Abstract
Background:
Ambient environmental pollutants have been shown to adversely affect respiratory health in susceptible populations. However, the role of simultaneous exposure to multiple diverse environmental pollutants is poorly understood.
Objective:
We applied a multidomain, multipollutant approach to assess the association between pediatric lung function measures and selected ambient air pollutants and pesticides.
Methods:
Using data from the US EPA and California Pesticide Use Registry, we reconstructed three months prior exposure to ambient air pollutants ((ozone (O3), nitrogen dioxide (NO2), particulate matter with a median aerodynamic diameter < 2.5μm (PM2.5) and < 10μm (PM10)) and pesticides (organophosphates (OP), carbamates (C) and methyl bromide (MeBr)) for 153 children with mild intermittent or mild persistent asthma from the San Joaquin Valley of California, USA. We implemented Bayesian kernel machine regression (BKMR) to estimate the association between simultaneous exposures to air pollutants and pesticides and lung function measures (forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), and forced expiratory flow between 25% and 75% of vital capacity (FEF25–75)).
Results:
In BKMR analysis, the overall effect of mixtures (pollutants and pesticides) was associated with reduced FEV1 and FVC, particularly when all the environmental exposures were above their 60th percentile. For example, the effect of the overall mixture at the 70th percentile (compared to the median) was a −0.12SD (−50mL, 95% CI: −180mL, 90mL) change in the FEV1 and a −0.18SD (−90mL, 95% CI: −240mL, 60mL) change in the FVC. However, 95% credible intervals around all of the joint effect estimates contained the null value.
Conclusion:
At this agricultural-urban interface, we observed results from multipollutant analyses, suggestive of adverse effects on some pediatric lung function measures following a cumulative increase in ambient air pollutants and agricultural pesticides. Given the uncertainty in effect estimates, this approach should be explored in larger studies.
Keywords: Pediatric Asthma, Complex Mixtures, Pesticides, Air Pollution, Lung Function
1. Introduction
The effects of exposure to ambient environmental agents on respiratory health and pulmonary function in children have been widely reported, particularly among children with asthma. These studies have significantly improved the understanding of the deleterious effects of individual pollutants and chemicals such as criteria air pollutants including particulate matter, ozone, and nitrogen dioxide,1,2 and additional ambient pollutants including polycyclic aromatic hydrocarbons3,4 and agricultural pesticides.5,6 To date, the preponderance of studies on environmental risk factors for respiratory disease have largely employed a risk-factor epidemiological framework, which attempts to isolate the impact of one pollutant (or total effect within one specific chemical group), though the general consensus is that individuals are rarely (if ever) affected by single chemical agents in isolation.7,8 As the field of environmental epidemiology moves towards multipollutant approaches, examining the effects of simultaneous exposure to multiple diverse environmental chemicals is increasingly feasible, especially when these ambient mixtures impact common receptors and pathophysiologic pathways and share similar endpoints.7,9,10 A multidomain approach considers the joint effect of multiple classes of environmental agents along the theme of the “total environment” paradigm which takes into consideration a more comprehensive range of concurrent exposures experienced by a population.11
Although chronic diseases of childhood are universally multifactorial and are challenged by adequate characterization of relevant exposures, the national burden of disease for pediatric asthma and the strong environmental antecedents associated with both asthma incidence and asthma-related morbidity result in a particularly salient example to conduct multidomain studies.12 Children with asthma who have spatially and temporally heterogeneous exposure to multiple domains of environmental chemicals may provide substantial insight into understanding the health effects of co-exposure to pollutants in a multidomain context.
The relationships between pediatric asthma morbidity and exposure to ambient environmental chemicals and pollutants are dependent on regional characteristics.13,14 The San Joaquin Valley (SJV) region in California is of particular environmental and public health interest given the relatively high prevalence of asthma and asthma hospitalizations compared to state and national averages.15,16 The region is also exposed to high levels of air pollution: topography, rapid population growth, a developing agricultural-urban interface (i.e. urban areas surrounded by intense agricultural operations), and two major interstate highways are the main reasons the SJV experiences some of the highest levels of air pollution in the United States.4,17 Several studies have found associations between increased exposure to ambient air pollutants (including traffic-related species) and asthma-related morbidity in the SJV. 3,4,16,18,19 Adverse health-related outcomes have also been linked to agricultural-related exposures (like pesticides and agricultural dust) in the region,20-23 though these studies focused on non-respiratory endpoints. Despite exposure to a distinctive mixture of ambient air pollutants and agricultural-related exposures observed in SJV, the nature of adverse multipollutant effects on pediatric asthma morbidity is unexplored in this region.
In this study, we examined the pulmonary health effects of ambient air pollutants (AAP) and agricultural pesticides for a group of children with asthma in the SJV region. We defined exposure to AAP and pesticides as region-specific, co-occurring quantitative metrics of interest based on the exposure prevalence in the source population, and demonstrated potency and adverse effects of both factors on asthma health in literature. We implemented Bayesian kernel machine regression (BKMR) models to explore the pulmonary health effects of this multipollutant exposure mixture. BKMR is a novel statistical method that estimates the cumulative or joint health effects of multiple pollutants,24,25 and addresses several statistical limitations associated with environmental mixtures analysis approaches by flexibly modeling exposures; handling complex structures of mixtures with highly correlated exposures, potentially non-linear (and non-additive) exposure-outcome associations and high-dimensional interactions.24,26,27 Specifically, we examined a) whether recent exposure (prior 3 months) to the mixture of AAP and pesticides jointly is associated with adverse pulmonary effects; b) the exposure–response relationships between combinations of environmental exposures and lung function; and c) whether the impact of an individual environmental exposure is more pronounced when it occurs as part of a mixture (i.e., whether the components of the mixture interact).
2. Materials and methods
2.1. Study Population
Data for this study were collected as part of the Fresno Asthmatic Children’s Environment Study (FACES), a longitudinal epidemiologic study of children with current asthma in Fresno, California. The details on recruitment and health evaluations have been reported previously.19,28 Briefly, the FACES study collected data on short- and long-term effects of ambient air pollution on children with asthma between 2000 and 2008. A total of 315 children between the ages of 6 and 11 years at baseline who had a primary residence within 20 km of the Fresno EPA Supersite Monitor29 were followed over this period. Study participants provided a detailed account of their general health history and exposures to several factors including secondhand tobacco smoke. Participants also performed scheduled spirometry throughout the study period and answered questions about asthma-related symptoms.
For this study, we conducted analyses of first available health evaluation (typically at the study baseline) for children with complete information on respiratory and overall health, demographic data and house addresses. The Institutional Review Boards of the participating institutions (University of California, Berkeley, and secondarily, Colorado State University) approved the study protocol, and written informed consent/assent was obtained from the parents or legal guardians of all participants.
2.2. Pulmonary Function Measures
Spirometry was performed using the EasyOne spirometer (Medical Technologies, Chelmsford, MA, USA). Children were asked to complete three (up to a maximum of eight attempts) acceptable flow-volume loop maneuvers, in accordance with recommendations for spirometry performance provided by the American Thoracic Society/European Respiratory Society. We assessed forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and forced expiratory flow between 25% and 75% of vital capacity (FEF25–75) as independent measures of pediatric lung function. In sensitivity analysis, we assessed the percentage of the FVC in the first second of forced expiration (FEV1/FVC ratio) to characterize the severity of airway obstruction among these children.
Our study made use of baseline pre-bronchodilator spirometry measures (one observation per participant), or the first available recorded measures when the baseline measurement was unavailable. We regressed FEV1, FVC, FEF25–75 and FEV1/FVC on sex, age, height and ethnicity using spirometric reference equations for the US population,30 and used the residual values as outcomes in our data analyses.31
2.3. Air Pollution Exposures
Air quality data were obtained from the EPA Air Quality System for individuals based on their geocoded residential address. Pollutant concentrations from the air monitoring stations located closest to the residential location (within 10 km) were obtained for 24-hr measurements of particulate matter with aerodynamic diameter < 2.5 μm (PM2.5) and <10 μm (PM10), 8-hour maximum concentration over 24 hours for ozone (O3), and 1-hour maximum concentration over 24 hours for nitrogen dioxide (NO2). We summarized concentrations as quarterly averages for exposures 3 months prior to pulmonary function measurements, to be consistent with pesticide exposures which are typically reported in ≥ 3-month cycles.32
2.4. Pesticide Exposures
We estimated agricultural pesticide exposure based on residential location and the California Pesticide Use Report (PUR) Data.32 The PUR, one of the most comprehensive databases of its kind, provides the amount (kg), date, and location (to one-square-mile sections) of specific pesticides (active ingredient) applied in the state quarterly. We characterized potential participant exposures to three classes of pesticides (carbamates (C), methyl bromide (MeBr), and organophosphates (OP)) with similar toxicity profiles relevant to asthma morbidity.33-35 Organophosphates and carbamates are acetylcholinesterase inhibitor pesticides implicated in exacerbation of respiratory disease and asthma due to their cholinergic action on airway smooth muscle and mucus-secreting epithelial cells.33 MeBr, a restricted-use fumigant, has also been implicated in adverse respiratory effects; the toxicological mechanism of action of MeBr is poorly understood, but believed to be due to the high reactivity associated with alkyl halides.34 Using the purexposure package in R,36 we created pesticide exposure measures corresponding to the 3-month period prior to spirometry, based on residential addresses using validated algorithms developed by Gunier et al.37 Pesticide exposure was estimated by calculating the percentage of land area for each section within a selected buffer (3km around geocoded residence); multiplying kilograms of active ingredient applied to each section (within the 3-month time frame) by the proportion of area within selected buffer, summing the area weighted mass (in kg) for all sections that intersect the buffer, and dividing by the buffer area. We assessed circular buffers with distances of 1.25km, 3km and 5km around each participant’s geocoded residence. The smallest buffer was based on drift models,38 and the largest buffer was selected based on the spatial scale most strongly correlated with MeBr fate and transport.39,40 As an intermediate distance for the range of drift from pesticide applications, we used 3km buffers.
2.5. Covariates
We developed a set of plausible confounders a priori based on previous studies of lung function and exposure to air pollution or pesticides, as well as directed acyclic graphs. As described above, the pre-analysis transformation of outcome variables using residual values included the use of age, height, sex and race/ethnicity as confounders; we also considered meteorological conditions (linear representations of temperature and precipitation averaged over three months prior to baseline spirometry measurement), season (fall/winter/spring/summer, based on evaluation of meteorological and air pollution patterns in the Fresno region); as well as subject-specific characteristics potentially associated with asthma and asthma exacerbation: body mass index (linear BMI in kg/m2), maternal education level (≤/> 12th grade education), household income (</≥ $30k), insurance status (yes/no), atopy (yes/no), and a measure of asthma severity based on Global Initiative for Asthma symptom severity guidelines (1 [mild intermittent]/2 [mild persistent]/3 [moderate persistent]/4 [severe persistent asthma]); and other competing deleterious exposures: smoker currently in home (yes/no), and self-reported residence proximity to a major roadway (</≥ 1 block away).
2.6. Statistical Analysis
All statistical analyses were performed in R version 3.5.0 (R Foundation for Statistical Computing, Vienna, Austria); BKMR analyses in R was performed using the bkmr package.41
2.6.1. Bayesian Kernel Machine Regression
We applied BKMR to estimate the association between exposure to a multipollutant mixture of air pollutants and pesticides and each outcome measure of lung function. Extensive details on the statistical approach are described elsewhere.24,27 BKMR offers two important advantages in the context of characterizing the risk from exposure to multiple pollutants. First, BKMR is able to estimate a flexible exposure-response surface for health-related effects of multipollutant exposures including potential nonlinear (such as threshold effect or polynomial effect) and non-additive interactive (for example synergism only beyond a particular copollutant exposure threshold) relationships, as are prevalent in environmental epidemiology studies.42-47 Second, BKMR performs a hierarchical variable selection (HVS) that can incorporate prior knowledge on the structure of the environmental mixture. In our setting, the HVS simultaneously estimates the posterior inclusion probability (PIP) for each domain (ambient air pollutants and pesticides) and for each individual pollutant within the domains. Observed exposure-response associations in environmental epidemiology studies may depend on only a subset of the mixture components;48,49 the HVS in BKMR uses methods within a Bayesian paradigm to identify important mixture components associated with the outcome while accounting for the overall structure of the mixture, and possible correlations between exposures.24
The hierarchical or multistep approach to variable selection first estimates the probability that each pre-specified pollutant group/domain be included in the model, and then assesses for evidence in the data that one of the components in a domain drives the group’s effect on the outcome. Variable selection yields posterior inclusion probabilities (PIP), representative of relative variable importance by magnitude. PIP values range between 0 and 1 indicating the level of certainty or uncertainty that the component is included in the model and is associated with the outcome.
For the primary analysis, the BKMR model is
For each subject i = 1, … , n, BKMR relates the health outcome (yi) to the M components of the exposure mixture xi = (x1i, … , xMi) through an unknown but smooth function h(·), which represents the exposure-response function that accommodates non-linearity and/or interaction among the mixture components, while controlling for C relevant confounders zi = (z1i, … , zCi). For our analyses, we implemented BKMR with the Gaussian kernel function, which captures an extensive range of underlying functional forms for h(·).
We ran three primary BKMR models to assess exposure association with regression-adjusted FEV1, FVC and FEF25-75. For each model, we used HVS formulation with exposures grouped into two domains: air pollutants (O3, NO2, PM2.5, PM10), and pesticides (C, MeBr, OP). HVS allows for variable selection both at the group level and individual pollutant level and provides models stability with highly correlated predictors. In sensitivity analysis, we created three exposure groups, splitting air pollutants into particles (PM2.5, PM10) and gases (O3, NO2). In addition, BKMR models were constructed to assess the effect of joint exposures on FEV1/FVC.
We ran the default Markov chain Monte-Carlo sampler (described in detail in Bobb et al.24) for 25,000 iterations after a burn in of 25,000 and every fifth sample was kept for inference. Convergence of the Markov chain was monitored by inspecting trace plots of model parameters.
We used square root and then centering and scaling (subtracting the vector mean and dividing by the standard deviation) transformation for the exposure measures to account for the severe right-skewedness typical of pesticide concentrations.22 All other continuous variables (lung function measures, temperature, precipitation, and BMI) were centered and scaled. To account for missing data (indicator for ‘any smoking in the home’ (16%)), we included an indicator for each missing observation.
2.6.2. Linear Regression
We used multivariable linear regression models to estimate associations of individual pollutant exposures and all pollutant exposures (multipollutant models) with each outcome measure of lung function. We also assessed potential interactions among mixture components based on BKMR results.
Variance inflation factors (VIF) were estimated for multipollutant models to assess the extent of collinearity between component pollutants, and all linear models were adjusted for all cofounders used in BKMR analyses.
3. Results
3.1. Study Population Characteristics
Of the 315 children recruited for the FACES study, complete exposure, outcome, and covariate data were available for 153 children (48.6%, Table 1). There were no significant differences in sociodemographic or exposure characteristics between children included and those excluded from the study (Supplementary Table 1).
Table 1.
Descriptive characteristics of study population
| Characteristic | N = 153 |
|---|---|
| Age years, mean ± SD | 9 ± 1.8 |
| Male Gender, n (%) | 92 (60.1) |
| Height (in), mean ± SD | 52.3 ± 4.8 |
| BMI (kg/m2), mean ± SD | 18.5 ± 4.6 |
| Ethnicity, n (%) | |
| Non-Hispanic Black | 21 (13.7) |
| Non-Hispanic White | 71 (46.4) |
| Hispanic | 61 (39.9) |
| Mother > 12th grade education, n (%) | 93 (60.8) |
| Insured, n (%) | 145 (94.8) |
| Atopy, n (%) | 120 (78.4) |
| Father/Mother Smokes (Current), n (%) | 8 (5.2) |
| Proximity to Freeway (< 1 block away), n (%) | 80 (52.3) |
| Asthma Severity, n (%) | |
| Mild intermittent or persistent asthma | 126 (82.3) |
| Moderate or severe persistent asthma | 27 (17.7) |
| FEV1 (L), mean ± SD | 1.7 ± 0.4 |
| FEV1 < 80% predicted, n (%) | 17 (11.1) |
| FVC (L), mean ± SD | 2.0 ± 0.5 |
| FVC < 80% predicted, n (%) | 12 (7.8) |
| FEF25-75 (L), mean ± SD | 1.8 ± 0.4 |
| FEF25-75 < 80% predicted, n (%) | 44 (28.8) |
| FEV1 /FVC, mean ± SD | 0.8 ± 0.0 |
| FEV1/FVC < 80% predicted, n (%) | 21 (14.9) |
Abbreviations: BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity.
The majority of children included in our study were non-Hispanic White (46.4%), male (60.1%), and on average 9 years (SD: 1.8); the mean BMI was 18.5 kg/m2 (SD: 4.6). Over three-quarters of participants were atopic (defined by positive skin reaction to one or more allergens or physician’s diagnosis of allergic rhinitis or eczema, 78.4%), and were also more like to have a diagnosis of mild intermittent or mild persistent asthma (severity category < 3, 82.3%), and be insured (94.8%). Over half (52.3%) of participants had addresses within one block of a freeway, and only 5.2% reported either parent as a current smoker. With regard to pulmonary function, 11.1%, 7.8% and 28.8% of participants had FEV1, FVC and FEF25-75 values below 80% of the predicted reference value, respectively.
3.2. Exposure Characteristics
The distributions of pollutant metrics are presented in Table 2. The concentration of exposure to daily (24-hr) PM2.5 exceeded the current National Ambient Air Quality Standard (NAAQS) 24-hr standard of 35 μg/m3 for 14 (9.2%) children, with an observed maximum of 40.2 μg/m3. All other ambient air pollutants had distributions below NAAQS standards, with average NO2 and O3 values well below the current NAAQS annual standard over the entire time period (NO2 mean: 15.5 ppb, SD: 3.3; O3 mean: 35.8 ppb, SD: 12.4). As expected, the distribution of pesticide exposure concentrations was right skewed, with several participants assigned low and zero exposure values (C: 45.1%, OP: 24.2%, MeBr: 77.8%) based on their residential addresses. Correlations between pairs of the exposure variables and meteorological conditions are presented in Figure 1. In general, the strongest correlations among AAP are seen between NO2 and PM2.5 (ρ = 0.9), which are both negatively correlated with O3 (ρ = −0.6 and −0.5, respectively). OPs were moderately positively correlated with O3 (ρ = 0.5) and moderately negatively correlated with NO2 (ρ = −0.5), while temperature and precipitation were, as expected, correlated with O3 (ρ = 0.8 and −0.7 respectively).
Table 2.
Ambient air pollutant and pesticide exposure data summary statistics
| Pollutant/Pesticide | Minimum | Mean | SD | Median | Interquartile Range |
Maximum |
|---|---|---|---|---|---|---|
| Nitrogen Dioxide (ppb) | 9.5 | 15.5 | 3.3 | 14.4 | 5.3 | 23.1 |
| Ozone (ppb) | 12.3 | 35.8 | 12.4 | 38.0 | 16.4 | 58.2 |
| Particulate Matter < 2.5 μm (PM2.5) (μg/m3) | 6.7 | 16.3 | 9.8 | 11.2 | 8.1 | 40.2 |
| Particulate Matter < 10 μm (PM10) (μg/m3) | 19.5 | 37.9 | 10.7 | 32.5 | 16.9 | 65.9 |
| Carbamates x 106(kg/3km2) | 0.0 | 0.1 | 0.3 | 0.0 | 0.1 | 2.4 |
| Methyl Bromides x 106(kg/3km2) | 0.0 | 3.9 | 9.9 | 0.0 | 0.0 | 48.9 |
| Organophosphates x 106(kg/3km2) | 0.0 | 0.9 | 1.1 | 1.1 | 1.2 | 5.4 |
Figure 1. Pairwise Pearson correlation among the seven environmental exposure measures and meteorological parameters.
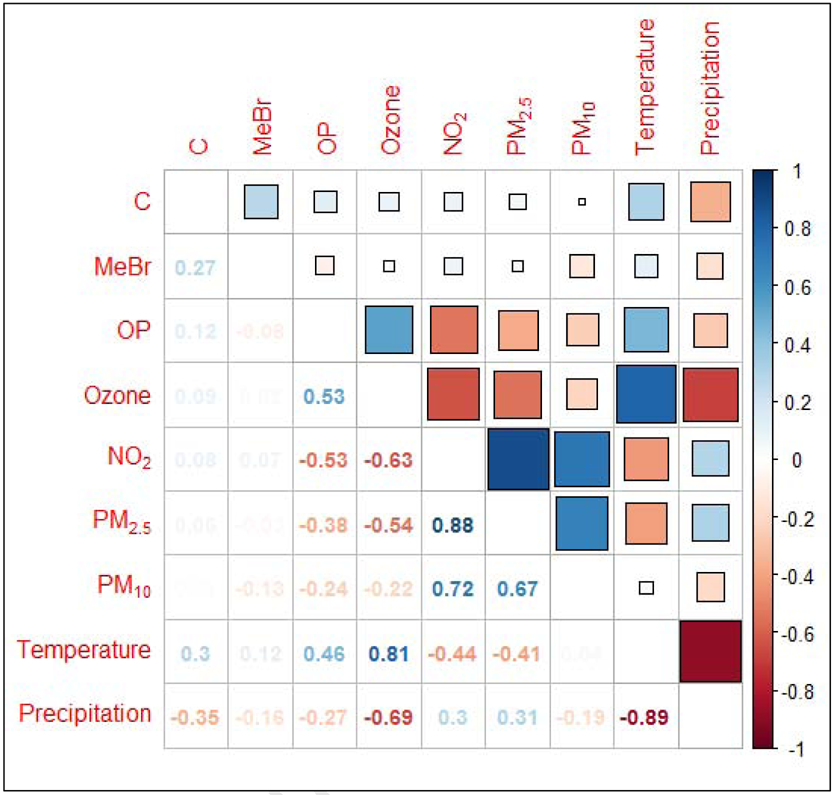
Note: NO2, nitrogen dioxide; PM2.5, particulate matter with a median aerodynamic diameter < 2.5μm; PM10, particulate matter with a median aerodynamic diameter < 10μm; OP, organophosphates; C, carbamates and MeBr, methyl bromide.
3.3. BKMR Analyses
The exposure domain PIPs (group PIP) and individual component PIPs (conditional PIP, the PIP of component inclusion conditional on domain inclusion) estimated from the BKMR models are presented in Table 3. We used a common threshold for “variable importance” of PIP > 0.5;27,50,51 values above 0.5 indicate individual component or domain importance in model inclusion and association with the outcome. The PIPs in each model indicated a clear separation between the domains, with AAPs driving the mixture in the FEV1 and FVC models (group PIP: 0.75 and 0.78 respectively), and pesticides driving the mixture in the FEF25-75 model (group PIP: 0.77). Within the exposure domains, NO2 (among AAP) and OP (among pesticides) were the most important drivers for both FEV1 and FVC models in our study (component PIP: 0.59 and 0.55 respectively). In the FEF25-75 model, MeBr was the most important pesticide and PM10 was the most important AAP, although both PIP values fell below 0.5 (component PIP: 0.46 and 0.28 respectively).
Table 3.
Group and conditional posterior inclusion probabilities (PIPs) from BKMR using exposure domain groups for hierarchical variable selection.
| PIPs (by outcome measure) | ||||||
|---|---|---|---|---|---|---|
| Exposure | FEV1 | FVC | FEF25-75 | |||
| Group | Conditional | Group | Conditional | Group | Conditional | |
| O3 | 0.75 | 0.10 | 0.78 | 0.10 | 0.51 | 0.27 |
| NO2 | 0.75 | 0.59 | 0.78 | 0.55 | 0.51 | 0.19 |
| PM2.5 | 0.75 | 0.25 | 0.78 | 0.29 | 0.51 | 0.25 |
| PM10 | 0.75 | 0.06 | 0.78 | 0.06 | 0.51 | 0.28 |
| C | 0.67 | 0.12 | 0.55 | 0.16 | 0.77 | 0.41 |
| MeBr | 0.67 | 0.11 | 0.55 | 0.15 | 0.77 | 0.46 |
| OP | 0.67 | 0.77 | 0.55 | 0.69 | 0.77 | 0.13 |
Group PIPs indicate the posterior probability of an exposure domain being included in the model; conditional PIPs indicate the posterior probability of a single exposure within the domain to be included in the model. Both provide an illustration of the relative ranking of variable importance for each exposure domain as well as each exposure within a particular domain.
Bold font indicates highest PIP in column.
Abbreviations: FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity.
In the HVS models fit with three groups (pesticides, ambient particles, and ambient gasses) in sensitivity analysis, results from the main analysis persisted. The gases (driven by NO2) were the main drivers of the FEV1 and FVC models, while pesticides (driven by MeBr) remained the main drivers of the FEF25-75 model (Supplementary Table 2).
Overall, we observed mostly small changes in lung function per unit increase in exposure, with considerable variability especially at higher concentrations (Figure 2). For FEV1 and FVC models, we observed negative and approximately linear associations with lung function for NO2 and O3, a nonlinear, almost J-shaped exposure response curve for OP, and largely null trends for the other exposures. For the FEF25-75 model, we observed approximately negative linear effects for carbamates and MeBr, with largely null effects for the other exposures.
Figure 2. The exposure-response relationship between exposure to environmental agents and lung function.
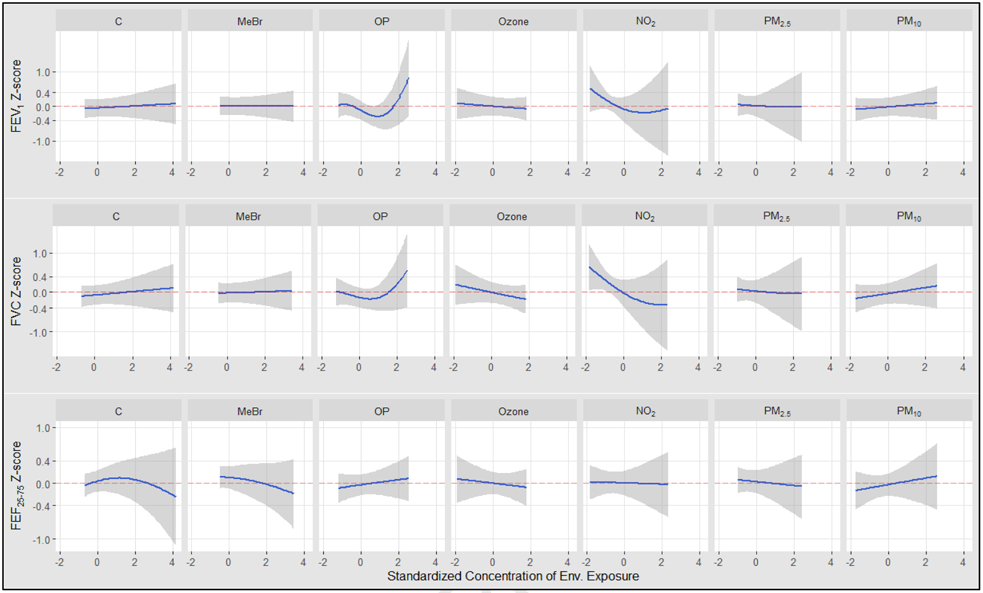
Univariate relation between each exposures and outcomes, with other exposures fixed at their median value. The results were assessed by the BKMR model adjusted for age, height, sex, race/ethnicity, temperature, precipitation, season, BMI, maternal education level, household income, insurance status, atopy, a measure of asthma severity (based on Global Initiative for Asthma symptom severity guidelines), smoker currently in home, and proximity to a major roadway.
Note. NO2, nitrogen dioxide; PM2.5, particulate matter with a median aerodynamic diameter < 2.5μm; PM10, particulate matter with a median aerodynamic diameter < 10μm; OP, organophosphates; C, carbamates and MeBr, methyl bromide.
Figure 3 displays summaries of the overall effect of the mixture on the lung function measures. Each panel represents a numeric summary of the change in lung function associated with a simultaneous change in each of the seven exposures from the 25th percentile to 75th percentile, as compared to their median value (50th percentile). Panels A and B showed a consistent decrease in FEV1 and FVC with increased exposure to the AAP and pesticide mixture. The joint effects were particularly deleterious when all exposures were above their 60th percentile, though 95% credible intervals around the effect estimates contained the null value. For example, the effect of the overall mixture at the 30th percentile (compared to the median) was a 0.41 SD change in the FVC (equivalent to a 210mL change, 95% CI: −40, 470mL), but a −0.18SD (−90mL, 95% CI: −240, 60mL) change in FVC at the 70th percentile (compared to the median).
Figure 3. Overall effect (95% CI) of mixtures on the 3 outcome measures.
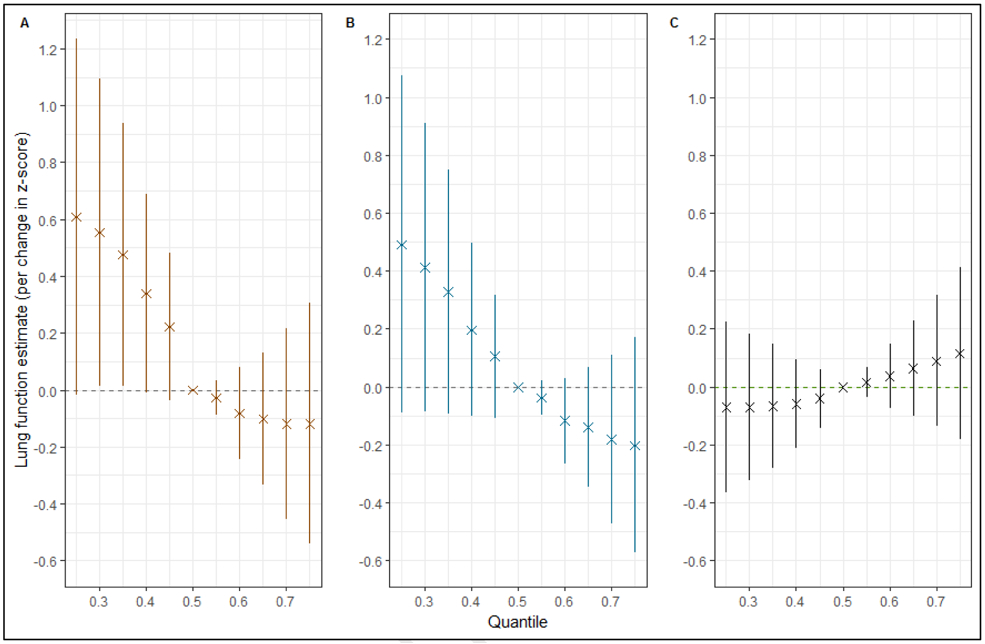
Figures depict the effect on lung function outcomes (A. forced expiratory volume in 1s (FEV1); B. forced vital capacity (FVC); and C. forced expiratory flow between 25% and 75% of vital capacity (FEF25-75)) when all the environmental exposures (ozone, nitrogen dioxide, particulate matter with median aerodynamic diameter < 2.5 μm, and < 10μm, organophosphates, carbamates and methyl bromide) at particular percentiles were compared to all the exposures at their 50th percentile.
The models were adjusted for age, height, sex, race/ethnicity, temperature, precipitation, season, BMI, maternal education level, household income, insurance status, atopy, a measure of asthma severity (based on Global Initiative for Asthma symptom severity guidelines), smoker currently in home, and proximity to a major roadway.
For the FEF25-75 model, we see ordered effects opposite those observed for the other outcome measures, with higher quantiles appearing less harmful (Figure 3C). The effect estimates are, however, relatively smaller (all within ± 0.12 SD of the median value), and credible intervals indicate very small differences between quantiles.
Interaction plots show mostly parallel patterns (no interaction) but suggest certain component exposure interactions on lung function. We summarize suggestive interactions between pairs of pollutants in Supplementary Figure 1. In FEV1 and FVC models, the negative effects of NO2 on lung function appeared to be diminished at higher levels of PM2.5. Similarly, the effects of NO2 on FEV1 and FVC appear to be less steep at higher levels of OP exposure. In addition, the negative effects on FEF25-75 observed for MeBr were greatest at the highest levels of C, and less harmful at higher levels of PM10 (Supplementary Figure 1). Notably, most of the observed interactions were in regions with fewer observations and relatively wide credible intervals.
3.4. Linear Regression Analyses
Summary of linear regression (no-interaction) models assessing the associations between exposure to environmental agents and lung function measures are displayed in Table 4. When examining one exposure at a time, most component pollutants were negatively associated with FEV1 and FVC; NO2 and OP had associations in opposite directions with confidence intervals that excluded the null value (βNO2 (95% CI): −0.31SD (−0.55, −0.07); βOP (95% CI): 0.21SD (0.03, 0.39)). In the single-pollutant FEF25-75 models, the strongest effect was observed for MeBr (βMeBr (95% CI): −0.15 (−0.29, −0.00)). In no-interaction multipollutant models that included all seven exposure mixture components, the association with NO2 persisted in FEV1 (β (95% CI): −0.74SD (−1.24, −0.24)) and FVC models (β (95% CI): −0.81SD (−1.30, −0.32)). VIF in multipollutant models ranged from 1.40 to 15.48, indicating some degree of multicollinearity between correlated predictors in the linear regression models.
Table 4.
Association between environmental agents and lung function based on linear regression models.
| FEV1 | FVC | FEV25-75 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Environmental Agent |
Single exposure | Multiple exposures | Single exposure | Multiple exposures | Single exposure | Multiple exposures | |||
| β (95% CI)1 | β (95% CI)1,2 | β (95% CI)1,2,3 | β (95% CI)1 | β (95% CI)1,2 | β (95% CI)1,2,3 | β (95% CI)1 | β (95% CI)1,2 | β (95% CI)1,2,3 | |
| O3 | −0.06 (−0.36, 0.25) | −0.19 (−0.49, 0.11) | −0.24 (−0.55, 0.04) | −0.09 (−0.39, 0.22) | −0.22 (−0.52, 0.07) | −0.29 (−0.58, 0.00) | 0.11 (−0.21, 0.42) | 0.03 (−0.30, 0.35) | −0.10 (−0.41, 0.22) |
| NO2 | −0.31 (−0.55, −0.07) | −0.74 (−1.24, −0.24) | −0.80 (−1.29, −0.30) | −0.35 (−0.59, −0.11) | −0.81 (−1.30, −0.32) | −0.87 (−1.35, −0.38) | −0.02 (−0.28, 0.24) | −0.10 (−0.64, 0.45) | −0.44 (−0.97, 0.09) |
| PM2.5 | −0.16 (−0.42, 0.11) | −0.01 (−0.46, 0.44) | −0.26 (−0.73, 0.22) | −0.18 (−0.44, 0.08) | −0.01 (−0.45, 0.44) | −0.26 (−0.73, 0.21) | 0.02 (−0.26, 0.30) | −0.03 (−0.52, 0.46) | −0.22 (−0.71, 0.27) |
| PM10 | −0.03 (−0.23, 0.18) | 0.44 (0.04, 0.84) | 0.40 (0.00, 0.81) | −0.05 (−0.26, 0.15) | 0.46 (0.07, 0.85) | 0.43 (0.03, 0.82) | 0.11 (−0.10, 0.32) | 0.13 (−0.30, 0.56) | 0.53 (0.08, 0.27) |
| C | 0.01 (−0.14, 0.16) | 0.10 (−0.07, 0.26) | −0.04 (−0.22, 0.14) | 0.02 (−0.13, 0.17) | 0.11 (−0.05, 0.27) | −0.03 (−0.21, 0.15) | −0.06 (−0.22, 0.10) | −0.02 (−0.20, 0.15) | 0.10 (−0.07, 0.26) |
| MeBr | −0.06 (−0.20, 0.08) | −0.01 (−0.16, 0.15) | 0.02 (−0.12, 0.17) | −0.04 (−0.18, 0.10) | 0.01 (−0.13, 0.16) | 0.04 (−0.11, 0.19) | −0.15 (−0.29, −0.00) | −0.11 (−0.28, 0.05) | −0.01 (−0.16, 0.15) |
| OP | 0.21 (0.03, 0.39) | 0.11 (−0.09, 0.31) | 0.14 (−0.13, 0.41) | 0.21 (0.03, 0.39) | 0.11 (−0.09, 0.31) | 0.14 (−0.13, 0.40) | 0.11 (−0.08, 0.30) | 0.09 (−0.12, 0.31) | 0.11 (−0.09, 0.31) |
| OP:NO2 | −0.13 (−0.31, 0.05) | −0.13 (−0.31, 0.05) | |||||||
| PM2.5:NO2 | 0.33 (0.08, 0.58) | 0.34 (0.09, 0.59) | |||||||
| C:MeBr | −0.29 (−0.46, −0.13) | ||||||||
| PM10:MeBr | 0.17 (−0.03, 0.38) | ||||||||
β estimates represent the mean change in the lung function measure per unit (z-score) increase in the exposure agent.
Bold font represents statistical significance.
Adjusted for BMI, maternal education, current smoking, insurance, proximity to freeway, atopy status, asthma severity at baseline, season of exposure temperature and precipitation.
Additionally adjusted for all the other environmental agents.
Additionally adjusted for interaction between selected environmental agents.
Abbreviations: CI, confidence interval; O3, ozone; NO2, nitrogen dioxide; PM2.5, particulate matter with a median aerodynamic diameter < 2.5μm; PM10, particulate matter with a median aerodynamic diameter < 10μm; OP, organophosphates; C, carbamates and MeBr, methyl bromide; FEV1, forced expiratory volume in 1s; FVC, forced vital capacity; FEF25-75, forced expiratory flow between 25% and 75% of vital capacity.
We tested for interaction between exposure components suggested by BKMR interaction plots in multivariable linear regression models including all the main exposure effects. We observed statically significant interaction terms for NO2 and PM2.5 in FEV1 (p = 0.01) and FVC (p = 0.01) models, and carbamates and MeBr in the FEF25-75 model (p < 0.01).
3.5. Sensitivity Analysis
Results for the FEV1/FVC models are shown in Supplementary materials. Similar to FEV1 and FVC models, AAPs were the main drivers of the effect of the mixture (group PIP: 0.65, Supplementary Table 3). However, O3 (among AAP) and MeBr (among pesticides) were the most important drivers (component PIP: 0.60 and 0.49 respectively) within exposure domains.
Supplementary Figure 2 indicates that an increase in exposure to the AAP and pesticide mixture was positively associated with participant FEV1/FVC levels. Similar to FEF25-75 models, effect estimates were relatively small (all within ± 0.17 SD of the median value), and credible intervals indicated minimal differences between quantiles.
4. Discussion
Short and long-term exposure to environmental chemicals including ambient air pollutants and pesticides have been shown to have negative respiratory health effects, particularly among people with asthma. We present a study examining the joint effects of AAPs and pesticides, as multipollutant exposures, on lung function among children with asthma in the San Joaquin Valley, a region with unique and diverse environmental exposure characteristics.
In this cross-sectional study, we observed a negative overall effect on FEV1 and FVC to exposure of a mixture of regionally prevalent AAPs and pesticides, and a slightly positive overall effect on FEF25-75. The associations with FEV1 and FVC were driven primarily by AAPs; NO2 in particular was identified as the most important contributor (highest conditional PIP). In contrast, pesticides (chiefly MeBr) were primarily driving the association with FEF25-75, though effect estimates were relatively smaller and less varied compared to the other models. Though these observations imply that the larger airways may be more sensitive to the environmental mixture characterized in this population compared to smaller airways, the null effect estimates from FEV1/FVC models suggest that the association between the AAP and pesticides and airway obstruction observed in this study was most likely marginal. In total, the analysis demonstrated substantial uncertainty (evidenced by credible intervals) with lack of statistical significance. However, it is critical to recognize the inverse relationship between the environmental pollutant mixture and lung volume measures, as persistently low lung function levels are associated with a risk of subsequent asthma morbidity, and future airway disease.52-54 These findings underscore the importance of reducing adverse early life environmental exposures with potential long-term respiratory health implications in this susceptible population.
The inconsistency in results based on outcome measure (lung volume (FEV1, FVC) versus expiratory flow (FEF25-75)) may be due to several factors, including small sample sizes, unmeasured or/and residual confounding. Differences between models could also be due to exposure pathway characteristics.55 For example, our findings may indicate differences in exposure window sensitivity: the effects of three month exposures may be different from traditional acute or chronic exposures.55,56 Differential findings may also be a function of asthma severity, as FEF25-75 and FEV1/FVC in the setting of a normal FEV1 have been linked to more severe asthma;18,57 a large proportion of our population had mild or moderate asthma at the time of outcome assessment. Finally, this difference in model results may be due to a difference in key exposures (pesticides vs. AAP vs. joint exposures). We observed that the main drivers for the FEF25-75 models differed from those for the FEV1 and FVC models. More refined epidemiological and toxicological analyses are required to conclusively relate effects of these kinds of exposures on specific spirometric measures.
Overall, our results are difficult to contextualize given the paucity of studies on ambient exposure mixtures and pediatric respiratory health in the multidomain context. However, there is some consistency between our results and several multipollutant air pollution health effect studies. For example, Ierodiakonou et al., recently reported negative associations between exposure to air pollution (O3, carbon monoxide, NO2, and sulfur dioxide concentrations) and lung function (including FEV1 and FVC) among children with asthma in a longitudinal study.58 These effects were observed with multiple short-term exposure windows including four-month averages. Similarly, in a longitudinal study of school children, Barraza-Villarreal and colleagues showed an inverse relationship between pulmonary function (FEV1 and FVC) and exposures to ozone and PM2.5 among children with asthma.59 Both multipollutant approaches used linear mixed models which tested the health effect of one pollutant (as the main predictor), while adjusting for exposure to the other pollutants, and fail to account for the nature of correlation between environmental exposures and possible nonlinear effects on the health outcome.24,27,60 By using BKMR, we evaluated for a potentially nonlinear and/or non-additive function of pollutant concentrations that effectively reflects the joint association of the exposure mixture with pediatric asthma outcomes.
The few pesticide-respiratory health effect studies among children adopt the single-pollutant approach, and have shown both negative and null effects of pesticide exposure on lung function.6,61,62 For example, a study by Raanan et al. provides evidence of significant FEV1 and FVC decreases with early life exposures to organophosphate pesticide in 7-year-old children in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) study.6 However, the authors explored early life exposure windows of children with no asthma, only in single-pollutant analyses. More recently, another study on children from the same CHAMACOS cohort showed that exposure to agricultural fumigants including MeBr resulted in no adverse respiratory effects.61 The authors again only conducted single-pollutant analyses, and do not explore complex and nonlinear synergistic effects that likely exist between other environmental exposures within and outside the domain of pesticides.
Our results contribute to the literature focused on exploring the association between multidomain exposures and health outcomes.63-65 In particular, it adds to our work on health effects of multidomain exposures as part of the Aggravating Factors of Asthma in a Rural Environment (AFARE) study. We previously observed significant associations between joint exposure to ozone, PM2.5 and OPs, and a biomarker of lung inflammation (leukotriene E4) among children with asthma65. We build on the multidomain approach by including more plausible exposures and using a validated clinical marker of respiratory health, while accounting for limitations associated with multipollutant analyses.
We highlighted high correlation between components of the mixture and nonlinear independent (main effects) associations with lung function parameters. In addition, there was evidence of complex and possibly non-synergistic interactions within the overall mixture, such that the potentiating effects of an exposure on another may be nullified by antagonizing effects from a third exposure (as evidenced by the relationship between MeBr, carbamates and ozone on FEF25-75). These are common types of complexities in the environmental epidemiology field, and while traditional models like generalized multivariable linear regression (using ordinary least squares) are easy to implement and interpret, these models have shortcomings in some regards. Results from our linear regression models provided quantification of the association between individual exposures and lung function in single exposure, co-pollutant and interaction models. In general, most of the results mirrored the exposure-response associations observed in BKMR models. However, linear regression models were unable to capture some of the non-linear associations; for example, the univariate effect of OP on FEV1 and FVC, or carbamates on FEF25-75. Further, the linear models were unable to quantitatively identify the pollutants driving the mixture, and the estimates obtained in multipollutant and interaction models may be unstable/unreliable in the presence of high correlation between exposure components, as observed in this study. Finally, linear regression models were also limited beyond quantifying the association between lung function and individual exposures; BKMR suitably provided answers to one of our main study objectives, evaluating the “overall” mixture effect on lung function.
The mechanisms for individual or joint effects of environmental exposures continue to be a subject of deliberation and research. Previously published studies of exposure to pesticides/AAPs and lung function have generally suggested that airway inflammation and hyperresponsiveness are important mechanistic features.2,35,66 However, toxicological mechanisms remain poorly understood. Given the limited understanding of the mechanistic pathways for these exposures, it may be that any joint effects are due to simultaneously present but differing mechanisms of action. Nevertheless, joint exposure to AAPs and pesticides is not uncommon, especially in regions such as the SJV where there is a high prevalence of children with asthma,16,17 and thus is relevant to consider in multipollutant frameworks.7
There are some limitations to the current study. First, we rely on area measurements of exposures from central ambient air quality monitors and the California Pesticide Use Report (PUR) data which fail to account for individual time-activity patterns and may result in exposure measurement error. This error would be expected to be non-differential with respect to the study outcome, and independent of any other biases, would likely drive effects toward null values. However, the use of residential addresses to determine exposures is a strength compared with studies using community-level data. We also use aggregated measures of exposures; the health effects of component chemicals (particularly for pesticides and particulate matter) may vary due to toxicological (and hence pathophysiologic) variations.67-69 More work may be needed to provide more accurate exposure estimates, although several studies have highlighted the health impacts using aggregated measures.5,6,22,70,71
We acknowledge that missing lung function data could result in bias, but the missing outcome data was likely random, and demographic characteristics of participants included in the study and those excluded were not appreciably different.
Although our study typifies the agricultural-urban interface, recruitment into the FACES cohort was geared towards capturing more urban exposures (within distance of the Fresno Super Site monitor) than agricultural pesticide exposures. Hence, it is likely that the effects of pesticide exposure in this cohort are less than representative in agricultural regions. Further, future studies should also focus on additional agricultural pesticides that have been shown to adversely affect respiratory health among children and adults.62,69 Similarly, extending the array of outcome measures to include self-reported respiratory symptoms, other measures of lung function, and biomarkers of exacerbation may help provide additional characterization of the respiratory effects of these multipollutant mixtures.
Lastly, as we are limited by PUR data to only 3-month exposures, we were unable to assess the effect of joint exposures at other short-term lags.
5. Conclusion
Our study addresses the respiratory health effects of exposure to a suite of important ambient air pollutants in the context of simultaneous exposure to regional agricultural pesticides, exploring multiple pollutant exposures in multiple exposure domains. Understanding how environmental exposures, particularly in the context of multidomain, multipollutant mixtures, influence pediatric asthma morbidity in uniquely exposed populations may ultimately inform regulatory policies to reduce these modifiable factors that contribute to disease burden.
Supplementary Material
Highlights.
Susceptible populations are exposed to a complex mixture of ambient pollutants.
We examined lung effects of simultaneous exposure to air pollutants and pesticides.
The pollutant mixture was associated with some reduced lung function measures.
Diverse multipollutant exposure mixtures may adversely influence asthma morbidity.
Acknowledgements
The authors acknowledge the support and contributions of the participating families, Liza Lutzker, the FACES staff and the community of Fresno.
Funding: This work was supported by the National Institutes of Health/ National Institute of Environmental Health Sciences grants K22ES023815 (PI: SM) and R01ES028811 (PI: Gennings).
Abbreviations:
- US EPA
United States Environmental Protection Agency
- SD
Standard Deviation
- CI
Confidence/Credible Interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary Material
Supplementary material to this article can be found online.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Snowden JM, Mortimer KM, Kang Dufour M-S, Tager IB. Population intervention models to estimate ambient NO2 health effects in children with asthma. J Expo Sci Environ Epidemiol 2015; 25: 567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet 2014; 383: 1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gale SL, Noth EM, Mann J, Balmes J, Hammond SK, Tager IB. Polycyclic aromatic hydrocarbon exposure and wheeze in a cohort of children with asthma in Fresno, CA. J Expo Sci Environ Epidemiol 2012; 22: 386–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padula AM, Balmes JR, Eisen EA, Mann J, Noth EM, Lurmann FW et al. Ambient polycyclic aromatic hydrocarbons and pulmonary function in children. J Expo Sci Environ Epidemiol 2015; 25: 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye M, Beach J, Martin JW, Senthilselvan A. Urinary Dialkyl Phosphate Concentrations and Lung Function Parameters in Adolescents and Adults: Results from the Canadian Health Measures Survey. Environ Health Perspect 2015; 124: 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raanan R, Balmes JR, Harley KG, Gunier RB, Magzamen S, Bradman A et al. Decreased lung function in 7-year-old children with early-life organophosphate exposure. Thorax 2016; 71: 148–153. [DOI] [PubMed] [Google Scholar]
- 7.Braun JM, Gennings C, Hauser R, Webster TF. What Can Epidemiological Studies Tell Us about the Impact of Chemical Mixtures on Human Health? Environ Health Perspect 2016; 124: A6–A9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vedal S, Kaufman JD. What Does Multi-Pollutant Air Pollution Research Mean? Am J Respir Crit Care Med 2011; 183: 4–6. [DOI] [PubMed] [Google Scholar]
- 9.Sexton K. Cumulative Risk Assessment: An Overview of Methodological Approaches for Evaluating Combined Health Effects from Exposure to Multiple Environmental Stressors. Int J Environ Res Public Health 2012; 9: 370–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menzie CA, MacDonell MM, Mumtaz M. A Phased Approach for Assessing Combined Effects from Multiple Stressors. Environ Health Perspect 2007; 115: 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. EPA. Using a Total Environment Framework (Built, Natural, Social Environments) to Assess Life-long Health Effects of Chemical Exposures. NCER Abstr. 2016. https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.rfatext/rfa_id/630 (accessed 25 Jan2019).
- 12.Health Effects Institute. Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. SPECIAL REPORT 17. Heal Eff Inst 2010.
- 13.Loftus C, Yost M, Sampson P, Arias G, Torres E, Vasquez VB et al. Regional PM2.5 and asthma morbidity in an agricultural community: A panel study. Environ Res 2015; 136: 505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keet CA, McCormack MC, Pollack CE, Peng RD, McGowan E, Matsui EC. Neighborhood poverty, urban residence, race/ethnicity, and asthma: Rethinking the inner-city asthma epidemic. J Allergy Clin Immunol 2015; 135: 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alcala E, Cisneros R, Capitman JA. Health care access, concentrated poverty, and pediatric asthma hospital care use in California’s San Joaquin Valley: A multilevel approach. J. Asthma 2017. doi: 10.1080/02770903.2017.1409234. [DOI] [PubMed] [Google Scholar]
- 16.Meng Y-Y, Rull RP, Wilhelm M, Lombardi C, Balmes J, Ritz B. Outdoor air pollution and uncontrolled asthma in the San Joaquin Valley, California. J Epidemiol Community Heal 2010; 64: 142–147. [DOI] [PubMed] [Google Scholar]
- 17.Tager IB, Lurmann FW, Haight T, Alcorn S, Penfold B, Hammond SK. Temporal and Spatial Patterns of Ambient Endotoxin Concentrations in Fresno, California. Environ Health Perspect 2010; 118: 1490–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortimer K, Neugebauer R, Lurmann F, Alcorn S, Balmes J, Tager I. Air Pollution and Pulmonary Function in Asthmatic Children. Epidemiology 2008; 19: 550–557. [DOI] [PubMed] [Google Scholar]
- 19.Mann JK, Balmes JR, Bruckner TA, Mortimer KM, Margolis HG, Pratt B et al. Short-Term Effects of Air Pollution on Wheeze in Asthmatic Children in Fresno, California. Environ Health Perspect 2010; 118: 1497–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee S, McLaughlin R, Harnly M, Gunier R, Kreutzer R. Community exposures to airborne agricultural pesticides in California: Ranking of inhalation risks. Environ Health Perspect 2002; 110: 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts EM, English PB, Grether JK, Windham GC, Somberg L, Wolff C. Maternal Residence Near Agricultural Pesticide Applications and Autism Spectrum Disorders Among Children in the California Central Valley. Environ Health Perspect 2007; 1482: 1482–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen AE, Gaines SD, Deschênes O. Agricultural pesticide use and adverse birth outcomes in the San Joaquin Valley of California. Nat Commun 2017; 8: 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang W, Carmichael SL, Roberts EM, Kegley SE, Padula AM, English PB et al. Residential Agricultural Pesticide Exposures and Risk of Neural Tube Defects and Orofacial Clefts Among Offspring in the San Joaquin Valley of California. Am J Epidemiol 2014; 179: 740–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2014; 16: 493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bobb JF, Claus Henn B, Valeri L, Coull BA. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Heal 2018; 17: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA et al. The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20–40 Months of Age: Evidence from Rural Bangladesh. Environ Health Perspect 2017; 125: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coull BA, Bobb JF, Wellenius GA, Kioumourtzoglou M-A, Mittleman MA, Koutrakis P et al. Part 1. Statistical Learning Methods for the Effects of Multiple Air Pollution Constituents. Res Rep Health Eff Inst 2015; : 5–50. [PubMed] [Google Scholar]
- 28.Margolis HG, Mann JK, Lurmann FW, Mortimer KM, Balmes JR, Hammond SK et al. Altered pulmonary function in children with asthma associated with highway traffic near residence. Int J Environ Health Res 2009; 19: 139–155. [DOI] [PubMed] [Google Scholar]
- 29.Watson JG, Chow JC, Bowen JL, Lowenthal DH, Hering S, Ouchida P et al. Air Quality Measurements from the Fresno Supersite. J Air Waste Manage Assoc 2000; 50: 1321–1334. [DOI] [PubMed] [Google Scholar]
- 30.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999; 159: 179–87. [DOI] [PubMed] [Google Scholar]
- 31.Kloog I, Chudnovsky AA, Just AC, Nordio F, Koutrakis P, Coull BA et al. A new hybrid spatio-temporal model for estimating daily multi-year PM2.5 concentrations across northeastern USA using high resolution aerosol optical depth data. Atmos Environ 2014; 95: 581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDPR (California Department of Pesticide Regulation). California Pesticide Use Reporting Data. 2015https://www.cdpr.ca.gov/docs/pur/purmain.htm (accessed 3 Dec2017).
- 33.Colovic MB, Krstic DZ, Lazarevic-Pasti TD, Bondzic AM, Vasic VM. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr Neuropharmacol 2013; 11: 315–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulathsinghala A, Shaw I. The toxic chemistry of methyl bromide. Hum Exp Toxicol 2014; 33: 81–91. [DOI] [PubMed] [Google Scholar]
- 35.Hernández AF, Casado I, Pena G, Gil F, Villanueva E, Pla A. Low Level of Exposure to Pesticides Leads to Lung Dysfunction in Occupationally Exposed Subjects. Inhal Toxicol 2008; 20: 839–849. [DOI] [PubMed] [Google Scholar]
- 36.Severson R. purexposure: Pull and Calculate Exposure to CA Pesticide Use Registry Records. 2018https://github.com/leighseverson/purexposure.
- 37.Gunier RB, Harnly ME, Reynolds P, Hertz A, Von Behren J. Agricultural Pesticide Use in California: Pesticide Prioritization, Use Densities, and Population Distributions for a Childhood Cancer Study. Environ Health Perspect 2001; 109: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teske ME, Bird SL, Esterly DM, Curbishley TB, Ray SL, Perry SG. AgDrift®: A model for estimating near-field spray drift from aerial applications. Environ Toxicol Chem 2002; 21: 659–671. [DOI] [PubMed] [Google Scholar]
- 39.Gemmill A, Gunier RB, Bradman A, Eskenazi B, Harley KG. Residential proximity to methyl bromide use and birth outcomes in an agricultural population in California. Environ Health Perspect 2013; 121: 737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Johnson B, Segawa R. Empirical relationship between use, area, and ambient air concentration of methyl bromide. J Environ Qual 2006; 34: 420–8. [PubMed] [Google Scholar]
- 41.Bobb JF. bkmr: Bayesian Kernel Machine Regression. 2017https://cran.r-project.org/package=bkmr.
- 42.Torrent M, Sunyer J, Garcia R, Harris J, Iturriaga MV, Puig C et al. Early-Life Allergen Exposure and Atopy, Asthma, and Wheeze up to 6 Years of Age. Am J Respir Crit Care Med 2007; 176: 446–453. [DOI] [PubMed] [Google Scholar]
- 43.Li S, Batterman S, Wasilevich E, Wahl R, Wirth J, Su F-C et al. Association of daily asthma emergency department visits and hospital admissions with ambient air pollutants among the pediatric Medicaid population in Detroit: Time-series and time-stratified case-crossover analyses with threshold effects. Environ Res 2011; 111: 1137–1147. [DOI] [PubMed] [Google Scholar]
- 44.Coker E, Liverani S, Su JG, Molitor J. Multi-pollutant Modeling Through Examination of Susceptible Subpopulations Using Profile Regression. Curr Environ Heal Reports 2018; : 59–69. [DOI] [PubMed] [Google Scholar]
- 45.Billionnet C, Gay E, Kirchner S, Leynaert B, Annesi-Maesano I. Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environ Res 2011; 111: 425–434. [DOI] [PubMed] [Google Scholar]
- 46.Coker E, Gunier R, Bradman A, Harley K, Kogut K, Molitor J et al. Association between Pesticide Profiles Used on Agricultural Fields near Maternal Residences during Pregnancy and IQ at Age 7 Years. Int J Environ Res Public Health 2017; 14: 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henn BC, Schnaas L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, Hernández-Avila M et al. Associations of Early Childhood Manganese and Lead Coexposure with Neurodevelopment. Environ Health Perspect 2012; 120: 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kupsco A, Kioumourtzoglou M-A, Just AC, Amarasiriwardena C, Estrada-Gutierrez G, Cantoral A et al. Prenatal Metal Concentrations and Childhood Cardiometabolic Risk Using Bayesian Kernel Machine Regression to Assess Mixture and Interaction Effects. Epidemiology 2019; 30: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu JC, Peng RD. Health effect of mixtures of ozone, nitrogen dioxide, and fine particulates in 85 US counties. Air Qual Atmos Heal 2018. doi: 10.1007/s11869-017-0544-2. [DOI] [Google Scholar]
- 50.Berger JO, Barbieri MM. Optimal predictive model selection. Ann Stat 2004; 32: 870–897. [Google Scholar]
- 51.Coker E, Chevrier J, Rauch S, Bradman A, Obida M, Crause M et al. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environ Int 2018; 113: 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tantisira K, Fuhlbrigge A, Tonascia J, Vannatta M, Zeiger R, Strunk R et al. Bronchodilation and bronchoconstriction: Predictors of future lung function in childhood asthma. J Allergy Clin Immunol 2006; 117: 1264–1271. [DOI] [PubMed] [Google Scholar]
- 53.Versiani Nunes Pinheiro de Queiroz M, Gonçalves Alvim C, Cruz ÁA, de Lima Belizário Facury Lasmar LM. Lung function in severe pediatric asthma: a longitudinal study in children and adolescents in Brazil. Clin Transl Allergy 2017; 7: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G et al. After asthma: redefining airways diseases. Lancet 2018; 391: 350–400. [DOI] [PubMed] [Google Scholar]
- 55.Götschi T, Heinrich J, Sunyer J, Künzli N. Long-Term Effects of Ambient Air Pollution on Lung Function. Epidemiology 2008; 19: 690–701. [DOI] [PubMed] [Google Scholar]
- 56.He Q-Q, Wong TW, Du L, Jiang Z-Q, Gao Y, Qiu H et al. Effects of ambient air pollution on lung function growth in Chinese schoolchildren. Respir Med 2010; 104: 1512–1520. [DOI] [PubMed] [Google Scholar]
- 57.Piccioni P, Tassinari R, Carosso A, Carena C, Bugiani M, Bono R. Lung function changes from childhood to adolescence: a seven-year follow-up study. BMC Pulm Med 2015; 15: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ierodiakonou D, Zanobetti A, Coull BA, Melly S, Postma DS, Boezen HM et al. Ambient air pollution, lung function, and airway responsiveness in asthmatic children. J Allergy Clin Immunol 2016; 137: 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barraza-Villarreal A, Sunyer J, Hernandez-Cadena L, Escamilla-Nuñez MC, Sienra-Monge JJ, Ramírez-Aguilar M et al. Air Pollution, Airway Inflammation, and Lung Function in a Cohort Study of Mexico City Schoolchildren. Environ Health Perspect 2008; 116: 832–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mauderly JL, Burnett RT, Castillejos M, Özkaynak H, Samet JM, Stieb DM et al. Is the air pollution health research community prepared to support a multipollutant air quality management framework? Inhal Toxicol 2010; 22: 1–19. [DOI] [PubMed] [Google Scholar]
- 61.Gunier RB, Raanan R, Castorina R, Holland NT, Harley KG, Balmes JR et al. Residential proximity to agricultural fumigant use and respiratory health in 7-year old children. Environ Res 2018; 164: 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raanan R, Gunier RB, Balmes JR, Beltran AJ, Harley KG, Bradman A et al. Elemental Sulfur Use and Associations with Pediatric Lung Function and Respiratory Symptoms in an Agricultural Community (California, USA). Environ Health Perspect 2017; 125: 087007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Czarnota J, Gennings C, Colt JS, De Roos AJ, Cerhan JR, Severson RK et al. Analysis of Environmental Chemical Mixtures and Non-Hodgkin Lymphoma Risk in the NCI-SEER NHL Study. Environ Health Perspect 2015; 123: 965–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y, Dong T, Hu W, Wang X, Xu B, Lin Z et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ Int 2019; 123: 325–336. [DOI] [PubMed] [Google Scholar]
- 65.Benka-Coker WO, Loftus C, Karr C, Magzamen S. Characterizing the joint effects of pesticide exposure and criteria ambient air pollutants on pediatric asthma morbidity in an agricultural community. Environ Epidemiol 2019; : 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fryer AD. Mechanisms of organophosphate insecticide-induced airway hyperreactivity. AJP Lung Cell Mol Physiol 2003; 286: L963–L969. [DOI] [PubMed] [Google Scholar]
- 67.Yang Y, Pun VC, Sun S, Lin H, Mason TG, Qiu H. Particulate matter components and health: a literature review on exposure assessment. J Public Heal Emerg 2018; 2: 14–14. [Google Scholar]
- 68.Adams K, Greenbaum DS, Shaikh R, van Erp AM, Russell AG. Particulate matter components, sources, and health: Systematic approaches to testing effects. J Air Waste Manage Assoc 2015; 65: 544–558. [DOI] [PubMed] [Google Scholar]
- 69.Hoppin J a., Umbach DM, London SJ, Henneberger PK, Kullman GJ, Alavanja MCR et al. Pesticides and atopic and nonatopic asthma among farm women in the agricultural health study. Am J Respir Crit Care Med 2008; 177: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect 1999; 107: 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benka-Coker W, Loftus C, Karr C, Magzamen S. Association of Organophosphate Pesticide Exposure and a Marker of Asthma Morbidity in an Agricultural Community. J Agromedicine 2019; : 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


