Abstract
In response to the coronavirus disease 2019 (COVID-19) pandemic, the U.S. Surgeon General advised all hospitals and ambulatory care centers to delay nonurgent medical procedures and surgeries. This recommendation, echoed by a multigastroenterology society guideline, led to the suspension of colonoscopies for colorectal cancer (CRC) screening and surveillance. Although this temporary suspension was necessary to contain COVID-19 infections, we as gastroenterologists, patient advocates, and CRC researchers have witnessed the downstream impact of COVID-19 and this recommendation on CRC screening, research, and advocacy. These effects are particularly noticeable in medically underserved communities where CRC morbidity and mortality are highest. COVID-19–related pauses in medical care, as well as shifts in resource allocation and workforce deployment, threaten decades worth of work to improve CRC disparities in medically underserved populations. In this perspective, we present the unique challenges COVID-19 poses to health equity in CRC prevention and provide potential solutions as we navigate these uncharted waters.
Abbreviations: CBPR, community-based participatory research; CRC, colorectal cancer; FIT, fecal immunochemical test; FOBT, fecal occult blood test; FQHC, federally qualified health centers
Graphical abstract
The coronavirus disease 2019 (COVID-19) has led to a global pandemic, with over 7.5 million infected and 420,000 deaths.1 This healthcare crisis has also underscored persistent inequities, including the highest rates of COVID-19 infections and deaths in African American and Hispanic communities.2 , 3 Although minimizing morbidity and mortality from the disease remains the top priority of clinicians, researchers, and policymakers, it is important to rigorously assess and address the profound clinical and public health impact of COVID-19 on other aspects of health and health care.
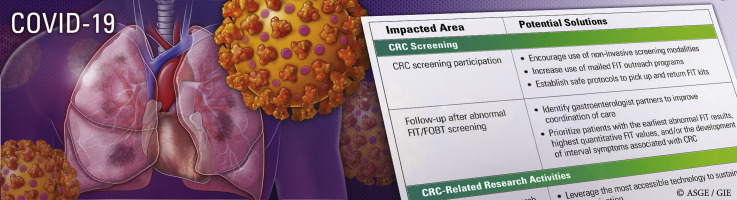
Colorectal cancer (CRC) provides a valuable lens through which we can view the deleterious effects of COVID-19 on non-COVID-19–related diseases in medically underserved populations, defined as groups that have experienced health and healthcare inequities because of social determinants of health.4, 5, 6, 7 CRC is the most common GI malignancy and disproportionately affects medically underserved populations. Specifically, African Americans and Native Americans have the highest incidence of CRC8; African Americans have the highest rate of CRC-related death,8 and Hispanics have CRC screening rates far below whites and African Americans.8 Some progress has been made to increase awareness and implement interventions that address inequities in CRC screening and outcomes, but there is more work to be done. In this perspective we highlight how COVID-19 threatens to undo this progress and offer potential solutions to these challenges during the COVID-19 era (Table 1 ).
Table 1.
Summary of areas related to CRC prevention in the medically underserved that have been impacted by COVID-19 and potential solutions
| Impacted area | Potential solutions |
|---|---|
| CRC screening | |
| CRC screening participation |
|
| Follow-up after abnormal FIT/fecal occult blood test screening |
|
| CRC-related research activities | |
| Community-based research |
|
| External factors |
|
| Engagement, advocacy, and policy | |
| Community outreach and engagement |
|
| Advocacy and policy |
|
CRC, Colorectal cancer; COVID-19, coronavirus disease 2019; FIT, fecal immunochemical test.
CRC screening for the underserved
COVID-19 has substantially impacted CRC screening programs in federally qualified health centers (FQHCs) and other resource-constrained settings. A mandatory shift in priorities to contain and mitigate COVID-19 sharply reduced clinical visits and the capacity to provide nonurgent primary care and endoscopic services.9 As a result, the volume of CRC screening dropped substantially—by up to 86% according to an early estimate.10 For medically underserved populations with already limited access to preventive health services, completing CRC screening is an even greater challenge now and will remain so for the foreseeable future.
Federally qualified health centers
FQHCs in the United States are funded by the federal government to provide primary care and preventive services to over 22 million low-income, uninsured, and underinsured individuals annually. In FQHCs and other resource-constrained settings, the fecal immunochemical test (FIT) and fecal occult blood test (FOBT) are the preferred screening modalities because of low cost, availability, and high patient participation.11 Because of social-distancing policies, clinics that previously required in-person pickup and/or return of FIT/FOBT for processing either have temporarily halted CRC screening or are determining new ways to screen without placing patients at risk for contracting COVID-19. Although mailed FIT outreach is an evidence-based strategy to improve CRC screening participation, such programs are sparse among FQHCs given upfront financial costs and hurdles to successful implementation.12, 13, 14
As we emerge from the immediate aftermath of the pandemic, FQHCs should consider implementing mailed FIT outreach programs, which can be funded internally or through external sources related to COVID-19 relief. In FQHCs where mailing noninvasive screening tests is not an option, mechanisms to establish safe protocols for patients to pick up and return FIT/FOBT kits, which might include contact-free drop-off boxes for FIT kits, should be used.
Follow-up after abnormal FIT/FOBT
CRC screening with FIT/FOBT is effective when patients with abnormal (ie, positive) results complete a colonoscopy to assess for precancerous and cancerous lesions.11 , 15 A longer time to colonoscopy after an abnormal FIT result is associated with a higher risk of CRC and more advanced stage of disease.16 For the 5% to 14% of FQHC patients with abnormal FIT results, colonoscopy coordination with outside gastroenterologists and health centers is currently challenging because of reductions in endoscopy capacity, strains on health systems, and inadequate health insurance coverage.11 , 17, 18, 19, 20, 21, 22 Until endoscopy units regain full capacity, FQHCs must identify gastroenterology partners willing to assist in care coordination until colonoscopy completion. FQHCs and gastroenterology partners can stratify patients with the highest CRC risk by prioritizing those with the earliest abnormal FIT results, the highest quantitative FIT values, and/or those who have developed interval symptoms associated with CRC.11 , 13 , 16
CRC research in the underserved
Research studies have highlighted that individuals from medically underserved populations are less likely to receive counseling to complete screening,23 have less access to preventive care and CRC treatment after diagnosis,24 are less likely to complete diagnostic colonoscopy after abnormal FIT,20 and are also less likely to receive colonoscopy surveillance after curative CRC treatment.25 26 Given the impact of COVID-19 on our research activities, we propose the following solutions for individuals facing similar challenges (Table 1).
Community-based research
Community-based participatory research is a frequently used research tool that builds strong partnerships between research teams (often within academic medical centers) and clinical practices in the community. Community-based participatory research ensures that the communities most affected by diseases of interest are represented through collaboration, shared decision-making power, and mutual ownership of the research process and products.27 However, increased use of telehealth to limit spread of COVID-19 has substantially limited community-based participatory research efforts and recruitment of patients for CRC screening studies. Among medically underserved populations, research participation is further challenged by less access to digital platforms, disproportionate unemployment, unstable housing, and, in some cases, the need to continue to work as an essential worker.28, 29, 30
To protect medically underserved populations from COVID-19 and maintain progress in CRC disparities research, we propose several recommendations. Whenever possible, research activities should use the most accessible form of technology (eg, prioritizing use of telephones over video conferencing for meetings) and conducted at times most convenient for participants (eg, before or after typical work hours). CRC investigators should also request signature waivers for projects that involve minimal risk as determined by local institutional review boards. As the number of COVID-19 cases decline, CRC researchers should use a tiered approach to re-engage community partners. An example might begin with communication to express solidarity and assess readiness followed by an offer to use available digital platforms to reignite research projects if interest remains. Engagement with community partners should acknowledge their time with reasonable incentives whenever possible. Our own work has signaled that community partners are eager to re-engage in prevention research because of the potential impact of COVID-19 screening interruptions on CRC outcomes.
External factors
Many specialists who are board-certified in internal medicine were called to assist healthcare systems during the peak of the pandemic. Although appropriately answering this call, doing so meant time away from usual research activities. Solutions to these challenges include informing funding agencies of changes in clinical practice that affect usual protected research time and developing budgetary scenarios should COVID-19 delay planned research activities by 6 months, 12 months, or more.
Engagement, advocacy, and policy
Community engagement in advocacy, policy, and outreach are critical to increasing public knowledge about CRC in medically underserved communities. COVID-19 has disrupted community engagement activities at local and national levels, raising concern that the momentum to address disparities will decline. We advocate for creative tactics from influential organizations, national medical societies, health systems, and policymakers to offset awareness and advocacy efforts affected by the pandemic (Table 1).
Community outreach and engagement
In 2000 the “Katie Couric effect” was described after Couric completed a screening colonoscopy on national television.31 , 32 Earlier this year, actor Will Smith shared a video about his colonoscopy experience and encouraged all, but particularly African American men, to do the same.33 Unfortunately, the impact of Smith’s video is unlikely to materialize because activities that would have cemented the importance of this act, including educational tours through inflatable colons and low-to-no cost screening colonoscopy programs during Colorectal Cancer Awareness Month, were canceled because of COVID-19.
The absence of community outreach and engagement events in 2020 presents an unforeseen challenge in relaying the importance of CRC screening to vulnerable communities. African Americans, Native Americans, and Hispanics, who are at highest risk for CRC, are also at highest risk of contracting and dying from COVID-19.2 , 3 , 34 In the midst of the COVID-19 pandemic, the death of George Floyd and many other unarmed African Americans has ignited civil unrest and activism around the country. It is of no surprise that these communities would be hesitant to engage in CRC screening at this time. However, as cancer persists despite these challenges, we must acknowledge these immediate apprehensions and work within our organizations to contribute solutions that address systemic racism while delivering a balanced message about the importance of CRC screening. Cognizant of this reality, the Colorectal Cancer Alliance transitioned financial assistance programs intended to provide CRC screening and care into a financial assistance fund for low-income individuals with COVID-19.35 Institutions and organizations involved in community outreach and engagement must also develop strategies to foster trust and re-engage medically underserved communities in CRC screening.
Advocacy and policy
Legislative support brings national visibility to CRC prevention and results in policy changes that are vital to decrease disparities. Several Washington, DC lobby events organized by gastroenterology societies, nonprofit organizations, and advocacy groups during Colorectal Cancer Awareness Month were canceled. Among these, the 2020 Fight CRC’s Call-on-Congress―a 3-day advocacy event to advocate for increased funding for expanded screening services in medically underserved communities―transitioned its event to a virtual forum. In light of COVID-19, we must harness the power of virtual platforms and social media for advocacy events and policy campaigns whenever possible. Although a physical presence on Capitol Hill has strong impact, we must continue to promote our messages through alternative avenues until we can safely resume in-person advocacy efforts.
Conclusion
As gastroenterologists, researchers, and patient advocates, we have observed the unequal impact that COVID-19 has had on medically underserved populations and now prepare for the likely downstream effects on CRC prevention through reduced access to care, suspended research efforts, and limited opportunities for community outreach and engagement and advocacy. The only way to avoid an exacerbation of CRC disparities because of COVID-19 is to devise a new way forward. It is critical that we take actionable steps to preserve existing efforts and cultivate new tactics that maintain momentum in CRC prevention. In the likely event that COVID-19 remains a backdrop for all healthcare until vaccines and treatments are available, we look forward to working with community partners, health institutions, and professional societies to develop strategies to improve long-standing disparities in CRC incidence, screening, and outcomes.
Disclosure
The following authors received research support for this study from the National Institutes of Health/National Cancer Institute: R. B. Issaka (award number K08CA241296), F. P. May (award number R03CA230947); National Institutes of Health/National Institute on Aging (award number R21AG061496) and the National Cancer Institute (award number UG3 CA233282): D. M. Gray II; and Tobacco-Related Disease Research Program (award number TRDRP 587791): F. P. May. All other authors disclosed no financial relationships.
References
- 1.Worldometer COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus/ Available at:
- 2.Choo E.K. COVID-19 fault lines. Lancet. 2020;395:1333. doi: 10.1016/S0140-6736(20)30812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibbins-Domingo K. This time must be different: disparities during the COVID-19 pandemic. Ann Intern Med. Epub 2020 Apr 28. [DOI] [PMC free article] [PubMed]
- 4.Siegel R.L., Miller K.D., Fedewa S.A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 5.Carethers J.M., Doubeni C.A. Causes of socioeconomic disparities in colorectal cancer and intervention framework and strategies. Gastroenterology. 2020;158:354–367. doi: 10.1053/j.gastro.2019.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Klerk C.M., Gupta S., Dekker E. Socioeconomic and ethnic inequities within organised colorectal cancer screening programmes worldwide. Gut. 2018;67:679–687. doi: 10.1136/gutjnl-2016-313311. [DOI] [PubMed] [Google Scholar]
- 7.Liss D.T., Baker D.W. Understanding current racial/ethnic disparities in colorectal cancer screening in the United States: the contribution of socioeconomic status and access to care. Am J Prev Med. 2014;46:228–236. doi: 10.1016/j.amepre.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 8.American Cancer Society . American Cancer Society; Atlanta, GA: 2020. Colorectal cancer facts & figures 2020-2022. [Google Scholar]
- 9.Mehrotra A, Chernew M, Linetsky D, et al. What impact has COVID-19 had on outpatient visits? To the Point (blog), Commonwealth Fund, April, 23, 2020. Available at: 10.26099/ds9e-jm36. [DOI]
- 10.EPIC Health Research Network Preventive cancer screenings during COVID-19 pandemic. https://ehrn.org/wp-content/uploads/Preventive-Cancer-Screenings-during-COVID-19-Pandemic.pdf Available at:
- 11.Robertson D.J., Lee J.K., Boland C.R. Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: a consensus statement by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2017;112:37–53. doi: 10.1038/ajg.2016.492. [DOI] [PubMed] [Google Scholar]
- 12.Singal A.G., Gupta S., Tiro J.A. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: a randomized controlled trial in a safety-net health system. Cancer. 2016;122:456–463. doi: 10.1002/cncr.29770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selby K., Baumgartner C., Levin T.R. Interventions to improve follow-up of positive results on fecal blood tests: a systematic review. Ann Intern Med. 2017;167:565–575. doi: 10.7326/M17-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coronado G.D., Petrik A.F., Vollmer W.M. Effectiveness of a mailed colorectal cancer screening outreach program in community health clinics: the STOP CRC Cluster randomized clinical trial. JAMA Intern Med. 2018;178:1174–1181. doi: 10.1001/jamainternmed.2018.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bibbins-Domingo K., Grossman D.C., Curry S.J. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2016;315:2564–2575. doi: 10.1001/jama.2016.5989. Accessed April 27, 2020. [DOI] [PubMed] [Google Scholar]
- 16.Corley D.A., Jensen C.D., Quinn V.P. association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA. 2017;317:1631–1641. doi: 10.1001/jama.2017.3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin J., Halm E.A., Tiro J.A. Reasons for lack of diagnostic colonoscopy after positive result on fecal immunochemical test in a safety-net health system. Am J Med. 2017;130:93. doi: 10.1016/j.amjmed.2016.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarfaty M., Doroshenk M., Hotz J. Strategies for expanding colorectal cancer screening at community health centers. CA Cancer J Clin. 2013;63:221–231. doi: 10.3322/caac.21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oluloro A., Petrik A.F., Turner A. Timeliness of colonoscopy after abnormal fecal test results in a safety net practice. J Commun Health. 2016;41:864–870. doi: 10.1007/s10900-016-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Issaka R.B., Singh M.H., Oshima S.M. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net system. Am J Gastroenterol. 2017;112:375–382. doi: 10.1038/ajg.2016.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thamarasseril S., Bhuket T., Chan C. The need for an integrated patient navigation pathway to improve access to colonoscopy after positive fecal immunochemical testing: a safety-net hospital experience. J Commun Health. 2017;42:551–557. doi: 10.1007/s10900-016-0287-2. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy A.M., Kim J.J., Beaber E.F. Follow-up of abnormal breast and colorectal cancer screening by race/ethnicity. Am J Prev Med. 2016;51:507–512. doi: 10.1016/j.amepre.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May F.P., Almario C.V., Ponce N. Racial minorities are more likely than whites to report lack of provider recommendation for colon cancer screening. Am J Gastroenterol. 2015;110:1388–1394. doi: 10.1038/ajg.2015.138. [DOI] [PubMed] [Google Scholar]
- 24.Yabroff K.R., Gansler T., Wender R.C. Minimizing the burden of cancer in the United States: Goals for a high-performing health care system. CA Cancer J Clin. 2019;69:166–183. doi: 10.3322/caac.21556. [DOI] [PubMed] [Google Scholar]
- 25.Salz T., Weinberger M., Ayanian J.Z. Variation in use of surveillance colonoscopy among colorectal cancer survivors in the United States. BMC Health Serv Res. 2010;10:256. doi: 10.1186/1472-6963-10-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neugut A.I., Zhong X., Lebwohl B. Adherence to colonoscopy at 1 year following resection of localized colon cancer: a retrospective cohort study. Therap Adv Gastroenterol. Epub. 2018 Mar 26 doi: 10.1177/1756284818765920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasick R., Oliva G., Goldstein E. Community-engaged research with community-based organizations: a resource manual for UCSF researchers. In: Fleisher P., editor. UCSF Clinical and Translational Science Institute (CTSI) resource manuals and guides to community-engaged research. University of California San Francisco; San Francisco, CA: 2010. p. 1-27. [Google Scholar]
- 28.United States Department of Labor, Washington DC, USA. U.S. Bureau of Labor Statistics . 2010. (Labor force statistics from the current population survey). Available at: https://www.bls.gov/cps/. Accessed July 2, 2020. [Google Scholar]
- 29.Honein-AbouHaidar G.N., Kastner M., Vuong V. Systematic review and meta-study synthesis of qualitative studies evaluating facilitators and barriers to participation in colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2016;25:907–917. doi: 10.1158/1055-9965.EPI-15-0990. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention COVID-19 in racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html Available at:
- 31.He J., Efron J.E. Screening for colorectal cancer. Adv Surg. 2011;45:31–44. doi: 10.1016/j.yasu.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Cram P., Fendrick A.M., Inadomi J. The impact of a celebrity promotional campaign on the use of colon cancer screening: the Katie Couric effect. Arch Intern Med. 2003;163:1601–1605. doi: 10.1001/archinte.163.13.1601. [DOI] [PubMed] [Google Scholar]
- 33.Balzora S., Gray D. Everyone needs to see this: Will Smith gets a colonoscopy. Healio Gastroenterology. https://www.healio.com/gastroenterology/interventional-endoscopy/news/online/%7B5352c995-4de5-4360-89e1-7d0f071aba0d%7D/everyone-needs-to-see-this-will-smith-gets-a-colonoscopy Available at:
- 34.Centers for Disease Control and Prevention Cases of coronavirus disease (COVID-19) in the U.S. www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Available at:
- 35.Colorectal Cancer Alliance Screening and financial assistance. https://www.ccalliance.org/patient-family-support/financial-assistance-programs Available at: