine (Mydrin P; Santen Pharmaceuticals, Osaka, Japan). The participants rested for 10 min in a quiet room prior to the examination. After dilation of the pupils, the subjects were required to rest in the sitting position for at least 5 min in a quiet dimly lit room, where the temperature was maintained at 25°C. Subsequently, their blood pressure and HR were measured in the left arm. The RBF measurements were assessed after the blood pressure, HR and IOP measurements. The temporal arteries and veins having relatively straight segments and sufficiently far from the bifurcations were selected for the measurement of RBF.
Statistical analysis
The data were analysed using JMP software V.13.0.2 (SAS, Tokyo, Japan). The distribution of data was examined using the Shapiro–Wilk Test. Paired t tests were used to compare between the hemispheres corresponding to the normal and damaged hemifields in the same eye of the same patient with NTG. One-way analysis of variance followed by Tukey–Kramer honestly significant difference post-hoc test was used to compare the variables between healthy hemispheres of healthy subjects and normal hemispheres of patients with NTG. Multivariate regression analysis was used to investigate the associations between structural and RBF measurements of the damaged/normal hemispheres and SAP parameters in the corresponding hemifields. Univariate linear regression models were built using temporal artery (TA) RBF or temporal vein (TV) RBF as the dependent variable, and RNFLT, GCCT, RS and demographic ocular and systemic characteristic variables as the independent variables. Gender, axial length and factors that were significant in the univariate analyses (p<0.05) were then further included in the multivariable linear regression model. Logistic regression analysis was conducted to examine the impact of using topical and systemic medications on the TA RBF and TV RBF in NTG patients and healthy subjects.
RESULTS
The demographics of the study population are summarised in table 1. Forty-three eyes of 43 patients (men, 19; women, 24; mean (SD) age, 63.3 (9.8) years) and 24 eyes of 24 patients (men, 11; women, 13; mean (SD) age, 66.2 (6.1) years) were studied. There were no significant differences in age, gender, spherical equivalent, axial length, IOP, SBP, DBP, OPP, HR, proportion of hypertension or OCT-derived disk area measurements between healthy and NTG eyes. The mean (SD) of the VF mean deviation was −5.01 dB (3.83; range, −13.95 to 0.28 dB), and the mean (SD) VF PSD was 9.92 dB (4.15; range, 2.22 to 16.05 dB) in NTG eyes.
Table 1.
Demographics of the study population
| Variable | Healthy eyes (n=24) | NTG eyes (n=43) | P value |
|---|---|---|---|
| Age, mean (SD; 95% CI), years | 66.2 (6.1; 63.6 to 68.7) | 63.3 (9.8; 60.3 to 66.3) | 0.201* |
| No of men/women | 11/13 | 19/24 | 0.897† |
| VF defect location, no of superior/inferior | – | 27/16 | |
| Spherical equivalent, mean (SD; 95% CI), D | −0.55 (2.13; −1.45 to 0.35) | −1.51 (2.44; −2.26 to−0.76) | 0.112* |
| Axial length, mean (SD; 95% CI), mm | 23.8 (1.1; 23.4 to 24.3) | 24.1 (1.3; 23.7 to 24.5) | 0.324* |
| IOP, mean (SD; 95% CI), mm Hg | 15.7 (3.0; 14.4 to 16.9) | 15.3 (2.5; 14.5 to 16.1) | 0.598* |
| SBP, mean (SD; 95% CI), mm Hg | 131.1 (9.5; 127.1 to 135.1) | 130.3 (9.6; 127.3 to 133.2) | 0.742* |
| DBP, mean (SD; 95% CI), mm Hg | 77.1 (9.6; 73.1 to 81.2) | 78.0 (11.3; 74.5 to 81.5) | 0.744* |
| MOPP, mean (SD; 95% CI), mm Hg | 47.7 (6.0; 45.2 to 50.3) | 48.3 (6.9; 46.2 to 50.5) | 0.726* |
| HR, mean (SD; 95% CI), beats/min | 65.8 (7.6; 62.5 to 69.0) | 65.5 (8.5; 62.9 to 68.1) | 0.892* |
| Hypertension, No. (%) | 5 (21) | 6 (14) | 0.466† |
| SAP MD, mean (SD; 95% CI), dB | 0.48 (1.33; −0.09 to 1.04) | −5.01 (3.83; −6.20 to −3.84) | <0.001* |
| SAP PSD, mean (SD; 95% CI), dB | 1.79 (0.41; 1.62 to 1.96) | 9.88 (4.13; 8.61 to 11.15) | <0.001* |
| Disc area, mean (SD; 95% CI), mm2 | 2.21 (0.43; 2.03 to 2.39) | 2.32 (0.60; 2.13 to 2.50) | 0.449* |
| Rim area, mean (SD; 95% CI), mm2 | 1.15 (0.50; 0.94 to 1.36) | 0.59 (0.30; 0.50 to 0.68) | <0.001* |
Boldface indicates statistically significant difference.
Calculated as unpaired t-test, with p<0.05 considered to be statistically significant.
Calculated as χ2 test, with p<0.05 considered to be statistically significant.
dB, decibel; DBP, diastolic blood pressure; HR, heart rate; IOP, intraocular pressure; MD, mean deviation; MOPP, mean ocular perfusion pressure; NTG, normal-tension glaucoma; PSD, pattern SD; SAP, standard automated perimetry; SBP, systolic blood pressure; VF, visual field.
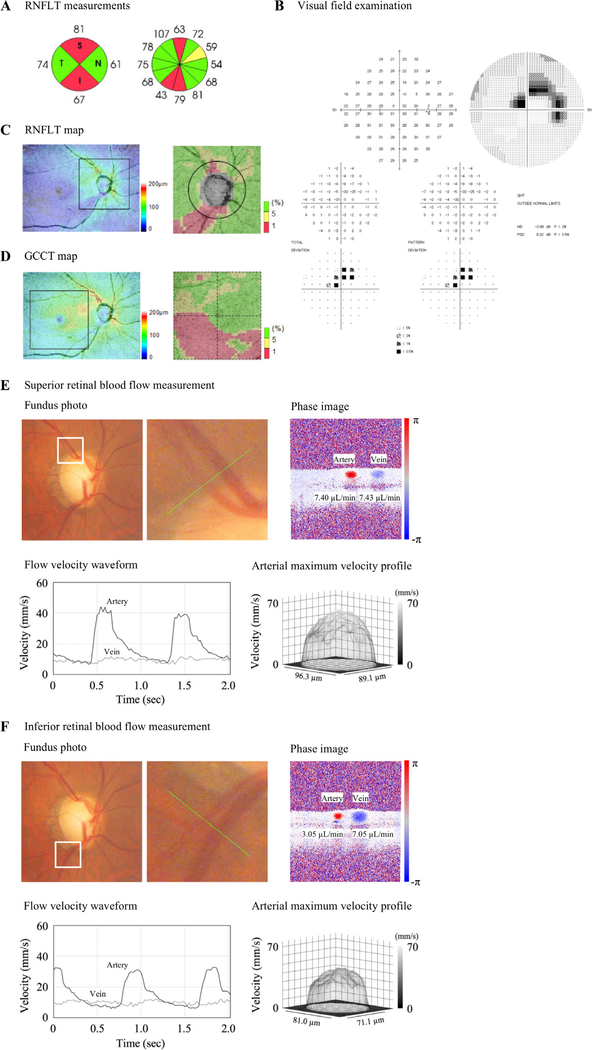
Figure 1 demonstrates a representative case of a patient with NTG with a single-hemifield VF defect (superior VF defect). It shows that reduced RNFLT (figure 1A,C) and GCCT (figure 1D), lower TA RBF and lower TV RBF (figure 1E,F) were observed in the inferior hemisphere compared with the superior hemisphere, which corresponded to the glaucomatous VF defects shown in the superior hemifield in the total and pattern deviation plots (figure 1B).
Figure 1.
A representative case of normal-tension glaucoma with superior visual field defect. (A) Retinal nerve fibre layer thickness (RNFLT) measurements. (B) Visual field examination. (C) RNFLT map. (D) Ganglion cell complex thickness (GCCT) map. (E) Superior retinal blood flow (RBF) measurement. The upper left image shows the fundus photograph, the white line square shows the next photograph and the green line shows the measurement location. The upper right image shows the phase image. The lower left image shows the velocity waveform of the average velocity in the blood vessel, the black line shows the artery and the grey line shows the vein. The lower right image shows the velocity profile at the maximum velocity in the artery. (F) Inferior RBF measurement. Reduced RNFLT, GCCT, lower temporal artery RBF, and temporal vein RBF were observed in the inferior hemisphere compared with the superior hemisphere, which corresponded to the glaucomatous visual field defects shown in the superior hemifield in the total and pattern deviation plots. In the Topcon RNFLT deviation map, areas with RNFLT thinner than the fifth percentile and the first percentile of the normative database were marked in yellow and in red, respectively. dB, decibel; GHT, glaucoma hemifield test; I, inferior; MD, mean deviation; N, nasal; PSD, pattern SD; S, superior; T, temporal.
Table 2 presents comparisons of the RNFLT, GCCT, RS and RBF parameters between the damaged and normal hemispheres, and normal hemispheres of NTG eyes and healthy hemispheres. Twenty-seven eyes with NTG showed VF defects confined to the superior hemifield. In eyes with NTG, the RNFLT was thinner in the damaged hemisphere than in the normal hemisphere (mean (SD), 76.6 (11.9) vs 92.7 (14.5) μm; p<0.001). The GCCT was thinner in the damaged hemisphere than the normal hemisphere (mean (SD), 69.0 (19.7) vs 99.7 (22.8) μm; p<0.001). The RS was reduced in the damaged hemisphere compared with the normal hemisphere (mean (SD), 25.2 (4.2) vs 29.3 (1.7) dB; p<0.001). Also, lower TA RBF (mean (SD), 3.61 (1.68) vs 5.86 (2.59) μL/min; p<0.001) and TV RBF (mean (SD), 5.61 (2.51) vs 6.94 (2.83) μL/min; p<0.001) were found in the damaged hemisphere compared with the normal hemisphere. We also compared the values in the hemisphere with apparently normal VF in NTG eyes with the healthy hemisphere in healthy subjects. We found that the RNFLT (mean (SD), vs 122.8 (20.3) μm; p<0.001), GCCT (mean (SD), vs 98.8 (6.5) μm; p=0.012), TA RBF (mean (SD), vs 8.40 (3.36) μL/min; p<0.001), and TV RBF (mean (SD), vs 9.28 (4.47) μL/min; p=0.002) were significantly reduced in the normal hemisphere of NTG eyes compared with the healthy hemisphere. The RS in the normal hemisphere of NTG eyes (mean (SD), 29.3 (1.7) dB) was reduced compared with the healthy hemisphere but did not reach a statically significant level (mean (SD), 30.0 (1.3) dB; p=0.187).
Table 2.
Comparisons of variables between normal and damaged hemispheres in normal-tension glaucoma (NTG) eyes; and normal hemisphere of NTG eyes and healthy hemisphere of healthy eyes
| NTG eyes, mean (SD) |
Healthy eyes, mean (SD) |
||||
|---|---|---|---|---|---|
| Variable | Damaged hemisphere, n=43 | Normal hemisphere, n=43 | P value* | Healthy hemisphere, n=48 | P value† |
| RNFLT, μm‡ | 69.0 (19.7) | 99.7 (22.8) | <0.001 | 122.8 (20.2) | <0.001 |
| GCCT, μm | 76.6 (11.9) | 92.7 (14.5) | <0.001 | 98.8 (6.5) | 0.012 |
| RS, dB | 25.2 (4.2) | 29.3 (1.7) | <0.001 | 30.0 (1.3) | 0.187 |
| TA RBF, μL/min | 3.61 (1.68) | 5.86 (2.59) | <0.001 | 8.40 (3.36) | <0.001 |
| TA diameter, μm | 76.0 (13.8) | 84.2 (13.5) | <0.001 | 97.1 (13.9) | <0.001 |
| TA average velocity, mm/s | 12.8 (4.1) | 15.4 (4.7) | 0.003 | 17.1 (5.7) | 0.111 |
| TA maximum velocity, mm/s | 26.5 (8.7) | 31.6 (10.6) | 0.007 | 33.7 (10.7) | 0.308 |
| TA minimum velocity, mm/s | 5.2 (2.1) | 6.7 (2.7) | 0.001 | 7.7 (3.5) | 0.109 |
| TV RBF, μL/min | 5.61 (2.51) | 6.94 (2.83) | 0.010 | 9.28 (4.47) | 0.002 |
| TV diameter, μm | 112.5 (15.2) | 115.1 (18.9) | 0.409 | 120.3 (19.1) | 0.168 |
| TV average velocity, mm/s | 9.5 (3.7) | 10.4 (3.9) | 0.184 | 12.7 (4.6) | 0.007 |
Boldface indicates statistically significant difference.
Paired t-tests were used to compare the variables between the normal hemisphere and the hemisphere corresponding to the visual field defect in the same eye with NTG. P<0.05 is considered to be statistically significant.
One-way analysis of variance followed by Tukey–Kramer Honestly Significant Difference post-hoc test was used to compare the variables between healthy hemispheres and normal hemispheres of NTG, with p<0.05 considered to be statistically significant.
Measured in superior (46°–135°) and inferior (226°–315°) quadrants.
RNFLT, retinal never fibre layer thickness; GCCT, ganglion cell complex thickness; RS, retinal sensitivity; TA, temporal artery; RBF, retinal blood flow; TV, temporal vein.
Table 3 demonstrates the associations between RBF and other measurements (RNFLT, GCCT and RS) in the normal and damaged hemispheres. The TA RBF and TV RBF were correlated with RNFLT (r=0.581, p<0.001 and r=0.311, p=0.043, respectively) and GCCT (r=0.463, p=0.002 and r=0.307, p=0.045, respectively) in the normal hemisphere but not with RNFLT (r=0.024, p=0.877 and r=0.087, p=0.578, respectively) and GCCT (r=0.007, p=0.963 and r=0.068, p=0.666, respectively) in the damaged hemisphere. There was no correlation between RS and TA or TV RBF in the damaged hemifield and corresponding hemisphere (r=0.147, p=0.347 and r = −0.108, p=0.489, respectively). Scatter plots illustrating the relationships between TA RBF and RNFLT for all hemispheres (r=0.628; 95% CI 0.507 to 0.717; p<0.001) are shown in online supplementary figure 1.
Table 3.
Associations between retinal blood flow (RBF) and other parameters in each hemisphere in normal-tension glaucoma eyes
| Variable | RNFLT, μm* | GCCT, μm | RS, dB | |||
|---|---|---|---|---|---|---|
| r (95% CI) | P value† | r (95% CI) | P value† | r (95% CI) | P value† | |
| Normal hemifield and corresponding hemisphere | ||||||
| TA RBF, μL/min | 0.581 (0.340 to 0.751) | <0.001 | 0.463 (0.189 to 0.670) | 0.002 | 0.348 (0.053 to 0.587) | 0.022 |
| TV RBF, μL/min | 0.311 (0.011 to 0.559) | 0.043 | 0.307 (0.007 to 0.556) | 0.045 | 0.104 (−0.203 to 0.392) | 0.509 |
| Damaged hemifield and corresponding hemisphere | ||||||
| TA RBF, μL/min | 0.024 (−0.278 to 0.322) | 0.877 | 0.007 (−0.294 to 0.307) | 0.963 | 0.147 (−0.161 to 0.428) | 0.347 |
| TV RBF, μL/min | 0.087 (−0.219 to 0.378) | 0.578 | 0.068 (−0.237 to 0.361) | 0.666 | −0.108 (−0.396 to 0.198) | 0.489 |
Boldface indicates statistically significant difference.
Measured in superior (46°–135°) and inferior (226°–315°) quadrants.
Calculated as Pearson correlation coefficient, with p<0.05 considered to be statistically significant.
RNFLT, retinal never fibre layer thickness; GCCT, ganglion cell complex thickness; RS, retinal sensitivity; TA, temporal artery; TV, temporal vein.
In the fully adjusted multivariable model, normal (ß = −1.70; p<0.001) and damaged hemisphere (ß = −2.94; p=0.005), and RNFLT (ß=0.33; p=0.021) were associated with TA RBF reduction (table 4). Similar results were observed in TV RBF of normal (ß = −1.75; p=0.022) and damaged hemisphere (ß = −2.37; p=0.028; online supplementary table 1).
Table 4.
Influence of factors on temporal artery retinal blood flow
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Factor | Coefficient | 95% CI | P value | Coefficient | 95% CI | P value |
| Age, per year | −0.01 | −0.08 to 0.06 | 0.781 | – | – | – |
| Gender, female | 0.68 | 0.12 to 1.24 | 0.018 | 0.73 | −0.22 to 1.68 | 0.129 |
| Axial length, mm | −0.67 | −1.12, to 0.21 | 0.005 | −0.22 | −0.61 to 0.17 | 0.263 |
| IOP, mm Hg | −0.02 | −0.23 to 0.19 | 0.194 | – | – | – |
| SBP, mm Hg | 0.03 | −0.05 to 0.07 | 0.855 | – | – | – |
| DBP, mm Hg | 0.01 | −0.05 to 0.06 | 0.783 | – | – | – |
| MOPP, mm Hg | 0.01 | −0.07 to 0.10 | 0.738 | – | – | – |
| HR, beats/min | −0.01 | −0.07 to 0.06 | 0.972 | – | – | – |
| Hypertension | 0.40 | −0.34 to 1.14 | 0.284 | – | – | – |
| RS, dB | 0.39 | 0.24 to 0.54 | <0.001 | −0.03 | −0.21 to 0.16 | 0.781 |
| RNFLT*, per 10 μm | 0.61 | 0.46 to 0.76 | <0.001 | 0.33 | 0.05 to 0.60 | 0.021 |
| GCCT, per 10 μm | 1.11 | 0.77 to 1.45 | <0.001 | 0.07 | −0.37 to 0.51 | 0.747 |
| Group | ||||||
| Healthy hemisphere | Reference | Reference | ||||
| Normal hemisphere | −2.54 | −3.64 to 1.43 | <0.001 | −1.70 | −2.87 to 0.52 | <0.001 |
| Damaged hemisphere | −4.79 | −5.90 to 3.68 | <0.001 | −2.94 | −4.61 to 1.26 | 0.005 |
Boldface indicates statistically significant difference.
Measured in superior (46°–135°) and inferior (226°–315°) quadrants.
IOP, intraocular pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; MOPP, mean ocular perfusion pressure; HR, heart rate; RS, retinal sensitivity; RNFLT, retinal never fibre layer thickness; GCCT, ganglion cell complex thickness.
DISCUSSION
In the present study, we observed that the RBF, RNFLT and GCCT were significantly reduced in the perimetrically normal hemisphere of NTG eyes compared with the healthy hemisphere values in the healthy eyes. Previous reports have evaluated the retinal vessel diameter or RBF in glaucomatous eyes and its association with RNFLT and VF defects.6–8,21 However, to the best of our knowledge, this is the first report evaluating RBF volumes of NTG eyes with single-hemifield damage. The finding of early structural loss in the hemisphere with apparently normal VF is consistent with the literature that demonstrated that eyes with SAP defects confined to a single hemifield have evidence of diffuse attenuation in RNFLT, GCCT and RS in apparently normal areas of the VF.21–23
Our study further supports previous data reported by Arend et al24 who examined the effect of asymmetric altitudinal VF damage on retinal microcirculation obtained through fluorescein angiography in NTG eyes. The report revealed that the arteriovenous passage time in the hemisphere with more severe glaucomatous VF damage is significantly reduced compared with the opposite hemisphere, indicating that it reduced RBF in the hemisphere with VF damage. Our results also showed that RBF was reduced both in TA RBF and TV RBF in the hemisphere with VF defect compared with the one without VF defect.
Previous reports have evaluated the retinal vessel diameter in NTG eyes and its association with RNFLT. Kim et al found a strong association between decreased peripapillary arteriole diameter and RNFLT in the corresponding hemifield in eyes with NTG.8 Our study also observed a significant correlation between RBF and structural measurements in the normal hemisphere (table 3), and in the hemisphere of total subjects (online supplementary figure 1). The loss of retinal ganglion cells may cause a decrease in the RBF via autoregulatory mechanisms by responding to a decreased regional demand for oxygen.6,7,25–27 This hypothesis is supported by the observation that retinal vessel narrowing is found in various forms of optic nerve damage and is not specific to glaucoma.25,26,28–30 There was no correlation between RBF, structural measurement and RS in the damaged hemisphere (table 3). Takusagawa et al31 reported that focal capillary dropout could be visualised in the superficial vascular complex (SVC) but not in the intermediate capillary plexus (ICP) and deep capillary plexus (DVP) in the glaucomatous eyes using a projection-resolved OCT-A. The lack of correlation with RNFLT, GCCT and RS in the damaged hemisphere suggests that measurements of RBF in the damaged hemisphere may mainly reflect the blood flow demands of the tissues that are less associated with glaucoma, for example, the ICP and DVP.
Previous reports have evaluated the relationship between total RBF, RNFLT and RS in glaucoma eyes using DOCT by a double circular scan method.9,21 Hwang et al9 reported that there was no correlation or paradoxical correlation between blood flow and structure. Sehi et al21 evaluated total RBF in both normal and damaged hemispheres of POAG with VF defects confined to a single hemifield, and reported that the reduced RBF in the abnormal hemisphere was significantly associated with thinner RNFLT and GCCT in the same hemisphere, but there was no significant association between these parameters in the normal hemisphere. These results are contrary to the results of our study. The differences in the DOCT measurement method (double circular scan and segmental scan method; total venous vessels and only temporal arteriovenular vessel measurement) and in the target patients (POAG and NTG) may have been the cause of this discrepancy. Further studies are needed to confirm the relationship between RBF and structural measurements in glaucoma.
Several studies applying OCT angiography (OCTA) into POAG with single-hemifield VF damage have documented a relationship between SAP RS and vessel density measurements in the overall peripapillary region.32,33 Yarmohammadi et al33 reported that circumpapillary and perifoveal vessel density significantly correlated with SAP RS in both the normal and damaged hemifield and corresponding hemisphere. In our study, RS and TA RBF were significantly correlated in the normal hemifield and corresponding hemisphere but not in the damaged hemifield and corresponding hemisphere. OCTA evaluates RNFL specific vascular morphology (SVC), whereas DOCT evaluates RBF in the inner retina (SVC +ICP + DCP). Therefore, RS may not correlate with RBF measured by DOCT in damaged hemispheres with thinner RNFLT. In cases of glaucoma in which the RNFL is thin enough to cause VF impairment, it is clinically useful to evaluate RNFL-specific blood factors, such as OCTA.
The multivariate regression analysis (table 4 and online supplementary table 1) revealed that the hemisphere in NTG was associated with blood flow reduction independent from structural loss (RNFLT and GCCT). Our results support previous reports.9,34 Yarmohammadi et al34 reported that OCTA vessel density was more strongly correlated with the severity of VF loss than RNFLT, and decreased vessel density was significantly associated with severity of VF damage independent of the structural loss in POAG. Similarly, Hwang et al9 reported that there was a close link between reduced RBF and VF loss in glaucoma that is largely independent of structural loss. Considering these reports and our results, blood flow factor may be independent of structural loss in glaucoma.
Sehi et al21 used DOCT by a double circular scan method to evaluate total venular RBF in both normal and damaged hemispheres of POAG with VF defects confined to a single hemifield. The results showed that both total venular RBF and OCT measurements (mean RNFLT and GCCT) were reduced in the hemisphere corresponding to the abnormal hemifields compared with the opposite hemisphere in glaucoma eyes. The double circular scan method can measure whole blood flow by measuring all venous blood vessels in the retina. Because the double circular scan method evaluates whole RBF, the method has an advantage, especially in situations when comparing whole RBF between individuals. On the other hand, the advantages of the segmental scan method are: (1) the arterial blood flow can be measured; (2) the retinal artery and vein pulsate, and the segmental scan method can obtain the waveform by continuously measuring one heartbeat, so that the blood flow can be measured more accurately; (3) measuring only the blood flow of the temporal arteriovenular with the thickest RNFL might be more indicative of glaucoma status. The double circular scan method measures whole blood flow, but also includes parts of nasal quadrant that are less likely to be associated with glaucoma. Meanwhile, the segmental scan method has several disadvantages: measurement of all retinal arteriovenular vessels is time consuming, and the reproducibility is poor for vessels<50 μm. Therefore, we selected four temporal vessels (TA and TV) per eye. Because our study measures only the largest retinal arteriovenular, the blood flow in the blood vessels branching in the optic disc region may have been underestimated.
The present study has several limitations. First, the use of topical and systemic medications during the course of this study may have potentially affected the RBF.35 Several reports have indicated that the use of topical beta blockers decreases RBF.36 Furthermore, another report suggested that the use of topical carbonic anhydrase inhibitors increased RBF in patients with glaucoma.37 The full list of the topical and systemic medications has been provided in online supplementary table 2. Logistic regression analysis showed that none of the topical and systemic medications were statistically associated with average TA RBF and TV RBF in NTG and healthy eyes (p>0.05). However, this might be due to the small sample size. The results of this study might not completely have eliminated the effects of eye drops. Second, we could not measure all retinal arteries and veins since the reproducibility is poor for vessels less than 50 μm. To calculate all arterial blood flow, technical problems must be solved.
CONCLUSION
In NTG eyes with single-hemifield damage, the RBF was found to be significantly reduced in the damaged hemifield and corresponding hemisphere. RBF reduction and thinner RNFLT and GCCT were also observed in the normal hemisphere of NTG eyes. The reduced RBF was associated with thinner RNFLT and GCCT in the normal hemifield and the corresponding hemisphere, but not in the damaged hemifield and the corresponding hemisphere. Multivariate regression analysis suggested that blood flow attenuation may be independent from structural loss in NTG.
Supplementary Material
Acknowledgements
The authors would like to thank Enago ( www.enago.jp) for the English language review.
Funding The work was supported by JSPS KAKENHI Grant Number 16k20296.
Footnotes
Competing interests Akitoshi Yoshida has a patent for the Doppler OCT system.
Patient consent for publication Not required.
Ethics approval This study was approved by the Institutional Review Boards of Asahikawa Medical University (approval number: 17114), which is in agreement with the tenets of the Declaration of Helsinki.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available in a public, open access repository. Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. All data in the study are available to share. Please contact Youngseok Song should you have any request/inquiry for data sharing.
REFERENCES
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet 2004;363:1711–20. [DOI] [PubMed] [Google Scholar]
- 2.European Glaucoma Society Terminology and Guidelines for Glaucoma, 4th Edition - Chapter 2: Classification and terminologySupported by the EGS Foundation: Part 1: Foreword; Introduction; Glossary; Chapter 2 Classification and Terminology. Br J Ophthalmol 2017;101:73–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonas JB, Gründler AE, Gonzales-Cortés J. Pressure-dependent neuroretinal rim loss in normal-pressure glaucoma. Am J Ophthalmol 1998;125:137–44. [DOI] [PubMed] [Google Scholar]
- 4.Flammer J, Orgül S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res 2002;21:359–93. [DOI] [PubMed] [Google Scholar]
- 5.Plange N, Remky A, Arend O. Colour Doppler imaging and fluorescein filling defects of the optic disc in normal tension glaucoma. Br J Ophthalmol 2003;87:731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonas JB, Nguyen XN, Naumann GO. Parapapillary retinal vessel diameter in normal and glaucoma eyes. I. morphometric data. Invest Ophthalmol Vis Sci 1989;30:1599–603. [PubMed] [Google Scholar]
- 7.Jonas JB, Naumann GO. Parapapillary retinal vessel diameter in normal and glaucoma eyes. II. correlations. Invest Ophthalmol Vis Sci 1989;30:1604–11. [PubMed] [Google Scholar]
- 8.Kim JM, Sae Kim M, Ju Jang H, et al. The association between retinal vessel diameter and retinal nerve fiber layer thickness in asymmetric normal tension glaucoma patients. Invest Ophthalmol Vis Sci 2012;53:5609–14. [DOI] [PubMed] [Google Scholar]
- 9.Hwang JC, Konduru R, Zhang X, et al. Relationship among visual field, blood flow, and neural structure measurements in glaucoma. Invest Ophthalmol Vis Sci 2012;53:3020–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Bower BA, Izatt JA, et al. Retinal blood flow measurement by Circumpapillary Fourier domain Doppler optical coherence tomography. J Biomed Opt 2008;13:064003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doblhoff-Dier V, Schmetterer L, Vilser W, et al. Measurement of the total retinal blood flow using dual beam Fourier-domain Doppler optical coherence tomography with orthogonal detection planes. Biomed Opt Express 2014;5:630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leitgeb RA, Werkmeister RM, Blatter C, et al. Doppler optical coherence tomography. Prog Retin Eye Res 2014;41:26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang XJ, Milner TE, Nelson JS. Characterization of fluid flow velocity by optical Doppler tomography. Opt Lett 1995;20:1337–9. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y, Chen Z, Saxer C, et al. Phase-resolved optical coherence tomography and optical Doppler tomography for imaging blood flow in human skin with fast scanning speed and high velocity sensitivity. Opt Lett 2000;25:114–6. [DOI] [PubMed] [Google Scholar]
- 15.Nagaoka T, Tani T, Song Y-S, et al. Evaluation of retinal circulation using Segmental-Scanning Doppler optical coherence tomography in anesthetized cats. Invest Ophthalmol Vis Sci 2016;57:2936. [DOI] [PubMed] [Google Scholar]
- 16.Tani T, Song Y-S, Yoshioka T, et al. Repeatability and reproducibility of retinal blood flow measurement using a Doppler optical coherence tomography flowmeter in healthy subjects. Invest Ophthalmol Vis Sci 2017;58:2891–8. [DOI] [PubMed] [Google Scholar]
- 17.Tani T, Takahashi A, Nagaoka T, et al. Abnormality of retinal arterial velocity profiles using Doppler Fourier-domain optical coherence tomography in a case of Takayasu’s arteritis with aortic regurgitation. Am J Ophthalmol Case Rep 2017;5:134–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagaoka T, Mori F, Yoshida A. Retinal artery response to acute systemic blood pressure increase during cold pressor test in humans. Invest Ophthalmol Vis Sci 2002;43:1941–5. [PubMed] [Google Scholar]
- 19.Kim T-W, Park U-C, Park KH, et al. Ability of Stratus OCT to identify localized retinal nerve fiber layer defects in patients with normal standard automated perimetry results. Invest Ophthalmol Vis Sci 2007;48:1635. [DOI] [PubMed] [Google Scholar]
- 20.Araie M, Murata H, Iwase A, et al. Differences in relationship between macular inner retinal layer thickness and retinal sensitivity in eyes with early and progressed glaucoma. Invest Ophthalmol Vis Sci 2016;57:1588–94. [DOI] [PubMed] [Google Scholar]
- 21.Sehi M, Goharian I, Konduru R, et al. Retinal blood flow in glaucomatous eyes with single-hemifield damage. Ophthalmology 2014;121:750–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagga H, Greenfield DS, Knighton RW. Macular symmetry testing for glaucoma detection. J Glaucoma 2005;14:358–63. [DOI] [PubMed] [Google Scholar]
- 23.Chang M, Yoo C, Kim S-W, et al. Retinal vessel diameter, retinal nerve fiber layer thickness, and intraocular pressure in Korean patients with normal-tension glaucoma. Am J Ophthalmol 2011;151:100–5. [DOI] [PubMed] [Google Scholar]
- 24.Arend O, Remky A, Cantor LB, et al. Altitudinal visual field asymmetry is coupled with altered retinal circulation in patients with normal pressure glaucoma. Br J Ophthalmol 2000;84:1008–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonas JB, Fernández MC, Naumann GO. Parapapillary atrophy and retinal vessel diameter in nonglaucomatous optic nerve damage. Invest Ophthalmol Vis Sci 1991;32:2942–7. [PubMed] [Google Scholar]
- 26.Rader J, Feuer WJ, Anderson DR. Peripapillary vasoconstriction in the glaucomas and the anterior ischemic optic neuropathies. Am J Ophthalmol 1994;117:72–80. [DOI] [PubMed] [Google Scholar]
- 27.Papastathopoulos KI, Jonas JB. Follow up of focal narrowing of retinal arterioles in glaucoma. Br J Ophthalmol 1999;83:285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frisén L, Claesson M. Narrowing of the retinal arterioles in descending optic atrophy. A quantitative clinical study. Ophthalmology 1984;91:1342–6. [DOI] [PubMed] [Google Scholar]
- 29.Rankin SJ, Drance SM. Peripapillary focal retinal arteriolar narrowing in open angle glaucoma. J Glaucoma 1996;5:22–8. [PubMed] [Google Scholar]
- 30.Zheng Y, Cheung N, Aung T, et al. Relationship of retinal vascular caliber with retinal nerve fiber layer thickness: the Singapore Malay eye study. Invest Ophthalmol Vis Sci 2009;50:4091. [DOI] [PubMed] [Google Scholar]
- 31.Takusagawa HL, Liu L, Ma KN, et al. Projection-Resolved optical coherence tomography angiography of macular retinal circulation in glaucoma. Ophthalmology 2017;124:1589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C-L, Bojikian KD, Wen JC, et al. Peripapillary retinal nerve fiber layer vascular microcirculation in eyes with glaucoma and Single-Hemifield visual field loss. JAMA Ophthalmol 2017;135:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Peripapillary and macular vessel density in patients with glaucoma and Single-Hemifield visual field defect. Ophthalmology 2017;124:709–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yarmohammadi A, Zangwill LM, Diniz-Filho A, et al. Relationship between optical coherence tomography angiography vessel density and severity of visual field loss in glaucoma. Ophthalmology 2016;123:2498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa VP, Harris A, Stefánsson E, et al. The effects of antiglaucoma and systemic medications on ocular blood flow. Prog Retin Eye Res 2003;22:769–805. [DOI] [PubMed] [Google Scholar]
- 36.Yoshida A, Ogasawara H, Fujio N, et al. Comparison of short- and long-term effects of betaxolol and timolol on human retinal circulation. Eye 1998;12 (Pt 5):848–53. [DOI] [PubMed] [Google Scholar]
- 37.Feke GT, Rhee DJ, Turalba AV, et al. Effects of dorzolamide-timolol and brimonidine-timolol on retinal vascular autoregulation and ocular perfusion pressure in primary open angle glaucoma. J Ocul Pharmacol Ther 2013;29:639–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.