Abstract
The human gastrointestinal tract is home to a vibrant, diverse ecosystem of prokaryotic and eukaryotic microorganisms. The gut fungi (mycobiota) have recently rose to prominence due to their ability to modulate host immunity. Colonization of the gut occurs through a combination of vertical transmission from the maternal mycobiota, environmental and dietary exposure. Human and animal data demonstrate that nutrition strongly affects the mycobiota composition and that changes in the fungal communities can result in the aggravation of metabolic diseases. The mechanisms pertaining to the mycobiota’s influence on host health, pathology, and resident gastrointestinal communities through intra-kingdom, trans-kingdom and immune crosstalk are beginning to come into focus, setting the stage for a new chapter in microbiota-host interactions. Herein we present an examination of the inception, maturation, and dietary modulation of gastrointestinal and nutritional fungal communities with an inspection on their impact on metabolic diseases in humans.
Keywords: Mycobiota, early-life, nutrition, metabolism, fungi, trans-kingdom
Introduction
A diverse community of fungi coexists in the gut with other members of the microbiota (75, 99, 103, 128). In humans and mice, the gut mycobiota is dominated by members of the Ascomycota phylum that includes – among the most prevalent taxa – the genera Candida and Saccharomyces. A growing number of studies are uncovering how gut commensal fungi can influence host immunity in a number of immune-mediated diseases (75). However, recent findings implicate a role for gut fungi beyond their direct effect on the host immune responses. A complete understanding of the role of gut fungi in health and disease requires an effort to integrate different aspects of their interaction with the host immunity, the gut microbiota, and the host metabolism. Herein we review recent and historical perspectives on the reciprocal effects between fungi and human nutrition with a focus on the mycobiota’s ability to influence the course of gastrointestinal metabolic disease.
Early-life human mycobiota: Engraftment, breast milk and the transition to solid foods
While the establishment of a stable bacterial microbiota early in life has been linked to health later in life (4, 93), key questions about the pediatric sources of mycobiota engraftment and the importance of intestinal fungi in the context of disease development are just beginning to be investigated. A trend of increased fungal 18s rRNA in vaginal versus cesarean section birth has been observed, persisting until about one-year post delivery and mimicking what is seen for bacterial diversity(123). Principal coordinate analysis based on 18S rRNA sequencing revealed a cluster of exclusively vaginally delivered children with higher relative abundance of Candida spp., indicating that birthing method plays a role in setting mycobiota diversity on a genus level in infants. In very low birth weight neonatal infants, a population susceptible to gastrointestinal candidiasis, vaginal delivery is correlated with triple the risk of C. albicans colonization within one week of birth(15). Moreover, 76% of the mothers of these early-colonized infants were themselves colonized (compared to 56% of other infants), suggesting direct maternal transfer of fungi. Vertical transmission was confirmed in 65% of early colonization instances at birth by DNA fingerprinting, which dropped to only 41% of total colonizations 2 weeks after birth, indicating that mothers can be vectors for vertical mycobiota transfer with environmental transmission playing an increasingly significant role after the first few weeks of birth. Subsequent work has confirmed similar vertical transmission of obligate anaerobic bacteria(57), establishing a common, validated route for maternal microbial transfer.
Following birth, children are offered their first nutritional choice, namely: breast milk or formula, providing another opportunity for pediatric inoculation of microbes including fungi (Fig. 1). Several studies have investigated the fungi residing in breast milk, offering differing perspectives on the composition of the breast milk mycobiota based on both culture-independent sequencing approaches and fungal culturomics. Callado and colleagues reported a milk mycobiota consisting of Malassezia, Davidella, Sistotrema and Penicillium genera which varied only slightly across the four nations sampled (Finland, South Africa, China and Spain)(17). Additionally, 18S quantification of fungal loads were consistent geographically. This collection of genera is in contrast with an earlier investigation by the same laboratories which identified Candida, Malassezia and Saccharomyces as the highest abundance genera across breast milk samples(16). A more recent study of mothers of preterm infants corroborated a portion of these results showing a predominance of Candida spp. (C. albicans, C. parapsilosis, C. krusei and C. glabrata), and Saccharomyces cerevisiae by ITS2 amplification and sequencing(50). Breast milk was found to be devoid of cell-free fungal DNA, indicating that isolated sequences represent a fungal cell-derived community. Samples from this study were completely devoid of the genus Malassezia, common skin commensal fungi, suggesting a difference either in the donor populations or in the collection techniques across studies that might influence the extent of Malassezia shedding from the skin where this fungus is highly abundant. Furthermore, fungal isolates obtained from culturing breast milk were dominated by C. parapsilosis and Rhodotorula mucilaginosa. Interestingly, researchers have been unable to isolate Malassezia from breast milk samples despite high abundance by culture-independent identification. This suggests that live Malassezia is either not present in the breast milk or may be reflective of the culture and collection conditions implemented, although the latter is less likely as Malassezia culturing techniques are historically very well established (16, 17). Controlled studies comparing the milk, nipple and areola mycobiomes with a wider range of culture media and temperatures are needed to further delineate genuine inhabitants of lactation from serendipitous dermis carryover organisms and cell-free DNA. For bona fide inhabitants of breast milk, questions remain as to carbon and nitrogen sources available to fungi in this biological medium(16); their recognition by maternal immunity; and the time course for fungal expansion throughout pregnancy, after delivery, and after cessation of breast feeding.
Figure 1: Dietary factors affecting the gastrointestinal mycobiota throughout life.
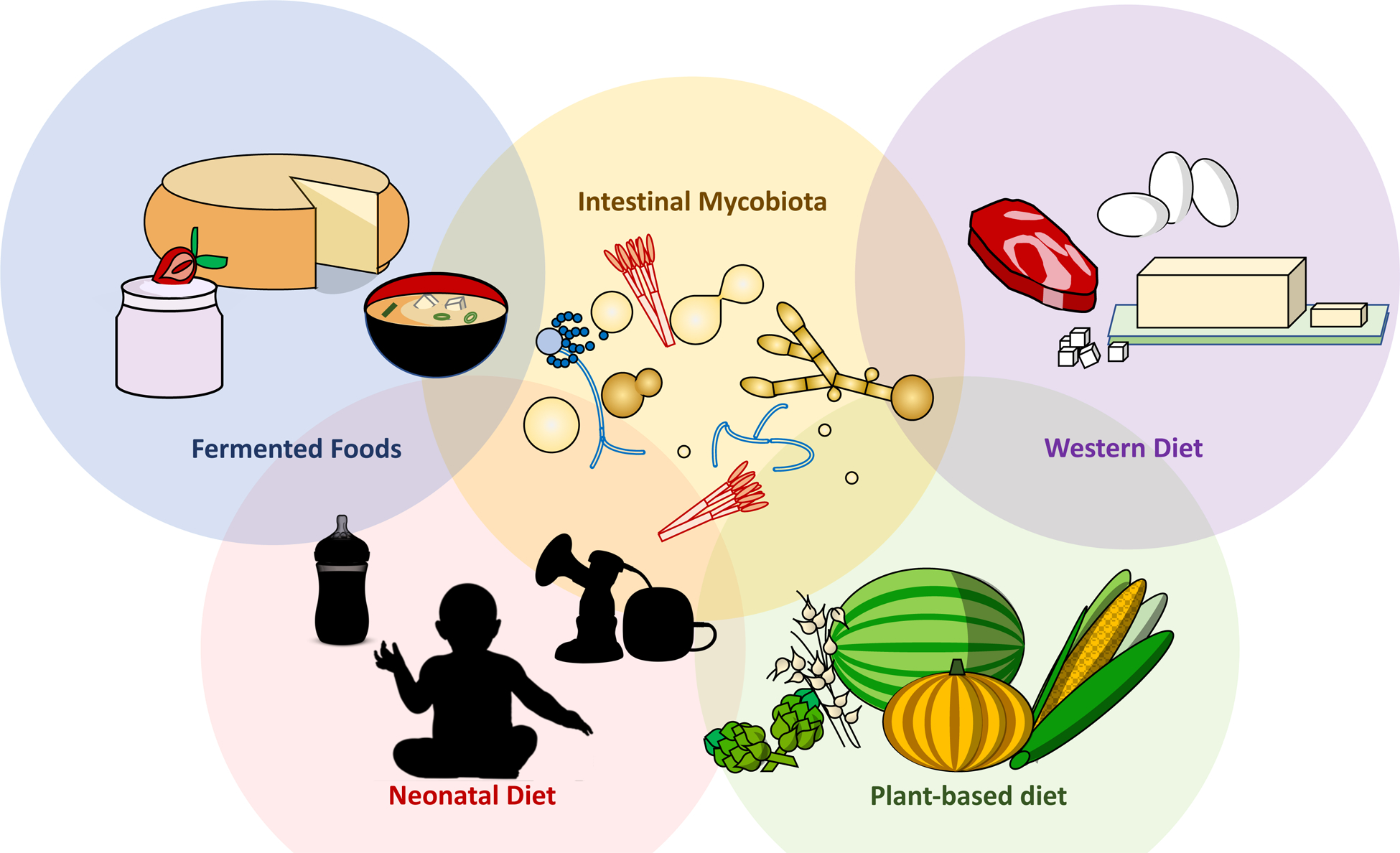
Dietary modulation of the mycobiota begins at birth, where formula or breast milk serve as primary fungal inoculum. Transition to solid food and adherence to either a plant-based or ‘Western’ diet (high in simple carbohydrates, fat and protein) result in drastic alteration of both bacterial and fungal communities within the gut. Finally, the production of a range of fermented foods, or their contamination by fungi often leads to selection of a specific nutritional microbiota prior to ingestion, altering the consumer’s native intestinal microbiota.
The mycobiota transition from neonate to toddler occurs during a formative life period, when nutrition, metabolism, and immunity undergo rapid change and development. A progression from a high Malasseziales abundance to an increase in Saccharomycetales is observed between 1–5 months of age, a common timeframe for the introduction of soft, non-milk foods(40). In terms of bacterial populations, a decrease in both Enterobacteriaceae and Bifidobacteriaceae and increase in Lachnospiraceae is observed during the same time period, suggesting a concerted, trans-kingdom shift. A distinct gut microbiota state was identified in neonates with higher Candida spp. abundance and this was associated with an increase in allergen-specific IgE concentrations at 2 years of age. This suggests that a neonatal gut conducive to or containing Candida spp. expansion may influence atopy severity and asthma development later in life, an ability previously established for a defined group of intestinal bacteria(4). These findings are further corroborated by studies in mice showing that fungal dysbiosis and colonization with specific fungi can negatively influence the outcome of allergic airway inflammation both early and later in life(74, 104, 106, 118). Another cohort showed a steady increase of fungal alpha-diversity from 10 days to two years of age(102). The OTUs with the most relative abundance during this time period transitioned from Debaryomyces spp. (during formula or breastfeeding) to Saccharomyces spp. (with weaning and solid food). Detection of fungal DNA was correlated between mother-child pairs, with children of fungi-positive mothers being more likely to also be positive. This is possibly indicative of a maternal, bacterial community transfer more conducive to fungal colonization later in life. Further inspection of this critical transition period will require additional longitudinal studies, with a particular focus on species/strain acquisition and persistence.
Dietary modulation of intestinal fungi in adulthood
In contrast to the similarities between individuals and the longitudinally consistent nature of the bacterial microbiota, the adult intestinal mycobiota has been documented as being extremely unique and variable as made evident through both geographically diverse and longitudinal fecal material sequencing (34, 43, 45, 88, 109). Dietary and nutritional differences in cohorts could potentially explain some of the interindividual variability. In a pair of studies in the same geographical region, Hallen-Adams and colleagues evaluated the mycobiota of healthy individuals on conventional (Western) and plant-based diets (45, 109). While conventional diet seems to support the existence of a preferred ‘core mycobiota’ consisting of Debaryomycetaceae (Debaryomyces and Candida spp.), Dipodascaceae (Galactomyces spp., Geotrichum spp.), Davidiellaceae (Cladosporium spp.), and Malasseziaceae (Malassezia spp.) family members; the vegetarian mycobiota favors a greater abundance of spore-forming and dietary fungi (Fusarium spp., Penicillium spp. and Aspergillus spp., Cladosporium spp.). This alteration could be explained by a reduction in yeast-like fungal loads in the intestine, allowing for the observed domination of dietary and environmental fungi; a genuine expansion of filamentous fungi in the gastrointestinal tract; or increased intake of allochthonous mycobiota from a plant-based diet. Turnbaugh and coworkers further explored the changes in the human mycobiota induced by a plant- or animal-based diet (32). While Penicillium species seemed to flourish after 2–3 days of the animal-based diet (rich in cheeses, meat and eggs), Debaryomyces and Candida spp. were drastically reduced in relative abundance. In contrast, a plant-based diet (chiefly fruit, grains and vegetables) showed a corresponding increase of a diet-associated Candida sp. While this study makes interesting connections between the diet-derived fungi and the fecal mycobiota, interpreting abundance changes is confounded by the lack of dietary controls with minimal fungal components. Lewis and coworkers followed up on their seminal study examining bacterial-diet interactions in healthy individuals(132) to investigate the archaea and fungal members of the gastrointestinal microbiota(52). Specifically, they revealed a positive correlation between recent dietary intake of carbohydrates and Candida species while saturated fatty acids resulted in a negative correlation. When expanded to long-term dietary choices, these patterns resulted in trends in the same directions without statistical significance, possibly reflecting subtle changes in the diet not equated for in the volunteer diet questionnaire and highlighting challenges involved in conducting and enforcing a controlled nutrition study for extended periods of time.
Examining the impact of controlled, therapeutic diets on the intestinal mycobiota offers a unique vantage point to gauge the potential therapeutic benefit or detriment of these organisms to select patient populations. A recent examination of pediatric patients with inflammatory bowel disease uncovered a significant negative correlation between Crohn’s disease (CD) patient initiation of exclusive enteral nutrition (EEN), a first-line dietary treatment for the disease, and the relative abundance of Candida albicans, Clavispora lusitaniae and Cyberlindnera jandini –organisms that are typically increased in CD patients(72). Although the relative abundance of fungi in this cohort was not informative in predicting patient response to anti-TNF treatment, EEN patients maintained significantly decreased abundances of these fungi at the 8-week post-dietary modulation follow-up. Enteral nutrition is a broad term encompassing both elemental (amino acid-based) and non-elemental (isolated protein-based, such as casein) which past studies have determined have equivalent efficacy in the treatment of CD(87). Notably the diet is routinely low in both complex carbohydrate and fiber sources, correlating well with past nutritional studies with the Candida genus(52). Whether the observations of these preliminary studies are due to direct depletion of pro-fungal nutrients or indirect mediation through host immunity or bacterial microbiota alterations remains to be discerned. Looking ahead to future studies, the impact of therapeutic diets such as low FODMAP (for IBS patients(46)) - as well as regional or cultural diets may lead to further insight into the precise mechanism by which distinct elements within the diet alter the gastrointestinal mycobiota.
To facilitate assessment of mycobiota changes in a more controlled setting and possibly reveal individual the dietary components responsible, murine controlled-diet experiments offer an attractive alternative to human nutrition studies. Gale and colleagues evaluated changes in ITS2-fungal abundances following a high-fat diet in mice(49). After 8 weeks of diet, high-fat diet mice showed a significant decrease in the genus Saccharomyces (specifically S. cerevisiae) while Candida showed no change compared to conventional murine diet. Quantitative PCR of both diets revealed that species present in murine stool were overwhelming (80–90%) absent from the diet, effectively controlling for changes simply due to transient microorganisms. Importantly, S. cerevisiae represented <0.01% of diet sequences in the diets (compared to >80% in feces), supporting the notion that a high-fat diet induces a genuine outgrowth of the species within the murine gastrointestinal tract. Another study carried out by Ludwig and coworkers(122) examined the effect of both a Western diet (high sugar, cholesterol and fat) and caloric restriction compared to a conventional murine diet. While they observed only a marginal change in beta-diversity between regimens, an expansion in Wallemia spp. and the Basidiomycota genus Sporobolomyces was observed with western diet and caloric restriction, respectively. Interestingly, Candida spp. were highest in abundance with conventional diet compared to both alternative treatments. Importantly, the ability to manipulate increasingly intricate dietary components (e.g. sources of fiber, phenolic compounds, phytosterols and terpenes) in future studies may yield a new level of mechanistic insight into the observed effect of whole foods in the human diet.
Dietary fungi and alteration of the microbiota
Just as nutrition and diet may impact the mammalian mycobiota, so too can fungal dietary components modulate the bacterial and fungal communities in food preparation and the gastrointestinal tract. An early investigation of antagonist dietary fungi-fungal symbiont interactions revealed a negative sequencing correlation (Spearman coefficient of -0.31) between Debaryomyces hansenii, a fungus common in fermented products such as cheese(12), and Candida yeasts in feces(11). Isolation from 44 different cheeses and coculture with a variety of Candida species revealed several D. hansenii strains that produced peptide antifungal natural products or yeast killer toxins (YKTs). Yeast killer toxins, also known as mycocins, are antimycotic peptides produced by a variety of fungi in a similar manner as bacteriocins in bacteria. Inhibitory activity was found to be strongly dependent on temperature (most potent at 25 °C) and acidity (favoring a pH of 4.5). Although these properties cast doubt on the role of D. hansenii-derived YKTs in the small and large intestines, they do point to a plausible mechanism by which fermented products exclude Candida species, diminishing the inoculum of these opportunistic pathogens from dietary sources. Moreover, YKTs from Wickerhamomyces anomalus, a yeast previously identified in the normal murine mycobiota(34), have very recently been shown to be potent against Candida mesorugosa over a wide range of physiologically-relevant acidities (pH of 3–6) and temperatures (5–40 °C), increasing the possibility that YKTs play a role in directing fungal-mycobiota interactions within the mammalian intestinal tract(115). YKT susceptibility in C. albicans was aggravated by deletion of Hog1, a gene encoding a member of the MAPK phosphorylation pathway(82). Interestingly, Hog1-deficient mutants were previously shown to be more resistant to Congo red and calcofluor white, agents that bind to chitin in both yeasts and filamentous fungi(3), suggesting that alteration of the fungal cell wall increases susceptibility to pore-forming YKTs from D. hansenii. Another study determined that Pichia, a commensal genus found in a variety of fermented products such as miso soup and alcoholic beverages, displayed an inverse correlation in terms of relative abundance with Candida spp. in the oral cavity of HIV patients(86). Spent culture broths from a mycological repository Pichia strain (MRL1458, putatively assigned as P. farinosa) were found to be inhibitory towards the growth of Candida, Fusarium and Aspergillus spp. The causative agent was found to be a proteinase K-sensitive, heat- and alkali-insensitive diffusible molecule: presumably an antifungal peptide. This result matches well with the 30-year-old literature surrounding the discovery of salt-mediated killer toxins from P. farinosa and elucidation of its inhibitory activity against a variety of fungi(111, 112). Together, these studies highlight the importance of YKT peptides in preventing fungal contamination and spoilage in fermentation processes and hint at a potential mechanism for direct dietary modulation of the mycobiota by fungi.
The mycobiota inhabiting the intestinal tract not only have the potential ability to manipulate their own community, but also to influence the vastly more numerous neighboring bacteria, possibly indirectly influencing the mammalian host in the process. The increasing interest in the use of fecal microbiota transplantation (FMT) for the treatment of various microbial-related disease highlights the complexity of gut trans-kingdom interactions. In Clostridioides difficile infections, high burdens of Candida are associated with a negative outcome of FMT that is otherwise highly efficacious in this context(92, 139). In contrast, high levels of Candida have been linked to an increased responsiveness in patients with Ulcerative Colitis (UC) (71). In CDI, where C. difficile is the predominant bacterium, Candida and C. difficile appear to have a mutualistic relationship, thriving in a pro-inflammatory environment and becoming refractory to FMT(92). In UC patients, that retain a complex microbial community, high Candida burden were associated with an increased bacterial diversity that persisted following FMT. In these patients, a reduction of Candida burden following FMT was associated with an ameliorated disease severity, again suggesting a pro-inflammatory role for Candida although the underlying mechanisms remain unclear (71). These recent studies suggest that the influence of Candida on the colonization and persistence of different bacterial species is highly influenced by the preexisting disease context within the intestine.
Bushman and colleagues reported the existence of a significant positive correlation between the presence of Candida spp., detected in 57% of the fecal samples from healthy volunteers, and the genus Bacteroides(52). This finding has been subsequently corroborated by multiple independent studies(45, 133). In an attempt to exploit the bacterial community-modifying properties of fungi, Pu and coworkers trained a strain of S. cerevisiae for probiotic usage in freshly weaned swine(133). Livestock are typically weaned at an early age and supplemented with a regimen of antibiotics to promote rapid growth and stave opportunistic pathogen infection due to an underdeveloped intestinal microbiota. Unfortunately, this has led to the rapid evolution of drug resistant bacteria through direct selection in agricultural animals and indirect effects from wastewater run-off from grazing fields and animal housing facilities. Piglets were treated with a diet incorporating S. cerevisiae alone, S. cerevisiae and fermented egg white, or a control diet; each without the addition of antibiotics during weaning. After weaning on the yeast-containing diets, the fecal bacterial communities of both groups of S. cerevisiae-fed piglets were altered in beta diversity with an increase in Bacteroides spp. as well as a decrease in several Firmicutes including the genera Roseburia, Faecalibacterium and Anaerovibrio. Strikingly, these findings are in stark contrast to the results obtained from feeding chitin-glucan to mice on a high fat diet, wherein Delzenne and coworkers observed a dramatic reversal of high fat diet-induced Firmicutes Roseburia spp. and Eubacterium spp. depletion accompanied by an aggravated reduction in Bacteroides spp.(89) This apparent discrepancy suggests that live fungi may have altered, or even opposing, effects on the gastrointestinal microbiota when compared to their fungal cell wall components. In piglets, S. cerevisiae supplementation increased daily weight gain, resulting in a more efficient feed intake while decreasing diarrhea rate and death rate substantially. Additionally, fungi with increased pathogenic potential may also affect the mammalian mycobiota. Murine gastrointestinal exposure to Mucor circinelloides, a food contaminant and culprit in yogurt poisonings, was found to induce significant changes in the bacterial alpha and beta diversity, resulting in the expansion of Bacteroides and Butyricimonas spp. and a reduction in Akkermansia spp. and the family Verrucomicrobiaceae(85). Clearly, intestinal exposure to resident, probiotic and pathogenic fungi broadly affects the host intestinal bacterial communities and their responsiveness to diseases and therapy. As a consequence, the impact of and mechanisms underlying alteration of the gut ecological landscape within the host has emerged as a burgeoning field of future research.
Diet-derived mycotoxins and their effect on the host
Far from being solely beneficial organisms, fungi (in particular filamentous, spore-forming fungi) have a long and notorious history of food contamination, causing harm to both humans and livestock via their toxic secondary metabolites. Though hundreds of mycotoxins have been reported in the past century, we will focus on three of the most common classes of secondary metabolites: bicyclic polyketide toxins, aflatoxins and trichothecenes (Fig. 2). These toxic small molecules are prototypically synthesized by Aspergillus, Penicillium and Fusarium spp. through elaborate, specialized enzymatic machinery and display toxicity through a variety of mechanisms. For more information on the plethora of additional mycotoxins derived from Alternaria spp. and other molds, we invite readers to explore the extensive preexisting body of literature on the subject(18, 53).
Figure 2: Diverse filamentous fungi-derived mycotoxins adversely impact health.
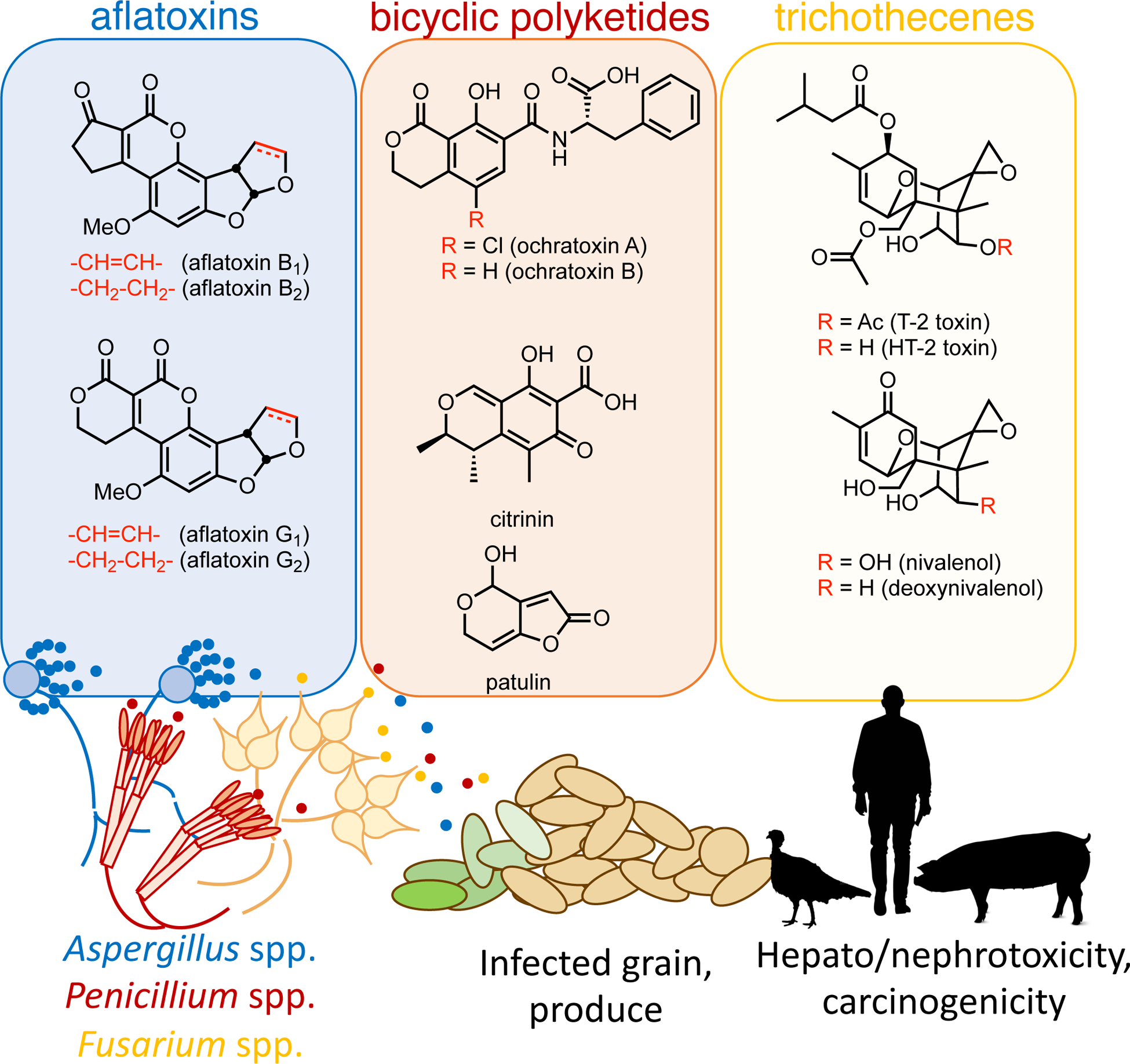
Ascomycota in food sources cause a range of deleterious health consequences in livestock and humans via mycotoxin production. The representative structures of three major classes of fungal toxins: aflatoxins, bicyclic polyketides and trichothecenes are highlighted in the figure and text, canonically derived from Aspergillus, Penicillium and Fusarium spp.
A series of simple, bicyclic polyketide products were isolated from Penicillium and Aspergillus molds beginning in the 1930s. Citrinin, the first such toxin, was discovered by Hetherington and Raistick from a characteristically yellow mold, P. citrinum, in 1931(51). The carbon backbone of crystalline, golden-colored citrinin is constructed through an iterative, non-reducing polyketide synthase (citS(pksCT)). The mature pentaketide intermediate undergoes a series of enzyme-catalyzed oxidation and reduction events followed by final cyclization and dehydration to the quinomethide toxin(48). Producers of citrinin typically infect nuts, cereals, fruit, rice and soybeans; posing a risk to both livestock and human health(91). Upon ingestion, citrinin accumulates in the mammalian kidney where it acts as a potent nephrotoxin, leading to oxidative damage through disruption of the mitochondrial respiratory chain(26, 119), although the precise mechanism of action is still under investigation.
Patulin, a natural product produced by Penicillium and Aspergillus spp., was discovered during a resurgence in fungal antibiotic discovery stoked by both Sir Alexander Fleming’s elucidation of penicillin in 1928 and the advent of World War II(38). As many as four independent laboratories may be credited with the first documentation of an antibiotic substance derived from an unknown Penicillium sp., P. claviforme, or A. clavatus from 1938–1942(6, 27, 117, 129, 130). Patulin’s bicyclic unsaturated lactone structure was deciphered by Woodward and Singh(131), and results from a fungal, non-reducing PKS that produces 6-methylsalicylic acid (6-MSA)(77). Critically, the study of this exact process in P. griseofulvum by A. J. Birch formed the basis of polyketide natural product chemistry(14). This product undergoes a decarboxylation reaction followed by a series of biosynthetic oxidation and cyclization steps to yield the mycotoxin patulin. Due to the intrinsic electrophilic nature of its dienoate moiety, patulin is reactive to Michael donors. Sulfhydryl groups of glutathione and cysteine efficiently form patulin adducts and are able to abolish the molecule’s toxic properties(78). The exact etiology of acute patulin mycotoxicosis is still debated, but recent studies suggest a role for mucosal tight junction disruption and bacterial translocation(78, 137). Apples, other fruit and fruit juices are the primary reservoirs for patulin, resulting in the strict regulation of mycotoxin levels in these consumer products in most Western countries(96).
Ochratoxin A was originally isolated from a bulk maize meal culture of A. ochraceus (K-804 strain) which was found to be acutely toxic to young mice, rats and ducklings(81). Bioassay-guided fractionation yielded a pure chlorophenol which induced sudden death in ducklings with an LD50 of 500 mg per kg. Histology of the liver revealed fatty infiltration of parenchymal cells throughout the gross structure of the organ. The 3,4-dihydroisocoumarin structure of ochratoxin A is furnished by a type I polyketide synthase(125). This pentaketide scaffold subsequently undergoes benzylic oxidation followed by adenylation, thiolation and condensation with phenylalanine catalyzed by a non-ribosomal peptide synthetase to afford ochratoxin B. Finally, a dedicated halogenase installs the aryl chloride moiety, delivering ochratoxin A. Ochratoxin A acts primarily as a nephrotoxic agent of multiple mechanisms of unclear ranking in importance and has been linked to historic cases of human endemic nephropathy in the Balkan states in the 1950s(94, 138). The possible food stocks harboring contamination are extremely varied, encompassing both plant-based (e.g. cereals, wine, vegetables, coffee, peanuts) and animal-based (e.g. dairy, meat) products which serve as suitable substrates for the ochratoxin-producing fungi(91).
In the spring and summer of 1960, England was subjected to a massive agricultural outbreak affecting domesticated turkeys, known only as Turkey “X” Disease, that swept the country devastating the poultry market. Animals presented with a characteristic liver disease resulting in compression of sinusoids and loss of granular appearance with regions of eosinophilic cells with homogenous cytoplasm, indicative of damage and possible initiation of carcinogenesis(126). The source of the disease was discovered to be a newly initiated groundnut supply from Brazil and the feedstocks were quickly altered sparing half a season of fowl(67). Over the course of the next five years, researchers elucidated the causative agents: the aflatoxins, a series of extremely potent mycotoxins arising from the secondary metabolism of Aspergillus flavus(5). The identity of four highly unsaturated scaffolds were revealed by Wogan and coworkers for two blue (aflatoxin B1 and B2) and two green fluorescing (aflatoxin G1 and G2) compounds. Their tetracyclic skeleton arises through the sequential action of two fatty acid synthases (aflA and aflB) and a type I, non-reducing fungal polyketide synthase (aflC (pksA)) resulting in the formation of norsolinic acid anthrone(28). An anthrone oxidase (hypC) catalyzes the aerobic conversion to the anthraquinone, norsolinic acid which undergoes a series of post-polyketide synthase tailoring steps yielding the mature aflatoxins(36). Interestingly, A. nidulans and A. versicolor produce only the anthraquinone sterigmatocystin, a toxic intermediate in the biosynthesis of the aflatoxins, through an otherwise homologous pathway(20). Aflatoxin-producing Aspergillus spp. are notorious for their ability to invade stores of peanuts, grain (e.g. corn, wheat, linseed) and dairy products(101), posing risks to humans and livestock alike. Aflatoxins produced by A. flavus, A. nomius and A. parasiticus are bioactivated by cytochrome P450 epoxidation in the liver forming genotoxins (e.g. aflatoxin B1 exo 8,9-epoxide). These highly electrophilic epoxides intercalate into DNA, by virtue of their unsaturated, planar scaffold, and rapidly alkylate N7-guanine residues(56). This, in turn, may result in errors in DNA-adduct replication or repair, leading to carcinogenesis in the liver.
The large family of trichothecene natural products arise from a collection of filamentous fungi typically from the genera Fusarium and Trichoderma. The trichothecene secondary metabolites were originally isolated from Trichothecium roseum, a typical phytopathogen found in fruits such as melons, apples and grapes(39). In 1968, Prentice and Dickson at the University of Wisconsin investigated the occasional instances of Fusarium spp. associated with a wheat disease known as ‘scab’(95). Upon ingestion of Fusarium-infected feed, cattle present with emesis and, upon gratuitous consumption, death. Similar cases of mycotoxicosis have been known for nearly one hundred years. In an incident from early Soviet Russia, contaminated cereal incorporation into rye bread resulted in human poisoning, with affected individuals displaying weakness, vertigo, headache and nausea(35). Prentice and Dickson were successful in isolating emetics from corn infected by several Fusarium spp. and the structures of T-2 toxin and HT-2 toxin (named for the T-2 strain identification code and the deacetylated hydroxy-derivative of the former, respectively) were disclosed by Strong and colleagues(9, 10). Around the same time of these discoveries, two closely related cyclohexenone-containing natural products from F. nivale and F. roseum were isolated and designated as nivalenol and deoxynivalenol, respectively(83, 114, 135). Collectively these fungal secondary metabolites arise from terpene biosynthesis involving the cationic cyclization of farnesyl diphosphate to form the sesquiterpenoid trichodiene. This committed intermediate undergoes an elaborate series of oxidations, a cyclization and a varying degree of acylation reactions to form an extremely diverse series of toxins from a common biosynthetic origin(33). The toxic properties of these compounds are dependent on the nucleophilic epoxide moiety adorning their intricate scaffold, as chemoenzymatic removal leads to a drastic loss in cytotoxicity(110). The established central mechanism for toxicity of many members of this family involves inhibition of ribosomal protein synthesis(31). Continued efforts examining their ability to activate innate immunity and affect intestinal homeostasis may prove as interesting avenue of future research(42, 58).
The gut mycobiota and metabolic diseases
Whether through mycotoxin, bacterial modulation or nutrient metabolism several studies suggest that select fungal strains have the ability to influence host metabolism. This influence might either be a direct effect on the metabolism of selected nutrients or an indirect modulation of the host microbiota. Microbial communities in the intestine are unavoidably connected and these microbial networks are crucial to various host metabolic pathways (52).(45, 133)(89). Despite this continuous cross-talk, recent studies suggest that gut fungi might be directly involved in disease progression independently from their interaction with gut bacteria. High levels of Candida and Saccharomyces species have been found in children with beta-cell autoimmunity that progressed to clinical type 1 diabetes (T1D) early in life(54). Children with beta-cell autoimmunity also presented with bacterial dysbiosis that was nonetheless not associated with early T1D development. In a HFD-induced obese mouse model, administration of a probiotic beverage containing high levels of yeasts (including Candida spp.) altered the gut bacterial composition and reduced both liver damage and the serum levels of cholesterol and IL-6(62). In leptin-resistant (db/db) mice, which develop fatty livers associated with severe obesity and type 2 diabetes, intensive gavage with S. boulardii altered the gut bacterial microbiota and resulted in decreased body weight, fat mass, hepatic steatosis, and inflammatory state. S. cerevisiae has also been shown to reduce levels of liver damage and alter the bacterial microbiota composition in mouse and rat models of acute liver injury(73, 136). Fungal-derived sugars might contribute to these metabolic changes(37, 89). Administration of fungal-derived mannan to mice with chemically induced acute lipemia reduced the levels of atherogenic LDL, cholesterol, and triglycerides. Mannan also causes labilization of lysosomal membranes and a decrease in the volume of the lipid droplets in hyperlipidemic mice(41). β-glucan of fungal origin has also been reported to reduce SGLT‐1 expression on the intestinal epithelium and to improve glucose control and fatty liver in genetic and high-fat diet obese rodent models(19, 25, 59, 65). Besides its immunological effect, β-glucan might also act as a dietary fiber by slowing gastric emptying; hindering triglyceride and cholesterol absorption and consequently reducing cholesterol levels(105). The ability of fungi to modulate host uptake of key nutrition components important to the development of obesity opens the door for future precise experiments investigating their effect on obesity-related diseases.
Akin to nutrients absorbed throughout the gastrointestinal tract, gut-derived microbial products – including metabolites and toxins – are constantly transported to the liver via the portal circulatory system where they may undergo metabolism prior to entering the systemic circulation (Fig. 3). This continuous exposure to immune-reactive substances has the potential to induce potent immune responses. In the healthy liver, such responses are effectively counteracted by unique regulatory mechanisms such as regulatory soluble mediators and local tolerogenic antigen-presenting cells (APCs). This immune tolerance is crucial to avoid unnecessary responses that can lead to the development of liver pathologies through detrimental inflammatory responses. In most cases, the etiology of these diseases appears to involve a failure of immune tolerance possibly incited by environmental triggers in a genetically predisposed host. These environmental triggers could include xenobiotics, diet and components of the microbiota. Importantly, recent evidence suggests that intestinal fungi could affect the gut-liver axis and might play a critical role in the development of liver pathologies.
Figure 3: Fungi modulate host metabolism directly by secreting enzymes and toxins, and indirectly through their cell wall polysaccharides.
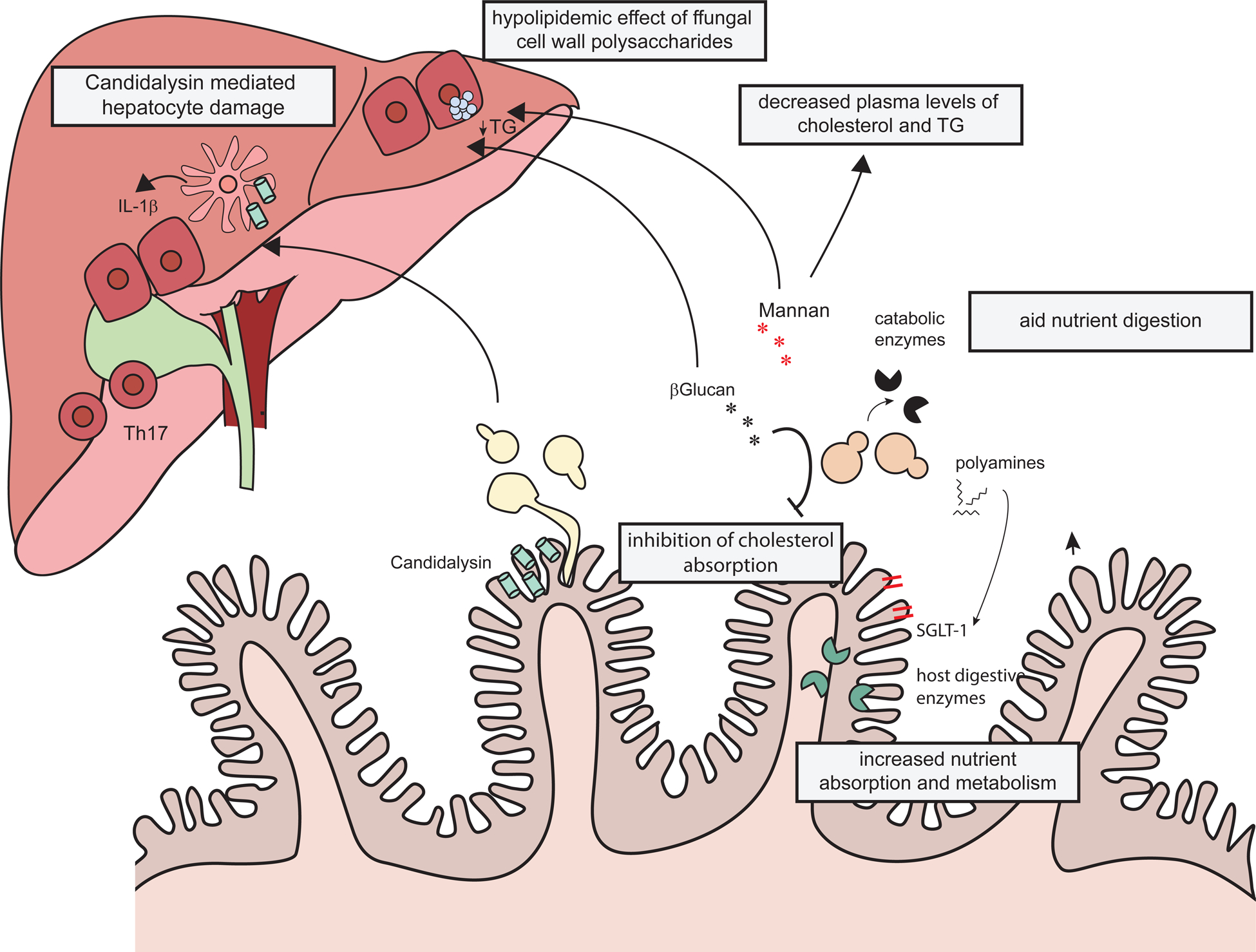
The toxin candidalysin secreted by C. albicans, induces liver damage by promoting the release of IL-1β by liver phagocytes. β-Glucans on the surface of the fungal cell wall decrease intestinal absorption of cholesterol and reduce epithelial expression of the glucose transporter SGLT‐1. β-Glucan might slow gastric emptying thus reducing triglycerides and cholesterol absorption. Mannan also exerts a hypolipidemic effect on the mouse liver. Fungi secrete enzymes that directly digest the luminal nutrients and polyamines that modulate the expression of host metabolic enzymes and nutrient transporters in the intestinal epithelium.
Fungal dysbiosis has been reported in cirrhosis patients with HCV, alcohol-related liver disorders and non-alcoholic fatty liver disease (NAFLD)(8, 68). In particular, cirrhosis patients had an increased burden of Candida and a lower Basidiomycota/Ascomycota ratio was observed in patients with advanced and infected cirrhosis(8). In cirrhosis patients, this increased Candida burden has been previously associated with increased mortality(63) and recent evidence suggests that intestinal fungi contribute to the development of alcohol‐associated liver disease(134). Similarly, an increased burden of Candida, including C. albicans, has been reported in the feces of alcohol-associated liver disease patients(29, 134). ASCA IgG, that can be generated in response to C. albicans, is increased in alcoholic hepatitis and is associated with increased mortality(134)(28). In mice, colonization with C. albicans led to a worsened outcome of ethanol-induced liver disease whereas treatment with the antifungal amphotericin resulted in lower levels of liver injury, inflammation, and hepatic steatosis (29, 134). A recent analysis showed that 30% of alcoholic hepatitis patients had fecal samples positive for C. albicans strains carrying the candidalysin expressing gene ECE1, whereas ECE1 was not detected in control patients. Importantly, these patients had an increased disease severity and were at higher risk of mortality(29). Candidalysin is a cytolytic enzyme that damages the host’s cell membrane by forming pore-like structures that lead to LDH release and calcium influx into the cytoplasm. These trigger a danger response signaling pathway that activates epithelial immunity(2, 84, 120, 121) as well as IL-1β secretion by macrophages (60, 100) (113). In mice, C. albicans exacerbation of alcoholic liver disease was dependent on ECE1 expression, leading to increased levels of IL-1β and inflammatory chemokines in the liver via the release of candidalysin(29). Several pattern recognition receptors (PRR) have been shown to recognize carbohydrates on the surface of the fungal cell wall and to be essential for the initiation of immuno-modulatory responses. In particular, C-type lectin receptors (CLRs) such has Dectin-1, Dectin-2, and macrophage-inducible C-type lectin (Mincle) signal via the tyrosine kinase (Syk)/Card9 pathway to induce NF-κB activation(107). In the gut, fungal-recognition and responses are mediated by a subset of myeloid cells, characterized by the expression of the chemokine receptor CX3CR1(70, 74, 75). While it remains to be established whether these cells and pathways play any role in influencing the host metabolic status in response to intestinal fungi, mice lacking Dectin-1 on hematopoietic cells are protected from ethanol induced liver damage(134). Systemic β-glucan injection has been shown to promote local IL-1β increase in the liver and, in vitro, β-glucan promotes the release of IL-1β by Kupffer cells(134). However, this in vitro release is Dectin-1 independent(134), and Dectin-1 appears to be dispensable for C. albicans-induced hepatic damage(29). This discrepancy could be due to insufficient levels of β-glucan released systemically by gut C. albicans, or by β-glucan signaling through other pro-inflammatory pathways such as TLR2/TLR4(29).
A further mechanism by which fungal colonization might contribute to liver damage is through the induction of adaptive T cell responses. Gut fungal colonization can induce strong Th17 responses both locally and systemically(69, 104). Th17 cells have been implicated in the development of immune-mediated liver diseases(66, 76, 90). Among such diseases, primary biliary cirrhosis, primary sclerosing cholangitis (PSC) and autoimmune hepatitis are the most common indications for liver transplant. Peripheral blood mononuclear cells isolated from PSC patients had an increased Th17 response to stimulation with C. albicans when compared to cells from healthy individuals(61). Biliary candida infections have been reported in PSC and are associated with a worsened disease prognosis(61, 64), providing one plausible locale for origination of a T cell interaction. Whether intestinal Candida can also contribute to the development of immune-mediated liver diseases remains unclear.
The C-type lectin receptor Mincle, an essential component of the innate immune response to systemic C. albicans(127), is highly expressed in hepatic innate inflammatory cells and endothelial cells of autoimmune hepatitis (AIH) patients(44). AIH patients also present with increased levels of Syk phosphorylation, a tyrosine kinase immediately downstream in the CLR signaling pathway. These findings could be replicated in the mouse model of concanavalin A-induced hepatitis, and both chemical and genetic blockade of the Mincle pathway protected against the development of the disease. However, SAP130, the only endogenous, non-pathogen–derived Mincle ligand was also found to be increased suggesting that the role of Mincle in the development of autoimmune-liver diseases might be fungal-independent(44).
The clinical presentation of NAFLD is very similar to the pathology spectrum observed in patients with alcoholic liver disease. The disease presentation ranges from simple steatosis and non-alcoholic steatohepatitis to the development of cirrhosis and hepatocellular carcinoma. NAFLD is characterized by the accumulation of fat in hepatocytes. Furthermore, NAFLD is associated with obesity and with a high risk of type 2 diabetes (T2D), hypertension, dyslipidemia, and hypertension. Supporting a role of the intestinal microbiota in the disease pathogenesis, germ-free mice are protected from diet-induced obesity and insulin resistance(97). Further, microbiota transplant has been shown to transfer NAFLD features from high-fat diet fed mice to GF animals and the gut microbiota appears to modulate lipid metabolism in the liver, independently of obesity(29, 60). In humans, gestational weight gain is associated with changes in the metabolic potential of the infant microbiota, including abnormalities in vitamin synthesis and carbohydrate utilization pathways of the infant microbiota(13, 30, 108). These data suggest that these metabolic imbalances can be affected by the gut microbiota. Although the role of fungi in the development of NAFLD has not been studied, sequencing of the intestinal fungal communities has demonstrated fungal dysbiosis in obese subjects with a less diverse mycobiota characterized by an increased abundance of the phylum Ascomycota and class Saccharomycetes(80). Interestingly, the mycobiota composition in these individuals also correlated with various metabolic indexes including levels of fasting triglycerides and HDL-cholesterol suggesting a potential link between gut fungi and lipid metabolism. As mentioned above, the differences in fungal composition might be driven by the different dietary intake of obese patients and further studies are needed to clarify the driving forces behind the observed changes.
Fungi also secrete various digestive enzymes and small molecules that can influence host metabolism. Secretion of catabolic enzymes is a unique feature that fungi, being eukaryotes devoid of digestive organs, rely on to obtain nutrients from their environmental niches. S. cerevisiae boulardii administration to healthy volunteers resulted in an increased activity of lactase, α-glucosidase, and alkaline phosphatase activity measured in the intestinal brush border(55). These properties have been successfully used to treat enzymatic deficiency in patients with sucrase–isomaltase deficiency, an inherited disaccharidase deficiency that hinders sucrose digestion leading to diarrhea and abdominal cramps(47, 116). The unabsorbed sucrose is converted to hydrogen by gut bacteria leading to an increased concentration in the breath that can be used for diagnosis. S. boulardii can relieve this enzymatic deficiency, reducing gastrointestinal hydrogen production and clinical symptoms in these patients while consuming a sucrose-containing diet via the production of the enzyme sucrase(47, 116). A similar mechanism is also thought to underlie the protective effect of S. boulardii in patients with drug-induced disaccharidase inhibition and trehalose intolerance(24, 98). Loss of epithelial surface caused by mechanical injury can also profoundly affect nutrient absorption. In mice, intestine resection causes an increased circumference of the intestinal wall, mucosal hyperplasia and reduced enzymatic activity. Several studies have shown that S. boulardii can restore gut-associated enzymatic activity (including disaccharidases, sucrose, lactase and maltase activity while rescuing the levels of D-glucose uptake) by increasing expression of the sodium/glucose cotransporter 1 (SGLT-1) in the brush border membrane (21, 23). The effect is likely mediated by the release of polyamines from S. boulardii cells that are able to directly promote synthesis of intestinal glycoproteins, nutrient transporters and digestive enzymes(1, 21–23). Studies on S. boulardii highlight the unexplored role that intestinal fungi and their enzymatic machinery have as direct modulators of intestinal metabolic activity.
Concluding remarks
The gut mycobiota is comprised of a diverse community of fungi inhabiting the human gastrointestinal tract. This collection of intestinal fungi plays a key role in several aspects of human nutrition. Inoculation at birth and through breastfeeding introduces neonates to their foundational GI mycobiota(15, 16, 50, 123). This community shifts as infants begin transitioning to soft and solid foods, and later adapts to the dietary choices of adults(40, 45, 102, 109). Changes within the mycobiota correlate with the much larger bacterial community dynamics, resulting in increases in obligate anaerobic genera such as Bacteroides(52, 133). Additionally, fungi are central elements in fermentation processes. More than simply providing a unique source of nutrition, colonizing fungi alter the mycobiota of these food sources by producing selective antimycotic peptides, providing a unique selection mechanism for gastrointestinal inoculation. In a darker turn, infection of produce, grain and silage by filamentous fungi presents a challenge to livestock and humans alike. Mycotoxins biosynthesized by Fusarium, Aspergillus, Penicillium and other molds, varying in their intricate structures, induce genotoxic, nephrotoxic and hepatotoxic effects(56, 78, 138).
The gastrointestinal mycobiota is broadly recognized by innate and adaptive immunity, serving to prevent opportunistic pathogens from causing systemic infection. Mucosal fungal immunity has been further implicated in the genesis and exacerbation of a broad spectrum of metabolic diseases and liver pathologies (7, 29, 70, 75, 79, 124). Future studies investigating the exact mechanism of how fungi promote these disease states and their role in other metabolic disease etiologies remain under investigation.
Summary Points List:
The human gastrointestinal fungal community, or mycobiota, is a diverse facet of the larger microbial community of eukaryotes and prokaryotes thriving within the host.
With the advent of modern sequencing technologies, a sharper image of the early-life acquisition and variability throughout life of the mycobiota is beginning to emerge.
Nutritional sources of fungi (e.g. fermented foods and plant-association) as well as general dietary choices appear to manipulate the intestinal communities.
Fungal contaminations within food sources have a global impact on human and livestock health through the production of hepatotoxic, nephrotoxic and carcinogenic mycotoxins.
The gastrointestinal mycobiota broadly affects immunity and physiology, resulting in aggravation of gastrointestinal cancers, obesity and other metabolic disorders. ·
Intra-kingdom (fungal-fungal) and trans-kingdom (fungal-bacterial) interactions can shape the gut microbiota to affect host immunity and disease.
Further research is needed to understand whether other members of the gut mycobiota can directly modulate host immunity, metabolism, and cancer development by secreting enzymes, toxins or metabolites.
Acknowledgements
The authors are supported by the National Institutes of Health (R01 DK113136, R01 DK121977, R21 AI146957 and F32DK120228), Kenneth Rainin Foundation, the Leona M. and Harry B. Helmsley Charitable Trust, Pilot Project Funding from the Center for Advanced Digestive Care (CADC), Irma Hirschl Research Scientist and Crohn’s and Colitis Foundation awards.
Glossary
- Intra-kingdom interaction
interaction that occur within the same kingdom of life. Examples relevant to this review include fungal–fungal and bacterial-bacterial interactions.
- Trans-kingdom interaction
interaction that occur within the different kingdoms of life. Example relevant to this review include fungal-bacterial interactions.
- Xenobiotics
substances that are foreign to the body
- Steatohepatitis
a type of fatty liver disease characterized by concurrent inflammation and fat accumulation in the liver.
- Dyslipidemia
an imbalance in the concentrations of triglycerides and HDL cholesterol in the plasma
- FODMAP
Fermentable Oligo-, Di- and Mono-saccharides And Polyols, highly fermentable but poorly absorbed short-chain carbohydrates and polyols that might exacerbate intestinal symptoms by increasing small intestinal water retention, colonic gas production, and intestinal motility.
- ITS
The nuclear ribosomal Internal Transcribed Spacer region of fungal DNA, widely used to characterize the diversity and composition of fungal communities.
Literature cited
- 1.Zaouche A Loukil JPCC, Lagausie P de, Peuchmaur M, Macry J, Fitoussi F, Bernasconi P, Bingen E. 2000. Effects of Oral Saccharomyces boulardii on Bacterial Overgrowth, Translocation, and Intestinal Adaptation after Small-Bowel Resection in Rats. Scandinavian Journal of Gastroenterology. 35(2):160–65 [DOI] [PubMed] [Google Scholar]
- 2.Allert S, Förster TM, Svensson C-M, Richardson JP, Pawlik T, et al. 2018. Candida albicans-Induced Epithelial Damage Mediates Translocation through Intestinal Barriers. mBio. 9(3):e00915–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arana DM, Nombela C, Alonso-Monge R, Pla J. 2005. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology,. 151(4):1033–49 [DOI] [PubMed] [Google Scholar]
- 4.Arrieta M-C, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, et al. 2015. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Science Translational Medicine. 7(307):307ra152–307ra152 [DOI] [PubMed] [Google Scholar]
- 5.Asao T, Büchi G, Abdel-Kader MM, Chang SB, Wick EL, Wogan GN. 1965. The Structures of Aflatoxins B and G1. J. Am. Chem. Soc 87(4):882–86 [DOI] [PubMed] [Google Scholar]
- 6.Atkinson N 1942. Antibacterial Substances Produced by Moulds. Australian Journal of Experimental Biology and Medical Science. 20(4):287–88 [Google Scholar]
- 7.Aykut B, Pushalkar S, Chen R, Li Q, Abengozar R, et al. 2019. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature. 574(7777):264–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj JS, Liu EJ, Kheradman R, Fagan A. Fungal dysbiosis in cirrhosis. Gut. 67:1146–54 [DOI] [PubMed] [Google Scholar]
- 9.Bamburg JR, Riggs NV, Strong FM. 1968. The structures of toxins from two strains of Fusarium Tricinctum. Tetrahedron. 24(8):3329–36 [DOI] [PubMed] [Google Scholar]
- 10.Bamburg JR, Strong FM. 1969. Mycotoxins of the trichothecane family produced by fusarium tricinctum and trichoderma lignorum. Phytochemistry. 8(12):2405–10 [Google Scholar]
- 11.Banjara N, Nickerson KW, Suhr MJ, Hallen-Adams HE. 2016. Killer toxin from several food-derived Debaryomyces hansenii strains effective against pathogenic Candida yeasts. International Journal of Food Microbiology. 222:23–29 [DOI] [PubMed] [Google Scholar]
- 12.Banjara N, Suhr MJ, Hallen-Adams HE. 2015. Diversity of Yeast and Mold Species from a Variety of Cheese Types. Curr Microbiol. 70(6):792–800 [DOI] [PubMed] [Google Scholar]
- 13.Baumann-Dudenhoeffer AM, D’Souza AW, Tarr PI, Warner BB, Dantas G. 2018. Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat Med. 24(12):1822–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birch AJ, Massy-Westropp RA, Moye CJ. 1955. Studies in relation to biosynthesis. VII. 2-Hydroxy-6-methylbenzoic acid in Penicillium griseofulvum Dierckx. Aust. J. Chem 8(4):539–44 [Google Scholar]
- 15.Bliss JM, Basavegowda KP, Watson WJ, Sheikh AU, Ryan RM. 2008. Vertical and Horizontal Transmission of Candida albicans in Very Low Birth Weight Infants Using DNA Fingerprinting Techniques. The Pediatric Infectious Disease Journal. 27(3):231. [DOI] [PubMed] [Google Scholar]
- 16.Boix-Amorós A, Martinez-Costa C, Querol A, Collado MC, Mira A. 2017. Multiple Approaches Detect the Presence of Fungi in Human Breastmilk Samples from Healthy Mothers. Sci Rep. 7(1):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boix-Amorós A, Puente-Sánchez F, Toit E du, Linderborg KM, Zhang Y, et al. 2019. Mycobiome Profiles in Breast Milk from Healthy Women Depend on Mode of Delivery, Geographic Location, and Interaction with Bacteria. Appl. Environ. Microbiol 85(9): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bräse S, Encinas A, Keck J, Nising CF. 2009. Chemistry and Biology of Mycotoxins and Related Fungal Metabolites. Chem. Rev 109(9):3903–90 [DOI] [PubMed] [Google Scholar]
- 19.Brockman DA, Chen X, Gallaher DD. 2013. Consumption of a high β-glucan barley flour improves glucose control and fatty liver and increases muscle acylcarnitines in the Zucker diabetic fatty rat. Eur J Nutr. 52(7):1743–53 [DOI] [PubMed] [Google Scholar]
- 20.Brown DW, Yu JH, Kelkar HS, Fernandes M, Nesbitt TC, et al. 1996. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc Natl Acad Sci U S A 93(4):1418–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buts J-P, Keyser ND, Marandi S, Hermans D, Sokal EM, et al. 1999. Saccharomyces boulardii upgrades cellular adaptation after proximal enterectomy in rats. Gut. 45(1):89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buts J-P, Keyser ND, Raedemaeker LD. 1994. Saccharomyces boulardii Enhances Rat Intestinal Enzyme Expression by Endoluminal Release of Polyamines. Pediatr Res. 36(4):522–27 [DOI] [PubMed] [Google Scholar]
- 23.Buts J-P, Keyser ND, Stilmant C, Sokal E, Marandi S. 2002. Saccharomyces boulardii Enhances N -Terminal Peptide Hydrolysis in Suckling Rat Small Intestine by Endoluminal Release of a Zinc-Binding Metalloprotease. Pediatr Res. 51(4):528–34 [DOI] [PubMed] [Google Scholar]
- 24.Buts J-P, Stilmant C, Bernasconi P, Neirinck C, Keyser ND. 2008. Characterization of α,α-trehalase released in the intestinal lumen by the probiotic Saccharomyces boulardii. Scandinavian Journal of Gastroenterology. 43(12):1489–96 [DOI] [PubMed] [Google Scholar]
- 25.Cao Y, Zou S, Xu H, Li M, Tong Z, et al. 2016. Hypoglycemic activity of the Baker’s yeast β-glucan in obese/type 2 diabetic mice and the underlying mechanism. Molecular Nutrition & Food Research. 60(12):2678–90 [DOI] [PubMed] [Google Scholar]
- 26.Chagas GM, Campello AP, Klüppel MLW. 1992. Mechanism of citrinin-induced dysfunction of mitochondria. I. Effects on respiration, enzyme activities and membrane potential of renal cortical mitochondria. Journal of Applied Toxicology. 12(2):123–29 [DOI] [PubMed] [Google Scholar]
- 27.Chain E, Florey HW, Jennings MA. 1942. An Antibacterial Substance Produced by Penicillium claviforme. Br J Exp Pathol. 23(4):202–5 [Google Scholar]
- 28.Chang P-K, Cary JW, Yu J, Bhatnagar D, Cleveland TE. 1995. TheAspergillus parasiticus polyketide synthase genepksA, a homolog ofAspergillus nidulans wA, is required for aflatoxin B1 biosynthesis. Molec. Gen. Genet 248(3):270–77 [DOI] [PubMed] [Google Scholar]
- 29.Chu H, Duan Y, Lang S, Jiang L, Wang Y, et al. 2019. The Candida albicans exotoxin Candidalysin promotes alcohol-associated liver disease. Journal of Hepatology [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collado MC, Isolauri E, Laitinen K, Salminen S. Effect of mother’s weight on infant’s microbiota acquisition, composition, and activity during early infancy: a prospective follow-up study initiated in early pregnancy | The American Journal of Clinical Nutrition | Oxford Academic. The American Journal of Clinical Nutrition. 92(5):1023–30 [DOI] [PubMed] [Google Scholar]
- 31.Cundliffe E, Cannon M, Davies J. 1974. Mechanism of Inhibition of Eukaryotic Protein Synthesis by Trichothecene Fungal Toxins. PNAS. 71(1):30–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, et al. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 505(7484):559–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desjardins AE, Hohn TM, McCormick SP. 1993. Trichothecene biosynthesis in Fusarium species: chemistry, genetics, and significance. Microbiol Rev. 57(3):595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dollive S, Chen Y-Y, Grunberg S, Bittinger K, Hoffmann C, et al. 2013. Fungi of the Murine Gut: Episodic Variation and Proliferation during Antibiotic Treatment. PLOS ONE. 8(8):e71806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dounin M 1926. The Fusariosis of Cereal Crops in European Russia in 1923. Phytopathology. 16:305–8 [Google Scholar]
- 36.Ehrlich KC, Li P, Scharfenstein L, Chang P-K. 2010. HypC, the Anthrone Oxidase Involved in Aflatoxin Biosynthesis. Appl. Environ. Microbiol 76(10):3374–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Everard A, Matamoros S, Geurts L, Delzenne NM. 2014. Saccharomyces boulardii Administration Changes Gut Microbiota and Reduces Hepatic Steatosis, Low-Grade Inflammation, and Fat Mass in Obese and Type 2 Diabetic db/db Mice. mBio. 5(3):e01011–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fleming A 1929. On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to their Use in the Isolation of B. influenzæ. Br J Exp Pathol 10(3):226–36 [Google Scholar]
- 39.Freeman GG, Morrison RI. 1949. The isolation and chemical properties of trichothecin, an antifungal substance from Trichothecium roseum Link. Biochem J. 44(1):1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, et al. 2016. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nature Medicine. 22(10):1187–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goncharova NV, Khrapova MV, Pupyshev AB, Korolenko ETs, Nešéáková Z, Korolenko TA. 2016. Hypolipidemic Effect of Mannan in Mice with Acute Lipemia Induced by Poloxamer 407. Bull Exp Biol Med. 162(1):18–22 [DOI] [PubMed] [Google Scholar]
- 42.Goossens J, Pasmans F, Verbrugghe E, Vandenbroucke V, De Baere S, et al. 2012. Porcine intestinal epithelial barrier disruption by the Fusariummycotoxins deoxynivalenol and T-2 toxin promotes transepithelial passage of doxycycline and paromomycin. BMC Veterinary Research. 8(1):245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gouba N, Raoult D, Drancourt M. 2014. Eukaryote Culturomics of the Gut Reveals New Species. PLoS One. 9(9): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greco SH, Torres-Hernandez A, Kalabin A, Whiteman C, Rokosh R, et al. 2016. Mincle Signaling Promotes Con A Hepatitis. The Journal of Immunology. 197(7):2816–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hallen-Adams HE, Kachman SD, Kim J, Legge RM, Martínez I. 2015. Fungi inhabiting the healthy human gastrointestinal tract: a diverse and dynamic community. Fungal Ecology. 15:9–17 [Google Scholar]
- 46.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. 2014. A Diet Low in FODMAPs Reduces Symptoms of Irritable BowelSyndrome. Gastroenterology. 146(1):67–75.e5 [DOI] [PubMed] [Google Scholar]
- 47.Harms H-K, Bertele-Harms R-M, Bruer-Kleis D. 1987. Enzyme-Substitution Therapy with the Yeast Saccharomyces cerevisiae in Congenital Sucrase-Isomaltase Deficiency. New England Journal of Medicine. 316(21):1306–9 [DOI] [PubMed] [Google Scholar]
- 48.He Y, Cox RJ. 2016. The molecular steps of citrinin biosynthesis in fungi. Chem. Sci 7(3):2119–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heisel T, Montassier E, Johnson A, Al-Ghalith G, Lin Y-W, et al. 2017. High-Fat Diet Changes Fungal Microbiomes and Interkingdom Relationships in the Murine Gut. mSphere. 2(5): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heisel T, Nyaribo L, Sadowsky MJ, Gale CA. 2019. Breastmilk and NICU surfaces are potential sources of fungi for infant mycobiomes. Fungal Genetics and Biology. 128:29–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hetherington AC, Raistrick H. 1931. Studies in the Biochemistry of Micro-organisms. Part XIV.—On the production and chemical constitution of a new yellow colouring mater, citrinin, produced from glucose by Penicillium. Philosophical Transactions of the Royal Society of London. Series B, Containing Papers of a Biological Character 220(468–473):269–95 [Google Scholar]
- 52.Hoffmann C, Dollive S, Grunberg S, Chen J, Li H, et al. 2013. Archaea and Fungi of the Human Gut Microbiome: Correlations with Diet and Bacterial Residents. PLOS ONE. 8(6):e66019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffmeister D,P Keller N 2007. Natural products of filamentous fungi: enzymes, genes, and their regulation. Natural Product Reports. 24(2):393–416 [DOI] [PubMed] [Google Scholar]
- 54.Honkanen J, Vuorela A, Muthas D, Orivuori L, Luopajärvi K, et al. 2020. Fungal Dysbiosis and Intestinal Inflammation in Children With Beta-Cell Autoimmunity. Front. Immunol 11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jahn H-U, Ullrich R, Schneider T, Liehr R-M, Schieferdecker HL, et al. 1996. Immunological and Trophical Effects of Saccharomycesboulardii on the Small Intestine in Healthy Human Volunteers. DIG. 57(2):95–104 [DOI] [PubMed] [Google Scholar]
- 56.Johnson WW, Guengerich FP. 1997. Reaction of aflatoxin B1 exo-8,9-epoxide with DNA: Kinetic analysis of covalent binding and DNA-induced hydrolysis. PNAS. 94(12):6121–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C. 2014. Vertical mother–neonate transfer of maternal gut bacteria via breastfeeding. Environmental Microbiology. 16(9):2891–2904 [DOI] [PubMed] [Google Scholar]
- 58.Kankkunen P, Rintahaka J, Aalto A, Leino M, Majuri M-L, et al. 2009. Trichothecene Mycotoxins Activate Inflammatory Response in Human Macrophages. The Journal of Immunology. 182(10):6418–25 [DOI] [PubMed] [Google Scholar]
- 59.Karumuthil-Melethil S, Gudi R, Johnson BM, Perez N, Vasu C. 2014. Fungal β-Glucan, a Dectin-1 Ligand, Promotes Protection from Type 1 Diabetes by Inducing Regulatory Innate Immune Response. The Journal of Immunology. 193(7):3308–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasper L, König A, Koenig P-A, Gresnigt MS, Westman J, et al. 2018. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun. 9(1):1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katt J, Schwinge D, Schoknecht T, Quaas A, Sobottka I, et al. 2013. Increased T helper type 17 response to pathogen stimulation in patients with primary sclerosing cholangitis. Hepatology. 58(3):1084–93 [DOI] [PubMed] [Google Scholar]
- 62.Kim D-H, Kim H, Jeong D, Kang I-B, Chon J-W, et al. 2017. Kefir alleviates obesity and hepatic steatosis in high-fat diet-fed mice by modulation of gut microbiota and mycobiota: targeted and untargeted community analysis with correlation of biomarkers. The Journal of Nutritional Biochemistry. 44:35–43 [DOI] [PubMed] [Google Scholar]
- 63.Krohn S, Zeller K, Böhm S, Chatzinotas A, Harms H, et al. 2018. Molecular quantification and differentiation of Candida species in biological specimens of patients with liver cirrhosis. PLOS ONE. 13(6):e0197319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulaksiz H, Rudolph G, Kloeters-Plachky P, Sauer P, Geiss H, Stiehl A. 2006. Biliary candida infections in primary sclerosing cholangitis. Journal of Hepatology. 45(5):711–16 [DOI] [PubMed] [Google Scholar]
- 65.KUSMIATI Dhewantara FXR. 2016. Cholesterol-Lowering Effect of Beta Glucan Extracted from Saccharomyces cerevisiae in Rats. Scientia Pharmaceutica. 84(1):153–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lan RYZ, Salunga TL, Tsuneyama K, Lian Z-X, Yang G-X, et al. 2009. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. Journal of Autoimmunity. 32(1):43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lancaster MC, Jenkins FP, Philp JM. 1961. Toxicity associated with Certain Samples of Groundnuts. Nature. 192(4807):1095–96 [Google Scholar]
- 68.Lang S, Liu J, Torralba MG, Kuelbs C. Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients With Alcoholic Hepatitis. Hepatology. 0(0): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leonardi I, Li X, Iliev I. 2018. Macrophage interactions with fungi and bacteria in inflammatory bowel disease. Current Opinion in Gastroenterology. 34(6):392–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leonardi I, Li X, Semon A, Li D, Doron I, et al. 2018. CX3CR1+ mononuclear phagocytes control immunity to intestinal fungi. Science. 359(6372):232–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leonardi I, Paramsothy S, Doron I, Semon A, Kaakoush NO, et al. 2020. Fungal Trans-kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host & Microbe. 27(5):823–829.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis JD, Chen EZ, Baldassano RN, Otley AR, Griffiths AM, et al. 2015. Inflammation, Antibiotics, and Diet as Environmental Stressors of the Gut Microbiome in Pediatric Crohn’s Disease. Cell Host & Microbe. 18(4):489–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li M, Zhu L, Xie A, Yuan J. 2015. Oral Administration of Saccharomyces boulardii Ameliorates Carbon Tetrachloride-Induced Liver Fibrosis in Rats via Reducing Intestinal Permeability and Modulating Gut Microbial Composition. Inflammation. 38(1):170–79 [DOI] [PubMed] [Google Scholar]
- 74.Li X, Leonardi I, Semon A, Doron I, Gao IH, et al. 2018. Response to Fungal Dysbiosis by Gut-Resident CX3CR1+ Mononuclear Phagocytes Aggravates Allergic Airway Disease. Cell Host & Microbe. 24(6):847–856.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li XV, Leonardi I, Iliev ID. 2019. Gut Mycobiota in Immunity and Inflammatory Disease. Immunity. 50(6):1365–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang M, Liwen Z, Yun Z, Yanbo D, Jianping C. 2018. The Imbalance between Foxp3. Journal of Immunology Research [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lynen F, Tada M. 1961. Die biochemischen Grundlagen der „Polyacetat-Regel”β. Angewandte Chemie. 73(15):513–19 [Google Scholar]
- 78.Mahfoud R, Maresca M, Garmy N, Fantini J. 2002. The Mycotoxin Patulin Alters the Barrier Function of the Intestinal Epithelium: Mechanism of Action of the Toxin and Protective Effects of Glutathione. Toxicology and Applied Pharmacology. 181(3):209–18 [DOI] [PubMed] [Google Scholar]
- 79.Malik A, Sharma D, Malireddi RKS, Guy CS, Chang T-C, et al. 2018. SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer. Immunity. 49(3):515–530.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mar Rodríguez M, Pérez D, Javier Chaves F, Esteve E, Marin-Garcia P, et al. 2015. Obesity changes the human gut mycobiome. Sci Rep. 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Merwe KJVD, Steyn PS, Fourie L, Scott DB, Theron JJ 1965. Ochratoxin A, a Toxic Metabolite produced by Aspergillus ochraceus Wilh. Nature. 205(4976):1112–13 [DOI] [PubMed] [Google Scholar]
- 82.Morales-Menchén A, Navarro-García F, Guirao-Abad JP, Román E, Prieto D, et al. 2018. Non-canonical Activities of Hog1 Control Sensitivity of Candida albicans to Killer Toxins From Debaryomyces hansenii. Front. Cell. Infect. Microbiol 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morooka N, Uratsuji N, Yoshizawa T, Yamamoto H. 1972. Studies on the Toxic Substances in Barley Infected with Fusarium spp. Food Hygiene and Safety Science (Shokuhin Eiseigaku Zasshi). 13(5):368–75 [Google Scholar]
- 84.Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, et al. 2016. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature. 532(7597):64–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mueller KD, Zhang H, Serrano CR, Billmyre RB, Huh EY, et al. 2019. Gastrointestinal microbiota alteration induced by Mucor circinelloides in a murine model. J Microbiol. 57(6):509–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mukherjee PK, Chandra J, Retuerto M, Sikaroodi M, Brown RE, et al. 2014. Oral Mycobiome Analysis of HIV-Infected Patients: Identification of Pichia as an Antagonist of Opportunistic Fungi. PLOS Pathogens. 10(3):e1003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Narula N, Dhillon A, Zhang D, Sherlock ME, Tondeur M, Zachos M. 2018. Enteral nutritional therapy for induction of remission in Crohn’s disease. Cochrane Database of Systematic Reviews [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nash AK, Auchtung TA, Wong MC, Smith DP, Gesell JR, et al. 2017. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome. 5(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Neyrinck AM, Possemiers S, Verstraete W, De Backer F, Cani PD, Delzenne NM. 2012. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin–glucan fiber improves host metabolic alterations induced by high-fat diet in mice. The Journal of Nutritional Biochemistry. 23(1):51–59 [DOI] [PubMed] [Google Scholar]
- 90.Oo YH, Banz V, Kavanagh D, Liaskou E, Withers DR, et al. 2012. CXCR3-dependent recruitment and CCR6-mediated positioning of Th-17 cells in the inflamed liver. Journal of Hepatology. 57(5):1044–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ostry V, Malir F, Ruprich J. 2013. Producers and Important Dietary Sources of Ochratoxin A and Citrinin. Toxins. 5(9):1574–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Panpetch W, Somboonna N, Palasuk M, Hiengrach P, Finkelman M, et al. 2019. Oral Candida administration in a Clostridium difficile mouse model worsens disease severity but is attenuated by Bifidobacterium. PLoS One. 14(1): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, et al. 2006. Factors Influencing the Composition of the Intestinal Microbiota in Early Infancy. Pediatrics. 118(2):511–21 [DOI] [PubMed] [Google Scholar]
- 94.Pfohl‐Leszkowicz A, Manderville RA. 2007. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Molecular Nutrition & Food Research. 51(1):61–99 [DOI] [PubMed] [Google Scholar]
- 95.Prentice N, Dickson AD. 1968. Emetic material associated with Fusarium species in cereal grains and artificial media. Biotechnology and Bioengineering. 10(4):413–27 [Google Scholar]
- 96.Puel O, Galtier P, Oswald IP. 2010. Biosynthesis and Toxicological Effects of Patulin. Toxins (Basel). 2(4):613–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rabot S, Membrez M, Blanchard H, Berger B. 2016. High fat diet drives obesity regardless the composition of gut microbiota in mice. Scientific Reports. 6:32484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Remenova T, Morand O, Amato D, Chadha-Boreham H, Tsurutani S, Marquardt T. 2015. A double-blind, randomized, placebo-controlled trial studying the effects of Saccharomyces boulardii on the gastrointestinal tolerability, safety, and pharmacokinetics of miglustat. Orphanet Journal of Rare Diseases. 10(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richard ML, Sokol H. 2019. The gut mycobiota: insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat Rev Gastroenterol Hepatol 16(6):331–45 [DOI] [PubMed] [Google Scholar]
- 100.Rogiers O, Frising UC, Kucharíková S, Jabra-Rizk MA, Loo G van, et al. 2019. Candidalysin Crucially Contributes to Nlrp3 Inflammasome Activation by Candida albicans Hyphae. mBio. 10(1): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rustom IYS. 1997. Aflatoxin in food and feed: occurrence, legislation and inactivation by physical methods. Food Chemistry. 59(1):57–67 [Google Scholar]
- 102.Schei K, Avershina E, Øien T, Rudi K, Follestad T, et al. 2017. Early gut mycobiota and mother-offspring transfer. Microbiome. 5(1):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schirmer M, Garner A, Vlamakis H, Xavier RJ. 2019. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 17(8):497–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shao T-Y, Ang WXG, Jiang TT, Huang FS, Andersen H, et al. 2019. Commensal Candida albicans Positively Calibrates Systemic Th17 Immunological Responses. Cell Host & Microbe. 25(3):404–417.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sima P, Vannucci L, Vetvicka V. 2018. β-glucans and cholesterol (Review). International Journal of Molecular Medicine. 41(4):1799–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skalski JH, Limon JJ, Sharma P, Gargus MD, Nguyen C, et al. 2018. Expansion of commensal fungus Wallemia mellicola in the gastrointestinal mycobiota enhances the severity of allergic airway disease in mice. PLOS Pathogens. 14(9):e1007260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Speakman EA, Dambuza IM, Salazar F, Brown GD. 2020. T Cell Antifungal Immunity and the Role of C-Type Lectin Receptors. Trends in Immunology. 41(1):61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stanislawski MA, Dabelea D, Wagner BD, Sontag MK. 2017. Pre-pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome. 5(113): [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suhr MJ, Banjara N, Hallen‐Adams HE. 2016. Sequence-based methods for detecting and evaluating the human gut mycobiome. Letters in Applied Microbiology. 62(3):209–15 [DOI] [PubMed] [Google Scholar]
- 110.Sundstøl Eriksen G, Pettersson H, Lundh T. 2004. Comparative cytotoxicity of deoxynivalenol, nivalenol, their acetylated derivatives and de-epoxy metabolites. Food and Chemical Toxicology. 42(4):619–24 [DOI] [PubMed] [Google Scholar]
- 111.Suzuki C, Ando Y, Machida S. 2001. Interaction of SMKT, a killer toxin produced by Pichia farinosa, with the yeast cell membranes. Yeast. 18(16):1471–78 [DOI] [PubMed] [Google Scholar]
- 112.Suzuki C, Nikkuni S. 1989. Purification and Properties of the Killer Toxin Produced by a Halotolerant Yeast, Pichia farinosa. Agricultural and Biological Chemistry. 53(10):2599–2604 [Google Scholar]
- 113.Swidergall M, Khalaji M, Solis NV, Moyes DL, Drummond RA, et al. 2019. Candidalysin Is Required for Neutrophil Recruitment and Virulence During Systemic Candida albicans Infection. J Infect Dis. 220(9):1477–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tatsuno T, Saito M, Enomoto M, Tsunoda H. 1968. Nivalenol, a Toxic Principle of Fusarium nivale. Chemical & Pharmaceutical Bulletin. 16(12):2519–20 [DOI] [PubMed] [Google Scholar]
- 115.Tay S-T, Lim S-L, Tan H-W. 2014. Growth inhibition of Candida species by Wickerhamomyces anomalus mycocin and a lactone compound of Aureobasidium pullulans. BMC Complementary and Alternative Medicine. 14(1):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Treem WR, Ahsan N, Sullivan B, Rossi T, Holmes R, et al. 1993. Evaluation of liquid yeast-derived sucrase enzyme replacement in patients with sucrase-isomaltase deficiency. Gastroenterology. 105(4):1061–68 [DOI] [PubMed] [Google Scholar]
- 117.van Luijk A 1938. Antagonism of Penicillium spe. versus Pythium debaryanum. Chronica Botanica. 4(3):210–11 [Google Scholar]
- 118.van Tilburg Bernardes E, Pettersen VK, Gutierrez MW, Laforest-Lapointe I, Jendzjowsky NG, et al. 2020. Intestinal fungi are causally implicated in microbiome assembly and immune development in mice. Nature Communications. 11(1):2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vanacloig-Pedros E, Proft M, Pascual-Ahuir A. 2016. Different Toxicity Mechanisms for Citrinin and Ochratoxin A Revealed by Transcriptomic Analysis in Yeast. Toxins. 8(10):273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Verma AH, Richardson JP, Zhou C, Coleman BM, Moyes DL. 2017. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin | Science Immunology. Science Immunology. 2(17):eaam8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Verma AH, Zafar H, Ponde NO, Hepworth OW, Sihra D, et al. 2018. IL-36 and IL-1/IL-17 Drive Immunity to Oral Candidiasis via Parallel Mechanisms. The Journal of Immunology. 201(2):627–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vorobyev A, Gupta Y, Sezin T, Koga H, Bartsch YC, et al. 2019. Gene-diet interactions associated with complex trait variation in an advanced intercross outbred mouse line. Nat Commun. 10(1):1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wampach L, Heintz-Buschart A, Hogan A, Muller EEL, Narayanasamy S, et al. 2017. Colonization and Succession within the Human Gut Microbiome by Archaea, Bacteria, and Microeukaryotes during the First Year of Life. Front. Microbiol 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang T, Fan C, Yao A, Xu X, Zheng G, et al. 2018. The Adaptor Protein CARD9 Protects against Colon Cancer by Restricting Mycobiota-Mediated Expansion of Myeloid-Derived Suppressor Cells. Immunity. 49(3):504–514.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang Y, Wang L, Wu F, Liu F, Wang Q, et al. 2018. A Consensus Ochratoxin A Biosynthetic Pathway: Insights from the Genome Sequence of Aspergillus ochraceus and a Comparative Genomic Analysis. Appl. Environ. Microbiol 84(19):e01009–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wannop CC. 1961. The Histopathology of Turkey “X” Disease in Great Britain. Avian Diseases. 5(4):371–81 [Google Scholar]
- 127.Wells CA, Salvage-Jones JA, Li X, Butcher S, Murray RZ. The Macrophage-Inducible C-Type Lectin, Mincle, Is an Essential Component of the Innate Immune Response to Candida albicans. The Journal of Immunology. 180(11):7404–13 [DOI] [PubMed] [Google Scholar]
- 128.Wheeler ML, Limon JJ, Underhill DM. 2017. Immunity to Commensal Fungi: Detente and Disease. Annual Review of Pathology: Mechanisms of Disease. 12(1):359–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wiesner BP. 1942. Bactericidal Effects of Aspergillus clavatus. Nature. 149(3778):356–57 [Google Scholar]
- 130.Wilkins WH, Harris GCM. 1942. Investigation into the Production of Bacteriostatic Substances by Fungi. I. Preliminary Examination of 100 Fungal Species. Br J Exp Pathol. 23(4):166–69 [Google Scholar]
- 131.Woodward RB, Singh Gurbakhsh. 1949. THE STRUCTURE OF PATULIN. J. Am. Chem. Soc 71(2):758–59 [DOI] [PubMed] [Google Scholar]
- 132.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, et al. 2011. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science. 334(6052):105–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Xu J, Li Y, Yang Z, Li C, Liang H, et al. 2018. Yeast Probiotics Shape the Gut Microbiome and Improve the Health of Early-Weaned Piglets. Front. Microbiol 9: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang A-M, Inamine T, Hochrath K, Chen P, Wang L, et al. 2017. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 127(7):2829–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yoshizawa T, Morooka N. 1973. Deoxynivalenol and Its Monoacetate: New Mycotoxins from Fusarium roseum and Moldy Barley. Agricultural and Biological Chemistry. 37(12):2933–34 [Google Scholar]
- 136.Yu L, Zhao X, Cheng M, Yang G, Wang B, et al. 2017. Saccharomyces boulardii Administration Changes Gut Microbiota and Attenuates D-Galactosamine-Induced Liver Injury. Sci Rep. 7(1):1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhai Q, Gong X, Wang C, Zhao J, Zhang H, et al. 2019. Food-borne patulin toxicity is related to gut barrier disruption and can be prevented by docosahexaenoic acid and probiotic supplementation. Food Funct. 10(3):1330–39 [DOI] [PubMed] [Google Scholar]
- 138.Zhang B, Shen XL, Liang R, Li Y, Huang K, et al. 2014. Protective role of the mitochondrial Lon protease 1 in ochratoxin A-induced cytotoxicity in HEK293 cells. Journal of Proteomics. 101:154–68 [DOI] [PubMed] [Google Scholar]
- 139.Zuo T, Wong SH, Cheung CP, Lam K, Lui R, et al. 2018. Gut fungal dysbiosis correlates with reduced efficacy of fecal microbiota transplantation in Clostridium difficile infection. Nat Commun. 9(1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]


