Abstract
Purpose
To evaluate the risk/risk factors for exudative retinal detachment (ERD) in ocular inflammatory diseases.
Design
Retrospective cohort study.
Methods
Patients with non-infectious ocular inflammation had been followed longitudinally between 1978–2007 at four US subspecialty uveitis centers. The main outcome measures were ERD occurrence and its predictive factors.
Results
One hundred seventy-six of 14,612 eyes with ocular inflammation presented with ERD. Among uveitis cases, Vogt-Koyanagi-Harada syndrome (VKH) (OR=109), undifferentiated choroiditis (OR=9.18), sympathetic ophthalmia (OR=8.43), primary or secondary panuveitis (OR=7.09), multifocal choroiditis with panuveitis (OR=4.51), and “other” forms of posterior uveitis (OR=16.9) were associated with a higher prevalence of ERD. Among the 9,209 uveitic or scleritic eyes initially free of ERD and followed-up, 137 incident ERD cases were observed over 28,949 eye-years at risk (incidence rate=0.47% (0.40%–0.56%)/eye-year). VKH (HR=13.2), sympathetic ophthalmia (HR=5.82), undifferentiated choroiditis (HR=6.03), primary or secondary panuveitis (HR=4.21), and rheumatoid arthritis (HR=3.30) were significantly associated with incident ERD. A significant dose-response relationship with ERD prevalence and incidence was observed for anterior chamber cells and vitreous cell activity. African Americans had significantly higher prevalence and incidence of ERD.
Conclusions
Other ocular inflammatory conditions besides VKH and posterior scleritis were associated with increased ERD risk, indicating that ERD does not necessarily dictate a diagnosis of VKH or posterior scleritis. In addition, the relationship of ERD with inflammatory severity factors implies that inflammation is a key predictive factor that is associated with developing ERD and requires early and vigorous control.
Introduction
Uveitis is a major cause of visual impairment worldwide and can cause devastating visual consequences. It has been estimated that in the United States, 30,000 new cases of legal blindness occur annually as a result of uveitis, 10% of new cases of blindness in the United States.1 Others have estimated that uveitis - including infectious causes – accounts for 25% of blindness worldwide.1,2 While these estimates may now be high in an era with more effective treatments for uveitis, it is clear that uveitis is an important cause of visual loss globally. Furthermore, it may cause a disproportionately large decrease in workforce productivity as it often affects working age adult and younger individuals,leading to an economic impact exceeding the proportion of blindness caused.3Because structural complications of uveitis can give rise to ocular injury and visual loss, characterization of the risk of and risk factors for the various structural complications would be valuable to identify high-risk individuals for clinical interventions.
Exudative retinal detachment is potential complication of ocular inflammation. It is a classic feature of Vogt-Koyanagi-Harada (VKH) syndrome, and also has been known to occur in posterior scleritis.1 The frequency with which it occurs in other conditions is unclear. Here, we report the results of an evaluation ofthe risk of and risk factors for exudative retinal detachment in a large cohort of patients with ocular inflammatory diseases, including the association with various forms of ocular inflammatory diagnoses.
Methods
Data Collection
The Systemic Immunosuppressive Therapy for Eye Disease (SITE) Cohort Study is a large retrospective cohort study of patients with non-infectious ocular inflammation managed at five tertiary care academic ocular inflammation centers in the United States.4 One of these centers was omitted from this analysis due to a consultative approach to patient management in which many follow-up visits were conducted at the referring center. The remaining centers primarily followed patients longitudinally. The patient data included in this analysiswere derived from patient visits, which occurred between 1978–2007 inclusive. Institutional Review Board (IRB) approval was obtained and maintained at all centers throughout the study under the governing IRBsat the University of Pennsylvania, the Massachusetts Eye and Ear Infirmary, the Johns Hopkins University School of Medicine, the Oregon Health and Sciences University and the National Eye Institute. IRB approval permitted data entry by medical record review and linkage to the National Death Index; permission was granted for waiver of informed consent based on minimal risk with no patient contact. In order to avoid limiting the risk set according to presuppositions, all inflammatory conditions studied in the SITE Cohort Study were included in an initial look at the risk of exudative retinal detachment. These conditions included anterior, intermediate, posterior, and panuveitis (sites of uveitis defined according to the International Uveitis Study Group approach4), scleritis, mucous membrane pemphigoid (MMP) with ocular involvement, inflammatory orbital disease, peripheral ulcerative keratitis, autoimmune cicatricial keratitis and others. “Secondary” eye inflammation referred to inflammation in the presence of a systemic disease diagnosis, whereas “primary” eye inflammation was localized to the eye without a known associated systemic disease. Subsequently, categories with trivial numbers of exudative retinal detachments were removed from further analysis. Patients with infectious ocular inflammatory diseases and human immunodeficiency virus infection had beenexcluded from the parent study. In addition, ERD associated with choroidal neovascularization (CNV) was excluded. All clinicians evaluating these patients had undergone an internal medicine residency, rheumatology fellowship and/or an ocular immunology and inflammation fellowship training programand emphasizedthat a primary goal in the workup of ocular inflammatory diseases cases was the identification of systemic diseases that might be associated with ocular inflammation.
The methodology for retrospective data collection and quality assurance has been described previously in detail.5,6 Briefly, extensive demographic and clinical information was collected for all patients seen at all participating clinical sites and followed longitudinally over time. Systemic inflammatory diseases were identified among these patients as well as certain markers for diseases (i.e. HLA-B27 and HLA-A29, which were tested when indicated per clinical judgment). A database was developedwith strict quality control measures with data entered into a standardized form.5Trained and certified chart reviewers entered the data regarding each eye of every patient at every visit manually into a custom Microsoft Access (Redmond, Washington, USA) database. Data collected included visual acuity, intraocular pressure, slit lamp and ophthalmoscopic clinical findings including the presence or absence of complications described in Table 1, among others. Quality assurance techniques and cross checks were utilized. Exudative retinal detachment was ascertained primarily ophthalmoscopically supplemented by diagnostic tests such as ultrasound, OCT, and/or FA when ophthalmoscopic diagnosis was uncertain. Anterior chamber cells and vitreous haze were graded in the standard numerical fashion approximating the methods specified by the Standardization of Uveitis Nomenclature Groupas closely as possible in a retrospective study.7 Measures of current ocular inflammation (AC cells, vitreous cells, vitreous haze, inflammation activity) were assessed in a time-updated manner for the incidence analysis in order to better capture the relationship between current inflammation and ERD risk. Overall activity of inflammation was graded as: active, slightly active. inactive (no signs of inflammation); or missing (unable to tell from the medical record). “Active” was defined as “When there are recorded statements of observing clear signs of inflammation at that particular visit in the ocular tissues. Examples would be statements that the inflammation is “active”, that anterior chamber or vitreous cells were observed (1+ or higher), the vitreous haze 1+ or higher was observed, etc.” “Slightly active” was defined as “activity that is minimally present, described also by terms such as mild, few, trace etc.” “Inactive” was defined as having no signs of inflammation and/or explicitly stated as inactive.
Table 1:
Prevalence of Exudative Retinal Detachment in the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study.
| Characteristic (at time of presentation) | Value | ERD Cases (%) | *Adjusted OR of ERD (95% CI) |
|---|---|---|---|
| Age | ≥ 35 | 108 (61.36) | 1.00 |
| < 35 | 68 (38.64) | 1.06 (0.72 – 1.58) | |
| Gender | Male | 78 (44.32) | 1.00 |
| Female | 98 (55.68) | 0.72 (0.50 – 1.04) | |
| Race | White | 81 (46.02) | 1.00 |
| African American | 50 (28.41) | 2.83 (1.80 – 4.44) | |
| Hispanic | 32 (18.18) | 4.90 (2.88 – 8.33) | |
| Type of inflammation, compared with anterior uveitis*,1 | VKH | 69 (39.20) | 109.4 (55.67 – 218.3) |
| Retinochoroiditis | 4 (2.27) | 9.18 (1.52 – 29.65) | |
| Sympathetic ophthalmia | 2 (1.14) | 8.43 (0.60 – 32.64) | |
| Primary or secondary panuveitis | 26 (14.77) | 7.09 (3.16 – 14.65) | |
| Multifocal choroid panuveitis | 5 (2.84) | 4.51 (1.36 – 16.52) | |
| Other posterior uveitis | 15 (8.52) | 16.91 (5.63 – 35.73) | |
| Posterior scleritis | 7 (3.98) | 41.63 (10.04 – 104.7) | |
| Necrotizing scleritis | 3 (1.70) | 8.11 (0.96 – 27.35) | |
| AC cells | No cell | 91 (51.70) | 1.00 |
| 0.5+ | 30 (17.05) | 1.54 (0.98 – 2.41) | |
| 1.0+ | 14 (7.95) | 1.07 (0.57 – 2.01) | |
| 2.0+ | 25 (14.20) | 2.31 (1.33 – 4.02) | |
| 3.0+ and greater | 16 (9.09) | 2.64 (1.30 – 5.38) | |
| Vitreous cells* | No cell | 77 (46.39) | 1.00 |
| 1.0+ | 36 (21.69) | 1.65 (0.99 – 2.77) | |
| 2.0+ | 25 (15.06) | 1.43 (0.80 – 2.55) | |
| 3.0+ and greater | 12 (7.23) | 2.04 (0.89 – 4.69) | |
| Vitreous haze* | None | 117 (74.05) | 1.00 |
| 1.0+ | 29 (18.35) | 1.57 (0.95 – 2.60) | |
| 2.0+ | 8 (5.06) | 1.14 (0.48 – 2.71) | |
| 3.0+ | 4 (2.53) | 1.18 (0.34 – 4.12) | |
| Inflammation activity | Inactive | 18 (10.34) | 1.00 |
| Slightly active | 22 (12.64) | 2.93 (1.40 – 6.15) | |
| Active | 134 (77.01) | 5.06 (2.85 – 9.00) | |
| Band keratopathy | No | 164 (93.18) | 1.00 |
| Yes | 12 (6.82) | 3.14 (1.59 – 6.22) |
Adjusted analysis adjusts for: type of inflammation, bilateral inflammation, smoking status, age category, and gender. 95% CI produced using Profile. Exudative retinal detachment (ERD); Odds ratio (OR), Confidence interval (CI), Vogt-Koyanagi-Harada (VKH); Profile log likelihood ratio confidence interval computed using only one eye per patient. “Other posterior uveitis” includes acute posterior multifocal placoid pigment epitheliopathy (APMPEE), multiple evanescent white dot syndrome (MEWDS), serpiginous choroiditis, and neuroretinitis. Other forms of uveitis and scleritis not associated with significantly higher risk than anterior uveitis are not listed as potential risk factors for but were included in the analysis.
The rest of the cases were kinds of inflammation unassociated with ERD. These are: anterior uveitis 21 (11.93%), intermediate uveitis 11 (6.25%), retinal vasculitis 4 (2.27%), anterior scleritis 8 (4.55%), and other 1 (0.57%).
Other conditions included in this study as potentially predictive factors for ERD can be divided into specific diseases and classes of disease. Specific diseases include eye diseases (e.g., posterior scleritis, multifocal choroid with panuveitis, sympathetic ophthalmia) and systemic diseases (e.g., Behçet Disease, Vogt-Koyanagi-Harada disease,polyarteritis nodosum, systemic lupus erythematosus, scleroderma, Sjögren’ssyndrome, sarcoidosis, Crohn’s disease, ulcerative colitis, dermatomyositis, polymyositis, juvenile idiopathic arthritis, and relapsing polychondritis). Conditions that do not match established specific disease diagnoses were categorized into classes of diseases using descriptive names such as undifferentiated choroiditis, panuveitis, and necrotizing scleritis.
The project was conducted in accordance with the principles of the Declaration ofHelsinki, with the approval of the governing Institutional Review Boards (IRBs) of eachinstitution, each of which has recognized the protocol as a minimal risk retrospective study with no patient contact, and approved waiver of informed consent, allowing all living anddeceased patients to be included.
Statistical Methodology and Outcome Measures
Simple and multivariate logistic regression models were utilized to determine risk factor associationsfor exudative retinal detachment at presentation by calculating crude and adjusted odds ratios. Generalized estimating equations (GEE)-derived methods were utilized to account for inter-eye correlation among the same patient. The prevalence of exudative retinal detachment at baseline was evaluated as the proportion within each at risk group using a per eye perspective.
The incidence of new exudative retinal detachments wasevaluated as the number of events per eye-year of time at risk of the event among those free of the event at the initial visit. Potential risk factors were evaluated using hazard ratios (HRs) and adjusted HRs with 95% confidence intervals [CI]) which were calculated using univariate and multivariateCox proportional hazard models. Smokingwas hypothesized to be a risk factor and therefore was included as an adjustment covariate because it has been reported to be associated with an increased likelihood of bilateral ocular inflammation.8 The outcome of cases of exudative retinal detachment versus other cases of ocular inflammation from the same diagnostic groups was compared using summary statistics and comparison of incidence rates. The 95% confidence intervals for the hazard ratios and odds ratios are listed in a subscript form in the results section. As recommended for observational epidemiological studies,9 p values and confidence intervals reported are nominal (not adjusted for multiple comparisons). SAS version 9.3 (SAS Corporation, Cary, NC) was utilized for all statistical analysis. The confidence intervals on the odds or hazard ratios were notated as a subscript in order to simplify the presentation of the many numbers reported. For example, odds ratio=2.83, 95% confidence interval: 1.80–4.44 would be notated as (OR = 1.802.834.44).
Results
Prevalence of Exudative Retinal Detachment (ERD)
Among a total of 14,612 eyes in the SITE Cohort seen for an initial visit, 176 eyes were identified as having an exudative retinal detachment,yielding acrude prevalence of 1.2%. ERD cases were rare among mucous membrane pemphigoid cases (0/898) and other forms of inflammation (1/323)compared to uveitis and scleritis, so these subpopulations of eyes of patients with ocular inflammation were not studied further.
The majority of patients with ERD in one or both eyes (61.4%) were older than 35 years of age and were female (see Table 1). Racial distributions were as follows: White (46.0%), African American (28.4%), and Hispanic (18.2%) among others.
Factors associated withERD at presentation included African American and Hispanic race/ethnicity (odds ratio (OR) = 1.802.834.44, p<0.0001 and OR = 2.884.908.33, p<0.0001respectively, each with respect to white participants). Age and sex were not associated with prevalent ERD (OR = 0.721.061.58, p=0.76 and OR = 0.500.721.04,p=0.082 respectively).
At the initial visit, among 265 Vogt-Koyanagi-Harada syndrome (VKH) cases, 69 (26%) had ERD (see Table 1) and 196 did not. Similarly, 2/61 (3%) of sympathetic ophthalmia cases, 26/1203 (2%) of primary or secondary panuveitis cases, 4/138 (3%) of retinochoroiditis cases, 5/356 (1%) of multifocal choroid panuveitis cases, 0/339 (0%) of birdshot retinochoroidopathy cases, 4/638 (1%) of retinal vasculitis cases, 8/1488 (0.5%) of anterior scleritis cases, 3/106 (3%) of necrotizing scleritis cases, 7/54 (13%) of posterior scleritis cases, and 15/263 (6%) of other posterior uveitis cases presented at the initial visit with ERD. The distribution of inflammatory conditions associated with ERD at initial presentation is given in Figure 1a.
Figures 1a and b:
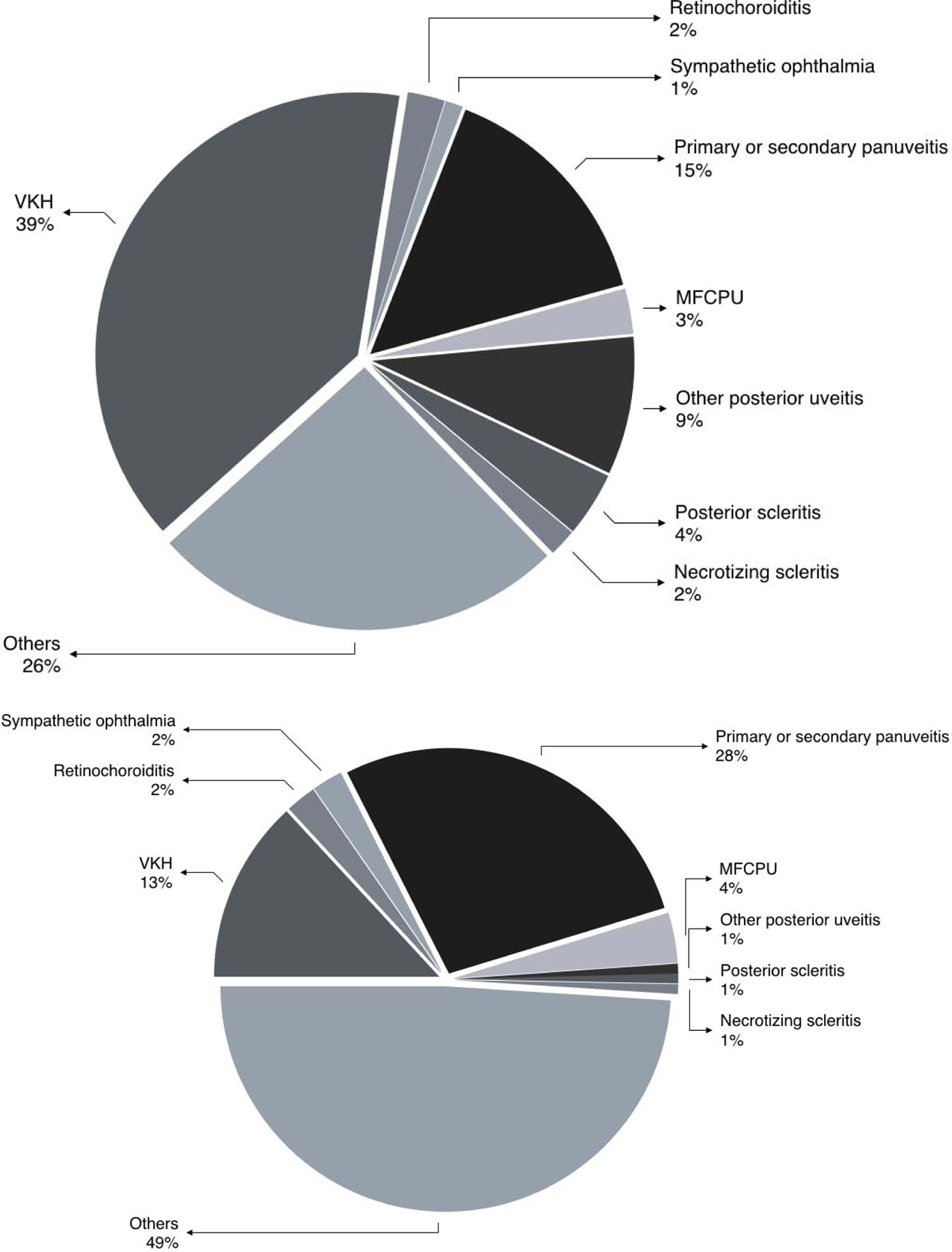
Pie chart indicating the percentage of prevalent (present at initial visit; Figure 1a) and incident (Figure 1b) exudative retinal detachments associated with various inflammatory disease diagnoses.
Among uveitis cases, VKH (OR=55.67109.4218.3),undifferentiated choroiditis(OR=1.529.1829.65), sympathetic ophthalmia (OR=0.608.4332.64),primary or secondary panuveitis (OR=3.167.0914.65), multifocal choroiditis with panuveitis (OR=1.364.5116.52), and “other” forms of posterior uveitis (OR=5.6316.9135.73) were associated with a higher prevalence of ERD (see Table 1). Posterior scleritis (OR 10.0441.63104.7), and necrotizing scleritis(OR 0.968.1127.35) also were associated with a higher prevalence of ERD than anterior scleritis cases.
No significant associations were found between prevalent ERD and spondyloarthropathies (including HLA-B27+ status), Behçet Disease, polyarteritis nodosum, granulomatosis with polyangiitis (previouslyknown as Wegener’s granulomatosis),other forms of arteritis, systemic lupus erythematosus, Sjögren’ssyndrome, scleroderma, juvenile idiopathic arthritis, rheumatoid arthritis, polymyositis, and dermatomyosititis. Additionally, no significant associations were found between ERD and other systemic conditions including Crohn’s disease, ulcerative colitis, hypertension, diabetes, and smoking.
Regarding clinical signs of inflammation, a dose-response relationship with ERD prevalence was observed for anterior chamber cells and vitreous cell activity. Slightly active (OR 1.402.936.15) and active inflammation (OR2.855.069.00)alsowere associated with a higher prevalenceof ERD. A similar pattern was seen when examining vitreous cells; higher levels of vitreous cells also tended to have similarly higher odds of ERD, but with the available power there was no statistically significant association. The presence of band keratopathy also was associated with higher odds of presentation with ERD (OR 1.593.146.22). On the other hand, vitreous hazewasnot associated with prevalent ERD. Neither were clinical complications of ocular inflammation—including macular edema, epiretinal membrane, iris synechiae, keratic precipitates and pre-retinal neovascularization (data not shown)—associated with increased prevalence.
Incidence and Risk Factors for Developing Exudative Retinal Detachment (ERD)
Among the 9,209 uveitic or scleriticeyeswith follow-up that initially free of ERD, 137 incident ERD cases were observed over 28,949eye-years at risk (incidence rate=0.0040.00470.0056%/eye-year) (Figure 2). AfricanAmerican racewas associated significantly with higher incidence of ERD (hazard ratio (HR)=1.051.622.48). However, age less than 35 years old (HR=0.991.502.28) and sex (female vs male; HR=0.711.091.66,respectively) were not associated with an altered incidence of ERD.
Figure 2:
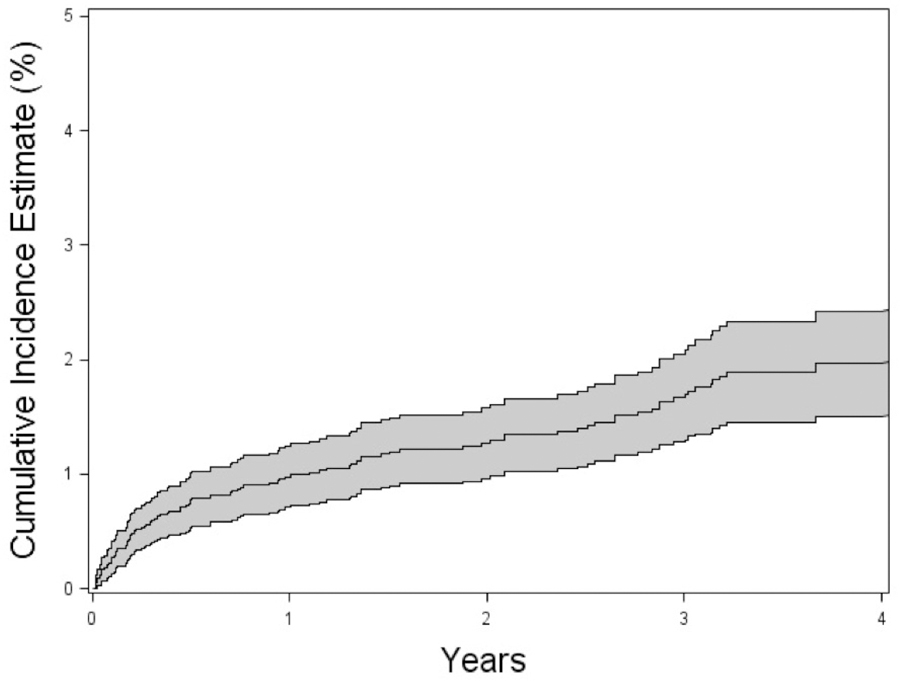
Cumulative incidence of exudative retinal detachment (ERD)in patients with non-infectious uveitis and/or scleritis as a percentage over time; 95% confidence intervals are indicated in gray. 0.3 logMAR is equivalent to doubling of the visual angle.
The distribution of inflammatory conditions associated with incident ERD is given in Figure 1b. Among the ocular inflammatory diagnoses, onlyVKH (HR=5.8713.1529.47, p<0.0001), sympathetic ophthalmia(HR=1.735.8219.54, p=0.0012),undifferentiated choroiditis(HR=1.876.0319.46, p=0.0042),and primary or secondary panuveitis(HR=2.394.217.40, p<0.0001) were significantly associated with incident ERD. Other ocular diagnoses and clinical features, including scleritis, multifocal choroiditis, birdshot retinochoroiditis, retinal vasculitis, and epiretinal membrane, choroidal neovascularization, retinal vascular sheathing, and retinal vascular occlusion were not significantly associated with incident ERD.
Among all the systemic inflammatory diagnoses examined, only rheumatoid arthritis (HR=1.433.307.63, p=0.0042) was associated with ahigher incidence of ERD, after adjusting for other factors, and this association was not replicated in the prevalence analysis; further assessment of this issue is needed before determining this is a robust association. The spondyloarthropathies (including HLA-B27+ status), Behçet Disease, polyarteritis nodosum, granulomatosis with polyangiitis, other forms of arteritis, systemic lupus erythematosus, scleroderma, Sjögren’ssyndrome, sarcoidosis, Crohn’sdisease, ulcerative colitis, dermatomyositis, polymyositis, juvenile idiopathic arthritis, and relapsing polychondritis were not significantly associated with the incident ERD. Neither were hypertension, diabetes and smoking significantly associated.
The incidence analysis demonstrated an even stronger relationship between (time-updated) inflammatory activity and ERD risk than the prevalent ERD analysis, with a clear dose-response relationship, with increasing ERD incidence observed with both increasingtime-updated anterior chamber and vitreous cell grade (Table 2). Slightly active (HR1.863.105.18, p<0.001) and active (HR1.732.834.64, p<0.0001) inflammation again both were associated with ERD when examining incidence. In contrast with the prevalent analysis results, vitreous haze (any level) (HR 1.672.754.53, p<0.0001) and macular edema (HR 1.181.973.29, p=0.0007) alsowere associatedwith a higher incidence of ERD.
Table 2:
Incidence and Risk Factors for Exudative Retinal Detachment (ERD) in the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. All values are those present at cohort entry, except AC cells, vitreous cells, vitreous haze, and inflammation activity are time-updated to better assess the relationship between current inflammation and exudative retinal detachment risk.
| Characteristic | Value | ERD Cases | Incidence rate per Eye-Year (95% CI) |
*Adjusted HR of ERD (95% CI) |
|---|---|---|---|---|
| Age | ≥ 35 | 72 | 0.0038 (0.0030 – 0.0048) | 1.00 |
| < 35 | 65 | 0.0065 (0.0050 – 0.0083) | 1.50 (0.99 – 2.28) | |
| Gender | Male | 46 | 0.0045 (0.0033 – 0.0061) | 1.00 |
| Female | 91 | 0.0048 (0.0039 – 0.0059) | 1.08 (0.71 – 1.65) | |
| Race | White | 84 | 0.0039 (0.0031 – 0.0049) | 1.00 |
| African American | 43 | 0.0086 (0.0062 – 0.0116) | 1.62 (1.05 – 2.48) | |
| Hispanic | 6 | 0.0060 (0.0022 – 0.0131) | 0.99 (0.37 – 2.64) | |
| Type of inflammation* | VKH | 18 | 0.0460 (0.0272 – 0.0726) | 13.15 (5.87 – 29.47) |
| Sympathetic ophthalmia | 3 | 0.0172 (0.0036 – 0.0504) | 5.82 (1.73 – 19.54) | |
| Retinochoroiditis | 3 | 0.0165 (0.0034 – 0.0483) | 6.03 (1.87 – 19.46) | |
| Primary or secondary | 38 | 0.0132 (0.0093 – 0.0181) | 4.21 (2.39 – 7.40) | |
| panuveitis | ||||
| Multifocal choroid panuveitis | 5 | 0.0057 (0.0019 – 0.0133) | 2.25 (0.76 – 6.68) | |
| Other posterior uveitis | 1 | 0.0028 (0.0001 – 0.0158) | 1.05 (0.14 – 7.97) | |
| Posterior scleritis | 1 | 0.0093 (0.0002 – 0.0519) | 3.03 (0.56 – 29.05) | |
| Necrotizing scleritis | 1 | 0.0043 (0.0001 – 0.0241) | 1.47 (0.20 – 10.96) | |
| AC cells | No cell | 67 | 0.0029 (0.0022 – 0.0037) | 1.00 |
| 0.5+ | 30 | 0.0099 (0.0067 – 0.0141) | 2.54 (1.56 – 4.13) | |
| 1.0+ | 22 | 0.0135 (0.0084 – 0.0204) | 3.46 (1.97 – 6.06) | |
| 2.0+ | 13 | 0.0165 (0.0088 – 0.0282) | 3.61 (1.72 – 7.59) | |
| 3.0+ | 3 | 0.0116 (0.0024 – 0.0339) | 2.63(0.76 – 9.17) | |
| 4.0+ | 2 | 0.0694 (0.0084 – 0.2506) | 13.59(3.22 – 57.28) | |
| Vitreous cells* | No cell | 63 | 0.0028 (0.0022 – 0.0036) | 1.00 |
| 0.5+ | 26 | 0.0117 (0.0076 – 0.0171) | 2.53 (1.45 – 4.39) | |
| 1.0+ | 17 | 0.0085 (0.0049 – 0.0136) | 1.86 (1.00 – 3.47) | |
| 2.0+ | 15 | 0.0145 (0.0081 – 0.0239) | 2.70 (1.35 – 5.39) | |
| 3.0+ | 6 | 0.0273 (0.0100 – 0.0594) | 4.77(1.83 – 12.43) | |
| 4.0+ | 2 | 0.0646 (0.0078 – 0.2333) | 13.73(3.98 – 47.35) | |
| Vitreous haze* | No | 89 | 0.0035 (0.0028 – 0.0043) | 1.00 |
| Yes | 36 | 0.0159 (0.0111 – 0.0220) | 2.75 (1.67 – 4.53) | |
| Inflammation activity | Inactive | 57 | 0.0026 (0.0019 – 0.0033) | 1.00 |
| Slightly active | 30 | 0.0116 (0.0078 – 0.0165) | 3.10 (1.86 – 5.18) | |
| Active | 50 | 0.0123 (0.0091 – 0.0162) | 2.83 (1.73 – 4.64) | |
| Rheumatoid arthritis | Yes | 10 | 0.0088 (0.0042 – 0.0161) | 3.30 (1.43 – 7.63) |
| Macular edema | Yes | 27 | 0.0143 (0.0094 – 0.0208) | 1.97(1.18 – 3.29) |
Adjusted analysis adjusts for: type of inflammation, bilateral inflammation, smoking status, age category, and gender. 95% CI produced using Profile Likelihood CI method. Exudative retinal detachment (ERD); Hazard ratio (HR), Confidence interval (CI), Anterior chamber (AC). “Other posterior uveitis” includes acute posterior multifocal placoid pigment epitheliopathy (APMPEE), multiple evanescent white dot syndrome (MEWDS), serpiginous choroiditis, and neuroretinitis.
Impact of Exudative Retinal Detachment on Visual Acuity
From the visit preceding to the first visit with ERD, mean visual acuity dropped 2.8 ETDRS-equivalent lines, then improved 1.9 lines at the first visit following resolution (Figure 3). These changes were noted to be statistically significant (p<0.0001).
Figure 3:
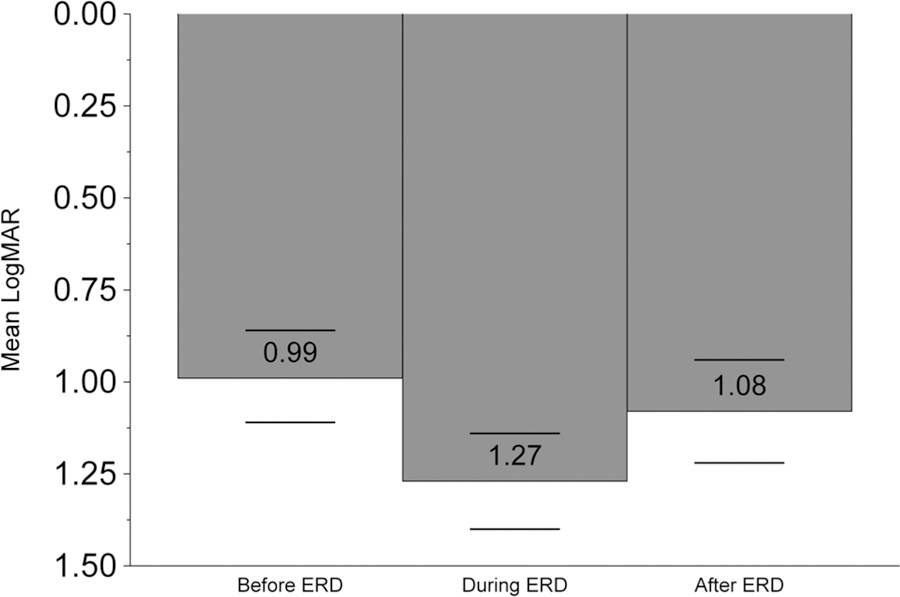
Mean logMAR visual acuity in cases of non-infectious uveitis and/or scleritis are given at the visit preceding incident exudative retinal detachment, at the time exudative retinal detachment was diagnosed, and at the visit following exudative retinal detachment diagnosis.
Discussion
Our results indicate that ERD is a rare finding in patients with ocular inflammatory disease, which occurs almost exclusively in the setting of posterior uveitis, panuveitis and/or scleritis. As expected, presentation with ERD is most strongly associated with VKH and posterior scleritis and is strongly associated with active inflammation—generally following a dose response relationship with increasing inflammation. However, several other forms of uveitis and scleritis also are associated with ERD to a lesser degree, and other demographic and clinical characteristics are associated with this finding. Thus, an important number of ERD cases are unrelated to VKH or posterior scleritis, especially ERD cases following initial presentation. The results also confirm the importance of controlling inflammation to avoid or resolve ERD.
Exudative retinal detachments tend to be less common and often have a better prognosis compared to other types of retinal detachment.10ERD is caused by fluid accumulation underneath the sensory retina leading to detachment, providing a straightforward explanation as to why ERD is associated with inflammation in or around this region of the eye. Retinal detachments caused by traction or a retinal breaktend to have a poor prognosis if the macula is involved, which is less true for ERD involving the macula. In contrast to exudative retinal detachments, treatment of rhegmatogenous or traction retinal detachment typically is surgical rather than medical. Medical treatment of ERD is possible due to the underlying pathophysiology of exudative retinal detachments which occurs when either retinal blood vessels or retinal pigment epithelium have suffered damage allowing fluid to accumulate underneath the retina. This is generally due to inflammatory or occasionally neoplastic diseases.10
As also previously reported by Rao,11Vogt-Koyanagi-Harada syndrome (VKH) had a strongassociation with ERD when examining prevalence; to a lesser extent there is an association with incident ERD after initial presentation as well. VKH is a multi-system disease, which is thought to result from an immune trigger and genetic predisposition leading to an attack on pigment resulting in diffuse choroiditis—typically with accompanying intraocular inflammation. The severe effect on the posterior segment leads to nonrhegmatogenous exudative/serous retinal detachment, vitreous inflammation, and RPE changes, including focal leakage at the RPE. The localized exudative retinal detachmentresults from subretinal fluid accumulation and can be the presenting sign in the acute phase in a large percentage of patients, as confirmed in our study.12,13Histopathological analysis shows lymphocytes underneath the RPE, epitheliod histiocytes and multinucleated giant cells.14It is important to differentiate true serous retinal detachments from intraretinal fluid accumulation or neurosensory retinal detachment, which all are well demonstrated by OCT, becausethis distinction has important treatment implications. Anti-inflammatory treatmentslead to resolution of intraretinal fluid earlier than subretinal fluid.15,16Given the presence of an exudative phase soon after onset of the disease, most VKH cases are diagnosed at presentation as a result of the presence of ERD, and the accompanyingsigns and visual symptoms. The pattern of having an early exudative phase followed by a chronic phase usually without ERDpresumably explains the much higher risk of prevalence compared to incidenceof ERD in VKH patients that was observed in our results.
Sympathetic ophthalmia, a condition sharing many attributes with VKH, also was associated with lesser degree of excess risk of ERD. Sympathetic ophthalmia isan immune mediateddisease.17It results from a diffuse granulomatous non-necrotizing inflammatory response that involves the entire uveal tract, which leads to thickening of the posterior choroid. This choroidal, as well as retinal, inflammation presumably leads to ERD in a minority of cases,18,19,20perhaps less often than in VKH because the onset may be less abrupt.
Inflammation in posterior scleritis can extend anteriorly and has been reported to cause several chorioretinal sequelae including cilioretinal artery occlusion, retinochorodial infarction, and serous retinal detachment.21,22 Leakage in the retinal pigment epithelium can lead to subretinal fluid and resulting ERD; breakdown of the blood-retinal barrier also may contribute.23 Presumably a more exuberant inflammatory response in necrotizing scleritisexplainsthe increased prevalence of exudative retinal detachment in necrotizing scleritis cases.
A dose response relationship between most clinical markers of inflammatory activity and risk of ERD was observed, confirming the expectation that ERD is associated with active inflammation and suggesting that it is likely to improve with control of inflammation (which validates clinical impressions). This pattern also was noted in a previous study in which the authors concluded that shorter duration of systemic corticosteroids and/or immunosuppressive drug therapy at first and second recurrence of VKH was associated with more clinical features of ERD.24
We also found that the site of inflammation is important in predicting the riskof presenting ERD with inflammation around the subretinal area being associated with a higher risk. For example, posterior scleritis, posterior uveitis, and panuveitis, which involves the posterior uvea, had a high association with ERD. Necrotizing scleritis was also highly associated. Although smoking has been reported to be associated with an increased likelihood of bilateral ocular inflammation, we found no association between smoking status and the prevalence or incidence of ERD.8Finally, certain systemic diseases, such as RA (associated with scleritis), and markers of severe intraocular inflammation, such as band keratopathy, also were associated with increased risk of ERD in some analyses, which other studies have demonstrated as well.25The association between RA and ERD may reflect a higher risk of severe scleritis in RA representing some residual confounding upon adjustment for scleritis.
Our observation of an average drop bynearly three lines in visual acuity in association with incident ERD (see Figure 3), and the nearly two line improvement in visual acuity on average at the visit after ERD was first diagnosed (after anti-inflammatory treatment in the large majority of cases) is consistent with the existing concept that ERD is a clinically important complication of inflammatory eye disease that benefits substantially from treatment.
The limitations of this study are mostly related to its retrospective design. Misclassification of ERD may have occurred; if so, misclassification would have tended to dilute true associations, which would lead to underestimates of risk ratios with true risk factors. In particular, choroidal neovascularization (CNV)may lead to localized retinal detachment, which could be confused with inflammatory exudative retinal detachment. In our study, we excluded ERD associated with CNV. Furthermore, CNV was not associated statistically with ERD in our study in a sensitivity analysis (data not shown),suggestingthat inflammatory ERD indeed was identified correctly in most cases, such that misclassification is unlikely to have led to qualitatively inaccurate conclusions. Confusion of ERD with central serous chorioretinopathy could have happened in a small number of cases, but since the two entities typically have different findings it is unlikely that such misdiagnosis happened to a degree sufficient to qualitatively affect results. However, when ERD cases don’t respond as expected to corticosteroid therapy, the possibility of central serous chorioretinopathy should be considered. Also, in a retrospective study, subtle cases of ERD may have been missed occasionally, which (if so) would lead to a slight underestimate of the absolute risk of ERD, but is not likely to affect predictive factor associations given that false negatives would be very few compared to true negatives. Losses to follow-up also can lead to bias for longitudinal analyses if loss to follow-up is more or less likely in cases at higher risk than other cases; however, given that associations were mostly similar in the incidence and prevalence analyses, it is unlikely that differential follow-up played an important role for qualitative interpretation of results. The results are derived from a tertiary center-based cohort, so results may be more representative of a tertiary than a primary ophthalmology practice experience; however, most ERD cases would be referred for tertiary care. While we do not directly assess the effects of corticosteroid and other anti-inflammatory treatment on the incidence of ERD, we may infer that treatments sufficient to suppressive active inflammation would be beneficial in avoiding ERD. Older retrospective observations may have underestimated disease activity given that newer imaging technologies were not used in the early periods of the study, which might have led to underestimation of the extent of the significant disease activity-outcome associations. Finally, the disease definitions in this cohort were based on earlier description and may not include the latest consensus about defining these diseases.
In summary, exudative retinal detachment, although rare, is an important finding in patients with uveitis and scleritis, which often is associated with substantially reduced visual acuity. Fortunately, however, it is also prone to substantial visual recovery with treatment. In this study, we found that in addition toVKH and posterior scleritis (the diagnoses most commonly associated with ERD), also sympathetic ophthalmia, undifferentiated choroiditis, primary or secondary panuveitis, necrotizing scleritis, multifocal choroiditis with panuveitis, and “other” forms of posterior uveitis were associated with a higher prevalence of ERD at presentation for tertiary care, indicating that ERD does not necessarily dictate a diagnosis of VKH or posterior scleritis (although ERD is more common in the latter entities). On the other hand, among the ocular inflammatory diagnoses, only VKH, sympathetic ophthalmia, undifferentiated choroiditis, and primary or secondary panuveitis were significantly associated with incident ERD, perhaps because first attacks of inflammatory eye disease may be more severe and/or because inflammation tends to be better managed after initial presentation for tertiary care. African American and Hispanic race/ethnicity also were associated with higher risk of ERD after adjusting for other variables, which requires further investigation. In addition, a dose-response relationship with ERD prevalence and incidence was observed for anterior chamber cells and vitreous cell activity and also a higher risk of presentation with ERD in association with band keratopathy, confirming a strong relationship of ERD with inflammatory severity. Thus, we demonstrate that inflammation is a key predictive factor that is associated with developing ERD and early and vigorous control of inflammation is necessary to reverse ERD and prevent sequelae as much as possible.
We estimated the risk of and risk factors for exudative retinal detachment (ERD) in a large multicenter cohort of patients with non-infectious ocular inflammation. The estimated overall incidence was 0.0047%/eye-year. Vogt-Koyanagi-Harada Disease and posterior scleritis were the most common associated conditions, especially early after onset, but ERD was observed with several other kinds of ocular inflammation as well. Active inflammation was a key predictive factor associated with ERD risk, requiring prompt and vigorous treatment.
Acknowledgments:
a. Funding/Support: This study was supported primarily by National Eye Institute Grant EY014943 (JHK). Additional support was provided by Research to Prevent Blindness, the Paul and Evanina Mackall Foundation, and the Lois Pope Life Foundation, Sight for Souls, and the Massachusetts Eye and Ear Global Surgery Program. JHK was an RPB James S Adams Special Scholar Award recipient, JET was an RPB Harrington Special Scholar Award recipient, and DAJ and JTR were Research to Prevent Blindness Senior Scientific Investigator Award recipients during the course of the study. GAL-C and RBN previously were supported by and HNS continue to be supported by intramural funds of the National Eye Institute. EBS receives support from the Department of Veterans’ Affairs. None of the sponsors had any role in the design and conduct of the report; collection, management, analysis, and interpretation of the data; or in the preparation, review, and approval of this manuscript.
b. Financial Disclosures:Deepika N Shah: no financial disclosure. Ahmad Al-Moujahed: no financial disclosure. Craig W. Newcomb: no financial disclosure. C. Stephen Foster: (equity owner) Eyegate, (consultant, lecturer) Allergan; (consultant, lecturer) Bausch & Lomb; (consultant) Sirion; (lecturer) Alcon; (lecturer) Inspire; (lecturer) Ista; (lecturer) Centocor; Douglas A. Jabs:no financial disclosure; John H. Kempen: (consultant) Santen, (DSMC Chair) Gilead, advisory board (Clearside); James Rosenbaum: (consultant) Abbvie, Roche, Janssen, Gilead, Santen, UCB, Novartis, Eyevensys; (grant support) Pfizer; (royalty) UpToDate; Jennifer E. Thorne: Consultant for AbbVie, Gilead, Nightstar, Advisory Board for Clearside; Grants from Allergan, NEI, Santen; Eric B. Suhler: (consultant, research support) Abbvie, Clearside, EyeGate, EyePoint, Gilead, (consultant only) Eyevensys, Santen, (research support only) Bristol Meyers Squibb, Genentech; Oktay Kaçmaz: no financial disclosure; Ebenezer Daniel: no financial disclosure; Grace A. Levy-Clarke: no financial disclosure; Robert B. Nussenblatt: no financial disclosure; H. Nida Sen: no financial disclosure; Nirali P. Bhatt: no financial disclosure.
Biographies

Dr. Ahmad Al-Moujahed, a first-year ophthalmology resident at Byers Eye Institute, Stanford University, has research interests in retinal diseases. He completed a research fellowship in the field of retinal diseases at Massachusetts Eye and Ear (MEE), Harvard Medical School, followed by a PhD in experimental pathology from Boston University School of Medicine. He carried out his PhD research at the Angiogenesis Laboratory, MEE. He has contributed multiple peer-reviewed publications and academic ophthalmology conference presentations.

Dr. Shah is a fellowship-trained and board-certified cataract and cornea surgeon with expertise in anterior segment inflammation and uveitis. Dr. Shah served as a Heed and Doris Duke Fellow and is a Diplomate of the American Board of Ophthalmology. She is a graduate of the University of Pennsylvania, Johns Hopkins University and Massachusetts Eye and Ear, Harvard Medical School. Her research interests include ocular inflammation, telemedicine and health disparities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol 1990;14:303–8. 10.1007/BF00163549 [DOI] [PubMed] [Google Scholar]
- 2.Bodaghi B, Cassoux N, Wechsler B, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore) 2001;80:263–70. 10.1097/00005792-200107000-00005 [DOI] [PubMed] [Google Scholar]
- 3.Rothova a, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra a. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996;80:332–6. 10.1136/bjo.80.4.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloch-Michel E, Nussenblatt RB. International Uveitis Study Group recommendations for the evaluationof intraocular inflammatory disease. Am J Ophthalmol. 1987;103(2):234–235. 10.1016/S0002-9394(14)74235-7 [DOI] [PubMed] [Google Scholar]
- 5.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol 2008;15:47–55. 10.1080/09286580701585892 [DOI] [PubMed] [Google Scholar]
- 6.Pasadhika S, Kempen JH, Newcomb CW, et al. Azathioprine for ocular inflammatory diseases. Am J Ophthalmol 2009;148:500–509.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jabs DA, Nussenblatt RBRJ. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509–516. 10.1016/j.ajo.2005.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galor A, Feuer W, Kempen JH, et al. Adverse effects of smoking on patients with ocular inflammation. Br J Ophthalmol. 2010. July; 94(7):848–53. 10.1136/bjo.2009.174466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine--reporting of subgroup analyses in clinical trials. NEnglJMed. 2007;357(21):2189–2194. 10.1056/NEJMsr077003 [DOI] [PubMed] [Google Scholar]
- 10.AAO. Retina and Vitreous. In: BCSC Section 12; :292–299. [Google Scholar]
- 11.Rao N a, Gupta A, Dustin L, et al. Frequency of distinguishing clinical features in Vogt-Koyanagi-Harada disease. Ophthalmology 2010;117:591–9, 599.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan D, Hirose T. Vogt-Koyanagi-Harada syndrome: review of clinical features. Semin Ophthalmol 2011;26:312–5. 10.3109/08820538.2011.588654 [DOI] [PubMed] [Google Scholar]
- 13.Bordaberry MF. Vogt-Koyanagi-Harada disease: diagnosis and treatments update. Curr Opin Ophthalmol 2010;21:430–5. 10.1097/ICU.0b013e32833eb78c [DOI] [PubMed] [Google Scholar]
- 14.Rao N a. Pathology of Vogt-Koyanagi-Harada disease. Int Ophthalmol 2007;27:81–5. 10.1007/s10792-006-9029-2 [DOI] [PubMed] [Google Scholar]
- 15.Ishihara K, Hangai M, Kita M, Yoshimura N. Acute Vogt-Koyanagi-Harada disease in enhanced spectral-domain optical coherence tomography. Ophthalmology 2009;116:1799–807. 10.1016/j.ophtha.2009.04.002 [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Park S, Lee J. Edema of the photoreceptor layer in Vogt-Koyanagi-Harada disease observed using high-resolution optical coherence tomography. Korean J Ophthalmol 2009;23:74–79. 10.3341/kjo.2009.23.2.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaithanyaa N, Devireddy SK, Kishore Kumar R V, et al. Sympathetic ophthalmia: a review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol 2012;113:172–6. 10.1016/j.tripleo.2011.01.036 [DOI] [PubMed] [Google Scholar]
- 18.Chang GC, Young LH. Sympathetic ophthalmia. Semin Ophthalmol 2011;26:316–20. 10.3109/08820538.2011.588658 [DOI] [PubMed] [Google Scholar]
- 19.Croxatto J, Rao N, McLean I, Marak G. Atypical histopatholigc featues in sympathetic ophthalmia. A study of a hundred cases. Int Ophthalmol 1982;4:129–135. 10.1007/BF00161902 [DOI] [PubMed] [Google Scholar]
- 20.Lubin J, Albert D, Weinstein M. Sixty-five years of sympathetic ophthalmia. A clinicopathologic review of 105 cases (1913–1978). Ophthalmology 1980;87:109–121. 10.1016/S0161-6420(80)35270-6 [DOI] [PubMed] [Google Scholar]
- 21.Shukla D, Agrawal D, Dhawan a, Ramchandani B. Posterior scleritis presenting with simultaneous branch retinal artery occlusion and exudative retinal detachment. Eye (Lond) 2009;23:1475–7. 10.1038/eye.2008.217 [DOI] [PubMed] [Google Scholar]
- 22.McCluskey P, Watson P, Lightman S, et al. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology 1996;106:2380–2386. 10.1016/S0161-6420(99)90543-2 [DOI] [PubMed] [Google Scholar]
- 23.Benson E Posterior scleritis. Surv Ophthalmol 1988;32(5), 297–316. 10.1016/0039-6257(88)90093-8 [DOI] [PubMed] [Google Scholar]
- 24.Errera M-H, Fardeau C, Cohen D, et al. Effect of the duration of immunomodulatory therapy on the clinical features of recurrent episodes in Vogt--Koyanagi--Harada disease. Acta Ophthalmol 2011;89:e357–66. 10.1111/j.1755-3768.2010.02055.x [DOI] [PubMed] [Google Scholar]
- 25.Kim R, Lowenstein J. Systemic diseases manifesting as exudative retinal detachment. Int Ophthalmol Clin 1998;38:177–95. 10.1097/00004397-199803810-00015 [DOI] [PubMed] [Google Scholar]


