Abstract
Introduction
Early excision and grafting remains the standard of care after burn injury. However, in a resource-limited setting, operative capacity often limits patient access to surgical intervention. This study sought to describe access to excision and grafting for adult burn patients in a sub-Saharan African burn unit and its relationship with burn-associated mortality.
Methods
We analyzed patients recorded in the Kamuzu Central Hospital Burn Registry in Lilongwe, Malawi from 2011–2019. We examined patient characteristics, interventions, and outcomes for adults aged ≥ 16 years. Modified Poisson regression modeling was used to identify risk factors for mortality.
Results
573 patients were included. Median age was 30 years (IQR 23–40) with a male preponderance (63%). Median percent total body surface area burned (%TBSA) was 15% (IQR 8–26) and 68% of burns were caused by flame. 27% (n=154) had burn excision with skin grafting, with a median time to operation of 18 days (IQR 9–38). When adjusted for age, %TBSA, and time to presentation, operative intervention conferred a survival benefit for patients with flame burns with a RR 0.16 (95% CI 0.06, 0.42).
Conclusions
In a resource-limiting setting, access to the operating room is inadequate, and burn patients are not prioritized. While many scald burn patients may be managed with wound care alone, patients with flame burn require surgical intervention to improve clinical outcomes. Burn injury in this region continues to confer a high risk of mortality, and more investment in operative capacity is imperative.
Keywords: burn, critical care, global surgery, adult
Introduction
Burns are tissue damage resulting from overexposure to heat, radiation, chemical, or electrical contact. They are a cause of significant mortality, morbidity from disability, and financial loss. Annually, burns are estimated to cause approximately 180,000 deaths worldwide, mostly in low- to middle-income countries (LMICs), which lack sufficient healthcare resources, both human and financial, to manage the complex needs of burn patients.[1, 2] Nearly two-thirds of the total burden of burns in LMICs occur in sub-Saharan Africa (SSA), which is disproportionally affected due to the high use of floor-level open fire for cooking, leading to a majority of burn injuries occurring in the home and putting both children and adults at high risk.[3–7]
Partial- and full-thickness burns, such as those commonly suffered after flame injury, present a special challenge in resource-limited environments as these burns often require surgical intervention rather than simple wound care. Evidence has demonstrated that early excision of burn wound eschar followed by coverage with a temporary biological dressing or autograft is superior to local wound care for patient outcomes.[8–10] Consequently, excision of non-viable tissue with surgical coverage has become the standard of care for burn management. With burn-associated mortality in SSA as high as 15–20%, interventions such as excision and grafting that improve morbidity and mortality are essential.[6] Unfortunately, available evidence suggests that access to this procedure is minimal in many LMICs.[11, 12]
Limited data exists from SSA on the epidemiology of adult burn patients and most published studies focus on pediatric patients.[6] In addition, there is minimal literature on the effect that limited access to operative intervention has on burn injury outcomes in adult patients. Consequently, we sought to describe the relationship between surgical excision and skin grafting and burn-associated mortality in an adult burn cohort in a resource-limited setting. We hypothesize that access to surgical intervention would reduce adult burn-related mortality at our center.
Methods
This study is a retrospective analysis of prospectively collected burn registry data at Kamuzu Central Hospital (KCH), a public, 900-bed tertiary care hospital in the capital city of Lilongwe, which serves as a referral center for approximately 6 million people in the central region of Malawi. The KCH Burn Unit was established in 2011 and has an average of 25–40 admissions per month.[13] Pediatric and adult patients are admitted to separate areas of the same unit. The KCH Burn Unit is a 31-bed unit with five full-time nurses, two trained clinical officers, and a consultant plastic surgeon. The burn registry was established in June 2011 to record patient information, including demographics, burn injury characteristics, type of operative intervention, and clinical outcomes.
In this study, patients were included if they were ≥ 16 years old and were admitted to the KCH Burn Unit between June 2011 and December 2019. The primary aim of this study was to describe the relationship between operative intervention and mortality among adult burn patients. The primary outcome was all-cause, in-hospital mortality among adult burn patients admitted to the KCH Burn Unit. Operative intervention was defined as those who underwent burn wound excision with skin grafting.
We initially examined the characteristics of the study cohorts, comparing patients who underwent operative intervention to those who did not. To identify potential confounders of the relationship between the use of excision and grafting of burn injury and mortality, we examined available independent variables. We used bivariate analysis to evaluate the relationship between each of the independent variables and in-hospital mortality. Chi-squared tests were utilized for the categorical variables and 2-sample t-tests for continuous variables. Medians of non-normally distributed continuous variables were tested using a Kruskal-Wallis test. Means were reported with standard deviations (SD) and medians with interquartile ranges (IQR). Overall crude mortality was calculated using all deaths in the study population.
We used a modified Poisson regression model to estimate the risk ratio for burn-associated mortality for patients who received burn excision and grafting versus those who did not, adjusting for confounders.[14, 15] After creating the initial model with mortality and potential confounders to the outcome, we used a change-in-effect method to remove covariates that did not substantially alter the relationship between the operative intervention and mortality. In addition, we tested for interaction in the model and reported those results. An unadjusted and adjusted risk ratio and 95% confidence interval are reported from the estimates of the final model. We also used a logistic regression model to estimate the predicted probability of death for each of the exposure groups.
All statistical analysis was performed using Stata/SE 16.0 (Stata- Corp LP, College Station, TX). Ethics approval was obtained from the University of North Carolina Institutional Review Board and the Malawi National Health Sciences Research Committee.
Results
During the study period, 573 adult patients were admitted to the KCH Burn Unit. The median age was 30 years (IQR 23–40), with a male preponderance (63%). Most patients had flame-associated burns (n=388, 68.0%) compared to either scald (n=152, 26.6%) or other mechanisms (n=31, 5.4%). Median percent total body surface area burned (%TBSA) was 15% (IQR 8–26%). 154 patients had burn excision and skin grafting (26.9%).
Table 1 compares those who underwent burn operative intervention with those who did not. The median age and the proportion of male to female patients were very similar between the two groups, but patients who had operative intervention presented later compared to those who did not. The etiology of burn injury was different between the two cohorts, as noted in Table 1. Among the operative group, flame burns were significantly more common at 81.8% (n=126) versus 62.5% (n=262, p<0.001). Scald burns were less common for operative patients (n=24, 15.6%) compared to non-operative patients (n=128, 30.6%). However, despite differences in burn etiology and mechanism, the %TBSA was very similar, with a median of 14–15% (IQR 7–20% and 9–30%, respectively) in both groups (p=0.2). Length of stay in the burn unit was significantly longer in the operative group with a median of 70 days (IQR 44–102) compared to just 12 days (IQR 5–30, p<0.001) in the non-operative group.
Table 1.
Characteristics of patients who underwent burn excision and grafting versus those who did not
| Operative Patients (n=154, 26.9%) | Non-Operative Patients (n=419, 73.1%) | p value | |
|---|---|---|---|
| Patient Age (years) | |||
| Median (IQR) | 30 (22–38) | 30 (23–43) | 0.3 |
| Gender: N (%) | |||
| Female | 59 (38.3) | 152 (36.3) | 0.7 |
| Male | 95 (61.7) | 267 (63.7) | |
| Missing | 0 (0.0) | 0 (0.0) | |
| Time to Presentation | |||
| 0–24 hours | 54 (35.1) | 254 (60.6) | <0.001 |
| 24–48 hours | 7 (4.5) | 33 (7.9) | |
| > 48 hours | 91 (59.1) | 120 (28.6) | |
| Missing | 2 (1.3) | 12 (2.9) | |
| Cause of Burn Injury: N (%) | |||
| Cooking Related | 21 (13.6) | 97 (23.2) | <0.001 |
| Clothes Caught Fire | 24 (15.6) | 50 (11.9) | |
| Fell into Fire | 79 (51.3) | 122 (29.2) | |
| House Fire | 4 (2.6) | 27 (6.4) | |
| Explosion | 4 (2.6) | 25 (6.0) | |
| Mob Justice | 3 (2.0) | 12 (2.9) | |
| Other | 19 (12.3) | 79 (18.9) | |
| Missing | 0 (0.0) | 7 (1.7) | |
| Type of Burn: N (%) | |||
| Scald Burn | 24 (15.6) | 128 (30.6) | <0.001 |
| Flame Burn | 126 (81.8) | 262 (62.5) | |
| Other | 4 (2.6) | 27 (6.4) | |
| Missing | 0 (0.0) | 2 (0.5) | |
| % Total Burn Surface Area (TBSA) | |||
| Burn | |||
| Median (IQR) | 14 (7–20) | 15 (9–30) | 0.2 |
| Length of Stay | |||
| Median (IQR) | 70 (44–102) | 12 (5–30) | <0.001 |
The median time to an operation was different between those with flame burns and those with non-flame burns at 18 days (IQR 9–38) and 11 days (IQR 5–19, p=0.008), respectively. Among the 154 patients who had excision and skin grafting, 63 (40.9%) only had excision or debridement at their first operation, while 82 (53.3%) also had skin grafting. 95 (62.9%) patients had more than one operation, with 46 patients (31.3%) having at least three separate procedures. Patients who required a second operation had a similar %TBSA compared to those who did not require a second procedure (12%, IQR 6–20) versus 14.5%, IQR 7–20, p=0.9). Patients requiring a second operation also had a similar proportion of flame burns at 84.2% (n=80) compared to 76.8% (n=43, p=0.3) in those patients who did not have a second procedure.
Crude mortality was markedly lower in the operative group at 3.3% (n=5) versus 27.9% (n=117, p<0.001) in the non-operative group with an unadjusted risk ratio of death of 0.11 (95% CI 0.05, 0.28, p<0.001) for the operative group. (Table 2) While burn depth was not available, the presence of flame burn served as a surrogate for burn depth given that flame burns are usually deeper than scald burns.[16] In testing, flame burn was determined to be a significant interaction term for our model (p=0.0002). Among patients with flame burn, crude mortality after an operation was 3.2% (n=4/126) compared to 39.7% (n=104/262, p<0.001) in those who did not have an operation. The difference in mortality was non-significant among non-flame burn patients who did and did not have an operative intervention at 3.6% (n=1/26) and 8.4% (n=13/138, p=0.7), respectively. The unadjusted risk ratio for mortality in patients having an operation after flame burn was 0.08 (95% CI 0.03, 0.21, p<0.001). The unadjusted risk ratio of mortality for patients with non-flame burn was not significant for those who underwent operative intervention. There was no benefit for patients who received excision only with no eventual skin grafting.
Table 2.
Clinical outcomes comparing patients who underwent burn excision and grafting versus those who did not.
| Operative Patients (n=154, 26.9%) | Non-Operative Patients (n=419, 73.1%) | p value | |
|---|---|---|---|
| Crude Mortality (In-hospital) | |||
| All Patients: N (%) | 5/154 (3.3) | 117/419 (27.9) | <0.001 |
| Patients with Flame Burn: N(%) | 4/126 (3.2) | 104/262 (39.7) | <0.001 |
| Patients with Non-Flame Burn: N(%) | 1/26 (3.6) | 13/138 (8.4) | 0.7 |
| Unadjusted Risk of Death | |||
| All Patients: Risk Ratio (95% CI) | 0.11 (0.05, 0.28) | -- | <0.001 |
| Patients with Flame Burn: Risk Ratio (95% CI) | 0.08 (0.03, 0.21) | <0.001 | |
| Patients with Non-Flame Burn: Risk Ratio (95% CI) | 0.43 (0.06, 3.17) | 0.4 | |
| Adjusted Risk of Death | |||
| Patients with Flame Burn: Risk Ratio (95% CI) | 0.16 (0.06, 0.42) | -- | <0.001 |
| Patients with Non-Flame Burn: Risk Ratio (95% CI) | 1.39 (0.13, 15.2) | -- | 0.8 |
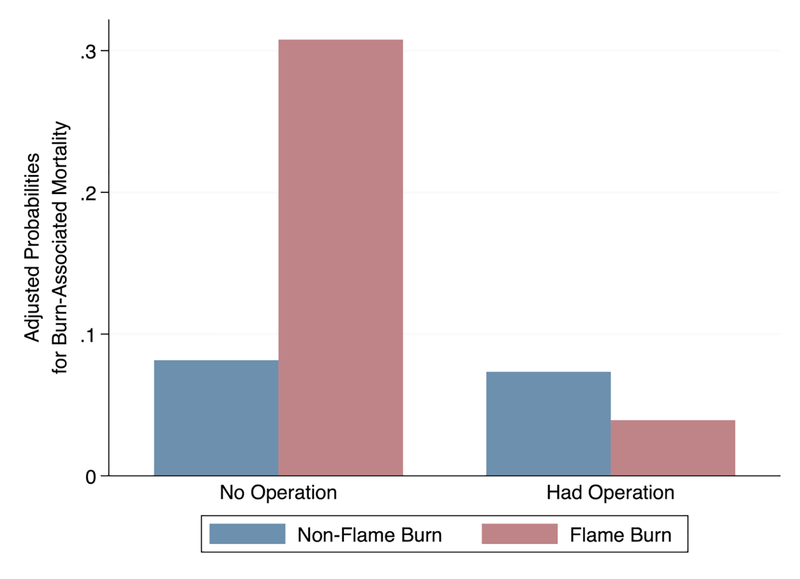
A modified Poisson model controlling for relevant confounders of burn-associated mortality showed an adjusted risk ratio of death after operative intervention for patients with flame burn to be 0.16 (95% CI 0.06, 0.42, p<0.001) when controlling for age, %TBSA, and time to presentation. For patients with a non-flame burn, the adjusted risk ratio was not significant. The adjusted predicted probability of death stratified by flame burn and surgical intervention is shown in Figure 1. The adjusted predicted probability of death after flame burn with surgical intervention was 0.04 (95% CI 0.01, 0.11) and 0.31 (95% CI 0.24, 0.39) without surgery. For those with non-flame burns, the adjusted predicted probability of death with surgery was 0.07 (95% CI 0.01–0.38) and 0.08 (95% CI 0.04, 0.16) without surgery. The median time to death for patients with flame burns who did not have surgery was 35 days (IQR 15–54 days) compared to 43.5 days (IQR 37–50 days, p=0.5) among patients with scald burns.
Figure 1.
The adjusted predicted probability of burn-associated mortality based on whether the patient underwent operative intervention, stratified by patients with and without flame burns.
Discussion
We report the first known description of the association between access to operative intervention and risk-adjusted clinical outcomes after burn injury in adult patients in sub-Saharan Africa. Overall crude mortality was very high at our center in Malawi, but there was a clear benefit to burn excision with grafting for patients after flame burn injury with a nearly 85% reduction in the adjusted risk of death. On the other hand, patients with non-flame burns did not have a significant mortality benefit after operative intervention. Excision without skin grafting had no mortality benefit for any group.
This study highlights the dearth of data available on adult burn outcomes in SSA. Most recent hospital-based data published from the region has focused on pediatric patients.[17–22] A recent systematic review of the epidemiology of burn injuries in the WHO Africa region found that young children were at the most risk, with a 2016 study calculating that over 80% of reported burns had occurred in children less than 10 years old.[6, 7] However, based on these studies, it is clear that adults are also at high risk despite the lack of available data, with similarly poor outcomes as children. In addition, as in non-burn related traumatic injury, young adults are often the victim. A 2013 study from Kenya found that adults were more likely to be involved in burn injury associated with kerosene stove explosions, a common mechanism associated with cooking.[23] In 2014, a population-based cross-sectional study from Sierra Leone showed that adults comprised at least half of the population that had suffered burns, and flame burns were more common among adults than children.[24] A similar study from Kenya determined that men were more likely to be hospitalized among adults, which is consistent with our study findings.[25] More data is needed on the epidemiology and outcomes of adult burn patients in SSA but the current literature suggests that burn centers must be prepared to treat adults, in addition to the large pediatric population, and adults may sustain deeper or more severe burn injuries.
The benefit of excision and skin grafting for deeper burns has been well described for both children and adults, with most data coming from high-income countries (HICs). Early excision of burn eschar helps prevent the release of inflammatory mediators and has been shown to reduce hospital length of stay, contracture, and organ failure, among other important clinical outcomes.[8, 26, 27] Unfortunately, the necessary surgical infrastructure to provide timely burn care is lacking in most LMICs with burn surgery substantially under-resourced and not prioritized in most countries in SSA. [28–31] In 2014, a systematic review of burn management capacity in LMICs demonstrated that only about a third of hospitals were able to provide skin grafting or treat common burn complications.[12] Even when hospitals have the capability of offering these services, resources are limited, and not all patients have access. This is exhibited in our center as only a third of patients had an operative intervention, and there was a substantial delay for those who did. Consequently, access to surgery is often very limited for burn patients who may benefit from intervention, resulting in many patients being treated with wound care alone, increasing the hospital length of stay, and a delay in wound healing.[32, 33] Our study also shows that patients with flame burns, which are more likely to be deeper compared to scald burns, benefited significantly from surgical intervention. The lack of mortality benefit for patients with scald burns may demonstrate that a wound care approach is more reasonable for patients with more superficial burns, although long-term outcomes are unknown. Additionally, patients who had excision and debridement either cohort did not have a mortality benefit suggesting that in an environment where temporary biological coverings are not available, skin grafting is necessary for wound cover to prevent complications such as sepsis and fluid loss.
Our study provides evidence that improvement in surgical infrastructure must be a priority in any strategy targeting victims of burn injury, especially those with deeper burns. Patients in our study clearly had access to burn care because they were able to reach a burn center and were admitted for burn care. Unfortunately, in our center and in many throughout the region, access to a burn unit does not guarantee access to operative intervention. It is much easier to admit a patient to the burn center than to organize the staffing and equipment needed to proceed with an operation. In a setting with severe resource limitations, even a few cases can be challenging to coordinate and complete, despite the clear survival advantaged afforded to patients whose burn was excised and grafted. Patients are triaged for surgery based on a number of factors including nutritional status, clinical stability, and the timing of when the operating room is actually available. We plan to use this study to improve patient selection for operative intervention and advocate for greater operating room access from the hospital administration.
Of course, the task of providing reliable surgical access to burn patients is only a part of the challenge. Burn management necessitates a comprehensive clinical strategy that includes initial resuscitation, intensive wound care, and nutrition optimization, in addition to resources needed for safe operative intervention. Each step requires skilled nurses, surgeons, wound care supplies, and sufficient space to treat patients with appropriate isolation.[34] Early excision may be detrimental to patients without this infrastructure as they require adequate preoperative resuscitation and available robust transfusion service.[35] Our study also shows that patients who presented later to the burn center had better outcomes. This may represent a patient selection bias, whereas patients who are very sick are unable to make it to the hospital or are triaged out of surgery. It is also possible that some very sick patients who were able to reach the burn center with flame burns were triaged out of surgery in favor of others who were more stable. However, given that the median time of death for patients with flame burns who did not have an operation was over four weeks, this seems less likely with the data suggesting that most of these people suffered a later death, not an early one. So, while improvements in burn care must include greater access to the operating room for patients, there must be parallel enhancements in perioperative care, including monitored resuscitation, anesthesia support, and targeted transfusion when needed.[36]
Another implication of our study is that resource optimization at centers in LMICs is imperative to improve patient outcomes. Specific patient populations clearly benefited more from surgery than others. Consequently, burn surgeons must be thoughtful in triaging patients who would most benefit from excision and grafting, prioritizing those with deeper wounds. Burn care is expensive, and costs increase with larger burns, so optimal utilization of available resources is imperative to improve burn-associated mortality.[34] This will require careful deliberation on ethical frameworks to help surgeons make difficult decisions on patient selection in resource-limited environments.[37]
Outside of improving surgical access for burn patients, burn injury prevention programs are also essential. While a review of prevention efforts is outside the scope of this study, most published programs have focused on improving safety for children. However, evidence suggests that efforts focusing on both adults and children are beneficial and may have a more significant impact.[38, 39]
Our study is limited by only including patients that survived long enough to present to the hospital. Consequently, patients in our catchment area with substantial burn injury may not have been captured in our data if they were too sick to survive until evaluation. We have attempted to mitigate our retrospective methodology by controlling for risk factors commonly identified as contributing to mortality such as age and %TBSA. Our study also lacks more detailed information on common outcomes associated with burn injuries such as pneumonia, acute kidney injury, and sepsis. Unfortunately, due to severe resource constraints, these data are not available.
Conclusion
In a resource-limiting setting, access to the operating room is inadequate, and burn patients are not prioritized. While many scald burn patients may be managed with wound care alone, patients with flame burn require surgical intervention to improve clinical outcomes. Burn injury in this region continues to confer a high risk of mortality and more investment in operative capacity is imperative.
Acknowledgements
Study data were collected and managed using REDCap electronic data capture tools hosted at UNC. REDCap (Research Electronic Data Capture) is a secure, web-based application designed to support data capture for research studies, providing: (1) an intuitive interface for validated data entry; (2) audit trails for tracking data manipulation and export procedures; (3) automated export procedures for seamless data downloads to common statistical packages; and (4) procedures for importing data from external sources.
Financial Support
Financial support was provided by the North Carolina Jaycee Burn Center in the Department of Surgery at the University of North Carolina for all aspects of the study including: design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Conflict of Interest
The authors have no conflict of interest to disclose. The authors have no financial relationships to disclose.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Peck MD (2011) Epidemiology of burns throughout the world. Part I: Distribution and risk factors. Burns 37(7):1087–1100. [DOI] [PubMed] [Google Scholar]
- 2.Mock CPM, Peden M, Krug E, eds (2008) A WHO plan for burn prevention and care. World Health Organization. [Google Scholar]
- 3.Ahuja RB, Bhattacharya S (2004) Burns in the developing world and burn disasters. Bmj 329(7463):447–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stokes M, Johnson W (2017) Burns in the Third World: an unmet need. Ann Burns Fire Disasters 30(4):243. [PMC free article] [PubMed] [Google Scholar]
- 5.Charles AG, Gallaher J, Cairns BA (2017) Burn Care in Low- and Middle-Income Countries. Clin Plast Surg 44(3):479–483. [DOI] [PubMed] [Google Scholar]
- 6.Nthumba PM (2016) Burns in sub-Saharan Africa: a review. Burns 42(2):258–266. [DOI] [PubMed] [Google Scholar]
- 7.Rybarczyk MM, Schafer JM, Elm CM, et al. (2017) A systematic review of burn injuries in low- and middle-income countries: epidemiology in the WHO-defined African Region. Afr J Emerg Med 7(1):30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ong YS, Samuel M, Song C (2006) Meta-analysis of early excision of burns. Burns 32(2):145–150. [DOI] [PubMed] [Google Scholar]
- 9.Janzekovic Z (1970) A new concept in the early excision and immediate grafting of burns. J Trauma 10(12):1103–1108. [PubMed] [Google Scholar]
- 10.Nguyen TT, Gilpin DA, Meyer NA, et al. (1996) Current treatment of severely burned patients. Ann Surgery 223(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph K, Trehan A, Cherian M, et al. (2016) Assessment of acute burn management in 32 low- and middle-income countries. World J Surg 40(4):791–800. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Wong EG, Mahmood U, et al. (2014) Burn management capacity in low and middle-income countries: A systematic review of 458 hospitals across 14 countries. Int J Surg 12(10):1070–1073. [DOI] [PubMed] [Google Scholar]
- 13.Kiser M, Beijer G, Mjuweni S, et al. (2013) Photographic assessment of burn wounds: a simple strategy in a resource-poor setting. Burns 39(1):155–161. [DOI] [PubMed] [Google Scholar]
- 14.Zou G (2004) A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology 159(7):702–706. [DOI] [PubMed] [Google Scholar]
- 15.Chen W, Qian L, Shi J, et al. (2018) Comparing performance between log-binomial and robust Poisson regression models for estimating risk ratios under model misspecification. BMC medical research methodology 18(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hettiaratchy S, Dziewulski P (2004) Pathophysiology and types of burns. Bmj 328(7453):1427–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallaher JR, Banda W, Lachiewicz AM, et al. (2018) Colonization with Multidrug-Resistant Enterobacteriaceae is Associated with Increased Mortality Following Burn Injury in Sub-Saharan Africa. World J Surg 42(10):3089–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chelidze KI, Lim CC, Peck RN, et al. (2016) Predictors of mortality among pediatric burn patients in East Africa. J Burn Care Res 37(2):e154–e160. [DOI] [PubMed] [Google Scholar]
- 19.Parizh D, Kuijs A, Nkumbi U, et al. (2018) A Pediatric Burn Unit in Sub-Saharan Africa. J Burn Care Res 39(suppl_1):S206–S206. [Google Scholar]
- 20.Grudziak J, Snock C, Mjuweni S, et al. (2017) The effect of pre-existing malnutrition on pediatric burn mortality in a sub-Saharan African burn unit. Burns 43(7):1486–1492. [DOI] [PubMed] [Google Scholar]
- 21.Mutiso V, Khainga S, Muoki A, et al. (2014) Epidemiology of burns in patients aged 0–13 years at a paediatric hospital in Kenya. East and Central African Journal of Surgery 19(3):12–21. [Google Scholar]
- 22.Karan A, Amado V, Vitorino P, et al. (2015) Evaluating the socioeconomic and cultural factors associated with pediatric burn injuries in Maputo, Mozambique. Pediatr Surg Int 31(11):1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ombati AN, Ndaguatha PL, Wanjeri JK (2013) Risk factors for kerosene stove explosion burns seen at Kenyatta National Hospital in Kenya. Burns 39(3):501–506. [DOI] [PubMed] [Google Scholar]
- 24.Wong EG, Groen RS, Kamara TB, Stewart K-A, Cassidy LD, Samai M, Kushner AL, Wren SM: Burns in Sierra Leone: a population-based assessment. Burns 2014, 40(8):1748–1753. [DOI] [PubMed] [Google Scholar]
- 25.Wong JM, Nyachieo DO, Benzekri NA, et al. (2014) Sustained high incidence of injuries from burns in a densely populated urban slum in Kenya: an emerging public health priority. Burns 40(6):1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hultman CS, Cairns BA, deSerres S, et al. (1995) Early, complete burn wound excision partially restores cytotoxic T lymphocyte function. Surgery 118(2):421–430. [DOI] [PubMed] [Google Scholar]
- 27.Rousseau A-F, Massion PB, Laungani A (2014) Toward targeted early burn care: lessons from a European survey. J Burn Care Res 35(4):e234–e239. [DOI] [PubMed] [Google Scholar]
- 28.Qureshi JS, Samuel J, Lee C, et al. (2011) Surgery and global public health: the UNC-Malawi surgical initiative as a model for sustainable collaboration. World J Surg 2011, 35(1):17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gutnik LA, Dielman J, Dare AJ, e tal (2015) Funding flows to global surgery: an analysis of contributions from the USA. Lancet 385:S51. [DOI] [PubMed] [Google Scholar]
- 30.Ozgediz D, Riviello R (2008) The “other” neglected diseases in global public health: surgical conditions in sub-Saharan Africa. PLoS Med 5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meara JG, Leather AJ, Hagander L, et al. (2015) Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet 386(9993):569–624. [DOI] [PubMed] [Google Scholar]
- 32.Puri V, Khare NA, Chandramouli M, et al. (2016) Comparative analysis of early excision and grafting vs delayed grafting in burn patients in a developing country. J Burn Care Res 37(5):278–282. [DOI] [PubMed] [Google Scholar]
- 33.Botman M, Beijneveld J, Negenborn V, et al. (2019) Surgical Burn Care in sub-Saharan Africa: A Systematic Review. Burns Open July 23. [Google Scholar]
- 34.Gallaher JR, Mjuweni S, Cairns BA et al. (2015) Burn care delivery in a sub-Saharan African unit: a cost analysis study. Int J Surg 19:116–120. [DOI] [PubMed] [Google Scholar]
- 35.Gallaher JR, Mjuweni S, Shah M, et al. (2015) Timing of early excision and grafting following burn in sub-Saharan Africa. Burns 41(6):1353–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gallaher JR, Molyneux E, Charles AG (2016) Sub-Saharan African hospitals have a unique opportunity to address intentional injury to children. Afr J Emerg Med 6(2):59–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wall S, Allorto N, Weale R (2018) Ethics of burn wound care in a low-middle income country. AMA journal of ethics 20(6):575–580. [DOI] [PubMed] [Google Scholar]
- 38.Scheven D, Barker P, Govindasamy J (2012) Burns in rural Kwa-Zulu Natal: Epidemiology and the need for community health education. Burns 38(8):1224–1230. [DOI] [PubMed] [Google Scholar]
- 39.Rybarczyk MM, Schafer JM, Elm CM, et al. (2016) Prevention of burn injuries in low-and middle-income countries: a systematic review. Burns 42(6):1183–1192. [DOI] [PubMed] [Google Scholar]